Abstract
Myocardial infarction and heart failure remain leading causes of mortality worldwide. Human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) represent a promising approach to regenerating damaged myocardium and restoring cardiac function. This review highlights advancements in hPSC-CM differentiation, scale-up, and clinical-grade manufacturing; delivery approaches; and insights from preclinical and clinical studies. We also examine mechanisms of repair, key challenges and mitigation strategies, and future directions to advance hPSC-CM therapies toward clinical translation.
Similar content being viewed by others
Introduction
Each year, more than 735,000 people in the United States suffer from myocardial infarction (MI), leading to the loss of up to one billion cardiomyocytes in the left ventricle and a substantial decline in cardiac contractile function1,2. While revascularization strategies such as percutaneous coronary intervention (PCI) reduce acute mortality, many patients still progress to heart failure (HF) due to the heart’s limited regenerative capacity3,4.
Cell therapies have been investigated to address this unmet need. Early trials using non-cardiac cells, including bone marrow-derived mesenchymal stem cells (MSCs), showed only modest functional benefit, largely attributed to paracrine effects rather than true remuscularization5,6. These limitations shifted focus towards cardiomyocytes derived from human pluripotent stem cells (hPSC-CMs), from both embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs), which have demonstrated robust remuscularization and functional cardiac recovery in preclinical studies7,8,9,10,11,12,13. With growing evidence from preclinical studies and increasingly efficient protocols for hPSC-CM differentiation, hPSC-CMs have emerged as a leading candidate for next-generation cardiac cell therapy14,15.
This review highlights recent advances in manufacturing, including hPSC-CM differentiation, purification, and clinical-scale production; delivery approaches; and insights from preclinical and clinical studies. We also discuss key remaining challenges: cell survival, engraftment arrhythmias (EAs), and immune rejection; and strategies to mitigate them. Finally, we outline future directions for advancing hPSC-CM therapies toward clinical translation.
Manufacturing of clinical-grade hPSC-CMs
Differentiation and purification
Primary cardiomyocytes have limited therapeutic potential due to challenges in their isolation and cultivation16. The advent of hPSCs enabled the derivation of unlimited numbers of functional human cardiomyocytes for therapeutic applications. Early spontaneous differentiation protocols led to less than 1% hPSC-CMs, insufficient for therapy17. Directed differentiation protocols using BMP4 and Activin A improved yields to >30% hPSC-CMs18,19. Sequential Wnt/β-catenin activation (days 0-1) followed by inhibition (days 3–5) now routinely yields >90% hPSC-CMs18,19,20.
To further enhance purity, lactate metabolic selection leverages differences in glucose and lactate metabolism between cardiomyocytes and non-cardiomyocytes, resulting in hPSC-CM populations with a purity of up to 99%15. Despite these advances, single cell RNA sequencing studies have revealed the heterogeneous nature of hPSC-CMs, composed of subpopulations including atrial-specific cells expressing MYL7 and NPPA; ventricular-specific cells expressing MYL2 and IRX4; and nodal-like cells expressing HCN4, SHOX2, and TBX321,22,23,24.
Subtype specific hPSC-CM differentiation protocols have therefore been gaining attention. For example, ventricular specification is promoted by retinoic acid (RA) inhibition or modifying BMP4 and Activin A levels, while atrial specification is enhanced by stimulating the RA pathway22,23,25,26. By integrating efficient differentiation protocols, metabolic purification, and subtype-directed approaches, hPSC-CMs can be developed into standardized therapeutic products with defined clinical functionality.
Clinical-scale production
The loss of 1 billion cardiomyocytes in an MI necessitates efficient and robust methods for hPSC-CM differentiation and expansion. Large scale expansion after differentiation enables a ~250-fold increase in hPSC-CM numbers within 4–5 passages27. Efficient cryopreservation further allows pooling of hPSC-CMs from multiple batches to generate the quantities required for therapeutic application28. Bioreactors have emerged as a promising approach to increase hPSC-CM yields. For example, stirred-tank reactors generate 1.8*106 hPSC-CMs per mL, achieving ~94% viable cells after cryopreservation, requiring a minimal footprint29. Similarly, Chen et al leveraged canonical Wnt signaling in a bioreactor, producing 1.5–2*109 hPSC-CMs per liter with 91–92% purity and 85% recovery post-cryopreservation30. Dhahri et al. applied the BMP4-Activin A protocol in a PDMS lined 1 liter roller bottle yielding 1.2*108 mature hPSC-CMs per liter31. Together, advances in bioreactor platforms, large-scale expansion, and cryopreservation are enabling the production of clinically relevant numbers of hPSC-CMs for transplantation.
Transplantation methods
A variety of approaches, including intracoronary, systemic intravenous, and retrograde coronary venous injections, were initially tested for hPSC-CM transplantation into infarcted myocardium. However, these methods resulted in poor cell retention and had limited functional recovery32. Consequently, current hPSC-CM delivery strategies have shifted towards intramyocardial injections and epicardial patches, which demonstrate improved engraftment and therapeutic potential.
Intramyocardial injection
Intramyocardial injections deliver cells directly into the myocardium using syringes or specialized catheters (Fig. 1a). Although overall cell retention is modest (~1–10%), robust engraftments of hPSC-CMs within the infarcted left ventricle of non-human primate (NHP) hearts have been demonstrated (Fig. 1b). For example, Chong et al. reported mean engraftments of 2.1% (0.7–5.3%) of the infarcted region in NHPs33. Engrafted hPSC-CMs form electromechanical connections with host cardiomyocytes, contributing to functional recovery in infarcted hearts33,34,35,36,37,38,39,40,41.
a Intramyocardial injections can be single cell solutions or 3D microtissues. b Inspired by a figure of a large hPSC-CM engraftment by Liu et al.41. (Top) control heart. (Bottom) hPSC-CM treated heart. The blue regions represent collagen 1, the pink is cTnT, and the green is human cTnI. c Fukuda et al. specialized delivery system. (Top left) spheroids in phase contrast42. (Top right) schematic of spheroid distribution upon injection42. (Bottom) distribution of tissue marking dye delivered through the injection device (left) long axis (right) short axis42. d Nanowired organoid by Tan et al. (Top left) stained for alpha sarcomeric actinin (green), Vimentin (red) nanowires (yellow) and DAPI (blue) (top right) stained for alpha sarcomeric actinin (green), nanowires (yellow) and DAPI (blue) (Bottom left) stained for alpha sarcomeric actinin (green), Vimentin (red) and DAPI (blue) (bottom right) stained for alpha sarcomeric actinin (green), von Willebrand Factor (red) and DAPI (blue)9. c Reprinted from ref. 42 Copyright (2019), with permission from Elsevier. d From Tan et al. Nanowired human cardiac organoid transplantation enables highly efficient and effective recovery of infarcted hearts. Science Advances 9, eadf2898 https://doi.org/10.1126/sciadv.adf2898. Reprinted with permission from AAAS.
Beyond single-cell suspensions, intramyocardial injections have been used to deliver 3D hPSC-CM microtissues (Fig. 1a). Compared to single cell suspensions, microtissues demonstrate improved cell retention, as demonstrated by a study comparing the retention of 20 μm (32.4 ± 10.8%) and 175 μm (48.7 ± 14.3%) fluorescent beads following transplantation42. Building on this, Fukuda et al. developed a multicomponent delivery system consisting of a specialized syringe attachment, a gelatin hydrogel, and purified hPSC-CM spheroids42 (Fig. 1c). The syringe attachment featured six needles, with multiple holes on the side of each needle, reducing spheroid backflow and dispersing cells more evenly in the myocardium. Delivery of hPSC-CM spheroids using this system improved cardiac function, including increased ejection fraction, in rat and pig heart failure models10,43.
3D hPSC-CM microtissues also enable the incorporation of supportive cell types and biomaterials. For example, vascular cells, such as endothelial cells, have been incorporated into 3D microtissues to enhance graft survival and maturation9,44 (Fig. 1d). Additional supporting cells, including fibroblasts and pericytes, have been explored to further promote engraftment. For example, Min et al. designed a microtissue system incorporating multiple cell types, cardiac extracellular matrix, and fluid flow to create macroscale tissue aggregates45. Delivery of these microtissues into a rat ischemia-reperfusion model improved cardiac function, evidenced by increased left ventricular ejection fraction and fractional shortening.
Epicardial patches
Epicardial patches provide another viable strategy to engraft hPSC-CMs onto the surface of infarcted myocardium (Fig. 2a). Zimmermann and Eschenhagen pioneered this approach in 2006 by implanting large engineered cardiac tissues composed of primary rat CMs onto infarcted rat hearts7 (Fig. 2b, c) and later demonstrated successful engraftment in a human heart46. Compared with intramyocardial injection, epicardial patches offer greater structural support, enhancing hPSC-CM retention (>10%).
a Epicardial patches are applied to the external layer of the heart. b, c Scaffolded patch composed of hPSC-CMs and a fibrin scaffold7. b Multiloop scaffolded patch. c Patches are secured to the heart by 6 sutures. d–f Scaffold free patch with hPSC-CMs + human umbilical vein endothelial cells + fibroblasts48. d beta myosin heavy chain expression in the patch. e Lumen structures form in the patch. f Patch attached to the outside of the heart. g Schematic of scaffold-based epicardial patch fabrication with hPSC-CMs, biomaterials, and bioactive factors. h Patch CMs’ retention (red) in infarcted mice hearts without bioactive factors (left) or with CHIR99021 + FGF1 nanoparticles (right)80. b, c Reproduced from ref. 7. d–f Reproduced from ref. 48. With permission from PNAS. h Reprinted from ref. 80 Copyright (2020), with permission from Elsevier.
There are two main approaches to patch fabrication, scaffold-free and scaffolded. Scaffold free patches rely on self-organized tissue sheets. For example, thermoresponsive poly(N-isopropylacrylamide) has been used to generate sheets of hPSC-CMs that detach from culture substrates at low temperatures and then stacked two to three layers thick47. Alternatively, Stevens et al developed scaffold free hPSC-CM patches using suspension culture on a rotating orbital plate, incorporating vascular supporting cells to promote vascularization and improve the functions of the patches48,49 (Fig. 2d–f).
Scaffold-based patches embed hPSC-CMs within biomaterials such as fibrin or collagen, often supplemented with bioactive factors (Fig. 2g). In cryoinjury guinea pig models, functional recovery has been demonstrated, although electrical integration between the patch and the host was limited, with 3 out of 10 subjects demonstrating coupling across two studies12,50. Importantly, transplantation of a clinically relevant size fibrin-based patch composed of hPSC-CMs, smooth muscle cells (SMCs), and endothelial cells into pig models increased ejection fraction and decreased infarct size8. Querdel et al. further demonstrated that patch engraftment and functional recovery was dependent on the dose of cells delivered by the patch51.
Preclinical models of myocardial infarction and heart failure
Rodent models were essential for initial proof-of-concept studies; however their high heart rates (HR) (400 and 600 beats per minute (bpm)) obscure engraftment arrhythmias (EAs). Guinea pigs exhibit action potentials more similar to humans, but their high collateral coronary blood flow makes creating a severe enough infarct by ligation difficult. Pig hearts are anatomically and electrophysiologically similar to humans (HR 80–100 bpm), while non-human primates (HR 120–150 bpm) provide the closest electrophysiological match, enabling more accurate arrhythmia assessment. EAs have been shown to be present in these models, with fatal arrhythmias observed in pig models52.
Three main approaches are used to induce myocardial injury. Permanent ligation of coronary arteries, particularly the left anterior descending artery (LAD), models a transmural MI7,53. Ischemia reperfusion (IR) models mimic a PCI treated MI, typically requiring 60–180 min of ligation followed by reperfusion9,13,22,41,54,55,56. Cryoinjury models create precise infarct borders, commonly used in guinea pig models12,38,50.
The dosing of transplanted hPSC-CMs varies widely between studies and between animal models. The timing of hPSC-CM delivery has been studied across acute7,12,13,22,38,41,50,57, subacute9,39,56, and chronic phases54,55. While most studies focus on acute and subacute treatment phases of MI, increasing attention is shifting toward chronic HF, especially with the initiation of human clinical trials in HF patients54,58,59. Table 1 summarizes models, injury induction, cell doses, and endpoints.
Clinical translation
Building on the promising preclinical findings, several human clinical trials have been initiated using single cell suspensions, spheroids, and epicardial patches. Table 2 summarizes ongoing and completed human trials including therapy type, inclusion criteria, and primary outcomes.
Intramyocardial injection trials
Allogenic hPSC-CMs have been delivered via intramyocardial injection during scheduled coronary artery bypass grafting (CABG) in two trials: one targeting patients with worsening ischemic heart disease (NCT05566600) and another enrolling patients with severe ischemic heart disease (NCT06340048). The HECTOR trial (NCT05068674) is evaluating transendocardial delivery of hPSC-CMs through cardiac catheterization.
The LAPiS trial (NCT04945018) administers allogeneic hPSC-CM spheroids intramyocardially to patients with severe HF. Five patients have been recruited into both a low dose and high dose cohort, 50 and 150 million hPSC-CMs, respectively, with early reports showing improved left ventricular ejection fraction (LVEF), decreased New York Heart Association (NYHA) classification, and reduced levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) (https://heartseed.jp/en/news/assets/2023/07/aa5fdd0940390720c758960ae066298f1c35d66c.pdf) (https://heartseed.jp/en/news/assets/2023/09/230911-Press%20Release-Heartseed_LAPiS_JCCvF.pdf).
Epicardial patch trials
The BioVAT-HF trial (NCT04396899) employs epicardial patches composed of hPSC-CMs and stromal cells in a collagen 1 matrix to treat HF patients. Few adverse effects have been reported, and functional benefits at the maximal dose (800 M hPSC-CM/patient) include reductions in NYHA classification (from stage III to II) and increased ejection fraction60. Notably, the graft remained detectable after 3 months post-transplantation in one patient later undergoing heart transplantation46.
Another ongoing clinical trial in Japan (jRCT2053190081) uses hPSC-CM cell sheets61,62. Similar to BioVAT-HF trial, few adverse events were causally linked to treatment. Published results from three patients indicate functional recovery in two cases, with increased LVEF and decreased left ventricular end systolic and diastolic diameters observed at both 6 months and 1 year post-treatment62.
Mechanism of repair
Engrafting hPSC-CMs into the myocardium to remuscularize damaged hearts was initially assumed to be the primary mechanism of cardiac repair. Murry’s group first demonstrated that hPSC-CMs functionally integrated into infarcted hearts, forming gap junctions with host cardiomyocytes via connexin-4356. Optogenetic silencing experiments confirmed this contribution, as contractile benefits were immediately lost when grafts were inhibited63.
Paracrine signaling has increasingly been recognized as another key mechanism of hPSC-CM mediated cardiac repair64,65,66. In particular, extracellular vesicles (EVs), including exosomes, have emerged potent mediators of cardiac recovery. Exosomes deliver bioactive cargos, proteins, messenger RNAs (mRNAs), microRNAs (miRNAs) and bioactive lipids, that modulate intercellular signaling67,68,69,70. In porcine MI models, EV injections improved cardiac function to a degree comparable with transplanted cells, underscoring their critical role in cardiac repair66.
Karbassi et al. further dissected this mechanism by generating noncontractile hPSC-CMs by knocking out slow skeletal TNNI1 and cardiac TNNI3, key components of the contractile machinery of hPSC-CMs65. Remarkably, these noncontractile hPSC-CMs preserved heart function after IR injury to a similar extent as wild-type hPSC-CMs, highlighting the importance of paracrine effects.
Both remuscularization and paracrine effects act in parallel. From a translational perspective, cell-free therapies may reduce risks associated with cell transplantation, but their rapid clearance may not confer long-term improvements to heart function as would a direct cell replacement therapy. By contrast, hPSC-CMs can act as a “living drug” after transplantation, providing contractile force, secreting pro-survival signals, and dynamically responding to host injury71.
Challenges and mitigation strategies
hPSC-CM population heterogeneity
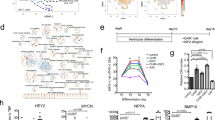
Single cell sequencing studies have highlighted the heterogeneous nature of hPSC-CM cultures22 (Fig. 3d). Thorough characterization of hPSC-CMs is critical to ensure reproducible therapeutic efficacy and to prevent aberrant in vivo differentiation of residual stem cells40. Atrial, ventricular, and nodal hPSC-CMs differ in electrophysiology, contractile function, and gene expression22,72,73,74,75. One study reported that populations with increased atrial and pacemaker-like cells led to increased rates of EA, with all animals demonstrating nearly sustained arrhythmia by day 8 post-transplantation22. The mechanisms underlying this increased arrhythmogenicity has not been fully explored. By contrast, ventricular specific hPSC-CMs have been differentiated23,25,26 and transplanted23, but their arrhythmia risk has not yet been investigated. A deeper mechanistic understanding of how hPSC-CM subtypes contribute to efficacy and safety is needed, and the optimal cell population for therapy remains undefined.
a Morphological differences in hPSC-CMs as they progressively matured with palmitate (Pal), Dex and T3 (DT) or PPar agonist + palmitate + Dex and T3 (mature)23. b ECG traces of arrhythmias present in a minipig injury model NSR normal, VT ventricular tachycardia, AJR accelerated junctional rhythm, AIVR accelerated idioventricular82. c Anti-arrhythmia drugs decrease the arrhythmogenic burden in hPSC-CM treated pigs22. d scRNAseq data of hPSC-CMs showing the multiple number of subpopulations contained within one culture22. e Diagram of gene edits made in MEDUSA hPSC-CMs to reduce automaticity. Red channels are knockouts, and green channels were knocked in. f Arrhythmogenic burden was decreased in the gene edited hPSC-CM treatment group81. a Modified from ref. 23 originally published under CC-BY 4.0 https://creativecommons.org/licenses/by/4.0/. b Modified from ref. 82 originally published under CC-BY 4.0 https://creativecommons.org/licenses/by/4.0/. c, d Modified from ref. 22 originally published under CC-BY 4.0. f Reprinted from ref. 81 with permission from Elsevier.
hPSC-CM maturity
Compared to adult cardiomyocytes, hPSC-CMs display an immature phenotype characterized by differences in 1) morphology, 2) electrophysiology, 3) calcium handling, 4) contractility, 5) metabolism, and 6) proliferative capacity76. Immature hPSC-CMs are typically round, exhibit depolarized resting potentials, reliance on glycolysis, generate low contractile force, and lack T-tubules.
Most transplantation studies have used hPSC-CMs with an age of two to three weeks post-differentiation in vitro (Table 1). This immaturity may confer advantages, including increased proliferation and enhanced ischemic tolerances. However, the automaticity of hPSC-CMs has been implicated in EAs due to interference with host action potential propagation76. Interestingly, there is evidence of in situ maturation, with spontaneous resolution of EAs observed around 30 days after hPSC-CM transplantation33,35,40. It has been demonstrated that in vitro matured hPSC-CMs form grafts with improved structure and function in injured hearts31.
Numerous strategies have been explored to promote hPSC-CM maturity prior to transplantation. Biophysical approaches include modifying culture substrates, electrical pacing (1–2 Hz), and mechanical stretch have been explored76,77. Modifying culture substrates has been shown to have an effect on maturity: aligned fibers led to a more mature cell population at earlier time points78. In addition, substrates coated with PDMS of ~400 kPa stiffness increased hPSC-CM maturation31. When transplanted into cryoinjured guinea pigs, PDMS-cultured hPSC-CMs exhibited decreased arrhythmic burden relative to tissue culture plastic cultured hPSC-CMs.
In parallel, biochemical approaches such as supplementing fatty acids with hormones and peroxisome proliferator-activated nuclear receptor agonists to culture media have also been used to shift metabolism from glycolysis to fatty acid oxidation, yielding hPSC-CMs with a compact ventricular phenotype (Fig. 3a)23. Although these matured grafts were smaller, they contained more structurally and functionally mature hPSC-CMs.
Notably, the ideal maturation state for hPSC-CMs remains undefined. Optimal cells should maximize graft quality, such as increased contractility and calcium handling, while minimizing risks such as the enhanced arrhythmogenicity and reduced survival or proliferation. Determining this balance represents one of the field’s greatest challenges and opportunities.
Cell survival and engraftment
Ensuring robust survival and integration of transplanted hPSC-CMs is essential for achieving lasting therapeutic benefits in patients. After transplantation, hPSC-CMs face multiple stressors including; hypoxia, oxidative stress, inflammation, and mechanical washout48,56,79. To address these challenges, Laflame et al. developed a pro-survival cocktail containing components that mitigate anoikis, apoptosis, necrotic, and mitochondrial cell death, while also enhancing ischemic tolerance56. This cocktail has enabled robust hPSC-CM engraftment in rodent and NHP models. In addition, microtissues such as spheroids have been employed to overcome mechanical washout and promote hPSC-CM engraftments10. Incorporation of electrically conductive silicon nanowires has been used to increase microtissue integration and enhanced functional recovery of host myocardium9.
Epicardial patches often suffer from low survival without sufficient vascularization. The addition of vascular supporting cells, particularly endothelial cells, has been used in patches to promote engraftment and vascular recruitment48. Other supporting vascular cells such as SMCs, have been shown to improve patches vascularization and functional recovery in pig models8. Beyond cellular composition, modulation of biochemical and physical cues within epicardial patches offers a powerful strategy to improve survival and engraftment. For example, Fan et al. engineered hPSC-CM patches to incorporate nanoparticles loaded with CHIR99021 and FGF14780 (Fig. 2g). These patches increased engraftment fourfold and stimulated hPSC-CM proliferation in a mouse model.
Engraftment arrhythmia
Large animal studies have reported EAs that occur shortly after hPSC-CM injections, which typically resolve spontaneously after 1 month (Fig. 3b, c)22,52,81. EAs are thought to arise from the heterogenicity and immaturity of transplanted hPSC-CMs. Compared with intramyocardial injections, fewer EAs have been observed following transplantation of epicardial hPSC-CM patches, likely due to their physical insulation from host myocardium50. However, this same limited electrical integration can lead to unsynchronized contractions of transplanted patches and suboptimal therapeutic benefit39.
A straightforward and clinically translatable strategy for reducing arhythmic burden after hPSC-CM transplantation is the use of anti-arrhythmia drugs. Nakamura et al. screened several clinically relevant antiarrhythmic drugs and identified two effective options: amiodarone, a class III antiarrhythmic drug primarily a potassium channel inhibitor, and ivabradine, a HCN4 channel antagonist82. Amiodarone was delivered continuously, while ivabradine was administered during sustained tachycardia when porcine subjects reached heart rates greater than 150 bpm. This regimen eliminated fatal arrhythmias and reduced overall arrhythmic burden. Selvakumar et al. further confirmed the effectiveness of combined ivabradine and amiodarone, demonstrating decreased arrhythmia duration (in hours per day) and frequency (days with arrhythmia)22 (Fig. 3c). In addition, they evaluated catheter ablation, successfully mapping EAs to hPSC-CM injection sites, and decreasing the arrhythmogenic burden following catheter ablation. Notably, one porcine subject treated with hPSC-CMs composed of a higher atrial subpopulation, had recurrent EAs traced to secondary locations beyond the original ablation target.
To further probe EA mechanisms, Marchiano et al. conducted systematic genome editing to reduce hPSC-CMs automaticity81. By knocking out HCN4, Cav3.2, and NCX1, while overexpressing of Kir2.1, they generated cells capable of responding to action potentials without spontaneous firing. When transplanted into porcine models, these engineered hPSC-CMs substantially reduced arrhythmic burden (Fig. 3e, f).
Immune rejection
Immune rejection remains a critical barrier to achieving long term hPSC-CM engraftment. While immunocompromised rodents (e.g., athymic rats) are widely used for transplantation studies, immunosuppression is currently the only viable option for large-animal and human trials.
Autologous hPSC-CMs are impractical for clinical use in acute MI treatment due to the 3–6 months required for manufacturing and their high cost83,84. In contrast, allogenic hPSC-CMs are more readily available and are being tested in all current clinical trials. However, they require either immunosuppressive regimens or human leukocyte antigen (HLA) matching to avoid rejection. Immunosuppressive drugs carry significant risks, particularly in vulnerable HF patients85,86. HLA class matching is a feasible alternative in relatively genetically homogenous populations such as Japan, where as few as 140 cell lines could match 90% of individuals87. By contrast, in genetically diverse populations such as United States, substantially larger HLA-matched cell banks are needed to achieve broad coverage, particularly for underrepresented ethnic groups88.
Emerging hypoimmune technologies offer a promising strategy to overcome these challenges. By genetically editing hPSC-CMs to eliminate expression of HLA class I and/or II molecules, these cells evade CD8+ and/or CD4+ T cell mediated killing83. To prevent natural killer (NK) cell mediated lysis, immune evasive factors such as CD47, and HLA-E/G can be knocked in89. Notably, hypoimmune gene-edited hPSC cardiac organoids have demonstrated the ability to restore contractile function in infarcted rat hearts and to improve graft retention and immune evasion in humanized mice relative to wild-type controls90.
Conclusions and future perspectives
Over the past decade, hPSC-CM therapies advanced significantly, culminating in ongoing clinical trials. Progress in hPSC-CM differentiation and purification has enabled the production of clinical-grade hPSC-CMs, while intramyocardial injections and epicardial patches have emerged as promising delivery strategies. Early clinical trial results suggest these approaches improve cardiac function.
Despite this progress, several key challenges must be addressed before hPSC-CM therapies transform MI and HF treatment. Major obstacles include limited cell survival, low engraftment efficiency, and the risk of EAs. Pro-survival cocktails and co-transplantation with supporting cells have shown promise in enhancing hPSC-CM survival, but the optimal cell type and composition to maximize engraftment remains undefined. To mitigate EAs, anti-arrhythmic drugs, catheter ablation, and ion channels gene editing have been explored. While anti-arrhythmic drugs and catheter ablation are clinically feasible, further investigation is needed to minimize EA risk.
Optimizing the composition and maturity of transplanted hPSC-CMs is another critical challenge. Evidence suggests that hPSC-CM populations enriched in atrial-like subpopulations may increase arrhythmogenic risk, whereas whether ventricular-specific populations reduce EA remains undetermined. Striking the right balance between the proliferative and stress-tolerant properties of immature hPSC-CMs and the contractile and electrophysiological competence of mature hPSC-CMs will be essential to defining the ideal therapeutic cell product.
Immune rejection also remains a major barrier. Although current immunosuppression regimes are effective, they pose significant risks, highlighting the need for alternative approaches. Hypoimmune technologies, pioneered in hPSC-derived pancreatic islet transplantation, offer promising strategies for cardiac regenerative medicine.
Lastly, a deeper mechanistic understanding of hPSC-CM-mediated cardiac repair is essential. The relative contributions of remuscularization compared to paracrine effects remain incompletely understood, and resolving this will be critical for refining therapeutic strategies.
Looking ahead, integrating emerging innovations in cell engineering, immunomodulation, and tissue engineering will be key to overcoming these challenges and realizing the full potential of hPSC-CM therapies for treatment of MI and HF.
References
CDC. Underlying Cause of Death 1999-2013 on CDC WONDER Online Database, released 2015. Data are from the Multiple Cause of Death Files, 1999-2013, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program (2015).
Mozaffarian, D. et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation 131, e29–e322 (2015).
O’Gara, P. T. et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127, e362–e425 (2013).
Levine, G. N. et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 67, 1235–1250 (2016).
Behfar, A., Crespo-Diaz, R., Terzic, A. & Gersh, B. J. Cell therapy for cardiac repair—lessons from clinical trials. Nat. Rev. Cardiol. 11, 232–246 (2014).
Sanz-Ruiz, R. et al. Phases I-III Clinical Trials Using Adult Stem Cells. Stem Cells Int. 2010, 579142 (2010).
Zimmermann, W.-H. et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 12, 452–458 (2006).
Gao, L. et al. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell–Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation 137, 1712–1730 (2018).
Tan, Y. et al. Nanowired human cardiac organoid transplantation enables highly efficient and effective recovery of infarcted hearts. Sci. Adv. 9, eadf2898 (2023).
Kawaguchi, S. et al. Intramyocardial Transplantation of Human iPS Cell-Derived Cardiac Spheroids Improves Cardiac Function in Heart Failure Animals. JACC Basic Transl. Sci. 6, 239–254 (2021).
Liu, Y. W. et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 36, 597–605 (2018).
Weinberger, F. et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med. 8, 363ra148–363ra148 (2016).
Kobayashi, H. et al. Regeneration of Nonhuman Primate Hearts With Human Induced Pluripotent Stem Cell–Derived Cardiac Spheroids. Circulation 150, 611–621 (2024).
Lian, X. et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 109, E1848–E1857 (2012).
Tohyama, S. et al. Distinct Metabolic Flow Enables Large-Scale Purification of Mouse and Human Pluripotent Stem Cell-Derived Cardiomyocytes. Cell Stem Cell 12, 127–137 (2013).
Zhou, B. et al. Functional isolation, culture and cryopreservation of adult human primary cardiomyocytes. Sign. Transduct. Target. Ther. 7, 254 (2022).
Doevendans, P. A. et al. Differentiation of Cardiomyocytes in Floating Embryoid Bodies is Comparable to Fetal Cardiomyocytes. J. Mol. Cell. Cardiol. 32, 839–851 (2000).
Sa, S. & McCloskey, K. Activin A and BMP4 Signaling for Efficient Cardiac Differentiation of H7 and H9 Human Embryonic Stem Cells. J. Stem Cells Regen. Med. 8, 198–202 (2012).
Kattman, S. J. et al. Stage-Specific Optimization of Activin/Nodal and BMP Signaling Promotes Cardiac Differentiation of Mouse and Human Pluripotent Stem Cell Lines. Cell Stem Cell 8, 228–240 (2011).
Lian, X. et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 8, 162–175 (2013).
Biendarra-Tiegs, S. M. et al. Single-Cell RNA-Sequencing and Optical Electrophysiology of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Reveal Discordance Between Cardiac Subtype-Associated Gene Expression Patterns and Electrophysiological Phenotypes. Stem Cells Dev. 28, 659–673 (2019).
Selvakumar, D. et al. Cellular heterogeneity of pluripotent stem cell-derived cardiomyocyte grafts is mechanistically linked to treatable arrhythmias. Nat. Cardiovasc. Res. 3, 145–165 (2024).
Funakoshi, S. et al. Generation of mature compact ventricular cardiomyocytes from human pluripotent stem cells. Nat. Commun. 12, 3155 (2021).
Yechikov, S. et al. NODAL inhibition promotes differentiation of pacemaker-like cardiomyocytes from human induced pluripotent stem cells. Stem Cell Res. 49, 102043 (2020).
Dark, N. et al. Generation of left ventricle-like cardiomyocytes with improved structural, functional, and metabolic maturity from human pluripotent stem cells. Cell Rep. Methods 3, 100456 (2023).
Lee, J. H., Protze, S. I., Laksman, Z., Backx, P. H. & Keller, G. M. Human Pluripotent Stem Cell-Derived Atrial and Ventricular Cardiomyocytes Develop from Distinct Mesoderm Populations. Cell Stem Cell 21, 179–194.e174 (2017).
Maas, R. G. C. et al. Massive expansion and cryopreservation of functional human induced pluripotent stem cell-derived cardiomyocytes. STAR Protoc. 2, 100334 (2021).
Preininger, M. K., Singh, M. & Xu, C. Cryopreservation of Human Pluripotent Stem Cell-Derived Cardiomyocytes: Strategies, Challenges, and Future Directions. Adv. Exp. Med. Biol. 951, 123–135 (2016).
Prondzynski, M. et al. Efficient and reproducible generation of human iPSC-derived cardiomyocytes and cardiac organoids in stirred suspension systems. Nat. Commun. 15, 5929 (2024).
Chen, V. C. et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 15, 365–375 (2015).
Dhahri, W. et al. In Vitro Matured Human Pluripotent Stem Cell–Derived Cardiomyocytes Form Grafts With Enhanced Structure and Function in Injured Hearts. Circulation 145, 1412–1426 (2022).
Li, J. et al. All Roads Lead to Rome (the Heart): Cell Retention and Outcomes From Various Delivery Routes of Cell Therapy Products to the Heart. J. Am. Heart Assoc. 10, e020402 (2021).
Chong, J. J. H. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014).
Guan, X. et al. Transplantation of human induced pluripotent stem cell-derived cardiomyocytes improves myocardial function and reverses ventricular remodeling in infarcted rat hearts. Stem Cell Res. Ther. 11, 73 (2020).
Funakoshi, S. et al. Enhanced engraftment, proliferation and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 6, 19111 (2016).
van Laake, L. W. et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 1, 9–24 (2007).
Xue, T. et al. Functional Integration of Electrically Active Cardiac Derivatives From Genetically Engineered Human Embryonic Stem Cells With Quiescent Recipient Ventricular Cardiomyocytes. Circulation 111, 11–20 (2005).
Shiba, Y. et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322–325 (2012).
Gerbin, K. A., Yang, X., Murry, C. E. & Coulombe, K. L. K. Enhanced Electrical Integration of Engineered Human Myocardium via Intramyocardial versus Epicardial Delivery in Infarcted Rat Hearts. PLOS One 10, e0131446 (2015).
Caspi, O. et al. Transplantation of Human Embryonic Stem Cell-Derived Cardiomyocytes Improves Myocardial Performance in Infarcted Rat Hearts. J. Am. Coll. Cardiol. 50, 1884–1893 (2007).
Liu, Y.-W. et al. Human embryonic stem cell–derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 36, 597–605 (2018).
Tabei, R. et al. Development of a transplant injection device for optimal distribution and retention of human induced pluripotent stem cell‒derived cardiomyocytes. J. Heart Lung Transplant. 38, 203–214 (2019).
Moon, S.-H. et al. The use of aggregates of purified cardiomyocytes derived from human ESCs for functional engraftment after myocardial infarction. Biomaterials 34, 4013–4026 (2013).
Giacomelli, E. et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 26, 862–879.e811 (2020).
Min, S. et al. Versatile human cardiac tissues engineered with perfusable heart extracellular microenvironment for biomedical applications. Nat. Commun. 15, 2564 (2024).
Jebran, A.-F. et al. Engineered heart muscle allografts for heart repair in primates and humans. Nature https://doi.org/10.1038/s41586-024-08463-0 (2025).
Zhang, J. Engineered Tissue Patch for Cardiac Cell Therapy. Curr. Treat. Options Cardiovasc. Med. 17, 399 (2015).
Stevens, K. R. et al. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc. Natl. Acad. Sci. 106, 16568–16573 (2009).
Stevens, K. R., Pabon, L., Muskheli, V. & Murry, C. E. Scaffold-Free Human Cardiac Tissue Patch Created from Embryonic Stem Cells. Tissue Eng. Part A 15, 1211–1222 (2008).
Pecha, S. et al. Human iPS cell-derived engineered heart tissue does not affect ventricular arrhythmias in a guinea pig cryo-injury model. Sci. Rep. 9, 9831 (2019).
Querdel, E. et al. Human Engineered Heart Tissue Patches Remuscularize the Injured Heart in a Dose-Dependent Manner. Circulation 143, 1991–2006 (2021).
Romagnuolo, R. et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Rep. 12, 967–981 (2019).
Mattapally, S. et al. Spheroids of cardiomyocytes derived from human-induced pluripotent stem cells improve recovery from myocardial injury in mice. Am. J. Physiol. Heart Circ. Physiol. 315, H327–h339 (2018).
Fernandes, S. et al. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J. Mol. Cell. Cardiol. 49, 941–949 (2010).
Riegler, J. et al. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ. Res. 117, 720–730 (2015).
Laflamme, M. A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 25, 1015–1024 (2007).
Ye, L. et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15, 750–761 (2014).
von Bibra, C. et al. Human engineered heart tissue transplantation in a guinea pig chronic injury model. J. Mol. Cell. Cardiol. 166, 1–10 (2022).
Shiba, Y. et al. Electrical Integration of Human Embryonic Stem Cell-Derived Cardiomyocytes in a Guinea Pig Chronic Infarct Model. J. Cardiovasc. Pharmacol. Therapeutics 19, 368–381 (2014).
Ensminger, S. et al. Safety and Efficacy of Induced Pluripotent Stem Cell-derived Engineered Human Myocardium as Biological Ventricular Assist Tissue in Terminal Heart Failure (BioVAT-DZHK20): Trial Design and Experience at UKSH Lübeck. Thorac. Cardiovasc. Surg. 72, DGTHG–V045 (2024).
Miyagawa, S. et al. Case report: Transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front. Cardiovasc. Med. 9, 950829 (2022).
Kawamura, T. et al. Safety confirmation of induced pluripotent stem cell-derived cardiomyocyte patch transplantation for ischemic cardiomyopathy: first three case reports. Front. Cardiovasc. Med. 10, 1182209 (2023).
Stüdemann, T. et al. Contractile Force of Transplanted Cardiomyocytes Actively Supports Heart Function After Injury. Circulation 146, 1159–1169 (2022).
Hu, Y. et al. Extracellular vesicle therapeutics for cardiac repair. J. Mol. Cell. Cardiol. 199, 12–32 (2025).
Karbassi, E. et al. Noncontractile Stem Cell-Cardiomyocytes Preserve Post-Infarction Heart Function. Circ. Res. 135, 967–969 (2024).
Gao, L. et al. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci. Transl. Med. 12, eaay1318 (2020).
Ibrahim, A. & Marban, E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu. Rev. Physiol. 78, 67–83 (2016).
Gallet, R. et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 38, 201–211 (2017).
Balbi, C. & Vassalli, G. Exosomes - Beyond Stem Cells for Cardiac Protection and Repair. Stem Cells https://doi.org/10.1002/stem.3261 (2020).
Dougherty, J. A. et al. Potential Role of Exosomes in Mending a Broken Heart: Nanoshuttles Propelling Future Clinical Therapeutics Forward. Stem Cells Int. 2017, 5785436 (2017).
Levy, O. et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 6, eaba6884 (2020).
Nakanishi-Koakutsu, M. et al. CD151 expression marks atrial- and ventricular- differentiation from human induced pluripotent stem cells. Commun. Biol. 7, 231 (2024).
Chapotte-Baldacci, C.-A., Pierre, M., Djemai, M., Pouliot, V. & Chahine, M. Biophysical properties of NaV1.5 channels from atrial-like and ventricular-like cardiomyocytes derived from human induced pluripotent stem cells. Sci. Rep. 13, 20685 (2023).
Hoff, K. et al. DNA methylation profiling allows for characterization of atrial and ventricular cardiac tissues and hiPSC-CMs. Clin. Epigenet. 11, 89 (2019).
Moretti, A. et al. Patient-Specific Induced Pluripotent Stem-Cell Models for Long-QT Syndrome. N. Engl. J. Med. 363, 1397–1409 (2010).
Karbassi, E. et al. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 17, 341–359 (2020).
Gomez-Garcia, M. J., Quesnel, E., Al-attar, R., Laskary, A. R. & Laflamme, M. A. Maturation of human pluripotent stem cell derived cardiomyocytes in vitro and in vivo. Semin. Cell Dev. Biol. 118, 163–171 (2021).
Kalkunte, N. G. et al. Engineering Alignment Has Mixed Effects on Human Induced Pluripotent Stem Cell Differentiated Cardiomyocyte Maturation. Tissue Eng. Part A 29, 322–332 (2023).
Anderl, J. N., Robey, T. E., Stayton, P. S. & Murry, C. E. Retention and biodistribution of microspheres injected into ischemic myocardium. J. Biomed. Mater. Res. Part A 88A, 704–710 (2009).
Fan, C. et al. CHIR99021 and fibroblast growth factor 1 enhance the regenerative potency of human cardiac muscle patch after myocardial infarction in mice. J. Mol. Cell. Cardiol. 141, 1–10 (2020).
Marchiano, S. et al. Gene editing to prevent ventricular arrhythmias associated with cardiomyocyte cell therapy. Cell Stem Cell 30, 396–414.e399 (2023).
Nakamura, K. et al. Pharmacologic therapy for engraftment arrhythmia induced by transplantation of human cardiomyocytes. Stem Cell Rep. 16, 2473–2487 (2021).
Hu, X. et al. Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01784-x (2023).
Huang, C.-Y. et al. Human iPSC banking: barriers and opportunities. J. Biomed. Sci. 26, 87 (2019).
Ruiz, R. & Kirk, A. D. Transplantation of the Liver (Third edition). The chapter being cited is 97- Long term toxicityof immunosuppressive therapy. Transplant. Liver 1354–1363 (2015).
Elezaby, A., Dexheimer, R. & Sallam, K. Cardiovascular effects of immunosuppression agents. Front. Cardiovasc. Med. 9, 981838 (2022).
Umekage, M., Sato, Y. & Takasu, N. Overview: an iPS cell stock at CiRA. Inflamm. Regen. 39, 17 (2019).
Gourraud, P. A., Gilson, L., Girard, M. & Peschanski, M. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cells 30, 180–186 (2012).
Hotta, A., Schrepfer, S. & Nagy, A. Genetically engineered hypoimmunogenic cell therapy. Nat. Rev. Bioeng. https://doi.org/10.1038/s44222-024-00219-9 (2024).
Silver, S. E. et al. Hypoimmunogenic hPSC-derived cardiac organoids for immune evasion and heart repair. Preprint at https://doi.org/10.1101/2025.04.09.648007 (2025).
Shiba, Y. et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391 (2016).
Guragain, B. et al. Implanted Human Cardiac Spheroids Electrically Couple With Infarcted Swine Myocardium. Circulation 149, 1855–1857 (2024).
Acknowledgements
The author’s work is funded by the National Institutes of Health (1R01HL175050, 1R01HL133308, 1U01HL169361, 1R01HL168255, 1R21HL167211). This work was also supported by American Heart Association Grant # 23IPA1054426/Ying Mei/2023 and the National Science Foundation (NSF) Award #OIA-2242812. The figures were created in https://BioRender.com.
Author information
Authors and Affiliations
Contributions
J.D.B. and Y.M. contributed to the conception, writing and editing of this manuscript. R.W.B. contributed to the revision of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bain, J.D., Barrs, R.W. & Mei, Y. Progress and challenges in transplantation of human pluripotent stem cell derived cardiomyocytes for cardiac therapy. npj Biomed. Innov. 3, 2 (2026). https://doi.org/10.1038/s44385-025-00048-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44385-025-00048-4