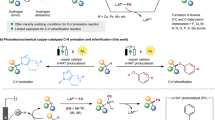
Abstract
Non-metallic catalysis has been known as a remarkable development strategy for hydrofunctionalization of unsaturated hydrocarbons. Herein, we report a unique chemically active method of BF3·OEt2 promoted multi-component, highly regioselective, and chemoselective hydrothio(seleo)phosphonylation of unsaturated hydrocarbons, which exhibits high yield and good substrate universality. The reaction mechanism was further elucidated to be Markovnikov addition by controlling experiments, 31P and 19F NMR spectra tracking experiments, X-ray diffraction analysis, and DFT calculations. Furthermore, the gram-scale attempt and the application of the reaction on the derivatization of natural products have been successfully conducted, leading to the discovery of 3as with potential anti-Parkinson's disease (PD) activities at 1 μM. This streamlined and efficient methodology has established a new platform for non-metallic Lewis acids-promoted hydrofunctionalization of unsaturated hydrocarbons and its application on new drug research.

Similar content being viewed by others
Introduction
Unsaturated hydrocarbons are abundant in the natural environment and play an important role in chemical production and organic synthesis1,2,3,4,5,6,7,8. In recent years, the hydrofunctionalization of unsaturated hydrocarbons has continued to develop rapidly, and different types of hydrogenation reagents have been applied to this field, such as S-H, P-H, Si-H, N-H, and B-H. The direct coupling of E-H (E=P, S, B, Si, Se, N, etc.)9,10,11,12,13,14,15,16 with unsaturated hydrocarbons to produce C-E compounds is more in line with the requirements of “efficient and atomically economical synthetic chemistry”17 than the conventional synthetic methods. Carbon heteroatoms play important roles in organic chemistry, medicinal chemistry, and life sciences. The presence of these carbon heteroatoms gives organic molecules diversity and complexity, thereby influencing the properties and functions of molecules. In medicinal chemistry, carbon heteroatoms can affect the activity, selectivity, and efficacy of drug molecules. In life sciences, carbon heteroatoms also play crucial roles, influencing the structure, function, and interactions of biological molecules. For example, phosphorus is an essential element in genetic material and it is also widely used in anti-cancer, anti-virus, and anti-inflammatory fields, of which, dexamethasone sodium phosphate is a famous P-containing drug18 (Scheme 1a). Sulfur is also widely present in various drugs, such as the marine-derived anti-tumor drug19 Lubitidine (Scheme 1a). Selenium is a necessary trace element in the human body with antioxidant functions, regulation of thyroid hormone function, immune function, and prevention of cancer20 (Scheme 1a). Se element has important medicinal value, but there are few selenium-containing pharmaceuticals on the market, mainly because there are relatively few strategies for the construction of C-Se bonds and it is difficult to synthesize. Therefore, it is particularly important to enrich and develop C-Se construction methods. Addition reaction is an efficient way to construct carbon heteroatoms21, which are usually achieved with simple organic acids (e.g., hydrochloric acid, sulfuric acid), but they are relatively difficult for the synthesis of highly regioselective, highly chemoselective C-E compounds with a single conformation. Transition metal catalysis9,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 usually effectively controls the selectivity of these reactions (Scheme 1b). Transition metal catalysts can often catalyze a wide range of reactions, and with their unique spatial configuration, they can efficiently control regioselectivity, stereoselectivity, and chemoselectivity, allowing for the formation of specific products36,37,38. At the same time, numerous scholars39,40,41 are also facing and attempting to address challenges such as the initiation of free radical reactions relying mainly on flammable and explosive peroxides and azo compounds, the preparation of complex metal catalysts in coordination chemistry, and the preparation and screening of complex ligands. Based on the above situation, de-metalization of highly selective constructed carbon heteroatoms has become the focus of our attention.
Given the challenges associated with transition metal catalysis, how to find a “one stone two birds” (more efficient42,43 and simpler44,45) way to achieve highly selective carbon-heteroatom bonds formation of unsaturated hydrocarbons has become the focus of attention in the organic chemistry community. In fact, compared to transition metals, promoting carbon-heteroatom bond formation by non-metallic elements is still relatively slow in organic synthesis46. However, this also precisely provides a great development space for the formation of research ideas on the selective realization of hydrofunctionalization reactions of alkynes and olefins by non-metallic elements47,48,49,50,51,52,53,54,55. The methodological strategies such as high chemoselectivity and high regioselectivity of unsaturated hydrocarbons for multi-component reactions (Scheme 1c) with non-metallic elemental promoters can well demonstrate the charm of atomic efficient and simple chemistry and can solve some challenges accompanying transition metal catalysis. In this work, “one stone four birds” becomes a reality: high regioselectivity, chemoselectivity, multicomponent reaction, and Markovnikov additions. Under the same Lewis acid promoter (BF3·OEt2) conditions, a simple and efficient synthetic strategy for constructing P-S-C and P-Se-C bonds was developed. Some natural products were derivatized, especially marine natural product derivatives, which exhibited potential anti-Parkinson's disease activity.
Results and discussion
Reaction development
To validate the above ideas, phenylacetylene (1a), sulfur powder (S8), and diphenylphosphine oxide (2a) were chosen as the model substrates, and the commercially available BF3·OEt2 was used as a regulator. Triethylamine was added as the base, which had been an efficient promotor in the formation of S=P-OH reagent (Table 1, entry 2). To our delight, the desired Markov product 3a was obtained in a tractable yield (78%, Table 1, entry 3). This result encouraged us to systematically investigate the interesting reaction. Extensive reaction condition screening, including solvents, various bases, and the amount of regulators, was conducted. Intriguingly, when the reaction was performed in toluene at 90 oC, the desired Markovnikov product, 3a, was exclusively obtained in 91% yield (Table 1, entry 1). The experiments have shown that the solvent considerably influences the efficiency of this transformation (Table 1, entries 3–6). From entries 7-11, it can be seen that different temperatures and bases have a great effect on the reaction. BF3·2MeOH and BF3·2AcOH do not work in the reaction due to the complexation of boron trifluoride with a large polar solvent. 0.5 equiv (eq.) of BF3·Et2O will complex with triethylamine and will not promote the reaction. 2.0 eq. of BF3·Et2O allows the ether to react preferentially with the P reagent, resulting in the formation of by-products. 1.0 eq. of BF3·OEt2 was screened as the optimal dosage. After the extensive screening of reaction factors, we found that treatment of 1a, S8, and 2a in the presence of BF3·OEt2 as regulator and triethylamine as the base in toluene at 90 °C afforded the desired Markovnikov product, 3a, in 91% yield with high α-regioselectivity.
Evaluation of substrate scope
With the optimal reaction conditions in hand, a variety of different aromatic terminal alkyne substrates, P(O)-H reagents were tested and afforded corresponding Markovnikov products in moderate to good yields and excellent regioselectivity. As shown in Fig. 1, the transformation system was applicable to various terminal alkynes. Aromatic alkynes, bearing various functional groups at the para position including halides (F, Cl, Br), n-butyl, methoxy, and tert-butyl, reacted smoothly with sulfur powder and diphenylphosphine oxide to give the desired products (3a-3g). Interestingly, aromatic alkynes with substituents at the meta-position also proceeded smoothly to afford the α-vinyl product in moderate to good yields (3h-3k).
More importantly, the reaction is compatible with amides giving the corresponding products (3i and 3j), which are difficult to synthesize by transition metal catalysis owing to the coordination of amino group to metals. This feature can be introduced to the synthesis of useful compounds bearing a coordination group. Notably, 1-ethynylnaphthalene and heteroaromatic alkyne were also efficient coupling partners, delivering 3l and 3m in 80% and 83% yields, respectively. In addition, the substrate’s scope of P(O)-H reagents was evaluated. Notably, 9,10-Dihydro-9-oxa-10-phosphaphenanthrene 10-oxide (DOPO), being widely used in flame retardant materials, was well suited to this reaction (3n-3p) and gave moderate yields (47–74%). To further explore the broad applicability of the methodology, the sulfur powder was replaced with selenium powder to evaluate the reaction system. As shown in Fig. 1, aromatic alkynes with substituents at the para- and meta-positions (3t-3y), naphthalene (3z), heteroaromatic alkyne (3aa) were found to be suitable for the transformation and gave the α-vinyl compounds in moderate yields (See SI for method optimization). Remarkably, 3ab and 3ac were also successfully prepared by P(O)-H reagent. Finally, it is noteworthy that the yields of all the selenium target compounds decreased because of the selenium atomic large π electron space56. It is more interesting to note that in the P-Se-C series of compounds, the small symmetric satellite peaks on both sides of the main peak of the 31P NMR spectrum may be produced by the coupling of the P and Se atoms.
As can be seen from Fig. 1, a series of vinyl thi(selen)ophosphonates were synthesized by multi-component Markovnikov addition reaction in highly regioselective. However, aromatic terminal alkenes were not suitable for this reaction system. Furthermore, it was found that triethylamine inhibited the catalytic activity of BF3·OEt2 in the transformation, and it promoted the reaction of S8 with P-H reagents. After X-ray single-crystal diffraction (XRD) and density functional theory (DFT) calculation studies, the structure of 2d was determined. The results show a P=S double bond instead of a P-S single bond (Scheme 3b), which is quite different from the previously reported structures57 (O=P-SH). Next, two components of 1a with 2d in the absence of base conditions were tested. To our delight, high regioselective Markovnikov product 3a complexed with BF3·OEt2 (3A, 96% yield) was obtained in a short time. This structure was further confirmed by XRD analysis (Scheme 2).
Phenylacetylene (1.2 mmol, 122.6 mg), 2d (1.0 mmol, 234.3 mg) and toluene (5 mL) were added to the tube and sealed. Then BF3·OEt2 (1.0 mmol, 265 μL) was injected into the sealed tube by micro-syringe. After reacting at 90 °C for 5 min, the reaction solution was warmed to room temperature, and the mixture was left to stand overnight at 0 °C. A large amount of colorless transparent solid precipitated. A small portion of the solid is taken out for X-ray single crystal diffraction analysis.
Then the substrate scope with regard to phosphate ester, and aromatic terminal alkenes was evaluated. As shown in Fig. 2, the new two-component Markovnikov addition protocol was applicable to a series of phosphonic acids, giving the Markovnikov products efficiently (3ad, 3ae, 3af). It is noteworthy that the yields of target compounds (3n, 3t) were greatly improved compared to the multi-component protocol. Most importantly, the substrates of terminal alkenes also proceeded smoothly to afford the Markovnikov products in excellent yields (3ag-3ak).
In the light of the excellent reactivity of BF3·OEt2 in promoting the reaction of internal alkynes and terminal olefins with S=P-OH reagent, our investigation was further extended to the selective reaction of unsaturated hydrocarbons with S=P-OH reagent. A comparative study revealed that the substrate has both endo-alkyne and terminal olefins fragments, and the S=P-OH reagent can be highly selective for Markov addition reactions with terminal olefins, yielding products in 53–83% (3am-3aq). As shown in Fig. 3, endoalkenes, terminal olefins, and terminal olefins fragments exist in the same structure, and terminal olefins show good chemical activity and give good yields. This method is also applicable to different S=P-OH reagents such as phosphorothioates.
Based on the above mentioned results, we further investigated the reaction of internal alkynes with S=P-OH reagents to discover whether excellent cis-trans isomerization ratios exist. By further optimization of the reaction conditions, compounds with high regioselectivity were obtained in 33–90% yields (Fig. 4). Significantly, it was found that the method could produce compounds with high regioselectivity 5c with E/Z ratios up to 95:5. As can be seen from Fig. 4, for the internal alkynes, E/Z ratios were generally above 91:9. While for the aromatic-alkyne-alkane substrates, the E/Z ratio was only 79:21, such as compound 5e, and the spatial site resistance may be the main reason for the reduced ratio. In the presence of Lewis acid, BF3 at high temperature and under prolonged conditions promotes the occurrence of side reactions of ether with S=P-OH and esterification of S=P-OH with the product, resulting in degradation of the product and a decrease in the raw material of S=P-OH, and thus low yields, but this does not affect the high chemoselectivity and regioselectivity of the reaction. The E/Z ratio was not detected in compound 5f, perhaps it was the non-equivalent proportional degradation that rendered it undetectable.
Investigations of mechanism
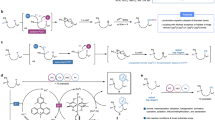
In order to better elucidate the unique chemical properties of the method, a series of experiments have been conducted to investigate the mechanism. As shown in Schemes 3, 3a was not detected in three-component reaction without triethylamine (Scheme 3a). S=P-OH reagent 2d was readily available in the presence of triethylamine for 1 h (Scheme 3b) and this structure was confirmed by XRD. The single-crystal data show an O-H bond length of 0.83995 Å and a S-H bond length of 2.95749 Å, which clearly confirms compound 2d. The two components reaction of 1a and 2d proceeded smoothly to reach almost 100% conversion in just 30 min with 96% yield of 3A (Scheme 2) in the presence of BF3·OEt2 in toluene at 90 °C. By growing a single crystal, the product was confirmed to be a complex of 3a with BF3·OEt2. In Schemes 3c, 2d and BF3·OEt2 dissolved in toluene, slowly adding n-hexane can precipitate complex 2D. We got the solid complex and characterized the compound with 1H NMR, 13C NMR, 31P NMR, and 19F NMR spectra (see SI), meanwhile, in the DFT calculations, we found that the compound 2D formed by this complexation mode is the lowest energy (species Int_1 in Fig. 6). Deuterium substitution experiments show that hydrogen is derived from the S=P-OH reagent (Scheme 3e).To investigate the turnover limiting step, we also conducted a kinetic isotope effect (KIE) study using an internal competition experiment (Scheme 3f). The isotope labeling experiment showed a high value of kinetic isotope effect (KIE = 2.66). Such a high value of KIE unambiguously established the reaction as the first-order kinetic isotope effect, and this step is considered to be the rate-limiting step of the whole reaction. Taking the aforementioned considerations into account, we anticipated that the conversion of terminal alkyne bond from sp to sp2 hybridization change would likely be the rate-limiting step of the whole reaction, and this conclusion will also be verified in DFT calculations (Fig. 6).
To shed more light on the role of BF3·OEt2, 31P NMR and 19F NMR experiments were carried out to trace the standard reaction. In the 31P NMR spectrum (Fig. 5), a singlet at δ = 74.4 ppm is observed which is assigned to 2d in toluene. As expected, the 31P NMR chemical shift associated with the 2d changed significantly as a function of added BF3·OEt2. And no 31P NMR chemical shift was found, which response fits well with a 1:1 binding model. 3A chemical shift of δ = 55.4 ppm is observed for P2+1a as compared to that of δ = 58.6 ppm (2D) of P1+BF3·OEt2. We could also observe two new phosphorus signals accompanied with the addition of methanol, which matches the signals of the free products 2d (δ = 70.5 ppm) and 3a (δ = 42.2 ppm). This is presumably because of the competitive complexation of methanol to boron and the release of 2d and 3a. The 19F NMR tracking experiment also gave similar results. Therefore, we speculate that BF3·OEt2 may undergo a complexation-activation in this reaction system.
(P1) and (F1): The spectra of 2d and BF3·OEt2 in toluene, respectively. (P2) and (F2): The spectra of the product obtained by adding BF3·OEt2 in 2d in toluene, respectively. (P3) and (F3): The spectra of the product obtained by adding 1a in the product of (P2)/(F2), respectively. (P4) and (F4): The spectra of the product obtained by adding CH3OH in the product of (P3)/(F3), respectively.
To explore the reaction mechanism and to understand the course of the selective reaction, DFT calculations58 were performed to probe the reaction between 2d and 1a promoted by BF3·OEt2 at the ωB97X-D(SMD)/def2-TZVPP//B3LYP-D3BJ(SMD)/6-31G(d,p) level59,60,61,62,63 of theory in toluene, molecular graphics have been produced with CYLview64 (Fig. 6). The lowest-lying reaction free energy paths (at 298K and 1 mol/L reference state) are shown in Fig. 6. In the first stage, 2d and 2d' are resonance structures, and the Gibbs free energy of 2d' is 5.2 kcal/mol above 2d, which allows for rapid transformations in the reaction system. 2d' substitutes the ether and complexes with boron trifluoride to produce intermediate Int_1 by TS_1, with activation barrier 15.2 kcal/mol. By isolated characterization of intermediate Int_1, specifically, the change from compound 2d (δ = 77.4 ppm) to intermediate Int_1 (δ = 58.6 ppm) is observed in 31P NMR, and this solid complex also characterized with 1H NMR, 13C NMR, and 19F NMR spectra. Then, the proton transfers through the S-H bond of Int_1 to the phenylethylene terminus to form the intermediate Int_265 (with free energy 24.5 kcal/mol above 2d) with high selectivity by TS_2, which is the biggest energy barrier in this reaction. This data further confirmed that the conversion of the hybridization orbital from sp to sp2 is the rate-limiting step of the whole reaction. In the meantime, NCI analysis66,67,68 explains that weak interaction between benzene rings stabilizes the structure of TS_2, and interaction between benzene rings and H-S hydrogen bonding stabilizes the structure of Int_2 (Fig. 7A). The high regioselectivity is mainly due to the stabilization of the cationic intermediate at the α-position, in contrast, DFT calculations show that the cationic intermediate at the β-position cannot exist stably. However, the calculation gives the result that dissociation of intermediate Int_2 requires a very high energy barrier, which is obviously more difficult to achieve in this reaction system. Int_2 is easy to react with each other in solution to form product 3A. In addition, the presence of trace amounts of A+ or B− will also lead to the rapid production of 3A from Int_2, and 3A has the lowest thermodynamic energy in the whole reaction.
Similarly, we performed a DFT calculation study using 2d and 1ag as model substrates (Fig. 7B) and gave essentially similar results. The sulfur negative ion attacks the α-site carbon positive ion, and the target compounds 3AG and 3AG' can be obtained. The calculations showed that the generation of 3AG was easier compared to 3AG' with a difference of 0.3 kcal/mol, and this finding was further verified by 13C-NMR. In addition, we note that styrene (1ag) has a lower reaction energy barrier for the reaction with 2d than phenylethynyl (1a) (21.1 kcal/mol vs 24.5 kcal/mol), this means that olefins substrate has better reactivity.
Applied research on the late-stage functionalization
To demonstrate the applicability of this methodology the hydrofunctionalization of S=P-OH regents with olefins/alkynes, and considering the potential application of vinyl thi(selen)ophosphonates, the phenylacetylene reaction was scaled up to nearly 17 times. The procedure proceeded smoothly, offering the desired product 3a in excellent yield (94% yield, Scheme 4), which laid a good foundation for the application of these compounds. For substrates containing acryl ketone fragments, we can obtain antimartensitic thiophosphonates in extremely excellent yields under mild conditions.
In addition, polybrominated diphenyl ether (PBDE) analogs of marine origin, steroids, and natural products Lonone and Carvone have been applied to this protocol (Fig. 8). According to the above established methodology, the PBDE thiophosphorylated derivative 3as could be obtained in 76% yield. For modification of steroidal derivatives, the target product was obtained in the antimartensitic position due to the presence of a strong electron-withdrawing group carbonyl. Particularly for the endoene steroid substrates we could obtain compound 3at in up to 95% yield, and similarly for the remaining different types of S=P-OH reagents, compounds 3au and 3av, respectively. For the sterols containing acrylate structures, the product (3ax-3az) yield was in the range of 45–60%, a yield which is perhaps very much related to the presence of the ester bond. The natural product, Lonone, could be similarly derivatized to give compound 3aw in moderate yield (53%). Notably, for watercress ketone, the martensitic position gave the quaternary carbon-derivatives 3ba-3bc good yields in the absence of the aromatic ring.
In order to explore the pharmacological value of these natural product derivatives, we have conducted biological activity studies on some of the synthesized natural product derivatives. PBDE derivatives of marine origin have potential applications in cerebral neurological disorders, and in recent years, our group69 has reported relevant studies in this area. In view of the fact that 3as also contains its similar structure, we have studied this compound for treating brain neurodegenerative diseases, such as Parkinson’s disease (PD), which is the second most common neurodegenerative disease after Alzheimer's disease (AD). Current treatments for PD mainly focus on improving the symptoms such as movement disorders, and cannot block the development of the disease. There is an urgent need to find effective drugs to reduce the loss of dopaminergic neuron degeneration. Here, we further explored the anti-PD activities of 1as, 3as, 3asR, and 3asS (3asR and 3asS are a pair of enantiomers, which have been separated from 3as). MPP+-induced neuronal damage model has been widely used in PD research because it elicits a severe PD-like syndrome characterized by elevation of intracellular reactive oxygen species level and apoptotic death70,71. N-acetylcysteine (NAC) or Paeoniflorin (PF), which act as antioxidants were used as positive controls. NAC at 1 mM concentration and PF at 5 μM concentration attenuated the MPP+-induced SH-SY5Y apoptotic cells per mm2 from 53.48 (±5.48 SEM) to 21.67 (±1.57 SEM) and 29.86 (±3.52 SEM), respectively. To our surprise, the original compound 1as had no protective effect (Fig. 9A, B), whereas 3as, 3asR, and 3asS showed much better decreased MPP+-induced neuronal apoptosis than the positive control, since their effects at 1 μM are comparable as NAC at 1 mM and PF at 5 μM. Statistically, 3as, 3asR, and 3asS reduced significantly apoptotic cells per mm2 compared with the MPP+ group, from 53.48 (±5.48 SEM) to 34.90 (±3.84 SEM), 33.39 (±2.55 SEM), 23.62 (±2.34 SEM), respectively, at 1 μM concentration (Fig. 9A, B). Such intriguing findings inspired us to explore the mechanism of action of 3as against mitochondrial apoptotic pathway. Apoptotic marker proteins Bax, Cytochrome C, Cleaved caspase-3, and Caspase-3 were detected by western blotting, and the results indicated that 3as significantly reversed the apoptotic protein levels up-regulated by MPP+ at 1 μM (Fig. 9C–F, The uncropped blot images could be found in SI). 3as could probably suppress the MPP+-induced SH-SY5Y mitochondrial apoptotic pathway (Fig. 10). This study provides evidence of supporting 3as as a potential therapeutic agent for the treatment of PD, and meanwhile indicates that the late-stage functionalization can sometimes lead to the discovery of new bioactive compounds.
A TUNEL staining images in MPP+-induced SH-SY5Y cells treated with 1as, 3as, 3asR, 3asS, NAC, PF, scale bar = 100 μm. Blue: DAPI, staining nuclei; Green: TUNEL-positive nucleus. B Statistics of MPP+-induced apoptotic cells treated with the compounds. C Representative images of Bax/Cytochrome C/Cleaved caspase-3/Caspase-3 immunoblot detection in the MPP+-induced SH-SY5Y treated with 3as. Quantification of (D) Bax/β-actin, (E) Cytochrome C/β-actin and (F) Cleaved caspase-3/β-actin ratio normalized to that of the Ctrl group. Data are mean ± SEM, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, as determined by Student's t test.
Upon MPP+ stimuli, pro-apoptotic proteins Bax are activated at the mitochondrial outer membrane to mediate its permeabilization, leading to release of Cytochrome C into the cytoplasm to activate caspases. Apoptotic marker proteins Bax, Cytochrome C, and Cleaved caspase-3 were downregulated by 3as in the MPP+-induced cells.
In summary, the first example of a nonmetallic Lewis acid-promoted hydrothio(selen)ophosphonylation of olefins/alkynes has been developed and studied in detail. A controlled three-component P-H, sulfur powder, and terminal alkynes/olefins with chemoselective and regioselective approach have been established. This strategy was simultaneously applied to the addition of internal alkynes, showing good regioselectivity. The desired products with excellent yields and selectivity were promoted with non-metallic Lewis acid BF3·OEt2. Moreover, the insertion of sulfur powder into P-H, the conversion of hydrogen ions, the complexation of 2D, and the generation of complexation end products were further elucidated by 31P NMR and 19F NMR spectrum tracking experiments, control experiments, XRD experiments, and DFT calculations. Finally, this intriguing methodology has been successfully applied for the late-stage functionalization of several natural products, such as PBDE, Steroids, Carvone, and Lonone derivatives. Specifically, we found that 3as attenuated MPP+-induced SH-SY5Y cell damage by inhibiting apoptosis through our anti-Parkinson’s disease study, which better illustrates the value of the application of synthetic chemistry. We expect the findings reported herein to contribute to the development of hydrofunctionalization with non-metallic Lewis acids for achieving high selectivity in new synthetic methods and their application on the late-stage functionalization towards potential drug leads.
Methods
General procedure for three-component reaction
The test tube with magnetron has added a mixture of the aryl-terminal alkyne (0.36 mmol), S8 (0.30 mmol, 11.5 mg) or Se (0.36 mmol, 28.4 mg), P-H reagent (0.30 mmol), toluene (2 mL), triethylamine (0.30 mmol, 30.3 mg) and sealed. Then, a micro-syringe injected BF3·OEt2 (0.30 mmol, 80 μL) into the sealed tube. After stirring for 4 h, the reaction solution was transferred to a round bottom flask and concentrated in vacuum. The crude residue was purified by silica gel column chromatography eluting with PE/EA to give the target compound.
General procedure for the reaction of alkynes/olefins with S(Se)=P-OH
In the dry tube with magnetron was added a mixture of alkyne/olefins (0.36 mmol), S(Se)=P-OH reagent (0.30 mmol), toluene (2 mL), and sealed. Then BF3·OEt2 (0.3 mmol, 80 μL) was injected into sealed tube by micro-syringe. After stirring for 30 min, the reaction solution was transferred to a round bottom flask and concentrated in vacuum. The crude residue was purified by silica gel column chromatography eluting with PE/EA to give the target compound.
General procedure for the reaction of endoalkenes with S=P-OH
In the dry tube with magnetron was added a mixture of aryl ethylenes (0.54 mmol), S=P-OH reagent (0.30 mmol), toluene (2 mL), and sealed. Then, a micro-syringe injected BF3·OEt2 (0.30 mmol, 80 μL) into the sealed tube. After stirring for 4 h at 100 °C, the reaction solution was transferred to a round bottom flask and concentrated in a vacuum. The crude residue was purified by silica gel column chromatography eluting with PE/EA to give the target compound.
General procedure for the reaction of natural products with S=P-OH
In the dry tube with magnetron was added a mixture of aryl ethylenes (0.36 mmol), S=P-OH reagent (0.30 mmol), toluene (2 mL), and sealed. Then, a micro-syringe injected BF3·OEt2 (0.30 mmol, 80 μL) into the sealed tube. After stirring for 4 h at room temperature, the reaction solution was transferred to a round bottom flask and concentrated in a vacuum. The crude residue was purified by silica gel column chromatography eluting with PE/EA to give the target compound.
Data availability
CCDC 2320434 (3A) and CCDC 2336190 (2d) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033. The authors declare that all other data supporting the findings of this study are available within the main text and its supplementary information files (Supplementary Information and Supplementary Data File 1).
References
Dhungana, R. K., KC, S., Basnet, P. & Giri, R. Transition metal-catalyzed dicarbofunctionalization of unactivated olefins. Chem. Rec. 18, 1314–1340 (2018).
Siu, J. C., Fu, N. & Lin, S. Catalyzing electrosynthesis: a homogeneous electrocatalytic approach to reaction discovery. Acc. Chem. Res. 53, 547–560 (2020).
Blieck, R., Taillefer, M. & Monnier, F. Metal-catalyzed intermolecular hydrofunctionalization of allenes: easy access to allylic structures via the selective formation of C-N, C-C, and C-O bonds. Chem. Rev. 120, 13545–13598 (2020).
Li, Z.-L., Fang, G.-C., Gu, Q.-S. & Liu, X.-Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 49, 32–48 (2020).
Jiang, H., Yu, X., Daniliuc, C. G. & Studer, A. Three-component aminoarylation of electron-Rich alkenes by merging photoredox with nickel catalysis. Angew. Chem. Int. Ed. 60, 14399–14404 (2021).
Cai, B. et al. Asymmetric hydrophosphinylation of alkynes: facile access to axially chiral styrene-phosphines. Angew. Chem. Int. Ed. 62, e202215820 (2023).
Zhuang, Q. B. et al. Catalytic asymmetric polycyclization of tertiary enamides with silyl enol ethers: total synthesis of (−)-cephalocyclidin A. J. Am. Chem. Soc. 145, 26550–26556 (2023).
Liu, C.-X. et al. Rhodium-catalyzed asymmetric C-H functionalization reactions. Chem. Rev. 123, 10079–10134 (2023).
Carney, J. R., Dillon, B. R., Campbell, L. & Thomas, S. P. Manganese‐catalyzed hydrofunctionalization of alkenes. Angew. Chem. Int. Ed. 57, 10620–10624 (2018).
Yang, X. H. & Dong, V. M. Rhodium-catalyzed hydrofunctionalization: enantioselective coupling of indolines and 1,3-dienes. J. Am. Chem. Soc. 139, 1774–1777 (2017).
Song, L., Yuan, W. & Ma, S. Copper-catalyzed highly selective approach to 2-boroallylic silanes from allenylsilanes. Org. Chem. Front. 4, 1261–1265 (2017).
Degtyareva, E. S., Borkovskaya, E. V. & Ananikov, V. P. Applying reen metrics to eco-riendly synthesis of sulfur-substitutced onjugated dienes based on atom-economic hydrothiolation. ACS Sustain. Chem. Eng. 7, 9680–9689 (2019).
Cheng, Z. et al. Cobalt-catalyzed regiodivergent double hydrosilylation of arylacetylenes. Angew. Chem. Int. Ed. 62, e202215029 (2023).
Dong, W. N. et al. Copper-catalyzed umpolung reactivity of propargylic carbonates in the presence of diboronates: one stone four birds. J. Am. Chem. Soc. 145, 27539–27554 (2023).
Hejna, B. G. et al. Knowles: “catalytic asymmetric hydrogen atom transfer: enantioselective hydroamination of alkenes”. J. Am. Chem. Soc. 145, 16118–16129 (2023).
Yang, P. C. et al. Syntheses of bufospirostenin A and ophiopogonol A by a conformation-controlled transannular prins cyclization. J. Am. Chem. Soc. 144, 17769–17775 (2022).
Trost, B. The atom economy-a search for synthetic efficiency. Science 254, 1471–1477 (1991).
Muhammed, J., Özer, A. Y., Ercan, M. T. & Hincal, A. A. In-vivo studies on dexamethasone sodium phosphate liposomes. J. Microencapsul. 13, 293–306 (1996).
Lurbinectedin is safe and active in relapsed small-cell lung cancer. Cancer Discov. 10, 757 (2020).
Zheng, X.-Q. et al. The antimetastatic effect and underlying mechanisms of thioredoxin reductase inhibitor ethaselen. Free Radic. Bio. Med. 162, 171–173 (2021).
Chen, J.-P., Wei, W.-T., Lia, Z.-C. & Lu, Z. Metal-catalyzed Markovnikov-type selective hydrofunctionalization of terminal alkynes. Chem. Soc. Rev. 53, 7566–7589 (2024).
Ji, D. et al. Palladium-catalyzed asymmetric hydrophosphination of internal alkynes: atroposelective access to phosphine-functionalized olefins. Chem 8, 3346–3362 (2022).
Huang, Q., Wang, W.-N. & Zhu, S.-F. Iron-catalyzed alkylzincation of terminal alkynes. ACS Catal. 12, 2581–2588 (2022).
Almenara, N., Barquin, M., Huertos, M. A. & Garralda, M. A. Oxidative addition of secondary phosphine oxides through Rh(I) center: hydrido-phosphinito-Rh(III) complexes and their catalytic activity in hydrophosphinylation of alkynes. Eur. J. Inorg. Chem. 47, 4935–4945 (2021).
Ying, J. L., Tan, Y. & Lu, Z. Cobalt-catalyzed hydrothiolation of alkynes for the diverse synthesis of branched alkenyl sulfides. Nat. Commun. 15, 8057 (2024).
Schuhknecht, D., Spaniol, T. P., Maron, L. & Okuda, J. Regioselective hydrosilylation of olefins catalyzed by a molecular calcium hydride cation. Angew. Chem. Int. Ed. 59, 310–314 (2020).
Saptal, V. B., Wang, R. & Park, S. Recent advances in transition metal-free catalytic hydroelementation (E = B, Si, Ge, and Sn) of alkynes. RSC Adv. 10, 43539–43565 (2020).
Zhou, X. L. et al. Cobalt-catalyzed intermolecular hydrofunctionalization of alkenes: evidence for a bimetallic pathway. J. Am. Chem. Soc. 141, 7250–7255 (2019).
Iwamoto, T., Nishikori, T., Nakagawa, N., Takaya, H. & Nakamura, M. Iron-catalyzed anti-selective carbosilylation of internal alkynes. Angew. Chem. Int. Ed. 56, 13298–13301 (2017).
Lu, X.-Y. et al. 1,1-Disubstituted olefin synthesis via Ni-catalyzed Markovnikov hydroalkylation of alkynes with alkyl halides. Chem. Commun. 52, 5324–5327 (2016).
Li, X.-W. & Nay, B. Transition metal-promoted biomimetic steps in total syntheses. Nat. Prod. Rep. 31, 533–549 (2014).
Zhang, X. et al. Ni(II)/Zn catalyzed reductive coupling of aryl halides with diphenylphosphine oxide in water. Org. Lett. 13, 3478–3481 (2011).
Corma, A., González-Arellano, C., Iglesias, M. & Sánchez, F. Efficient synthesis of vinyl and alkyl sulfides via hydrothiolation of alkynes and electron-deficient olefins using soluble and heterogenized gold complexes catalysts. Appl. Catal. A-Gen. 375, 49–54 (2010).
Niu, M., Fu, H., Jiang, Y. & Zhao, Y. Copper-catalyzed addition of H-phosphine oxides to alkynes forming alkenylphosphine oxides. Chem. Commun. 272–274 (2007).
Ananikov, V. P., Malyshev, D. A., Beletskaya, I. P., Aleksandrov, G. G. & Eremenko, I. L. Nickel(II) chloride-catalyzed regioselective hydrothiolation of alkynes. Adv. Synth. Catal. 347, 1993–2001 (2005).
Crossley, S. W. M., Obradors, C., Martinez, R. M. & Shenvi, R. A. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of clefins. Chem. Rev. 116, 8912–9000 (2016).
Wen, H., Liu, G. & Huang, Z. Recent advances in tridentate iron and cobalt complexes for alkene and alkyne hydrofunctionalizations. Coord. Chem. Rev. 386, 138–153 (2019).
Guo, J., Cheng, Z., Chen, J., Chen, X. & Lu, Z. Iron- and cobalt-catalyzed asymmetric hydrofunctionalization of alkenes and alkynes. Acc. Chem. Res. 54, 2701–2716 (2021).
Fan, Y. et al. Hydrofunctionalization of olefins to value-added chemicals via photocatalytic coupling. Green. Chem. 20, 3450–3456 (2018).
Zhan, Y. et al. Chemo- and regioselective nucleophilic hydrofunctionalization of unactivated aliphatic alkenes under transition-metal-free catalysts. Green. Chem. 23, 3250–3255 (2021).
Ramani, A., Desai, B., Dholakiya, B. Z. & Naveen, T. Recent advances in visible-light-mediated functionalization of olefins and alkynes using copper catalysts. Chem. Commun. 58, 7850–7873 (2022).
Huang, S. et al. Metal-free difunctionalization of alkynes to access tetrasubstituted olefins through spontaneous selenosulfonylation of vinylidene ortho-quinone methide (VQM). Org. Biomol. Chem. 17, 1121–1129 (2019).
Qin, H. et al. Electrochemical-induced hydrogenation of electron-deficient internal olefins and alkynes with CH3OH as hydrogen donor. Adv. Synth. Catal. 363, 2104–2109 (2021).
Margrey, K. A. & Nicewicz, D. A. A general approach to catalytic alkene anti-markovnikov hydrofunctionalization reactions via acridinium photoredox catalysis. Acc. Chem. Res. 49, 1997–2006 (2016).
Chalotra, N., Kumar, J., Naqvi, T. & Shah, B. A. Photocatalytic functionalizations of alkynes. Chem. Commun. 57, 11285–11300 (2021).
Stephan, D. W. The broadening reach of frustrated Lewis pair chemistry. Science 354, aaf7229 (2016).
Oderinde, M. S. & Organ, M. G. Studies on the Mechanism of B(C6F5)3-catalyzed hydrostannylation of propargylic alcohol derivatives. Angew. Chem. Int. Ed. 51, 9834–9837 (2012).
Soltani, Y., Wilkins, L. C. & Melen, R. L. Stoichiometric and catalytic C−C and C−H bond formation with B(C6F5)3 via cationic intermediates. Angew. Chem. Int. Ed. 56, 11995–11999 (2017).
Wu, L., Chitnis, S. S., Jiao, H., Annibale, V. T. & Manners, I. Non-Metal-Catalyzed heterodehydrocoupling of phosphines and hydrosilanes: mechanistic studies of B(C6F5)3-mediated formation of P–Si bonds. J. Am. Chem. Soc. 139, 16780–16790 (2017).
Kumar, G., Qu, Z.-W., Ghosh, S., Grimme, S. & Chatterjee, I. Boron lewis acid-catalyzed regioselective hydrothiolation of conjugated dienes with thiols. ACS Catal. 9, 11627–11633 (2019).
Wang, G. et al. Chemoselective borane-catalyzed hydroarylation of 1,3-dienes with phenols. Angew. Chem. Int. Ed. 58, 1694–1699 (2019).
Long, P.-W., He, T. & Oestreich, M. B(C6F5)3-catalyzed hydrosilylation of vinylcyclopropanes. Org. Lett. 22, 7383–7386 (2020).
Tang, H.-D. et al. Direct synthesis of thioesters from feedstock chemicals and elemental sulfur. J. Am. Chem. Soc. 145, 5846–5854 (2023).
Chen, H., Gao, L., Liu, X., Wang, G. & Li, S. B(C6F5)3-catalyzed hydroarylation of aryl alkynes for the synthesis of 1,1-diaryl and triaryl substituted alkenes. Eur. J. Org. Chem. 5238–5242 (2021).
Burykina, J. V. et al. Intermolecular photocatalytic chemo-, stereo- and regioselective thiol-yne-Ene coupling reaction. Angew. Chem. Int. Ed. 61, e202116888 (2022).
Hasegawa, M., Takahashi, K., Inoue, R., Haga, S. & Mazaki, Y. Selenacalix[4]dithienothiophene: synthesis, structure, and complexation of a cyclic tetramer of selenide-bridging dithienothiophene. Chem. Asian J. 14, 1647–1650 (2019).
Zhang, X. H. et al. Three-component coupling reaction in water: a one-pot protocol for the construction of P–S–C(sp3) and P–Se–C(sp3) bonds. Eur. J. Org. Chem. 14, 1884–1888 (2017).
Gaussian 16, Revision C.01Frisch, M. J. et al. Gaussian, Inc.; Wallingford CT, 2016.
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Petersson, G. A. et al. A complete basis set model chemistry. I. the total energies of closed‐shell atoms and hydrides of the first‐row elements. J. Chem. Phys. 89, 2193–2218 (1988).
CYLview, 1.0b; C. Y. Legault, Université de Sherbrooke (http://www.cylview.org) (2009).
Shi, Q. et al. Diradical generation via relayed proton-coupled electron transfer. J. Am. Chem. Soc. 144, 3137–3145 (2022).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
William, H., Andrew, D. & Klaus, S. VMD: visual molecular dynamics. J. Mol. Grap. 14, 33–38 (1996).
Johnson, E. R. et al. Revealing noncovalent interactions. J. Am. Chem. Soc. 132, 6498–6506 (2010).
Liu, L.-X. et al. Diversity-oriented synthesis of marine polybrominated diphenyl ethers as potential KCNQ potassium channel activators. Bioorg. Chem. 126, 105909 (2022).
Blum, D. et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 65, 135–172 (2001).
Rani, L., Ghosh, B., Ahmad, M. H. & Mondal, A. C. Evaluation of potential neuroprotective effects of vanillin against MPP+/MPTP-induced dysregulation of dopaminergic regulatory mechanisms in SH-SY5Y cells and a mouse model of Parkinson's Disease. Mol. Neurobiol. 60, 4693–4715 (2023).
Acknowledgements
We are grateful of the National Key Research and Development Program of China (No. 2021YFF0502400), the Natural Science Foundation of China (Nos. 82022069, 42076099, 21871182, 82104140), Shandong Laboratory Program (SYS202205), the Fundamental Research Projects of Science & Technology Innovation and Development Plan in Yantai City (No. 2023JCYJ057), Taishan Scholars Program (tsqn2023- 12302), and the CAS Youth Interdisciplinary Team. We sincerely acknowledge Dr. Zhijian Xu from Drug Discovery and Design Center, Shanghai Institute of Materia Medica, Chinese Academy of Sciences for the help of DFT calculations on our mechanism study.
Author information
Authors and Affiliations
Contributions
C.W.S., P.F.W., and Q.X. conceived and designed the experiments; C.W.S. carried out most of the experiments; P.F.W. carried out biological activity studies. Q.X. and Z.N.Y. carried out the DFT calculations; X.W.L., X.H.Z., and Y.Z. analyzed data; X.W.L., X.H.Z., and Y.Z. directed the project and wrote the paper. All authors participated in data analysis and interpretation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shao, CW., Wan, PF., Xu, Q. et al. Phosphinothio(seleno)ation of alkynes/olefins and application on the late-stage functionalization of natural products. Commun Chem 7, 290 (2024). https://doi.org/10.1038/s42004-024-01326-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42004-024-01326-9