Abstract
This study examined the longitudinal association of metabolic dysfunction-associated fatty liver disease (MAFLD) with distinct cognitive function trajectories, and determine whether and to what extent this association was mediated by MAFLD-related metabolites among 845 participants. Two cognitive function trajectories were identified as normal (n = 714, 84.50%) or large decrease (n = 131, 15.50%) pattern over 7 years. Participants with MAFLD (N = 277, 32.78%) had an 81% higher risk of developing a large decrease in cognitive function (odds ratio, 1.81; 95% confidence interval, 1.16–2.94) than non-MAFLD. Three MAFLD-related metabolites were identified as lysoPC(20:3(5z,8z,11z)), lysoPE(18:1(9z)/0:0), and valine, of which lysoPE(18:1(9z)/0:0) and valine played a partially mediated role in the association of MAFLD with a large decrease in cognitive function (mediation proportion = 9.93% and 11.04%, respectively). The results indicated that MAFLD was associated with a higher risk of developing a large decrease in cognitive function, which was partially mediated by lipid and amino acid metabolism.
Similar content being viewed by others
Introduction
With the escalation of the aging population, age-associated cognitive decline has been emerged as a widespread and crucial public health concern worldwide1. The decline in cognitive abilities not only impacts the individual’s quality of life and functional independence, but also gives rise to significant societal and economic implications2,3,4. Prevention was of utmost importance as no causal therapy existed for cognitive decline, therefore, identifications of potential modifiable risk factors for cognitive decline remains a pressing issue.
Metabolic dysfunction-associated fatty liver disease (MAFLD), which incorporates the pathogenic role of metabolic dysfunction in the development and progression of nonalcoholic fatty liver disease, is one the most common chronic liver diseases globally and affects approximately 25% of the adult population5,6,7. The occurrence of MAFLD is often accompanied by obesity, abnormal metabolism of blood lipids and glucose, and microecological imbalance. Additionally, MAFLD has been shown to be associated with a series of metabolic diseases8,9,10. These metabolic disorders associated with MAFLD are also known to be related to extrahepatic manifestations involving the central nervous system, such as cognitive impairment. A connection between MAFLD and cognitive decline has been proposed due to frequent reports of attention, forgetfulness, and memory issues in patients with MAFLD11,12,13,14. However, previous studies generated inconsistent conclusions regarding the association between MAFLD and cognitive function, with some failed to demonstrate a significant result15,16. One of the possible reasons for the inconsistencies may be the single measurement of cognitive function, which neglected the nature change of cognitive function over time. As acknowledged, characterizing longitudinal patterns of cognitive function may provide a more accurate and robust assessment for the association.
Therefore, this prospective cohort study aimed to identity the different trajectories of cognitive function over time, and investigate a possible link of MAFLD with distinct trajectories of cognitive function. When the association was constructed, we further aimed to assess whether and to what extent the association is mediated by MAFLD-related metabolites among Chinese adults.
Results
Baseline characteristics
A total of 845 participants were enrolled, a comparison of baseline characteristics between excluded and included participants was presented in Supplementary Table 1. Among the enrolled participants, 277 (32.78%) participants had MAFLD, the median age was 49.10 (interquartile range, 40.56–59.62) years and 300 (35.50%) were men. Compared with non-MAFLD participants, those with MAFLD were more likely to be older, men, less educated, have a higher proportion of current smokers, current drinkers, a higher prevalence of hypertension, diabetes, dyslipidemia, more likely to take relative medications, have more cardiometabolic risk factors (Table 1).
Trajectories of MMSE
A total of four times of MMSE measurements during a median follow of 6.21 (interquartile range: 6.08–6.31) years. The number of participants with 1, 2, 3, and 4 times of MMSE measurements was 121, 206, 330, and 180. Two-class model was selected as the preferred model according to the criteria mentioned in the method, with Bayesian Information Criterion of −5193.89, Akaike Information Criterion of −5165.24, average posterior probability of assignment of 78.66% and 21.34%, respectively (Supplementary Table 2). The number of participants in class 1 (normal decrease pattern) was 714 (84.50%) participants, which was 131 (15.50%) in class 2 (large decrease pattern) (Fig. 1).
Association of MALFD with trajectories of MMSE
A total of 72 (25.99%) participants in the MAFLD developed large decrease in MMSE score, which was significantly higher than that in non-MAFLD participants (59, 10.39%). After adjusted for potential covariates, MAFLD had an 81% higher risk of developing large-decrease in MMSE (OR, 1.81; 95% CI, 1.16–2.84; P = 0.0090) (Table 2). Subgroup analyses showed that the associations between MAFLD and the risk of developing large decrease in MMSE were consistent across different subgroups, all the P values for interaction were >0.05 (Supplementary Table 3).
Differentially expressed metabolites
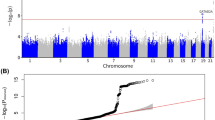
The comparison of positive ions, negative ions, and NMR metabolites between non-MAFLD and MAFLD group was presented in Supplementary Tables 4–6. The results showed that among 90 kinds of positive ions, the concentration of lysophosphatidylcholine (lysoPC) (20:3(5z,8z,11z)) in MAFLD group was significantly lower than that in non-MAFLD group (FC = 0.31; FDR = 0.02; Fig. 2A). Among 23 kinds of negative ions, the concentration of lysophosphatidylethanolamine (lysoPE) (18:1(9z)/0:0) in MAFLD group was significantly higher than that in non-MAFLD group (FC = 4.34; FDR < 0.001; Fig. 2B). Among 274 kinds of NMR-measured metabolites, the concentration of valine in MAFLD group was significantly lower than that in non-MAFLD group (FC = 0.47; FDR < 0.001; Fig. 2C).
The outcome was each metabolite identified. The horizontal dotted line represented the significance threshold (FDR-adjusted P = 0.05). The scatter denoted the up-regulated (red) or down-regulated (blue) metabolites for correlations with MAFLD. Each plot represents a metabolite identified. FDR false discovery rate, MAFLD metabolic dysfunction-associated fatty liver disease.
Mediation analysis
The results of mediation analysis were presented in Fig. 3, which showed that the association of MAFLD with large decrease in MMSE pattern was partially mediated by LysoPE(18:1(9z)/0:0) (mediated proportion = 9.93%) and valine (mediated proportion = 11.04%). However, lysoPC(20:3(5z,8z,11z)) did not play a mediated role in the association.
The outcome in the figure indicates a large decrease pattern of MMSE. A Contribution of LysoPC; B Contribution of LysoPE; C Contribution of Valine. Adjusted for age, sex, body mass index, education, physical activity, smoking, and drinking, hypertension, dyslipidemia, estimated glomerular filtration rate, and alanine transaminase. MAFLD metabolic dysfunction-associated fatty liver disease *P < 0.05; **P < 0.01; ***P < 0.001
Discussion
In this prospective cohort study, we identified two longitudinal patterns of cognitive function change over time. The status of MAFLD was associated with a higher risk of developing a large decrease in cognitive function over time. Metabolites of lysoPC(20:3(5z,8z,11z)), lysoPE(18:1(9z)/0:0), and valine were differently expressed in patients with MAFLD. Additionally, the association between MAFLD and the pattern of a large decrease in cognitive function was partial mediated by lysoPE(18:1(9z)/0:0) and valine. These findings indicated that MAFLD might increase cognitive impairment risk by disturbing lipid and amino acid metabolism.
Accumulative evidence with inconsistent results has investigated the associations of MAFLD with cognitive function. Cross-sectional analysis using data from the National Health and Nutrition Examination Survey showed that MAFLD was significantly associated with increased risk of cognitive impairment measured by serial digit learning test17. Similarly, the Rotterdam Study reported that MAFLD was associated with structural and hemodynamic brain markers in a population-based cross-sectional setting18. On the contrary, anther cross-sectional study conducted among patients with severe obesity showed that the cognitive impairment was not associated with the presence of MAFLD19. Post hoc analyses using data from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Systolic Blood Pressure Intervention Trial (SPRINT) also demonstrated that markers of chronic liver disease were not associated with cognitive impairment or related brain imaging markers among individuals with diabetes and hypertension15. These conflicting results were limited to the cross-sectional design. One prospective study investigated the role of NAFLD on the risk of incident cognitive impairment, and the result showed that patients with NAFLD had higher 4-year incidence of cognitive impairment than non-NAFLD patients did20. However, whether the longitudinal association persisted for MAFLD was not well-demonstrated, as the cognitive function declined with age.
In our study, we used latent class mixture models to identify distinct trajectories of cognitive function with aging. Two class of cognitive function trajectories were identified with approximately 15.50% participants had the large decline in cognitive function. Thes rate was a little smaller than the reported among older Mexican Americans (20.0%)21, which may be attributable to the younger adults enrolled in our study. Further analysis added longitudinal evidence on the association of MAFLD and cognitive function by demonstrating that participants with MAFLD had a higher risk of developing a large decline trajectory of cognitive function over time. Subgroup analysis showed that this positive association was consistent across different age, sex, and metabolic syndrome groups. Taken together, these findings suggested that strategies on management of metabolic risk factors and liver disease may brought additional benefit in prevention cognitive decline or even the development of dementia.
Another finding of our study was that we identified three metabolites differentially expressed in participants with MAFLD: lysoPC(20:3(5z,8z,11z)), lysoPE (18:1(9z)/0:0), and valine. The results were supported by previous investigations in vivo and in vitro. One review showed that accumulated altered lysoPCs have been implicated in the tissue impairment and dysfunction underlying metabolic disorders, including MAFLD22. One population-based study on MAFLD adults showed that lysoPC levels as a potential noninvasive biomarker for MAFLD23. In the context of lysoPE, Yamamoto et al found that NAFLD patients have significantly elevated levels of LysoPE in their bodies24. Valine was a kind of essential amino acid, and fattening animal experiments showed that supplementing valine could change the fatty acid composition and reduce lipid accumulation in liver25. Additionally, our study further performed the mediation analysis to explore the role of the three metabolites in the association between MAFLD and cognitive function trajectories. The result showed that approximately 10% of the pathway from MAFLD to a large decrease in cognitive function was mediated through lysoPE and valine, which may provide a possible mechanism underlying the association between MAFLD and cognitive impairment.
Although the pathological mechanisms by which MAFLD affects cognitive function have not yet been fully elucidated, several key components contributing to the association have been proposed, including insulin resistance, systemic inflammation, lipotoxicity, vascular dysfunction, and dysbiosis26,27. First, the liver was considered as the central organ responsible for metabolizing sugars, and the occurrence of MAFLD often accompanied by insulin resistance28. The disruption of insulin signaling in the brain can lead to cognitive impairment by downregulating blood–brain barrier insulin receptors and reducing the transport of insulin into the brain. Second, MAFLD exhibited chronic low-grade inflammation, which extended systemically. The proinflammatory cytokines could increase blood–brain barrier permeability and initiating a complex immune response the central nervus system, resulting in a neurotoxic effect and cognitive impairment29. Third, lipotoxicity emerged as accumulation of lipids in non-adipose tissues, particularly manifests in the liver. Dysregulation caused by lipotoxicity in the brain involves loss of orexin signaling, which was associated with memory impairment, learning deficits, and neuroinflammation30. Additionally, one study also showed that MAFLD accelerated the signs of Alzheimer’s disease in central nervous system by inducing neuronal apoptosis and decreased the expression of lipid metabolites which were responsible for beta amyloid plaque clearance in the disease31.
The longitudinal design with repeated measurements of MMSE allowed us to characterize the longitudinal trajectories of cognitive function over time. However, several limitations also needed to be addressed. First, we used abdominal ultrasound to identify fatty liver, which could not detect hepatic steatosis when fat content is <20%. Therefore, misclassification of MAFLD with mild steatosis would underestimate the effect of MAFLD on cognitive function trajectories. Second, cognitive function was measured by MMSE in the present study, which may underestimate the rate of cognitive decline for older adults with low cognitive function. However, MMSE was treated as continuous variable in the analysis, the aforementioned limitation made a relatively small influence on the association. Third, the selection bias maybe existed due to our inclusion and exclusion criteria, further studies are eagerly needed. Fourth, the relatively small sample size may lead to a lower statistical power for the subgroup analysis, hence, the findings of subgroup analysis were interpreted only as explanatory results. Fifth, a limitation of our mediation analysis was that both the exposure and mediator were measured at baseline. However, we thought MAFLD may represent a history health problem with hepatic steatosis, while metabolites represent the state around the time of sampling or at least a narrower time period than MAFLD. We did not expect a substantial alteration in the causal ordering of the exposure and mediation. The findings needed to be validated in future studies with a time-lagged between exposure and mediators. Sixth, due to the observational design, our study could not establish the causality between MAFLD and cognition, thus further research is warranted. Finally, findings of this study may not be generalizable to other populations, as results were based on Chinese population.
In conclusion, two distinct cognitive function trajectories over time were identified, and MAFLD was associated with a higher risk of developing a large decline of cognitive function. Additionally, the association was partially mediated by lysoPEs and valine. The results provided a novel insight for the association between MAFLD and cognitive decline, although large prospective studies are warranted to confirm our findings in different populations and to investigate the biological mechanisms for the observed associations.
Methods
Study population
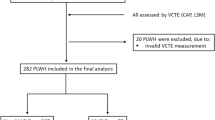
Data were obtained from the China Suboptimal Health Cohort Study (COACS), which was a prospective community-based study conducted in Jidong Oilfield Staff Hospital, China. The detailed information on the study has been descried previously32. In brief, a total of 1011 participants aged 18–65 years with data on metabolites were enrolled in the baseline survey from September 2013 to June 2014. All the participants completed the questionnaire interview, clinical and laboratory examines, and were followed up visits in year 2015, 2016, 2018, 2019, 2020, and 2021 to updated the afore-mentioned information. In our current study, we analyzed the association of baseline MAFLD, metabolites, with the risk of trajectories of cognitive function based on MMSE score were developed using data in 2015, 2018, 2019, and 2021. We excluded participants with cognitive impairment at baseline (n = 48), with missing data on the assessment of MAFLD (n = 118), leaving 845 participants in the current analysis (Fig. 4). The study was performed according to the guidelines of the Helsinki Declaration and was approved by Ethical Committee of the Jidong Oil-field Hospital of China National Petroleum Corporation. All participants were agreed to take part in the study and provided informed written consent.
Definition of MAFLD
MAFLD was defined as the presence of hepatic steatosis with one of three metabolic dysfunctions: overweight or obese (body mass index [BMI] ≥ 23 kg/m2), type 2 diabetes, or other metabolic abnormalities ≥233,34. Other metabolic abnormalities included (1) waist circumference ≥90 cm in men and ≥80 cm in women; (2) blood pressure (BP) ≥ 130/85 mm Hg or use of antihypertensives; (3) triglyceride ≥150 mg/dL or use of lipid-lowering agents; (4) high‐density lipoprotein cholesterol (HDL‐C) <40 mg/dL in men and <50 mg/dL in women or use of lipid-lowering agents; (5) fasting blood glucose (FBG) 5.6–6.9 mmol/L; (6) homeostasis model assessment of insulin resistance score ≥2.5. For lack of information on homeostasis model assessment of insulin resistance, we used triglyceride glucose index over the 75th percentile as an alternative to the 6th diagnostic criteria35.
Measurement of cognitive function
Cognitive function was assessed using the Chinese version of the Mini-Mental State Examination (MMSE) questionnaire, which comprises 30 items. The MMSE was administered by well-trained personnel. The total possible score ranges from 0 to 30, with a lower score indicates a poor cognitive ability. Following previous studies, participants with a MMSE score of <27 were considered cognitive impairment36.
Metabolomics analysis
Plasma samples were thawed at 4 °C. Once, thawed, plasma metabolomics analyses were performed with untargeted UHPLC-Q-TOF-MS (Shimadzu, Kyoto, Japan) and 1H-NMR (Varian VNMRS 600 MHz spectrometer, Agilent Technologies, USA), respectively. The plasma samples were analyzed by the UHPLC-Q-TOF-MS method in both positive and negative iron modes. Analytes with detection rates <80% or inter-or intra-assay coefficients of variation >20% were excluded. More details on the sample collection and measurements were described previously37.
Assessment of covariates
Information on demographics characteristics, lifestyle, personal medical history was collected using structured questionnaires. Anthropometric measurements were performed by trained staff. After at least a 12-h fating, blood samples were collected using venipuncture in the morning. The plasma samples were separated in the laboratory after centrifugation at 4 °C, for 10 min at 3000 × g. Then, the samples were stored at −80 °C immediately, and freeze–thaw cycles were strictly avoided until metabolomic analysis. The biochemical tests were analyzed using an auto-analyzer (Hitachi 747, Hitachi, Tokyo, Japan). Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.
Statistical analysis
The trajectories of MMSE were determined using latent class mixture models. The models were fitted with MMSE as the dependent variable, age as the time-scale mixed with the ‘lcmm’ R package. The analysis models were checked for between 1 and 4 trajectory classes based on a quadratic adherence trajectory function. The optimal number of classes was determined by Bayesian Information Criteria, Akaike Information criterion, sample size in each class, and clinical interpretation.
Baseline characteristics are presented as mean ± standard deviation, median with interquartile range, or frequency with percentages, as appropriate. Differences in baseline characteristics across non- and MAFLD groups were compared using the t test or Wilcoxon test for continuous variables and chi-square test for categorical variables. The association of MAFLD with different trajectories of MMSE was assessed by logistic regressions. Four models were constructed progressively. Model 1 was unadjusted; model 2 was adjusted for age and sex; model 3 was further adjusted for body mass index, education, physical activity, smoking, and drinking; model 4 was further adjusted for hypertension, dyslipidemia, estimated glomerular filtration rate, and alanine transaminase. To test the consistency of the findings, subgroup analysis stratified by age (≤60 vs >60 years), sex, body mass index (≤25 vs >25 kg/m2), depression status, and metabolic syndrome was performed. A multiplicative term between subgroups and MAFLD status was added into the model and the interaction was tested by a likelihood ratio test.
Metabolites differentially expressed between non-MAFLD and MAFLD participants were defined as meeting the following criterion: |Log2 Fold Change|≥ 1 and false discovery rate (FDR) < 0.05, using univariate analysis of t-test and Wilcoxon rank sum test, as appropriate. Additionally, mediation analyses were performed to evaluated whether and to what extent MAFLD-related metabolites may explain the association between MAFLD and the trajectories with MMSE by using the ‘mediation’ package in R. The mediated proportion was computed as indirect effect/total effect on the log scale × 100%. The models were also adjusted for covariates in model 4 above.
All the analyses were performed using R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria), and SAS version 9.4 (SAS Institute, Cary, NC, USA). All the statistical tests were 2-sided, and P < 0.05 was considered statistical significance.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The code used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Costa, A. Charting age-associated cognitive decline. CMAJ 189, E1470–e1471 (2017).
World Health Organization. Dementia Key Facts, vol. 2023 (World Health Organization, 2023).
Yoshida, K., Hata, Y., Ichimata, S., Okada, K. & Nishida, N. Argyrophilic grain disease is common in older adults and may be a risk factor for suicide: a study of Japanese forensic autopsy cases. Transl. Neurodegeneration 12, 16 (2023).
Stites, S. D., Karlawish, J., Harkins, K., Rubright, J. D. & Wolk, D. Awareness of Mild Cognitive Impairment and Mild Alzheimer’s Disease Dementia Diagnoses Associated With Lower Self-Ratings of Quality of Life in Older Adults. J. Gerontol. Ser. B Psychological Sci. Soc. Sci. 72, 974–985 (2017).
Eslam, M., Sanyal, A. J. & George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 158, 1999–2014.e1 (2020).
Kawano, Y. & Cohen, D. E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 48, 434–441 (2013).
Lekakis, V. & Papatheodoridis, G. V. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. 122, 3–10 (2024).
Trovato, F. M., Castrogiovanni, P., Malatino, L. & Musumeci, G. Nonalcoholic fatty liver disease (NAFLD) prevention: role of Mediterranean diet and physical activity. Hepatobiliary Surg. Nutr. 8, 167–169 (2019).
Hassani Zadeh, S., Mansoori, A. & Hosseinzadeh, M. Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 36, 1470–1478 (2021).
Li, X. et al. Non-alcoholic fatty liver disease is associated with brain function disruption in type 2 diabetes patients without cognitive impairment. Diab. Obes. Metab. 26, 650–662 (2024).
Yilmaz, Y. & Ozdogan, O. Liver disease as a risk factor for cognitive decline and dementia: an under-recognized issue. Hepatology 49, 698 (2009).
Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 88–106 (2019).
Mavrodaris, A., Powell, J. & Thorogood, M. Prevalences of dementia and cognitive impairment among older people in sub-Saharan Africa: a systematic review. Bull. World Health Organ. 91, 773–783 (2013).
Bai, W. et al. Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: a meta-analysis and systematic review of epidemiology studies. Age Ageing. 51, afac173 (2022).
Basu, E. et al. Association of chronic liver disease with cognition and brain volumes in two randomized controlled trial populations. J. Neurological Sci. 434, 120117 (2022).
Shang, Y. et al. Non-alcoholic fatty liver disease does not increase dementia risk although histology data might improve risk prediction. JHEP Rep. 3, 100218 (2021).
Yu, Q. et al. Association between Metabolic Dysfunction-associated Fatty Liver Disease and Cognitive Impairment. J. Clin. Transl. Hepatol. 10, 1034–1041 (2022).
Yilmaz, P. et al. Subclinical liver traits are associated with structural and hemodynamic brain imaging markers. Liver Int. 43, 1256–1268 (2023).
Wernberg, C. W. et al. The prevalence and risk factors for cognitive impairment in obesity and NAFLD. Hepatol. Commun. 7, e00203 (2023).
Liu, Q., Liu, C., Hu, F., Deng, X. & Zhang, Y. Non-alcoholic Fatty Liver Disease and Longitudinal Cognitive Changes in Middle-Aged and Elderly Adults. Front. Med. 8, 738835 (2021).
Howrey, B. T., Raji, M. A., Masel, M. M. & Peek, M. K. Stability in Cognitive Function Over 18 Years: Prevalence and Predictors among Older Mexican Americans. Curr. Alzheimer Res. 12, 614–621 (2015).
Chaurasia, B. & Summers, S. A. Ceramides - Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol. Metab. 26, 538–550 (2015).
Mazzini, F. N. et al. Plasma and stool metabolomics to identify microbiota derived-biomarkers of metabolic dysfunction-associated fatty liver disease: effect of PNPLA3 genotype. Metabolomics 17, 58 (2021).
Yamamoto, Y. et al. Analysis of serum lysophosphatidylethanolamine levels in patients with non-alcoholic fatty liver disease by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 413, 245–254 (2021).
Zhou, X. et al. Branched-chain amino acid modulation of lipid metabolism, gluconeogenesis, and inflammation in a finishing pig model: targeting leucine and valine. Food Funct. 14, 10119–10134 (2023).
Lombardi, R., Fargion, S. & Fracanzani, A. L. Brain involvement in non-alcoholic fatty liver disease (NAFLD): A systematic review. Digestive Liver Dis. 51, 1214–1222 (2019).
Robea, M. A. et al. Coagulation Dysfunctions in Non-Alcoholic Fatty Liver Disease-Oxidative Stress and Inflammation Relevance. Medicina 59, 1614 (2023).
Pal, S. C. & Méndez-Sánchez, N. Insulin resistance and adipose tissue interactions as the cornerstone of metabolic (dysfunction)-associated fatty liver disease pathogenesis. World J. Gastroenterol. 29, 3999–4008 (2023).
Fricker, Z. P. et al. Liver Fat Is Associated With Markers of Inflammation and Oxidative Stress in Analysis of Data From the Framingham Heart Study. Clin. Gastroenterol. Hepatol. 17, 1157–1164.e4 (2019).
Wasilewska, N. & Lebensztejn, D. M. Non-alcoholic fatty liver disease and lipotoxicity. Clin. Exp. Hepatol. 7, 1–6 (2021).
Kim, D. G. et al. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J. Neuroinflammation. 13, 1–18 (2016).
Wang, Y. et al. China suboptimal health cohort study: rationale, design and baseline characteristics. J. Transl. Med. 14, 291 (2016).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79, 1542–1556 (2023).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209 (2020).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351 (2010).
Zhou, W. et al. Carotid Intervention Improves Cognitive Function in Patients With Severe Atherosclerotic Carotid Disease. Ann. Surg. 276, 539–544 (2022).
Wang, H. et al. Machine learning of plasma metabolome identifies biomarker panels for metabolic syndrome: findings from the China Suboptimal Health Cohort. Cardiovasc. Diabetol. 21, 288 (2022).
Acknowledgements
We thank all study participants, their relatives, the members of the survey teams. This work was supported by Natural Science Foundation of China (U21A20414, 82374175, and 22338004) and Beijing Nova Program of Science & Technology (20220484162).
Author information
Authors and Affiliations
Contributions
A.W., Y.T., Q.H. contributed to the conception; A.W. and X.T. contributed to the manuscript drafting and the statistics analysis; Q.D., M.Z., X.X., Y.Z. contributed to the acquisition of data; Y.T., Q.H. contributed to critical revisions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, A., Tian, X., Deng, Q. et al. Longitudinal association of metabolic dysfunction-associated fatty liver disease, serum metabolites, with cognitive function trajectories. npj Metab Health Dis 3, 11 (2025). https://doi.org/10.1038/s44324-025-00055-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44324-025-00055-4