Abstract
Intercellular mitochondria transfer is an evolutionarily conserved process in which one cell delivers some of their mitochondria to another cell in the absence of cell division. This process has diverse functions depending on the cell types involved and physiological or disease context. Although mitochondria transfer was first shown to provide metabolic support to acceptor cells, recent studies have revealed diverse functions of mitochondria transfer, including, but not limited to, the maintenance of mitochondria quality of the donor cell and the regulation of tissue homeostasis and remodelling. Many mitochondria-transfer mechanisms have been described using a variety of names, generating confusion about mitochondria transfer biology. Furthermore, several therapeutic approaches involving mitochondria-transfer biology have emerged, including mitochondria transplantation and cellular engineering using isolated mitochondria. In this Consensus Statement, we define relevant terminology and propose a nomenclature framework to describe mitochondria transfer and transplantation as a foundation for further development by the community as this dynamic field of research continues to evolve.
Similar content being viewed by others
Main
Mitochondria are functionally and structurally diverse organelles that are essential for the health of nearly all eukaryotic cells1,2. These organelles are frequently associated with their roles in energy metabolism as the sites where the citric acid cycle, β-oxidation, electron transport chain, oxidative phosphorylation and other metabolic pathways occur. However, mitochondria have many other important functions, including cell signalling, viral sensing, cellular calcium homeostasis, redox homeostasis, iron handling, haem and lipid synthesis, cell division and cell death1. Phylogenetic and fossil record studies indicate that mitochondria evolved through an ancient endosymbiotic process in which alphaproteobacteria capable of aerobic respiration were engulfed by an anaerobic archaeal cell. The transition from an endosymbiosed bacteria to an ancestral organelle that gave rise to modern respiring mitochondria involved the development of complex protein transport systems, massive loss of thousands of alphaproteobacterial genes to the host nuclear genome, highly integrated organelle division processes and bioenergetic flexibility and function3,4,5. This process enabled complex multicellular life to form, as evidenced by fossil records dating to approximately 1.6 billion years ago and the evolution of all extant eukaryotic organisms today6,7,8,9.
Over the past approximately 20 years, we have come to understand that mitochondria can be transferred from one cell to another, a process that is termed horizontal or intercellular mitochondria transfer. Although the origins of mitochondria transfer are unclear, it may be a preserved relic of their alphaproteobacterial ancestry that confers a selective advantage to multicellular eukaryotes. Indeed, mitochondria transfer has been observed in evolutionarily diverse eukaryotes, including yeast10,11,12, molluscs13, fish14 and rodents15 as well as human cells16, and mtDNA transfer has been reported between cells in plants17,18,19. We are just beginning to understand the physiological functions of mitochondria transfer, how alterations in this process contribute to disease pathogenesis and how to harness mitochondria transfer biology to develop new therapies.
ICMTTN process and scope of review
The rapid pace of research on mitochondria transfer has created the need for clarity about terminology used in this field. To address this, we organized an International Committee on Mitochondria Transfer and Transplantation Nomenclature (ICMTTN), consisting of an international team of 31 investigators (working in 13 countries) in the field or highly relevant topics to assimilate a contemporary understanding of mitochondria transfer pathways and therapeutic approaches and provide guidance about recommended terminology and characterization standards (Fig. 1). The ICMTTN convened as a full group twice and operated in two geographically defined groups working in parallel. Each group met three times and each participant was afforded dedicated time for presentation of their ideas followed by group discussion. The chair (J.R.B.) and co-chair (K.K.S.) participated in both working groups to provide continuity but presented their ideas once. An initial draft consensus document was created, and each participant was invited to provide comments and suggestions over a 4-week period. Following incorporation of these improvements, we held a 30-day public commenting period hosted by MitoWorld (www.mitoworld.org) and considered suggestions and critiques before peer review.
In phase I, an international committee was formed and divided into two groups based on geography. After an introductory meeting with all members, groups A and B met three times. Each co-author held the floor to present nomenclature considerations, followed by group discussions. In phase II, an outline was presented to all co-authors for discussion before preparing the article. In phase III, a preliminary version of the article was posted on MitoWorld’s website for a public commenting period. In phase IV, the committee finalized the manuscript before formal peer review.
We begin by providing an abbreviated history of the field, highlighting some of the important milestones that have advanced our understanding of mitochondria transfer and transplantation. We then establish definitions of basic terms and concepts regarding mitochondria transfer and transplantation (Box 1). We recommend nomenclature for cell contact-dependent and contact-independent mitochondria transfer processes and for therapeutic approaches involving mitochondria transplantation. We recognize that this field is evolving quickly and that these recommendations may not always fully conform to future insights and conceptual advances. Therefore, we present this nomenclature framework below as a guide and set of recommendations that will probably need to be revisited in future years.
A brief history of the field
Foundational early discoveries
The earliest discoveries about intercellular mitochondria transfer were based on foundational observations about the ability of cells to acquire mitochondria and their components from their environment (Fig. 2). In 1977, it was reported that mitochondrial genotypes could be obtained by ρ0 yeast protoplasts, which lacked mtDNA, by fusing them with protoplasts of wild-type cells20. These results were extended a few years later using isolated mitochondria or enucleated cells10,11,12. In 1982, it was shown that isolated mitochondria from chloramphenicol and efrapeptin-resistant mouse tumour cells could be endocytosed by drug-sensitive tumour cells in culture and confer resistance, indicating that uptake of extracellular mitochondria could genetically transform drug-sensitive cells21. These results were later recapitulated by microinjection of isolated mitochondria into human tumour cell lines22.
It was then shown that injection of myoblast mitochondria harbouring a pathogenic mtDNA tRNALys mutation into mtDNA-deficient cell lines formed cytoplasmic hybrid cells called ‘cybrids’ and that the donor mtDNA conferred defects in mitochondrial protein translation and cytochrome c oxidase activity23. A few years later, it was shown that platelets24 and sperm25 from healthy individuals can deliver mtDNA to ρ0 cells to form cybrids with rescued aerobic respiration, and later breast cancer cybrids harbouring the G10398A mtDNA variant were reported to have apoptotic resistance and enhanced metastatic potential26. Although horizontal mtDNA gene transfer was found to occur between cells in flowering plants17,18, broadening the diversity of organisms known to exchange mitochondrial components, there is not yet direct evidence for the transfer of intact mitochondria between plant cells.
Recent studies on mitochondria transfer
In 2006, it was reported that human bone-marrow-derived mesenchymal stem cells or fibroblasts could deliver mitochondria to neighbouring A549 ρ0 lung carcinoma cells in culture to rescue cell-intrinsic defects in aerobic respiration16, providing the first evidence that mitochondria transfer could confer metabolic properties to acceptor cells. This was confirmed shortly thereafter using isolated mouse mitochondria taken up by human ρ0 cancer cells27, although we recognize that in healthy cells mouse mitochondria can fuse with human mitochondria but are not maintained over long periods of time due to mito-nuclear conflict28.
A series of studies have since demonstrated that mitochondria transfer occurs in vivo in complex animal systems. In 2011, it was reported that canine transmissible venereal tumour cells obtain mitochondria from host cells29, and the next year it was shown that bone-marrow stromal cells transfer mitochondria to pulmonary alveoli to dampen endotoxin-induced acute lung injury in mice15. Several years later it was shown that ρ0 tumours obtain mtDNA30 via intact mitochondria31 from host cells in mice to support tumorigenesis by promoting de novo pyrimidine synthesis32. In 2016, it was also shown that mouse oocyte differentiation is regulated in part by mitochondria transfer from sister cyst germ cells33. That same year, retinal ganglion cells were shown to shed damaged mitochondria and deliver them to adjacent astrocytes for degradation, establishing the concept of transmitophagy or licensed mitophagy, where one cell type borrows the degradative function of another34. Transmitophagy is now known to occur in several organ systems such as the heart35,36, brown adipose tissue37 and retina14,34 to preserve mitochondrial homeostasis of the donor cells38. Furthermore, platelets were shown to release mitochondria upon activation39, a process that was recently linked to wound healing40.
We now know that many cell types participate in mitochondria transfer in the central nervous system41,42,43,44, peripheral nervous system45, lung15,46,47,48, heart35,49, white adipose tissue50,51, brown adipose tissue37,51, bone52,53,54, haematopoietic system39,55,56,57 and the tumour microenvironment30,31,58,59. In some cases, respiration-competent mitochondria are released into the blood of mice and humans39,60, serving as a conduit through which cells in one organ can transfer mitochondria to cells in another organ (interorgan mitochondria transfer). This was shown with the delivery of adipocyte-derived mitochondria to the heart in obesity51,61; however, it is likely that many cell types contribute the pool of circulating extracellular mitochondria (ex-Mito) in blood. Mitochondria transfer has been found to regulate a diverse set of physiological processes, including regulation of adipose tissue homeostasis, cardiovascular health, wound healing, angiogenesis, haematopoiesis and inflammation, topics that were recently reviewed in more detail elsewhere38,62,63,64,65.
Development of therapeutic approaches
There has been considerable effort to harness the biology of mitochondria-transfer pathways for therapeutic purposes. These approaches involve some aspects of mitochondria-transfer biology, such as cellular uptake mechanisms, but are procedures to deliver mitochondria to an intended organ and/or cell type. One of the earliest studies attempting to use isolated mitochondria for therapeutic purposes was the isolation and transplantation of cardiac mitochondria into the hearts of rabbits subjected to ischaemia followed by reperfusion, an intervention that reduced ischaemia-reperfusion injury and improved functional recovery66. A similar finding was later observed in pigs67. In subsequent clinical trials, skeletal muscle mitochondria were isolated from paediatric patients requiring extracorporeal membrane oxygenation (ECMO) for autologous transplantation, allowing most of the patients to be successfully separated from ECMO faster68,69. Mitochondria transplantation is now being developed for other therapeutic indications, including, but not limited to, ischaemic stroke, ischaemic limb injury, spinal cord injury, neurodegeneration, cardiac resuscitation, refractory dermatomyositis or polymyositis, and inherited mitochondrial diseases43,70,71,72,73,74,75,76,77,78,79,80.
Furthermore, isolated mitochondria can be modified or implanted in gel matrices to improve mitochondria uptake efficiencies or therapeutic benefits74,81,82. Other groups have used isolated mitochondria to metabolically engineer cells in culture. For example, it was shown that culturing haematopoietic stem cells (HSCs) with isolated mitochondria leads to improved engraftment in mice receiving bone-marrow transplants83. This method was used in a clinical trial where HSCs from patients with large-scale mtDNA deletion syndromes were co-cultured with maternal mitochondria in vitro before autologous HSC transplantation84. These patients had reduced anaemia and improved quality of life. These encouraging studies suggest that therapies involving mitochondria transfer biology have the potential to treat a variety of diseases, calling for robust nomenclature that can empower and harmonize academic and industrial efforts to understand and leverage this biology to improve human health.
Basic concepts and terms
Mitochondria transfer
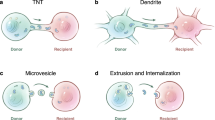
As cells differentiate and divide, their mitochondria are passed on to the two daughter cells. This process is referred to as ‘vertical inheritance of mitochondria,’ a term that specifically relates to mitochondria sorting during cell division, which can be asymmetric in some cases such as stem cell differentiation85,86. This vertical process is not mitochondria transfer. By contrast, horizontal or intercellular ‘mitochondria transfer’ is a process in which one cell donates some of its mitochondria to another cell in the absence of cell division (Fig. 3a). The ‘donor cell’ is the cell from which the transferred mitochondria originate, and the cell that obtains the transferred mitochondria is referred to as an ‘acceptor cell’. The term ‘acceptor cell’ applies equally well when that cell retains, degrades or re-exports the transferred mitochondria and is intended to be agnostic of fate or processing. The donor and acceptor cells are frequently but not necessarily developmentally distinct cell types.
a, Vertical inheritance of mitochondria occurs during cell division to pass on mitochondria to daughter cells. Horizontal or intercellular mitochondria transfer occurs in the absence of cell division and may occur between developmentally distinct cell types. b, The cell of origin is known as the donor cell, and the cells that obtain the transferred mitochondria are known as acceptor cells. When the donor and acceptor cells are defined in vivo, this is referred to as a mitochondria transfer axis. c, A mitochondria transfer network is the co-occurrence of multiple mitochondria transfer axes in parallel or in series. d, Interorgan mitochondria transfer occurs when cells in one organ release their mitochondria into a circulating body fluid, such as blood, for delivery to cells in another organ. e, Contact-dependent mechanisms of mitochondria transfer. Tunnelling nanotube-mediated mitochondria transfer occurs when two or more cells form connections that permit direct delivery of mitochondria from the cytoplasm of one cell to that of another. Dendritic structure-mediated mitochondria transfer is a process in which one cell extends a tubular structure that makes contact to acceptor cell via end feet. The cytoplasms of the two cells are not directly connected, as is the case with tunnelling nanotubes. Adhesion-mediated mitochondria transfer occurs when two cells make direct physical contact, sometimes mediated by a gap junctional channel such as Cx43. The mitochondria are transferred to the acceptor cell not via the channel pore, which is too small to permit passage of large cargo, but by an endocytic mechanism at the cell-to-cell interface. f, Contact-independent mechanisms of mitochondria transfer. Donor cells can release mitochondria into the extracellular space in various forms, and these can then be imported by acceptor cells. All these forms can be referred to as extracellular mitochondria (ex-Mito). One subset of ex-Mito is known as free mitochondria, which are not enclosed within an extracellular vesicle (EV). There are several subsets of EVs containing mitochondria (EV-Mito) that differ based on their size, type of mitochondrial cargo, and mechanisms of release. Cargo can include intact mitochondria or mitochondria-derived vesicles (MDVs). The mechanisms that regulate the release and uptake of ex-Mito remain the subject of ongoing investigation.
A ‘mitochondria transfer axis’ can be defined when both the donor and acceptor cell types are established in vivo (Fig. 3b). As one of many possible examples, adipocytes transfer mitochondria to macrophages in white and brown adipose tissues in mice, establishing an adipocyte-to-macrophage mitochondria transfer axis37,50. Adipocytes also seem to transfer some of their mitochondria to several other cell types, evoking the concept of a ‘mitochondria transfer network’ to reflect that cells may participate in multiple mitochondria transfer axes that can occur in parallel or stacked in series51 (Fig. 3c).
Furthermore, adipocytes can release their mitochondria into the blood, allowing them to be transported to other organs, such as the heart51,61. These studies establish the concept of ‘interorgan mitochondria transfer,’ a process that is defined as long-distance mitochondria transfer axis between a donor cell in one organ and an acceptor cell in another organ (Fig. 3d). In this case, the blood39,51,60,61, lymph or cerebrospinal fluid87 may serve as a conduit to distribute ex-Mito from the donor cell to the acceptor cell. The ex-Mito population is heterogeneous, containing multiple different types and from different cellular sources, a topic that is discussed in more detail below.
Some studies have used the term ‘mitochondria transfer,’ whereas others have used ‘mitochondrial transfer.’ The term ‘mitochondria transfer’ uses mitochondria as a noun, reflecting that a cell can transfer mitochondria (noun) to another cell. The term ‘mitochondrial transfer’ uses the adjective form ‘mitochondrial’ to describe a property or characteristic of the transfer. From a practical perspective, these two terms are synonymous and have been used interchangeably. Therefore, we suggest that ‘mitochondria transfer’ and ‘mitochondrial transfer’ are both acceptable and that the choice of which term to use is an investigator-specific preference.
Therapeutic approaches
There are also several emerging therapeutic approaches that involve mitochondria transfer pathways or mechanisms. One is called ‘mitochondria transplantation,’ a procedure in which mitochondria are isolated from a cellular or tissue source and then administered directly to an animal with the intent of eliciting a therapeutic response. Many studies have alternatively used the term ‘mitochondrial transplant,’ which is also acceptable. In this article, we use the term ‘mitochondria transplant’ to conform with well-established transplant terminology, where the noun form of the transplanted organ is used (for example heart transplant). As discussed in more detail later in this review, mitochondria transplantation has been employed in preclinical studies using model organisms70,88,89,90 and in recent clinical trials with promising early results66,68,69,91, including ongoing clinical trials that have not yet been published.
Another therapeutic approach involves in vitro cell engineering where cells obtained from a patient are exposed to isolated mitochondria or co-cultured with cells that deliver mitochondria as part of the cell-manufacturing process83. The latter approach was used in a recent clinical trial with encouraging findings, as discussed below84 These approaches have also been used to support the survival92 and boost the efficacy of engineered cells, such as chimeric antigen receptor (CAR) T cells93.
Methods to define mitochondria transfer
Mitochondria reporter proteins
These basic terms raise a methodological question about how mitochondria transfer can be defined at a practical level. This topic has been discussed recently94 so is covered here only briefly. There are multiple methodological approaches to detect mitochondria transfer. The most robust method is to use fluorescent reporter proteins attached to mitochondria localization signals, which are short polypeptide sequences that direct the fluorescent protein to the inner or outer mitochondrial membrane or the mitochondrial matrix. There are several mitochondria reporter protein constructs that can be used under the control of a stop-flox system, allowing one to define a donor cell based on the cell type specificity of a Cre recombinase14,50,95. One can then define a mitochondria transfer axis or network by identifying the labelled mitochondria in other cell types50,51. Similarly, one can express other mitochondrially targeted proteins (for example Halo96,97) that allow the detection of mitochondria transfer.
An important limitation of this approach is it can detect mitochondria transfer only if the mitochondria reporter protein is contained within the transferred mitochondrial structure. This is not always the case. One example is that a fraction of mitochondria-derived vesicles (MDVs) contain highly selected cargo and are generated as either single, outer membrane vesicles or double-membrane vesicles98,99; therefore, a fluorescent reporter protein targeted to the inner mitochondrial membrane (IMM) would not report all MDV transfer events. MDVs are transported into multivesicular bodies and can be released as exosomes. There is indeed evidence of selective sorting of mitochondrial components in MDVs that are ejected from cells. For example, brown adipocytes transfer MDVs to macrophages, and those MDVs lack the quintessential brown adipocyte IMM protein uncoupling protein 1 (ref. 37).
Mitochondrial dyes
An alternative fluorescence-based method used in many studies is to stain donor cells with fluorescent mitochondrial dyes and track the transferred mitochondria in another cell type in vitro. Although this method may initially seem attractive due to the low effort and cost required to test an idea related to mitochondria transfer, these dyes are leaky and highly susceptible to producing false-positive results, even after extensive washing100,101. All mitochondrial dyes probably efflux from the donor cell in situ during co-culture, creating dye transfer artifacts that drastically over-estimate mitochondria transfer efficiency or suggest that mitochondria transfer occurs when in fact it does not. As one of many examples, mature red blood cells, which lack mitochondria, are able to transfer MitoTracker dyes to 293T cells100.
Therefore, we strongly recommend against the use of mitochondrial dyes to define mitochondria transfer. If dyes are used, it is critical that many controls are used to reduce concern about dye leak and titrating the dye to identify the lowest possible concentration with the goal of reducing off-target staining of other organelles. Great caution should be exercised when interpretating mitochondria transfer results obtained using dyes, and any such findings should be validated using a dye-independent method to avoid reporting false-positive results.
Other methods
Other approaches to define mitochondria transfer include using donor cell types with divergent mtDNA sequence variants16,30,31,32,102,103, effectively serving as an unambiguous molecular barcode detectable with DNA sequencing or PCR; however, detection of transferred mtDNA on its own is not sufficient evidence of mitochondria transfer because the mitochondria could be stuck to the surface of the cell without internalization and because free mtDNA by itself can be transferred between cells. Alternatively, one may detect an acquired mitochondria-associated protein or property conferred to the acceptor cell, such as rescue of respiratory competence in cells that are deficient in oxidative phosphorylation16,21.
Methods to enforce mitochondria transfer
There are also several useful in vitro methods to study specific steps involved in mitochondria transfer (for example, mechanisms of mitochondria uptake or release). Examples include the addition of isolated mitochondria to cells in culture or the detection of released mitochondria into the medium, although in the latter case it is important to control for death that occurs in cell culture conditions. Some cell types are efficient at taking up ex-Mito, whereas others are not. There are several strategies to enhance the efficiency of uptake by acceptor cells, such as the addition of a centrifugation step (for example, MitoCeption)104,105, pressure (for example, MitoPunch)102,103,106,107 or conjugation with a cell penetrating peptide (for example, Pep-1)108. Cell culture methods can also be used with Transwell systems to physically separate the donor and acceptor cells, allowing one to distinguish between contact-dependent and contact-independent transfer mechanisms59.
Fates of mitochondria after cell entry
Another important consideration when studying mitochondria transfer axes is to consider the fate of the transferred mitochondria. Are the transferred mitochondria quickly degraded and how? Do transferred mitochondria gain access to the cytoplasm and get incorporated into the acceptor cell’s pool of endogenous mitochondria? Do the transferred mitochondria gain access to the nucleus and contribute to insertions of mtDNA fragments into the nuclear genome (numtogenesis109)? Is there durable genetic transformation of the acceptor cell from the mtDNA contained within the transferred mitochondria? Are the transferred mitochondria repackaged and later ejected from the cell for delivery to another cell type?
The fates of internalized mitochondria probably vary by donor cell type, acceptor cell type, physiological context or disease state. We caution against assuming that the fate of transferred mitochondria in one cell type is the same as in another. We also suggest that determining the fate and processing of the transferred mitochondria is not required to establish whether mitochondria transfer occurs, though addressing questions about fate and processing are likely to reveal important insights about the functions of mitochondria transfer in the context under investigation. Similarly, in the context of mitochondria transplantation, understanding the biodistribution, fate and processing of the transplanted mitochondria may shed light on the mechanisms of action of this therapeutic modality.
Mechanism-based nomenclature
Current findings suggest that cells use several distinct mechanisms to transfer mitochondria. These transfer mechanisms can be roughly grouped into two categories: ‘contact-dependent mitochondria transfer’ and ‘contact-independent mitochondria transfer.’ The identification of one transfer mechanism is not mutually exclusive of other mechanisms, as cells may participate in multiple mitochondria-transfer mechanisms simultaneously. Several recent reviews have discussed the molecular mechanisms of these mitochondria transfer pathways63,64,65, therefore this section focuses on the nomenclature used to describe these pathways.
Contact-dependent mitochondria transfer
Tunnelling nanotubes
In the most general sense, contact-dependent mitochondria transfer occurs when the donor and acceptors are required to be in physical contact with each other for the transfer event to occur. The most frequently reported contact-dependent mechanism is via tunnelling nanotubes (TNTs), which are long, thin membranous structures that form between two cells with support of filamentous (F)-actin110 (Fig. 3e). Mitochondria are transported along a microtubular highway using motor–adaptor complexes that involve the mitochondrial Rho GTPase 1 (Miro1)111,112.
TNT formation is a complex process but seems to be mediated in part by growth-associated protein 43 (GAP43), a protein that neurons utilize at the axonal growth cone to facilitate outgrowth of axons113, or the M-Sec protein, which triggers re-arrangement of plasma membrane proteins before TNT formation30. Hallmark features of TNTs are their thin diameter, typically 0.5–1.5 μm, the presence of F-actin, and the direct continuity of cytoplasm between cells. However, not all contact-dependent mitochondria transfer occurs via TNTs.
Dendritic structures
Cells can also make contact via dendritic structures to facilitate the mitochondria transfer, which we define as ‘dendritic structure-mediated mitochondria transfer’ (Fig. 3e). Three examples of this process involve the delivery of mitochondria from osteocytes to other osteocytes52, from osteocytes to endothelial cells114, and from astrocytes to neurons41. These dendritic structures tend to be 1.5–3 μm in diameter and form contact with other cells via end feet, which are widened termini of the dendritic structure. The end feet contact but do not fuse with the acceptor cell’s plasma membrane. The models propose that the end feet structures spatially determine the site where ex-Mito are released in a CD38-, mitofusin 2- and/or Miro1-dependent manner for delivery to the acceptor cell41,52,114, although other transfer mechanisms are also possible and could involve the release of ex-Mito for delivery to the cell in contact with the end feet. At a practical level, it is difficult to distinguish between TNT- and dendrite-mediated mitochondria transfer. This can be addressed at least partially by demonstrating that there is a lack of continuity between the cytoplasm of one cell and that of another, determining the width of the tubular structure extending from the donor cell and identifying end feet at the junction of the dendritic structure and the acceptor cell.
Adhesion via gap junctional channels
Another contact-dependent transfer mechanism involves connexin 43 (Cx43), a process that we define as ‘adhesion-mediated mitochondria transfer’15 (Fig. 3e). This gap junction protein has a very small pore that is too small to permit the passage of mitochondria from one cell type to another. Rather, Cx43 mediates the adhesion of two cells, and the acceptor cell can then obtain mitochondria from the donor cell through the formation and internalization of annular gap junction vesicles115. This process is analogous to trogocytosis in the immune system116. Alternatively, Cx43-mediated gap junctional channels require that the two cells be directly adjacent to each other, which may enable mitochondria transfer via other mechanisms, including the transfer of ex-Mito. If a donor cell ejects ex-Mito, those mitochondria are statistically more likely to be taken up by their closest neighbours. TNTs or dendritic structures can also theoretically form at the Cx43 contact site. These possibilities should be distinguished if feasible when adhesion-mediated mitochondria transfer is observed and will be further clarified as the field evolves.
Cell fusion is not mitochondria transfer
A frequent question that the field has encountered is whether cell fusion leading to the formation of multinucleated cells is a type of mitochondria transfer. Giant cells and osteoclasts are examples of multinucleated cells that form by cell fusion117. We suggest that this process does not constitute mitochondria transfer because all cellular components fuse into a single cellular entity. Therefore, cell fusion has no definable donor or acceptor cell. This process is better characterized as a form of vertical inheritance of mitochondria occurring in reverse directionality.
Contact-independent mitochondria transfer
Release of extracellular mitochondria
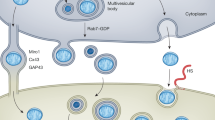
Cells do not need to be in direct physical contact to engage in mitochondria transfer. Rather, donor cells can eject their mitochondria to produce ex-Mito that can be captured by another cell type. Contact-independent mitochondria transfer processes can be described based on the structural characteristics of the mitochondria that are transferred (Fig. 3f). We recommend a tiered nomenclature framework. Ex-Mito is a general term that includes all forms of mitochondria or structurally intact mitochondrial fragments (for example, MDVs) found in extracellular fluid. By contrast, EVs that contain free-floating, cytosolic mtDNA and that are devoid of mitochondria or MDVs are not ex-Mito.
Extracellular vesicles with mitochondria
Some of these ex-Mito are enclosed within EVs, which can vary dramatically in terms of both size and cargo. EV-Mito can range in diameter from about 120 nm to 3–4 μm and can contain MDVs or intact mitochondria, with some EV-Mito containing multiple mitochondria35,37,41,60,61,118. There is a great deal of heterogeneity in these structures, and cells can release more than one type of EV-Mito51,61. Although we considered whether the types of mitochondrial cargos should be used to define EV-Mito, we felt there was insufficient information to make cargo-based classifications at this time. However, we do encourage investigators to characterize EV-Mito cargo when possible.
There are also multiple release mechanisms, including exosomal release via the multivesicular body49 and ectosomal release pathways through vesicle formation from the plasma membrane39. However, after EV-Mito are released from the cell, there is no reliable marker to distinguish between the exosomal and ectosomal release pathways, therefore these release mechanisms are not currently factored into EV-Mito nomenclature. In accordance with recent guidance by the International Society for Extracellular Vesicles (MISEV2023)119, we discourage the use of the terms ‘exosome’ and ‘ectosome’ unless these release mechanism can be clearly defined. Therefore, we recommend that EVs containing mitochondria may be called ‘EV-Mito’ and that they should be characterized at least in terms of their size, as this is a physical characteristic that can be measured using a variety of methods. EV-Mito should otherwise be defined using the nomenclature conventions established by MISEV2023 (ref. 119).
Free mitochondria
In addition, cells can release ex-Mito that are not enclosed in an EV membrane. We recommend that these ex-Mito be referred to as ‘free mitochondria.’ Although free mitochondria have been observed in tissues and in circulation42,51,65,120,121, the mechanisms by which cells release free mitochondria are unclear and require further study. Technically, free mitochondria meet the MISEV2023 definition of being an EV because they are membrane-bound structures released by cells, but referring to free mitochondria as EVs is confusing and discouraged. Electron microscopy and small-particle flow cytometry suggest that free mitochondria are typically 0.5–1.1 μm in diameter39,42,51,60,122. If electron microscopy is not feasible, free mitochondria can be defined by immunoprecipitation using antibodies that bind outer mitochondrial membrane proteins (for example, anti-TOM22) and that they lack EV markers (for example, the tetraspannins CD9, CD63 and CD81)51. However, there are also small free mitochondria about 100–200 nm in diameter found at least in cerebrospinal fluid123,124, which may or may not be MDVs or small mitochondria generated by fission. Electron microscopy and the presence of monoamine oxidase-B, which is an outer mitochondrial membrane protein, on the surface of these structures suggest that that they are free mitochondria with inner and outer mitochondrial membranes but that lack cristae123. We recommend that these structures be referred to as ‘small free mitochondria’.
Circulating extracellular mitochondria
Both EV-Mito and free mitochondria have been detected in plasma of mice and humans39,51,60,61. Indeed, many studies have reported the existence of cell-free mtDNA in plasma125,126, and the vast majority is located within ex-Mito127. Evidence for this includes size-exclusion chromatography indicating that cell-free mtDNA can be removed from plasma using 0.45-μm filters128. Furthermore, centrifugation of plasma at 15,000–16,000g depletes plasma of the mtDNA signal and eliminates the ability of plasma to respire39,60,128. Although detection of mtDNA in plasma may reflect the presence of circulating ex-Mito, measuring mtDNA is not sufficient on its own to identify this blood component, as mtDNA can also circulate without being contained in ex-Mito121,122,129,130. If the mtDNA is not contained within ex-Mito, then it will be susceptible to hydrolysis in a DNase I digestion assay. By contrast, mtDNA contained in ex-Mito should be susceptible to DNase I digestion after the addition of detergent122.
Circulating ex-Mito can be described in terms of their size, the proportions that are EV-Mito or free mitochondria, the donor cell type, morphology or other relevant characteristics. These circulating mitochondria can be delivered from cells in one organ to cells in another, as has been described between adipocytes and cells in the heart51,61 and between platelets and cells in the vessel wall40. It is likely that many more donor cell types release mitochondria into blood, including immune cells. This raises the intriguing possibility that interorgan mitochondria transfer is a ubiquitous phenomenon involving numerous donor cell types and destinations. An exciting future direction for the field will be to determine the diversity, connectivity and functions of interorgan mitochondria transfer networks.
Therapeutic approaches
Mitochondria-transfer biology has inspired the development of some exciting therapeutic approaches. There are several companies that have been recently founded to develop therapies or technologies focused on mitochondria transfer or transplantation. These companies and academic investigators are spearheading efforts to develop and evaluate the safety and efficacy of these therapeutic approaches, including mitochondria transplantation and the use of isolated mitochondria during cell engineering or manufacturing (Fig. 4). There is also an emerging concept of developing drugs that induce or inhibit mitochondria transfer to treat disease.
Mitochondria can be isolated from a cellular source or biofluid and used for therapeutic purposes. In mitochondria transplantation, the mitochondria are directly administered to a patient or model organism to elicit a therapeutic response. This procedure is distinct from MRT, which is an in vitro fertilization procedure that modifies the germline during reproduction. Isolated mitochondria can also be administered to cultured cells during cell manufacturing or processing in preparation for subsequent cellular therapies. In this scenario, the excess or unincorporated mitochondria are removed before infusion of the cells to the recipient.
Mitochondria transplantation
Defining mitochondria transplants
As described above, mitochondria transplantation is a procedure in which mitochondria are isolated from a cellular source and delivered postnatally to an individual with the specific purpose of eliciting a therapeutic response131. The mitochondria can be administered via various routes, including, but not limited to, direct injection into a tissue or systemic delivery via intravascular routes. This procedure is fundamentally distinct from mitochondria replacement therapy (MRT), which is an in vitro fertilization procedure that is used to correct or reduce the heteroplasmy of pathogenic mtDNA mutations in the offspring and that involves microinjection to bypass the plasma membrane132. In contrast to MRT, mitochondria transplantation is a procedure performed after birth, and the mitochondria are not expected to genetically modify the germline. The mitochondria are not directly injected into cells like in MRT. Rather, they are taken up using similar mechanisms as contact-independent mitochondria transfer. Mitochondria transplantation can also be used to treat a wide variety of diseases (not just heritable diseases caused by mtDNA mutations), can be autologous or allogeneic, and can be carried out multiple times in each patient, unlike MRT.
Mitochondria transplantation has been reported to improve disease outcomes in many preclinical models, including ischaemia-reperfusion injury in the heart66,69,91, lung88, brain70,87,133, limbs80 and kidney134; spinal cord injury74,75,90; cardiac resuscitation77; inherited mitochondrial diseases such as Leigh Syndrome79 and others. The first clinical trial reporting autologous mitochondria transplantation was a case-series of neonates and infants requiring ECMO support due to cardiac ischaemia-reperfusion injury, suggesting that the procedure was well tolerated and associated with improvements in ventricular function69. Subsequently, paediatric patients requiring ECMO were treated with or without autologous mitochondria transplants, with those who received mitochondria transplantation being more likely to be successfully separated from ECMO and less likely to experience another cardiovascular event compared with patients who did not receive mitochondria transplantation68. These and other studies have stimulated considerable interest in this therapeutic modality and inspired other clinical trials that are underway.
Types of mitochondria transplants
‘Autologous mitochondria transplants’ involve isolating mitochondria from the same individual who later receives the ex-Mito product as a therapy. This approach carries the least risk because all antigens and mtDNA in the mitochondria product are self-derived. ‘Heterologous or allogeneic mitochondria transplants’ involve isolating mitochondria from another individual or a cell line for direct administration to a recipient. As the mtDNA genome is inherited via the maternal lineage in mammals, a variant of heterologous mitochondria transplantation involves preparing the ex-Mito product from an individual’s biological mother or an individual from the same maternal lineage. Although there has not yet been a published heterologous mitochondria transplant clinical trial, at least one is currently in progress for refractory polymyositis or dermatomyositis (ClinicalTrials.gov ID NCT04976140) based on encouraging preclinical data in mice135.
Some evidence suggests heterologous mitochondria transplants are likely to be well tolerated. Specifically, there are approximately 3–12 billion ex-Mito per unit of platelets39, a heterologous blood product that is routinely and safely transfused to patients intravenously, even without known mtDNA haplogroup matching. If there is persistence of mtDNA from the donor mitochondria, mtDNA haplogroups and the degree of achieved mtDNA heteroplasmy should be taken into consideration136. Mice engineered to have high proportions of mtDNA heteroplasmy from two divergent but healthy mDNA genomes develop cardiopulmonary dysfunction and become frail137, although it is unlikely that this degree of mtDNA heteroplasmy could be achieved with mitochondria transplantation80,83. It will be important to consider the level of mtDNA heteroplasmy achieved in mitochondria transplant studies and to better understand the long-term stability and effects of any detected heteroplasmy on the recipient organism.
‘Xenogenic mitochondria transplants’ involve isolating mitochondria from one organism (for example human) and administering them to another (for example mouse). This scenario can be a useful experimental model but has unclear translational potential due to interspecies mito-nuclear conflict and the likely immunogenic response from exposure to foreign antigens in mitochondria from another species.
Durability of mitochondria transplants
The term ‘transplant’ implies that there is an intent for the administered mitochondria to be taken up by acceptor cells and to engraft the acceptor cells with those mitochondria. There are many examples in which cells in culture take up ex-Mito from the medium, use them for cellular respiration and replicate the donor-derived mtDNA for propagation to daughter cells51,104,138,139,140. Metabolically compromised cells seem to be more efficient at retaining and using ex-Mito in animal models, suggesting that the degree of engraftment might depend on the metabolic context of the acceptor cells51. Animal models involving ischaemia-reperfusion injury suggest that ex-Mito can be detected for up to 28 days after administration, though longer time frames were not tested67. These studies support the concept that administered mitochondria can escape the endocytic compartment and engraft in, or at least be used by, acceptor cells.
On the other hand, recent studies suggest that endothelial cells quickly degrade ex-Mito, with split-green fluorescent protein experiments indicating that these mitochondria are not incorporated into the endothelial cell’s endogenous pool of mitochondria80. This is consistent with other studies showing that healthy macrophages quickly engulf and degrade ex-Mito in tissues35,37. Taken together, these studies suggest that transplanted mitochondria can engraft, but this does not always occur even if a therapeutic response is observed. Indeed, it is possible that the therapeutic effects of mitochondria transplantation are due to the stimulation of mitophagy and mitochondrial biogenesis80, not necessarily direct effects of the transplanted mitochondria.
The degree and durability of engraftment may depend on the acceptor cell type, source of the ex-Mito, route of administration and the context in which the transplant is performed. Although there is debate about the degree or durability of engraftment with mitochondria transplantation, we point out that there are many examples where transplanted material does not successfully engraft (for example rejection after a solid organ transplant). A transplant still occurred if the grafted material failed or was removed. For this reason, we contend that no accepted definitions of the term ‘transplant’ require that engraftment occurs, though the term does imply the intent to engraft. Therefore, the term ‘mitochondria transplant’ is consistent with established definitions of the word ‘transplant,’ as defined by other bodies such as the World Health Organization.
Mitochondria transplant heterogeneity
An important consideration is that not all mitochondria transplants are the same or should be expected to produce the same results. Mitochondria can differ dramatically depending on their cell of origin141,142, and the methods of isolation and route of administration may differ from one study to another. Furthermore, isolated mitochondria can be complexed with other factors to alter their bioavailability and internalization by certain types of cells, as demonstrated by adding an asialoglycoprotein-based carrier to mitochondria for targeted delivery to the liver143. There is not yet enough information for us to provide guidance on mitochondria transplantation nomenclature that recognizes the heterogeneity of these factors.
Therefore, we suggest that mitochondria transplant studies should provide a transparent description of at least five factors: (1) the source material; (2) isolation method; (3) size of the isolated mitochondria; (4) whether the ex-Mito product is free or Mito-EVs; and (5) whether and how the ex-Mito were modified after isolation. When possible, we also encourage ex-Mito products to be characterized in terms of their inner and outer membrane integrity, capacity to respire and enrichment. Purity of an ex-Mito product is a challenging concept and may not be feasible to define because mitochondria are generally associated with other cellular components, including but not limited to endoplasmic reticulum, peroxisomes and lysosomal membranes. We recommend that enrichment is the relevant term and can be defined as the proportion of particles that contain ex-Mito using small-particle flow cytometry51 or other techniques, such as the degree of enrichment for mitochondrial proteins or mtDNA using immunoblots or quantitative PCR, respectively. Additional considerations that need to be evaluated are the unique pharmacokinetics and pharmacodynamics of transplanted mitochondria, which are fundamentally different from that of small or large molecule therapies.
Cell engineering using extracellular mitochondria
As described above, many cell types can obtain ex-Mito from culture medium or other cells. Some of the earliest observations in the field demonstrated that ex-Mito can endow cells with chloramphenicol and efrapeptin resistance, establishing the concept that cells can be engineered using ex-Mito. Although many cell types are highly efficient at importing and using ex-Mito, several methods have been developed to further enhance this process. One of these methods has been referred to as MitoCeption104,105, a technique in which the mitochondria capture process is enhanced through centrifugation at 4 °C followed by incubation at 37 °C. This method is analogous to ‘spinfection’ or ‘spinoculation,’ in which centrifugation enhances viral infection of cells in culture144,145. Pressure has also been used to enhance mitochondria uptake and associated mtDNA genetic transformation of ρ0 cells in vitro, a process called MitoPunch102,103,106,107. These and other methods allow the introduction of mitochondria from one source into cells in culture.
Cell engineering using ex-Mito has been used therapeutically in a recent clinical trial. It was reported that HSCs exposed to isolated mitochondria from peripheral blood mononuclear cells engraft better in lethally irradiated mice compared with unmanipulated HSCs and lead to improved haematopoietic reconstitution83. Based on this study, HSCs were isolated from patients with primary mitochondrial disease with large-scale mtDNA deletions and then cultured in vitro with isolated mitochondria from the patient’s biological mother84. The mitochondria-engineered HSCs were then autologously transplanted back to the patient. This trial demonstrated that the HSCs loaded with maternal mitochondria led to reduced anaemia and improved quality of life. It remains unknown, however, how long the donor mitochondria and mtDNA persist following the autologous HSC transplant. Furthermore, other studies have demonstrated that TNT-mediated delivery of mitochondria to CAR T cells or exposure of CAR T cells to isolated mitochondria exhibit enhanced capacity to kill tumour cells in vitro and in vivo93,146. These studies suggest that eliciting mitochondria transfer or adding ex-Mito to cells during manufacturing processes can improve therapeutic responses to cell therapies.
Again, there is a lack of information to recommend specific terminology about methods to engineer cells using ex-Mito. Like for mitochondria transplantation, we recommend that studies provide detailed methods to clarify the procedures used to engineer cells using ex-Mito and to characterize those mitochondria in terms of source material, isolation method, size of the isolated mitochondria, whether the ex-Mito are free or Mito-EVs, and enrichment for mitochondria. If the ex-Mito product has itself been engineered through expression of a mitochondria-targeted protein or other modification147, as was recently described, these features should also be clearly defined.
Drugs affecting mitochondria transfer
Several studies suggest that eliciting or inhibiting mitochondria transfer pathways with small molecules can treat a wide range of diseases. As one example, cancer cells obtain mitochondria from macrophages59, T cells58, fibroblasts148 and other cell types97,149,150 in the tumour microenvironment to support their own metabolic requirements, stimulate cell proliferation, suppress the antitumour immune response and resist chemotherapeutic agents. In the future, it may be possible to use pharmacological agents inhibit mitochondria transfer to cancer cells, which might slow cancer progression and/or make tumours more susceptible to other therapies. On the other hand, mitochondria transfer pathways also support the maintenance of mitochondrial homeostasis in several tissues, and these pathways are impaired in cardiometabolic diseases such as obesity and heart failure35,50. Restoring these pathways might reduce metabolic disease severity. As another example, a recent study suggested that pharmacological induction of mitochondria transfer can slow the progression of pulmonary fibrosis46. Further research is needed to understand how to precisely modify relevant mitochondria transfer axes for therapeutic purposes.
Outlook
In this Consensus Statement, we propose a nomenclature framework to describe mitochondria transfer and transplantation. We have categorized mitochondria transfer processes based on broad mechanisms of mitochondria transfer and the structural characteristics of ex-Mito. We also provide guidance regarding the minimal criteria needed to define mitochondria transfer axes or networks. Therapeutic approaches, such as mitochondria transplantation and cell engineering using ex-Mito, should be described in terms of the procedure being performed and characteristics of the ex-Mito used. The goal of this proposed nomenclature is to reduce the confusion that can be caused by the introduction of different names for similar processes or ex-Mito subsets as this field has evolved. We recognize that mitochondria transfer and transplantation are very active areas of research and that it is possible that new findings and insights may necessitate updates to the proposed nomenclature. For this reason, this Consensus Statement should be viewed as a work in progress, but we encourage researchers in the field to use the terms defined here.
References
Monzel, A. S., Enríquez, J. A. & Picard, M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat. Metab. 5, 546–562 (2023).
McBride, H. M., Neuspiel, M. & Wasiak, S. Mitochondria: more than just a powerhouse. Curr. Biol. 16, R551–R560 (2006).
Friedman, J. R. & Nunnari, J. Mitochondrial form and function. Nature 505, 335–343 (2014).
Roger, A. J., Muñoz-Gómez, S. A. & Kamikawa, R. The origin and diversification of mitochondria. Curr. Biol. 27, R1177–r1192 (2017).
Srinivasainagendra, V. et al. Migration of mitochondrial DNA in the nuclear genome of colorectal adenocarcinoma. Genome Med. 9, 31 (2017).
Geiger, O., Sanchez-Flores, A., Padilla-Gomez, J. & Degli Esposti, M. Multiple approaches of cellular metabolism define the bacterial ancestry of mitochondria. Sci. Adv. 9, eadh0066 (2023).
Miao, L., Yin, Z., Knoll, A. H., Qu, Y. & Zhu, M. 1.63-billion-year-old multicellular eukaryotes from the Chuanlinggou Formation in North China. Sci. Adv. 10, eadk3208 (2024).
Pennisi, E. The power of many. Science 360, 1388–1391 (2018).
Pennisi, E. Tiny fossils upend timeline of multicellular life. Science 383, 352–353 (2024).
Fukuda, H. & Kimura, A. Transfer of mitochondria into protoplasts of saccharomyces cerevisiae by mini-protoplast fusion. FEBS Lett. 113, 58–60 (1980).
Gunge, N. & Sakaguchi, K. Fusion of mitochondria with protoplasts in Saccharomyces cerevisiae. Mol. Gen. Genet 170, 243–247 (1979).
Yoshida, K. Interspecific and intraspecific mitochondria-induced cytoplasmic transformation in yeasts. Plant Cell Physiol. 20, 851–856 (1979).
Luo, Y. & Wang, W.-X. Increasing intercellular communication and directional organelle transfer in oyster hemocytes under copper stress. Environ. Sci. Technol. Lett. 10, 831–837 (2023).
Hutto, R. A. et al. Cone photoreceptors transfer damaged mitochondria to Müller glia. Cell Rep. 42, 112115 (2023).
Islam, M. N. et al. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 18, 759–765 (2012).
Spees, J. L., Olson, S. D., Whitney, M. J. & Prockop, D. J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl Acad. Sci. USA 103, 1283–1288 (2006).
Bergthorsson, U., Adams, K. L., Thomason, B. & Palmer, J. D. Widespread horizontal transfer of mitochondrial genes in flowering plants. Nature 424, 197–201 (2003).
Won, H. & Renner, S. S. Horizontal gene transfer from flowering plants to Gnetum. Proc. Natl Acad. Sci. USA 100, 10824–10829 (2003).
Gurdon, C., Svab, Z., Feng, Y., Kumar, D. & Maliga, P. Cell-to-cell movement of mitochondria in plants. Proc. Natl Acad. Sci. USA 113, 3395–3400 (2016).
Ferenczy, L. & MarÁZ, A. Transfer of mitochondria by protoplast fusion in Saccharomyces cerevisiae. Nature 268, 524–525 (1977).
Clark, M. A. & Shay, J. W. Mitochondrial transformation of mammalian cells. Nature 295, 605–607 (1982).
King, M. P. & Attardi, G. Injection of mitochondria into human cells leads to a rapid replacement of the endogenous mitochondrial DNA. Cell 52, 811–819 (1988).
Chomyn, A. et al. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol. Cell. Biol. 11, 2236–2244 (1991).
Chomyn, A. et al. Platelet-mediated transformation of mtDNA-less human cells: analysis of phenotypic variability among clones from normal individuals–and complementation behavior of the tRNALys mutation causing myoclonic epilepsy and ragged red fibers. Am. J. Hum. Genet 54, 966–974 (1994).
Manfredi, G., Thyagarajan, D., Papadopoulou, L. C., Pallotti, F. & Schon, E. A. The fate of human sperm-derived mtDNA in somatic cells. Am. J. Hum. Genet. 61, 953–960 (1997).
Kulawiec, M., Owens, K. M. & Singh, K. K. mtDNA G10398A variant in African-American women with breast cancer provides resistance to apoptosis and promotes metastasis in mice. J. Hum. Genet. 54, 647–654 (2009).
Katrangi, E. et al. Xenogenic transfer of isolated murine mitochondria into human rho0 cells can improve respiratory function. Rejuv. Res 10, 561–570 (2007).
Yoon, Y. G., Haug, C. L. & Koob, M. D. Interspecies mitochondrial fusion between mouse and human mitochondria is rapid and efficient. Mitochondrion 7, 223–229 (2007).
Rebbeck, C. A., Leroi, A. M. & Burt, A. Mitochondrial capture by a transmissible cancer. Science 331, 303 (2011).
Tan, A. S. et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 21, 81–94 (2015).
Dong, L. F. et al. Horizontal transfer of whole mitochondria restores tumorigenic potential in mitochondrial DNA-deficient cancer cells. eLife 6, e22187 (2017).
Bajzikova, M. et al. Reactivation of dihydroorotate dehydrogenase-driven pyrimidine biosynthesis restores tumor growth of respiration-deficient cancer cells. Cell Metab. 29, 399–416.e310 (2019).
Lei, L. & Spradling, A. C. Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science 352, 95–99 (2016).
Davis, C. H. et al. Transcellular degradation of axonal mitochondria. Proc. Natl Acad. Sci. USA 111, 9633–9638 (2014).
Nicolás-Ávila, J. A. et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109.e123 (2020).
Zhang, K. et al. TREM2hi resident macrophages protect the septic heart by maintaining cardiomyocyte homeostasis. Nat. Metab. 5, 129–146 (2023).
Rosina, M. et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 34, 533–548.e512 (2022).
Nicolás-Ávila, J. A., Pena-Couso, L., Muñoz-Cánoves, P. & Hidalgo, A. Macrophages, metabolism and heterophagy in the heart. Circ. Res. 130, 418–431 (2022).
Boudreau, L. H. et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 124, 2173–2183 (2014).
Levoux, J. et al. Platelets facilitate the wound-healing capability of mesenchymal stem cells by mitochondrial transfer and metabolic reprogramming. Cell Metab. 33, 283–299.e289 (2021).
Hayakawa, K. et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555 (2016).
Joshi, A. U. et al. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat. Neurosci. 22, 1635–1648 (2019).
Zhou, J. et al. Astrocytic LRP1 enables mitochondria transfer to neurons and mitigates brain ischemic stroke by suppressing ARF1 lactylation. Cell Metab. 36, 2054–2068.e14 (2024).
Liu, D. et al. Regulation of blood-brain barrier integrity by Dmp1-expressing astrocytes through mitochondrial transfer. Sci. Adv. 10, eadk2913 (2024).
van der Vlist, M. et al. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron 110, 613–626.e619 (2022).
Huang, T. et al. Efficient intervention for pulmonary fibrosis via mitochondrial transfer promoted by mitochondrial biogenesis. Nat. Commun. 14, 5781 (2023).
Scozzi, D. et al. Mitochondrial damage–associated molecular patterns released by lung transplants are associated with primary graft dysfunction. Am. J. Transplant. 19, 1464–1477 (2019).
Huang, T. et al. Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy. Sci. Adv. 7, eabj0534 (2021).
Liang, W. et al. Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired. Nat. Commun. 14, 5031 (2023).
Brestoff, J. R. et al. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab. 33, 270–282.e278 (2021).
Borcherding, N. et al. Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into blood. Cell Metab. 34, 1499–1513.e1498 (2022).
Gao, J. et al. Endoplasmic reticulum mediates mitochondrial transfer within the osteocyte dendritic network. Sci. Adv. 5, eaaw7215 (2019).
Suh, J. et al. Mitochondrial fragmentation and donut formation enhance mitochondrial secretion to promote osteogenesis. Cell Metab. 35, 345–360.e347 (2023).
Ding, P. et al. Mitochondria from osteolineage cells regulate myeloid cell-mediated bone resorption. Nat. Commun. 15, 5094 (2024).
Yang, C. et al. Mitochondria transfer mediates stress erythropoiesis by altering the bioenergetic profiles of early erythroblasts through CD47. J. Exp. Med. 219, e20220685 (2022).
Court, A. C. et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 21, e48052 (2020).
Luz-Crawford, P. et al. Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Res. Ther. 10, 232 (2019).
Saha, T. et al. Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells. Nat. Nanotechnol. 17, 98–106 (2022).
Kidwell, C. U. et al. Transferred mitochondria accumulate reactive oxygen species, promoting proliferation. eLife 12, e85494 (2023).
Dache, Z. A. A. et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 34, 3616–3630 (2020).
Crewe, C. et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 33, 1853–1868.e1811 (2021).
Dong, L. F. et al. Mitochondria on the move: Horizontal mitochondrial transfer in disease and health. J. Cell Biol. 222, e202211044 (2023).
Borcherding, N. & Brestoff, J. R. The power and potential of mitochondria transfer. Nature 623, 283–291 (2023).
Liu, D. et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct. Target Ther. 6, 65 (2021).
Al Amir Dache, Z. & Thierry, A. R. Mitochondria-derived cell-to-cell communication. Cell Rep. 42, 112728 (2023).
McCully, J. D. et al. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 296, H94–h105 (2009).
Kaza, A. K. et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J. Thorac. Cardiovasc. Surg. 153, 934–943 (2017).
Guariento, A. et al. Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 162, 992–1001 (2021).
Emani, S. M., Piekarski, B. L., Harrild, D., del Nido, P. J. & McCully, J. D. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 154, 286–289 (2017).
Norat, P. et al. Intraarterial transplantation of mitochondria after ischemic stroke reduces cerebral infarction. Stroke Vasc. Interv. Neurol. 3, e000644 (2023).
McCully, J. D., del Nido, P. J. & Emani, S. M. Mitochondrial transplantation: the advance to therapeutic application and molecular modulation. Front. Cardiovasc. Med. 10, 1268814 (2023).
Liu, Z., Sun, Y., Qi, Z., Cao, L. & Ding, S. Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases. Cell Biosci. 12, 66 (2022).
Gollihue, J. L. & Rabchevsky, A. G. Prospects for therapeutic mitochondrial transplantation. Mitochondrion 35, 70–79 (2017).
Patel, S. P. et al. Delivery of mitoceuticals or respiratory competent mitochondria to sites of neurotrauma. Mitochondrion 68, 10–14 (2023).
Xu, J. et al. Targeted transplantation of engineered mitochondrial compound promotes functional recovery after spinal cord injury by enhancing macrophage phagocytosis. Bioact. Mater. 32, 427–444 (2024).
Nakamura, Y., Park, J.-H. & Hayakawa, K. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp. Neurol. 324, 113114 (2020).
Hayashida, K. et al. Exogenous mitochondrial transplantation improves survival and neurological outcomes after resuscitation from cardiac arrest. BMC Med 21, 56 (2023).
Walker, M., Federico, E., Sancak, Y. & Levitt, M. R. Mitochondrial transplantation in ischemic stroke: insights from a first-in-human brain trial. Curr. Transplant. Rep. https://doi.org/10.1007/s40472-024-00428-6 (2024).
Nakai, R. et al. Mitochondria transfer-based therapies reduce the morbidity and mortality of Leigh syndrome. Nat. Metab. https://doi.org/10.1038/s42255-024-01125-5 (2024).
Lin, R. Z. et al. Mitochondrial transfer mediates endothelial cell engraftment through mitophagy. Nature 629, 660–668 (2024).
Nakano, T., Nakamura, Y., Park, J. H., Tanaka, M. & Hayakawa, K. Mitochondrial surface coating with artificial lipid membrane improves the transfer efficacy. Commun. Biol. 5, 745 (2022).
Park, J. H. et al. O-Glc N acylation is essential for therapeutic mitochondrial transplantation. Commun. Med. 3, 169 (2023).
Jacoby, E. et al. Mitochondrial augmentation of CD34+ cells from healthy donors and patients with mitochondrial DNA disorders confers functional benefit. NPJ Regen. Med. 6, 58 (2021).
Jacoby, E. et al. Mitochondrial augmentation of hematopoietic stem cells in children with single large-scale mitochondrial DNA deletion syndromes. Sci. Transl. Med. 14, eabo3724 (2022).
Hinge, A. et al. Asymmetrically segregated mitochondria provide cellular memory of hematopoietic stem cell replicative history and drive HSC attrition. Cell Stem Cell 26, 420–430.e426 (2020).
Döhla, J. et al. Metabolic determination of cell fate through selective inheritance of mitochondria. Nat. Cell Biol. 24, 148–154 (2022).
Chou, S. H.-Y. et al. Extracellular mitochondria in cerebrospinal fluid and neurological recovery after subarachnoid hemorrhage. Stroke 48, 2231–2237 (2017).
Cloer, C. M. et al. Mitochondrial transplant after ischemia reperfusion promotes cellular salvage and improves lung function during ex-vivo lung perfusion. J. Heart Lung Transpl. 42, 575–584 (2023).
Alway, S. E. et al. Mitochondria transplant therapy improves regeneration and restoration of injured skeletal muscle. J. Cachexia Sarcopenia Muscle 14, 493–507 (2023).
Zhu, Z. et al. Photobiomodulation augments the effects of mitochondrial transplantation in the treatment of spinal cord injury in rats by facilitating mitochondrial transfer to neurons via connexin 36. Bioeng. Transl. Med. 8, e10473 (2023).
Masuzawa, A. et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol. 304, H966–H982 (2013).
Court, A. C. et al. Survival advantage of native and engineered T cells is acquired by mitochondrial transfer from mesenchymal stem cells. J. Transl. Med. 22, 868 (2024).
Baldwin, J. G. et al. Intercellular nanotube-mediated mitochondrial transfer enhances T cell metabolic fitness and antitumor efficacy. Cell 187, 1–17 (2024).
Tiash, S., Brestoff, J. R. & Crewe, C. A guide to studying mitochondria transfer. Nat. Cell Biol. 25, 1551–1553 (2023).
Pham, A. H., McCaffery, J. M. & Chan, D. C. Mouse lines with photo-activatable mitochondria to study mitochondrial dynamics. Genesis 50, 833–843 (2012).
Los, G. V. et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 3, 373–382 (2008).
López-Andarias, J. et al. Cell-penetrating streptavidin: a general tool for bifunctional delivery with spatiotemporal control, mediated by transport systems such as adaptive benzopolysulfane networks. J. Am. Chem. Soc. 142, 4784–4792 (2020).
König, T. et al. MIROs and DRP1 drive mitochondrial-derived vesicle biogenesis and promote quality control. Nat. Cell Biol. 23, 1271–1286 (2021).
König, T. & McBride, H. M. Mitochondrial-derived vesicles in metabolism, disease, and aging. Cell Metab. 36, 21–35 (2024).
Chen, C., Li, H., Zhang, J. & Cheng, S. C. Exploring the limitations of mitochondrial dye as a genuine horizontal mitochondrial transfer surrogate. Commun. Biol. 7, 281 (2024).
de Almeida, M. J., Luchsinger, L. L., Corrigan, D. J., Williams, L. J. & Snoeck, H. W. Dye-independent methods reveal elevated mitochondrial mass in hematopoietic stem cells. Cell Stem Cell 21, 725–729.e724 (2017).
Patananan, A. N. et al. Pressure-driven mitochondrial transfer pipeline generates mammalian cells of desired genetic combinations and fates. Cell Rep. 33, 108562 (2020).
Sercel, A. J. et al. Generating stable isolated mitochondrial recipient clones in mammalian cells using MitoPunch mitochondrial transfer. STAR Protoc. 2, 100850 (2021).
Caicedo, A. et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci. Rep. 5, 9073 (2015).
Nzigou Mombo, B. et al. MitoCeption: transferring isolated human MSC mitochondria to glioblastoma stem cells. J. Vis. Exp. https://doi.org/10.3791/55245 (2017).
Sercel, A. J. et al. Stable transplantation of human mitochondrial DNA by high-throughput, pressurized isolated mitochondrial delivery. eLife 10, e63102 (2021).
Dawson, E. R., Patananan, A. N., Sercel, A. J. & Teitell, M. A. Stable retention of chloramphenicol-resistant mtDNA to rescue metabolically impaired cells. Sci. Rep. 10, 14328 (2020).
Chang, J. C. et al. Treatment of human cells derived from MERRF syndrome by peptide-mediated mitochondrial delivery. Cytotherapy 15, 1580–1596 (2013).
Singh, K. K., Choudhury, A. R. & Tiwari, H. K. Numtogenesis as a mechanism for development of cancer. Semin. Cancer Biol. 47, 101–109 (2017).
Ljubojevic, N., Henderson, J. M. & Zurzolo, C. The ways of actin: why tunneling nanotubes are unique cell protrusions. Trends Cell Biol. 31, 130–142 (2021).
Ahmad, T. et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 33, 994–1010 (2014).
Zhang, Y. et al. iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-α yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Rep. 7, 749–763 (2016).
Watson, D. C. et al. GAP43-dependent mitochondria transfer from astrocytes enhances glioblastoma tumorigenicity. Nat. Cancer 4, 648–664 (2023).
Liao, P. et al. Osteocyte mitochondria regulate angiogenesis of transcortical vessels. Nat. Commun. 15, 2529 (2024).
Norris, R. P. Transfer of mitochondria and endosomes between cells by gap junction internalization. Traffic 22, 174–179 (2021).
Joly, E. & Hudrisier, D. What is trogocytosis and what is its purpose. Nat. Immunol. 4, 815–815 (2003).
Brukman, N. G., Uygur, B., Podbilewicz, B. & Chernomordik, L. V. How cells fuse. J. Cell Biol. 218, 1436–1451 (2019).
Huang, Y. et al. TP53/p53 facilitates stress-induced exosome and protein secretion by adipocytes. Diabetes 72, 1560–1573 (2023).
Welsh, J. A. et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 13, e12404 (2024).
Stephens, O. R. et al. Characterization and origins of cell-free mitochondria in healthy murine and human blood. Mitochondrion 54, 102–112 (2020).
Caicedo, A. et al. The diversity and coexistence of extracellular mitochondria in circulation: a friend or foe of the immune system. Mitochondrion 58, 270–284 (2021).
Melki, I. et al. Platelets release mitochondrial antigens in systemic lupus erythematosus. Sci. Transl. Med. 13, eaav5928 (2021).
D’Acunzo, P. et al. Mitovesicles secreted into the extracellular space of brains with mitochondrial dysfunction impair synaptic plasticity. Mol. Neurodegener. 19, 34 (2024).
D’Acunzo, P. et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci. Adv. 7, eabe5085 (2021).
Trumpff, C. et al. Stress and circulating cell-free mitochondrial DNA: a systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion 59, 225–245 (2021).
Knez, J. et al. Correlates of peripheral blood mitochondrial DNA content in a general population. Am. J. Epidemiol. 183, 138–146 (2015).
Roch, B. et al. Plasma derived cell-free mitochondrial DNA originates mainly from circulating cell-free mitochondria. Preprint at bioRxiv https://doi.org/10.1101/2021.09.03.458846 (2021).
Chiu, R. W. K. et al. Quantitative analysis of circulating mitochondrial DNA in plasma. Clin. Chem. 49, 719–726 (2003).
Caicedo, A. et al. Decoding the nature and complexity of extracellular mtDNA: types and implications for health and disease. Mitochondrion 75, 101848 (2024).
McArthur, K. et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, eaao6047 (2018).
McCully, J. D., Levitsky, S., Del Nido, P. J. & Cowan, D. B. Mitochondrial transplantation for therapeutic use. Clin. Transl. Med 5, 16 (2016).
Herbert, M. & Turnbull, D. Progress in mitochondrial replacement therapies. Nat. Rev. Mol. Cell Biol. 19, 71–72 (2018).
Huang, P.-J. et al. Transferring xenogenic mitochondria provides neural protection against ischemic stress in ischemic rat brains. Cell Transplant. 25, 913–927 (2016).
Rossi, A. et al. Mitochondria transplantation mitigates damage in an in vitro model of renal tubular injury and in an ex vivo model of DCD renal transplantation. Ann. Surg. 278, e1313–e1326 (2023).
Yu, S. H. et al. Human umbilical cord mesenchymal stem cell-derived mitochondria (PN-101) attenuate LPS-induced inflammatory responses by inhibiting NFκB signaling pathway. BMB Rep. 55, 136–141 (2022).
Lechuga-Vieco, A. V., Justo-Méndez, R. & Enríquez, J. A. Not all mitochondrial DNAs are made equal and the nucleus knows it. IUBMB Life 73, 511–529 (2021).
Lechuga-Vieco, A. V. et al. Heteroplasmy of wild-type mitochondrial DNA variants in mice causes metabolic heart disease with pulmonary hypertension and frailty. Circulation 145, 1084–1101 (2022).
Gäbelein, C. G. et al. Mitochondria transplantation between living cells. PLoS Biol. 20, e3001576 (2022).
Kim, M. J., Hwang, J. W., Yun, C.-K., Lee, Y. & Choi, Y.-S. Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci. Rep. 8, 3330 (2018).
Coon, H. G. & Ho, C. Transformation of cultured cells to chloramphenicol resistance by purified mammalian mitochondrial DNA. Brookhaven Symp. Biol. 29, 166–177 (1977).
Fecher, C. et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat. Neurosci. 22, 1731–1742 (2019).
Kuznetsov, A. V. & Margreiter, R. Heterogeneity of mitochondria and mitochondrial function within cells as another level of mitochondrial complexity. Int. J. Mol. Sci. 10, 1911–1929 (2009).
Liu, X., Khouri-Farah, N., Wu, C. H. & Wu, G. Y. Targeted delivery of mitochondria to the liver in rats. J. Gastroenterol. Hepatol. 35, 2241–2247 (2020).
Ye, L. et al. Centrifugal enhancement of hepatitis C virus infection of human hepatocytes. J. Virol. Methods 148, 161–165 (2008).
Guo, J., Wang, W., Yu, D. & Wu, Y. Spinoculation triggers dynamic actin and cofilin activity that facilitates HIV-1 infection of transformed and resting CD4 T cells. J. Virol. 85, 9824–9833 (2011).
Harada, S. et al. Intercellular mitochondrial transfer enhances metabolic fitness and anti-tumor effects of CAR T cells. Blood 140, 2356–2357 (2022).
Suh, J. & Lee, Y.-S. Mitochondria as secretory organelles and therapeutic cargos. Exp. Mol. Med. 56, 66–85 (2024).
Ippolito, L. et al. Cancer-associated fibroblasts promote prostate cancer malignancy via metabolic rewiring and mitochondrial transfer. Oncogene 38, 5339–5355 (2019).
Marlein, C. R. et al. NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood 130, 1649–1660 (2017).
Moschoi, R. et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood 128, 253–264 (2016).
Acknowledgements
The authors completed this work as the ICMTTN. We extend our thanks and appreciation to D. Brierton (Washington University School of Medicine) for her exceptional administrative support of this international effort. We also thank G. Freedman (MitoWorld and the National Laboratory for Education Transformation) for hosting the public commenting period on www.mitoworld.org and Z. Kirshner (Kirshner Design, kirshnerdesign.com) for website design and support. We thank the individuals and groups who submitted comments during the public commenting period. Costs associated with this publication were paid for by a grant from the Burroughs Wellcome Fund (CAMS, 1019648) to J.R.B.
Author information
Authors and Affiliations
Contributions
J.R.B. conceived of the article, organized the ICMTTN, chaired all meetings, wrote the initial draft of the manuscript, and led revisions. K.K.S. contributed to the organization process, served as co-chair for all meetings, and assisted with writing the first draft and revisions. All authors participated in the introductory, working group and summation meetings; contributed to the conceptual content and organization of this article; contributed to writing, editing and/or revising this manuscript; and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
J.R.B. is a member of the Scientific Advisory Board of LUCA Science, Inc.; receives research support from LUCA Science and Edgewise Therapeutics; is a consultant for Columbus Instruments, Inc.; has consulted for DeciBio within the past 12 months; receives royalties from Springer Nature Group; is an inventor on technology licensed to Columbus Instruments, Inc. with royalty rights; is an inventor on pending patent applications related to the treatment of metabolic diseases (63/625,555) and allergic diseases (US20210128689A1) and mitochondria transfer (018984/US); and is a co-founder and holds equity in Symbiogenix, Inc. K.K.S. is a co-founder, holds equity in, and serves on the Scientific Advisory Board for YUVA Biosciences. E.B. holds patents on detection of extracellular mitochondria in inflammatory conditions (WO2015051466A1) and on mitochondrial autoantibodies in autoimmune diseases (WO2022/246565A1), and is a principal investigator and scientific advisor to MitrixBio. L.B. receives grant support from Philips Medical Systems, ZOLL Medical Corp, Nihon Kohden, PCORI, BrainCool and United Therapeutics; is on the Scientific Advisory Board for Nihon Kohden, HP and Philips; holds seven issued patents and several pending patent applications involving the use of medical slurries as human coolant devices, the creation of slurries, reperfusion cocktails, and measurement of respiratory quotient; and serves on committees for the American Heart Association, which has a financial interest in the outcome of resuscitation studies being conducted and that sells training materials worldwide on resuscitation techniques. A.C. is a founder of and scientific advisor to Dragon Biomed and a scientific advisor to Luvigix. J.A.E. has collaborated with Minova Therapeutics. Å.B.G. is a consultant for Lexeo Therapeutics. J.D.M. has pending patents for the isolation and use of isolated mitochondria and is a founder and member of the Scientific Advisory Board and Board of Directors for cellvie. M.K. is the chief scientific officer of Cells for Cells, EVast Bio, and Regenero (Chilean consortium for regenerative medicine) with Corporación de Fomento de la Producción support; has received research support from ANID (National Agency for Research and Development) basal FB210024 and Cells for Cells; is an inventor on patents related to mesenchymal stem cells (pending patents WO2014135924A1, WO20170646770A2, WO2017064672A1 and WO2019051623). R.N. receives research support from LUCA Science, Inc. A.J.S. is an employee of MitoWorld. M.A.T. is a co-founder and shareholder for NanoCav, a private start-up company with licences for mitochondria-transfer techniques and applications. A.R.T. is an inventor of a pending patent related to the diagnostic detection of cf-mtDNA (WO 2016/063122 A1). R.T. is a scientific advisor for Cytokinetics, Inc. and GenKardia, Inc. M.W. has a pending patent (US20210085713A1) related to compositions and methods for treating stroke. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Rubén Quintana-Cabrera and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brestoff, J.R., Singh, K.K., Aquilano, K. et al. Recommendations for mitochondria transfer and transplantation nomenclature and characterization. Nat Metab 7, 53–67 (2025). https://doi.org/10.1038/s42255-024-01200-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-024-01200-x
This article is cited by
-
Mitochondrial transfer in cancer: mechanisms, immune evasion, and therapeutic opportunities
Genomics & Informatics (2026)
-
Recent Advances in Mitochondrial Transplantation for Ischemic Stroke: A Review of in Vitro and in Vivo Studies
Translational Stroke Research (2026)
-
The role of mitochondrial transfer in lung disease
Respiratory Research (2025)
-
From powerhouse to modulator: regulating immune system responses through intracellular mitochondrial transfer
Cell Communication and Signaling (2025)
-
Expanding research and care for Leigh syndrome: efforts of a patient-led advocacy organization
Research Involvement and Engagement (2025)