Abstract
Several transitions, or new patterns and dynamics in the contributors and health outcomes, have altered the character and burden of the multi-decade, worldwide growth in prevalence of type 2 diabetes (T2DM). These changes have led to different needs for prevention and care. These dynamics have been driven by diverse demographic, socio-economic, behavioural, and health system response factors. In this Perspective, we describe these transitions and how their attributes have set the stage for multimorbidity, or multiple long-term conditions (MLTCs), to be the next major challenge in the diabetes epidemic. We also describe how the timing and character of these stages differ in high-, middle-, and low-income countries. These challenges call for innovation and a stronger focus on MLTCs across the spectrum of cause, effectiveness, and implementation studies to guide prevention and treatment priorities.
Similar content being viewed by others
Introduction
The global pandemic of type 2 diabetes (T2DM) is often viewed as a long-term by-product of the great epidemiologic transition, wherein in the previous century, human patterns of mortality and disease exchanged high rates of mortality due to infections and maternal and child mortality for lower mortality rates driven by chronic conditions such as cardiovascular disease (CVD), cancer, and T2DM1,2. However, there have been differing changes in incidence for specific chronic conditions. For example, deaths due to CVD decreased beginning in the 1970s and 1980s whereas T2DM, lacking effective policy or health care options for prevention, accelerated in the 1990s2,3,4,5. Although recent signs of a decrease in T2DM incidence in some countries have emerged6,7, most countries of the world continue to experience unrelenting growth of the problem6,7,8.
The T2DM pandemic has itself been complex and multi-phasic. Underlying the overall increases in prevalence, have been several transitions across distinct periods of the T2DM epidemic that impacted the burden and approaches to care, prevention, and research9,10,11,12. In this Perspective, we explore how these transitions have set the stage for multiple long-term conditions (MLTC, or multimorbidity) impacting morbidity, health services, and approaches to prevent T2DM.
Seeding and steady growth of the problem
The first phase of growth in the T2DM epidemic, evident in the 1970s and 1980s, consisted of steady, gradual, increases in prevalence in high income countries (HICs) and more rapid increases in selected low- and middle-income countries (LMICs) undergoing rapid urbanization, nutritional and other sociocultural changes associated with westernization5,13,14,15 (Fig. 1). This was associated with a global nutrition transition, with dietary shifts toward refined carbohydrates, added sweeteners and edible oils, and away from legumes and fresh fruits and vegetables. Declining physical activity levels contributed further and collectively they are cited as driving an environment conducive to increases in obesity and T2DM16,17. However, where data were available, diagnosed diabetes prevalence rates were quite low, at around 2–4% in the US, UK, and Northern Europe5,18,19 compared to recent global estimates, which have surpassed 10% of adults5,8. Further, this low diabetes prevalence contrasted with very high rates of severe complications, including a 20-fold increased rate of lower extremity amputation, 10 times the rate of end stage renal disease (ESRD), and three times the risk of CVD20,21,22,23,24,25,26. These high complication risks were attributed to suboptimal diabetes care, risk factors and self-management practices. For example, estimates from the some HICs in the mid-1990s showed that large segments of the population with diagnosed diabetes had HbA1c levels well over 9% (75 mmol/mol) and blood pressure levels > 140/80, levels considered to be a sign of inadequate management by current standards27,28,29. Thus, perception of the problem centred more on the complications accompanying T2DM rather than the incidence and potential prevention of T2DM.
Targeting quality of care
The juxtaposition of low diabetes prevalence but high incidence of complications among people with diabetes, combined with new evidence for the preventability of diabetes complications with glycemic, blood pressure, and multifactorial CVD risk factor management for both type 1 diabetes and T2DM, spurred a second, distinct period between 1995–201030,31,32. A focus on improving quality of care became the primary public health response, including concerted attention to improve the structure and organization of health services for chronic disease care9,28,29,33 (Fig. 1). Collectively, these improvements in care delivery are credited with leading to broad reductions in rates of incidence of myocardial infarction, stroke, lower extremity amputations, and ESRD during the 2000s and contributing to a step change in the quality and length of life for people with the condition in selected high income countries21,34,35,36,37. However, these advances did not bring care close to optimal levels, as only a minority of people with diabetes met guideline-directed target levels of risk factor control and preventive care practices38. Further, in the background of these improvements, increases in diagnosed T2DM incidence accelerated,4,5,7,8 accompanied by a dramatic growth in obesity and T2DM in youth and young adulthood, disproportionately affecting sub-populations of lower socio-economic and non-white populations in the US, UK, and Europe that continue today11,39,40.
Stagnation, diversification, and divides
Following the encouraging improvements in diabetes care and reduction in complications, the period from 2010 to the present reveals new, unanticipated dynamics. First, the disproportionate decrease in CVD mortality of the prior decades was accompanied by diversification and relative increases in other forms of morbidity12,41. (Fig. 1). For example, among the populations with diabetes in the US and UK, hospitalizations and deaths due to vascular causes declined precipitously while other common causes, including kidney disease, respiratory infections and deaths due to cancers, liver disease and dementia have increased37,42. A similar analysis of hospitalizations in Australia among adults with diabetes shows that admissions due to severe stress and adjustment disorders, iron deficiency anaemia, kidney stones, and gastroenteritis have increased43. These studies are a reminder that diabetes is associated with many other conditions not traditionally regarded as complications of diabetes. Although less specific, and with weaker magnitudes of association than classic complications, the large number of conditions alongside their relatively high frequency in older adults, means they can have an important overall impact on diabetes-associated morbidity.
A second, important observation from this period has been an apparent stagnation of improvements in care and reductions in complications44,45,46. In the US, this has included a plateau in levels of glycemic, blood pressure, and lipid control and a resurgence in complications in adults with diagnosed diabetes and an increase in mortality rates in the general population of middle-aged white men. Increases in hospitalizations for acute hyperglycaemia and lower extremity amputations have been reported in the US, UK, and Australia, although there remains considerable international variation in these trends47,48,49,50,51. More broadly, there has been a slowing of improvements in CVD mortality in many countries, with notable effects on overall life expectancy in middle age men in the US44. In LMICs, trends in care and risk factor management are unclear but have been shown to be at a generally suboptimal level and worse than in HICs52. Further, drivers of a slowdown in improvements are also unclear but socio-economic recession and inequalities, as well as the challenges of heterogenous, early onset disease, have each been cited as possible factors53,54.
A third, unexpected trend has been an apparent peak and decrease in diabetes incidence itself in some settings. This has been paired with a plateau in prevalence in many HICs, although there is little evidence that this reflects broader global trends6,45,47 The decrease in incidence seen in high-income countries remains unexplained6,7. Some countries, including the US, UK, and Finland have invested in structured community-based prevention programmes. While these programmes have promising potential to reduce incidence in high-risk participants, their reach across the population needs to be substantial, such as that seen in England, to alter trends in incidence55. It is also possible that an accompanied emphasis on screening and testing for pre-diabetes, health promotion efforts and increased education and awareness of the scale of the diabetes threat, is helping turn the tide on the underlying risk behaviours. However, there has been limited data on trends in risk factors to confirm this. It is also possible that shifts in diagnostic criteria to HbA1c from fasting plasma glucose, or other changes in testing, have affected incidence of diagnosis56.
Increased burden in the young
Many elements of the transitions described above were influenced or exacerbated by an age divide, cutting across aetiology, care, prevention and complications57,58. For example, the greatest relative increase in incidence and prevalence has been among the young. Among cases, the risk profiles at diagnosis and the excess risk of morbidity and mortality is greater in young than older populations compared to same-aged counterparts without diabetes59,60,61,62,63. Earlier age-at-onset of T2DM is associated with higher proportions of obesity, a more adverse behavioural risk profile, proportionately more people from racial/ethnic minorities, and worse risk factor control than those with later-onset T2DM and worse pregnancy outcomes compared to women with type 1 diabetes59,60,61,62,64,65. Where improvements have occurred in care, prevention, and outcomes, they have been driven by the older population, leading to a reduction in the age distribution of the population with diabetes-related complications21. The factors driving this shifting burden to the young are unclear, but socio-economic inequalities and a disproportionate vulnerability of high risk youth to obesogenic environments may play a large role6,47,53,57,58.
The global high-to-low income divide
The transitions described above are mainly evident in HICs, as a large data gap in complications and morbidity among populations with diabetes leaves the status of LMICs unclear36. Where data exists, LMICs appear to have followed similar paths5,66,67. However, periods of acceleration of increases in diabetes incidence and prevalence were accompanied by even greater magnitudes and longer periods of increases in South Asia, the Middle East, North Africa, and Oceania8. In China, increases in burden have generally paralleled those of the US, but given the population size, disproportionately affected the overall global burden68. The greater growth in diabetes prevalence in LMICs is consistent with previously described nutritional and obesity transitions, as well as economic and commercial influences affecting the availability and affordability of unhealthy compared to healthy foods, and environmental risks affecting levels of physical activity16,17,69. These changes have also included a shift from persons of high socio-economic status (SES) to low SES having highest risk even in countries with histories of undernutrition alongside the long-term migration of people from rural to urban settings70,71,72,73. Despite the long-term role of urbanization, recent evidence also suggests that the current growth in obesity prevalence is being influenced more by increases in rural than urban settings73. Populations with diabetes in LMICs also have lower levels of preventive care services and risk factor management and higher relative risk of complications and mortality67,72,74,75.
The reductions in overall mortality rates across LMICs should be expected to benefit adults with diagnosed diabetes, but the lack of continuous diabetes surveillance systems with the ability to track denominators with diagnosed diabetes has limited published reports on causes-specific mortality or complications among populations with diabetes76. Thus, whether the long-term reductions selected complications and the apparent diversification of outcomes seen in HICs are also occurring in LMICs is unknown. Reports from India and Mexico showing considerably higher relative risks of mortality, CVD, and kidney disease than that shown in HICs raises the question of whether population trends and dynamics could also be different67,77,78.
The emergence of MLTCs as the next diabetes epidemiologic transition
We have described a succession of major transitions in the diabetes epidemic, from seeding and growth of the problem to successes in care and prevention of complications with an underlying shifting burden to the young to an era of stagnating care, diversification of outcomes, and persistent socio-economic disparities. These transitions, accompanied by the co-factors of decreasing CVD mortality, early onset and greater heterogeneity of T2DM have set the stage for the next transition of the evolving diabetes epidemic in HICs, with MLTCs representing the next major challenge (Fig. 2).
Multimorbidity, or MLTCs, has been defined as the co-occurrence of two or more chronic conditions within a single individual, including non-communicable diseases of long duration like CVD or cancer, a mental health condition and mood disorders, and infections of long duration, such as HIV or hepatitis C79. The UK National Institute for Health and Care Excellence (NICE), definition also includes alcohol or substance abuse, symptom complexes such as frailty or chronic pain, sensory impairments and ongoing conditions that affect function, such as learning disability80. MLTCs may be further classified as concordant if they share a pathophysiological pathway with the index condition (e.g. diabetes and chronic kidney disease), or discordant if no obvious shared pathway exists, (e.g. T2DM and asthma), and complex if three or more conditions affect multiple bodily systems. Although the concept of concurrent conditions has historically been intrinsic T2DM, leading many to report concordance and cross-prediction of microvascular and macrovascular complications, public health agencies in the US, UK, as well as the WHO have prioritised the problem of MLTCs for chronic diseases as a whole81,82,83,84,85,86,87.
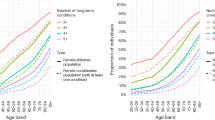
The transitions in the diabetes epidemic could increase MLTCs by acting at both ends of the age distribution. In older adults, gains in longevity and diversifying complications will likely drive a persistent and higher prevalence of MLTC and cumulative morbidity even if incidence rates of complications decline (Fig. 3). In younger populations, the heterogeneous and adverse risk profile at diagnosis of obesity, and early onset comorbid conditions, particularly in ethnic minority populations, results in an earlier and longer exposure to adverse metabolic risk with potentially long-term effects on health service use, and work productivity during the life stage when optimal work productivity is expected88. The net effect of obesity, metabolic heterogeneity, and the changing environment on lifestyle and mental health before onset could also change the initial context. The lack of available new systems, structures, guidelines, or therapeutic approaches to reduce and effectively manage MLTCs or mitigate the influential effect of wider social determinants of health on MLTCs will create greater challenges.
Figure is hypothetical and not drawn from a specific population or data set. However, the estimates of median and 25th percentiles in the distribution of age at diagnosis are based on data from the US National Diabetes Surveillance System108. The estimates of change in average age of death are based on published modelled estimates that are also based on US national data24. T2DM: Type 2 Diabetes Mellitus.
The study of MLTCs has been limited by the complexity of their measurement and the heterogeneity of conditions, which has often relied on crude metrics89. Although a diverse taxonomy of MLTC metrics exists, quantified by diagnostic categories, drug use, physiologic measures90, and several weighted summary scores, the most commonly used approach is to sum the number of conditions from a pre-selected list of diagnosis codes. Although easily interpretable, this is also a blunt way of estimating the variation, severity, and impact of MLTCs. More recently, clustering approaches, including machine learning (ML)-based statistical methods, have attempted to identify distinct clusters or phenotypes. To date, these studies have studied distinct phenotypes based on clinical characteristics and health status at diagnosis, to predict risk for diabetes complications91. At least one study has shown that ML-derived clusters, in turn, are highly predictive of subsequent outcomes, possibly serving as a precursor for their incorporation into treatment models92. Distinct clusters of risk factors may also act earlier, such as in a pre-diabetes phase, or later, as MLTCs increase. A rapidly growing literature is attempting to understand how clusters of MLTCs develop longitudinally but the complexity involved has limited both interpretation and clinical utility to date93,94. Advances in identifying the underlying mechanisms and molecular drivers are likely to influence characterisation of prominent MLTC phenotypes in the near future95.
The epidemiology and characteristics of diabetes and multimorbidity
The lack of standardized MLTC metrics leads to highly disparate prevalence estimates across studies96. One systematic review of 193 studies estimated the pooled prevalence of MLTC at 42.4%, with the prevalence being higher in older adults and in studies that included large numbers of conditions. Barnett et al. showed that the proportion with at least three conditions doubles roughly every decade of life, from about 5% at age 40 to more than 10%, 20%, and 40% at age 50, 60, and 70 years respectively97. Further, people in the lowest decile of social deprivation have a prevalence of three or more conditions 10 to 15 years earlier than those living in more affluent areas. Studies in the UK suggest that the prevalence of having three or four or more MLTCs has increased over time98, although the degree to which shifts in screening, diagnoses, or coding have affected trends over time is not clear.
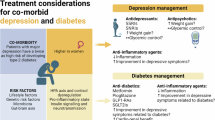
Diabetes has consistently been shown to be one of the central components of MLTCs. Population-based studies have shown that at diagnosis of T2DM, 26% of men and 34% of women already have two additional conditions while 10–15% have at least three conditions99. In UK National Health System data, by age 50, one-third of persons with diabetes have at least three conditions, spend >20 years with them and die 11 years earlier than the general population100. The most common contributing conditions to MLTC in people with T2DM are hypertension, depression, coronary heart disease, chronic kidney disease, anxiety, arthritis, and atrial fibrillation. Analyses of clustering have suggested that MLTCs may follow particular, distinct patterns, including classic cardio-metabolic precursors (e.g. obesity, hypertension), later stage vascular conditions (atrial fibrillation, stroke, peripheral vascular disease), mental health conditions with a young onset, and mental health conditions clustered with chronic obstructive pulmonary disease and asthma99,101,102. The patterns of prominent comorbid conditions also vary considerably by age. Among older adults, classic complications like myocardial infarction, stroke, heart failure, and peripheral vascular disease play major roles, whereas in young adulthood mental health conditions and asthma also play large roles100, Although these increases are thought to be driven by an actual increase in burden of disease due to persistent prevalence and declining competing risk of cardiovascular disease mortality, the impact of changing diagnostic and coding practices and the assembly of more data sources have not been clarified.
Aetiologic drivers and prospects for prevention and management
The aetiology of MLTCs in persons with diabetes stems from numerous factors and pathways. Studies in the general population have shown that age, socio-economic status (SSE), material deprivation (an inability to afford basic resources), obesity, physical inactivity, and smoking, are all important predictors of MLTCs89. The cardinal drivers of diabetes complications, which are hyperglycaemia, insulin resistance, hypertension, dyslipidemia, and inflammation, may affect non-traditional conditions contributing to MLTCs, such as liver disease, respiratory disorders, infections, cancers, mental health, and musculoskeletal problems103. Similarly, the toxic effects of excess glucose, dysfunction of arterial walls, and chromosomal variation affecting the aging process, may also play a role in MLTC development. A UK study showed that obesity dramatically increases risk of increased severity of MLTCs104. It remains unclear, though, whether the risk factors are fundamentally different from those of the constituent components or whether MLTCs represent incremental levels of severity along the same aetiological continuum.
It is important to understand what these transitions mean for the future of research and public health programmes. The rapid developments in precision medicine, multi-omics, artificial intelligence, and data science will each play a role in clarifying the diabetes-associated pathways and unique modifiable risk factors for prevention and management of MLTCs. However, if MLTCs are the next major transition in the diabetes epidemic, and the key drivers lie at both ends of the age spectrum as we suggest, the most practical priorities will lie in prevention, ongoing or routine care models and coordination, and globally, population monitoring. The key questions for prevention and management are whether the approaches (e.g., lifestyle interventions, self-management, better risk stratification, pharmaceutical interventions; better-integrated care) are distinct from what works for its constituent component or whether distinct, bespoke approaches are needed for MLTCs. The search for promising prevention approaches has centred around lifestyle interventions with physical activity to prevent functional decline and the use of multi-disciplinary teams to target the pharmacological, medical and self-management challenges of MLTCs, but consensus on the degree of effectiveness is still lacking105. Observational studies have suggested that weight loss in obese people can have beneficial effects in some concordant and discordant conditions but few weight loss intervention studies have evaluated the impact on MLTCs106,107. Better population monitoring, and with it, the need for more efficient and textured metrics, alongside simple measurements that can be applied internationally, are needed. There is also a need to expand the prevention agenda beyond HICs while improving the ability to monitor the problem using new metrics.
Concluding remarks
In this Perspective, we have described several major transitions within the global T2DM pandemic that have shaped its long-term impact and now set the stage for the coming challenges. Underlying the long-term growth of global T2DM prevalence, declining CVD mortality, a period of improvements in care and outcomes, a diversification of complications in older adults, potential stagnation in long-term care improvements, and a shifting burden from older adults to middle-aged adults have all shaped a changing profile of type 2 diabetes morbidity. Collectively, these dynamics have set the stage for a high prevalence of MLTCs being an extensive and persistent challenge for care and prevention in the future. Whether LMICs follow the patterns seen in HIC will have enormous implications for the global burden of diabetes because most of the world’s individuals with diabetes reside in LMICs. It will be essential for future research in health services research, epidemiology, and implementation research to innovate to facilitate an effective population health response to the MLTC challenge.
References
Omran, A. R. The epidemiologic transition: a theory of the epidemiology of population change. 1971. Milbank Q 83, 731–757 (2005).
Mackenbach, J. P. The rise and fall of diseases: reflections on the history of population health in Europe since ca. 1700. Eur. J. Epidemiol. 36, 1199–1205 (2021).
Ford, E. S. et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N. Engl. J. Med. 356, 2388–2398 (2007).
Geiss, L. S. et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. Jama 312, 1218–1226 (2014).
Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387, 1513–1530 (2016).
Benoit, S. R., Hora, I., Albright, A. L. & Gregg, E. W. New directions in incidence and prevalence of diagnosed diabetes in the USA. BMJ Open Diabetes Res. Care 7, e000657 (2019).
Magliano, D. J. et al. Trends in the incidence of diagnosed diabetes: a multicountry analysis of aggregate data from 22 million diagnoses in high-income and middle-income settings. Lancet Diabetes Endocrinol. 9, 203–211 (2021).
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 404, 2077–2093 (2024).
Vinicor, F. Is diabetes a public-health disorder? Diabetes Care 17, 22–27 (1994).
Zimmet, P. Z., Magliano, D. J., Herman, W. H. & Shaw, J. E. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2, 56–64 (2014).
Fagot-Campagna, A. et al. Type 2 diabetes among North adolescents: an epidemiologic health perspective. J. Pediatrics 136, 664–672 (2000).
Gregg, E. W., Sattar, N. & Ali, M. K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 4, 537–547 (2016).
Zimmet, P., Taft, P., Guinea, A., Guthrie, W. & Thoma, K. The high prevalence of diabetes mellitus on a Central Pacific Island. Diabetologia 13, 111–115 (1977).
Bennett, P. H., Burch, T. A. & Miller, M. Diabetes mellitus in American (Pima) Indians. Lancet 2, 125–128 (1971).
West, K. M. Epidemiology of diabetes and its macrovascular complications. Diabetes Care 2, 63–64 (1979).
Popkin, B. M. The nutrition transition and its health implications in lower-income countries. Public Health Nutr. 1, 5–21 (1998).
Jaacks, L. M. et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 7, 231–240 (2019).
Trends in the prevalence and incidence of self-reported diabetes mellitus—United States, 1980-1994. MMWR Morb. Mortal Wkly. Rep. 46, 1014–1018 (1997).
Geiss, L. S. et al. Surveillance for diabetes mellitus–United States, 1980-1989. MMWR CDC Surveill. Summ. 42, 1–20 (1993).
Wetterhall, S. F. et al. Trends in diabetes and diabetic complications, 1980-1987. Diabetes Care 15, 960–967 (1992).
Gregg, E. W. et al. Changes in diabetes-related complications in the United States, 1990-2010. N. Engl. J. Med. 370, 1514–1523 (2014).
Klein, R. & Klein, B. E. Relation of glycemic control to diabetic complications and health outcomes. Diabetes Care 21, C39–C43 (1998).
Haffner, S. M., Lehto, S., Ronnemaa, T., Pyorala, K. & Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 339, 229–234 (1998).
Koyama, A. K. et al. Trends in lifetime risk and years of potential life lost from diabetes in the United States, 1997-2018. PLoS One 17, e0268805 (2022).
Nelson, R. G. et al. Incidence of end-stage renal disease in type 2 (non-insulin-dependent) diabetes mellitus in Pima Indians. Diabetologia 31, 730–736 (1988).
Currie, C. J., Morgan, C. L., Gill, L., Stott, N. C. & Peters, J. R. Epidemiology and costs of acute hospital care for cerebrovascular disease in diabetic and nondiabetic populations. Stroke 28, 1142–1146 (1997).
Beckles, G. L. et al. Population-based assessment of the level of care among adults with diabetes in the U.S. Diabetes Care 21, 1432–1438 (1998).
Saaddine, J. B. et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988-2002. Ann. Intern. Med. 144, 465–474 (2006).
Saaddine, J. B. et al. A diabetes report card for the United States: quality of care in the 1990s. Ann. Intern. Med. 136, 565–574 (2002).
Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 317, 703–713 (1998).
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 837–853 (1998).
Gaede, P. et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 348, 383–393 (2003).
Fleming, B. B. et al. The diabetes quality improvement project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care 24, 1815–1820 (2001).
Burrows, N. R., Li, Y., Geiss, L. S. & Gregg, E. W. Declining rates of hospitalization for selected cardiovascular disease conditions among adults aged ≥35 years with diagnosed diabetes, US, 1998-2014. Diabetes Care 2018;41:293–302 Response. Diabetes Care 41, E59-E59 (2018).
Ali, M. K. et al. Achievement of goals in U.S. diabetes care, 1999-2010. N. Engl. J. Med. 368, 1613–1624 (2013).
Harding, J. L., Pavkov, M. E., Magliano, D. J., Shaw, J. E. & Gregg, E. W. Global trends in diabetes complications: a review of current evidence. Diabetologia 62, 3–16 (2019).
Pearson-Stuttard, J. et al. Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 10, 46–57 (2022).
Ali, M. K., Bullard, K. M., Gregg, E. W. & Del Rio, C. A cascade of care for diabetes in the United States: visualizing the gaps. Ann. Intern. Med. 161, 681–689 (2014).
Dabelea, D. et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 311, 1778–1786 (2014).
Mayer-Davis, E. J. et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N. Engl. J. Med. 376, 1419–1429 (2017).
Tomic, D., Shaw, J. E. & Magliano, D. J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 18, 525–539 (2022).
Gregg, E. W. et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 391, 2430–2440 (2018).
Tomic, D., Craig, M. E., Magliano, D. J. & Shaw, J. E. Reasons for hospitalisation in youth with type 1 diabetes, 2010-2019. Diabet. Med. 41, e15218 (2024).
Mehta, N. K., Abrams, L. R. & Myrskylä, M. US life expectancy stalls due to cardiovascular disease, not drug deaths. Proc. Natl Acad. Sci. USA 117, 6998–7000 (2020).
Fang, M., Wang, D., Coresh, J. & Selvin, E. Trends in diabetes treatment and control in U.S. adults, 1999-2018. N. Engl. J. Med. 384, 2219–2228 (2021).
Geiss, L. S., et al. Resurgence of diabetes-related nontraumatic lower extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care 42, 50–54 (2018).
Gregg, E. W., Hora, I. & Benoit, S. R. Resurgence in diabetes-related complications. Jama-J. Am. Med. Assoc. 321, 1867–1868 (2019).
Quigley, M. et al. Trends in diabetes-related foot disease hospitalizations and amputations in Australia, 2010 to 2019. Diabetes Res. Clin. Pract. 194, 110189 (2022).
Morton, J. I., Lazzarini, P. A., Shaw, J. E. & Magliano, D. J. Trends in the incidence of hospitalization for major diabetes-related complications in people with type 1 and type 2 diabetes in Australia, 2010-2019. Diabetes Care 45, 789–797 (2022).
Lazzarini, P. A. et al. Global trends in the incidence of hospital admissions for diabetes-related foot disease and amputations: a review of national rates in the 21st century. Diabetologia 66, 267–287 (2023).
Maheswaran, R., et al. Time trends and geographical variation in major lower limb amputation related to peripheral arterial disease in England. BJS Open 8, zrad140 (2024).
Manne-Goehler, J. et al. Health system performance for people with diabetes in 28 low- and middle-income countries: A cross-sectional study of nationally representative surveys. PLoS Med. 16, e1002751 (2019).
Misra, S. et al. Characteristics and care of young people with type 2 diabetes included in the national diabetes audit datasets for England. Diabetes Med. 40, e14940 (2023).
Woolf, S. H., Chapman, D. A., Sabo, R. T. & Zimmerman, E. B. Excess deaths from COVID-19 and other causes in the US, March 1, 2020, to January 2, 2021. Jama 325, 1786–1789 (2021).
McManus, E., Meacock, R., Parkinson, B. & Sutton, M. Population level impact of the NHS Diabetes Prevention Programme on incidence of type 2 diabetes in England: an observational study. Lancet Reg. Health Eur. 19, 100420 (2022).
Knudsen, J. S. et al. Changes in type 2 diabetes incidence and mortality associated with introduction of HbA1c as diagnostic option: A Danish 24-year population-based study. Lancet Reg. Health Eur. 14, 100291 (2022).
Saydah, S., Bullard, K. M., Imperatore, G., Geiss, L. & Gregg, E. W. Cardiometabolic risk factors among US adolescents and young adults and risk of early mortality. Pediatrics 131, e679–e686 (2013).
Cheng, Y. J., et al. Secular changes in the age-specific prevalence of diabetes among U.S. adults: 1988-2010. Diabetes Care 36, 2690–2696 (2013).
Dibato, J. E. et al. Association of cardiometabolic multimorbidity and depression with cardiovascular events in early-onset adult type 2 diabetes: a multiethnic study in the U.S. Diabetes Care 44, 231–239 (2021).
Saydah, S. H., Siegel, K. R., Imperatore, G., Mercado, C. & Gregg, E. W. The cardiometabolic risk profile of young adults with diabetes in the U.S. Diabetes Care 42, 1895–1902 (2019).
Tryggestad, J. B. & Willi, S. M. Complications and comorbidities of T2DM in adolescents: findings from the TODAY clinical trial. J. Diabetes Complicat. 29, 307–312 (2015).
Dabelea, D. et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. Jama 317, 825–835 (2017).
Life expectancy associated with different ages at diagnosis of type 2 diabetes in high-income countries: 23 million person-years of observation. Lancet Diabetes Endocrinol. 11, 731–742 (2023).
Xie, J. et al. Global burden of type 2 diabetes in adolescents and young adults, 1990-2019: systematic analysis of the Global Burden of Disease Study 2019. Bmj 379, e072385 (2022).
Murphy, H. R. et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. Lancet Diabetes Endocrinol. 9, 153–164 (2021).
Chen, L. et al. A systematic review of trends in all-cause mortality among people with diabetes. Diabetologia 63, 1718–1735 (2020).
Anjana, R. M. et al. Contrasting associations between diabetes and cardiovascular mortality rates in low-, middle-, and high-income countries: cohort study data from 143,567 individuals in 21 countries in the PURE study. Diabetes Care 43, 3094–3101 (2020).
Liu, X., et al. Trends in the incidence and mortality of diabetes in China from 1990 to 2017: a joinpoint and age-period-cohort analysis. Int. J. Environ. Res. Public Health 16, 08 (2019).
Miranda, J. J. et al. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat. Med. 25, 1667–1679 (2019).
Deepa, M., Anjana, R. M., Manjula, D., Narayan, K. M. & Mohan, V. Convergence of prevalence rates of diabetes and cardiometabolic risk factors in middle and low income groups in urban India: 10-year follow-up of the Chennai Urban Population Study. J. Diabetes Sci. Technol. 5, 918–927 (2011).
Zabetian, A., Sanchez, I. M., Narayan, K. M., Hwang, C. K. & Ali, M. K. Global rural diabetes prevalence: a systematic review and meta-analysis covering 1990-2012. Diabetes Res. Clin. Pract. 104, 206–213 (2014).
Shivashankar, R. et al. Quality of diabetes care in low- and middle-income Asian and Middle Eastern countries (1993-2012): 20-year systematic review. Diabetes Res. Clin. Pract. 107, 203–223 (2015).
Bixby, H. et al. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature 569, 260-+ (2019).
Flood, D. et al. Health system interventions for adults with type 2 diabetes in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 17, e1003434 (2020).
Gregg, E. W. et al. Improving health outcomes of people with diabetes: target setting for the WHO Global Diabetes Compact. Lancet 401, 1302–1312 (2023).
Ali, M. K., Siegel, K. R., Laxy, M. & Gregg, E. W. Advancing measurement of diabetes at the population level. Curr. Diabetes Rep. 18, 108 (2018).
Jagannathan, R., Patel, S. A., Ali, M. K. & Narayan, K. M. V. Global updates on cardiovascular disease mortality trends and attribution of traditional risk factors. Curr. Diabetes Rep. 19, 44 (2019).
Alegre-Diaz, J. et al. Diabetes and cause-specific mortality in Mexico City. N. Engl. J. Med. 375, 1961–1971 (2016).
Academy of Medical Scienes. Multimorbidity (MM): a priority for global health research. (2018).
Kernick, D., Chew-Graham, C. A. & O’Flynn, N. Clinical assessment and management of multimorbidity: NICE guideline. Br. J. Gen. Pract. 67, 235–236 (2017).
Whitty, C. J. M. et al. Rising to the challenge of multimorbidity. Bmj 368, l6964 (2020).
Brownrigg, J. R. et al. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population-level cohort study. Lancet Diabetes Endocrinol. 4, 588–597 (2016).
Morgan, C. L. et al. The prevalence of multiple diabetes-related complications. Diabetes Med. 17, 146–151 (2000).
Tesfaye, S. et al. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 352, 341–350 (2005).
Vinik, A. I., Maser, R. E. & Ziegler, D. Neuropathy: the crystal ball for cardiovascular disease? Diabetes Care 33, 1688–1690 (2010).
Xu, X. H. et al. Diabetic retinopathy predicts cardiovascular mortality in diabetes: a meta-analysis. BMC Cardiovasc. Disord. 20, 478 (2020).
Yun, J. S. et al. Cardiovascular disease predicts severe hypoglycemia in patients with type 2 diabetes. Diabetes Metab. J. 39, 498–506 (2015).
Magliano, D. J., Martin, V. J., Owen, A. J., Zomer, E. & Liew, D. The productivity burden of diabetes at a population level. Diabetes Care 41, 979–984 (2018).
Ho, I. S. et al. Variation in the estimated prevalence of multimorbidity: systematic review and meta-analysis of 193 international studies. BMJ Open 12, e057017 (2022).
Stirland, L. E. et al. Measuring multimorbidity beyond counting diseases: systematic review of community and population studies and guide to index choice. Bmj 368, m160 (2020).
Ahlqvist, E., Prasad, R. B. & Groop, L. 100 years of insulin: towards improved precision and a new classification of diabetes mellitus. J. Endocrinol. 252, R59–r70 (2021).
Krauth, S. J. et al. Association of latent class analysis-derived multimorbidity clusters with adverse health outcomes in patients with multiple long-term conditions: comparative results across three UK cohorts. EClinicalMedicine 74, 102703 (2024).
Kuan, V. et al. Identifying and visualising multimorbidity and comorbidity patterns in patients in the English National Health Service: a population-based study. Lancet Digit Health 5, e16–e27 (2023).
Jensen, A. B. et al. Temporal disease trajectories condensed from population-wide registry data covering 6.2 million patients. Nat. Commun. 5, 4022 (2014).
Langenberg, C., Hingorani, A. D. & Whitty, C. J. M. Biological and functional multimorbidity-from mechanisms to management. Nat. Med. 29, 1649–1657 (2023).
Violan, C. et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One 9, e102149 (2014).
Barnett, K. et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380, 37–43 (2012).
Head, A. et al. Inequalities in incident and prevalent multimorbidity in England, 2004-19: a population-based, descriptive study. Lancet Healthy Longev. 2, e489–e497 (2021).
Nowakowska, M. et al. The comorbidity burden of type 2 diabetes mellitus: patterns, clusters and predictions from a large English primary care cohort. BMC Med. 17, 145 (2019).
Gregg, E. W. et al. The burden of diabetes-associated multiple long-term conditions on years of life spent and lost. Nat. Med. 30, 2830–2837 (2024).
Cicek, M., Buckley, J., Pearson-Stuttard, J. & Gregg, E. W. Characterizing multimorbidity from type 2 diabetes: insights from clustering approaches. Endocrinol. Metab. Clin. North Am. 50, 531–558 (2021).
Aguado, A., Moratalla-Navarro, F., López-Simarro, F. & Moreno, V. MorbiNet: multimorbidity networks in adult general population. Analysis of type 2 diabetes mellitus comorbidity. Sci. Rep. 10, 2416 (2020).
Khunti, K. et al. Diabetes and multiple long-term conditions: a review of our current global health challenge. Diabetes Care 46, 2092–2101 (2023).
Kivimaki, M. et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2, e277–e285 (2017).
Smith, S. M., Wallace, E., Clyne, B., Boland, F. & Fortin, M. Interventions for improving outcomes in patients with multimorbidity in primary care and community setting: a systematic review. Syst. Rev. 10, 271 (2021).
Khunti K. F. et al. Weight change and risk of obesity-related complications: a retrospective population-based cohort study of a UK primary care database. Diabetes Obes. Metab. 25, 2669–2679 (2023).
Espeland, M. A. et al. Eight-year changes in multimorbidity and frailty in adults with type 2 diabetes mellitus: associations with cognitive and physical function and mortality. J. Gerontol. A Biol. Sci. Med Sci. 77, 1691–1698 (2022).
Centers for Disease Control and Prevention. U.S. Diabetes Surveillance System and Diabetes Atlas. (2019).
Acknowledgements
E.W.G. is funded by the SFI Converge Centre [Grant 22/RP/10091]. S.M. is funded by a Wellcome Trust Career Development Award (223024/Z/21/Z) and is supported by the NIHR Imperial Biomedical Research Centre. K.K. is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM), NIHR Global Research Centre for Multiple Long Term Conditions, NIHR Cross NIHR Collaboration for Multiple Long Term Conditions and the NIHR Leicester Biomedical Research Centre (BRC). J.V. is supported by the North West London NIHR Applied Research Collaboration, the Imperial NIHR Biomedical Research Centre, and CW+, the official charity of Chelsea and Westminster Hospital NHS Foundation Trust.
Author information
Authors and Affiliations
Contributions
E.W.G., J.V., and K.K. conceptualized the article; E.W.G. led writing of the manuscript; N.H., M.S., S.M., and J.P.S. each contributed to literature search, review and revision of the manuscript. M.S. and K.K. also contributed to construction of graphics.
Corresponding author
Ethics declarations
Competing interests
S.M. has received speaker Honoraria from Lilly and Sanofi, UK. K.K. has acted as a consultant, speaker or received grants for investigator-initiated studies for Astra Zeneca, Bayer, Novo Nordisk, Sanofi-Aventis, Servier, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Oramed Pharmaceuticals, Pfizer, Roche, Daiichi-Sankyo, Applied Therapeutics, Embecta and Nestle Health Science. J.P.S. is a Partner at Lane Clark & Peacock LLP and Chair of the Royal Society for Public Health and reports consultancy fees from Novo Nordisk outside of this work. J.V. was the National Clinical Director for Diabetes and Obesity at NHS England from April 2013 to September 2023 and currently is the National Clinical Lead for Multiple Long Term Conditions at NHS England.
Peer review
Peer review information
Communications Medicine thanks Sanjay Kinra and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gregg, E.W., Holman, N., Sophiea, M. et al. Multiple long-term conditions as the next transition in the global diabetes epidemic. Commun Med 5, 42 (2025). https://doi.org/10.1038/s43856-025-00742-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-00742-9