Abstract
Evaluating diagnostic test accuracy during epidemics is difficult due to an urgent need for test availability, changing disease prevalence and pathogen characteristics, and constantly evolving testing aims and applications. Based on lessons learned during the SARS-CoV-2 pandemic, we introduce a framework for rapid diagnostic test development, evaluation, and validation during outbreaks of emerging infections. The framework is based on the feedback loop between test accuracy evaluation, modelling studies for public health decision-making, and impact of public health interventions. We suggest that building on this feedback loop can help future diagnostic test evaluation platforms better address the requirements of both patient care and public health.
Similar content being viewed by others
Introduction
Newly emerging infectious agents present a particular challenge for diagnostic test development and evaluation. These agents often surface in the form of an outbreak, an epidemic or a pandemic with high urgency for targeted infection control, but minimal knowledge about the infectious agents themselves. Rapid availability of diagnostic tests, along with information on their accuracy, however limited, is critical in these situations.
Traditional diagnostic study designs and quality assessment tools developed for individual patient care, as proposed in existing guidelines for diagnostic tests1,2,3, are difficult to apply in a volatile environment in which there are continuously evolving research questions, infectious agents, and intervention options. These challenges were particularly apparent during the SARS-CoV-2 pandemic, where the quality of diagnostic studies available in the field was generally limited. The Cochrane review on rapid, point-of-care (POC) antigen and molecular-based tests for diagnosing SARS-CoV-2 infection found a high risk of bias in different domains in 66 of the 78 studies considered (85%)4. The most frequent potential source of bias was identified in the reference standard domain, including potential of imperfect gold/reference standard bias, incorporation bias, and diagnostic review bias (an explanation of these biases is given in Table 1). In the Cochrane review on antibody tests for identification of current and past infection with SARS-CoV-25, the most frequent potential source of bias was identified in the patient selection domain, due to selection or spectrum bias (48 of 54 studies, 89%). A particular issue is that biases with regard to the reference standard or the index test can lead to an overestimation or underestimation of sensitivity and specificity.
In both application fields, the differences between the diagnostic test accuracy estimates reported by the manufacturers and those estimated later in the Cochrane meta-analyses were enormous. The mean sensitivity reported by manufacturers for antigen tests was 89% (as of 22/06/2022)6. In comparison, the sensitivity estimated in the meta-analysis on antigen tests4 was 72% in symptomatic and 58% in asymptomatic individuals. This discrepancy shows that the timely evaluation of newly developed laboratory tests under real-life conditions is crucial and should be planned and started before market launch.
This Perspective article is the result of an interdisciplinary workshop which we conducted as part of a research project funded by the German Research Foundation. This brought together expertise from all disciplines relevant to diagnostic test development and evaluation, ranging from molecular test development to public-health decision-making. Firstly, the project members gave presentations on the respective sub-areas of the project to create a common basis for the following moderated panel discussions, integrating the expertise and experience from the individual workshop participants. Subsequently, a previously created draft of the framework was further developed and the next steps were planned. We describe the challenges and potential solutions that were discussed for implementing state-of–the-art diagnostic test development and evaluation processes, based on accuracy studies performed at different phases of an epidemic or pandemic.
This Perspective is divided into three key sections. First, we discuss the relevance of diagnostic studies for public health decision-making based on mathematical models. Second, we describe the challenges in developing diagnostic tests and propose study designs to accelerate the evaluation of their diagnostic accuracy. Third, considering the challenges mentioned above, we propose a unified framework for rapid diagnostic test development and clinical evaluation. This highlights that multiple and perhaps different study designs will be necessary to build a convincing portfolio of evidence for various stakeholders during outbreaks of emerging infections.
Diagnostic tests and testing strategies during the COVID-19 pandemic
For SARS-CoV-2, three types of tests can be distinguished according to their target: the polymerase chain reaction (PCR) test, the antigen test and the antibody test. The PCR test detects viral particles, the antigen test viral surface proteins and the antibody test SARS-CoV-2 specific antibodies. POC tests refer to those that can be evaluated directly on site. They are available for all three test types. While PCR and antibody tests are usually only performed by trained staff in hospitals and testing centers or similar, there are antigen tests for trained staff (rapid antigen test) but also as a home testing kit (antigen self-test, freely accessible)7. The cost of testing varied widely across phases of the pandemic, countries, type of test, and manufacturer. Rapid antigen tests now cost $1 in the United States of America (USA), PCR tests cost $5, and antibody tests cost $50 (test kit only with no personnel costs or similar; average approximate based on internet research and Du et al.8). PCR and antigen tests use nasal or throat swabs as specimen material, antibody tests use a blood sample. The PCR test takes up to 48 h to give results, whilst the antigen and the antibody test give results within 15 min. All tests are performed once, and a second test is often performed to confirm the test result (e.g., a PCR test to confirm a positive antigen test). A recently published network meta-analysis showed a mean sensitivity of 93% and specificity of 98% for PCR tests, 75% sensitivity and 99% specificity for antigen tests9, and a Cochrane review reported a sensitivity and specificity of 94.3% and 99.8% for total antibody tests10. Throughout the pandemic, these tests were used in different combinations as part of various population-level testing strategies. Rapid antigen tests were used as part of screening and isolation programmes to detect asymptomatic infections in the community, and especially in key workers and workplaces. This was often combined with follow-up testing with PCR tests to minimise unnecessary isolation due to false positives.
Different testing strategies were used to fulfil various aims, e.g. full population screening programmes to break infection chains and studies such as the Real-time Assessment of Community Transmission (REACT) study for high-quality, real-time surveillance. Their strategies characteristics and costs differed accordingly. No public data on costs is available for most of these strategies, but modelling studies have been used to assess their cost-effectiveness under certain assumptions, taking the context in which the testing strategies were used into account and synthesizing all available evidence8,11,12,13.
Population-level information as a key input for public health decision-making
While diagnostic tests are usually developed for individual diagnosis and patient care, their results also play a crucial role in public health decision-making. Population-level case data, collected based on the number of positive diagnostic tests in surveillance systems worldwide, are a central input parameter for decision-making processes in public health policy. Cases might in this situation represent different outcomes of contact with an infectious agent (e.g., infections or deaths), and also different types of measures of this contact (e.g., incident or cumulative cases derived from seroprevalence studies).
Surveillance systems for infectious diseases provide reports on the number of cases associated with specific pathogens using standardized case definitions based on pre-defined rules (including diagnostic test results) and legal obligations. These surveillance systems run constantly for notifiable diseases associated with high public health risks14. Surveillance-related case data (based on diagnostic test results) are directly used for public health decision-making. They enable the development and parameterization of infectious disease models (e.g., for early warning and monitoring) and for decision-analytic models (e.g., for assessing the benefit-harm, cost-effectiveness or other trade-offs when guiding public health interventions). This is especially true in epidemic or pandemic situations when reducing harm at a population level becomes a crucial aspect of the decision-making philosophy15,16, and high consequence decisions must be made under uncertainty and time pressure. In such scenarios, two fundamental and extremely relevant quantities are some measure of the presence of the infection in the population (e.g., prevalence or incidence data) and a measure of existing immunity to the infection in the population, i.e. seroprevalence data.
An important decision supported by dynamic infectious disease modelling studies focusing on predicting infection dynamics is the timing of interventions. Interventions are most effective when deployed in time17 and may cease to be effective if implemented too late18. Therefore, it is imperative that decisions about implementing interventions are made in a timely manner and sometimes with incomplete evidence, but with all relevant information being collected and reported appropriately. Monitoring population-level data from as soon as possible is essential since it can be used to set thresholds for starting interventions19 and determine when intervention measures are no longer necessary and can be ended20.
Due to reporting delays and the fact that the decision-making process is not instantaneous, decisions can come too late when relying solely on current population-level data. This is where infectious disease modelling comes in. Models help decision-makers obtain reasonable estimates of how the epidemic is likely to progress and what impact different interventions may have. This enables timely and informed decision-making21,22,23. Combined with benefit-harm and health economic models to account for unintended effects and costs of interventions, infectious disease models enable decision-makers to make optimal decisions given the available evidence and resources24,25,26.
The points discussed above are exemplified by decision-making during the SARS-CoV-2 pandemic. Even during the early phases of the pandemic, decisions about interventions were made from population-level data. In the United Kingdom (UK), the timing of the first nationwide lockdown was determined based on the predicted number of people treated for SARS-CoV-2 in intensive care units (ICUs)19. In Australia, more targeted lockdowns were implemented based on regional prevalence data27,28, and local lockdowns were also implemented in the UK during later phases of the pandemic29. Prevalence data became even more important when contact tracing and test-intervention strategies were implemented, because the predictive value of diagnostic tests depends on the infection prevalence. As vaccines became available, subpopulations most at risk of severe COVID-19 were prioritised and given the opportunity to be vaccinated first30,31. In Germany, vaccination and testing control rules for access to parts of public life varied from region to region. Again, the region-specific thresholds were based on the number of hospitalised patients testing positive for SARS-CoV-2 in the respective region32.
Mathematical models were used throughout to support the decision-making process. The threshold for applying the first nationwide lockdown in the UK was set based on the number of people estimated to be potentially needing ICU treatment based on different modelling scenarios19. In Austria, the decision to prioritise vaccinating elderly and vulnerable groups was based on decision-analytic modelling aiming to minimise hospitalisations and deaths33. In general, infectious disease and decision-analytic models contributed substantially to the type and intensity of interventions implemented34,35,36. Once tests became widely available, they were also used to devise effective mass testing and isolating strategies37,38.
The current pandemic has thus demonstrated the need for accurate and timely population-level case data and clinical case data (requiring different diagnostic tests and testing strategies), to allow public health policy decisions to be as well-informed as possible. Diagnostic tests, as the primary tool to obtain these population-level data, are therefore at the heart of all modelling efforts during an epidemic or pandemic, and early and precise knowledge about their accuracy is crucial for interpreting and further applying these case data.
Challenges for diagnostic test evaluation in an epidemic setting
Diagnostic tests developed for emerging infections should serve various purposes, including individual clinical diagnosis, screening, and surveillance. These purposes demand distinct strategies and, in theory, require separate approval mechanisms39. However, test development, evaluation of technical validity, clinical validity and utility, as well as test validation currently do not account for the different uses in a generalized way. The challenges and potential solutions in this article and the framework proposed therein have been described with all these purposes in mind and are summarised in Box 1.
In the initial phase of an outbreak of an emerging infection, the main focus of diagnostic test development is providing a diagnostic test that can identify infected individuals with high sensitivity, so that they can be isolated and treated as soon as possible. This is especially important because the effectiveness of contact tracing depends directly on the quality and timeliness of case identification. Of course, a high specificity is also important, to prevent unnecessary isolation or treatment. This is usually achieved by direct detection of the pathogen, e.g., by molecular genetic tools such as PCR, microscopy, antigen tests or cultivation of the microorganisms involved. Later, a better understanding of the immune protection caused by contact with the agent is required, leading to the development of indirect pathogen detection tools such as antibody tests. Here, sensitivity and specificity are equally important to evaluate proxies of long-term immune protection and to detect past low severity infections which would have been missed otherwise. However, from the perspective of population-level modelling, some accuracy may be sacrificed if the true diagnostic accuracy of the test is known so that aggregate correction methods can be applied. Knowledge of the specificity of the direct detection tools developed earlier can also come into play in the case of reported reinfections, when it becomes important to understand whether these are true reinfections or due to false positives in a time of intensified testing. High specificity is also important once treatment options are available, but possibly come with relevant side effects, high costs or limited availability. Different population-level uses also require different diagnostic characteristics. Although PCR tests with a noticeable delay between testing and communication of results were used for population-level testing during the early phases of the COVID-19 pandemic, tests used for population screening generally need to be easily and quickly administered as POC tests, and lower accuracies, especially in specificity, are accepted as a trade-off for this. However, relatively high sensitivity is still important to make testing-and-isolation strategies effective. Deficiencies in specificity may be compensated for by confirmatory follow-up testing with highly specific tests to minimise unnecessary isolation. Furthermore, target populations, testing aims and prioritised estimators (e.g. sensitivity or specificity) can change rapidly, necessitating constant test evaluation and re-evaluation.
During an epidemic or pandemic, direct and indirect tests are thus used for different purposes and require different study designs, with different sample size calculations and study populations, to provide critical information with high precision and validity.
During epidemics with emerging infections, all new tests must, in general, quickly go through three steps: the test must be developed, its clinical performance assessed, and then information on its performance incorporated into infectious disease modelling to inform public health decision-making. Each step has potential sources of various biases that must be considered. Next we describe potential challenges during these steps and how these challenges might affect the submission process to regulatory agencies, also considering the perspective of test developers from industry.
Diagnostic test development
Diagnostic tests for emerging infections typically are in the in vitro diagnostic (IVD) test category, as they examine human body specimens (e.g., nasopharyngeal swabs, nasal swabs, blood or saliva39). IVDs are generally considered medical devices40. Consequently, their development has to adhere to the rules of regulatory agencies and a pre-defined complex legal framework. Currently, the European Union (EU) IVD Regulation 2017/746 covers IVD medical devices, and focuses on a legislative process that prioritises individual safety, which means that different types of clinical data must be collected before submission. If a test is deemed capable of distinguishing infected individuals from non-infected ones, it has to be shown not to produce a one-off result41.
There are several phase models for the development of diagnostic tests described in the literature. We discuss using the frequently used four-phase model2,42,43. The four phases for this are: I, evaluation of analytical performance; II, diagnostic accuracy estimation and determination of threshold; III, clinical performance estimation; and IV, evaluation together with diagnostic and/or therapeutic measures with regard to a patient-relevant endpoint.
Inter-rater agreement, analytical sensitivity (minimally detectable levels)41 and cross-reactivity have to be investigated in the phase I studies to verify the technical validity, repeatability and reproducibility of laboratory tests (on a lot-to-lot, instrument group, and day-to-day basis). However, in the early phase of an epidemic or pandemic, there are often not enough samples from infected individuals. Sharing data and using a common infrastructure by, for instance, collecting samples at national reference centres, could solve this problem, if they are made accessible to IVD developers. A possible limitation of this approach is the risk of spectrum bias due to the particular mix of individuals, e.g. there may be more severe cases in the samples than in the population. Furthermore, regulatory agencies do not allow the use of (frozen) biobank samples for approval.
After having shown good technical performance, clinical performance in phase II and III studies must be demonstrated. An integral part of assessing the sensitivity and specificity of a continuous diagnostic test is the determination of the threshold at which it should be used41. This must be fixed before moving on to diagnostic test evaluation, to avoid bias caused by a data-driven threshold selection44,45. The optimal threshold for a diagnostic test depends on the prevalence and consequences associated with misclassification38,46, which may both change over time. Thus a new study is needed every time the threshold changes, requiring extensive resources (particularly time and money).
Phase II studies are initial, so-called proof-of-concept studies covering clinical performance and are often carried out in a two-gate design47, where sensitivity is estimated in diseased individuals and specificity in healthy samples from a different source. However, this design can lead to spectrum bias (Table 1). Sensitivity and specificity have been shown to be generally overestimated in such studies47. Likewise, a meta-analysis showed that a two-gate case-control design can lead to an overestimation of diagnostic accuracy48. In most situations outside an epidemic or pandemic, individuals tested are symptomatic and suspect they have the infection of interest, if the test is to be used to guide therapy or decide about isolation. However, during epidemics or pandemics, tested individuals can also be asymptomatic if the test is intended as a contact tracing or screening test41. In both cases, real-world samples may not be as perfect as in a laboratory situation41 because testing can also be performed at POC, in the community, at the workplace, school, or home39. A test may require different performance characteristics if it is the first test in line, used to triage who will be tested further, compared to when the test is used to confirm infection. For instance, in a confirmation setting, most individuals who clearly do not have the infection of interest will be excluded41.
Diagnostic test evaluation
IVDs must be evaluated in phase III diagnostic accuracy studies that ideally start by including all individuals who will be tested in clinical practice to avoid selection bias (all-comer studies). Individuals fulfilling the inclusion criteria should be enrolled consecutively, without judging how likely this person is to test positive or negative41. In such prospective diagnostic studies, to minimise variability and thus increase statistical power, all study participants ideally undergo all tests under investigation (index tests) as well as the reference standard to assign their final diagnosis.
The reference standard must be sufficiently reliable to differentiate between people with and without the target condition, but it is usually not perfect41. This imperfectness has to be taken into account when interpreting the results. Suppose a POC antigen test for SARS-CoV-2 is evaluated with a PCR test as reference standard resulting in a sensitivity of 90%. This does not mean that 90% of people with SARS-CoV-2 will be detected but that the POC test will be positive in 90% of cases with a positive PCR test. Solutions to this may include follow-up data or composite reference standards, which use all tests or clinical criteria available for a diagnosis. However, if the test under evaluation is part of this composite reference standard, this may lead to incorporation bias49.
Depending on the phase of the epidemic or pandemic, recruitment speed can vary considerably due to changes in incidence. The guideline on clinical evaluation of diagnostic agents of the European Medicine Agency2 demands sample size specification in a confirmatory diagnostic accuracy study in the study protocol. The required sample size is highly dependent on the prevalence of the target condition, which may change during the recruitment phase, making a priori sample size calculations inappropriate at the time of recruitment.
Submission to regulatory agencies
Studies for industry face rigorous regulatory and ethics requirements as clinical trials follow strict processes and regulatory guidelines which are assessed in the regulatory submission process and are potentially controlled by audits. Clinical studies must be transparent, traceable and reproducible. Special attention must be paid to data quality and privacy. This leads to very detailed study preparation, documentation, quality control, and long and less flexible study processes.
When the SARS-CoV-2 pandemic began, the need for diagnostic tests grew with the rising number of cases. Regulatory bodies (like the U.S. Food & Drug Administration, FDA) established country-specific emergency use authorization guidelines50,51to make it easier and faster to bring a test for SARS-CoV-2 to the market and make it accessible during the pandemic. The WHO declared the end of the COVID-19 pandemic as a Public Health Emergency on 5 May 202352. However, the FDA did not set a specific end date for the use of diagnostic tests authorized under Emergency Use Authorization (EUA), and they still remain valid under section 564 of the Federal Food, Drug, and Cosmetic Act, enabling uninterrupted use of EUA-authorized COVID-19 tests until further notice of regulatory transition requirements53,54.
Submission process requirements such as sample size, inclusion criteria for subjects, and properties of the reference test differ between countries and may change during an epidemic or pandemic. Therefore, it is not always possible for a single study to be the basis for submissions to different countries or certificates, and several studies must be planned.
The different and changing requirements are not the only challenges submission teams face. The changing prevalence of infection makes adequate project management and timeline planning difficult. Recruitment of positive cases fulfilling the recruitment requirements can be slow which leads to a longer study duration and, subsequently, longer time to market. New mutations make re-evaluations of statistical properties necessary. Considering regulatory changes during pandemics and possible mutations, (pre)planning such a study is complicated and time-consuming.
Potential solutions for the challenges presented
The challenges discussed previously are multidimensional but can be addressed by three countermeasures in several areas. First, test developers should use methodological approaches to address study designs and statistical analyses, increasing study efficiency and reducing the risk of bias. Second, strategic approaches and regulatory guidance for the industry should be deployed to clearly define opportunities but also limitations in the development and approval process. Third, results and feedback from population-level mathematical modelling should inform test development and validation for deriving optimal study designs based on formal value-of-information analyses.
Methodological solutions
Methodological solutions fall into two categories; statistical methods to control bias, and those to increase speed and efficiency.
The different biases in diagnostic studies have been described extensively, both in general55,56,57 and also specifically in the context of the SARS-CoV-2 pandemic58 and POC tests for respiratory pathogens59. From a methodological standpoint, the problem of bias can be addressed in two ways: either by choosing a study design in the planning stage that minimises the risk of bias, or by using analytical methods that correct for potential bias.
An excellent overview of how to avoid bias through an appropriate design can be found in Pavlou et al.60. Important for the planning phase is the work of Shan et al.61, who present an approach to calculate the sample size in the presence of verification bias (i.e., partial or differential verification bias).
In terms of bias reduction methods during the analysis phase, most studies focus on the correction of verification bias. Bayesian approaches are mainly proposed for differential verification bias62,63, while there are a variety of methods for partial verification bias64.
Time to market has to be reduced significantly in pandemics to find an optimal trade-off between misclassification and missed opportunities for action. From a statistical point of view, the methods and processes must be reconsidered. One possibility to improve study designs and statistical analysis is adaptive designs, that can increase efficiency. These approaches have been long established in therapeutic studies and are also anchored in guidelines3,65. With adaptive designs, it is possible to make pre-specified modifications during the study. For example, inclusion and exclusion criteria can be changed, the trial can be terminated early due to futility or efficacy, or the sample size can be recalculated. The characteristics and typical adaptive designs have been very clearly summarised66. A review of published studies with adaptive designs showed that the pharmaceutical industry in particular increasingly uses simple adaptive designs, with more complex adaptive designs still being rare67.
In diagnostic studies, however, experience in using adaptive designs in diagnostic clinical trials for submissions is limited. A summary of the current state of research is available for diagnostic accuracy studies68, for randomised test-treatment studies69 and for adaptive seamless designs70. Methods for blinded and unblinded sample size re-calculations for diagnostic accuracy studies have been published recently71,72,73, as well as adaptive designs for test-treatment studies74 and adaptive seamless designs. The diagnostic industry heavily depends on regulatory guidelines worldwide. If regulatory bodies emphasise more efficient diagnostic trials that include, e.g., adaptive designs, the implementation of modern study designs will be incentivised.
In the following, concrete possible solutions to the above-mentioned challenges are explained as examples. For details, please refer to the corresponding articles.
Firstly, the problem of setting a threshold in an early study that may later turn out not to be optimal can be addressed by selecting a limited pool of promising thresholds75. These are then evaluated simultaneously in the validation study, with the type I error adjusted accordingly. Another approach is to use mixture modelling without defining a threshold76. Prevalence-specific cut-offs might be developed and defined a priori.
Secondly, if the testing strategy and thus the target population change during the study, adaptive designs offer the possibility to re-estimate the sample size in a blinded manner based on the prevalence estimated in the interim analysis71.
Thirdly, a seamless enrichment design can be chosen to address the problem of biased diagnostic accuracy in two-gate designs, in which proof-of-concept and confirmation are performed together in one study. However, it is apparent that regulatory authorities are cautious of the possible shortcomings of these innovative designs, and a lot of work needs to be done to get them approved77. This, in turn, results in the manufacturers of diagnostic tests being conservative in their study designs.
Solutions for political decision-making based on mathematical modelling
The accuracy, accessibility, and costs of diagnostic tests all play a role in decisions about testing programmes, and the decision on whether, for instance, a more sensitive test with lower accessibility and higher costs (in the context of SARS-CoV-2, e.g. a real-time PCR test) should be administered with low frequency or a less sensitive test with better accessibility and lower costs (in the context of SARS-CoV-2, e.g. a rapid antigen test) should be administered with higher frequency is context-specific. Mathematical models can and should support these decisions in real-time, as was the case during the COVID-19 pandemic78.
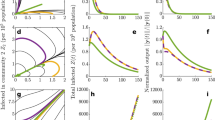
When considering model input data, one key aspect that has to be taken into account by modelling studies is the deliberate parameterization of accuracy for case numbers based directly or indirectly on the results of a diagnostic test79. This typically includes incidence rates as well as seroprevalence estimates. Knowledge about the diagnostic accuracy of the tests is critical as biased estimates of sensitivity and specificity can lead to biased estimates in modelling results used for health decision making. The textbook example for this is an overestimation of the specificity of an antibody test used for seroprevalence studies in low-prevalence settings which leads to an overestimation of the proportion of the population which has had already contact with the emerging infectious agent. As a consequence, population immunity would be overestimated, underestimating the risk associated with an uncontrolled spread in the population. If true diagnostic accuracy is known, population-level estimates on e.g. seroprevalence can be corrected, either before the modelling study or within the modelling framework80. If it is not known that the proposed diagnostic accuracy is biased, modelling can help in detecting implausibilities, especially if parameter fitting is carried out regularly. Here, modelling can inform diagnostic test evaluation with respect to potential biases, but also in the context of value of information analyses. During the first three months of the SARS-CoV-2 pandemic, only a minority of modelling studies in the field accounted for test accuracy estimates; the remaining used incidence and later seroprevalence data as if they represented the ground truth. This approach would be appropriate if incidence or seroprevalence data were already corrected for imperfect test accuracy estimates. However, in this case, the correction procedure should still be reported in the modelling study to enable a transparent evaluation of model parameterization, and the model(s) should be reparametrized once updated information on diagnostic test accuracy is available. Earlier decision making based on updated information increases the impact of these decisions on population health (Fig. 1). Decisions just a few weeks or even a couple of days earlier can make a huge difference, offering a critical time window for accelerated diagnostic studies. Fig. 2 shows the sensitivity of model-based assessments of interventions to diagnostic test accuracy parameters. The results show that even relatively small biases in the estimation of test accuracy (much smaller than those found in the Cochrane reviews) for an antibody test used to derive the proportion of undetected cases in a population have an enormous effect on the predicted further course of the epidemic (the mechanism for this impact is that the proportion of undetected cases is used to correct reported case numbers before they are used to calibrate transmissibility estimates and other parameters). The results are enough to change public health decision-making from, for example, not implementing population-level contact reduction measures to introducing a hard lockdown if the defined outcome of interest crosses a set decision-analytic threshold.
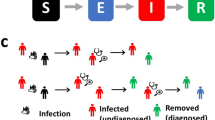
Time course of the number of individuals requiring intensive care therapy if measures are taken at different time points to reduce the effective reproduction number below 1 (reproduction number (R) at the start set to R = 2, reduced to R = 0.9 after 7, 14, 21, 28 days). Assumptions of the Susceptible-Exposed-Infectious-Recovered (SEIR) model: pre-infection time: 3 days, infectious time: 7 days, proportion of individuals requiring intensive care unit out of all infectious patients: 2%, length of stay in intensive care unit: 15 days, population size: 80.000.000, number of susceptible individuals at the start: 79.950.000, number of exposed individuals at start: 40.000, number of infectious individuals at the start: 10.000, number of immune individuals at the start: 0, number of individuals requiring intensive care at the start: 0.
Forecast for number of individuals requiring intensive care therapy if measures as described for Fig. 1 are taken after 28 days, with the model parametrised using different assumptions for antibody test specificity. SEIR model with Infectious compartment split into ‘Detected’ and ‘Undetected’ compartments. Parametrisation as described in Fig. 1, but with 2% of detected infectious cases requiring intensive care. Proportion of detection obtained from data from the ‘Heinsberg’ seroprevalence study86, corrected for antibody test sensitivity of 0.9, and specificity of 0.9, 0.93, 0.96, 0.99, 1.
Longitudinal panels as a platform for diagnostic accuracy studies
Given the rapidly changing research questions during an epidemic or pandemic, there is a huge practical challenge in setting up diagnostic studies even with the modern study designs described above, because the acceptable time spans for recruiting study participants and for conducting the actual studies are very short. The availability of a study platform that allows immediate initiation of diagnostic studies reflecting the current research question and infection dynamics is indispensable for timely studies in the field. One way to ensure this is the sustainable implementation of a longitudinal panel within existing cohorts (e.g., as the NAKO Health Study81) that is tested regularly for the presence or absence of the pathogen by a defined test (or several) under evaluation. Another approach is to use data from hospitals, health insurance or public health agencies. For example, a platform comparable to the UK Office for National Statistics (ONS) panel82 or the REACT study83 can be built and used to evaluate the tests or testing strategies under study, and for real-time communication of the results of the respective tests representing current or past infection dynamics. In this setting, flexible and fast study designs can fulfil both, equally important, purposes at the same time.
Feedback triangle at the centre of a unified framework
As discussed above, the development and evaluation of diagnostic tests in an epidemic or pandemic setting is closely linked to modelling studies used to inform political and public health decision-making. This link is at the centre of the unified framework we propose based on experiences during the SARS-CoV-2 pandemic (Fig. 3). The execution of diagnostic studies for new tests or new application areas of existing tests depends heavily on current test strategies and those potentially applied in the future. Results from diagnostic studies are a direct input in mathematical modelling studies, and in turn results from these models are used for decision-making based on a defined decision-making framework. However, modelling studies can also give crucial feedback to those responsible for planning and analysing diagnostic accuracy studies. Here, so-called value-of-information analyses can help identify those gaps in knowledge regarding diagnostic test accuracy that need to be tackled first or require the greatest attention84. This can directly affect sample size estimations, for instance if more precision is needed to estimate the test’s specificity (as is often the case with antibody tests). Therefore, the optimal strategy to deal with these constant feedback loops is to establish continuous collaboration between the disciplines representing the three parts of this loop (in green in Fig. 3). This collaboration platform can use the longitudinal panel with complementary perspectives described above to create a unified diagnostic test development and evaluation framework during an epidemic or pandemic. The modern study designs and bias reduction methods described above can be applied to obtain the best potentially available evidence about diagnostic test accuracy in different settings. When creating such a framework for developing and evaluating diagnostic tests and considering the corresponding results in modelling studies, both infection-specific (e.g. transmission rates, case fatality ratios) and test-specific characteristics (e.g. test type, costs, availability) must be considered, especially for the collection of population-level data from testing programmes.
Diagnostic test-intervention studies using a cluster-randomised approach
In many situations, diagnostic test accuracy estimates should only be seen as surrogate information since the actual outcome of interest during an ever-changing pandemic, especially in the later phases, is the effect of an application of this test on clinical or population-level outcomes. Here, it is possible, as has been discussed during the SARS-CoV-2 pandemic, to take a step further and move test evaluation to phase IV or diagnostic test-intervention studies. In this phase, individuals or clusters of individuals are randomised to a diagnostic strategy (e.g., regular testing of the entire population or testing only in case of symptoms). The relevant clinical endpoint is then compared between randomised groups42. Thus, the test strategy is treated as an intervention evaluated for its effectiveness and safety. Diagnostic test accuracy helps to reach this endpoint but is not the only factor under evaluation. The practicability of the strategy, as well as real-world effectiveness and interaction with other interventions (e.g., the case isolation and quarantine of close contacts), are also assessed indirectly in this approach. In a dynamic infectious disease setting where an intervention can have indirect effects on people other than the target population, only cluster-randomised approaches allow for a reasonable estimation of population-level effects of the intervention under study. In infectious disease epidemiology, similar designs are applied when assessing the effectiveness of vaccination programs on a population level, often combined with a staggered entry approach to allow all clusters to benefit from the intervention over time (so-called stepped-wedge design). During the pandemic, small-scale pilot studies were discussed, trying to mirror such an approach in a non-randomised way, often claiming to be a natural experiment. However, most of them did not follow guidelines and recommendations available for diagnostic test-intervention studies that would have improved the quality of the results and their usefulness for evidence-based public health. Rigorous application of cluster-randomised diagnostic test-intervention studies to implement testing strategies can support decision-making processes in the later stages of an epidemic or pandemic.
Conclusion
The development and evaluation of diagnostic tests for emerging infectious agents during an epidemic or pandemic come with many serious challenges. We propose integrating diagnostic studies in a unified framework representing the triangle of diagnostic test evaluation, predictive or decision-analytic public health modelling and the testing strategy applied in this population. This framework can use modern, flexible and fast study designs and should incorporate a longitudinal panel as a continuous study platform. Diagnostic test-intervention studies need to be planned early and should be used for evidence-based public health during later phases of an epidemic or pandemic, when research questions become more complicated and testing strategies serve as interventions to counteract infectious disease dynamics.
References
Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU. http://data.europa.eu/eli/reg/2017/746/oj (2017).
European Medicines Agency. Guideline on clinical evaluation of diagnostic agents (European Medicines Agency, 2009).
FDA. Statistical Guidance on Reporting Results from Studies Evaluating Diagnostic Tests - Guidance for Industry and FDA Staff. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/statistical-guidance-reporting-results-studies-evaluating-diagnostic-tests-guidance-industry-and-fda (2007).
Dinnes, J. et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, CD013705 (2021).
Deeks, J. J. et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, CD013652 (2020).
DxConnect Test Directory. https://finddx.shinyapps.io/testdirexplorer_beta/ (2022).
Röhrig, B. The Diagnostic Test: Goodness, Characteristics, and Interpretation: Under the Impact of the Corona Pandemic and Different SARS-CoV-2 Tests. Gesundheitswesen 85, 578–594 (2023).
Du, Z. et al. Comparative cost-effectiveness of SARS-CoV-2 testing strategies in the USA: a modelling study. Lancet Public Health 6, e184–e191 (2021).
Veroniki, A. A. et al. Rapid antigen-based and rapid molecular tests for the detection of SARS-CoV-2: a rapid review with network meta-analysis of diagnostic test accuracy studies. BMC Med 21, 110 (2023).
Fox, T. et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2022, CD013652 (2022).
Hurtado, A. V. et al. The economic cost of implementing antigen-based rapid diagnostic tests for COVID-19 screening in high-risk transmission settings: evidence from Germany. Health Econ. Rev. 12, 15 (2022).
Pighi, L. et al. Cost-effectiveness analysis of different COVID-19 screening strategies based on rapid or laboratory-based SARS-CoV-2 antigen testing. Clin. Chem. Lab. Med. 61, E168–E171 (2023).
Nguyen, H. T. et al. Cost and cost-effectiveness of four different SARS-CoV-2 active surveillance strategies: evidence from a randomised control trial in Germany. Eur. J. Health Econ. 24, 1545–1559 (2023).
ECDC. Surveillance Atlas of Infectious Diseases. http://atlas.ecdc.europa.eu/public/index.aspx (2024).
Daugherty, B. L. et al. Ethical considerations: care of the critically ill and injured during pandemics and disasters. Chest Consens. Statement Chest 146, e145S–e155S (2014).
Emanuel, E. J. et al. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N. Engl. J. Med. 382, 2049–2055 (2020).
Kelso, J. K., Milne, G. J. & Kelly, H. Simulation suggests that rapid activation of social distancing can arrest epidemic development due to a novel strain of influenza. BMC Public Health 9, 117 (2009).
Faes, C., Hens, N. & Gilbert, M. On the timing of interventions to preserve hospital capacity: lessons to be learned from the Belgian SARS-CoV-2 pandemic in 2020. Arch. Public Health 79, 164 (2021).
Ferguson, N. M. et al. Report 9: Impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand. https://doi.org/10.25561/77482 (2020).
Ngonghala, C. N. et al. Mathematical assessment of the impact of non-pharmaceutical interventions on curtailing the 2019 novel Coronavirus. Math. Biosci. 325, 108364 (2020).
van Kerkhove, M. D. & Ferguson, N. M. Epidemic and intervention modelling – a scientific rationale for policy decisions? Lessons from the 2009 influenza pandemic. Bull. World Health Organ 90, 306 (2012).
Heesterbeek, H. et al. Modeling infectious disease dynamics in the complex landscape of global health. Science 347, aaa4339 (2015).
Kretzschmar, M. E. et al. Challenges for modelling interventions for future pandemics. Epidemics 38, 100546 (2022).
Mauskopf, J. et al. Economic Analysis of Vaccination Programs: An ISPOR Good Practices for Outcomes Research Task Force Report. Value Health 21, 1133–1149 (2018).
Siebert, U. When should decision-analytic modeling be used in the economic evaluation of health care? Eur. J. Health Econ. 4, 143–150 (2003).
Ultsch, B. et al. Methods for Health Economic Evaluation of Vaccines and Immunization Decision Frameworks: A Consensus Framework from a European Vaccine Economics Community. Pharmacoeconomics 34, 227–244 (2016).
ABC News. Melbourne lockdown extended by seven days as Victoria records 20 local COVID-19 cases. https://www.abc.net.au/news/2021-08-11/victoria-covid-cases-melbourne-lockdown-extension/100366822 (2021).
Al Jazeera. Australia’s Canberra extends COVID-19 lockdown. https://www.aljazeera.com/news/2021/8/31/australias-canberra-extends-covid-19-lockdown (2021).
The Institute for Government. Coronavirus: local lockdowns. https://www.instituteforgovernment.org.uk/explainers/coronavirus-local-lockdowns (2020).
Federal Government: Federal and Länder Governments consult on the coronavirus situation. https://www.bundeskanzler.de/bk-en/news/corona-state-premier-conference-1983156 (2021).
World Health Organisation. WHO SAGE Roadmap for Prioritizing Uses of COVID-19 Vaccines in the Context of Limited Supply: An Approach to Inform Planning and Subsequent Recommendations Based on Epidemiological Setting and Vaccine Supply Scenarios, First Issued 20 October 2020, Latest (WHO, 2021).
Gesetz zur Verhütung und Bekämpfung von Infektionskrankheiten beim Menschen (IfSG). https://www.gesetze-im-internet.de/ifsg/ (2000).
Jahn, B. et al. Targeted COVID-19 Vaccination (TAV-COVID) Considering Limited Vaccination Capacities—An Agent-Based Modeling Evaluation. Vaccines 9, 434 (2021).
Moore, S., Hill, E. M., Tildesley, M. J., Dyson, L. & Keeling, M. J. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect. Dis. 21, 793–802 (2021).
Chowdhury, R. et al. Dynamic interventions to control COVID-19 pandemic: a multivariate prediction modelling study comparing 16 worldwide countries. Eur. J. Epidemiol. 35, 389–399 (2020).
Giordano, G. et al. Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy. Nat. Med. 26, 855–860 (2020).
Nussbaumer-Streit, B. et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst. Rev 4, CD013574 (2020).
Nussbaumer-Streit, B. et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review [Update]. Cochrane Database Syst. Rev. 9, CD013574 (2020).
Mina, M. J. & Andersen, K. G. COVID-19 testing: One size does not fit all. Science 371, 126–127 (2021).
Commission Implementing Regulation (EU) 2022/1107 of 4 July 2022 laying down common specifications for certain class D in vitro diagnostic medical devices in accordance with Regulation (EU) 2017/746 of the European Parliament and of the Council. http://data.europa.eu/eli/reg_impl/2022/1107/oj (2022).
Leeflang, M. M. G. & Allerberger, F. How to: evaluate a diagnostic test. Clin. Microbiol. Infect. 25, 54–59 (2019).
Sackett, D. L. & Haynes, R. B. The architecture of diagnostic research. BMJ 324, 539–541 (2002).
Koebberling, J., Trampisch, H. & Windeler, J. Memorandum: Evaluation of diagnostic measures. J. Clin. Chem. Clin. Biochem. 28, 873–880 (1990).
Leeflang, M. M. G., Moons, K. G. M., Reitsma, J. B. & Zwinderman, A. H. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values: mechanisms, magnitude, and solutions. Clin. Chem. 54, 729–737 (2008).
Ewald, B. Post hoc choice of cut points introduced bias to diagnostic research. J. Clin. Epidemiol. 59, 798–801 (2006).
Jahn, B. et al. On the role of data, statistics and decisions in a pandemic. AStA Adv. Statistical Anal 106, 349–382 (2022).
Rutjes, A. W. S., Reitsma, J. B., Vandenbroucke, J. P., Glas, A. S. & Bossuyt, P. M. M. Case-control and two-gate designs in diagnostic accuracy studies. Clin. Chem. 51, 1335–1341 (2005).
Lijmer, J. G. et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 282, 1061–1066 (1999).
Karch, A., Koch, A., Zapf, A., Zerr, I. & Karch, A. Partial verification bias and incorporation bias affected accuracy estimates of diagnostic studies for biomarkers that were part of an existing composite gold standard. J. Clin. Epidemiol. 78, 73–82 (2016).
Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU. http://data.europa.eu/eli/reg/2017/746/2022-01-28 (2022).
FDA. In Vitro Diagnostics EUAs. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas (2023).
WHO. Coronavirus disease (COVID-19) pandemic. https://www.who.int/europe/emergencies/situations/covid-19 (2024).
Roche Diagnostics. COVID-19. https://diagnostics.roche.com/us/en/landing-pages/roche-covid-19-updates.html (2024).
FDA. COVID-19 Emergency Use Authorizations for Medical Devices. https://www.fda.gov/medical-devices/emergency-use-authorizations-medical-devices/covid-19-emergency-use-authorizations-medical-devices (2023).
Hall, M. K., Kea, B. & Wang, R. Recognising bias in studies of diagnostic tests part 1: patient selection. Emerg. Med. J. 36, 431 (2019).
Kea, B., Hall, M. K. & Wang, R. Recognising bias in studies of diagnostic tests part 2: interpreting and verifying the index test. Emerg. Med. J. 36, 501–505 (2019).
Kohn, M. A., Carpenter, C. R. & Newman, T. B. Understanding the direction of bias in studies of diagnostic test accuracy. Acad. Emerg. Med. 20, 1194–1206 (2013).
Suchá, D., van Hamersvelt, R. W., van den Hoven, A. F., de Jong, P. A. & Verkooijen, H. M. Suboptimal quality and high risk of bias in diagnostic test accuracy studies at chest radiography and CT in the acute setting of the COVID-19 pandemic: a systematic review. Radiol. Cardiothorac. Imaging 2, e200342 (2020).
Hughes, J. M., Penney, C., Boyd, S. & Daley, P. Risk of bias and limits of reporting in diagnostic accuracy studies for commercial point-of-care tests for respiratory pathogens. Epidemiol. Infect. 146, 747–756 (2018).
Pavlou, A., Kurtz, R. M. & Song, J. W. Diagnostic accuracy studies in radiology: how to recognize and address potential sources of bias. Radio. Res. Pr. 2021, 5801662 (2021).
Shan, G., Zhang, H. & Jiang, T. Determining sample size for a binary diagnostic test in the presence of verification bias. J. Biopharm. Stat. 28, 1193–1202 (2018).
De Groot, J. A. H. et al. Adjusting for differential-verification bias in diagnostic-accuracy studies: a Bayesian approach. Epidemiology 22, 234–241 (2011).
Lu, Y., Dendukuri, N., Schiller, I. & Joseph, L. A Bayesian approach to simultaneously adjusting for verification and reference standard bias in diagnostic test studies. Stat. Med. 29, 2532–2543 (2010).
de Groot, J. A. H. et al. Correcting for partial verification bias: a comparison of methods. Ann. Epidemiol. 21, 139–148 (2011).
European Medicines Agency. Adaptive Pathways. https://www.ema.europa.eu/en/human-regulatory-overview/research-development/adaptive-pathways (2016).
Thorlund, K., Haggstrom, J., Park, J. J. & Mills, E. J. Key design considerations for adaptive clinical trials: a primer for clinicians. BMJ 360, k698 (2018).
Cerqueira, F. P., Jesus, A. M. C. & Cotrim, M. D. Adaptive design: a review of the technical, statistical, and regulatory aspects of implementation in a clinical trial. Ther. Innov. Regul. Sci. 54, 246–258 (2020).
Zapf, A. et al. Adaptive trial designs in diagnostic accuracy research. Stat. Med. 39, 591–601 (2020).
Hot, A. et al. Randomized test-treatment studies with an outlook on adaptive designs. BMC Med. Res. Methodol. 21, 110 (2021).
Vach, W. et al. A potential for seamless designs in diagnostic research could be identified. J. Clin. Epidemiol. 129, 51–59 (2021).
Stark, M. & Zapf, A. Sample size calculation and re-estimation based on the prevalence in a single-arm confirmatory diagnostic accuracy study. Stat. Methods Med. Res. 29, 2958–2971 (2020).
Stark, M. et al. Blinded sample size re-estimation in a comparative diagnostic accuracy study. BMC Med Res Methodol 22, 115 (2022).
Köster, D., Hoyer, A. & Zapf, A. Adaptive designs with unblinded sample size re-estimation for diagnostic accuracy studies. 66. Jahrestagung der Deutschen Gesellschaft für Medizinische Informatik, Biometrie und Epidemiologie e. V. (GMDS), 12. Jahreskongress der Technologie- und Methodenplattform für die vernetzte medizinische Forschung e.V. (TMF) (2021) https://doi.org/10.3205/21GMDS079.
Hot, A. et al. Sample size recalculation based on the prevalence in a randomized test-treatment study. BMC Med Res Methodol 22, 205 (2022).
Westphal, M., Zapf, A. & Brannath, W. A multiple testing framework for diagnostic accuracy studies with co-primary endpoints. Stat. Med. 41, 891–909 (2022).
Bouman, J. A., Riou, J., Bonhoeffer, S. & Regoes, R. R. Estimating the cumulative incidence of SARS-CoV-2 with imperfect serological tests: Exploiting cutoff-free approaches. PLoS Comput. Biol. 17, e1008728 (2021).
Pepić, A. et al. A diagnostic phase III/IV seamless design to investigate the diagnostic accuracy and clinical effectiveness using the example of HEDOS and HEDOS II. Stat. Methods Med. Res. 33, 433–448 (2024).
Krumkamp, R. et al. Negative SARS-CoV-2 PCR or rapid antigen test result and the subsequent risk of being infectious: a mathematical simulation study. BMC Med. Res. Methodol. 21, 165 (2021).
Trikalinos, T. A., Siebert, U. & Lau, J. Decision-analytic modeling to evaluate benefits and harms of medical tests: Uses and limitations. Med. Decision Making 29, E22–9 (2009).
Rogan, W. J. & Gladen, B. Estimating prevalence from the results of a screening test. Am. J. Epidemiol. 107, 71–76 (1978).
Peters, A. et al. Framework and baseline examination of the German National Cohort (NAKO). Eur. J. Epidemiol. 37, 1107–1124 (2022).
Office for National Statistics. COVID-19 Infection Survey. https://www.ons.gov.uk/surveys/informationforhouseholdsandindividuals/householdandindividualsurveys/covid19infectionsurvey (2023).
Imperial College London. Real-time Assessment of Community Transmission (REACT) Study. https://www.imperial.ac.uk/medicine/research-and-impact/groups/react-study/ (2024).
Siebert, U., Rochau, U. & Claxton, K. When is enough evidence enough? - Using systematic decision analysis and value-of-information analysis to determine the need for further evidence. Z. Evid. Fortbild. Qual. Gesundhwes 107, 575–584 (2013).
Leeflang, M. M. G. Systematic reviews and meta-analyses of diagnostic test accuracy. Clin. Microbiol. Infect. 20, 105–113 (2014).
Streeck, H. et al. Infection fatality rate of SARS-CoV2 in a super-spreading event in Germany. Nat. Commun. 11, 5829 (2020).
Acknowledgements
This work was supported by unrestricted grants from the Deutsche Forschungsgemeinschaft (German Research Council, grant numbers KA 5361/1-1 and ZA 687/3-1, project number 458526380).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.C. contributed to writing the first draft, attended the workshop and participated in the discussions, and contributed to and approved the final version of the manuscript. D.K. contributed to writing the first draft, attended the workshop and participated in the discussions, and contributed to and approved the final version of the manuscript. P.B. attended the workshop, participated in the discussions, and contributed to and approved the final version of the manuscript. O.G. attended the workshop, participated in the discussions, and contributed to and approved the final version of the manuscript. A.J. attended the workshop, participated in the discussions, and contributed to and approved the final version of the manuscript. M.K. attended the workshop, participated in the discussions, and contributed to and approved the final version of the manuscript. M.L. participated in the discussions and contributed to and approved the final version of the manuscript. R.M. attended the workshop, participated in the discussions, and contributed to and approved the final version of the manuscript. J.R. attended the workshop, participated in the discussions, and contributed to and approved the final version of the manuscript. N.S.-M. attended the workshop, participated in the discussions, and contributed to and approved the final version of the manuscript. U.S. participated in the discussions and contributed to and approved the final version of the manuscript. C.S. participated in the discussions and contributed to and approved the final version of the manuscript. C.E. participated in the discussions and contributed to and approved the final version of the manuscript. N.R. contributed to writing the first draft, attended the workshop and participated in the discussions, and contributed to and approved the final version of the manuscript. A.K. contributed to writing the first draft, attended the workshop and participated in the discussions, and contributed to and approved the final version of the manuscript. A.Z. contributed to writing the first draft, attended the workshop and participated in the discussions, and contributed to and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Kathy Leung and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaturvedi, M., Köster, D., Bossuyt, P.M. et al. A unified framework for diagnostic test development and evaluation during outbreaks of emerging infections. Commun Med 4, 263 (2024). https://doi.org/10.1038/s43856-024-00691-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-024-00691-9