Abstract
Non-alcoholic steatohepatitis (NASH) is characterized by lipotoxicity, inflammation and fibrosis, ultimately leading to end-stage liver disease. The molecular mechanisms promoting NASH are poorly understood, and treatment options are limited. Here, we demonstrate that hepatic expression of bone morphogenetic protein 8B (BMP8B), a member of the transforming growth factor beta (TGFβ)–BMP superfamily, increases proportionally to disease stage in people and animal models with NASH. BMP8B signals via both SMAD2/3 and SMAD1/5/9 branches of the TGFβ–BMP pathway in hepatic stellate cells (HSCs), promoting their proinflammatory phenotype. In vivo, the absence of BMP8B prevents HSC activation, reduces inflammation and affects the wound-healing responses, thereby limiting NASH progression. Evidence is featured in primary human 3D microtissues modelling NASH, when challenged with recombinant BMP8. Our data show that BMP8B is a major contributor to NASH progression. Owing to the near absence of BMP8B in healthy livers, inhibition of BMP8B may represent a promising new therapeutic avenue for NASH treatment.
Similar content being viewed by others
Main
Non-alcoholic fatty liver disease (NAFLD) is characterized by excessive accumulation of lipids within the liver. The prevalence of NAFLD is high and progressively increasing, especially in the westernized world1, primarily as a consequence of increasing obesity and metabolic syndrome prevalence2,3. Despite the prognosis of isolated steatosis (non-alcoholic fatty liver, NAFL) being relatively benign per se, exposure to sustained metabolic insults and lipotoxicity contributes to the development of hepatocyte injury, inflammation and liver fibrosis; these histological features define NASH4,5. Moreover, NASH may progress to advanced liver fibrosis or cirrhosis, which is associated with increased mortality and is a leading cause of liver transplantation1. A full understanding of NASH pathophysiology is needed, but is lacking so far. Furthermore, given the limited range of currently approved therapies, the identification of new targetable pathways to ameliorate and/or reverse NASH is a global health priority.
Bone morphogenetic proteins (BMPs) are members of the TGFβ–BMP superfamily. BMPs contribute to liver function in health and disease, and are involved in regulation of iron and lipid metabolism6,7, angiogenesis8,9, the liver macrophage niche10, liver regeneration and chronic liver disease progression (fibrosis and hepatocellular carcinogenesis)11,12,13. Depending on their liver-cell-specific roles and their capability to activate SMAD1, SMAD5 and SMAD9 (SMAD1/5/9), or SMAD2 and SMAD3 (SMAD2/3) downstream effectors and SMAD-independent pathways, TGFβ–BMP ligands exert counteracting effects on inflammation and compensatory proliferation (promoted by most BMPs, and inhibited by TGFs), HSC activation and fibrosis (promoted by TGFs and BMP9, and inhibited by BMP7) and cell differentiation (BMPs promote differentiation; TGFs promote epithelial–mesenchymal transition)11,14,15. A comprehensive understanding of the differential action played by each superfamily member in liver disease may thus reveal unique diagnostic and therapeutic opportunities.
Here, we studied BMP8B, a poorly characterized member of the superfamily, that has been implicated in regulating energy expenditure16,17, germline cell proliferation and maturation18 and cancer19,20. The contribution of BMP8B in liver pathophysiology is in its infancy and is limited to some incidental observations: single-nucleotide polymorphisms (SNPs) in the BMP8B locus have been associated with increased necroinflammation in people with viral hepatitis21, and upregulation of Bmp8b can be found in murine models of diet-induced NASH (for example, choline- and folate-deficient diet, GSE62362)22, and in murine genetic models of NASH and chronic cholestasis (for example, MDR2-knockout (KO), or in IKKγ/NEMO-KO mice; GSE33161)23,24. Currently, the biological consequences of the upregulation of BMP8B in these models are unknown, in light of the fact that BMP8B exhibits two unusual characteristics. First, whereas most BMPs signal via either the branch of the SMAD pathway involving ALK receptor tyrosine kinase (ALK) proteins ALK4, ALK5 and ALK7 (signalling via SMAD2/3) or that involving ALK1, ALK2, ALK3 and ALK6 (signalling via SMAD1/5/9), BMP8B signals via both17,18. Given the key contrasting roles of SMAD branches in regulating liver pathophysiology11, we posited that BMP8B may be tuning TGFβ–BMP signalling during the wound-healing response, modulating NASH development and progression. Second, BMP8B messenger RNA is almost undetectable in healthy livers of mice17 and of humans (https://www.proteinatlas.org/ENSG00000116985-BMP8B/tissue; https://gtexportal.org/home/gene/BMP8B). However, screening publicly available databases, Bmp8b mRNA expression is elevated in experimental models of NAFLD/NASH, with a degree of upregulation proportional to the entity of damage (Supplementary Table 1).
Here, we demonstrate that the expression of BMP8B in hepatocytes and HSCs in people and in murine models of NASH is proportional to the disease stage. BMP8B activates both SMAD2/3 and SMAD1/5/9 in primary murine HSCs, promoting their activation and proinflammatory behaviour. In diverse in vivo and in vitro models of wound healing and NASH, the absence of BMP8B prevents HSC activation and/or the activation of inflammatory pathways, thus affecting wound-healing responses and preventing NASH progression. Furthermore, using new human primary three-dimensional microtissues challenged with fatty acids to mimic NASH25 and treated with recombinant BMP8, we show that BMP8B is a major contributor to human NASH progression and that our findings can be translated into human NASH pathophysiology.
RESULTS
BMP8B expression is induced in liver injury and NASH
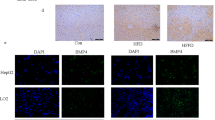
Levels of Bmp8b mRNA (Extended Data Fig. 1a) and protein (Extended Data Fig. 1b,c) expression are very low in normal mouse liver. However, BMP8B is upregulated at the mRNA and protein level in both the western diet (WD)-induced NASH mouse model, and in the livers of acute or chronic carbon tetrachloride (CCl4)-injured mice. In diseased livers, Bmp8b is co-localized with albumin (Extended Data Fig. 1b; produced by hepatocytes) and alpha smooth muscle actin (αSMA; Extended Data Fig. 1c; a marker of activated HSC and pericytes), but is not expressed in other liver cell types (Supplementary Fig. 1).
Clinical evidence from two independent cohorts of people with biopsy-proven NASH showed that BMP8B mRNA expression in liver biopsies increases with the NASH disease stage (Fig. 1a,f), level of hepatocellular ballooning (Fig. 1c,h) and fibrosis (Fig. 1e,j). Consistent with its regulation by estrogens26, BMP8B is expressed at higher levels in females; it appears reduced in patients with type 2 diabetes mellitus (T2DM) under medical treatment (metformin with/without biguanides, incretins or thiazolidinediones; details in Fig. 1a–j). Full details of the population are available in Supplementary Table 2. As observed in rodents, human BMP8B protein is expressed in keratin 18 (KRT18, also known as K18)+ hepatocytes and αSMA+ HSCs and pericytes (Fig. 1k). These results indicate that BMP8B is induced in HSC and hepatocytes in response to acute or chronic liver damage, raising the question of whether BMP8B signalling may contribute to liver disease progression.
a–j, Relative mRNA expression levels of BMP8B, measured by quantitative real-time polymerase chain reaction (RTqPCR) (Cambridge cohort, TaqMan, a–e; Newcastle/Paris cohort, NanoString, f–j) in the RNA extracted from liver biopsies of two independent cohorts of people with NASH. The levels of BMP8B transcript increase with disease progression according to the NASH activity score (NAS) (a,f). The components of the NAS score show that the main drivers of BMP8B expression are hepatocellular ballooning (c,h), fibrosis stage (e,j), sex and the diagnosis/treatment of T2DM. Results are shown as mean ± s.e.m. (n = 40 Cambridge cohort; n = 113 Newcastle/Paris cohort); expression data of biological replicates are represented as dot plots. BK, BestKeeper housekeeping genes. Statistical significance (P < 0.05) was assessed by multivariate analysis of variance (MANOVA) with histological variables, sex and T2DM as covariates; n.s., not significant. Full details of the population are in Supplementary Table 2. k, Representative immunofluorescence of BMP8B, K18 (mainly hepatocytes) and αSMA (activated HSC/pericytes) protein expression in FFPE liver biopsies of people with NASH at different stages of the disease (n = 6 people; 2–3 needle biopsy specimens per person; magnification, ×20). BMP8B is expressed in NASH fibrosis, and co-localizes with both K18 and αSMA (white arrows indicate co-localization with αSMA).
HSC and primary hepatocytes upregulate Bmp8b in vitro
Consistent with the in vivo observations, levels of Bmp8b mRNA expression in primary mouse hepatocytes (PHs) were low under basal conditions at baseline, and comparable to those in liver tissue (Extended Data Fig. 2). However, Bmp8b mRNA was strongly upregulated in murine PHs when cultured in vitro (Extended Data Fig. 2a), preferentially when cultured at lower density, a condition known to favour PH proliferation and dedifferentiation (Extended Data Fig. 2b,c)27. Interestingly, data from publicly available databases (GSE122660 (ref. 28) and Extended Data Fig. 2d) suggested that fatty acids and/or proinflammatory factors did not further modulate BMP8B levels either in human PHs or in hepatocyte cell lines. In primary murine HSCs, BMP–TGFβ receptors and intracellular effectors (SMADs) were abundant but their expression was relatively stable during trans-differentiation into activated myofibroblasts (next-generation sequencing, NGS); Extended Data Fig. 3a,b and Supplementary Table 5). Ingenuity upstream regulator analysis (URA; Extended Data Fig. 3c) confirmed the activation of the TGFβ signalling pathway, as was also suggested by the transcriptional regulation of multiple BMP–TGFβ ligands during the HSC activation process (Extended Data Fig. 3b). Bmp8b upregulation was transient and peaked at day 4; its upregulation was enhanced by palmitic acid, but not by other modulators of HSC activation (Extended Data Fig. 3d). Bmp8b mRNA was undetectable in Kupffer cells (KC) and in circulating inflammatory cells.
BMP8B activates both branches of BMP–TGFβ signalling in primary HSCs
We next studied which hepatic cells sense BMP8B and its mechanism of action. Previous reports have shown that BMP8 proteins act as pan-BMP–TGFβ-receptor agonists and activate SMAD2/3 and SMAD1/5/9 (refs. 17,18,29), but this information has never been confirmed in the liver.
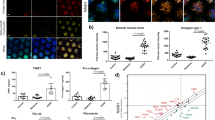
We first studied the capability of primary murine HSCs (Fig. 2) and alpha mouse liver 12 (AML12) cells (Supplementary Fig. 2) to sense BMP8B; in line with previous evidence showing that BMP8 proteins bind all type 1 and type 2 receptors18, BMP8 activated both SMAD2/3 and SMAD1/5/9 in an ALK-dependent manner (Fig. 2a–d), also promoting the expression of SMAD targets (Fig. 2e) in HSCs. Conversely, BMP8 did not activate SMADs in AML12 cells (Supplementary Fig. 2a,b). Furthermore, markers of proliferation, apoptosis, differentiation, epithelial–mesenchymal transition and proinflammatory pathways were not affected in BMP8-stimulated primary murine hepatocytes (Supplementary Fig. 3). These results suggest that hepatocyte-derived BMP8B might not signal in an autocrine fashion on hepatocytes, but instead could potentially modulate other cell types.
a–d, Primary murine BMP8B-KO HSCs (confluence 35,000 cells per cm2) activated in vitro (day 7) were treated, after 3-h FBS starvation, with or without recombinant human BMP8 protein (30–75 ng mL–1) and/or ALK1/2/3/6 inhibitor (i) (K02288, 1 µM) or ALK4/5/7 inhibitor (i) (A-8301, 5 µM) to study phosphorylation (p) of SMAD2 (a,c) or SMAD1/5/9 (b,d) phosphorylation after 30-min incubation. TGFβ (5 ng mL–1) or BMP7 (5 ng mL–1) was used as positive control. Immunofluorescence (magnification, ×20) was used to study phosphorylated SMAD (green); αSMA (red) was used to stain the cytoplasm and Hoechst 33342 (blue) was used to stain the nucleus. The percentage of positive nuclei was assessed by Harmony High Content Imaging and Analysis Software. e, Gene expression (relative mRNA expression levels) of BMP–TGFβ targets measured by RTqPCR was assessed after 5 h of incubation with ligands and inhibitors. All the results are shown as mean ± s.e.m.; expression data of biological replicates are represented as dot plots. One-way analysis of variance (ANOVA) plus Fisher’s least significant difference multiple-comparison test was used to estimate the statistical significance among treatments (4 biological replicates per group; each biological replicate is a pool of 3 livers). Lowercase letters indicate post hoc analysis significance: a, reference group; groups with different letters are statistically different per post hoc comparison; differences between groups with the same letter are statistically not significant per post hoc comparison.
BMP8B is an autocrine and paracrine signal promoting HSC activation in vitro
Given the expression profile of Bmp8b during HSC activation, we investigated its involvement in this process. Using primary HSCs freshly isolated from BMP8B-KO and wild-type (WT) mice and cultured in vitro, we observed that the absence of BMP8B delayed the time-associated increase in αSMA mRNA (Fig. 3a) and protein (Fig. 3b,c). Genetic ablation of BMP8B also decreased the transcription of collagens, proinflammatory cytokines and multiple downstream effectors of SMAD signalling without changing Tgfβ1 expression (Fig. 3a). The defective production of monocyte chemoattractant protein 1 (Mcp1, also known as Ccl2) and ‘regulated upon activation, normal T cell expressed and presumably secreted’ (Rantes, also known as Ccl5) is relevant to liver pathophysiology given that these chemokines drive the recruitment of inflammatory cells and promote wound-healing responses, and their downstream receptors are candidate targets for NASH treatment30. Ingenuity pathway analysis (IPA) and URA from fully activated HSCs (day 8) confirmed that BMP8B ablation reduced the activation of proinflammatory pathways and TGFβ signalling (Fig. 3d; expanded TGFβ network in Fig. 3e; NGS data analysis in Supplementary Table 6). Moreover, analysis of the medium from cultured HSCs (Fig. 3f) revealed a defective extracellular matrix production and processing (as shown by the reduced conentration in the culturing medium of PRO-C3, activated MMP9 and C3M) and a reduced release of proinflammatory mediators such as interleukin-6 (IL-6) and MCP1.
a–c, Freshly isolated HSCs from BMP8B-KO mice and WT littermate mice were cultured for 4, 6 and 8 d at a density of 35,000 cells per cm2. Gene expression (a; RTqPCR) and αSMA protein expression (b,c; immunofluorescence magnification, ×10) were studied to investigate HSC activation status (4 biological replicates per group; each biological replicate is a pool of 3 livers). d, Prediction of canonical pathways and upstream regulators significantly enriched and predicted to be activated (red) or inhibited (blue) according to IPA/URA in fully activated (day 8) HSCs (for details, see the NGS analysis in Supplementary Table 6). e, Graphical representation in a network format of the changes of TGFβ-target levels suppressed in the NGS dataset in KO (versus WT) cells. f, Profiling of the culturing medium (WT, 3 biological replicates per group; KO, 4 biological replicates per group; each biological replicate is a pool of cells from 3 murine livers): collagen-3 secretion and processing (proposed as marker of NASH fibrosis36) was assessed studying PRO-C3, MMP9 and C3M assays; secretion of proinflammatory mediators was assessed quantifying IL-6 and MCP1 (RANTES was below detectability) in the culturing medium at multiple time points (days 4, 8 and 11 of culturing). Relative mRNA expression of genes measured by RTqPCR or NGS; αSMA protein quantification was performed by immunofluorescence and quantified using ImageJ 1.8.0. Sequencing data are shown in a heat map format representing the ‘activation’ (Z) score (inhibition (blue) or activation (red)) of pathways/upstream regulators predicted by IPA/URA. All the other results are shown as mean ± s.e.m.; biological replicates are represented by dots. Statistical significance was assessed by MANOVA using time point and genotype as covariates.
Although direct BMP8 treatment (alone or in combination with lipopolysaccharide, LPS) did not affect murine bone marrow-derived macrophages (BMDMs; Supplementary Fig. 4a,b; suggesting that BMP8 does not activate the inflammasome directly, or indirectly via TLR4), conditioned medium from HSCs induced expression in BMDMs of SMAD2/3-target genes (Socs3) and proinflammatory genes such as Mcp1 (Supplementary Fig. 4c); this effect was less pronounced in challenging BMDMs with BMP8B-KO HSC-conditioned medium. These data suggest that BMP8B, mediating the secretion of proinflammatory cytokines in HSC, might indirectly modulate inflammatory-cell behaviour, with repercussions on the wound-healing responses to liver injury.
Recombinant BMP8 rescued αSMA mRNA and protein levels, as well as the expression of genes involved in ECM remodelling (Extended Data Fig. 4a–c), inflammation (Extended Data Fig. 4d) and SMAD signalling (Extended Data Fig. 4e) in the BMP8B-KO HSC. Intriguingly, BMP8 accelerated some features of HSC activation in WT cells, suggesting that BMP8B produced by HSCs themselves is not saturating their intracellular signalling, and that BMP8B produced by other cells may also be modulating HSC behaviour.
Taken together, these results confirm that both endogenous BMP8B production, and the ectopic administration of the recombinant protein (mimicking a paracrine effect), are able to promote HSC trans-differentiation into myofibroblasts. Furthermore, the defective activation of BMP8B-KO HSCs leads to reduced ECM deposition and, most importantly, to impaired inflammatory pathways in HSCs, further implicating BMP8B in the pathogenesis of liver disease.
Genetic ablation of BMP8B tunes the wound-healing response in the acute CCl4 model
We thus investigated whether the absence of BMP8B also modulated the wound-healing response in vivo following acute CCl4 treatment. In WT mice, acute CCl4 injury induced Bmp8b mRNA in the liver, peaking 3 d after the insult, but remaining elevated at day 5 (Fig. 4a). At day 3 after CCl4, Bmp8b was one of the most strongly upregulated TGFβ/BMP family ligands (Fig. 4b). Bmp8b was produced by hepatocytes and HSCs (Fig. 4c), in agreement with our in vitro and human observations. BMP8B absence prevented the CCl4-driven increase in liver weight (Fig. 4d) and tended to decrease alanine aminotransferase (ALT) 3 d after CCl4 injection (Fig. 4e). Despite showing similar levels of hepatocellular damage to that in WT littermates (Fig. 4f and Supplementary Fig. 5a), at 3 d post CCl4 injection, BMP8B-KO livers showed reduced inflammatory infiltrates (Fig. 4g and Supplementary Fig. 5a; semi-quantitative histological score in Supplementary Table 3), and a defective infiltration of lymphocytes (CD3+) and myeloid (LY6CG+) cells (Fig. 4h and Supplementary Fig. 5b; immune cell deconvolution on NGS is shown in Fig. 4i). Moreover, at both time points (more predominantly at day 5), BMP8b-KO mice exhibited reduced αSMA-positive myofibroblasts (Fig. 4j and Supplementary Fig. 5c). Because this is a model of wound-healing response to acute hepatic injury, also featuring compensatory hepatocyte regeneration, and given that these processes are heavily modulated by inflammatory pathways30 and BMP–TGFβ signalling11, we next investigated the effect of BMP8B on hepatocyte proliferation: the absence of BMP8B resulted in reduced hepatocyte proliferation (Fig. 4k and Supplementary Fig. 5d) and dedifferentiation (Fig. 4l). Gene expression analysis (Fig. 4m,n) confirmed the anticipated effects of CCl4 in promoting inflammation, HSC activation and compensatory regeneration in WT mice. However, all these changes were attenuated in BMP8B-KO mice. Taken together, these results confirmed that the absence of BMP8B limited HSC activation, preventing immune cell infiltration; during the resolution phase of the wound-healing response (day 5) to acute injury, BMP8B-KO livers showed a faster recovery from hepatic damage and reduced tissue-repair responses.
BMP8B-KO and littermate mice received a single olive oil (OO) or CCl4 intraperitoneal (i.p.) injection, and were culled 3 d or 5 d later (n for each group, applicable to all comparisons): OO, WT = 6; CCL4 3 d, WT = 4; CCL4 3 d, KO = 6; CCL4 5 d, WT = 7; CCL4 KO, 5 d = 5). a, Relative Bmp8b mRNA expression measured by RTqPCR (whole liver). b, Relative mRNA expression TGFβ–BMP family ligands (3 d postinjection), measured by NGS (the heat map represents relative basal expression level, from blue (lowest) to yellow to orange (highest); the dotted graph represents log2(fold change)) (see Supplementary Table 8 for details). c, In situ hybridization (two replicates; staining repeated twice) reveals that, upon CCl4 challenge, Bmp8b mRNA is expressed by K18+ cells (mainly hepatocytes) and αSMA+ cells (mainly activated HSCs and pericytes; magnification: ×40). d, Liver to body weight ratio (LW/BW (%)) is increased by CCl4; BMP8B-KO mice show less-pronounced liver enlargement. e, CCl4-treated BMP8B-KO mice show a trend towards reduced serum ALT. f,g, HALO imaging software analysis on whole-tissue (haematoxylin and eosin; H&E) specimens suggests that CCl4 induces a substantial increase of hepatocellular damage (f) and inflammation (g), peaking 3 d after the injection; BMP8B-KO mice showed reduced inflammation at the same time point. h,i, Further IHC immune cell profiling (h; day 3) suggests a reduced infiltration in the BMP8B-KO livers by CD3+ (mostly lymphocytes) and of LY6CG+ (mostly myeloid) cells in the KO liver. This result is also confirmed by the immune cell-type deconvolution analysis performed on the NGS data (i). We also profiled by IHC αSMA staining (j) and proliferating cell nuclear antigen (PCNA) staining (k), which appeared to be reduced in BMP8B-KO mice compared with WT littermates, especially at day 5. l, Furthermore, in light of decreased compensatory proliferation, circulating levels of AFP appeared to be reduced in the BMP8B-KO mice. m,n, Relative mRNA expression (whole-liver mRNA) levels of genes measured by RTqPCR and IPA on the NGS data (day 3) confirm reduced inflammation, stellate cell activation and proliferation in BMP8B-KO mice. Representative images of H&E and IHC are shown in Supplementary Fig. 5. Supplementary Table 4 shows that the lipid composition of the livers did not differ between WT and KO mice. All the results are shown as mean ± s.e.m.; biological replicates are represented as dot plots. Statistical significance was assessed either by one-way ANOVA plus Fisher’s least significant difference multiple-comparison test, or by two-sided Student’s t-test (and false-discovery rate (FDR) correction for NGS data). Lowercase letters indicate post hoc analysis significance: a, reference group; groups with different letters are statistically different per post hoc comparison; differences between groups with the same letter are statistically not significant per post hoc comparison.
Genetic ablation of BMP8B delays liver regeneration after partial hepatectomy
We next investigated whether genetic ablation of BMBP8B delayed liver regeneration irrespective of the challenge used to promote compensatory hepatocyte proliferation. We used the partial hepatectomy (PHx) model, wherein the regeneration is primed predominantly by resident immune cells (compared with the acute CCl4 model that is characterized by a large component of inflammatory infiltrates). PHx induces a peak in hepatocyte proliferation 3 d after surgery; this process is dependent on inflammatory priming and modulated by TGFβ–BMP signals11,31. BMP8B mRNA and protein expression was significantly increased during the regenerative phase and, consistent with other models, hepatocytes and pericytes/HSCs were identified as the cellular source of BMP8B; in this model, the protein staining appeared to be more intense in hepatocytes (Fig. 5a–d). Bmp8b was the most significantly upregulated BMP–TGFβ family member following PHx (Fig. 5b), and its absence led to defective liver regrowth (Fig. 5e). Hepatocyte proliferation was reduced in BMP8B-KO mice (versus WT) (Fig. 5f–i). Results from gene expression analysis (Fig. 5j,k) were consistent with reduced hepatocyte proliferation (reduced Ccnd1 and Ccne1 mRNA; increased p21 mRNA) and impaired ECM remodelling (reduced Mmp9). As with CCl4 treatment, BMP8B-KO livers showed defects in pathways involved in inflammation (IL-6 and STAT3 are also crucial for the priming of liver regeneration31) and proliferation after PHx (Fig. 5k).
BMP8B-KO and littermate mice received PHx and were culled 3 d later (n per group for all the comparisons): day 0, WT = 5; day 0, KO = 4; day 3, WT = 5; day 3, KO = 4). a, Relative Bmp8b mRNA expression measured by RTqPCR. b, Relative mRNA expression of TGFβ family ligands (3 d postinjection) measured by NGS (the heat map represents relative basal expression level from blue (lowest) to yellow to orange (highest); the dotted graph represents log2(fold change); see Supplementary Table 8 for details). c,d, FISH (magnification, ×40; 2 replicates; staining repeated twice) and immunofluorescence (two replicates; staining repeated twice) reveal that after PHx, Bmp8b mRNA and protein are expressed by K18+ cells (mainly hepatocytes) and αSMA+ cells (mainly activated HSC and pericytes). Arrowheads point to cells that most express Bmp8b mRNA (green dots). e, Liver to body weight ratio shows that BMP8B-KO mice have reduced regenerative potential compared with WT littermates (resected liver in red as reference). f–i, Defective hepatocyte proliferation was also confirmed by reduced PCNA IHC staining (PCNA quantified by HALO imaging software analysis on whole tissue; staining was repeated once; magnification ×10) (f,h), and a reduced number of mitotic figures (H&E staining repeated once; magnification, ×20) (g,i). j,k, Relative mRNA expression levels (whole-liver RNA; resected livers from the same mice were used as ‘day 0’ reference) of genes measured by RTqPCR (j) and IPA analysis (k; NGS) confirm reduced inflammation and compensatory proliferation in BMP8B-KO mice during the proliferative phase of liver regeneration. All the results are shown as mean ± s.e.m.; replicates are represented as dot plots. Statistical significance was assessed either by one-way ANOVA plus Fisher’s least significant difference multiple-comparison test, or by two-sided Student’s t-test (and FDR correction for NGS data). Lowercase letters indicate post hoc analysis significance: a, reference group; groups with different letters are statistically different per post hoc comparison; differences between groups with the same letter are statistically not significant per post hoc comparison.
These results confirmed in the PHx model that the absence of BMP8B11,32 is associated with defective hepatic inflammatory pathways and compensatory hepatocyte proliferation.
BMP8B promotes NASH in human 3D in vitro NASH microtissues
We next investigated in vitro whether BMP8B contributed to NASH pathophysiology and progression. We used a new in vitro microphysiological system (MPS) consisting of three-dimensional perfused microtissues of primary human cells (PH; Kupffer cells, KCs; and HSCs) challenged with a mix of free fatty acids (FFA) to mimic NASH25,33. This in vitro model displayed high homology with in vivo NASH models (Fig. 6a; ~80% of expressed genes overlapping with the WD model) and contained highly differentiated hepatocytes expressing high levels of albumin, apolipoproteins, clotting factors and metabolic genes (top 250 genes and differential analysis in Supplementary Table 7); AFP was expressed at low levels. The MPS expressed a repertoire of BMP–TGFβ effectors (Fig. 6b) and ligands (Fig. 6c) and developed lipid droplets when challenged with FFA (Fig. 6d). Finally, BMP8B was expressed in this model; FFA challenge promoted a modest and not significant upregulation of BMP8B (Extended Data Fig. 5a). To mechanistically study the contribution of BMP8B to NASH, we administered recombinant BMP8 with or without ALK inhibitors (TGFβ and BMP7 were used as positive controls) to the FFA-challenged MPS, and performed phospho-proteomics (Extended Data Fig. 5b) and transcriptomics (5-h incubation; Fig. 6e,f and Supplementary Table 7). As previously observed in murine HSCs, BMP8 induced the phosphorylation of SMAD1 and SMAD3 (Extended Data Fig. 5b) and the transcription of their targets; these effects were prevented by ALK inhibitors (Fig. 6f). Using IPA/URA (Fig. 6e), we focused on biological mechanisms differentially regulated by BMP8, which were clustered into three groups. The first cluster (‘BMP-like’) included pathways and upstream regulators promoted by BMP8 and BMP7 with modulation reduced or prevented by the ALK1/2/3/6 inhibitor K02288; this cluster included proinflammatory pathways (IL-1, NFκB, CCR3) and drivers of cell proliferation and/or survival (CSF2, telomerase signalling, AMPK, mTOR). The ‘TGFβ-like’ cluster (modulated by BMP8 and TGFβ; modulation reduced/prevented by the ALK4/5/7 inhibitor A-8301) featured known TGFβ effectors (ERK1/2, SMAD2/4, P38 MAPK, JUNB), growth factors (FGF, EGFR, NGF, HGF, VEGFA) and proinflammatory and profibrotic pathways. Finally, the ‘BMP/TGFβ-like’ cluster (modulated by BMP8, BMP7 and TGFβ; modulation reduced/prevented by both ALK inhibitors) featured inflammatory pathways (STAT4, FOS, INFG, JAK/STAT), growth-factor signalling (CSF1/3, PDGF, GM-CSF) and proliferation, survival and carcinogenesis (JNK, AKT, FOS). The change of the phosphorylation status of some of these hits (marked in bold in Fig. 6e) was also confirmed experimentally by targeted phospho-proteomics (Extended Data Fig. 5b). These results indicate that BMP8 rapidly activated both TGFβ–BMP-dependent signalling, promoting multiple aspects of the wound-healing response driving NASH progression30,34.
Human primary liver cells (PHs, KCs and HSCs) were cultured in an in vitro NASH model (LiverChip, CN Bio Innovations) consisting of 3D perfused microtissues of primary human cells challenged with a medium containing a mixture of FFAs, in the presence of physiologically relevant quantities of insulin and sugars to induce a NASH-like phenotype. a, Comparison of the transcriptomic data (NGS) of this in vitro model with the in vivo WD model of NASH (in Figs. 7 and 8) shows high homology (~80% overlapping genes). Details on differentially expressed genes are in Supplementary Table 7. b–d, This model expresses a plethora of TGFβ–BMP effectors (b) and ligands (c), comparable to in vivo NASH models, and develops lipid droplets as a consequence of FFA challenge, as observed through Oil Red O staining (example picture, magnification, ×10; staining repeated multiple times with similar results) (d). CPM, counts per million. e, One week after fat challenge, cells were treated with or without recombinant human BMP8 protein (75 ng mL–1), with or without ALK 1/2/3/6 inhibitor (K02288, 1 µM) or ALK 4/5/7 inhibitor (A-8301, 5 µM) to study TGFβ–BMP-related gene expression changes at 5 h (NGS data). Those with changes in phosphorylation status (Extended Data Fig. 5b) are shown in bold. f,g, RTqPCR confirmation of TGFβ–BMP targets (long-term consequences) at 48 h (RTqPCR; NGS data are in Extended Data Fig. 5e). Treatment with TGFβ (5 ng mL–1) or BMP7 (5 ng mL–1) was used as positive control. All the results are shown as mean ± s.e.m. (biological replicates are represented as dot plots) or in a heat map matrix representing the activation Z-score of canonical pathways and upstream Regulators significantly (P < 0.05) enriched and predicted as activated (red) or inhibited (blue) according to IPA. To provide a framework of interpretation, the data have been clustered in BMP-like (modulated, significantly enriched and with –2 ≤ Z-score ≥ 2, by both BMP8 and BMP7; modulation reduced or prevented by K02288), TGFβ-like (modulated by both BMP8 and TGFβ; modulation reduced/prevented by A-8301) and BMP/TGFβ-like (modulated by BMP8, BMP7 and TGFβ; modulation reduced/prevented by both ALK inhibitors). h,i, Two days after the culture with challenges, we studied the cell secretome in the culturing medium for ALT (h) and inflammatory factors and markers of collagen deposition and remodelling (i); additional data are in Extended Data Fig. 5d; PRO-C3 was undetectable). Statistical significance (4 biological replicates per group) was assessed by two-sided t-test (e, each treatment versus control) for the NGS data, or by one-way ANOVA plus Fisher’s least significant difference multiple-comparison test (f–i). Lowercase letters indicate post hoc analysis significance. a, reference group; groups with different letters are statistically different per post hoc comparison; differences between groups with the same letter are statistically not significant per post hoc comparison.
We next studied the effects of long-term (48 h) stimulation by daily administration of BMP8, TGFβ or BMP7 (RTqPCR in Fig. 6g and Extended Data Fig. 5c; secretome in Fig. 6h,i and Extended Data Fig. 5d; NGS in Extended Data Fig. 5e and Supplementary Table 7). We confirmed that BMP8 promotes the transcription (Fig. 6g) of proinflammatory chemokines (RANTES and MCP1), and of drivers of cell proliferation (MYC, CCNE1) featuring a ‘BMP-like’ behaviour11. However, in this chronic setting, BMP8 showed a neutral effect on canonical markers of HSC activation (as shown by the lack of modulation of αSMA, TIMP1 and COL1A1; Fig. 6g) and had a weak promoting effect on ID1 (while having a neutral effect, or even a suppressive effect, on SMAD2/3 targets) (Extended Data Fig. 5c). The analysis of the secretome in the culturing medium (48 h) (Fig. 6h,i and Extended Data Fig. 5d) demonstrated that BMP8 promoted the release of ALT, M-CSF, RANTES and MCP1, and suppressed the release of anti-inflammatory cytokines IL-10 and IL-13, while only mildly (and not significantly) inducing TIMP1 and C3M excretion. We next used NGS to compare the effects of BMP8 at 5 h versus 48 h (Extended Data Fig. 5e). These analyses confirmed a consistent modulation at both time points of pathways involved in inflammatory responses and cell cycle control. On the other hand, in agreement with the BMP–TGFβ targets (Extended Data Fig. 5c) and fibrotic genes (Fig. 6g) at 48 h, TGFβ-like pathways and profibrotic signals either ceased to be regulated at 48 h (ERK1/2) or followed a biphasic response (activation at 5 h, suppression at 48 h: SMAD2, JUNB, PDGFB), thus suggesting that these pathways were only transiently modulated by BMP8.
Together, these results confirmed, in an advanced MPS human 3D in vitro NASH model, that ectopic BMP8 treatment activated BMP–TGFβ pathways to promote wound-healing responses; however, in these settings, only mild and transient effects on HSC activation and fibrosis processes were observed.
Absence of BMP8B attenuates NASH progression in vivo
To determine whether the aforementioned mechanisms were pathophysiologically relevant, we next studied the contribution of BMP8B to NASH progression in vivo in the WD model of NASH F1 fibrosis (a model of NASH fibrosis that recapitulates the hepatic lipid profile characteristic of people with NASH)35. As observed in the acute injury models, BMP8B was one of the most upregulated TGFβ–BMP ligands (Fig. 7a–c: RTqPCR, NGS and fluorescence in situ hybridization (FISH)), and was produced in K18+ hepatocytes and αSMA+ HSCs (GDF3 upregulation is also confirmed in human NASH, Supplementary Fig. 6). We used male BMP8B-KO mice because, in contrast to female BMP8B-KO mice, they exhibit a similar energy balance to that of WT controls16,17, thus avoiding confounding effects of obesity and adipose tissue function on NASH progression. As previously described16,17, male BMP8B-KO mice were indistinguishable from WT littermates with respect to body weight and body composition (Supplementary Fig. 7a), metabolic biochemistry (Supplementary Fig. 7b), adipose tissue weight (Supplementary Fig. 7c), food intake (Supplementary Fig. 7d), energy expenditure (Supplementary Fig. 7e), liver weight (Fig. 7d) and hepatic lipid composition (Supplementary Fig. 7f) on both WD and low-fat control diet (LFD). However, the livers of BMP8B-KO mice fed WD exhibited less NASH activity than did their WT littermates, as indicated by (1) reduced ALT (Fig. 7e); (2) decreased activity component of the SAF score, with both hepatocyte ballooning and lobular inflammation scores reduced (Fig. 7f,g; details in Supplementary Fig. 8a,b); and (3) diminished fibrosis (Fig. 7h,i); a trend toward reduced fibrosis was also observed in circulating PRO-C3 (Fig. 7j), a non-invasive circulating biomarker of hepatic fibrosis in NASH36. In both genotypes, the pattern of fibrosis was peri-sinusoidal5. Further immuno-profiling of the inflammatory infiltrates in WD-fed mice (Fig. 7k,l) and immune cell-type deconvolution analysis (NGS; Fig. 7m) was indicative of a defective infiltration of B cells (CD45R+), lymphocytes (CD3+) and myeloid cells (LY6CG+), whereas KC were unchanged (F4/80+; Supplementary Fig. 8c). Also while on WD, BMP8B-KO mice had reduced expression of proinflammatory (Rantes) and profibrotic (collagens and Timp1) genes in response to WD, whereas αSMA mRNA (Fig. 7n) and protein (IHC; Supplementary Fig. 8d) were unchanged. IPA (NGS; Fig. 7o) confirmed that BMP8B-KO mice on WD exhibited defective upregulation of multiple proinflammatory/profibrotic/proliferative pathways when compared with WT littermates. Taken together, these data show that the absence of BMP8B attenuated NASH in mice with mild fibrosis.
BMP8B-KO and wild-type littermate mice were treated for 32 weeks with WD (9 WT and 8 KO) or low-fat diet (LFD; 8 WT and 6 KO) as control. a,b, Relative mRNA expression levels of Bmp8b and other TGFβ ligands were measured by RTqPCR (a) or NGS (b) in murine livers. The heat map represents relative basal expression level, from blue (lowest) to yellow to orange (highest). The dotted graph represents log2(fold change). Detailed NGS analysis is available in in Supplementary Table 8. The levels of Bmp8b mRNA transcript are almost absent in the normal liver and highly expressed in WD-induced NASH in K18+ cells (mainly hepatocytes) and αSMA+ cells (mainly activated HSCs and pericytes) by FISH (c; 2 replicates; staining repeated twice; magnification: ×40) as was also observed at protein level (Extended Data Figure 1). d, Liver to body weight ratio is increased by WD with no genotype-associated effects. WD-treated BMP8B-KO mice show reduced serum alanine aminotransferase (ALT) (e), SAF activity score (f,g; details in Supplementary Fig. 8a,b) and fibrosis (h,i; PSR stain quantified using Indica Lab HALO imaging software on the whole-tissue scanned slide). j, A trend in reduced circulating PRO-C3, a non-invasive biomarker of fibrosis, is also observed in WD-challenged BMP8B-KO mice. k–m, IHC immune cell profiling (WD) suggests a reduced infiltration into BMP8B-KO livers by CD3+ cells (mostly lymphocytes), LY6CG+ cells (mostly myeloid) and CD45R+ cells (mostly B cells) (k,l); this result is also confirmed by the immune cell-type deconvolution analysis (m) performed on the NGS data. n,o, Relative mRNA expression levels (whole-liver RNA) of genes involved measured by RTqPCR (n) and IPA analysis (o; NGS) confirm reduced inflammation, fibrosis and stellate cell activation in WD-challenged BMP8B-KO mice. All the results are shown as mean ± s.e.m.; biological replicates are represented as dot plots. Statistical significance was assessed by either two-tailed Student’s t-test (b,j,l; plus FDR for NGS), one-way ANOVA plus Fisher’s least significant difference multiple-comparison test (a,d,e,i,n), or by Kruskal–Wallis one-way ANOVA on ranks plus Dunn’s multiple-comparison test (g), when relevant. Lowercase letters indicate post hoc analysis significance: a, reference group; groups with different letters are statistically different per post hoc comparison; differences between groups with the same letter are statistically not significant per post hoc comparison.
To investigate the effect of the absence of BMP8B in advanced NASH fibrosis, we challenged BMP8B-KO and WT mice with a choline-deficient high-fat diet (CDHFD; Extended Data Fig. 6), known to induce F2 fibrosis after 12 or more weeks of challenge37. As previously observed, BMP8B-KO mice were comparable to WT mice with respect to body weight (Extended Data Fig. 6a), liver weight to body weight ratio (Extended Data Fig. 6b) and metabolic profile (Extended Data Fig. 6c). In contrast to the WD model, we did not observe protection from liver damage in BMP8B-KO mice: liver histology and circulating ALT and PRO-C3 levels were similar between genotypes (Extended Data Fig. 6d–j). However, gene expression profiling of the whole liver (Extended Data Fig. 6k) and of freshly isolated HSCs (Extended Data Fig. 6l) confirmed our previous observations of an impaired activation of the TGFβ signalling, and a reduced expression of proinflammatory genes and markers of HSC activation. These results suggested that the absence of BMP8B had an impact on the activation of the TGFβ–BMP signalling and inflammation in HSCs, but either inhibition of BMP8B might not be powerful enough to interfere with the fibrotic processes once they have been chronically activated, or the protection from fibrosis might be context- or model-dependent.
Comparative NGS analysis identifies wound-healing responses as the key pathways modulated by BMP8B in vivo
Last, we characterized the BMP8B-mediated mechanisms common to acute CCl4, PHx and WD, three models with strong and diverse effects on the hepatic wound-healing responses, to identify model-independent functions of BMP8B. Using IPA/URA (Fig. 8a–c; details in Supplementary Table 8), we focused on processes modulated by all the three challenges, and by the presence or absence of BMP8B. Compared with WT littermates challenged with the three treatments, BMP8B-KO mice exhibited impaired activation of TGFβ downstream effectors, pathways/upstream regulators controlling inflammation, liver regeneration/hepatocellular carcinogenesis and fibrosis/HSC cell transactivation. Notably, the activation status of most of these pathways was mirrored by recombinant BMP8 in the MPS NASH model (Fig. 6e).
Gene expression profiled by NGS, analysed with two-sided t-test (sample size as defined in Figs. 4, 5 and 7), and analysed by IPA in BMP8B-KO mice and WT mice following CCl4 (3 d), PHx (3 d) and WD (32 weeks) challenges. Details are in Supplementary Table 8. a,b, Prediction of canonical pathways (a) and upstream regulators (b) significantly enriched and predicted as activated (red) or inhibited (blue) according to IPA/URA. Data are shown as a heat map matrix format representing the activation (Z) score prediction by IPA. c, Graphical representation in networks of differentially modulated genes (green, downregulated; red, upregulated in the WD, KO versus WT comparison) leading to the predicted (–2 ≤ Z-score ≥ 2) inhibition (blue) of the upstream regulators according to IPA. d, Comparing the genes differentially modulated in the treated BMP8B-KO mice (versus WT), a subset of 171 genes was differentially modulated in at least 2 of the 3 datasets (P < 0.05; –0.378 ≤ log2(fold change) ≥ 0.378). e, Of these genes, 36 hits show the same direction of regulation in all the 3 datasets, thus being most directly associated with BMP8B absence independently from the challenge. Data are shown in a heat map with a matrix format representing the regulation (log2(fold change), KO versus WT).
Comparing the genes significantly modulated in these models (Fig. 8d), we also retrieved a list of hits significantly modulated in at least two of the models (KO versus WT comparison) and showing the same direction of modulation in the three groups. We identified 36 shared genes (Fig. 8e) featuring modulators of inflammation and/or NASH progression (for example, MCP1 (Ccl2 (refs. 38,39)) or OPG (Tnfrsf11b40,41), TGFβ signalling/HSC activation/fibrosis (for example, Col4a1/2 (ref. 42) or MCP1 (Ccl2 (refs. 38,39)) and proliferation/cancer (such as the VEGF target Angpt2 (ref. 43) or Ngfr44). Similarly, genetic ablation of BMP8B prevented the suppression of genes usually expressed in healthy livers (FA elongation, sterol metabolism, bile acid transporters and P450 coenzymes), including PPARα targets (Acot1 (ref. 45), Cyp2c8 (ref. 46), Sult2a8 (ref. 47)). PPAR upregulation (also featured by IPA; Fig. 8a) is particularly intriguing, as PPARs exert anti-inflammatory and anti-fibrotic activities in NASH, and are candidate targets for NASH treatment48.
Taken together, these data confirm that preventing the de novo induction of BMP8B (or inhibiting its function) might be sufficient to attenuate inflammation and chronic wound-healing responses; BMP8B could thus serve as a candidate therapeutic strategy to delay NASH development and progression.
Discussion
The incidence and prevalence of NASH is rising owing to the twin epidemics of obesity and metabolic syndrome. This emerging trend is occurring just as the other great global driver of chronic liver disease, viral hepatitis, is waning because of advances in hepatitis B vaccination and hepatitis C virus treatments. NASH usually occurs in the context of hepatic lipotoxicity, leading to hepatocellular damage that in turn causes compensatory proliferation, a proinflammatory and profibrotic microenvironment, ultimately leading to cirrhosis and HCC3,49,50.
We found that BMP8B was marginally expressed in the normal liver, but it was the member of the TGFβ–BMP pathways most consistently upregulated among acute and chronic hepatic injury models; we also found that BMP8B upregulation was disease-stage-dependent in murine models (Supplementary Table 1) and also in people (Fig. 1) with NASH. Our results point to BMP8B being an important mediator of NASH progression, as it drives both branches of the SMAD signalling pathway, and it promotes inflammation, wound-healing responses and NASH progression. To untangle the effect of BMP8B upregulation in these processes, we first studied BMP8B-KO HSCs and treated them with BMP8 (Figs. 2 and 3 and Extended Data Fig. 4): we found that absence of BMP8B was sufficient to attenuate HSC activation and their inflammatory phenotype and that recombinant BMP8 rescues their proinflammatory and profibrotic phenotype. HSCs contribute to liver inflammation, facilitating the recruitment of inflammatory cells, and producing multiple cytokines and chemokines30; intriguingly the absence of BMP8B also reduced the potential of the HSCs to modulate the behaviour of inflammatory cells (Supplementary Fig. 4c)30. Bmp8b thus was a likely contributor to the early events mediating HSC activation, triggering proinflammatory and profibrotic responses30. Using BMP8B-KO mice, we have shown that the absence of BMP8B leads to defective HSC activation, inflammation and compensatory regeneration in acute models of liver injury and/or hepatocyte loss (CCl4 and PHx), indicating that BMP8B may be a crucial effector of wound-healing responses orchestrating liver repair30.
We investigated whether upregulation of BMP8B in NASH was pathophysiologically relevant for NASH progression. As HSC activation is a consequence of the transition from NAFL to NASH, their contribution to the wound-healing response in NASH might have implicated BMP8B in early phases of NASH progression48,49. We posited that the selective proinflammatory effect of BMP8B, together with its capacity to promote HSC activation, could exert a central regulatory function determining NASH progression. In support of this hypothesis, we demonstrated, in both human and murine pre-clinical NASH models, that BMP8B modulation directs the TGFβ–BMP signalling pathways towards promoting proinflammatory responses, cell proliferation (crucial for both liver regeneration and potentially hepatocellular carcinogenesis) and HSC activation (Figs. 6–8 and Extended Data Fig. 6). The comparative NGS analysis (Figs. 6 and 8) confirmed a comprehensive role for BMP8B in modulating TGFβ–BMP downstream effectors11, pathways and upstream regulators involved in inflammation, proliferation/carcinogenesis, HSC activation and fibrosis30. Whereas the effect of BMP8B is more evident in the early proinflammatory stages, its influence on relatively late events, such as fibrosis, may be less relevant as suggested by both the in vitro MPS and the CDHFD models. This may be due to compensatory mechanisms occurring in advanced NASH and/or the extreme redundancy of TGFβ–BMP signalling.
Given that BMP8B is a secreted protein, not required for the normal function of the liver and selectively induced in liver disease, we propose that preventing the induction of BMP8B and/or blocking its extracellular activity could be a safe strategy to limit the progression of NASH and, potentially, of other liver diseases. More studies are needed to identify at which stage humans could benefit the most from this treatment and to develop an efficient in vivo inhibition that selectively targets BMP8B without interfering with the beneficial functions of the TGFβ–BMP system in liver (patho)physiology. However, on the basis of our results, we speculate that the inhibition of BMP8B could be beneficial to prevent the shift from NAFL to NASH and in the early phases of NASH progression to prevent HSC activation and inflammation.
Methods
Study population (people with NASH)
The Cambridge Cohort consisted of 40 consecutive men recruited at the NASH Service at the Cambridge University Hospital. The Newcastle/Paris Cohort consisted of 113 people recruited at the Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, under ethical approval EPoS-UK (REC 15/NE/0150) and Hospital Pitié-Salpêtrière, Sorbonne Université. Each participant had a clinical diagnosis of NAFLD (those with alternate diagnoses and etiologies were excluded), histology scored by either D.T. or S.D. according to the NASH CRN Scoring System (NAS5), and snap-frozen tissue for research purposes. This study was approved by the relevant Ethics Committees in the participating institutions. All participants gave their informed consent for the use of data (biochemistry and clinical history) and samples for research purposes; the principles of the Declaration of Helsinki were followed. A description of the study population is given in Supplementary Table 2.
Animals
All data are from male mice. C57Bl6/J mice were purchased from Charles River. BMP8B-KO mice were generated as previously described51 on a C57Bl6/J background and compared with WT littermates. Mice were maintained in a temperature-controlled room (21 °C) with a 12-h light/dark cycle with free access to food and water in pathogen-free facilities compliant with the FELASA Guidelines52. This research was regulated under the Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012, following ethical review by the University of Cambridge Animal Welfare and Ethical Review Body (AWERB), and by the USC Bioethics Committee of Newcastle University. Mice were fed a chow diet (Safe Diets, code ds-105) until they were enrolled into specific procedures.
Western diet studies
Western (Teklad, TD88137) and low-fat control (Teklad, TD08485) diets were administered ad libitum to 9-week-old animals for a total duration of the challenge of 32 weeks. Fat and lean mass was calculated by time-domain nuclear magnetic resonance (TD-NMR) by using a minispec Live Mice Analyzer LF50 (Bruker) the day of the culling. Serum and tissue collection were performed in the fed condition the day of euthanization.
Choline-deficient high-fat diet studies
BMP8B-KO mice and WT littermates underwent 14 weeks of feeding with l-amino acid rodent diet with 60% kcal fat and modified levels of choline and methionine, formulated by Research Diets (product code, A06071302) to model NASH and fibrosis37. Serum and tissue collection were performed in the fed condition the day of euthanization. The lateral left lobe of the liver was used for whole-tissue analyses, and the rest of the liver was used for HSC isolation. HSC GE analysis was studied in freshly isolated cells (that is, not cultured).
High-fat diet studies
Five-week-old WT mice were purchased from Harlan Laboratory. Temperature was maintained at 21 °C with a 12-h light/dark cycle. Seven lean controls were fed a regular chow diet (Rat & Mouse No. I Maintenance; Special Diet Services; diet code 801151) and 7 in 55% high-fat diet (energy composition: 55% fat, 29% protein and 16% carbohydrate; diet code: 829197; Special Diet Services) for 14 d.
Carbon tetrachloride studies
Sixteen-week-old BMP8B-KO or WT mice underwent i.p. injection of 2 µL g–1 body weight of the CCl4:olive oil (1:1 (vol/vol)) mix. Mice were humanely euthanized under isoflurane terminal anaesthesia, 3 or 5 d after the i.p. injection, by cutting the heart and IVC. Blood was collected from the chest cavity. These experiments were approved by the Animal Welfare and Ethical Review Board and carried out at Newcastle University Comparative Biology Centre under a UK Home Office license.
Partial hepatectomy
Twelve-week-old mice underwent two-thirds partial hepatectomy following the method of Higgins and Anderson31. The left lateral and median lobes were completely excised, and mice were humanely killed 3 d after the surgery during the expected peak of proliferation. Resected livers were used as a day 0 control for gene expression analyses.
Isolation and culture of primary hepatocytes
Hepatocytes were isolated using a two-step collagenase perfusion method: after cervical dislocation of mice, liver was exposed by midline incision and perfused at 37 °C in situ by cannulation of cava vein with a 26-G needle (Gilson 500-flux flow peristaltic pump at 6.4 mL min–1) with GIBCO Liver Perfusion Medium (cat. no. 17701, Invitrogen) containing 2% penicillin–streptomycin (P/S; cat. no. 15140) for approximately 10 min and, then GIBCO Liver Digestion Medium (cat. no. 17703, Invitrogen) for an additional 8–10 min. The digested liver was then kept in Leibovitz’s L-15 medium (cat. no. L1518, Sigma) containing 5% FBS in ice until the hepatocytes were detached and filtered using a plastic mesh with 100-µm pores. Hepatocytes were the centrifuged at 1,500 r.p.m for 2 min at 4 °C, washed twice with GIBCO hepatocyte wash medium (HWM) (cat. no. 17704, Invitrogen) containing 1% P/S, and then Percoll 42% (cat. no. P1644, Sigma Aldrich) in sterile PBS was used to remove dead cells. Live cells were then washed once with GIBCO hepatocyte wash medium (HWM) containing 1% P/S, and once with hepatocyte attachment medium (HAM) and Williams medium E (WME) in the presence of 5% FBS, 1% P/S and 1% glutamine (pH 7.4). Cells were then seeded in BD Biocoat Collagen I 12-well Multiwell Plates (BD Biosciences, Cat 356500) at a density or 20,000 or 100,000 cells per cm2 in HAM for additional 2 h, and then were maintained in the hepatocytes maintenance medium Williams medium E (WME) in the presence of 5% FBS, 1% P/S, 1% glutamine plus primary hepatocyte maintenance supplements (4% ITS and 70 µL dexamethasone; cat. no. CM 4000, Invitrogen) with or without BMP8 (30 ng mL–1) recombinant protein (R&D cat. no. 1073-BP).
Isolation, culture and activation of HSCs
Primary mouse HSCs were isolated from livers of BMP8B-KO or WT littermate controls. Liver tissue was digested with pronase and collagenase B (Roche) and the cell suspension was subsequently separated by an 11.5% Optiprep gradient (Sigma). HSCs were cultured into plastic (Corning) using DMEM supplemented with pyruvate (1%), glutamine (1%), penicillin–streptomycin (1%) and heat-inactivated FBS (during the activation process: 16%; in fully activated HSCs: 10%); all reagents were from Life Technologies. Freshly isolated (day 0) cells were considered quiescent and ‘day 7+’ cultures were regarded as fully activated. Cells were maintained in an incubator at 37 °C with 5% CO2. For the time-course experiments, HSCs were grown at a confluence of 35,000 cells per cm2 either on Corning well 6 plates (RNA) or (for IF) in Nunc Lab-Tek Permanox plastic Chamber Slide system (Sigma, C7182-1PAK) for the time-course during the activation process. The growing medium (with/without hBMP8 protein (30 ng mL–1); R&D cat. no. 1073-BP), or 100 μM oleic acid, or 100 μM palmitic fatty acid, or 30 ng mL–1 TNFα, or 50 ng mL–1 LPS, or 10 ng mL–1 PDGF was refreshed the day after the isolation, and every 2–3 d afterwards. Experiments involving fully activated HSCs were performed after HSC activation (day 8) in T75 plastic Flasks (Corning); sub-confluent (70%) cells were then plated at a confluence of 35,000 cells per cm2 either on Corning 6-well plates (RNA) or Perkin Elmer 96-well Cell-carrier Ultra Plate (pSMAD); after overnight (ON) culture, and 3 h FBS starvation, the cells where treated (for 0.5 h for SMAD phosphorylation; 5 h for RNA) in FBS-free culturing medium with or without recombinant human BMP8 protein (30–75 ng mL–1; R&D Cat 1073-BP), and/or ALK1/2/3/6 inhibitor (K02288, 1 µM), and/or ALK 4/5/7 inhibitor (A-8301, 5 µM), or TGFβ (5 ng mL–1), or BMP7 (5 ng mL–1).
AML12 culturing, maintenance and treatment
AML12 cells (ATCC CRL-2254) were maintained in DMEM/F12 high glucose (Invitrogen, cat. no. 11330-057) supplemented with 10% FBS, 1% penicillin–streptomycin antibiotics, 1% ITS (Insulin-transferrin-selenium supplement; Life technologies, cat. no 41400-045), 40 ng mL–1 dexamethasone (Sigma, cat. no. D4902-25MG). For the experiment, the cells were plated at a density of 75,000 cells per cm2 in growth medium on Corning 6-well plates (RNA) or Perkin Elmer 96-well Cellcarrier Ultra Plate (pSMAD). After 24 h of FBS and dexamethasone starvation, cells were treated for 30 min (pSMAD) or 5 h (RNA) with human BMP8 protein (30–75 ng mL–1; R&D cat. no.1073-BP), and/or ALK 1/2/3/6 inhibitor (K02288, 1 µM), and/or ALK 4/5/7 inhibitor (A-8301, 5 µM), or TGF-β (5 ng mL–1), or BMP7 (5 ng mL–1).
Microphysiological system—3D human in vitro NASH model
Cell cultures were performed in the microphysiological system (MPS), LiverChip (CN Bio Innovations), as previously described25,33. Briefly, 3D microtissue cultures were performed with cryopreserved primary human cells (hepatocytes, KC and HSCs), purchased from Life Technologies. Cells were cultured for one week in HEP–FAT medium (CN Bio Innovations) which contains a mixture of saturated and unsaturated FFAs, as well as physiologically relevant quantities of insulin and sugars. Complete medium changes were performed on all wells every 48 h. After 8 d, microtissues were cultured for up to 48 h in the presence of either: BMP8 (75 ng mL–1), ALK1/2/3/6 inhibitor (K02288, 1 µM), ALK4/5/7 inhibitor (A-8301, 1 µM), TGF-β (5 ng mL–1) or BMP7 (5 ng mL–1).
Isolation, culture, differentiation and treatment of primary bone marrow-derived macrophages
Femur and tibia bones from WT mice were isolated and cleaned, and 10 mL of RPMI-1640 (Sigma) was flushed through each bone using a syringe. Bone marrow cells were counted manually, pelleted by centrifugation, and resuspended in RPMI-1640 with 20–30% L929-conditioned medium, 10% heat-inactivated FBS (Gibco, Thermo Fisher Scientific) and 100 units mL–1 penicillin–streptomycin (Thermo Fisher Scientific) (macrophage differentiation medium). To differentiate into macrophages, cells were seeded in 10-cm non-culture-treated plates (Falcon) at a density of 5 × 106 cells per plate per 10 mL of macrophage differentiation medium and cultured for 7 da at 37 °C in 5% CO2. On day 5 of differentiation, medium was removed, and 10 mL of fresh macrophage differentiation medium was added to each plate. On day 7 of differentiation, macrophages were detached using ice-cold PBS containing 1 mM EDTA, counted manually, centrifuged at 500g, room temperature for 5 min and resuspended in macrophage differentiation medium at the concentration of 5 × 105 cells mL–1. Immediately after, cells were plated for experiments at the following densities: 100 µL of cell suspension per well of 96-well plate, 500 µL per well of 24-well plate, 1 mL per well of 12-well plate, 2 mL per well of 6-well plate and 10 mL per 10-cm plate.
To make L929-conditioned medium, L929 cells (CCL-1, ATCC) were seeded in DMEM supplemented with 10% heat-inactivated FBS, 100 units mL–1 penicillin–streptomycin and 2 mM l-glutamine (Sigma) at a density of 500,000 cells per 50 mL medium per T175 tissue culture flask. Medium was collected after 1 week of culture, and then 50 mL of fresh DMEM was supplemented with 10% heat-inactivated FBS, 100 units mL–1 penicillin–streptomycin and 2 mM l-glutamine was added onto cells and collected 1 week later. Batches obtained after the first and second weeks of culture were mixed at a 1:1 ratio and stored at –20 °C. Treatments were performed in FBS-free medium, with human BMP8 protein at 75 ng mL–1 (R&D Cat 1073-BP). Duration of the treatment: RNA, 1, 4, 8, 24 h; IL-1β secretion, 24 h; HSC conditional medium 25%, 24 h.
Energy expenditure
Energy Expenditure was measured by indirect calorimetry in a home-cage calorimetry chamber with 10-L capacity, as previously described53. Briefly, the calorimetry chamber was housed within a larger temperature-controlled room. Room air to the chamber was 21 °C, continuously monitored and fixed. Oxygen consumption and carbon dioxide production were measured using a custom built oxygen and carbon dioxide monitoring system. Measurements of oxygen concentration and carbon dioxide concentration in room air and air leaving each cage were measured every 11 minutes. Mice were kept in the calorimetry chamber for 48 h, and water/food consumption were also measured. Energy expenditure was then calculated from oxygen consumption and carbon dioxide by using a modified Weir equation54.
Analyses in serum and culturing media (biochemistry and proinflammatory panels)
Metabolic biochemistry and transaminases were measured on the Dimension RXL analyser (Siemens Healthcare) or Perkin Elmer DELFIA using reagents and calibrators purchased from Siemens. Free fatty acids were measured using the Roche Free Fatty Acid Kit (code 11383175001) modified to run in MicroTitre plate format. Insulin was measured using electrochemical luminescence immunoassay on the MesoScale Discovery immunoassay platform. Inflammatory markers were studied by MesoScale Discovery (MSD) multiplex assay kits. Assays were run in technical duplicates. A minimum of two quality-control samples were run in each assay. All these measurements were performed by the Biochemistry Assay Lab (CBAL) of the Metabolic Research Laboratories, University of Cambridge. PRO-C3, C3M and MMP9 were analysed by Nordic Bioscience A/S using competitive enzyme-linked immunosorbent assays (ELISAs) for the measurement of formation of type III collagen in human and rodents (PRO-C3 and rPRO-C3), MMP-9 mediated fragment of type III collagen (C3M) and circulating active MMP-9 (MMP9), as previously described for PRO-C3 and C3M55,56. The MMP-9 assay was run on a 96-well streptavidin plate coated with the appropriate biotinylated synthetic peptide dissolved in an optimized buffer and incubated for 30 min at 20 °C. Twenty microlitres of peptide calibrator or sample was added to appropriate wells, followed by 100 µL of conjugated monoclonal antibody raised against the amino-terminal part of activated MMP9. The plate was incubated for 20 h ± 1 h at 2–8 °C. Finally, 100 µL tetramethylbenzidine (TMB) Sens (Kem-En-Tec cat. no. 4850) was added and the plate was incubated for 15 min at 20 °C. All incubation steps were done with gentle shaking at 300 r.p.m. After each incubation step, the plate was washed five times in washing buffer (20 mM Tris, 50 mM NaCl, pH 7.2). The TMB Sens reaction was stopped by adding 100 µL of 1% H2SO4, and extinction was measured at 450 nm, subtracting the background at 650 nm. A calibration curve was calculated using a 4-paramtric fit model. All samples were measured within the detection range and with acceptable percent of coefficient of variation (CV%).
Tissue collection and histology
All animal tissues for protein or RNA extraction were frozen at time of collection unless otherwise stated. Samples for histology were placed in 10% buffered formalin overnight before transfer to 70% ethanol and later embedding in paraffin. Serial 4-µM sections were obtained from FFPE blocks and extra-coated with paraffin to preserve tissue integrity.
Histology
After overnight at 37 °C, sections were dewaxed with xylene and 100% ethanol, and washed in running water for minimum 2 min. The sections were stained with standard H&E or Sirius Red histochemical stains. For Sirius Red: Picro-Sirius Red (Pioneer Research Chemicals, PRC/R/109) was applied for 1 hh; sections were rinsed in 1% acetic acid (in dH2O) to ‘waterproof’ red staining and remove excess. Fast Green 1% in dH2O (Sigma, F7252-25G) was then incubated for 15 s and excess stain was rinsed in acetic acid 1% in dH2O (Fisher Scientific, A/0360/PB17) to remove excess stain. Sections were then dehydrated in graded alcohols, cleared in xylene and mounted.
Oil Red O staining of 3D cultures from the MPS in vitro model was completed as previously described33. Briefly, scaffolds containing microtissues were fixed in 4% PFA for 15 min, washed twice with 70% isopropanol and then stained for 1 h in Oil Red O solution (Sigma Aldrich). Tissues were washed three times in dH2O and twice in 70% isopropanol before colour bright-field images of stained scaffolds were taken using an inverted light microscope (Leica).
Immunohistochemistry
After overnight at 37 °C, sections were dewaxed with xylene and industrial methylated spirits, washed in running water for minimum 5 min, and kept always hydrated using TBST. The sections then underwent the following steps: (1) (Optional) target/antigen retrieval 25 min at 97.5 °C (pH 6.0, Vector Laboratories, H-3300 or pH 9.0, Vector Laboratories, H-3301); (2) washes in TBST; (3) blocking endogenous peroxidase for 5 min (DAKO Real Peroxidase Blocking solution, cat. no. S2023); (4) washes in TBST; (5) Blocking serum for 20–30 min (Animal-Free Protein Block, Vector Laboratories, SP-5030); (5) primary antibody incubation for 60 min at RT or ON at 4 °C (PCNA, DAKO cat. no. M0879—diluted 1:100; αSMA, SIGMA cat. no. A2547—diluted 1:100; CD3 (CD3–12), Abcam, cat no. ab11089—diluted 1:800; CD45R (RA3-6B2), Abcam, cat. no. ab64100—diluted 1:1,000; LY6C/G, Abcam, cat. no. ab2557—diluted 1:100 in antibody diluent DAKO, S3022; F4/80 (CI:A3-1) rat anti-mouse antibody, Bio-Rad, cat. no. MCA497—diluted 1:20); (6) washes in TBST for 5 min; (7) incubation for 30 min with MOM ImmPress Polymer Reagent (Vector Laboratories, cat. no. MP-2400) or ImmPRESS HRP Polymer (Vector Laboratories, MP-7451); (8) washes in TBST; (9) DAB solution (5–10 min), prepared following the manufacturer’s instruction (Vector Laboratories, peroxidase substrate kit DAB, cat. no. SK-5100; or, ImmPACT DAB, Vector Laboratories, SK-4105); (9) Washes in TBST; and (10) incubation (1 min) with Dako REAL haematoxylin (cat. no. S2020). The sections were then washed in tap water, dehydrated in graded alcohols, cleared in Xylene and mounted.
Immunofluorescence
HSCs and PHs were washed in cold, sterile PBS two times and fixated in 4 °C paraformaldehyde 4% for 10 min (cells)–20 min (tissues). All tissues were FFPE embedded. After being kept overnight at 37 °C, tissue sections were dewaxed with xylene and 100% industrial methylated spirits, washed in running water for a minimum of 5 min, and kept always hydrated using TBST; antigen revealing step (Citrate, pH 6) at 97% for 20 min was also performed in FFPE tissues. After PBS washing, cells/slides were treated with PBS + glycine 0.1 M for 15 min, membranes were permeabilized with TBS + Triton X-100 0.1% pH 7.4. After blocking in animal-free blocking solution (Vector Laboratories, SP-5030; 30 min at RT for HSCs; 2 h at RT for livers), tissues/cells were then incubated overnight (4 °C, in agitation) with primary antibody diluted in in blocking buffer (Vector Laboratories, SP-5035). Antibodies used were Sigma, anti-αSMA 1A4, cat. no. A2547, 1:200; R&D, anti-human/mouse BMP8B antibody, cat. no. AF6305, Concentration: 10 µg mL–1; Abcam, anti-cytokeratin 18, cat. no. ab181597, 1:100; Abcam, anti-cytokeratin 18 (C-04), cat. no. ab668, 1:100; Abcam, anti-mouse cytokeratin 19 antibody (EPNCIR127B), cat. no. ab133496, 1:100; R&D, anti-human/mouse albumin antibody, cat. no. AF3329-SP, 10 µg mL–1; Abcam, anti-CD68 antibody, cat. no. ab125212, 1:100; Thermo FIsher, anti-VE–cadherin polyclonal antibody, cat. no. 36-1900, 5 µg mL–1; Cell Signaling, anti-phospho-SMAD2 (Ser465/Ser467) (E8F3R), cat. no. 18338T, 1:300; Cell Signaling, anti-phospho-SMAD1 (Ser463/465)/SMAD5 (Ser463/465)/SMAD9 (Ser465/467) (D5B10), cat. no. 13820S, 1:100. After washing in TBST, and blocking as described in the previous sections, the secondary antibodies were incubated for 1 h at RT in blocking buffer, followed by Hoechst 33342 (Thermo, cat. no. H3570; 1:,2000 in TBS) for 5 min, and multiple TBST washes. Secondary antibodies used: Sigma, anti-rabbit highly cross-adsorbed CF 633, cat. no. SAB4600132, 1:500; Sigma, anti-mouse highly cross-adsorbed CF 568, cat. no. SAB4600075, 1:500; Thermo Fisher, anti-sheep cross-adsorbed Alexa Fluor 488, cat. no. A-11015, 1:1000; Thermo, anti-rabbit highly cross-adsorbed, Alexa Fluor 488, cat. no. A21206. For the FFPE tissues, True View Quenching Kit (Vector Laboratories, SP8400) was also applied for 3 min to reduce auto-fluorescence (murine studies). The slides where then mounted and kept at 4 °C. Pictures were taken with the Zeiss LSM 510 Meta (cells) confocal microscope, PerkinElmer Opera Phenix High Content Screening System (pSMADs) or Zeiss AxioScan Z1 (tissues) at ×10–×40, and analysis—percentage (%) of marked area or percentage (%) of marked nuclei—was performed using the ImageJ 1.8.0 software57, HALO (tissue slides) or using PerkinElmer Opera Phenix software (pSMADs).
FISH
Detection of mouse BMP8 was performed on FFPE sections using Advanced Cell Diagnostics (ACD) RNAscope 2.5 LS Multiplex Reagent Kit (cat. no. 322800), RNAscope 2.5 LS Probe Mm-BMP8B (cat. no. 570088) (ACD). Briefly, sections were cut at 4 µM thick, and baked for 1 h at 60*C before they were loaded onto a Bond RX instrument (Leica Biosystems). Slides were deparaffinized and rehydrated on board prior to pre-treatments using Epitope Retrieval Solution 2 (cat. no. AR9640, Leica Biosystems) at 95 °C for 15 min, and ACD Enzyme from the Multiplex Reagent kit at 40 °C for 15 min. Probe hybridization and signal amplification was performed according to manufacturer’s instructions. TSA plus fluorescein detection at 1:1,000 (Akoya Biosciences, cat. no. NEL741001KT) dilution of BMP8B were performed on the Bond Rx according to the ACD protocol. Slides were then removed from the Bond Rx and placed into TBS in preparation for standard immunofluorescence counterstaining (K18 and αSMA) that was performed as described in the previous sections.
Tissue imaging, quantification and scoring
All the tissue slides were scanned using a Zeiss AxioScan Z1 and analysed using HALO software (Indica Labs). The ‘Cytoplasmic & Nuclear IHC quantification’ module was used to quantify Nuclear PCNA (percentage of total nuclei stained with DAB), CD45R, CD3 and LY6CG; and nuclear roundness/area thresholds were used to avoid false positives (for example, PCNA staining of the nucleus of the inflammatory cells) when needed. αSMA and PSR staining (percentage stained area) were quantified using the ‘Area Quantification’ module. The ‘tissue Classifier module’, utilizing a state-of-the-art machine-learning algorithm to identify tissue types on the basis of colour, texture and contextual features, was used to distinguish damaged liver (red), inflammatory infiltrates (yellow), healthy liver (green) and vessels (blue). The analyses were performed in the whole scanned section to avoid selection bias; tissue edges and vessels were excluded using appropriate tissue annotation and optical density settings in all the quantifications. Halo was ‘trained by example’ on randomly selected images, and then the analysis was extended on the whole batch of sections with HALO’s fully automatized and unbiased pipeline. WD, CDHFD and CCl4 tissue slides were also scored by an expert liver pathologist (D.T.) while blinded, using NASH CRN and SAF scoring systems for NAFLD/NASH or a necro-inflammatory score (range 0–6) for CCl4-induced liver injury, as previously described4,5,58.
ELISA for IL-1β
BMDMs were treated as described in the legend of Supplementary Fig. 4, and culture supernatants were collected. IL-1β ELISA (DY401, R&D Systems) was performed on the culture supernatants according to manufacturer’s instructions.
Targeted phospho-proteomics
Protein phosphorylation in Extended Data Fig. 5b was evaluated on the lysates of the 3D co-cultures in Fig. 6 and Extended Data Fig. 5 treated for 30 min with BMP8; analysis was performed either with ELISA kits (SMAD1 (pS463/S465 + total), Abcam, cat. no. ab186035, and Total Human SMAD3 ELISA Kit, Novus Biologicals, cat. no. NBP2-76622) or by Luminex xMAP Custom Phospho-Panel multiplexed assays (cat. no. PR-CU060-KIT-17, Protavio); there were three biological replicates per treatment, and two (ELISA) or three (Luminex) technical replicates per analysis. pSMADs were normalized against their total proteins; all the other proteins have been normalized against the average of total SMAD1 and SMAD3.
RNA extraction and RNA integrity
RNA from cells was isolated using miRNAeasy Mini Kits (Qiagen), according to the manufacturer’s instructions. Tissue RNA (animal studies and human Cambridge cohort biopsies) was isolated using STAT-60 (AMS biotechnology, CS-502) according to the following procedure: tissue were homogenized in 1 mL of STAT-60 using a tissue homogenizer, mixed by vortexing and centrifuged at 13,000g (RT) for 5 min. The supernatant was then mixed by vortexing with chloroform (200 µL; Sigma, cat. no. 650471) and centrifuged at 12,000g for 15 min (4 °C). The supernatant was then mixed with isopropanol (500 µL; Sigma, cat. no. 33539) and centrifuged at 10,000g for 10 min at 4 °C to pellet RNA. The pellet was washed with 75% ethanol (1 mL) and allowed to dry until ethanol had completely evaporated, and was resuspended in RNAse-free water (Thermo Fisher). Human Newcastle/Paris cohort biopsies were lysed using Trizol (Sigma Aldrich), and mRNA was extracted with the Allprep DNA/RNA Micro kit (Qiagen). Samples were stored in aliquot at –80 °C prior to use. All reagents, plastic wares and supplies used were sterile, nuclease free and of molecular-biology grade. RNA purity (A260/A280 > 1.80) and concentration were determined using Nanodrop spectrophotometer (Thermo Fisher). RNA integrity was studied using the 2100 Bioanalyzer System (Agilent) and RNA 6000 Nano or Pico Kits (Agilent). A RNA Integrity Number (RIN) of 7 or 8 was considered the lowest cut-off for RTqPCR and NGS, respectively.
Lipid extraction and liquid chromatography mass spectrometry for the analysis of intact lipids
Tissue samples were extracted using approximately 50 mg of liver using an adaptation of the Folch methanol:chloroform extraction method59. Briefly, a metal bead (Qiagen) and 600 µL of chloroform:methanol 2:1 solution was added to each sample tube. Samples were homogenized using a Tissue Lyser (Qiagen) for 10 minutes at 30 Hz. Water (400 µL) was added, after which the samples were homogenized for a further 2 min. Samples were sonicated in a water bath for 10 min. Finally, samples were centrifuged (3,000g, 10 min) and the organic and aqueous layers were carefully separated and dried under nitrogen.
A 10-µL aliquot of the lipid extracts described above were diluted with 100 µL of 2:1:1 (vol/vol/vol) of HPLC-grade isopropanol/acetonitrile/water. The instrumentation comprised of a Thermo Scientific Accela Autosampler coupled to a Thermo Scientific Elite Iontrap-Orbitrap Hybrid mass spectrometer with a heated electrospray ionization (HESI) source (Thermo Scientific). Separation of the triglyceride lipid species was achieved by injecting 5 µL of each sample onto a Waters Acquity BEH (C18, 50 × 2.1 mm, 1.7 µm) column (Waters) maintained at 55 °C. Mobile phase A consisted of 10 mM ammonium formate in acetonitrile: water (6:4), and mobile phase B contained 10 mM ammonium formate in isopropanol: acetonitrile (9:1). The concentration of mobile phase B was increased from 40–99% in 4 min then equilibrated at 40% for 1 min with a flow rate of 1.0 mL min–1. The HESI source was operated in positive-ion mode with the source temperature maintained at 375 °C, the desolvation gas temperature was 380 °C and the gas-flow rate was set at 40 arbitrary units. Mass spectrometric data were collected in the range 100–2,000 m/z. Data processing was performed using the XCMS V 3.3 package (Bioconductor) within R (V 2.15.2, RStudio Inc, The R Foundation for Statistical Computing) to perform peak detection and alignment of the ions. The raw data were converted to the .mzML format using MSConvert V3 (ProteoWizard, http://proteowizard.sourceforge.net/news.shtml) and the script performed peak picking in the RT domain using the area under curve at full width half maximum using an inbuilt library for peak assignment and annotation. The data were normalized to the relevant internal standards for all annotated peaks.
Reverse transcription polymerase chain reaction
To generate cDNA, 1,000 ng (tissues) or 250–500 ng (cells) of RNA in 10 µL RNAse-free water was used according to the manufacturer’s protocol (Reverse Transcriptase System, Promega). All tubes were heated to 65 °C for 5 min and returned to ice. Ten microlitres of RT mix were then added, composed as follows: 4 µL M-MLV RT buffer (Promega M351A); 2 µL 25 mM MgCl2 (Promega A351B); 2.5 µL nucleotide triphosphate (dNTP, Promega U151B); 0.5 µL (100 mg mL–1) of random hexamers (Promega C118A); 1.0 µL of reverse transcriptase (RT; Promega M170b). Samples were incubated at 37 °C for 1 h. RT negatives were also used, as well as a titration curve from a pool of cDNAs (2 µL from each sample). Each sample was diluted 1:10 or 1:40 with RNAse-free water. Reverse transcription polymerase chain reaction was performed using TaqMan or SYBR green (Thermo Fisher) in a 13-µL real-time PCR reaction. For all experiments, the following PCR conditions were used: denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, then at 60 °C for 60 s. All primers were designed on Primer Express v3.0 and are available in Supplementary Table 9. Primers are available on request. Reactions were run in duplicate for each sample and quantified in the ABI QuantStudio 7 detection system (Applied Biosystems). Data were expressed as arbitrary units and expression of target genes corrected to the geometric average of three housekeeping genes (namely BestKeeper, BK): 18S, 36b4 and Tbp (murine data) or 18S, CYCA and GUSB (human data).
NanoString (Newcastle cohort)
Samples were run on the NanoString nCounter analysis system using a custom-made assay panel (Nanostring). Sample input was normalized to 100 ng where possible, or a maximum volume of 6 µL was used. Normalization to housekeeping genes RPL19, SRSF4 and YWHAZ was done using the nSolver 3.0 software (Nanostring).
Whole-transcriptome amplification and RNA sequencing (NGS)
RNA from tissues (2 µg RNA) or cells (300 ng RNA) were used to generate barcoded sequencing libraries using Illumina TruSeq Stranded mRNA Library Preparation Kit (Illumina) or, for Fig. 3 and Extended Data Figure 3, Takara Clontech SMARTer stranded total RNA-Seq Kit v2—Pico Input Mammalian (cat. no. 634412), following the manufacturer’s instructions. Samples failing to show amplification were removed from the experiment, and the amplification was repeated. The sequencing libraries were normalized for concentration and combined into pools of 96-plex. The pooled libraries were sequenced on 3 lanes of an Illumina HiSeq 4000 instrument at single-end 50-bp (SE50), yielding an average of >15 million reads per sample. Library preparation was performed by the Genomics and Transcriptomic Core at the Institute of Metabolic Science. The sequencing was performed at the Genomics Core, Cancer Research UK Cambridge Institute.
Statistics and reproducibility
WD, PH and CCL4 experiments were performed in two batches and the hypothesis that the two batches could perform differently was tested with multivariate approaches; this hypothesis was rejected and the data were merged using the whole cohort for the analysis. In vitro experiments were performed at least two times with four biological replicates (every biological HSC replicate is also a pool of HSC derived from three mouse livers). 3D microtissues and CDHFD experiments were performed once. All data are expressed as mean ± s.e.m. All analyses were performed using NCSS Statistical Package. Statistical significance (P ≤ 0.05) was assessed with two-sided Student’s t-test (two groups), paired t-test, one-way ANOVA plus Fisher’s least significant difference (multiple-comparisons), or MANOVA, when appropriate. Non-parametric analysis (Kruskal–Wallis one-way ANOVA on ranks plus Dunn’s multiple-comparison test) was used to assess significance of semi-quantitative measures (that is, histologic scoring)60. Data in Supplementary Table 1 were retrieved by publicly available databases available on NCBI GEO (https://www.ncbi.nlm.nih.gov/geo/) and analysed using the online tool GEO2R. RNA-sequencing data were aligned using TopHat V2.0.11 (ref. 61) to the mouse GRCm38 genome or ensembl v92, and genes were counted using HTseq-count (v0.8.0). The raw gene-level counts were then used for differential gene expression analysis (two-tailed t-test or GLM likelihood ratio, when appropriate) using LimmaR 3.28.14 and EdgeR 3.8.5.
The raw P values were then adjusted by the Benjamini–Hochberg procedure to control the FDR, the expected proportion of incorrectly rejected null hypotheses ("false discoveries")62.
Bioinformatics functional analyses
After statistical analysis, differentially expressed genes within groups were studied using the Ingenuity Pathway Analysis (Qiagen). We imputed in IPA the whole transcriptome and then filtered for analysis focused on only statistically significant (p<0.05) items with -0.378 ≤ log2(fold change) ≥ 0.378. Pathways and ‘Upstream Regulator’ networks showing relationships and interactions experimentally confirmed between differentially expressed genes and others that functionally interact with them, were generated and ranked in terms of significance of participating genes (p<0.05) and activation status (Z-score). A comparison analysis was then performed to focus only on those pathways significantly enriched in all the datasets of the same series (mice: WT – WD vs. LFD, WD – KO vs. WT; CCl4 vs. OO; CCl4 – KO vs. WT; cells: BMP8 vs. CTRL). We considered “biologically relevant” only those genes that are statistically significant (p<0.05), with a -0.378 ≤ log2(fold change) ≥0.378, and enriched in ‘significantly modulated’ pathways in the comparative analysis, and/or those genes with a FDR<0.05. We also performed a “deconvolution analysis” (ImmQuant Software) to for inferring immune cell-type composition from gene-expression data using average abundance (log2 CPM) of the whole transcriptome in the different groups tested, analysing the data against FACS-based and/or signature-based reference data from the ImmGem Consortium63.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. In vivo NGS data in this manuscript have been deposited in GEO, accession number GSE110404.
References
Younossi, Z. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20 (2018).
Azzu, V., Vacca, M., Virtue, S., Allison, M. & Vidal-Puig, A. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in non-alcoholic fatty liver disease. Gastroenterology 158, 1899–1912 (2020).
Vacca, M., Allison, M., Griffin, J. L. & Vidal-Puig, A. Fatty acid and glucose sensors in hepatic lipid metabolism: implications in NAFLD. Semin. Liver Dis. 35, 250–261 (2015).
Bedossa, P. et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 56, 1751–1759 (2012).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Andriopoulos, B. Jr. et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat. Genet. 41, 482–487 (2009).
Wang, W., Yang, Y., Meng, Y. & Shi, Y. GDF-3 is an adipogenic cytokine under high fat dietary condition. Biochem. Biophys. Res. Commun. 321, 1024–1031 (2004).
Cunha, S. I., Magnusson, P. U., Dejana, E. & Lampugnani, M. G. Deregulated TGF-beta/BMP signaling in vascular malformations. Circ. Res. 121, 981–999 (2017).
David, L., Feige, J. J. & Bailly, S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 20, 203–212 (2009).
Bonnardel, J., et al. Stellate cells, hepatocytes, and endothelial cells imprint the Kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity 51, 638–654 (2019).
Herrera, B., Addante, A. & Sanchez, A. BMP signalling at the crossroad of liver fibrosis and regeneration. Int. J. Mol. Sci. 19, E39 (2017).
Hernanda, P. Y. et al. SMAD4 exerts a tumor-promoting role in hepatocellular carcinoma. Oncogene 34, 5055–5068 (2015).
Zheng, Y. et al. Bone morphogenetic protein 2 inhibits hepatocellular carcinoma growth and migration through downregulation of the PI3K/AKT pathway. Tumour Biol. 35, 5189–5198 (2014).
Zhang, Y., Alexander, P. B. & Wang, X. F. TGF-beta family signaling in the control of cell proliferation and survival. Cold Spring Harb. Perspect. Biol. 9, a022145 (2017).
Kreidl, E., Ozturk, D., Metzner, T., Berger, W. & Grusch, M. Activins and follistatins: emerging roles in liver physiology and cancer. World J. Hepatol. 1, 17–27 (2009).
Martins, L. et al. A functional link between AMPK and orexin mediates the effect of BMP8B on energy balance. Cell Rep. 16, 2231–2242 (2016).
Whittle, A. J. et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149, 871–885 (2012).
Wu, F. J. et al. BMP8A sustains spermatogenesis by activating both SMAD1/5/8 and SMAD2/3 in spermatogonia. Sci. Signal 10, eaal1910 (2017).
Cheng, Z. et al. BMP8B mediates the survival of pancreatic cancer cells and regulates the progression of pancreatic cancer. Oncol. Rep. 32, 1861–1866 (2014).
Mima, K. et al. Gene expression of bone morphogenic protein 8B in the primary site, peripheral blood and bone marrow of patients with gastric cancer. Oncol. Lett. 6, 387–392 (2013).
Saito, T. et al. Genetic variations in humans associated with differences in the course of hepatitis C. Biochem. Biophys. Res. Commun. 317, 335–341 (2004).
Tryndyak, V. et al. Interstrain differences in the severity of liver injury induced by a choline- and folate-deficient diet in mice are associated with dysregulation of genes involved in lipid metabolism. FASEB J, 26, 4592–4602 (2012).
Stoyanov, E. et al. Chronic liver inflammation modifies DNA methylation at the precancerous stage of murine hepatocarcinogenesis. Oncotarget 6, 11047–11060 (2015).
Cubero, F. J. et al. TNFR1 determines progression of chronic liver injury in the IKKγ/Nemo genetic model. Cell Death Differ. 20, 1580–1592 (2013).
Kostrzewski, T. et al. Three-dimensional perfused human in vitro model of non-alcoholic fatty liver disease. World J. Gastroenterol. 23, 204–215 (2017).
Grefhorst, A. et al. Estrogens increase expression of bone morphogenetic protein 8b in brown adipose tissue of mice. Biol. Sex Differ. 6, 7 (2015).
Nakamura, T., Yoshimoto, K., Nakayama, Y., Tomita, Y. & Ichihara, A. Reciprocal modulation of growth and differentiated functions of mature rat hepatocytes in primary culture by cell–cell contact and cell membranes. Proc. Natl Acad. Sci. USA 80, 7229–7233 (1983).
Breher-Esch, S., Sahini, N., Trincone, A., Wallstab, C. & Borlak, J. Genomics of lipid-laden human hepatocyte cultures enables drug target screening for the treatment of non-alcoholic fatty liver disease. BMC Med. Genomics 11, 111 (2018).
Pellegrinelli, V. et al. Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nat Commun 9, 4974 (2018).
Pellicoro, A., Ramachandran, P., Iredale, J. P. & Fallowfield, J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 14, 181–194 (2014).
Fausto, N., Campbell, J. S. & Riehle, K. J. Liver regeneration. Hepatology 43, S45–S53 (2006).
Kan, N. G., Junghans, D. & Izpisua Belmonte, J. C. Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. FASEB J. 23, 3516–3525 (2009).
Kostrzewski, T. et al. A microphysiological system for studying nonalcoholic steatohepatitis. Hepatol. Commun. 4, 77–91 (2020).
Schuster, S., Cabrera, D., Arrese, M. & Feldstein, A. E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 15, 349–364 (2018).
Hall, Z. et al. Lipid zonation and phospholipid remodeling in nonalcoholic fatty liver disease. Hepatology 65, 1165–1180 (2017).
Boyle, M. et al. Performance of the PRO-C3 collagen neo-epitope biomarker in non-alcoholic fatty liver disease. JHEP Rep. 1, 188–198 (2019).
Matsumoto, M. et al. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Pathol. 94, 93–103 (2013).
Baeck, C. et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 61, 416–426 (2012).
Krenkel, O. et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 67, 1270–1283 (2018).
Bosselut, N. et al. Including osteoprotegerin and collagen IV in a score-based blood test for liver fibrosis increases diagnostic accuracy. Clin. Chim. Acta 415, 63–68 (2013).
Yilmaz, Y. et al. Serum levels of osteoprotegerin in the spectrum of nonalcoholic fatty liver disease. Scand. J. Clin. Lab. Invest. 70, 541–546 (2010).
Mann, J. & Mann, D. A. Transcriptional regulation of hepatic stellate cells. Adv. Drug Deliv. Rev. 61, 497–512 (2009).
Faillaci, F. et al. Liver angiopoietin-2 is a key predictor of de novo or recurrent hepatocellular cancer after hepatitis C virus direct-acting antivirals. Hepatology 68, 1010–1024 (2018).
Scheving, L. A., Zhang, X., Stevenson, M. C., Threadgill, D. W. & Russell, W. E. Loss of hepatocyte EGFR has no effect alone but exacerbates carbon tetrachloride-induced liver injury and impairs regeneration in hepatocyte Met-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G364–G377 (2015).
Franklin, M. P., Sathyanarayan, A. & Mashek, D. G. Acyl-CoA thioesterase 1 (ACOT1) regulates PPARα to couple fatty acid flux with oxidative capacity during fasting. Diabetes 66, 2112–2123 (2017).
Thomas, M. et al. Peroxisome proliferator-activated receptor alpha, PPARα, directly regulates transcription of cytochrome P450 CYP2C8. Front Pharmacol. 6, 261 (2015).
Feng, L. et al. Identification and characterization of a novel PPARalpha-regulated and 7alpha-hydroxyl bile acid-preferring cytosolic sulfotransferase mL-STL (Sult2a8). J. Lipid Res. 58, 1114–1131 (2017).
Hardy, T., Anstee, Q. M. & Day, C. P. Nonalcoholic fatty liver disease: new treatments. Curr. Opin. Gastroenterol. 31, 175–183 (2015).
Brunt, E. M. et al. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers 1, 15080 (2015).
Anstee, Q. M., Reeves, H. L., Kotsiliti, E., Govaere, O. & Heikenwalder, M. From NASH to HCC: current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 16, 411–428 (2019).
Zhao, G. Q., Deng, K., Labosky, P. A., Liaw, L. & Hogan, B. L. The gene encoding bone morphogenetic protein 8B is required for the initiation and maintenance of spermatogenesis in the mouse. Genes Dev. 10, 1657–1669 (1996).
FELASA working group on revision of guidelines for health monitoring of rodents and rabbits et al. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim. 48, 178–192 (2014)..
Virtue, S. et al. Peroxisome proliferator-activated receptor γ2 controls the rate of adipose tissue lipid storage and determines metabolic flexibility. Cell Rep. 21, 2005–2012.e7 (2018).
Elia, M. & Livesey, G. Energy expenditure and fuel selection in biological systems: the theory and practice of calculations based on indirect calorimetry and tracer methods. World Rev. Nutr. Diet 70, 68–131 (1992).
Nielsen, M. J. et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am. J. Transl. Res. 5, 303–315 (2013).
Barascuk, N. et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin. Biochem. 43, 899–904 (2010).
Girish, V. & Vijayalakshmi, A. Affordable image analysis using NIH Image/ImageJ 1.8.0. Indian J. Cancer 41, 47 (2004).
Wilson, C. L. et al. Ubiquitin C-terminal hydrolase 1: a novel functional marker for liver myofibroblasts and a therapeutic target in chronic liver disease. J. Hepatol. 63, 1421–1428 (2015).
Folch, J., Lees, M. & Stanley, G. H. S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Gibson-Corley, K. N., Olivier, A. K. & Meyerholz, D. K. Principles for valid histopathologic scoring in research. Vet. Pathol. 50, 1007–1015 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Benjamini, Y., Drai, D., Elmer, G., Kafkafi, N. & Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125, 279–284 (2001).
Frishberg, A., Brodt, A., Steuerman, Y. & Gat-Viks, I. ImmQuant: a user-friendly tool for inferring immune cell-type composition from gene-expression data. Bioinformatics 32, 3842–3843 (2016).
Acknowledgements
The authors are indebted to the following colleagues and institutions: MRC Metabolic Diseases Unit (MC_UU_00014/5): M. Dale, M. Campbell, R. Dias, the Disease Model Core (DMC; H. Webber, D. Hart, S. Grocott, C. Beresford, D. Jessop, E. Rasijeff and A. Warner), the Biochemistry Assay Lab (K. Burling and collaborators), the Genomics and Transcriptomics Core (M. Ma), the Histology Core (J. Warner) and the Imaging Core (G. Strachan). MRC Human Nutrition Research and Department of Biochemistry, University of Cambridge: F. Sanders, Z. Hall and J. West. Histopathology/ISH core facility of Cancer Research UK—Cambridge Institute: J. Jones for assistance with in situ hybridization. The human NASH histological samples come from the Human Research Tissue Bank of the Cambridge University Hospitals, which is supported by the NIHR Cambridge Biomedical Research Centre. M.V., J.L.G. and A.V.P. are supported by MRC programs (MRC MDU Programme Grant. PO 4050281695 ‘Lipotoxicity and the Metabolic Syndrome’ and MRC DMC MC UU 12012/2 to A.V.P.; Lipid Profiling and Signalling, MC UP A90 1006 to J.L.G.) and MRC adjunct funding as part of the Cambridge Initiative in Metabolic Diseases (Lipid Dynamics and Regulation: MC_PC_13030). M.V., M.A. and A.V.P. are also supported by the Cambridge NIHR Biomedical Research Center (Gastroenterology); M.V. is a recipient of the BRC Gastroenterology Pump-Priming award 2018/2019 that funded part of this study. F.O. is supported by MRC program Grants MR/K0019494/1 and MR/R023026/1. J.L. is supported by Medical Research Council PhD studentship and a CRUK program grant (C18342/A23390). Q.M.A., M.V., A.V.P., V.R., M.A. and D.T. are contributing members of the European NAFLD Registry. Q.M.A. is supported by the Newcastle NIHR Biomedical Research Centre (BRC). M.V. has been fellow of the Fondazione Umberto Veronesi in 2014. M.A., A.V.P. and J.L.G. received funding from the Evelyn Trust. M.V., O.G., D.T., M.A., F.O., Q.M.A., M.J.N., D.J.L. and A.V.P. are members of the EPoS (Elucidating Pathways of Steatohepatitis) consortium, which is funded by the Horizon 2020 Framework Program of the European Union under Grant Agreement 634413.
Author information
Authors and Affiliations
Contributions
M.V. and A.V.P. conceived and designed the study and wrote the manuscript. M.V., S.V. and V.P. designed and performed the WD experiment. J.L., F.O., M.V. and S.V. designed and performed the PHx and CCl4 experiments. M.V., S.S., T.K., Z.T. and K.P. performed the in vitro experiments. O.G., Q.M.A., V.R., M.E.D.A. and S.D. contributed with human data and samples. D.T. and S.D. scored the liver histology. Z.T. and W.L. contributed to the design of some of the in vitro experiments. M.J.N. and D.J.L. performed the collagen-3/MMP9 assays. Z.A. and J.L.G. performed liver lipidomics. B.Y.H.L. and M.V. analysed the NGS sequencing data. M.V. performed most of the analyses in all the models. All the authors provided useful criticism during the study, and critically reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
At the time of this study, T.K. and S.S. were employees of CN Bio Innovations; M.J.N. and D.J.L. of Nordic Bioscience and are among original inventors and patent holders for the PRO-C3, C3M and MMP9 assays. F.O. is a director of Fibrofind. J.L. and F.O. are shareholders in Fibrofind. The other authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Christoph Schmitt.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 In liver disease, BMP8b is overexpressed in Albumin and αSma positive cells.
a, Relative mRNA expression levels of Bmp8b measured by quantitative real-time polymerase chain reaction (RTqPCR) in murine livers. The levels of BMP8b mRNA transcript increase in Western Diet (WD)-induced NASH [(CTRL) n: 8; (WD) n:8], but not in High Fat Diet (HFD)-induced NAFL [(CTRL) n:5; (HFD) n:6], and in acute [3 days; (CTRL) n: 6; (CCl4) n:4] (3 days; n:4-5/group) and chronic [8 weeks; (CTRL) n: 5; (CCl4) n:5] carbon tetrachloride (CCl4) -induced liver damage. All the results are shown as mean ± s.e.m.; expression data of biological replicates represented as dot plots; statistical significance (vs. control treatment) assessed by two-sided Student’s t-test. b-c, Representative IF of Bmp8b and Albumin (Alb) or αSma protein expression: in Western Diet (WD)-induced NASH and following acute (3 days) CCl4 challenge Bmp8b is expressed in the liver, and co-localizes with both αSma and Albumin thus suggesting that Bmp8b is expressed in hepatocytes and activated HSC following hepatic damage (2 replicates / condition; staining repeated twice).
Extended Data Fig. 2 Bmp8b is overexpressed by primary hepatocytes when cultured in vitro.
a, Relative mRNA gene expression levels of Bmp8b measured by quantitative real-time polymerase chain reaction (RTqPCR) in PH cultured at low (20,000 cells/cm2) or high (100,000 cells/cm2) confluence to model a highly proliferative (b; Cyclin E1, Ccne1) or differentiated (c; Albumin, Alb) behavior, respectively. Bmp8b mRNA expression levels are very low at baseline, and induced from 24h after culturing. All the results are shown as mean ± s.e.m. Statistical significance was assessed by Multivariate Analysis of Variance (MANOVA; 5 replicates/group). d, Microarray data from publicly available database GSE122660 of human primary hepatocytes (PH) and human hepatocytes cell lines (HepG2 and Huh7) cultured in 2D and challenged for 72h with DMSO or a mixture of oleic (OA) and palmitic (PA) fatty acids (with/without TNFα) suggest that also human hepatocytes express BMP8B and that the challenges with fatty acids and/or pro-inflammatory factors do not influence BMP8B expression. Expression data were retrieved using the tool Geo2R from NCBI, and statistical significance was assessed using One-Way Analysis of Variance (ANOVA; 3 replicates/group).
Extended Data Fig. 3 Bmp8b is overexpressed by Primary Hepatic Stellate Cells when cultured in vitro.
Relative mRNA gene expression levels measured by RNA sequencing (a-c) or quantitative real-time polymerase chain reaction (RTqPCR; d) in murine primary hepatic stellate cells cultured at a density of 35,000 cells/cm2 and harvested before (day 0) and after culture (days 1, 4, 8, 12). Average mRNA abundance (Log2CPM) of BMP/TGFβ receptors and effectors (a), and of TGFβ/BMP family members (b): Artn, Bmp 2/8a, and Inhb b/c/e were suppressed; Bmp3/5/7/9/10 and Gdf9/10 were suppressed after a transient upregulation at day 1 of culture; Tgfβ2/3, Gdf6 and Inhba were upregulated (Nodal and Gdf3 were not expressed by HSC). c, IPA “upstream regulator” analysis based on time-dependent GE changes (NGS) in HSC at different stages of the trans-activation program compared to Day 1 (D1) of culturing shows activation of multiple TGFβ-related effectors Detailed NGS analysis (significantly modulated genes, IPA analysis) available in Supplementary Table 5; statistical significance was assessed by GLM likelihood ratio (edgeR) and then adjusted by the Benjamini-Hochberg procedure to control the False Discovery Rate (FDR). “Upstream Regulators” shown are all significantly enriched (P<0.05 - and with a 2≤Z-Score≥2) in at least one comparison. d, Time-dependent changes of Bmp8b expression in HSC treated with drivers of HSC activation (PDGFB 10ng/mL; Oleic Acid 100 μM; Palmitic Acid 100 μM; TNFα 30ng/mL; LPS 50 ng/mL). Bmp8b mRNA expression levels are very low at baseline and induced 24h after culturing (Area Under the Curve, AUC, in the small panel); Palmitic Acid significantly induces Bmp8b, while LPS reduces its expression over the time. All the results are shown as mean ± s.e.m. or in a heatmap format (representing gene abundance expressed as Log2CPM, or the degree of “Upstream Regulator” activation -Z-score- at the IPA “Upstream Regulator” Analysis). Sample Size: 4 biological replicates/group for panels a-c (each biological replicate is a pool of 3 livers); 3 replicates/time point for panel (d). Statistical significance was assessed by one-way analysis of variance (ANOVA) plus Fisher’s least significant difference test (d). Lowercase letters indicate post hoc analysis significance: a, reference group; groups with different letters are statistically different per post hoc comparison; differences between groups with the same letter are statistically not significant per post hoc comparison.
Extended Data Fig. 4 Recombinant BMP8 rescues the defect in HSC activation observed in Bmp8b KO HSC cultured in vitro.
Freshly isolated HSC from Bmp8b KO mice and WT littermates were cultured for 4 and 6 days at a density of 35K cells/cm2 in presence/absence of recombinant BMP8 (30 mg/mL) to study the effect of BMP8b gain of function/recovery in both KO and WT cells. αSMA protein expression (a,b, IF; staining repeated twice in each biological replicate; magnification: 10X), and gene expression (RTqPCR) of multiple markers of HSC transactivation (c), inflammation (d) and TGFβ/BMP targets (e) were checked to study HSC’s activation status (4 biological replicates/group; each biological replicate is a pool of three livers). All the results are shown as mean ± s.e.m. Statistical significance was assessed by Multivariate Analysis of Variance (MANOVA).
Extended Data Fig. 5 BMP8 stimulates TGFβ/BMP signaling in a human 3D in vitro NASH model promoting inflammation and proliferation (continues from Figure 6).
a, BMP8b mRNA expression (RTqPCR) in the cells cultured without/with medium containing a mixture of saturated and unsaturated FFAs (Lean vs. Fat; n: 6 replicates/group). b, Targeted phospho-proteomics data of cells studied 30 min after BMP8 challenge (vs. Control; 3 biological replicates/group) in cells cultured in “Fat” medium; c, TGFβ/BMP targets studied by BMP8b mRNA expression (RTqPCR) studied after 2 challenges (every 24h) of BMP8, TGFβ, or BMP7 (in “Fat” medium; cells and media were harvested 48h after commencing the challenges; n: 4 replicates/group). d, Secreted proteins quantified in the culturing media of 48h treated cells (n: 4 replicates/group); e, IPA “upstream regulator” analysis of the RNA sequencing of cells treated with recombinant BMP8 for 5h vs 48h (4 replicates/group; full list of genes differentially regulated and statistical design are provided in Supplementary Table 7). All the results are shown as mean ± s.e.m. (expression data of biological replicates are represented as dot plots), or in a heatmap format [representing the degree of “Upstream Regulator” activation (-2 ≤ Z-score ≥2) at the IPA “Upstream Regulator” Analysis]. To provide a framework of interpretation of the sequencing data, we clustered the results in “early response” regulators (modulated at 5h; not modulated at 48h), “persistent response” regulators (modulated both at 5h and 48h with the same direction), “late response” regulators (mildly/not regulated at 5h and modulated at 48h), “biphasic” regulators (showing opposite direction of regulation between 5h and 48h data). Statistical significance was assessed by two-sided Student T-Test (a,b), or by One-Way analysis of variance (ANOVA; c, d) plus Fisher’s least significant difference test (n: 4 replicates/group). Lowercase letters indicate post hoc analysis significance: a, reference group; groups with different letters are statistically different per post hoc comparison; differences between groups with the same letter are statistically not significant per post hoc comparison.
Extended Data Fig. 6 Bmp8b KO mice challenged with CDHFD model (NASH F2 fibrosis).
BMP8b KO and wild-type littermates mice were treated for 14 weeks with a choline deficient high-fat diet (CDHFD – N: 9WT & 6KO). CDHFD -treated Bmp8b KO mice show no difference in BW (a), Liver to body weight percent ratio (b: LW/BW%), glucose and lipid metabolism (c), ALT (d), NASH activity (e, H&E; f, NASH activity score) and Fibrosis (g, Picro-Sirius Red, PSR; h, “Kleiner” Fibrosis Stage; i, PSR quantification (% of stained area quantified using HALO imaging software, Indica Lab) on the whole-tissue scanned slide; j, Procollagen C3, PRO-C3). However, the relative mRNA expression levels of key genes measured by RTqPCR in the livers (k), and in freshly isolated HSC (l; Sample size: 6WT & 3 KO HSC pools) show impaired activation of TGFβ/BMP signaling, reduced inflammation, and defective HSC activation in Bmp8b KO mice compared to WT littermates. a-l, All the results are shown as mean ± s.e.m. (biological replicates are represented as dot plots). Statistical significance was assessed by two-sided Student T-Test.
Supplementary information
Supplementary Information
Supplementary Figures 1–8 and Supplementary Tables 1–4
Supplementary Data
Supplementary Tables 5–9
Rights and permissions
About this article
Cite this article
Vacca, M., Leslie, J., Virtue, S. et al. Bone morphogenetic protein 8B promotes the progression of non-alcoholic steatohepatitis. Nat Metab 2, 514–531 (2020). https://doi.org/10.1038/s42255-020-0214-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-020-0214-9
This article is cited by
-
Metabolic dysfunction-associated steatotic liver disease and steatohepatitis-associated hepatocarcinoma preclinical models
Nature Reviews Gastroenterology & Hepatology (2026)
-
A microphysiological model of human MASLD reveals paradoxical response to resmetirom
Communications Biology (2026)
-
Osteokines in Nonalcoholic Fatty Liver Disease
Current Obesity Reports (2024)
-
Inhibition of hepatic oxalate overproduction ameliorates metabolic dysfunction-associated steatohepatitis
Nature Metabolism (2024)
-
An unbiased ranking of murine dietary models based on their proximity to human metabolic dysfunction-associated steatotic liver disease (MASLD)
Nature Metabolism (2024)