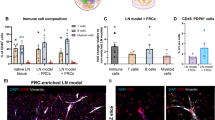
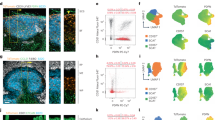
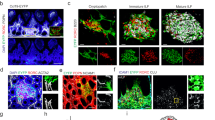
Abstract
Fibroblastic reticular cells (FRCs) are known to inhabit T cell–rich areas of lymphoid organs, where they function to facilitate interactions between T cells and dendritic cells. However, in vivo manipulation of FRCs has been limited by a dearth of genetic tools that target this lineage. Here, using a mouse model to conditionally ablate FRCs, we demonstrated their indispensable role in antiviral T cell responses. Unexpectedly, loss of FRCs also attenuated humoral immunity due to impaired B cell viability and follicular organization. Follicle-resident FRCs established a favorable niche for B lymphocytes via production of the cytokine BAFF. Thus, our study indicates that adaptive immunity requires an intact FRC network and identifies a subset of FRCs that control B cell homeostasis and follicle identity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mueller, S.N. & Germain, R.N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 9, 618–629 (2009).
Turley, S.J., Fletcher, A.L. & Elpek, K.G. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat. Rev. Immunol. 10, 813–825 (2010).
Gretz, J.E., Norbury, C.C., Anderson, A.O., Proudfoot, A.E. & Shaw, S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192, 1425–1440 (2000).
Roozendaal, R. et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30, 264–276 (2009).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Acton, S.E. et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity 37, 276–289 (2012).
Astarita, J.L., Acton, S.E. & Turley, S.J. Podoplanin: emerging functions in development, the immune system, and cancer. Front. Immunol. 3, 283 (2012).
Malhotra, D. et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 13, 499–510 (2012).
Link, A. et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 8, 1255–1265 (2007).
Khan, O. et al. Regulation of T cell priming by lymphoid stroma. PLoS ONE 6, e26138 (2011).
Lukacs-Kornek, V. et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol. 12, 1096–1104 (2011).
Siegert, S. et al. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS ONE 6, e27618 (2011).
Cyster, J.G. B cell follicles and antigen encounters of the third kind. Nat. Immunol. 11, 989–996 (2010).
Cyster, J.G. et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 176, 181–193 (2000).
Roozendaal, R. & Carroll, M.C. Complement receptors CD21 and CD35 in humoral immunity. Immunol. Rev. 219, 157–166 (2007).
Tew, J.G., Kosco, M.H., Burton, G.F. & Szakal, A.K. Follicular dendritic cells as accessory cells. Immunol. Rev. 117, 185–211 (1990).
Wang, X. et al. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. J. Exp. Med. 208, 2497–2510 (2011).
Katakai, T. et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J. Immunol. 181, 6189–6200 (2008).
Chai, Q. et al. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity 38, 1013–1024 (2013).
Katakai, T. Marginal reticular cells: a stromal subset directly descended from the lymphoid tissue organizer. Front. Immunol. 3, 200 (2012).
Buch, T. et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426 (2005).
Fletcher, A.L. et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front. Immunol. 2, 35 (2011).
Cervantes-Barragan, L. et al. Dendritic cell-specific antigen delivery by coronavirus vaccine vectors induces long-lasting protective antiviral and antitumor immunity. mBio 1, 4 (2010).
Cupovic, J. Low Avidity CD8+ T cells In Viral Infection from Neuroinflammation to Adoptive T Cell Therapy. PhD thesis, Eidgenössische Technische Hochschule Zürich (2014).
Gonzalez, S.F. et al. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat. Immunol. 11, 427–434 (2010).
Chyou, S. et al. Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J. Immunol. 181, 3887–3896 (2008).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 196, 65–75 (2002).
Mackay, F. & Browning, J.L. BAFF: a fundamental survival factor for B cells. Nat. Rev. Immunol. 2, 465–475 (2002).
Mackay, F. et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190, 1697–1710 (1999).
Gorelik, L. et al. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J. Exp. Med. 198, 937–945 (2003).
Garin, A. et al. Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity 33, 84–95 (2010).
Gonzalez, S.F. et al. Trafficking of B cell antigen in lymph nodes. Annu. Rev. Immunol. 29, 215–233 (2011).
Bajénoff, M. & Germain, R.N. B-cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells. Blood 114, 4989–4997 (2009).
Alimzhanov, M.B. et al. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc. Natl. Acad. Sci. USA 94, 9302–9307 (1997).
Boulianne, B. et al. AID-expressing germinal center B cells cluster normally within lymph node follicles in the absence of FDC-M1+ CD35+ follicular dendritic cells but dissipate prematurely. J. Immunol. 191, 4521–4530 (2013).
Browning, J.L. et al. Lymphotoxin-β receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 23, 539–550 (2005).
Koni, P.A. et al. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity 6, 491–500 (1997).
Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3, 292–303 (2003).
Jarjour, M. et al. Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J. Exp. Med. 211, 1109–1122 (2014).
Yang, C.Y. et al. Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proc. Natl. Acad. Sci. USA 111, E109–E118 (2014).
Junt, T., Scandella, E. & Ludewig, B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat. Rev. Immunol. 8, 764–775 (2008).
Koning, J.J. & Mebius, R.E. Interdependence of stromal and immune cells for lymph node function. Trends Immunol. 33, 264–270 (2012).
Malhotra, D., Fletcher, A.L. & Turley, S.J. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol. Rev. 251, 160–176 (2013).
Scandella, E. et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat. Immunol. 9, 667–675 (2008).
Acknowledgements
We thank L. Cameron for technical assistance at the Dana-Farber cancer Institute Confocal Imaging core. Supported by the US National Institutes of Health (5R01 DK074500-08, 2P01AI045757-15, R21 CA182598-01 to S.J.T.; R01 AI039246, P01 AI078897 and R37 AI054636 to M.C.C.; and T32 CA 070083-15 to V.C.), the Barr Foundation (S.J.T.), the Cancer Research Institute (V.C.), the Vontobel Foundation (to B.L.) and the Helmut Horten Foundation (to B.L.).
Author information
Authors and Affiliations
Contributions
V.C. and M.C.W. designed and performed experiments and analyzed results; L.O., J.Cu., J.M.N.-B., F.A.S., J.Ch., F.C. and C.J.H. performed experiments; K.W. provided reagents and critical input on the manuscript; B.L. provided Ccl19-Cre mice and critical input on the manuscript; M.C.C. and S.J.T. designed and supervised the study; and V.C. and S.J.T. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Generation and characterization of Ccl19-Cre × Rosa26-eYFP and Ccl19-Cre × iDTR mice.
(a) EYFP expression is achieved in Ccl19-Cre x Rosa26-EYFP mice following Cre-mediated excision of a loxP-flanked transcriptional “stop” sequence. (b) Representative flow cytometric profile of freshly isolated stromal cells from skin draining lymph nodes (n>10 mice from 3 independent experiments). (c) In Ccl19-Cre x iDTR mice, the DTxn receptor gene is expressed following Cre-mediated recombination. (d) Confocal analysis of popliteal lymph nodes from DTR+Cre+ animals 24 h following DTxn administration stained for IgD, RANK-L and MadCAM. Scale bars 100 μm (n=3 mice from 3 independent experiments). (e) Lymph nodes from Ccl19-Cre x iDTR mice were collected, lysed in trizol and analyzed for the presence of Rank-l transcript. Lines indicate mean. Data are normalized to cyclophillin. Each data point represents one lymph node from individual mice (n=4-10 mice/group from 2-3 separate experiments). NS non significant, * p<0.05
Supplementary Figure 2 Injection of DTxn does not result in systemic toxicity or inflammation.
(a) Animal weight was determined following DTxn administration (n=3-4 mice/group per experiment from two independent experiments). (b) Lymph nodes from Ccl19-Cre x iDTR mice were collected and processed into single cell suspensions. Neutrophils (CD45+CD11b+Gr1high), monocytes (CD45+CD11b+Gr1–/low) and dendritic cells (CD45+CD11c+MHCII+) were enumerated using flow cytometric analysis. Data are representative of at least three independent experiments (mean+/-sd, n=3 mice/group per experiment). (c) Lymph nodes from Ccl19-Cre x iDTR mice were collected, lysed in trizol and analyzed for expression of inflammatory mediator genes. Lines indicate mean. Data are normalized to cyclophillin. Each data point represents one lymph node from individual mice (n=4-10 mice/group from 2-3 separate experiments). NS non significant, *p<0.05, **p<0.01, ***p<0.001
Supplementary Figure 3 Effects of the ablation of FRCs on T cells and B cells.
(a,b) Lymph nodes from Ccl19-Cre x iDTR mice were collected and processed into single cell suspensions. CD4+ and CD8+ T cells were enumerated using flow cytometric analysis (CD45+B220–CD3+CD4+/–CD8+/–). Data are representative of at least three independent experiments (mean+/-sd, n=4 mice/group per experiment). (c-e) Schematic representation of the experimental protocols utilized to assess T cell immunity. (f) Ccl19 promoter activity was evaluated in B cells from Ccl19-Cre x Rosa26-EYFP mice. (g) B cells from DTR+Cre+ mice were cultured in vitro in the presence of DTxn and viability was determined over the course of 72 h (n=3 wells/group). NS non significant, * p<0.01, **p<0.001
Supplementary Figure 4 Ablation of FRCs disrupts the organization of splenic white pulp and humoral responses of the marginal zone.
(a) Confocal analysis of spleen sections from Ccl19-Cre x Rosa26-EYFP mice stained for GFP, B220 and CD4. Scale bars 200 μm (left) and 50 μm (right) (n=3 mice from two separate experiments). (b-e) Spleens from Ccl19-Cre x Rosa26-EYFP-iDTR mice were processed into single cell suspensions and EYFP+cells were enumerated using flow cytometry. (mean+/-sd, n=3 mice). (f,g) Weight and total cell numbers for spleens of Ccl19-Cre x iDTR mice are indicated. Data are representative of at least two independent experiments (mean+/-sd, n=3 mice/group per experiment). (h) Confocal analysis of splenic white pulp from Ccl19-Cre x iDTR animals 24 h following DTxn administration. Scale bars 200 μm (n=3 mice from two independent experiments). (i) B cells in the spleen were enumerated using flow cytometric analysis (CD45+B220+CD3–). Data are representative of at least three independent experiments (mean+/-sd, n=3 mice/group per experiment). (j) Flow cytometric analysis of lymph nodes and spleen in DTR+Cre– and DTR+Cre+ animals. Fold decrease in FRC numbers at indicated time points is shown. (k) Marginal zone humoral responses were assessed by determining antigen-specific IgM production via ELISA following TNP-ficoll immunization. NS non significant, *p<0.05, **p<0.01
Supplementary Figure 5 Peyer’s patches are affected only marginally by the ablation of FRCs.
(a) Confocal analysis of Peyer’s patches sections from Ccl19-Cre x Rosa26-EYFP mice stained for GFP, PDPN and RANK-L. Scale bar 150 μm (n=3 mice from two independent experiments). (b,c) Peyer’s patches from Ccl19-Cre x iDTR mice were processed into single cell suspensions and stromal and B cells were enumerated using flow cytometry 72 h after DTxn administration. The figure shows two combined experiments (mean+/-sd, n=3 mice/group per experiment). (d) Confocal analysis of Peyer’s patches from Ccl19-Cre x iDTR animals 72 h following DTxn administration. Scale bars 400 μm (n=3 mice from two independent experiments). NS non significant, *p<0.05
Supplementary Figure 6 FRCs promote B cell migration.
(a) Ccl19-Cre x iDTR mice were injected with CFSE-labeled, CD45.1+ congenic B cells 24 h after DTxn administration. Homing of B cells to lymph nodes was evaluated 90 min or 24 h later. Data are representative of three independent experiments (mean+/-sd, n=3 mice/group per experiment). (b) Lymph nodes from Ccl19-Cre x iDTR mice were collected, lysed in trizol and analyzed for the presence of Cxcl12 transcript. Lines indicate mean. Data are normalized to cyclophillin. Each data point represents one lymph node from individual mice (n=4-10 mice/group from 2-3 separate experiments). (c) Purified FRCs were cultured in a 24 well plate. CFSE-labeled B cells were seeded on top of a transwell insert and allowed to migrate towards the FRCs for 2 h. After that time, cells were recovered and B cells enumerated by flow cytometry. The figure shows three combined experiments (mean+/-sd, n= 3 wells/condition per experiment). (d) WT mice received one single dose of CD62L-specific antibody i.p. and B cell numbers were determined in lymph nodes at different time points. The figure shows two combined experiments (mean+/-sd, n=3 mice/group per experiment). (e) Ccl19-Cre x iDTR mice were injected with 1 mg/kg FTY720 i.p. and, 4 h later, with DTxn. B cell numbers were determined 24 or 72 h after DTxn administration. For analysis at 72 h, mice received an additional dose of FTY720. The figure shows two combined experiments (mean+/- sd, n= 3-4 mice/group per experiment). NS non significant, *p<0.05, **p<0.01, ***p<0.001
Supplementary Figure 7 Functional consequences of the ablation of FRCs.
(a) Ccl19-Cre x iDTR mice were injected with DTxn, followed by passive immunization with rabbit anti-phycoerythrin. 18 h later, mice received phycoerythrin (PE) in the footpad and the popliteal node was imaged 6 h later for B220, FDCM1 and PE. (n=3 mice). (b) Serum was collected from Ccl19-Cre x iDTR mice 3 days after DTxn administration and levels of circulating BAFF were determined by flow cytometry-based bead assay. Each dot represents a mouse. (c,d) Ccl19-Cre x iDTR mice were injected in the footpad with 0.5 ng/g DTxn and numbers of FRCs and B cells were determined by flow cytometry 72 h later. Data are representative of two independent experiments (mean+/-sd, n=3 mice/group per experiment). (e) After enzymatic digestion of lymph nodes, cell suspensions were left untreated in complete medium or treated with protease inhibitors at room temperature. BAFF staining was evaluated by flow cytometry at different time points. Data are representative of three independent experiments. (f) CFSE-labeled purified B cells were cultured in vitro for 5 days in contact with FRCs, separated from FRCs by a transwell filter (TW), or with FRC-conditioned culture supernatant. B cell proliferation was determined as CFSE dilution in B220+ cells. NS non significant, * p<0.001
Supplementary Figure 8 Model.
Juxtaposed to marginal reticular cells (MRCs) underneath the subcapsular sinus and at the boundary between T and B cell zones, FRCs nurture B cells by producing BAFF. Ablation of FRCs has detrimental effects on B cell homeostasis: the architectural integrity of primary follicles is lost and B cell viability is profoundly decreased, leading to a significant loss of B lymphocytes.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 5828 kb)
Source data
Rights and permissions
About this article
Cite this article
Cremasco, V., Woodruff, M., Onder, L. et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol 15, 973–981 (2014). https://doi.org/10.1038/ni.2965
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ni.2965
This article is cited by
-
Lymph node homeostasis and adaptation to immune challenge resolved by fibroblast network mechanics
Nature Immunology (2022)
-
Neonatal LTβR signaling is required for the accumulation of eosinophils in the inflamed adult mesenteric lymph node
Mucosal Immunology (2022)
-
Multitier mechanics control stromal adaptations in the swelling lymph node
Nature Immunology (2022)
-
Mesenchymal stem cells empower T cells in the lymph nodes via MCP-1/PD-L1 axis
Cell Death & Disease (2022)
-
LRRC15+ myofibroblasts dictate the stromal setpoint to suppress tumour immunity
Nature (2022)