Abstract
Advances in soft bioelectronics are crucial for personalized diagnostics and therapeutics, offering seamless integration with tissues through conformal, modulus-adaptive designs. This review highlights materials, fabrication strategies, and closed-loop systems enabling wearable, implantable, and injectable devices with strong adhesion, mechanical matching, and broad signal acquisition. These innovations support stable tissue-device interfaces, driving the future of personalized healthcare.
Similar content being viewed by others
Introduction
Bioelectronics involves the acquisition and processing of biosignals1,2,3 or biomarkers4,5 generated by the body, with applications in fields such as personalized diagnosis6,7,8,9,10,11, health monitoring12,13,14,15,16,17, and identification of new biological mechanisms18,19,20. To develop a precise and reliable bioelectronic system, it is essential to develop a new biosensing platform capable of accurately detecting biosignals21 and delivering visual feedback22 to users while seamlessly interfacing with the body23,24,25,26,27,28. However, issues associated with stiffness and shape mismatches between biological tissues and conventional rigid platforms often lead to discomfort, limiting the long-term stability, precise sensing29, and feedback therapy in a closed-loop manner30. Furthermore, other challenges inherent to humid environments caused by secretions, such as sweat or noise interference from body movements during daily activities, should be resolved31. Moreover, the mechanical properties and shapes of biological tissues vary significantly depending on the target region in the human body where the biosensor is applied32,33. To effectively address these limitations, individual biosensor platforms should be designed to closely reflect the unique properties of each target tissue34,35,36, maximize signaling performance37,38, ensure wearer comfort39, and maintain long-term operational stability40,41,42, even under implanted conditions43,44.
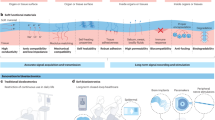
From this perspective, this review aims to discuss the various material strategies, device fabrication techniques, and closed-loop systemic approaches employed in recently reported bioelectronic devices, while also suggesting prospects for achieving stable tissue-interfacing bioelectronics. As shown in Fig. 1, essential requirements for optimal in vivo application of chronically stable wearable and implantable bioelectronics are presented (Fig. 1a). These include material selection (e.g., biocompatibility, biodegradability, shape adaptability, stretchability, tissue adhesion, and self-healing), sensing multifunctionality (e.g., real-time monitoring of electrophysiological signals, ions, movements, and thermal information), and deep-tissue access strategies (e.g., minimally invasive approaches such as syringe-aided injection and probe-type insertion) (Fig. 1b), which are reflected in the anatomical depth-dependent selection of device types across representative tissue targets (Fig. 1c). Considering the individual device configurations required for various types of biosensor applications, a flexible and ultrathin patch-type device for on-wearable sensing is essential to accommodate curvilinear surfaces and large areas of skin, as well as to minimize inflammation or discomfort during prolonged contact with the tissue (Fig. 1a, top). Therefore, adopting a skin-like substrate is highly beneficial for enhancing comfort and stability by matching the elasticity of dynamically moving tissues.
a Flexible and ultrathin patch-type devices enable seamless integration with the skin surface (Top). Stretchable serpentine patterning and ultrathin substrate provide enhanced mechanical compliance, stress dissipation, and self-healing capabilities. Implantable biosensing platforms and deformable biosensors are designed for adaptability to macroscopic tissue curvatures and maintain robust adhesion under shear and tensile stress (Middle). Minimally invasive approaches, including pre-folded devices and injectable hydrogel systems, reduce the need for surgical procedures and minimize scarring (Bottom). Conductive hydrogels, injected or molded to conform to tissue surfaces, further facilitate localized therapeutic and sensing functionalities. b Schematic illustration is shown for key features of implantable and wearable devices alongside their corresponding target tissues. c Schematic illustration (Created in BioRender. Son, D. (2025) https://BioRender.com/zx1a2k6) of depth-based classification of representative tissue targets and corresponding bioelectronic platforms. Target tissues are organized from superficial (e.g., skin) to deep (e.g., peripheral nerves and muscles) based on anatomical depth. For each depth level, proper types of functional devices (e.g., wearable patches, microneedles, implantable probes, and injectable hydrogels) are shown to reflect integration strategies. d Young’s modulus of biological tissues and materials commonly used in soft bioelectronics.
In cases where the desired biosignals need to be measured not through the skin but from organs deep within the body, an implantable biosensing platform is necessary. Such platforms can be positioned closer to deep, hard-to-access target tissues, such as peripheral nerves, the heart, and the brain, enabling the detection of subtle signals, such as neuronal spike signals (Fig. 1a, middle). However, implantable sensors pose challenges, including tissue damage and reduced long-term stability, owing to unavoidable issues such as inflammatory or immune responses. To resolve these problems, various researchers have begun exploring extremely soft patch-shaped biosensors that can dramatically reduce tissue damage by either reducing the intrinsic moduli of bioelectronic materials45 or injecting devices using syringe-like sheath guidance tools. For reliable and precise measurements, implantable patch-type platforms must conform to curved internal tissues, exhibit strong bioadhesion, remain fixed in biofluid environments, and remain stable over time. If biosignal measurement remains challenging, machine learning (ML) techniques based on artificial intelligence (AI) can be utilized to analyze personal information more effectively, even without directly positioning the implanted sensor on the target area46,47.
Despite these promising alternatives, large-scale patch-like devices require invasive surgery, which increases the risk of infection and surgical complications. To realize patient-friendly biosensor platforms, it is crucial to design minimally invasive devices that can be applied to deep tissues (Fig. 1a, bottom). Injectable hydrogels, which can be administered via syringes or molded to fit damaged or irregular tissue surfaces, are promising solutions for such applications. The development of optimized, minimally invasive biosensing platforms tailored to specific tissues and applications is essential to ensure safety and efficacy. This review explores various stable tissue-interfacing bioelectronics and rigorously investigates the mechanical characteristics and sensing platforms suitable for different biological tissues and organs. These are categorized based on essential functions, device types, and application strategies as illustrated in Fig. 1b, and organized based on anatomical target locations (Fig. 1c). Additionally, a comparative figure (Fig. 1d) highlights the mechanical properties of targeted soft tissues and commonly used substrates/electrodes. To complement these visual summaries, Table 1 outlines key features of wearable and implantable bioelectronic systems along with the enabling materials/technologies, advantages, and challenges. Furthermore, Table 2 provides a comprehensive comparison of representative functional materials (insulators, conductors, and semiconductors), detailing their properties, benefits, and limitations to guide optimal material selection for conformable electronics. In addition, future directions for developing patient-friendly biosensor platforms that meet the demands of various clinical and diagnostic scenarios are proposed.
Wearable patch-type bioelectronics
The skin is the outermost tissue of the body and is responsible for detecting physical stimuli from the external environment and protecting the internal tissues. Therefore, wearable patch-type bioelectronics can detect various biosignals, such as movements occurring on the skin or metabolic biomarkers48,49,50. Using either strain51,52,53 or pressure sensors54 attached to the epidermis, dynamic movements of the body can be precisely detected55,56. Similarly, temperature-sensitive57,58 devices can continuously monitor changes in body temperature. In addition, these patch-type wearable technologies can be applied in various ways, such as detecting pulse waves59,60,61 caused by heartbeats62,63, recognizing voice vibrations64,65,66 generated during speech, and monitoring body movements for motion recognition. Furthermore, the real-time detection of various biomarkers, such as metabolites67, ions68,69,70, nucleic acids71, and antigens72, generated from the sweat glands73,74 of the skin, enables real-time health monitoring and disease diagnosis75. Moreover, the wearable platform is capable of monitoring compound action potentials76 such as electroencephalograms77 (EEG), electrocardiograms (ECG)78,79, and electromyograms80,81 (EMG) which are generated by nerve or muscle activities and can be detected through the skin.
To capture these diverse biosignals, a patch-type wearable biosensor device capable of conformally attaching to specific skin locations and functioning effectively is required82,83,84,85. However, because the shape and curvature of the skin vary across different body parts, traditional rigid, flat sensors unavoidably detach, making precise sensing challenging. To address this issue, researchers have developed flexible patch-type sensors86,87. For example, by patterning and depositing biocompatible electrodes, such as Au or Pt, onto flexible polyethylene terephthalate (PET) substrates and incorporating chemical sensing materials, a flexible multifunctional sensing platform (e.g., sweat and temperature) can be fabricated. In addition, integrating a wireless communication module into a flexible printed circuit board (FPCB) enables the collection of biosignals without artifact intervention from the skin, including during movement (Fig. 2a)88. In addition to flexibility, conformal contact, which ensures complete adhesion to the skin tissue without causing unconformities, is crucial for obtaining accurate biosignals. To achieve high-fidelity and stable skin-interfaced bioelectronic measurements, ultrathin device geometries are employed to minimize bending stiffness, enabling van der Waals-driven conformal adhesion without external adhesives. The critical membrane thickness is determined by balancing bending, elasticity, and adhesion energies at the skin interface89. Additionally, placing stiff components along or near the neutral mechanical plane, or splitting it with soft adhesive layers, minimizes strain during bending and enhances mechanical durability70 Together, these strategies ensure intimate, reliable contact with skin while maintaining device performance under dynamic motion. Building on these strategies, ultrathin and flexible sensing materials particularly those based on nanofiber-driven network structures offer additional promise for achieving seamless conformal contact in biosensors. For instance, pressure-sensitive nanofibers have been developed by dispersing conductive nanomaterials such as carbon nanotubes (CNTs) and graphene and depositing them through an electrospinning process. When these nanofibers are deposited on a 1.4 μm-thick PET film, the resulting structure can be applied as a bending-insensitive pressure sensor capable of measuring resistance changes in response to pressure. Because of its extremely thin structure, the sensor exhibited transmittance over 90% and achieved conformal contact with curved surfaces. This technology can enable large-area (9 × 9 cm2) pressure-monitoring systems when integrated with an organic transistor-based 12 × 12 active matrix (Fig. 2b)90. Furthermore, amplification is essential for accurately detecting bioelectrical signals such as compound action potentials with ultralow amplitudes. Compared with traditional silicon-based transistors, organic semiconductor-based biosensors91,92 are more suitable for flexible or stretchable platforms. In this regard, integrating active components such as organic field-effect transistors93,94 (OFETs) or organic electrochemical transistors95,96 (OECTs) on ultrathin platforms is critical for amplifying biosignals and obtaining high-quality data. For instance, an ultrathin, flexible OECT was developed using a parylene-C substrate with transparent silver nanowire electrodes and an active layer composed of the flexible polymer poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS). The developed OECT device features an ultrathin thickness of under 5 μm, a high transconductance near 1 mS, and >90% optical transparency. This system demonstrated the capability of measuring ECG signals through conformal contact with the epidermis (Fig. 2c)97. Moreover, an advanced OECT design was fabricated by depositing Cr/Au (10 nm/50 nm) electrodes on a 1 μm parylene-C substrate and applying a 4-terminal vertical Corbino structure, which eliminates the influence of parasitic resistance in traditional vertical channel structures. The resulting OECT exhibited a remarkably high transconductance of >400 mS and an improved cutoff frequency of 2 kHz. This OECT demonstrated the ability to measure various bioelectrical signals, including ECG, electrooculograms (EOG), and EMG signals, through conformal contact with different skin regions (Fig. 2d)98. Ultrathin flexible patch-type bioelectronics can achieve conformal contact with curved skin tissue. However, to ensure the stable and long-term operation of the skin, they require tissue-like mechanical properties and additional stretchability to accommodate the deformations caused by skin movements.
a Photograph of a integrating the multiplexed sweat sensor array and the wireless FPCB wearable on a wrist. Reproduced with permission from ref. 87. b Photograph of the ultrathin large area (9 × 9 cm2) pressure sensor nanofibre sheet integrated with a active-matrix organic transistors. Reproduced with permission from ref. 90. c Schematic illustration of fully transparent ultrathin OECTs fabricated on parylene-C substrate. Reproduced with permission from ref. 97. d Photograph of skin-compatible 4-terminal vertical Corbino OECT (vcOECT) under tensile strain from 200 –0% (top) and images of physiological monitoring of the skin-attached vcOECTs (bottom). Reproduced with permission from ref. 98. e Schematic illustration of a multifunctional electronics with physical serpentine structured epidermal electronics (top) and photograph of pristine, compressed, stretched state of device on skin (bottom). Reproduced with permission from ref. 108. f Optical images of a wavy, single-crystal Si ribbons after transferred on PDMS (top) and angled-view scanning electron micrograph (SEM) image Si ribbons (bottom). Reproduced with permission from ref. 109. g Photograph and cross-sectional SEM image of nanomesh pressure sensor (top). Photograph of nanomesh sensor measuring the grip force and capacitance of sensor (bottom). Reproduced with permission from ref. 110. h Schematic illustration of the ultrathin EMG electrode module assembled through biphasic, nano-dispersed interface connections (top) and photograph of an 21channel EMG electrode array attached on human arm. Reproduced with permission from ref. 111. i Photograph of the epidermal sweat sensing and demonstrating under the bending or stretching state. Reproduced with permission from ref. 112. j Photograph of the stretchable multi-electrochemical sensor device under tensile strain and applied on the human wrist while excercising. Reproduced with permission from ref. 113. k Schematic illustration and photograph of liquid metal elastomer foam (LMEF) and capacitive sensor array demonstration. Reproduced with permission from ref. 116. l Schematic illustration and photograph of PVA-CA-MXene hydrogel attaching to very high bonding tape (VHB) tape for sensor monitoring the throat vibration. Reproduced with permission from ref. 124. m Schematic illustration and photograph of autonomous recovery and principle of the self-healing process with magnetic alignment of Nd2Fe14B. Reproduced with permission from ref. 131. n Schematic illustration of an tough, hydrophobic self-healing polymer film with network structure and photograph of 5 × 5 pressure-sensing array before and after self-healing. Reproduced with permission from ref. 132. o Schematic illustration of the fabrication process of the self-healable stretchable electrode by embedding a conductive network into self-healing polymer matrix (left). Photograph of the light emitting capacitor self-healed after a day and multifunctional self-healable electronic skin. Reproduced with permission from ref. 133.
To address these challenges, soft, stretchable, ultrathin patch-type biosensors with tissue-like mechanical moduli30 (1–1.5 MPa) have been developed99,100. To develop soft and stretchable patch-type electronics, thin and flexible conductive materials are often engineered into deformable structures and integrated onto soft substrates, allowing the entire system to stretch uniformly. A common strategy involves patterning flexible electrodes and interconnects into mechanically adaptive geometries such as serpentine101, kirigami102, or honeycomb103 layouts. These structural designs enable the system to undergo large deformations without compromising electrical performance. Building on this, the island-plus-serpentine104 and island-bridge strategies105 place rigid functional components on isolated micro-islands connected by stretchable interconnects like arc-shaped105 serpentine106, or fractal designs107. Arc-shaped interconnects use out-of-plane buckling to absorb strain, serpentine geometries leverage in-plane scissor-like deformations to enhance stretchability, and fractal layouts provide both high mechanical compliance and large-area coverage. Together, these approaches allow conformal integration with soft, curved biological surfaces while maintaining reliable function, making them highly effective for next-generation wearable and implantable bioelectronics. For example, a polyimide/Au/polyimide structure patterned with a serpentine design was developed as a transfer tattoo. When applied as strain sensors, temperature sensors, or electrodes to measure EMG or EEG signals, these structures functioned effectively as stretchable biosensors capable of achieving conformal contact with the epidermis (Fig. 2e)108. Alternatively, stretchable biosensors can be fabricated by transferring a flexible biosensor layer onto prestrained substrates. Once the pre-strain is released, the biosensor forms a wrinkled structure, allowing stretchability. A buckled-structure stretchable electronic system was reported with a thin ribbon-shaped silicon device bonded to pre-strained (3.5%) polydimethylsiloxane (PDMS) and yields a larger range of compressive and tensile deformability (-24% to 5.5%) (Fig. 2f)109. However, while stretchable structures mitigate some challenges, they can introduce mismatched mechanical properties between the stretchable substrate such as PDMS (0.5-3.5 Mpa)30, Ecoflex (200-500 kPa)30 and rigid metal wiring such as Au ( ~ 74 GPa)30, Silicon (130-185 GPa)30 potentially leading to distortions during stretching. By contrast, the use of intrinsically stretchable conductive materials can reduce these mismatches. Nanomesh structures, in which conductive nanowires are interwoven into a network, retain conductive pathways even under strain, enabling stretchability. When combined with materials such as polyvinyl alcohol (PVA), parylene, and polyurethane nanofibers via electrospinning, they can further enhance mechanical compliance. For instance, Au nanomesh electrodes can be integrated into ultrathin pressure sensor devices by utilizing PVA nanofibers as a sacrificial supporting layer and polyurethane nanofibers as an intermediate layer. These ultrathin, compliant nanoporous structures can be sequentially laminated onto objects and forming stretchable nanomesh systems capable of conformal contact with skin (Fig. 2g)110. Another approach involves the deposition of Au onto elastomers such as styrene-ethylene-butylene-styrene (SEBS). When Au nanoparticles are dispersed on the surface of a SEBS substrate, electrical conductivity is maintained despite microcrack formation in the Au layer during stretching, as continuous electrical pathways are formed. This method enabled the development of wearable patch-type biosensors with stretchable electrodes and wires. Moreover, by controlling the Au deposition speed, the interpenetrating network of polymer and Au can be modified, allowing for tunable conductivity and adhesion performance between the sensing and encapsulation layers. Depending on the SEBS substrate thickness, the electrodes and wires can be distinguished and selectively applied, enabling a plug-and-play approach for various applications such as EMG electrode arrays (Fig. 2h)111. Additionally, composite materials comprising conductive particles and stretchable polymers can serve as conductive stretchable sensors. Composite inks made from conductive metals, such as Ag, and stretchable polymers, such as SEBS or Ecoflex, can be patterned onto stretchable substrates using techniques such as screen printing or laser patterning. For instance, a composite of SEBS and silver flakes (AgFs) was dissolved in toluene and screen-printed layer-by-layer using the doctor blade technique (Fig. 2i)112. The stretchable composite ink was prepared by mixing Ecoflex and silver flakes with methyl isobutyl ketone (MIBK) (Fig. 2j)113. Both composite inks enabled the fabrication of sensors and the wiring of intrinsically stretchable epidermal sweat analysis patch devices. Unlike traditional elastic conductors, liquid metals with high conductivities and liquid-like properties are ideal for stretchable electronics114,115. Patterned liquid metals can serve as wiring, resistive sensors, or capacitor structures, and can be applied to devices such as pressure sensors. Eutectic gallium-indium (EGaIn) has a low melting point (15.5 °C) and high conductivity (3.5 × 106 S m−1)116. When combined with an elastomer in a foam form, EGaIn can be used to develop soft and highly compressible pressure sensors that five times more sensitive (0.992 pF kPa−1) than pure silicone foam sensors. As shown in Fig. 2k116, EGaIn was dispersed in the uncured Ecoflex and mixed with sugar particles. After curing, sugar was dissolved in water to create a liquid metal elastomer foam (LMEF). LMEF exhibited a unique property in which its permittivity increased (2.25–11.7) as the air gaps between the foam layers were compressed under pressure. Once the air gaps disappeared, the permittivity decreased as the liquid-metal droplets deformed within the elastomer. This property enables the LMEF to function as a capacitor-type pressure sensor that can detect body movements or be applied in sensor arrays. Similarly, hydrogels, which are soft and water-rich materials with properties resembling those of body tissues, can be functionalized for application to tissues to detect physiological changes or to interface between biological tissues and electronics, making them suitable for create stretchable biosensors. Conductive hydrogels can detect various biosignals117,118,119, including mechanical vibrations120,121, skin deformation122, and biochemical markers such as proteins or genes123. Owing to its high conductivity and two-dimensional (2D) material properties, MXenes are widely used as fillers in conductive hydrogels. However, MXenes are prone to oxidation when used as hydrogel fillers, leading to changes in their mechanical and electrical properties. To address this issue, a catechol-functionalized poly(vinyl alcohol) (PVA-CA)-based hydrogel was developed to prevent MXene oxidation and applied as a wearable strain sensor (Fig. 2l)124. The developed hydrogel strain sensor demonstrated applications, such as detecting finger movements when attached to the skin or detecting vocal cord vibrations for voice recognition.
Although intrinsically soft and stretchable wearable patch-type bioelectronic devices exhibit mechanical properties similar to those of skin, their soft nature makes them vulnerable to external damage. Self-healable patch sensors can address this limitation by recovering their mechanical and electrical performances after damage, thereby enhancing their durability and lifespan125,126,127,128,129,130. One approach for creating self-healing biosensors involves the use of magnetic microparticles, such as Nd2Fe14B, which attract each other. Circuits fabricated with the magnetic self-healing ink can be aligned to enable self-repair, which can be utilized to implement self-healing electrochemical wearable patch devices (Fig. 2m)131. Another method involves designing polymers with the reversible nature of their chemical bonds, glass transition temperature (Tg), and the ability of physical/chemical interactions to facilitate bond reformation. Designing self-healing polymers often involves balancing Tg and selecting suitable reversible bonding mechanisms to achieve optimal healing properties under specific conditions. By optimizing the composition of the hard (with strong hydrogen bonds) and soft segments (with weak hydrogen bonds), the mechanical properties of these polymers, including their self-healing ability and toughness, can be fine-tuned. When combined with liquid metals, these polymers can be used to create wearable patches with self-healing properties and sensors with resistive and capacitive structures (Fig. 2n)132. Furthermore, embedding self-healing polymers with conductive materials that form network structures, such as CNTs or Ag nanowires, can produce electrodes with stretchability and self-healing capabilities. These materials have been used to implement soft, stretchable, and self-healing ECG, as well as strain sensors. When applied to network structures, these devices can also be utilized in transparent displays such as light-emitting capacitors (LECs) and wearable patch systems (Fig. 2o)133. Although patch-type wearable sensors can measure and process various biosignals on the skin, they have limitations in detecting subtle electrical signals, such as compound action potential occurring inside the body. Implantable patch-type bioelectronics may be more suitable than wearable patch-type devices for long-term continuous monitoring of conditions such as chronic diseases or disabilities. Implantable biosensors can directly contact the surface of internal tissues, enabling real-time, long-term monitoring of biosignals in closed-loop systems that are difficult to detect in the skin. Consequently, extensive research has been conducted on implantable biosensors to develop such devices.
Implantable probe-type bioelectronics
Unlike the skin, the internal environment of the body is surrounded by biofluids, requiring devices to maintain effective encapsulation to prevent fluid penetration134. Organic semiconductors are particularly vulnerable to degradation in the presence of oxygen, moisture, and bodily fluids, which can cause charge trapping and reduced performance. To address this, strategies such as molecular design135 and encapsulation particularly hybrid barriers combining elastomers and inorganic materials134 are increasingly employed to ensure environmental stability and prevent leakage or contamination. Additionally, biocompatibility is essential for avoiding severe inflammatory or immune responses, necessitating the use of non-immunogenic coatings136, biodegradable materials137, or the incorporation of therapeutic agents138,139. These approaches are crucial for ensuring both the long-term functionality and safety of bioelectronic devices in harsh physiological environments. Organs, such as the brain, heart, muscles, and peripheral nerves, have distinct shapes, physical properties, and surrounding tissues based on their functions. Therefore, the implantable biosensor designs must be tailored to the specific physical, structural, and functional characteristics of the target organs and application sites140,141,142,143,144.
Biosignals are often attenuated as the distance between the signal generation site and biosensor increases because of factors such as body movement, metabolism, and biofluid interference. To address this issue, invasive needle-type devices have been developed to acquire more precise signals by directly inserting electrodes or sensors into the target locations145. A representative example is the Utah slanted electrode array (USEA), which consists of microneedle arranged in an 10 × 10 array to detect bioelectrical signals from specific locations. Each electrode shaft was insulated with parylene C (6 μm)146 and implanted in the ulnar nerve of human subjects (Fig. 3a)147. USEA have been applied to peripheral nerves and are widely used to achieve high selectivity of bioelectrical signals. However, microelectrode arrays, such as USEA, face challenges in achieving precise placement on curved surfaces owing to their rigid flat substrates. To overcome this limitation, researchers have developed platform devices that incorporate silicon microneedles on stretchable or flexible substrates such as PDMS, enabling the electrodes to conform to desired shapes or locations through substrate deformation. As shown in Fig. 3b148, microneedle electrodes were fabricated from a silicon-on-insulator (SOI) wafer using microelectromechanical system (MEMS) fabrication technology. The silicon-based 16 channel microneedle array was partially embedded in PDMS (Sylgard-184, 10:1) and implanted into the peroneal nerves of rats using cuffless implantation.
a Photograph of side view of explanted USEA (top) and representative image of implant location following removal of the array and SEM image of the tip of explanted USEA (bottom). Reproduced with permission from ref. 147. b Schematic illustration of the flexible microneedle nerve array (MINA) implanted in a rodent autonomic nerve and photograph of MINA implanted onto a vagus nerve. Reproduced with permission from ref. 148. c Schematic illustration of two microprobe designs applied in papillary muscle experiments (top) and photograph of a four-shank microprobe before and after insertion into the rabbit papillary muscle (bottom). Reproduced with permission from ref. 149. d Schematic illustration of probe tip, probe packaging, flex cable and headstage with checkerboard site layout of Neuropixel (dark squares). Reproduced with permission from ref. 150. e Schematic illustration and photograph of flexible probe electrode and implantation process with tungsten guide. Reproduced with permission from ref. 152. f Photograph of a single ultraflexible MEA and 16-filament assembled by helix structure (top). Photograph of implantation of the MEA into brain (bottom). Reproduced with permission from ref. 153. g Schematic illustrations of the PEDOT/PSS/pHEMA coated microelectrodes neural probe architecture and the photographs of the implantation into brain. Reproduced with permission from ref. 154. h Schematic illustration of the implantation process of TIME electrode and photograph of implantation into the sciatic nerve of rat. Reproduced with permission from ref. 155. i Left: Schematic illustration nanofabrication process of a multilayer neural probe encapsulated by PFPE-DMA elastomers(top). Photograph and bright field optical image of PFPE-DMA elastomer-encapsulated four layers neural probes (bottom). Right: Immunostaining of horizontal brain slices was performed at two (top), six (middle), and 12 weeks (bottom) post-implantation. The fluorescent signals are represented by different colors: green for neurons (NeuN), pink for astrocytes (GFAP), cyan for microglia (IBA1), blue for DAPI-stained nuclei, and red for rhodamine 6G-labeled implanted devices. Reproduced with permission from ref. 156. j Schematic illustration of a 3D brain neural mapping device that is consisted of ECoG, Utah array, and Michigan probe. Reproduced with permission from ref. 157.
While microelectrode arrays can collect biosignals over a wide area, bulky organs, such as the brain or heart, often require deeper tissue signals. Shank-type microprobes with electrodes arranged to penetrate tissues at varying depths are advantageous for these applications. For example, microprobes applied to the heart or papillary muscles have been shown to recording cardiac signals in ex vivo experiments (Fig. 3c)149. By positioning iridium microelectrodes on micromachined silicon shanks, researchers demonstrated that microprobes with precise electrode spacing (50–750 μm) can be effectively used to map the electrical properties of the heart. Similarly, in the brain, multichannel probes have been developed and integrated into various platforms to obtain signals from deep regions. One example is the Neuropixels probe, a high-density silicon probe measuring 70 × 10,000 μm, which integrates 960 channels with low-impedance TiN electrodes compatible with complementary metal-oxide-semiconductor (CMOS) processing. The compact design and comprehensive functionality of the Neuropixel probes enable the recording of large neuronal populations across multiple brain regions in freely moving animals (Fig. 3d)150. However, silicon-based probes, which are effective for measurement, pose the risks of tissue damage and foreign body responses owing to their rigid and non-bendable mechanical properties.
To address this issue, flexible probe sensors that can deform under an applied force while maintaining their functionality have been developed151. By fabricating microelectrodes on flexible, ultrathin polymer substrates, such as polyimide, researchers have created flexible probes capable of measuring brain activity. In Fig. 3e152, a polyimide-based Ti/Au flexible probe consisting of one stimulation electrode, one ground electrode, and three recording electrodes was fabricated by MEMS technology. The 20 μm-thick probe could be folded and easily inserted deep into the brain using temporary tungsten guide sticks. An additional ground electrode was included to prevent power leakage, and neural spike signals from the Subthalamic Nucleus (STh) located 7 mm deep in the rat brain, were recorded. Expanding the number of channels in these flexible microelectrode arrays enables long-term brain signal measurements. For example, a flexible microelectrode array composed of eight filaments with polyimide and gold electrodes was developed using an elastocapillary process to form a self-assembled 120-channel ultraflexible array. This device was implanted into the mouse brain, and neural activity was recorded for at least 12 weeks (Fig. 3f)153. However, when flexible probes are fabricated using polyimide and gold electrodes, integrating numerous channels into a narrow, elongated platform reduces the electrode area and increases the impedance. To overcome this challenge, electrode performance can be enhanced through surface coatings made of electrochemically superior materials. For example, coating a flexible gold electrode array on a parylene substrate with PEDOT:PSS, which has excellent electrochemical properties, improves performance and enables the detection of neuronal spike signals in the rat hippocampus (Fig. 3g)154. Flexible probe-type electrodes are not limited to brain applications but are also used for peripheral nerves. Microelectrodes that penetrate peripheral nerves enable the precise acquisition of sensory and motor-related signals from nerve bundles. One such design is the Transverse Intrafascicular Multichannel Electrode (TIME), which features platinum electrodes patterned on a flexible polyimide substrate capable of penetrating peripheral nerves. TIME demonstrated a more selective and precise recording and stimulation of peripheral nerves than cuff structures (Fig. 3h)155.
Despite their advantages, the mechanical properties of flexible probes remain relatively stiff compared to those of soft biological tissues, highlighting the need for even softer probes to achieve mechanical compliance. To address this issue, high-density soft probes with three-dimensional integration of metal electrodes on perfluorinated dielectric elastomers have been developed. Nanometer-thick lithographed electrode arrays in a 3D configuration with a cross-sectional density of 7.6 electrodes per 100 µm² were designed and packaged. These soft probes exhibited tissue-like mechanical properties and demonstrated long-term stability for 10 weeks, maintaining their dielectric encapsulation for over a year in environments similar to biofluids. They also reliably captured the electrical activity in the mouse brain or spinal cord for several months without interfering with the behavior of the animals (Fig. 3i)156. As described above, probe-type biosensors can obtain information at various depths by directly positioning the sensors deep within the tissue. However, their application over large areas remains challenging, necessitating the development of sensor platforms capable of covering wide regions to improve spatial resolution. To achieve this, the sensors must be positioned at multiple locations. As a solution, an electrode platform that enables 3D mapping was proposed by combining a Utah array (based on flexible polyimide and Au electrodes) for multisite signal acquisition, a Michigan probe for deep measurements, and an electrocorticogram (ECoG) electrode array for large-area surface measurements (Fig. 3j)157. Biosensors that use such invasive platforms can be positioned precisely at the target location, enabling highly accurate measurements. To improve the insertion of these sensors while minimizing tissue damage and maintaining long-term stability, several advanced strategies have been developed158. Microactuation techniques such as electrically159, ultrasonically160, or microfluidically161 driven methods temporarily enhance the stiffness of flexible electrodes during implantation, allowing for controlled and accurate penetration without the need for rigid carriers. Magnetic insertion162 offers a contactless alternative, ideal for ultrathin or delicate probes, enabling precise three-dimensional navigation and reducing contamination risks. Additionally, bioinspired approaches drawing from organisms like sea cucumbers, squid, mosquitoes, and woodpeckers introduce mechanisms for dynamic stiffening, graded flexibility, guided penetration, and impact mitigation, further enhancing implantation efficiency and safety163. Together, these techniques represent a significant step forward in developing minimally invasive, stable, and biocompatible neural interfaces. However, because these platforms penetrate tissues, they are prone to inflammatory or immune responses, and larger application areas result in greater tissue damage. In contrast, patch- or cuff-type platforms, which are applied to the tissue surface without penetration, cause less tissue damage but are less effective at obtaining deep signals. To address this limitation, advances in artificial intelligence and machine-learning techniques for bioelectronic signal processing and closed-loop systems have enabled the analysis and interpretation of surface-acquired signals to extract more comprehensive biometric information. By leveraging these advancements, implantable patch-type platforms offer a promising approach for maximizing information acquisition while minimizing tissue damage.
Implantable biosensors with stable tissue interfacing
Implantable deformable biosensors applied to tissue surfaces typically have a patch-type form factor that is effective for acquiring electrophysiological signals over areas of complex and curved tissues, such as the brain, heart164, and nerves165. For instance, ECoG signals can be recorded by implanting a sensor with multiple electrodes in the cerebral cortex; the number of electrode channels is critical for precise signal acquisition and advanced signal processing. Recently, high-density (800 μm vertical, 500 μm horizon pitch) 1024-channel platinum-nanorod-based electrodes with low electrochemical impedance have been integrated onto flexible substrates (e.g., polyimide), enabling the measurement of cerebral signals through thousands of channels in real time (Fig. 4a)166. However, expanding ECoG channels using passive electrodes still presents challenges, including crosstalk issues between the electrodes and increased electrode impedance as the spacing and size of the electrodes decrease, potentially leading to signal quality degradation. In addition, the real-time analysis of fine signals across numerous channels requires significant computational resources. To overcome these limitations, active-matrix sensors are the most promising for detecting signals over a large tissue area. For example, active-matrix ECoG sensor arrays with silicon transistors on polyimide-based flexible substrates were applied to the cortex of cats and various neural activities, such as sleep spindles, visually evoked responses, and seizures. (Fig. 4b)167. Such brain interfaces have the potential to be applied in closed-loop bioelectronic systems based on brain-wave monitoring. However, to implement more precise and multifunctional closed-loop bioelectronic systems, it is necessary to establish interfaces that connect the central nervous system to the peripheral nervous system, which is linked to the organs and muscles throughout the body. Therefore, neural interfaces that selectively stimulate nerve bundles such as the peripheral or vagus nerves or that analyze signals using multichannel electrodes are required. Flexible electrode platforms can be utilized for cuff-type interfaces that effectively wrap around the peripheral or vagus nerves. By depositing metal layers (Ti/Au/Pt) on parylene-C, a thin-film multichannel nerve cuff was fabricated and applied to the vagus nerve, demonstrating its capability of targeted selective stimulation (Fig. 4c)168. To achieve signals with a high signal-to-noise ratio, ultraconformable parylene-C/Au microelectrodes can be coated with biocompatible conducting polymers, such as PEDOT:PSS, to reduce impedance. This ultraconformable peripheral nerve interface demonstrated stable recording of ENG signals for 21 days post implantation. Additionally, the cuffs exhibited excellent biocompatibility, causing significantly less fibrotic scarring than the clinically comparable PDMS silicone cuffs. Beyond simple nerve signal recordings, they enabled the precise modulation of nerve activity at the sub-nerve level, which facilitated a diverse range of paw movements (Fig. 4d)169.
a Photographs of flexible μLED arrays integrated with 1024 channel platinum nanorod grid (PtNRGrids) for different area (32 × 32 mm2 and 5 × 5 mm2) and real-time recording on the cortical surface of pig brain. Reproduced with permission from ref. 166. b Photographs of 360-channel active electrode array implanted on cat brain. Reproduced with permission from ref. 167. c Schematic illustration of channel design and aprocess of thin film (TF) cuff wrapped around a nerve (top). Schematic illustration and images of TF cuff implanted on a infraorbital nerve of mouse (bottom). Reproduced with permission from ref. 168. d Schematic illustration of ultraconformable nerve cuff arrays with PEDOT:PSS microelectrodes and the photograph of the implantation into peripheral nerve of rat. Reproduced with permission from ref. 169. e Schematic illustration of stretchable ECoG patch device based on PI/Au/PI electrode and PDMS substrate. Reproduced with permission from ref. 177. f Photograph of PDMS-based stretchable ECoG patch electrode and stretched up to 100% strain, implanted on the brain surface. Reproduced with permission from ref. 178. g Schematic illustration of stretchable electronic patch constructed by nanocomposite and PEDOT:PSS (top) and photograph of stretchable bioelectronic patch attached during the dynamic movement of heart. Reproduced with permission from ref. 179. h Photographs of soft rubbery epicardiac bioelectronic patch under different kind of deformation (top) and implantation on the living porcine heart (bottom). Reproduced with permission from ref. 28. i Schematic illustration about morphing electronics based on viscoplastic electrode and photographs of implantation process wrapping the sciatic nerve. Reproduced with permission from ref. 181. j Schematic illustration of self-locking and stress relaxation property of adaptive self-healing electronic epineurium. Reproduced with permission from ref. 182. k Schematic illustration of fatigue resist property of dynamically recoverable AgF-SHP nanocomposite and soft peripheral neuroprosthesis. Reproduced with permission from ref. 183.
Despite their flexibility, these materials of sensors such as conductive polymer PPy, PANI (0.5 to 1.5 GPa)30 and PEDOT (2–5 GPa)30 remain rigid compared to the modulus of biological tissues (1 kPa to 40 kPa)30, often causing tissue damage owing to mechanical mismatches. To overcome this mechanical mismatch, conductive hydrogels based on PEDOT:PSS and PANI have emerged as promising alternatives due to their tissue-like softness and biocompatibility170,171. However, their widespread application is hindered by low conductivity stemming from limited polymer content and water loss, which affects electrical stability. Blending with additives, such as small-molecule plasticizers172 or soft polymers173, has proven effective in enhancing both conductivity and mechanical compliance, though trade-offs remain (e.g., additive leaching or reduced conductivity). More recently, intrinsically stretchable PEDOT derivatives have been developed by polymerizing EDOT directly onto elastic substrates like PU174 or PDMS175, offering improved stretchability without external additives, though challenges in processing and adhesion persist176. Together, these strategies provide a material design foundation for integrating PEDOT-based conductors into conformable bioelectronic systems. On the other hand, numerous studies have focused on developing soft and stretchable implantable sensors with mechanical properties similar to those of tissues. For instance, in Fig. 4e Son et al.177 demonstrated that serpentine-structured polyimide/Au/polyimide electrodes transferred onto PDMS achieved brain-like mechanical properties. The application of a soft, tough, and ionically conductive hydrogel (STICH) further improved its electrical and mechanical performances. The STICH material exhibited brain-compliant softness with a Young’s modulus of ~9.46 kPa, providing a conformal interface with the cortex, and high toughness ( ~ 36.85 kJ/mm³), ensuring structural integrity during interactions with wet brain tissue. Stretchable metal electrodes with wavy patterns printed on an elastomer were coated with STICH as an interfacial layer, significantly reducing impedance from 60 kΩ–10 kΩ at 1 kHz. Another approach involves depositing parylene-C onto PDMS, followed by the patterning of metal electrodes. The PDMS-based ECoG electrode arrays fabricated using parylene-treated PDMS were evaluated for their long-term stability and reliability. The mechanical and electrochemical properties were assessed over a period of up to 8 months through accelerated aging tests. When implanted in the primary somatosensory area of the brain, the ECoG electrode array successfully recorded somatosensory-evoked potentials (SEPs) in response to mechanical paw stimulation with sufficient spatial resolution to differentiate between forepaw and hind paw stimuli. Furthermore, the PDMS-based electrodes demonstrated stable chronic recording capability for up to 3 months in non-human primates, maintaining conformability with the brain tissue (Fig. 4f)178.
For cardiac tissue monitoring, implantable sensors must possess strain-resistant properties to withstand continuous contractions and relaxations of the heart muscle. Kong et al. developed 16-channel intrinsically stretchable implantable electrodes on a SEBS substrate, incorporating multi-walled carbon nanotubes (CNTs), liquid metal, and PEDOT:PSS. The microcracked PEDOT:PSS served as an interface with the biological tissue, whereas the liquid metal interconnects and CNT nanocomposites were used as electrodes. The sensor arrays endured a tensile strain of up to 400% and demonstrated high durability under 5000 times of repetitive deformation cycles. Additionally, they achieved stable conformal contact with the heart surface, enabling reliable monitoring and mapping of ECG signals on the epicardium (Fig. 4g)179. Furthermore, to enable multiplexing in soft stretchable patch sensor platforms, the integration of stretchable transistors is crucial. Stretchable wiring and resistive sensors can be fabricated by embedding Ag nanowires into soft substrates such as PDMS, whereas stretchable transistors can be created by patterning PDMS with P3HT-based semiconductor polymers and ion gels. These advancements supported the integration of ECG, strain, and temperature sensors into multifunctional epicardial patch devices with multiplexing capabilities (Fig. 4h)28.
Unlike wide-area applications, such as the brain or heart, applying sensors to nerve bundles requires not only soft and stretchable properties but also additional specialized features to meet the specific demands of neural interfaces. For cuff-type devices applied to nerves, wrapping the device along the surface of the nerve and securely fixing it is crucial. However, traditional fixation methods that use sutures or locking holes can cause tissue damage during surgical procedures and may not provide long-term stability. To address these limitations, cuff-type devices require not only soft and elastic properties but also self-locking capabilities for easier application180. Self-healing substrates can satisfy this requirement. For example, a polymer combining methylene bis (phenyl urea) (MPU) and isophorone bisurea (IU) with PDMS features dynamic hydrogen bonds and provides viscoelastic properties and self-healing capabilities. This enabled the cuff to self-lock via self-healing of the substrate (Fig. 4i)181. Additionally, the stress-relaxation properties of PDMS-MPU-IU derived from its weak and strong hydrogen bonds minimized the mechanical mismatch stresses on the tissue after application (Fig. 4j)182. Furthermore, in Fig. 4k183 Electrodes made from nanocomposite of AgF coated with gold nanoshells (AuNS) and self-healing polymers can re-establish the percolation network of AuNS coated AgF after damaged due to dynamic self-healing capability. As a result, device offer dynamic recovery of electrical and mechanical performance from the fatigue caused by repeated muscle contractions, enabling stable operation even under repeated deformation. However, in dynamic tissues like the heart or muscles, repeated movements can deform the device and cause it to shift or detach from the target site, making stable placement of multifunctional multichannel sensors particularly challenging. Thus, for patch- or cuff-type devices applied to tissue surfaces, the use of adhesive materials that can securely attach the device to the living tissues is critical.
Bioadhesive sensors for on-site signaling
Despite advancements in stretchable and flexible sensors, traditional electronics face significant challenges in achieving mechanical modulus matching with soft biological tissues that exhibit softness. Tissues such as the heart, muscles, and nerves, which contain >70% moisture and are in continuous motion, exacerbate these challenges. As a result, sensors experience low frictional forces and weak interfacial bonding with the tissues, leading to increased electrode impedance and degraded sensor performance over time. This can also result in acute or chronic inflammatory responses30,141,184.
In this regard, adhesive materials play a crucial role in mitigating these challenges by providing stable and conformal interfaces on these tissues185,186, resulting in strong interactions between bioelectronic devices and tissues187,188. Among them, soft and adhesive hydrogels can stabilize the biotic-abiotic interfaces through the formation of covalent and/or non-covalent bonds. For instance, hydrogel adhesives composed of k-carrageenan and acrylamide copolymers enabled successful adhesion on bladder surfaces, and a sensor integrated with the adhesives effectively detected strain and EMG signals in the bladder (Fig. 5a–c)189. In addition, it has been reported that nanoparticles exhibit strong tissue adhesion by themselves by modulating their shape and surface area. They exhibit electrostatic interactions, van der Waals forces, and hydrogen bonding with the polymeric chains of the extracellular matrix of tissues190,191. As shown in Figs. 5d–g, silica nanoparticles modified with carboxylic acid combined with a dissipative hydrogel substrate effectively displaced water molecules against tissue surfaces, forming stable interfaces for reliable ECG and heart rate monitoring192.
a The schematic illustration for in situ diagnosis process of overactive bladder rat model. b Photographs showing the attached ultra-soft hydrogel combined with structurally engineered islets (USH-SI) sensor onto the bladder. (Scale bar = 10 mm) c Plot accumulated urine volume and intra-bladder pressure (IBP), strain sensor resistance and EMG RMS. Reproduced with permission from ref. 189 (a–c). d Schematic illustration of the absorption of the interfacial water by densified activated nanoparticles (ANP) and the formation of nanohesion (top) and the interactions between ANP and surfaces through short-range forces (bottom). e The in vivo fixation procedure of strain sensor. (Scale bar = 2 mm) f Pulse signals acquired by a nanohesive-fixed strain sensor with simultaneous ECG signals. g Comparison of pulse rates from ECG and nanohesive-fixed strain sensors over 30 min. Reproduced with permission from ref. 192 (d–g). h Schematic illustration of the polymer hydrogel bioadhesvie (PHB) enabling robust biointerfaces for wireless sensors. i Measurements of f0 during squeezing and releasing of a rat flank validate the reliability of the PHB-based biointerface. Reproduced with permission from ref. 195 (h, i). j Schematic illustration a bioelectronic device’s physical attachment to tissue. k Images of epicardial ECG recordings at day 0, day 14, and on-demand detachment of bioadhesive electrodes. l Surface ECG signals from needle electrodes compared to epicardial ECG signals recorded by bioadhesive electrodes at day 0 and day 14. Reproduced with permission from ref. 196 (j–l). m Schematic illustration of 3D printing-based fabrication of the adhesive bioelectronic interface. n Images of the implanted all-hydrogel bioelectronic interfaces on rat heart. o Epicardial recordings from hydrogel interfaces at day 28 post-implantation. Reproduced with permission from ref. 170 (m–o). p Adhesive attachment using BASC channels with wet tissues, enabled by semiconducting and adhesive polymers for robust covalent bonding. q Schematic illustration of device structure of the adhesive OECT under strain. Scale bar, 5 mm. r Stable attachment of a adhesive OECT to gastrocnemius medialis (GM) muscle during mechanical agitation, compared to non-adhesive OECT, with corresponding EMG recordings. Reproduced with permission from ref. 197 (p–r). s Images of ultraconformal brain adhesion of shape-morphing cortex-adhesive (SMCA) sensors on curved surfaces of ex vivo bovine cortex. t–u Electrocorticogram recordings comparing PDMS (t) and SMCA (u) sensors under tFUS stimulation. Reproduced with permission from ref. 200 (s–u). v Wet-resistant adhesion of the electrospun self-healing polymer (SHP) network (E-SHN) with Alg-CA on cardiac tissue during washing. w Bidirectional interfacing via Alg-CA coatings between tissues and EGaIn composites, shown with ECG signals during rat heart stimulation. Reproduced with permission from ref. 201 (v–w).
However, interfaces formed by non-covalent bonds frequently exhibit mechanical failure upon excessive water infiltration and significant dynamic movement193,194. Adhesives incorporating carboxylic acid and N-hydroxysuccinimide (NHS), which can be covalently bound to tissues, address these issues, ensuring long-term stability in vivo. In particular, these adhesives have been effectively utilized for measuring intracranial pressures and monitoring stable ECG signals, even in the dynamic environments of cardiac tissue (Fig. 5h–l)195,196. Furthermore, integrating the adhesive layers into soft and flexible hydrogel systems ensures excellent mechanical stability and biocompatibility under physiological conditions, enabling long-term ECG signal acquisition (Fig. 5m–o)170. On-tissue conformal OECT films have also been employed for successful EMG sensing, highlighting their flexibility and adhesive properties in skeletal muscle tissue (Fig. 5p–r)197.
Aforementioned, the use of adhesive hydrogel interfaces in existing biosensors facilitates reliable signal sensing and minimizes tissue damage184,198. However, the adhesive strength can vary depending on the physicochemical features of the target tissues. To induce robust tissue adhesion, a certain amount of time and mild pressure on the tissue are also required. Critical challenges remain in achieving in vivo monitoring of electrophysiological signals for several months28,199. Recently, a self-healing and stretchable biosensor integrated with adhesive hydrogels containing polyphenol moieties (e.g., catechol) was developed (Fig. 5s-w). Catechol-modified hydrogels exhibit robust adhesion even in aqueous environments, inspired by the material-independent adhesiveness of the byssal threads of marine mussels. In this context, sensors with catechol-modified materials show rapid and conformal adhesion on the curved surfaces of the brain and heart tissues, enabling the successful and precise measurement of neural and cardiac signals (e.g., ECoG and ECG)200,201. Interestingly, the adhesive ECoG sensor recorded signals with a high signal-to-noise ratio against the mechanical vibration generated by the low-intensity focused ultrasound (Fig. 5s-u). Adhesive ECG sensors are composed of liquid-metal composite electrodes, where the oxidation layer of the liquid metal is stabilized by coordination bonds with the catechol-modified alginate polymer, ultimately enabling stable real-time monitoring of ECG signals in both static and dynamic environments (Fig. 5v, w). In future studies, advanced adhesive hydrogels that can be retained without extreme swelling and weak cohesion should be integrated with flexible bioelectronics to enhance the long-term performance of devices, which would be promising for personalized medicine.
Injectable bioelectronic platforms with sensing and stimulating functions
Syringe-injectable strategies for delivering materials into the internal environment of the body have attracted significant research interest. Among these approaches, injectable hydrogels have demonstrated remarkable potential. Injectable hydrogels have mechanical properties inferior to those of native biological tissues, enabling them to conform to the surrounding tissue shapes upon injection while maintaining their functional properties202,203,204.
Based on these properties, injectable hydrogels have been used for in vivo drug delivery applications. As shown in the study corresponding to Fig. 6a, a biocompatible nanocomposite hydrogel was engineered to enable injectability through a combination of Schiff base formation and ionic interactions, facilitating rapid in-situ gelation upon contact with physiological fluids202. This approach enables the hydrogel to form a stable interface with the target tissues without requiring external curing agents. In vivo, this system was applied to wound healing and liver hemostasis models in rats, demonstrating effective adhesion to tissue surfaces and the promotion of rapid hemostasis while supporting cellular regeneration. This study highlighted the potential of bioresponsive hydrogels for localized therapeutic applications and their ability to conform to irregular tissue structures after injection. Moreover, conductive hydrogels have been investigated for their ability to enable stimulus-responsive applications and facilitate targeted stimulation in vivo203. In Fig. 6b, an injectable thermo-responsive hydrogel nanocomposite was designed using PLA-PEG-PLA polymers, drug-loaded micelles, and superparamagnetic iron oxide nanoparticles (SPIONs). The injectability of this hydrogel was achieved through its shear-thinning behavior, which enabled it to pass through a syringe and subsequently recover its mechanical integrity. Once delivered, the system responds to external alternating magnetic fields to generate localized hyperthermia, which enhances drug diffusion into the surrounding tissues. In vivo experiments using glioblastoma models demonstrated that this method effectively promotes targeted drug delivery while minimizing systemic exposure, offering a promising strategy for controlled drug release in cancer therapy.
a A graphical illustration describing the injection process of a hydrogel-based nanocomposite carrying various nanoparticles, including PLGA-drug NPs (Nanoparticles) and FIONs (Ferrimagnetic iron oxide nanocubes). Reproduced with permission from ref. 202. b Intracortical hydrogel nanocomposite-mediated penetrative drug transport to deep brain tumors. (i) Illustration of drug diffusion into deep brain tumors enabled by magnetically triggered mild hyperthermia. (ii) Hydrogel nanocomposite injection. (iii) Hydrogel nanocomposite biodegradation process. Reproduced with permission from ref. 203. c Illustration of bidirectional electrophysiological interfacing facilitated by injectable tissue-interfacing prostheses composed of conductive hydrogels (IT-IC hydrogel). The hydrogel fills injured muscle or nerve sites, restoring tissue continuity and enabling neuromuscular signal transmission (red box). It forms a bioelectronic bridge (blue box) that allows bidirectional electrical signal transfer between muscles and peripheral nerves. This system integrates robotic assistance to enable real-time neuromuscular monitoring, facilitate targeted electrical stimulation, and support adaptive rehabilitation for enhanced functional recovery. Reproduced with permission from ref. 204. d (i) A composite material comprising a prepolymer carrier and conductive filler is injected near a target nerve, curing in place to establish an electrically conductive interface. (ii) Application of an injectable composite electrode in swine models, enabling stimulation of single or multiple nerves, including parallel nerve bundles and neural plexuses. Syringe-based delivery facilitated nerve cuff formation (1–3 mm diameter) and targeted encapsulation within the nerve sheath, utilizing its structure as a natural mold for uniform distribution. Reproduced with permission from ref. 205. e (i) Injectable deployment of elastin–gelatin–carbon nanotubes 20 (EGCx, where x is the concentration of carbon nanotubes (CNTs) in mg ml−1) scaffolds utilizing its water-activated shape-memory properties. The EGC20 cardiac patch was delivered onto a porcine heart via a commercial catheter under thoracoscopic guidance. (ii) Schematic representation of the minimally invasive catheter-based injection of the prefabricated EGC20 scaffold (top) and the thoracoscopic view of the surgical site projected onto a monitor (bottom). (iii) In vivo catheter-based delivery of the EGC20 scaffold onto a porcine heart, illustrating the sequential process of insertion, ejection, saline application, and shape recovery. Reproduced with permission from ref. 206. f (i) Conceptual schematic of an injectable mesh cardiac patch for atrial fibrillation treatment. (ii) Illustration showing the endoscope-assisted injection of the mesh patch into a living animal. (iii) Mesh patch deployment process, including injection from a glass tube, release, and shape recovery on the heart tissue surface. Reproduced with permission from ref. 207. g (i) Schematic illustration of injectable electronics, highlighting the overall mesh structure with red-orange lines and indicating supporting and passivating polymer mesh layers. Yellow lines depict metal interconnects between I/O pads and recording devices. (ii) Illustration of the stereotaxic-guided in vivo injection of mesh electronics into a mouse brain. (iii) Optical image of the injection process in an anesthetized mouse. Reproduced with permission from ref. 208. h (i) Photograph of a mouse immediately after the injection of mesh electronics. The inset illustrates the noncoaxial intravitreal injection targeting the RGC layer. Yellow dots represent multiplexed recording electrodes. (ii) In vivo through-lens fundus images of the same mouse eye, recorded at days 0 and 14 post-injection. Reproduced with permission from ref. 209. i (i) Illustration detailing the syringe-based injection process of electrode arrays and their subsequent unfolding in the subdural space, with through-hole sizes typically in the millimeter range. (ii) Schematic of a syringe-injectable electronic device preloaded in a glass syringe with a buffer solution such as PBS or artificial cerebrospinal fluid. Air pressure from a dispenser expels the buffer solution, allowing the fluid flow to carry the device through the syringe tip. Scanning electron microscopy (SEM) image of the fabricated sensor section of the injectable electrode array. Reproduced with permission from ref. 210. j (i) Conceptual schematic of a biodegradable electronic tent introduced into the intracranial space via a minimally invasive device. The inset provides an X-ray image of the device post-deployment. (ii) Sequential images illustrating the deployment process in a simulated structure. Reproduced with permission from ref. 211. k Photographic top-view images of the soft ECoG array prototype: (i) in a folded configuration and (ii) in its fully expanded state. (iii) Cross-sectional schematic illustrating the inner chamber containing aqueous solution. (iv) Post-placement image of the ECoG system on the brain (left) and a photograph of the extracted brain showing the electrode array positioned on the contralateral hemisphere (right). Reproduced with permission from ref. 212. l Conceptual illustration of minimally invasive ECoG (MI-ECoG): (i) A folded ECoG device implanted onto the cortical surface through a burr-hole craniotomy. (ii) Controlled expansion via fluidic actuation for large-area cortical signal acquisition. (iii) A top-down view showing the MI-ECoG unfolding inside a brain phantom model, driven by syringe-applied air pressure. (iv) X-ray images demonstrating the device’s expansion process, with white boxes indicating its structure before, during, and after deployment. Reproduced with permission from ref. 213. m (i) Conceptual illustration of shape-adaptive electrode arrays (SCEAs) engineered for minimally invasive implantation and large-area intracranial neural recording. The inset presents an image of an epidurally implanted SCEA in a rat brain, delivered via a minimally invasive surgical procedure. The skull was subsequently removed to provide a clear view of the implanted array. (ii) Optical images showing the SCEA in both its compressed and fully deployed states. Reproduced with permission from ref. 214. n (i) Conceptual schematic showing the adhesive implantation of the bioadhesive pacing lead onto the epicardium, highlighting its capabilities for sensing, stimulation, controlled detachment via reservoir-injected fluid, and subsequent retrieval. (ii) Image of an ex vivo porcine heart (400 g) being lifted by the attached bioadhesive pacing lead. (iii) Sequential images displaying the bioadhesive pacing lead affixed to a porcine heart (top) and the heart post-detachment (bottom). Reproduced with permission from ref. 215.
Injectable conductive hydrogels, with their ability to adapt to tissue morphology and their superior conductivity and structural reliability, have successfully reconnected several nerve pathways and restored nerve signal transmission (Fig. 6c)204. The hydrogel in this study utilized phenylborate-based dynamic covalent bonds and in-situ gold nanoparticle formation, which enhanced its self-healing and conductive properties while maintaining its mechanical integrity post-injection. In vivo tests in rodents demonstrated the ability of the hydrogel to fill nerve gaps, restore nerve conduction, and promote tissue repair. In addition, the hydrogel was integrated into a closed-loop rehabilitation system, where electromyographic (EMG) signals were monitored in real time to trigger electrical stimulation and assist motor recovery through robotic intervention.
Injectable conductive hydrogels were also explored as electrodes interfacing internal tissues (Fig. 6c, d). Specifically, in Fig. 6d, the hydrogels serve as neural interface devices, bridging the internal and external environments using minimally invasive techniques205. An injectable composite electrode, based on the in situ curing of a silicone-silver composite, was developed to form conductive and biocompatible interfaces post-injection. In vivo, this system was injected into the brachial plexus of rats and vagus nerve of swine for electrical stimulation, demonstrating effective neural signal transmission and stable functionality for up to 4 weeks. This minimally invasive approach offers a promising alternative to conventional neuromodulation electrodes, ensuring long-term biocompatibility and adaptability to complex neural structures.
In addition to hydrogel injections, the concept of hydrogel-based patches has been investigated. Researchers have successfully delivered conductive hydrogel patches that are significantly larger than the syringe diameter to the epicardium (Fig. 6e, f)206,207. As shown in Fig. 6e, a cryogel with shape-memory properties was used to achieve injectability, enabling the patch to be compressed within the syringe and then expanded to its original shape upon reaching body temperature206. This mechanism facilitates the minimally invasive delivery of large-area patches for cardiac applications. In vivo, this approach improved cardiac function in myocardial infarction models by enhancing electrical conductivity and supporting tissue repair. Similarly, the hydrogel patch shown in Fig. 6f employs a mesh-like structure to optimize the pressure distribution during injection, ensuring uniform expansion and adhesion to extensive areas, such as the atrium of the heart207. This study utilized a double-network hydrogel composed of PVA and PEDOT:PSS, which provided high electrical conductivity (~12.5 mS/cm) and excellent mechanical durability with a stretchability of ~125% and compressibility of ~70%. Additionally, the mesh-like structure of the hydrogel patch exhibited shape-memory properties and maintained its structural integrity over 10,000 cycles of systolic and diastolic deformation, ensuring long-term stability in dynamic cardiac environments. This approach successfully reduced atrial fibrillation duration in rabbit models. Both studies incorporated fibrin glue to ensure stable attachment of the patches to the heart, with the patches remaining securely affixed for 4 weeks, demonstrating their potential for long-term cardiac applications.
Although hydrogel patches have proven to be feasible as neural interfaces and injectable patches, challenges such as long-term stability and swelling-related issues remain in complex electronic applications204,205,206,207. To address these limitations, researchers have explored stiffer non-hydrogel materials for minimally invasive delivery to target tissues.
An ultrathin (<1 μm) gold and polymer composite mesh electronic device was developed for syringe injections into the rat brain to enable EEG signal acquisition (Fig. 6g)208. The open macroporous structure of the device enabled it to be loaded into a fine-gauge needle; upon injection, it unfolded and conformed to the surrounding neural tissue. This seamless integration facilitated stable, inflammation-free neural recordings for 5 weeks, demonstrating the feasibility of soft, injectable electronics for chronic neural monitoring.
To achieve minimally invasive neural interfacing in the visual system, 16-channel mesh-like electrodes were injected into a rat eye using a noncoaxial intravitreal injection scheme. This method allowed the device to unroll inside the eye and conformally coat the highly curved retina while reducing mechanical mismatch (Fig. 6h)209. The mesh established a stable interface with the retinal ganglion cell layer, ensuring long-term integration and stable in vivo recordings of retinal ganglion cells (RGCs) for at least 2 weeks. This study demonstrated the potential of injectable electronics for neural prosthetics and ophthalmic research, enabling continuous monitoring of RGC activity in response to natural visual stimulation and circadian rhythm changes.
For real-time multimodal physiological monitoring, a multifunctional mesh device integrating temperature, pressure, and neural sensors is implanted on the brain surface (Fig. 6i)210. The mesh was injected in a compressed state and expanded upon deployment, where the elastic restoring force of the mesh structure facilitated its unfolding, ensuring conformal contact with the cortical surface. The flexible polymer substrate embedded with graphene-based multi-channel electrodes and MoS2-based nanosensors enabled continuous, high-resolution monitoring of ECoG signals, intracranial temperature (ICT), and intracranial pressure (ICP), while also allowing electrical stimulation of neural tissues, demonstrating the potential of injectable bioelectronic platforms. In vivo experiments in a rabbit model confirmed that the device effectively recorded epileptic discharges from the cortical surface and provided real-time electrical stimulation to mitigate seizures, while simultaneously tracking intracranial temperature and pressure dynamics.
A biodegradable injectable mesh device was developed to eliminate the need for surgical removal (Fig. 6j)211. Constructed from PLCL-PLGA shape-memory polymers, the device remained compact during injection and subsequently expanded to ~200 times its original size, forming a conformal interface with the neural tissue. This self-deployable mechanism was enabled by programmable shape-memory properties, allowing the mesh to undergo controlled expansion upon reaching physiological temperatures. Over 150 days, the device gradually degraded through hydrolysis, with minimal inflammatory response observed in vivo. Throughout its functional lifetime, the device maintained high-fidelity ECoG signal acquisition with an SNR of ~12 dB, supporting stable neural recordings for at least 2 weeks. This study highlights the potential of transient bioresorbable implants that provide reliable monitoring before natural dissolution, thereby reducing the risks associated with permanent implantation.
Beyond mesh structures, strategies utilizing the internal forces within the device have been explored for in vivo expansion189,190,191,192. Devices that incorporate fluidic chambers use internal pressure to facilitate expansion within biological tissues. For example, an inverted patch was unfolded under pressure to cover the cortical surface for large-scale ECoG signal recording (Fig. 6k)212. This approach involves soft robotic actuation driven by fluid pressure, enabling the patch to be deployed through a minimally invasive burr hole (~20 mm in diameter) rather than requiring a large craniotomy. The eversion-based deployment allows the device to gently unfold and conform to the curvature of the brain, maximizing cortical coverage while minimizing surgical trauma. Furthermore, the system integrates built-in strain sensors that provide real-time feedback during deployment, ensuring precise positioning and stability of the electrode array. This scalable technique demonstrates the potential for large-area, high-resolution neural recording without extensive tissue disruption.
Similarly, an origami-structured device was designed to expand under pressure for ECoG acquisition (Fig. 6l)213. The device achieved injectability by being delivered in a compact, pre-folded configuration and subsequently unfolded upon the application of controlled pressurization. This approach enables the delivery of a large-area electrode array through a minimally invasive opening while maintaining a low profile during insertion. In vivo demonstrations using animal models confirmed that the unfolded electrode array effectively covered the cortical surface, ensuring stable and high-resolution electrophysiological recordings.
Shape memory alloys (SMAs) have also been utilized to facilitate in-situ expansion (Fig. 6m)214. In this study, SMA wires were embedded along the periphery of a crumpled electrode device, enabling it to remain compact during injection and then unfold upon activation by body temperature or an external stimulus. This technique enables precise placement of neural recording electrodes while ensuring minimal trauma during delivery. In vivo testing demonstrated that the expanded device successfully acquired ECoG signals, providing a scalable method for minimally invasive brain-computer interface applications.
Despite these advancements, one limitation of injectable electronic patches has been the lack of adhesive layers, which restricts stable long-term operation at the target site. Among the approaches that have addressed this limitation, a syringe-injectable bioadhesive pacing lead has been developed, enabling stable epicardial attachment via physical and covalent interactions for continuous cardiac monitoring (Fig. 6n)215. The bioadhesive pacing lead, fabricated using a 3D-printed bioadhesive interface composed of polyacrylic acid–grafted polyurethane (PAA-PU), demonstrated strong epicardial adhesion, with a shear strength of 49 kPa (nonconductive) and 36 kPa (conductive), capable of lifting 400 g of porcine heart ex vivo. Furthermore, it maintained stable sensing amplitude and capture threshold for 2 weeks in rodent and porcine models, while exhibiting robust electrophysiological performance with a charge injection capacity of over 450 μC/cm², significantly outperforming commercially available pacing leads (145 μC/cm²).
Taken together, these studies demonstrate the versatility of minimally invasive syringe-injectable devices tailored to specific tissue environments. Ongoing research in this field continues to push the boundaries of materials science, sensor integration, and device design toward broader applications in bioelectronics.
Conclusion and future perspectives
Recent advancements in bioelectronic devices have focused on addressing the challenges of mechanical mismatch with biological tissues, ensuring long-term adhesion, and enabling minimally invasive large-area signal acquisition. However, the existing technologies still face significant limitations. As shown in Fig. 7a, brain applications using rigid patch electrodes fail to achieve conformal contact on the curved surface of the brain, leading to compromised signal quality. Additionally, the lack of adhesive layers causes the devices to lose their initial positions over time. Invasive electrodes, while effective for signal acquisition, create sustained inflammatory responses owing to a large modulus mismatch with brain tissue, and their non-deployable nature restricts their application to the exposed area during open craniotomy. Similarly, as shown in Fig. 7b, the heart patches also suffer from the absence of adhesive layers, which require sutures for fixation. This leads to patch movement caused by the continuous cardiac motion and inflammation at the suture sites. Furthermore, its non-deployable design limits its application to the exposed epicardial area during open-heart surgery.
a Rigid brain patch electrodes fail to adapt to the brain’s curvature, leading to poor signal quality and instability. The absence of an adhesive layer results in displacement from the intended position, disrupting continuous monitoring. Modulus mismatching between implanted devices and brain tissue induces inflammatory responses. b Conventional cardiac patches require sutures for fixation, which can lead to displacement due to continuous cardiac motion and localized inflammation. Their non-deployable design further restricts their application to open-heart surgery. c A perspective schematic illustration of a minimally invasive brain patch that is injectable into the intracranial space and deployable upon delivery, enabling large-area expansion while maintaining modulus matching with brain tissue. The soft structure allows conformal contact, while the adhesive layer ensures precise positioning and stable interfacing for effective signal acquisition. d A perspective schematic illustration of a minimally invasive cardiac patch that is injectable into the thoracic cavity and deployable upon delivery, enabling large-area expansion while maintaining modulus matching with the heart tissue. The soft structure allows conformal contact, while the adhesive layer ensures precise positioning and stable interfacing, even during dynamic cardiac motion, leading to reliable electrophysiological monitoring.
To overcome these limitations, future bioelectronic device developments should prioritize designs that incorporate modulus matching, adhesive layers, minimally invasive approaches, and deployable configurations. As shown in Fig. 7c, minimally invasive strategies involving injectable and deployable patches enable devices to be inserted through small incisions using catheter-like conduits. These patches conformed to the curved surface of the brain, achieving intimate contact through the adhesive layers, which ensured positional stability over time. Deployable designs enable patches to expand over large areas, facilitating signal acquisition or stimulation across broader regions. Similarly, as shown in Fig. 7d, heart patches can adopt this approach, in which an injectable and deployable design permits insertion through minimally invasive procedures compatible with endoscopic surgery. Injectable systems utilizing internal expansion forces facilitate deployment upon delivery, with mechanisms such as fluidic channels and shape-memory materials enabling controlled expansion. Meanwhile, adhesive layers and modulus matching ensure conformal integration with dynamic tissue surfaces. These features maintain device stability even during continuous cardiac motion and prevent tissue damage caused by mechanical mismatches. These bioelectronic platforms represent a transformative step towards overcoming the shortcomings of existing technologies by offering improved long-term stability and high signal quality. These innovations are expected to play a pivotal role in the diagnosis and treatment of neurological, cardiac, and other complex diseases.
In parallel with these design considerations, wireless communication, robust power delivery, and biodegradability will be key areas of focus for future bioelectronic devices. Wireless communication can eliminate the risks of infection and mechanical stress often associated with wired connections, while providing reliable real-time data transmission. A stable and long-lasting power supply—such as wireless power transfer or on-device energy harvesting—is equally important to maintain device functionality without frequent battery replacements or cumbersome external wiring. Moreover, integrating biodegradable materials could allow implants to safely degrade over time, removing the need for additional surgical intervention to retrieve or replace the device. Together, these features will support more patient-friendly, clinically adaptable bioelectronic platforms that align with minimally invasive treatment paradigms.
While many tissue-interfacing bioelectronic platforms have demonstrated robust performance in controlled laboratory conditions, their clinical translation remains limited due to a variety of practical and systemic challenges. This gap often stems from insufficient integration of translational considerations during the early stages of device development. For instance, materials commonly used in research may not meet the reproducibility, purity, or regulatory traceability required for clinical-grade applications. Moreover, biocompatibility testing often focuses on short-term in vitro and in vivo assays, while chronic implantation demands long-term, physiologically relevant studies that assess immune response, degradation byproducts, and tissue remodeling. In addition, the complex architectures of soft, multi-material bioelectronics present challenges for large-scale manufacturing, requiring novel fabrication workflows that can ensure precision, scalability, and compatibility with sterilization and packaging requirements. Finally, clinical trial readiness (e.g., biomarker validation, patient cohort design, data interpretation strategies, and early interaction with regulatory agencies) must be built into the development timeline rather than added post hoc. To facilitate the clinical translation of tissue-interfacing bioelectronics, future efforts should focus on strategically establishing a holistic development pathway that integrates device innovation with material standardization, predictive safety and performance evaluation, scalable production, and clinical adoption strategies. Future research should focus on advancing material selection, device engineering, and integration strategies to develop versatile and adaptable bioelectronic devices capable of satisfying the diverse demands of various tissue environments, while ensuring that these developments are aligned with the practical requirements of clinical translation, including manufacturability, long-term biocompatibility, and regulatory readiness.
Data Availability
No datasets were generated or analysed during the current study.
References
Rivnay, J., Owens, R. M. & Malliaras, G. G. The rise of organic bioelectronics. Chem. Mat. 26, 679–685 (2014).
Rogers, J., Bao, Z. & Lee, T.-W. Wearable bioelectronics: opportunities for chemistry. Acc. chem. Res. 52, 521–522 (2019).
Li, Y., Veronica, A., Ma, J. & Nyein, H. Y. Y. Materials, structure, and interface of stretchable interconnects for wearable bioelectronics. Adv. Mater. https://doi.org/10.1002/adma.202408456 (2024).
Berggren, M. & Richter-Dahlfors, A. Organic bioelectronics. Adv. Mater. 19, 3201–3213 (2007).
Willner, I., Baron, R. & Willner, B. Integrated nanoparticle–biomolecule systems for biosensing and bioelectronics. Biosens. Bioelectron. 22, 1841–1852 (2007).
Zhang, X. et al. Flexible temperature sensor with high reproducibility and wireless closed-loop system for decoupled multimodal health monitoring and personalized thermoregulation. Adv. Mater. 36, 2407859 (2024).
Zhang, C. W. et al. Hydrogel-based soft bioelectronics for personalized healthcare. Med. X 2, 20 (2024).
Yao, G., Gan, X. & Lin, Y. Flexible self-powered bioelectronics enables personalized health management from diagnosis to therapy. Sci. Bull. 69, 2289−2306 (2024).
Yao, G. et al. Flexible bioelectronics for physiological signals sensing and disease treatment. J. Materiomics 6, 397–413 (2020).
Ghorbanizamani, F., Moulahoum, H., Guler Celik, E. & Timur, S. Material design in implantable biosensors toward future personalized diagnostics and treatments. Appl. Sci. 13, 4630 (2023).
Vaghasiya, J. V., Mayorga-Martinez, C. C. & Pumera, M. Wearable sensors for telehealth based on emerging materials and nanoarchitectonics. npj Flex. Electron. 7, 26 (2023).
Wang, Y. et al. Skin bioelectronics towards long-term, continuous health monitoring. Chem. Soc. Rev. 51, 3759–3793 (2022).
Li, J., Fang, Z., Wei, D. & Liu, Y. Flexible pressure, humidity, and temperature sensors for human health monitoring. Adv. Healthc. Mater. 13, 2401532 (2024).
Mirzajani, H. & Kraft, M. Soft bioelectronics for heart monitoring. Acs Sensors 9, 4328–4363 (2024).
Lyu, X. et al. Hydrogel bioelectronics for health monitoring. Biosens 13, 815 (2023).
Zhang, Y., Chen, H. & Song, Y. Wearable healthcare monitoring and therapeutic bioelectronics. Wearable Electron. 2, 18−22 (2025).
Yang, A. & Yan, F. Flexible electrochemical biosensors for health monitoring. ACS Appl. Electron. Mater. 3, 53–67 (2020).
Fang, Y. et al. Recent advances in bioelectronics chemistry. Chem. Soc. Rev. 49, 7978–8035 (2020).
Huang, Y. et al. Bioelectronics for electrical stimulation: materials, devices and biomedical applications. Chem. Soc. Rev. 53, 8632–8712 (2024).
Chavan, S. S., Pavlov, V. A. & Tracey, K. J. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 46, 927–942 (2017).
Pitsalidis, C. et al. Organic bioelectronics for in vitro systems. Chem. Rev. 122, 4700–4790 (2021).
Yakoh, A., Siangproh, W., Chailapakul, O. & Ngamrojanavanich, N. Optical bioelectronic device based on a screen-printed electroluminescent transducer. ACS Appl. Mater. Interfaces 12, 22543–22551 (2020).
Schiavone, G. & Lacour, S. P. Conformable bioelectronic interfaces: mapping the road ahead. Sci. Transl. Med. 11, eaaw5858 (2019).
Sunwoo, S.-H., Ha, K.-H., Lee, S., Lu, N. & Kim, D.-H. Wearable and implantable soft bioelectronics: device designs and material strategies. Annu Rev. Chem. Biomol. Eng. 12, 359–391 (2021).
Shin, Y., Lee, H. S., Jeong, H. & Kim, D.-H. Recent advances in conductive hydrogels for soft biointegrated electronics: materials, properties, and device applications. Wearable Electron. 1, 255–280 (2024).
Ohayon, D. & Inal, S. Organic bioelectronics: from functional materials to next-generation devices and power sources. Adv. Mater. 32, 2001439 (2020).
Park, J. et al. Conformal fixation strategies and bioadhesives for soft bioelectronics. Adv. Func. Mater 34, 2313728 (2024).
Sim, K. et al. An epicardial bioelectronic patch made from soft rubbery materials and capable of spatiotemporal mapping of electrophysiological activity. Nat. Electron. 3, 775–784 (2020).
Li, Y. et al. Achieving tissue-level softness on stretchable electronics through a generalizable soft interlayer design. Nat. Commun. 14, 4488 (2023).
Cho, K. W. et al. Soft bioelectronics based on nanomaterials. Chem. Rev. 122, 5068–5143 (2021).
Yin, J., Wang, S., Tat, T. & Chen, J. Motion artefact management for soft bioelectronics. Nat. Rev. Bioeng. 2, 541–558 (2024).
Kwon, S. et al. Skin-conformal, soft material-enabled bioelectronic system with minimized motion artifacts for reliable health and performance monitoring of athletes. Biosens. Bioelectron. 151, 111981 (2020).
Fallegger, F., Schiavone, G. & Lacour, S. P. Conformable hybrid systems for implantable bioelectronic interfaces. Adv. Mater. 32, 1903904 (2020).
Zhao, C., Park, J., Root, S. E. & Bao, Z. Skin-inspired soft bioelectronic materials, devices and systems. Nat. Rev. Bioeng. 2, 671–690 (2024).
Shao, Y. et al. A universal packaging substrate for mechanically stable assembly of stretchable electronics. Nat. Commun. 15, 6106 (2024).
Tringides, C. M. et al. Viscoelastic surface electrode arrays to interface with viscoelastic tissues. Nat. Nanotechnol. 16, 1019–1029 (2021).
Picca, R. A. et al. Ultimately sensitive organic bioelectronic transistor sensors by materials and device structure design. Adv. Funct. Mater. 30, 1904513 (2020).
Babamiri, B., Bahari, D. & Salimi, A. Highly sensitive bioaffinity electrochemiluminescence sensors: recent advances and future directions. Biosens. Bioelectron. 142, 111530 (2019).
Someya, T., Bao, Z. & Malliaras, G. G. The rise of plastic bioelectronics. Nature 540, 379–385 (2016).
Mariello, M., Kim, K., Wu, K., Lacour, S. P. & Leterrier, Y. Recent advances in encapsulation of flexible bioelectronic implants: materials, technologies, and characterization methods. Adv. Mater. 34, 2201129 (2022).
Li, Y., Li, N., De Oliveira, N. & Wang, S. Implantable bioelectronics toward long-term stability and sustainability. Matter 4, 1125–1141 (2021).
Goding, J. A., Gilmour, A. D., Aregueta-Robles, U. A., Hasan, E. A. & Green, R. A. Living bioelectronics: strategies for developing an effective long-term implant with functional neural connections. Adv. Funct. Mater. 28, 1702969 (2018).
An, D. et al. Embracing plasticity: unlocking the full potential of flexible and stretchable electronics through the elastoplastic behavior of metallic materials. Adv. Funct. Mater. 35, 2412796 (2024).
Fang, Y. et al. Mechanical design principles of conductive gels applied for flexible electronics. Adv. Funct. Mater. 35, 2416398 (2024).
Choi, C., Lee, Y., Cho, K. W., Koo, J. H. & Kim, D.-H. Wearable and implantable soft bioelectronics using two-dimensional materials. Acc. Mater. Res. 52, 73–81 (2018).
Wang, Y. et al. Multifunctional nano-conductive hydrogels with high mechanical strength, toughness and fatigue resistance as self-powered wearable sensors and deep learning-assisted recognition system. Adv.Funct.Mater. 34, 2409081 (2024).
Xu, C., Solomon, S. A. & Gao, W. Artificial intelligence-powered electronic skin. Nat. Mach. Intel. 5, 1344–1355 (2023).
Someya, T. & Amagai, M. Toward a new generation of smart skins. Nat. Biotechnol. 37, 382–388 (2019).
Sempionatto, J. R., Lasalde-Ramírez, J. A., Mahato, K., Wang, J. & Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 6, 899–915 (2022).
Qiao, Y. et al. Wearable sensor for continuous sweat biomarker monitoring. Chemosensors 10, 273 (2022).
Souri, H. et al. Wearable and stretchable strain sensors: materials, sensing mechanisms, and applications. Adv. Intell. Syst. 2, 2000039 (2020).
Lu, Y., Biswas, M. C., Guo, Z., Jeon, J.-W. & Wujcik, E. K. Recent developments in bio-monitoring via advanced polymer nanocomposite-based wearable strain sensors. Biosens. Bioelectron. 123, 167–177 (2019).
Liu, X. et al. Recent progress on smart fiber and textile based wearable strain sensors: materials, fabrications and applications. Adv. Fiber Mater. 4, 361–389 (2022).
Han, W. B. et al. Ultra-stretchable and biodegradable elastomers for soft, transient electronics. Nat. commun. 14, 2263 (2023).
Song, E. et al. Miniaturized electromechanical devices for the characterization of the biomechanics of deep tissue. Nat. biomed. eng. 5, 759–771 (2021).
Reddy, P. G. et al. Biodegradable, self-adhesive, stretchable, transparent, and versatile electronic skins based on intrinsically hydrophilic poly (caproactone-urethane) elastomer. Adv. Eng. Mater. 26, 2401704 (2024).
Liu, L. et al. Recent advances in flexible temperature sensors: materials, mechanism, fabrication, and applications. Adv. Sci. 11, 2405003 (2024).
Kuzubasoglu, B. A. & Bahadir, S. K. Flexible temperature sensors: a review. Sensor Actuat A Phys. 315, 112282 (2020).
Meng, K. et al. Wearable pressure sensors for pulse wave monitoring. Adv. Mater. 34, 2109357 (2022).
He, J. et al. A Universal high accuracy wearable pulse monitoring system via high sensitivity and large linearity graphene pressure sensor. Nano Energy 59, 422–433 (2019).
Fu, Y., Zhao, S., Wang, L. & Zhu, R. A wearable sensor using structured silver-particle reinforced PDMS for radial arterial pulse wave monitoring. Adv. Healthc. Mater. 8, 1900633 (2019).
Kwak, Y. H., Kim, W., Park, K. B., Kim, K. & Seo, S. Flexible heartbeat sensor for wearable device. Biosens. Bioelectron. 94, 250–255 (2017).
Chen, S. et al. Flexible wearable sensors for cardiovascular health monitoring. Adv. Healthc. Mater. 10, 2100116 (2021).
Yang, Q. et al. Mixed-modality speech recognition and interaction using a wearable artificial throat. Nat. Mach. Intell. 5, 169–180 (2023).
Lee, S. et al. An ultrathin conformable vibration-responsive electronic skin for quantitative vocal recognition. Nat. Commun. 10, 2468 (2019).
Shen, Y. et al. A highly stretchable and breathable polyurethane fibrous membrane sensor for human motion monitoring and voice signal recognition. Sensor Actuat. A Phys. 331, 112974 (2021).
Kim, J. et al. Wearable bioelectronics: enzyme-based body-worn electronic devices. Acc. Mater. Res. 51, 2820–2828 (2018).
Parrilla, M., Cuartero, M. & Crespo, G. A. Wearable potentiometric ion sensors. TrAC 110, 303–320 (2019).
Li, G. & Wen, D. Wearable biochemical sensors for human health monitoring: sensing materials and manufacturing technologies. J. Mater. Chem. B 8, 3423–3436 (2020).
Huang, Y., Su, Y. & Jiang, S. Neutral layer design for flexible electronics. Flexible Electron. 321, 349 (2022).
Ye, C., Lukas, H., Wang, M., Lee, Y. & Gao, W. Nucleic acid-based wearable and implantable electrochemical sensors. Chem. Soc. Rev. 53, 7960−7982 (2024).
Ok, J., Park, S., Jung, Y. H. & Kim, T. i. Wearable and implantable cortisol-sensing electronics for stress monitoring. Adv. Mater. 36, 2211595 (2024).
Zhao, Y. et al. A wearable freestanding electrochemical sensing system. Sci. Adv. 6, eaaz0007 (2020).
Zhao, H. et al. An integrated wearable sweat sensing patch for passive continuous analysis of stress biomarkers at rest. Adv. Funct. Mater. 33, 2212083 (2023).
Yu, Y., Nyein, H. Y. Y., Gao, W. & Javey, A. Flexible electrochemical bioelectronics: the rise of in situ bioanalysis. Adv. Mater. 32, 1902083 (2020).
Liu, Z. et al. Wearable systems of reconfigurable microneedle electrode array for subcutaneous multiplexed recording of myoelectric and electrochemical signals. Adv. Sci. https://doi.org/10.1002/advs.202409075 (2024).
Mirbakht, S. S. Highly self-adhesive and biodegradable silk bioelectronics for all-in-one imperceptible long-term electrophysiological biosignals monitoring. Adv. Sci 12, 2405988 (2025).
Nemati, E., Deen, M. J. & Mondal, T. A wireless wearable ECG sensor for long-term applications. IEEE Commun. Mag. 50, 36–43 (2012).
Kim, C. S. et al. Self-powered wearable electrocardiography using a wearable thermoelectric power generator. ACS Energy Lett 3, 501–507 (2018).
Tan, P. et al. Solution-processable, soft, self-adhesive, and conductive polymer composites for soft electronics. Nat. Commun. 13, 358 (2022).
Liu, D. et al. A wearable in-sensor computing platform based on stretchable organic electrochemical transistors. Nat. Electron. 7, 1176–1185 (2024).
Ye, H., Wu, B., Sun, S. & Wu, P. Self-compliant ionic skin by leveraging hierarchical hydrogen bond association. Nat. commun. 15, 885 (2024).
Zhu, P. et al. Skin-electrode iontronic interface for mechanosensing. Nat. Commun. 12, 4731 (2021).
Yoon, H. et al. Decoding tissue biomechanics using conformable electronic devices. Nat. Rev. Mater. 10, 4–27 (2025).
Dagdeviren, C. et al. Conformable piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat. Mater. 14, 728–736 (2015).
Chen, Y. et al. Flexible inorganic bioelectronics. npj Flexible Electronics 4, 2 (2020).
Kim, H. et al. Fully integrated, stretchable, wireless skin-conformal bioelectronics for continuous stress monitoring in daily life. Adv. Sci. 7, 2000810 (2020).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Jeong, J. et al. Materials and optimized designs for human-machine interfaces via epidermal electronics. Adv. Mater. 25, 6839–6846 (2013).
Lee, S. et al. A transparent bending-insensitive pressure sensor. Nat. Nanotechnol. 11, 472–478 (2016).
Inal, S., Rivnay, J., Suiu, A.-O., Malliaras, G. G. & McCulloch, I. Conjugated polymers in bioelectronics. Acc. Mater. Res. 51, 1368–1376 (2018).
Malliaras, G. G. Organic bioelectronics: a new era for organic electronics. Biochimica. Biophysica. Acta (BBA) General Subjects 1830, 4286–4287 (2013).
Sugiyama, M. et al. An ultraflexible organic differential amplifier for recording electrocardiograms. Nat. Electron. 2, 351–360 (2019).
Koo, J. H. et al. Wearable electrocardiogram monitor using carbon nanotube electronics and color-tunable organic light-emitting diodes. ACS Nano 11, 10032–10041 (2017).
Wu, M. et al. Ultrathin, soft, bioresorbable organic electrochemical transistors for transient spatiotemporal mapping of brain activity. Adv.Sci. 10, 2300504 (2023).
Nishinaka, M. et al. High-transconductance organic electrochemical transistor fabricated on ultrathin films using spray coating. Small Structures 2, 2000088 (2021).
Takemoto, A. et al. Fully transparent, ultrathin flexible organic electrochemical transistors with additive integration for bioelectronic applications. Adv. Sci. 10, 2204746 (2023).
Lee, I. et al. Ultraflexible vertical corbino organic electrochemical transistors for epidermal signal monitoring. Adv. Mater. 37, e2410444 (2024).
Kim, C. H., Lee, D. H., Youn, J., Lee, H. & Jeong, J. Simple and cost-effective microfabrication of flexible and stretchable electronics for wearable multi-functional electrophysiological monitoring. Sci. Rep. 11, 14823 (2021).
Liang, X. et al. Advanced stretchable aerogels and foams for flexible electronics and beyond. Adv. Funct. Mater. 34, 2408707 (2024).
Ling, Y. et al. Bioinspired elastomer composites with programmed mechanical and electrical anisotropies. Nat. Commun. 13, 524 (2022).
Kim, S. H. et al. Mechanically and electrically durable, stretchable electronic textiles for robust wearable electronics. RSC adv 11, 22327–22333 (2021).
Pu, J., Wang, X., Xu, R. & Komvopoulos, K. Highly stretchable microsupercapacitor arrays with honeycomb structures for integrated wearable electronic systems. ACS Nano 10, 9306–9315 (2016).
Jung, D. et al. Multilayer stretchable electronics with designs enabling a compact lateral form. npj Flex. Electron. 8, 13 (2024).
Li, R., Li, M., Su, Y., Song, J. & Ni, X. An analytical mechanics model for the island-bridge structure of stretchable electronics. Soft Matter 9, 8476–8482 (2013).
Yan, Z. et al. Hierarchical serpentine-helix combination for 3D stretchable electronics. Adv. Mater. 35, 2210238 (2023).
Fan, J. A. et al. Fractal design concepts for stretchable electronics. Nat. Commun. 5, 3266 (2014).
Kim, D.-H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Khang, D.-Y., Jiang, H., Huang, Y. & Rogers, J. A. A stretchable form of single-crystal silicon for high-performance electronics on rubber substrates. Science 311, 208–212 (2006).
Lee, S. et al. Nanomesh pressure sensor for monitoring finger manipulation without sensory interference. Science 370, 966–970 (2020).
Jiang, Y. et al. A universal interface for plug-and-play assembly of stretchable devices. Nature 614, 456–462 (2023).
Yin, L. et al. A stretchable epidermal sweat sensing platform with an integrated printed battery and electrochromic display. Nat. Electron. 5, 694–705 (2022).
Kim, S. et al. An intrinsically stretchable multi-biochemical sensor for sweat analysis using photo-patternable ecoflex. npj Flex. Electron. 7, 33 (2023).
Liu, S., Shah, D. S. & Kramer-Bottiglio, R. Highly stretchable multilayer electronic circuits using biphasic gallium-indium. Nat. Mater. 20, 851–858 (2021).
Zhuang, Q. et al. Permeable, three-dimensional integrated electronic skins with stretchable hybrid liquid metal solders. Nat. Electron. 7, 598–609 (2024).
Yang, J. et al. Ultrasoft liquid metal elastomer foams with positive and negative piezopermittivity for tactile sensing. Adv. Funct. Mater. 30, 2002611 (2020).
Hui, Y. et al. Three-dimensional printing of soft hydrogel electronics. Nat. Electron. 5, 893–903 (2022).
He, H. et al. Strong and high-conductivity hydrogels with all-polymer nanofibrous networks for applications as high-capacitance flexible electrodes. npj Flex. Electron. 8, 56 (2024).
He, H. et al. Hybrid assembly of polymeric nanofiber network for robust and electronically conductive hydrogels. Nat. Commun. 14, 759 (2023).
Kim, S. et al. Injection-on-skin granular adhesive for interactive human–machine interface. Adv. Mater. 35, 202307070 (2023).
Roy, A. et al. A highly stretchable, conductive, and transparent bioadhesive hydrogel as a flexible sensor for enhanced real-time human health monitoring. Adv. Mater. 36, 202404225 (2024).
Zhang, S. et al. Constructing electronic/ionic-conductive hydrogel with soft compliance and high conductivity as flexible strain sensor. Adv. Eng. Mater. 26, 202400697 (2024).
Yuk, H., Lu, B. & Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 48, 1642–1667 (2019).
Chae, A. et al. Highly oxidation-resistant and self-healable MXene-based hydrogels for wearable strain sensor. Adv. Funct. Mater. 33, 202213382 (2023).
Guo, H. et al. Universally autonomous self-healing elastomer with high stretchability. Nat. Commun. 11, 2037 (2020).
Zhang, W. et al. Skin-like mechanoresponsive self-healing ionic elastomer from supramolecular zwitterionic network. Nat. Commun. 12, 4082 (2021).
Wang, J. et al. Fatigue-free artificial ionic skin toughened by self-healable elastic nanomesh. Nat. Commun. 13, 4411 (2022).
Boahen, E. K. et al. Ultrafast, autonomous self-healable iontronic skin exhibiting piezo-ionic dynamics. Nat. Commun. 13, 7699 (2022).
Jung, J. et al. Self-healing electronic skin with high fracture strength and toughness. Nat. Commun. 15, 9763 (2024).
Liu, M. et al. An extreme environment capable self-healing single active layered triboelectric sensors as fully recyclable and transparent human-machine interfaces. Adv. Funct. Mater. 35, 202414152 (2024).
Bandodkar, A. J. et al. All-printed magnetically self-healing electrochemical devices. Sci. Adv. 2, e1601456 (2016).
Ying et al. Waterproof, highly tough, and fast self-healing polyurethane for durable electronic skin. ACS Appl. Mater. Interfaces 12, 11072–11083 (2020).
Son, D. et al. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotechnol. 13, 1057–1065 (2018).
Song, E. et al. Materials for flexible bioelectronic systems as chronic neural interfaces. Nat. Mater. 19, 590–603 (2020).
Zheng, Y. et al. Environmentally stable and stretchable polymer electronics enabled by surface-tethered nanostructured molecular-level protection. Nat. Nanotechnol. 18, 1175–1184 (2023).
Lee, Y. et al. A lubricated nonimmunogenic neural probe for acute insertion trauma minimization and long-term signal recording. Adv. Sci. 8, 2100231 (2021).
Lee, J. et al. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat. Commun. 10, 5205 (2019).
Park, J., Lee, Y., Kim, T. Y., Hwang, S. & Seo, J. Functional bioelectronic materials for long-term biocompatibility and functionality. ACS Appl. Electron. Mater. 4, 1449–1468 (2022).
Lim, C. et al. Tissue-like skin-device interface for wearable bioelectronics by using ultrasoft, mass-permeable, and low-impedance hydrogels. Sci. Adv. 7, eabd3716 (2021).
Feron, K. et al. Organic bioelectronics: materials and biocompatibility. Int. J. Mol. Sci. 19, 2382 (2018).
Lin, M. et al. Soft wearable devices for deep-tissue sensing. Nat Rev Mater 7, 850–869 (2022).
Song, E. et al. Recent advances in microsystem approaches for mechanical characterization of soft biological tissues. Microsyst. Nanoeng. 8, 77 (2022).
Guimarães, C. F. et al. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
Yang, Q. et al. Photocurable bioresorbable adhesives as functional interfaces between flexible bioelectronic devices and soft biological tissues. Nat. Mater. 20, 1559–1570 (2021).
Cho, Y. H., Park, Y., Kim, S. & Park, J. 3D Electrodes for bioelectronics. Adv. Mater. 33, 2005805 (2021).
Xie, X. et al. Long-term reliability of Al2O3and Parylene C bilayer encapsulated Utah electrode array based neural interfaces for chronic implantation. J. Neural Eng. 11, 026016 (2014).
Christensen, M. B., Wark, H. A. C. & Hutchinson, D. T. A histological analysis of human median and ulnar nerves following implantation of Utah slanted electrode arrays. Biomater 77, 235–242 (2016).
Yan, D. et al. Ultraflexible and stretchable intrafascicular peripheral nerve recording device with axon-dimension, cuff-less microneedle electrode array. Small 18, 2200311 (2022).
Kim, C.-S. et al. Use of micromachined probes for the recording of cardiac electrograms in isolated heart tissues. Biosens. Bioelectron. 19, 1109–1116 (2004).
Jun, J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Yuk, H. et al. 3D printing of conducting polymers. Nat. Commun. 11, 1604 (2020).
Kim, J. H. et al. Flexible deep brain neural probe for localized stimulation and detection with metal guide. Biosens. Bioelectron. 117, 436–443 (2018).
Guan, S. et al. Self-assembled ultraflexible probes for long-term neural recordings and neuromodulation. Nat. Protoc. 18, 1712–1744 (2023).
Chik, G. et al. Flexible multichannel neural probe developed by electropolymerization for localized stimulation and sensing. Adv. Mater. Technol. 7, 2200143 (2022).
Boretius, T. et al. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens Bioelectron. 26, 62–69 (2010).
Le Floch et al. 3D spatiotemporally scalable in vivo neural probes based on fluorinated elastomers. Nat. Nanotechnol. 19, 319–329 (2024).
Lee, J. Y. et al. Foldable three dimensional neural electrode arrays for simultaneous brain interfacing of cortical surface and intracortical multilayers. npj Flex. Electron. 6, 86 (2022).
Apollo, N. V. et al. Gels, jets, mosquitoes, and magnets: a review of implantation strategies for soft neural probes. J. of Neural Eng. 17, 041002 (2020).
Muthuswamy, J., Okandan, M., Jain, T. & Gilletti, A. Electrostatic microactuators for precise positioning of neural microelectrodes. IEEE Trans. Biomed. Eng. 52, 1748–1755 (2005).
Chen, P.-C. & Lal, A. Detachable ultrasonic enabled inserter for neural probe insertion using biodissolvable polyethylene glycol. In 2015 Transducers—2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS) 125–128 (IEEE, 2015).
Vitale, F. et al. Fluidic microactuation of flexible electrodes for neural recording. Nano Lett. 18, 326–335 (2017).
Dryg, I. D. et al. Magnetically inserted neural electrodes: tissue response and functional lifetime. IEEE Trans. Neural Syst. Rehabilitation Eng. 23, 562–571 (2015).
Shoffstall, A. J. & Capadona, J. R. Bioinspired materials and systems for neural interfacing. Curr. Opin. Biomed. Eng. 6, 110–119 (2018).
Athanasiadis et al. Printed elastic membranes for multimodal pacing and recording of human stem-cell-derived cardiomyocytes. npj Flex. Electron. 4, 16 (2020).
Rocha-Flores, P. E. et al. Softening, conformable, and stretchable conductors for implantable bioelectronics interfaces. Adv. Mater. Technol. 10, 2401047 (2024).
Tchoe, Y. et al. An electroencephalogram microdisplay to visualize neuronal activity on the brain surface. Sci. Transl. Med. 16, eadj7257 (2024).
Viventi, J. et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 14, 1599–1605 (2011).
Bong, J. et al. Flexible, multichannel cuff electrode for selective electrical stimulation of the mouse trigeminal nerve. Biosens. Bioelectron. 142, 111493 (2019).
Carnicer-Lombarte, A. et al. Ultraconformable cuff implants for long-term bidirectional interfacing of peripheral nerves at sub-nerve resolutions. Nat. Commun. 15, 7523 (2024).
Zhou, T. et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 22, 895–902 (2023).
Yu, X. et al. Highly stretchable, ultra-soft, and fast self-healable conductive hydrogels based on polyaniline nanoparticles for sensitive flexible sensors. Adv. Funct. Mater. 32, 2204366 (2022).
Wang, Y. et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 3, e1602076 (2017).
Jiang, Y. et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 375, 1411–1417 (2022).
Hansen, T. S., West, K., Hassager, O. & Larsen, N. B. Highly stretchable and conductive polymer material made from poly(3,4-ethylenedioxythiophene) and polyurethane elastomers. Adv. Funct. Mater. 17, 3069–3073 (2007).
Duan, S., Wang, Z., Zhang, L., Liu, J. & Li, C. Three-dimensional highly stretchable conductors from elastic fiber mat with conductive polymer coating. ACS Appl. Mater. Interfaces 9, 30772–30778 (2017).
Li, W., Li, Y., Song, Z., Wang, Y.-X. & Hu, W. PEDOT-based stretchable optoelectronic materials and devices for bioelectronic interfaces. Chem. Soc. Rev. 53, 10575–10603 (2024).
Lee, S. et al. Stretchable surface electrode arrays using an alginate/PEDOT:PSS-based conductive hydrogel for conformal brain interfacing. Polymers 15, 84 (2022).
Moon, H. et al. Soft, conformal PDMS-based ECoG electrode array for long-term in vivo applications. Sens. Actuators B. Chem. 401, 135099 (2024).
Wang, S. et al. Intrinsically stretchable electronics with ultrahigh deformability to monitor dynamically moving organs. Sci. Adv. 8, eabl5511 (2022).
Yi, J. et al. Water-responsive supercontractile polymer films for bioelectronic interfaces. Nature 624, 295–302 (2023).
Liu, Y. et al. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotechnol. 38, 1031–1036 (2020).
Song, K. I. et al. Adaptive self-healing electronic epineurium for chronic bidirectional neural interfaces. Nat. Commun. 11, 4195 (2020).
Seo, H. et al. Durable and fatigue-resistant soft peripheral neuroprosthetics for in vivo bidirectional signaling. Adv. Mater. 33, 2007346 (2021).
Xie, C. et al. Mussel-inspired hydrogels for self-adhesive bioelectronics. Adv. Funct. Mater. 30, 1909954 (2020).
Wu, J. et al. Adhesive anti-fibrotic interfaces on diverse organs. Nature 630, 360–367 (2024).
Liu, S. et al. Strategies for body-conformable electronics. Matter 5, 1104–1136 (2022).
Yang, Y. et al. Development and applications of mussel-inspired composite hydrogels for flexible bioelectronics. Chem. Eng. J. 474, 145891 (2023).
Yang, Q., Hu, Z. & Rogers, J. A. Functional hydrogel interface materials for advanced bioelectronic devices. Acc. Mater. Res. 2, 1010–1023 (2021).
Oh, B. et al. Ultra-soft and highly stretchable tissue-adhesive hydrogel based multifunctional implantable sensor for monitoring of overactive bladder. Biosens. Bioelectron. 225, 115060 (2023).
Arno, M. C. et al. Exploiting the role of nanoparticle shape in enhancing hydrogel adhesive and mechanical properties. Nat. commun. 11, 1420 (2020).
Baik, J. S. et al. Colloidal supraballs of mesoporous silica nanoparticles as bioresorbable adhesives for hydrogels. Chem. Mater. 34, 584–593 (2022).
Pan, Z. et al. Designing nanohesives for rapid, universal, and robust hydrogel adhesion. Nat. Commun. 14, 5378 (2023).
Yang, J., Ruobing, B. & Zhigang, S. Topological adhesion of wet materials. Adv. Mater. 30, 1800671 (2018).
Yao, L. et al. Autonomous underwater adhesion driven by water-induced interfacial rearrangement. Nat. Commun. 14, 6563 (2023).
Lin, J. et al. Wireless bioelectronics for in vivo pressure monitoring with mechanically-compliant hydrogel biointerfaces. Adv. Mater. 36, 2400181 (2024).
Deng, J. et al. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 20, 229–236 (2021).
Li, N. et al. Bioadhesive polymer semiconductors and transistors for intimate biointerfaces. Science 381, 686–693 (2023).
Li, S., Yang, C. & Jun, F. Tissue adhesive hydrogel bioelectronics. J. Mater. Chem. B 9, 4423–4443 (2021).
Zhu, M. et al. Flexible electrodes for in vivo and in vitro electrophysiological signal recording. Adv. Health. Mater. 10, 2100646 (2021).
Lee, S. et al. A shape-morphing cortex-adhesive sensor for closed-loop transcranial ultrasound neurostimulation. Nat. Electron. 7, 800–814 (2024).
Choi, H. et al. Adhesive bioelectronics for sutureless epicardial interfacing. Nat. Electron. 6, 779–789 (2023).
Cha, G. D. et al. Multifunctional injectable hydrogel for in vivo diagnostic and therapeutic applications. ACS Nano 16, 554–567 (2022).
Kang, T. et al. Penetrative and sustained drug delivery using injectable hydrogel nanocomposites for postsurgical brain tumor treatment. ACS Nano 17, 5435–5447 (2023).
Jin, S. et al. Injectable tissue prosthesis for instantaneous closed-loop rehabilitation. Nature 623, 58–65 (2023).
Trevathan, J. K. et al. An injectable neural stimulation electrode made from an in-body curing polymer/metal composite. Adv. Healthc. Mater. 8, 1900892 (2019).
Wang, L. et al. Injectable and conductive cardiac patches repair infarcted myocardium in rats and minipigs. Nat. Biomed. Eng. 5, 1157–1173 (2021).
Dai, J. et al. Injectable mesh-like conductive hydrogel patch for elimination of atrial fibrillation. Adv. Healthc. Mater. 13, 202303219 (2024).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
Hong, G. et al. A method for single-neuron chronic recording from the retina in awake mice. Science 360, 1447–1451 (2018).
Kim, J. et al. Injectable 2D material-based sensor array for minimally invasive neural implants. Adv. Mater. 36, 202400261 (2024).
Bae, J. Y. et al. A biodegradable and self-deployable electronic tent electrode for brain cortex interfacing. Nat. Electron. 7, 815–828 (2024).
Song, S., Fallegger, F., Trouillet, A., Kim, K. & Lacour, S. P. Deployment of an electrocorticography system with a soft robotic actuator. Sci Robot 8, eadd1002 (2023).
Coles, L. et al. Origami-inspired soft fluidic actuation for minimally invasive large-area electrocorticography. Nat. Commun. 15, 6290 (2024).
Wei, S. et al. Shape-changing electrode array for minimally invasive large-scale intracranial brain activity mapping. Nat. Commun. 15, 715 (2024).
Deng, J. et al. A bioadhesive pacing lead for atraumatic cardiac monitoring and stimulation in rodent and porcine models. Sci. Transl. Med. 16, eado9003 (2024).
Acknowledgements
This study was supported by Institute for Basic Science (Nos. IBS-R015-D1 and IBS-R015-D2) and a grant of the Korea-US Collaborative Research Fund (KUCRF), funded by the Ministry of Science and ICT and Ministry of Health & Welfare, Republic of Korea (grant number: RS-2024-00467213). This study was also supported by the NAVER Digital Bio Innovation Research Fund, funded by NAVER Corporation (Grant No. [37-2023-0040]) and the Korean Fund for Regenerative Medicine(KFRM) grant funded by the Korea government(the Ministry of Science and ICT, the Ministry of Health & Welfare). (code: 23B0102L1).
Author information
Authors and Affiliations
Contributions
J.J., S.J., and S.Y. contributed equally to this work. J.J., S.J., and S.Y. contributed to sections of the article including figures and tables of the sections. D.S. and M.S. conceptualized and supervised the article. J.J., S.J., S.Y., M.S. and D.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jang, J., Jin, S., Yoon, S. et al. Materials strategy and device fabrication for stable closed-loop bioelectronics. npj Biosensing 2, 27 (2025). https://doi.org/10.1038/s44328-025-00045-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44328-025-00045-y
This article is cited by
-
Recent strategies for designing adhesive hydrogels to enhance muscle tissue regeneration
Biomedical Engineering Letters (2026)
-
Conforming strategies for bioelectronics on arbitrary surfaces
npj Biosensing (2025)