Abstract
Chronic liver diseases present complex diagnostic challenges requiring multimodal data integration. Current workflows rely on sequential specialist consultations, creating critical delays. Artificial intelligence agents autonomous systems capable of reasoning and collaborative decision-making represent a transformative approach through distributed problem-solving networks. Multi-agent architectures provide therapeutic decision support across metabolic, cholestatic, and malignant transformations. We present a conceptual framework for clinical implementation.
Similar content being viewed by others
Introduction
Chronic liver diseases encompass a complex continuum spanning metabolic dysfunction-associated steatotic liver disease (MASLD), steatohepatitis (MASH), alcohol-related liver disease (ALD), metabolic-ALD (met-ALD), primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), and their malignant transformations including hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA)1. These conditions share pathophysiological pathways involving hepatic inflammation, metabolic dysregulation, and progressive fibrosis, yet present unique diagnostic and therapeutic challenges requiring sophisticated clinical coordination.
MASLD affects approximately 25% of the global population, surpassing viral hepatitis as the leading chronic liver disease worldwide2. The complex interplay between metabolic and alcohol factors has established met-ALD as a distinct clinical entity separate from pure ALD, with unique prognostic implications3,4. Similarly, cholestatic diseases including PSC and PBC represent distinct autoimmune conditions with different pathophysiological mechanisms and surveillance requirements. Cholestatic diseases, while less prevalent, present particularly challenging scenarios, with PSC patients carrying a 400-fold increased CCA risk compared to the general population5. Traditional clinical workflows necessitate sequential coordination between multiple specialists radiologists, hepatologists, interventional radiologists, pathologists, and molecular geneticists each contributing isolated assessments requiring manual integration and clinical synthesis.
Unlike conventional artificial intelligence systems that operate as static databases or isolated analytical tools, AI agents represent autonomous software systems capable of reasoning, planning, and executing complex clinical tasks through continuous cycles of assessment, adaptation, and collaboration6. These systems form dynamic collaborative networks where individual agents communicate, negotiate solutions, and redistribute computational tasks based on case complexity. True multi-agent systems emerge when agents establish communication protocols enabling distributed problem-solving, where collective intelligence exceeds individual agent capabilities.
AI agents could demonstrate transformative potential for autonomous imaging triage, real-time clinical data integration, dynamic treatment selection, and continuous disease monitoring across the chronic liver disease spectrum. Through standardized inter-agent communication protocols, these systems would enable collaborative reasoning where multiple specialized agents contribute domain expertise to complex diagnostic challenges. When diagnostic uncertainty arises, agents engage in structured deliberation protocols, sharing evidence with quantified confidence scores until consensus emerges or appropriate escalation to human specialists occurs.
Chronic liver disease management challenges
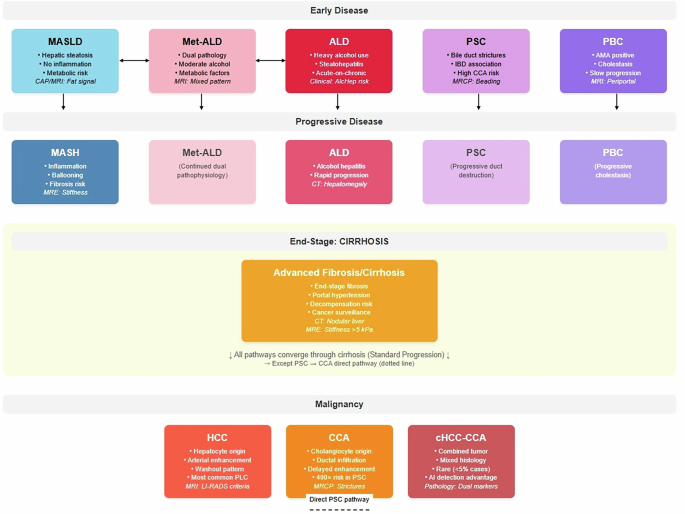
Chronic liver diseases present multifaceted diagnostic and therapeutic challenges that exemplify the need for coordinated clinical decision-making. These conditions share overlapping pathophysiological mechanisms while requiring disease-specific expertise, making them ideal candidates for AI agent coordination systems. Notably, MetALD and ALD represent distinct diagnostic categories rather than progressive stages, as do PSC and PBC among cholestatic diseases (Table 1) (Fig.1).
Comprehensive disease progression model demonstrating parallel and interconnected pathways of chronic liver diseases. The metabolic pathway (MASLD → MASH, blue) represents steatosis-to-steatohepatitis progression driven by obesity, diabetes, and metabolic dysfunction. MetALD (pink) and ALD (red) are shown as separate parallel conditions rather than sequential progression, reflecting current nomenclature where MetALD represents concurrent metabolic and alcohol-related factors with moderate alcohol consumption, while ALD involves heavy alcohol use with acute-on-chronic hepatitis risk. These conditions may transition bidirectionally based on drinking pattern changes but represent distinct diagnostic entities. Cholestatic diseases (PSC and PBC, purple shades) are presented as separate parallel autoimmune conditions, each with distinct pathophysiology PSC characterized by bile duct strictures and IBD association, PBC by AMA-positive status and slower progression. PSC carries exceptional malignant transformation risk (400-fold increased CCA risk). All pathways converge through cirrhosis (orange) to primary liver cancers, with the critical exception that PSC demonstrates direct cholangiocarcinoma development (dashed arrow) independent of cirrhosis. Malignant endpoints include hepatocellular carcinoma (HCC, arising from hepatocytes), cholangiocarcinoma (CCA, from cholangiocytes), and rare combined tumors (cHCC-CCA, <5% of cases) exhibiting mixed histology detectable through AI pathology. Molecular stratification spans metabolic risk genes (PNPLA3, TM6SF2), HCC drivers (TP53, CTNNB1), and CCA mutations (IDH1/2, FGFR2), enabling precision surveillance and therapeutic targeting. Clinical significance: Solid arrows indicate standard cirrhosis-mediated progression; dashed arrow represents direct malignant risk pathway. Color coding facilitates pathway recognition: blue (metabolic), pink (Met-ALD), red (alcohol-related), purple (cholestatic), orange (end-stage), dark red (malignant) supporting multidisciplinary care coordination and risk stratification across the chronic liver disease spectrum.
What are AI agents: from technology to healthcare
AI agents represent autonomous software systems that perceive their environment, reason about complex problems, and execute actions to achieve specific goals—fundamentally different from static AI tools that simply analyze data when prompted7. In the technology sector, AI agents have revolutionized customer service through chatbots that not only answer questions but actively escalate issues, coordinate with human agents, and learn from interactions to improve future responses. Financial institutions deploy AI agents for fraud detection that continuously monitor transactions, communicate with multiple security systems, and autonomously implement protective measures when suspicious patterns emerge7.
Healthcare applications of AI agents extend beyond single-task automation to collaborative networks where multiple specialized agents work together on complex clinical scenarios. Unlike conventional clinical AI systems that operate in isolation—such as a radiology AI that analyzes one image at a time AI agents form dynamic networks that communicate, share knowledge, and collectively solve problems that exceed individual capabilities7.
The framework we propose for chronic liver disease management represents one potential approach to organizing AI agents in hepatology, though alternative architectures could be equally effective depending on institutional needs and clinical workflows.
Current state of AI in hepatology: from individual tools to agent potential
Currently, AI applications in hepatology exist primarily as isolated, single-purpose tools rather than the collaborative agent networks we propose. Existing technologies include deep learning systems for hepatic steatosis quantification achieving 85–90% accuracy in research settings8,9, though clinical deployment remains limited. For cholangiocarcinoma detection, recent studies demonstrate AI systems achieving 80% accuracy for distinguishing malignant from benign biliary strictures in PSC patients10, but these operate as standalone diagnostic aids requiring manual integration with clinical workflows. Cross-institutional AI deployment in healthcare remains in early stages. The FDA has approved several AI tools for medical imaging, but these function independently without inter-system communication11 Epic’s electronic health record system has introduced basic AI decision support tools, though these lack the autonomous reasoning and collaborative capabilities that define true AI agents12,13. Google’s Medical Large Language Model (Med-PaLM) represents advances in medical AI reasoning, but deployment is limited to research contexts rather than clinical practice12. The transition from current isolated AI tools to the collaborative agent networks described in our framework requires significant technological and regulatory advancement. While individual components exist in preliminary form, the autonomous, communicating, and adaptive systems we propose represent a future vision rather than current reality.
AI agent fundamentals: architecture and interaction patterns
AI agents operate through three core components: perception modules that continuously monitor data streams, reasoning engines that process information and make decisions, and action interfaces that execute responses or communicate with other systems. Agent-to-agent interaction occurs through standardized messaging protocols, enabling autonomous coordination without human intervention. Human-agent interaction maintains hierarchical oversight where agents provide recommendations with confidence scores while clinicians retain final decision authority. This distributed architecture allows multiple specialized agents to collaborate on complex problems while maintaining individual accountability for specific tasks (Fig. 2).
The framework consists of A individual AI agent components with perception, reasoning, and action modules, B autonomous agent-to-agent communication through standardized protocols, C hierarchical human-clinician oversight maintaining final decision authority, and D distributed collaborative problem-solving with specialized agents (MASLD Assessment, PSC Surveillance, Imaging Analysis, and Pathology Integration) working together while maintaining individual task accountability.
AI agent architecture for chronic liver disease management: a conceptual framework
We propose a conceptual multi-agent framework for chronic liver disease management that demonstrates how AI agents could communicate dynamically, collaborate on complex clinical cases, and adapt organizational structures based on patient presentation complexity. This represents one potential approach among many possible architectures, designed to illustrate the transformative potential of agent-based systems in hepatology practice. Unlike traditional parallel processing systems, these networks would demonstrate emergent intelligence through real-time agent recruitment, consensus-building protocols, and distributed knowledge sharing across institutional boundaries (Fig. 3).
Comprehensive diagram illustrating how AI agent networks solve sophisticated clinical problems through distributed intelligence and collaborative reasoning. Starting with complex clinical presentation (45-year-old with Met-ALD and suspected malignancy), the system dynamically forms appropriate network topology progressing from simple (2–3 agents) through standard (4–8 agents) to complex swarm configurations (10+ agents) based on case complexity assessment. Parallel specialized analysis shows four primary agents: Metabolic Assessment Agent identifying MASLD risk factors, Alcohol History Agent detecting moderate ALD pattern, Imaging Analysis Agent recognizing mixed HCC/iCCA features, and Pathology Integration Agent requesting additional immunohistochemistry markers. Inter-agent communication protocols demonstrate structured messaging where agents share evidence with quantified confidence levels, triggering consensus-building mechanisms when uncertainty exceeds predetermined thresholds. The emergent solutions level demonstrates network-level intelligence producing integrated treatment approaches combining metabolic optimization, alcohol cessation protocols, surgical evaluation, and molecular targeting—solutions no single agent could independently generate.
Building upon these fundamental AI agent principles, we now present our proposed architecture concept for chronic liver disease management. This framework demonstrates how specialized agents could organize into collaborative networks, each contributing domain-specific expertise while maintaining autonomous decision-making capabilities.
Dynamic network topology
AI agent networks organize hierarchically across three operational levels. Primary Specialist Agents (Level 1) focus on disease-specific analysis including MASLD Assessment, PSC Surveillance, and Malignant Transformation Detection capabilities. Coordination Agents (Level 2) orchestrate workflow optimization, resource allocation, and timeline management across multiple specialist agents. Meta-Agents (Level 3) provide quality assurance, learning coordination, and ethical oversight across entire networks.
Network topology would adapt dynamically based on case complexity assessment. Simple MASLD surveillance cases activate 2–3 agents operating through basic communication patterns. Complex presentations such as met-ALD with suspected malignant transformation trigger network expansion, recruiting 8–10 specialized agents operating through structured collaboration protocols including evidence sharing, consensus building, and coordinated decision-making processes.
Specialized agent functions
Disease-Specific Triage Agents would serve as initial clinical interfaces, automatically analyzing incoming imaging studies and clinical data to identify high-risk cases requiring urgent attention across the liver disease spectrum. While current AI systems achieve promising results for individual tasks—such as hepatic steatosis detection with 85% accuracy8,9 and HCC surveillance with similar performance9—the proposed agents would integrate multiple data streams simultaneously, a capability not yet demonstrated in clinical practice.
Metabolic Assessment Agents focus on MASLD-to-MASH and met-ALD evaluations, integrating metabolic parameters, body composition analysis, and genetic risk factors. These agents distinguish simple steatosis from inflammatory MASH, predict fibrosis progression rates, and recommend optimal surveillance intervals based on individual risk profiles.
Alcohol-Related Disease Agents specialize in ALD evaluation, incorporating consumption patterns, withdrawal risk assessment, and treatment response monitoring. MetALD-specific agents distinguish this condition from pure MASLD or ALD by quantifying concurrent metabolic and alcohol contributions. These agents monitor potential transitions between MetALD, MASLD, and ALD based on evolving alcohol consumption patterns and metabolic parameters, recognizing these as distinct diagnostic entities rather than progressive stages.
Cholestatic Disease Agents operate as specialized systems for PSC and PBC, recognizing these as distinct autoimmune cholestatic conditions with different pathophysiologies and management requirements. PSC-specific agents focus on intensive CCA surveillance given the 400-fold increased risk and unique direct malignant transformation pathway independent of cirrhosis, while PBC-specific agents emphasize treatment response monitoring to ursodeoxycholic acid and autoimmune marker integration. Both incorporate cholangiographic findings while maintaining expertise in distinguishing benign from malignant biliary strictures.
Inter-agent communication protocols
Agents would communicate through standardized messaging protocols enabling real-time knowledge sharing and collaborative decision-making processes. When Metabolic Assessment Agents identify concerning fibrosis progression markers, they automatically transmit structured data packets containing risk parameters, confidence scores, and evidence summaries to Surveillance, Historical Analysis, and Treatment Planning Agents.
Consensus mechanisms activate when agents reach conflicting diagnostic conclusions. For distinguishing between MASLD, MetALD, and ALD where Metabolic and Alcohol Assessment Agents must classify the condition as distinct entities rather than stages, agents evaluate alcohol consumption thresholds, metabolic parameter contributions, and potential for bidirectional transitions. When uncertainty exists predetermined thresholds.
Collaborative problem-solving workflows
The transformative potential of AI agents emerges when facing clinical challenges that exceed individual agent capabilities. Consider a complex case of met-ALD with suspected malignant transformation: The Initial Triage Agent must first distinguish whether this represents true MetALD (concurrent metabolic and moderate alcohol factors) versus transitioning MASLD or established ALD. The Initial Triage Agent detects concerning patterns including elevated alpha-fetoprotein (425 ng/mL), new liver lesions, and combined diabetes/alcohol history8. Uncertainty recognition triggers multi-agent consultation protocols automatically spawning a specialized task force comprising Metabolic Risk Assessment, Alcohol History Analysis, Imaging Pattern Recognition, Pathology Correlation, and Oncology Protocol Agents.
Collaborative problem-solving proceeds through parallel analysis, evidence synthesis, consensus building, and solution emergence phases. The Metabolic Agent identifies MASLD progression indicators, the Alcohol Agent indicates moderate ALD risk, the Imaging Agent detects mixed HCC/iCCA features with quantified confidence levels, and the Pathology Agent requests additional immunohistochemistry markers. The Meta-Agent Mediator recognizes conflicting evidence requiring additional data, triggering automatic ordering of specialized tumor markers, genetic testing, and advanced magnetic resonance imaging protocols.
Technical implementation and clinical integration
AI agent systems employ microservices architecture ensuring scalability, maintainability, and regulatory compliance across multiple disease domains. Each agent operates as an independent service with clearly defined Application Programming Interfaces (APIs), enabling modular development and deployment strategies. This architecture supports Health Insurance Portability and Accountability Act (HIPAA) compliance through encrypted data transmission, comprehensive access logging, and detailed audit trails for all agent actions.
Inter-agent communication relies on standardized protocols including Agent Communication Language (ACL) specifications, Contract Net Protocol for task distribution, and consensus-building algorithms for collaborative decision-making. Network resilience mechanisms ensure continued operation during individual agent failures through redundancy protocols that automatically redistribute tasks when specialist agents become unavailable.
Current limitations and future requirements
The proposed multi-agent architecture requires technological capabilities that exceed current healthcare AI implementations. Existing clinical AI systems operate as isolated tools without inter-system communication protocols. Electronic health record integration remains limited, with most AI tools requiring manual data entry and separate workflow processes. The autonomous decision-making and collaborative reasoning described in our framework represent aspirational capabilities requiring advances in natural language processing, federated learning, and healthcare data interoperability standards that are currently under development but not clinically deployed.
Clinical workflow integration
Successful deployment requires careful attention to workflow integration design and user experience optimization across hepatological conditions. Implementation frameworks prioritize seamless integration with existing electronic health records and imaging systems while maintaining clinician autonomy in final decision-making processes.
Real-time agent coordination enables parallel processing of clinical workflows that traditionally occur sequentially. While Imaging Analysis Agents process radiological studies, Laboratory Integration Agents simultaneously correlate biochemical findings, and Historical Pattern Recognition Agents analyze longitudinal trends. These parallel analyses converge through structured integration protocols, reducing diagnostic timelines from weeks to days while maintaining comprehensive clinical assessment quality (Fig. 4).
Comparative analysis of traditional sequential workflow versus AI-enhanced parallel workflow for chronic liver disease management across the spectrum from MASLD/ALD to HCC and PSC to cholangiocarcinoma. The left panel illustrates conventional approach with sequential steps requiring 15–18 days total completion time, creating bottlenecks characteristic of current fragmented care models. The right panel demonstrates AI-enhanced parallel workflow featuring integrated clinical steps and AI processing columns. The clinical workflow maintains essential human oversight while AI agents operate continuously in parallel, providing real-time triage, simultaneous image analysis, evidence-based recommendations with quantified confidence metrics, adaptive monitoring during procedures, and integrated summary generation for multidisciplinary team decisions. The AI-enhanced workflow achieves 65% reduction in time, completing diagnostic and treatment pathway in 5–7 days compared to traditional 15–18 days, while maintaining clinical oversight and improving decision accuracy through intelligent assistance.
Federated learning approaches enable model improvement across institutions while maintaining patient privacy, with particular attention to diverse chronic liver disease presentations across different populations. Clinical feasibility is enhanced through key design principles including explainable AI recommendations using attention visualization and feature importance metrics, uncertainty quantification helping clinicians understand confidence levels, and human oversight mechanisms ensuring clinicians maintain final authority in all clinical decisions14.
Evidence base and validation requirements
Development builds upon substantial evidence from single-modality AI applications across hepatological conditions. Recent studies demonstrate AI systems achieving diagnostic accuracies exceeding 85% for MASLD detection and fibrosis staging, similar performance levels for HCC surveillance in cirrhotic patients, and over 80% accuracy for CCA detection in PSC patients9. Recent advances in uncertainty quantification methods14 and real-time diagnostic systems achieving 96% accuracy15 demonstrate the feasibility of confidence-based AI decision support across medical specialties. However, integration into autonomous AI agent systems requires comprehensive validation strategies including retrospective analysis using large institutional datasets, prospective pilot studies in controlled environments with extensive human oversight, and multi-center trials evaluating generalizability across different patient populations and institutional settings.
Challenges and future directions
Despite transformative promises, AI agents face significant deployment challenges. Regulatory approval processes must evolve to accommodate autonomous systems that adapt behavior based on clinical experience across multiple disease domains. Liability and accountability frameworks require urgent clarification when AI agents make erroneous recommendations. Consider a scenario where an AI agent misclassifies benign biliary strictures as malignant cholangiocarcinoma, leading to unnecessary surgical intervention—determining responsibility between the software developer, healthcare institution, and treating physician becomes legally complex. Similar liability challenges exist in autonomous vehicles where AI systems cause accidents, demonstrating that healthcare is not unique in facing these accountability dilemmas. In finance, algorithmic trading systems that make catastrophic decisions have established precedents for distributed liability models. Concrete safeguards must include comprehensive audit trails, explainable AI decision pathways with clear reasoning documentation, and clearly defined escalation protocols when agent confidence falls below predetermined thresholds. Training and education programs must prepare clinicians to effectively collaborate with AI agents while maintaining critical clinical skills across hepatology subspecialties. This includes understanding when to override AI recommendations, interpreting uncertainty metrics, and maintaining diagnostic competency independent of AI assistance.
Multi-agent coordination introduces novel technical challenges including communication protocol standardization, consensus mechanism optimization, and network security across inter-institutional collaborations. Ensuring reliable agent-to-agent communication while maintaining HIPAA compliance requires sophisticated encryption and authentication protocols that preserve collaborative functionality while protecting patient privacy.
Future evolution will progress through carefully staged implementation phases. Initial deployment will focus on decision support and workflow optimization, establishing clinician trust and regulatory acceptance. Intermediate phases will introduce predictive agents anticipating disease progression patterns, personalized medicine agents integrating genomic data with multimodal clinical findings, and quality assurance agents monitoring care consistency across institutions. Advanced implementations will feature research agents continuously analyzing institutional data for novel insights and pattern recognition that exceeds current clinical understanding.
Long-term vision encompasses sophisticated collective intelligence through swarm-based problem-solving capabilities where networks of 20–50 specialized agents collaborate on complex cases, generating emergent diagnostic insights that exceed individual human or agent capabilities16. However, achieving this vision requires resolving fundamental questions about human-AI collaboration boundaries, establishing international standards for medical AI agent deployment, and developing robust mechanisms for continuous learning while preventing algorithmic drift that could compromise patient safety.
Bias and accuracy limitations
AI agents inherit biases from training datasets, potentially perpetuating healthcare disparities across demographic groups. Chronic liver disease presents particular challenges as MASLD prevalence varies significantly across ethnic populations, while PSC predominantly affects Northern European descendants. Agents trained on demographically limited datasets may demonstrate reduced accuracy in underrepresented populations, potentially exacerbating existing healthcare inequities. Accuracy limitations arise from several sources including incomplete clinical data integration, rare disease presentations not adequately represented in training sets, and dynamic disease evolution that exceeds agent learning capabilities. Multi-agent systems compound these challenges as error propagation between communicating agents can amplify initial diagnostic mistakes. Mitigation strategies must include diverse training datasets, continuous performance monitoring across demographic subgroups, and uncertainty quantification mechanisms that flag cases requiring human review when confidence metrics indicate potential bias or accuracy concerns.
Conclusion
The proposed framework demonstrates one potential future approach to implementing AI agents in chronic liver disease management, illustrating how such systems could integrate complex clinical workflows when the necessary technologies mature beyond current capabilities, enhance diagnostic accuracy, and optimize treatment selection across the spectrum of metabolic, alcohol-related, and cholestatic liver diseases. Unlike conventional AI tools operating in isolation, AI agents provide comprehensive clinical decision support that adapts to individual patient presentations over time and according to institutional capabilities.
The interconnected nature of chronic liver diseases, exemplified by shared metabolic risk factors between MASLD and CCA17,18, highlights the critical need for integrated management approaches that AI agents uniquely provide. The ability to simultaneously monitor fibrosis progression, metabolic complications, and malignant transformation represents a significant advancement over current fragmented care models.
While this conceptual framework represents one potential implementation approach, alternative architectures may be equally viable depending on institutional capabilities and clinical needs.
Successful implementation requires collaborative development between clinicians and engineers, with careful attention to workflow integration, regulatory compliance, and maintenance of appropriate human oversight across multiple hepatology subspecialties. The future of chronic liver disease management lies not in replacing clinical expertise but in augmenting human capabilities through intelligent automation that handles routine tasks while enabling clinicians to focus on complex decision-making and patient interaction. Through careful development and validation, AI agents can illuminate the path toward more precise, efficient, and effective care for patients with these challenging conditions.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Banales, J. M. et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 17, 557–588 (2020).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Lekakis, V. & Papatheodoridis, G. V. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. 122, 3–10 (2024).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 29, 101133 (2024).
Karlsen, T. H. et al. Primary sclerosing cholangitis—a comprehensive review. J. Hepatol. 67, 1298–1323 (2017).
Wang, P. et al. Development and validation of a deep-learning algorithm for the detection of polyps during colonoscopy. Nat. Biomed. Eng. 2, 741–748 (2018).
Moritz, M., Topol, E. & Rajpurkar, P. Coordinated AI agents for advancing healthcare. Nat. Biomed. Eng 9, 432–438 (2025).
Tian, S., Chen, Y., Zhang, Y. & Xu, X. Clinical value of serum AFP and PIVKA-II for diagnosis, treatment and prognosis of hepatocellular carcinoma. J. Clin. Lab. Anal. 37, e24823 (2023).
Calderaro, J. et al. Artificial intelligence for the prevention and clinical management of hepatocellular carcinoma. J. Hepatol. 76, 1348–1361 (2022).
Singh, Y. et al. Deep learning analysis of magnetic resonance imaging accurately detects early-stage perihilar cholangiocarcinoma in patients with primary sclerosing cholangitis. Hepatology https://doi.org/10.1097/HEP.0000000000000123 (2025).
Benjamens, S. et al. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit. Med. 3, 118 (2020).
Singhal, K. et al. Large language models encode clinical knowledge. Nature 620, 172–180 (2023).
Nowrozy, R., Ahmed, K. & Wang, H. Artificial Intelligence in enhancing electronic health record systems: a comprehensive survey. in Health Information Science (eds Siuly, S., Xing, C., Li, X. & Zhou, R.) 1–15 (Springer, 2025).
Singh, Y. et al. Deep learning-based uncertainty quantification for quality assurance in hepatobiliary imaging-based techniques. Oncotarget 16, 249 (2025).
Urban, G. et al. Deep learning localizes and identifies polyps in real time with 96% accuracy in screening colonoscopy. Gastroenterology 155, 1069–1078 (2018).
Chen, R. J. et al. Multimodal co-attention transformer for survival prediction in gigapixel whole slide images. In Proc. IEEE/CVF Int. Conf. Comput. Vis. 4015-4025 (IEEE, 2021).
Wongjarupong, N. et al. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 17, 149 (2017).
Saillard, C. et al. Predicting survival after hepatocellular carcinoma resection using deep learning on histological slides. Hepatology 72, 2000–2013 (2020).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol. 73, 202–209 (2020).
Trépo, E. & Valenti, L. Update on NAFLD genetics: from new variants to the clinic. J. Hepatol. 72, 1196–1209 (2020).
Hirschfield, G. M. et al. Primary sclerosing cholangitis. Lancet 382, 1587–1599 (2013).
Izquierdo-Sanchez, L. et al. Cholangiocarcinoma landscape in Europe: diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J. Hepatol. 76, 1109–1121 (2022).
Kather, J. N. et al. Deep learning-based classification of primary liver cancer subtypes on routine histopathology. Nat. Med. 30, 891–899 (2024).
Calderaro, J. et al. Molecular and histological correlations in liver cancer. J. Hepatol. 71, 616–630 (2019).
Acknowledgements
This study was developed under the Precision-BTC Scientific Mentorship Program 2024–25 as part of the COST Action Precision-BTC Network (CA22125). The authors thank Mayo Clinic Comprehensive Cancer Center for their institutional support. This research received no specific grant funding from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Y.S. and J.B.A. conceived the study and wrote the manuscript. Q.A.H., D.V.V.-G., D.P., S.S., Y.W., N.H., A.C., A.M.K., V.K., K.N., E.Q., B.J.E., and G.J.G. contributed to manuscript preparation and critical revision. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
J.B.A. declares consultancies for Flagship Pioneering, QED Therapeutics, and AstraZeneca Nordic. J.B.A. has received research funding from Incyte Corporation (EU-DK-ST-21122), Adcendo, and the AMMF UK-charity for cholangiocarcinoma, but not related to this study. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Singh, Y., Hathaway, Q.A., Vera-Garcia, D.V. et al. Artificial Intelligence-based agents in chronic liver disease: transforming diagnostic and therapeutic workflows through clinical decision-making. npj Gut Liver 2, 34 (2025). https://doi.org/10.1038/s44355-025-00049-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44355-025-00049-5