Abstract
Anxiety, a behavioral consequence of stress, has been characterized in humans and some vertebrates but remains largely unexplored in invertebrates. Here, we demonstrate that after being exposed to fish water, which simulates the presence of predators, pond snails (Lymnaea stagnalis) exhibit a series of sustained fear responses. These include increased aerial respiration, changes in righting behavior, and reduced escape responses. Notably, these behaviors persist even after the stressor (fish water) is removed, indicating that they likely represent an anxiety-like state rather than a simple conditioned reflex. Additionally, exposure to fish water enhances long-term memory formation for the operant conditioning of aerial respiration, suggesting that the predator scent potentially induces a state of heightened alertness, which enhances memory consolidation processes. Furthermore, when snails experience fish water alongside an appetitive stimulus (carrot), they form configural learning—a higher form of learning – where the appetitive stimulus now triggers a fear response instead of eliciting feeding. Importantly, the anxiolytic drug alprazolam prevents these anxiety-like responses. Through dose-response experiments, we found that alprazolam at a concentration of 0.1 µM for 15 min effectively counteracts predator-induced anxiety without causing sedation. This treatment also prevents the effects of predator cues on learning and memory. However, consistent with data from vertebrates - alprazolam induces anterograde amnesia, impairing the formation of new memories for up to 3 h after treatment, though it does not cause long-term memory deficits. Overall, this is the first study showing that a molluscan model organism exhibits anxiety-like behaviors similar to those seen in vertebrates, and these behaviors can be mitigated by an anti-anxiety drug. This suggests that fundamental anxiety mechanisms are evolutionarily conserved across species. By using this simple invertebrate model, our research offers new insights into the biological basis of anxiety and sets the stage for future pharmacological studies.
Similar content being viewed by others
Introduction
Fear and anxiety are complex behavioral responses to environmental threats characterized by increased arousal, expectancy, autonomic and neuroendocrine activation, and behavioral reduction of typical behaviors (like exploration and feeding) to facilitate coping with adverse or unexpected situations [1, 2]. Fear is an adaptive mechanism that typically arises in response to a real or identifiable threat, triggering an immediate protective reaction to avoid harm [3]. The amount of risk that an animal perceives is associated with the degree of fear and the response of the animal to any fear state depends on the different factors associated with risk. In the case of predation threat for instance the predator size, distance from a predator, and safe refuges around would all serve as stimuli that an animal assesses before responding to the risk and which contribute to the state of fearfulness in the animal [4]. Anxiety, instead, occurs even in the absence of a real threat or risk, leading to an exaggerated response, which can cause unnecessary physiological stress [5]. Anxiety is thus a state that allows for prediction and preparedness for uncertainty [6]. Anxiety and fear go hand in hand as both have the same evolutionary root wherein fear is transient, requires the presence of a threat cue, is context-dependent and anxiety is a continuation of this fear state in the absence of the threat stimulus. Evolution of anxiety has thus occurred because survival is more important than the quality of life and over-preparedness for an uncertain threat can increase chances of survival under dynamically risky environments [7]. However, when anxiety becomes excessive, it can be maladaptive and lead to anxiety-related disorders, which may interfere with the ability to cope with stressful events [8] and affect cognitive functions [9]. Anxiety-related disorders are among the most prevalent psychiatric conditions, impacting 7–10% of the global population, with their incidence steadily rising each year [10]. Despite their widespread nature, the precise etiopathogenetic mechanisms underlying these disorders remain unclear [11].
Animal models are essential for unraveling the pathogenic mechanisms of anxiety disorders, offering critical insights that drive the development of safe and effective therapies [11]. Although non-human organisms cannot fully replicate the complexity of human fear and anxiety disorders, these behaviors can be broken down into fundamental components, enabling the study of their underlying biological mechanisms [12]. In particular, anxiety can be dissected into three primary components: (1) the perception and evaluation of a threatening stimulus, (2) its cognitive and physiological processing, and (3) the resulting behavioral response [13]. These elements can be investigated across a wide range of species, as most animals possess an innate ability to perceive danger, adapt their behavior accordingly, and learn to associate specific cues with threats, allowing them to respond effectively to harmful stimuli [14]. Thus, the anxiety and fear that have evolved under strong survival selection pressure must be present across taxa in the Tree of Life. In rodent models, anxiety is often assessed through behavioral tests that exploit the approach-avoidance conflict, measuring the animals’ drive to explore novel environments (approach) against their instinct to avoid potential dangers (avoidance) [15, 16].
In recent years, studies on zebrafish have broadened the concept of anxiety to include all vertebrates [17], while research on Aplysia and bees has suggested that some aspects of the anxiety response are even conserved in invertebrates [18,19,20,21,22]. Notably, in 2014, Fossat and colleagues demonstrated that crayfish exhibit anxiety-like behaviors similar to those seen in vertebrates, indicating that several underlying mechanisms have been conserved from invertebrates to humans [22]. In particular, using a dark/light maze, which is similar to the elevated plus-maze used with rodents, it has been shown that crayfish exposed to electric shocks develop a context-independent and long-lasting avoidance behavior that mirrors the anxiety responses observed in rodents [22]. Additionally, this anxiety-like behavior can be mitigated by chlordiazepoxide, a potent anxiolytic drug that modulates GABA type A receptors, suggesting that GABA plays a role in regulating anxiety-like behavior in crayfish as in mammals [18, 22,23,24]. Overall, these ground-breaking findings paved the way for additional studies aimed at investigating the ability of other invertebrates to exhibit anxiety-like behaviors [25] akin to those observed in mammals. Research utilizing invertebrate models in translational psychiatry offers notable experimental efficiency due to several advantages. These include shorter generation times, higher reproductive rates, and reduced costs for animal care [26] compared to mammals. Moreover, invertebrates are more amenable to experimental manipulation, enabling streamlined studies and rapid generation of data, which can significantly accelerate the research pipeline [27,28,29,30,31,32,33,34,35,36].
Building on these foundations, this study examined whether the pond snail Lymnaea stagnalis (L. stagnalis, Linnaeus, 1758) exhibits anxiety-like behaviors and whether these behaviors can be modulated by anxiolytic treatments [28, 34, 37]. Since the 1970s, L. stagnalis has been widely used to study conserved neurobiological mechanisms, showcasing advanced forms of learning [38, 39] and memory once thought to be exclusive to mammals, such as the Garcia effect—taste-specific conditioned aversion requiring visceral sickness [40, 41]—and configural learning [42, 43]. Additionally, prior research has demonstrated that various stressors, including severe fasting [29, 44,45,46,47], bacterial endotoxins (lipopolysaccharide) [48, 49], predator scent [50, 51], thermal shock [52, 53], and overcrowding [54, 55], can significantly alter the snails’ behavior and memory formation, either enhancing or impairing them depending on the intensity and timing of the stressor [55]. Remarkably, laboratory-bred L. stagnalis snails, maintained under controlled conditions for over 250 generations, retain the ability to detect and respond to historically sympatric predators, despite never encountering them [51]. This unique feature allows the use of predator stress to explore maladaptive and adaptive responses to threats in preclinical settings. For example, we demonstrated that exposing snails to a noxious stimulus followed by the scent of a predator (e.g., crayfish) induces a state of emotional arousal reminiscent of fear and anxiety observed in rodents exposed to predator cues [51, 56].
Another advantage of L. stagnalis as a model organism for pharmacological studies is its open circulatory system and lack of a blood-brain barrier, which facilitates drug administration [57,58,59].
Compounds can be delivered by immersing the snails in a drug-containing tank, ensuring efficient delivery to the central ring ganglia. This approach has been used successfully with various drugs, including acetylsalicylic acid (an anti-inflammatory) [49, 60, 61], propranolol (a β-adrenergic receptor blocker) [25], and bioactive compounds like carnosine [62] and the flavonoid quercetin [42, 48, 63, 64]. Notably, administering propranolol before operant conditioning training selectively impaired the consolidation of emotional memories enhanced by nociception and predator exposure [25]. This provided the first evidence in a molluscan model that stressor-evoked memories can be categorized as emotional or non-emotional and selectively modulated pharmacologically [25].
Based on these findings, in this study, we developed and validated a behavioral paradigm to investigate, for the first time in a molluscan model, the anxiogenic effects of predator exposure on behavior. Next, we investigated whether the benzodiazepine alprazolam could prevent the anxiogenic effects of predator exposure in L. stagnalis. Alprazolam, an anti-anxiety drug, enhances GABA’s action at GABA-A receptors, increasing chloride ion influx into neurons [24, 65,66,67]. Pharmacological and molecular evidence supports the presence of functional GABA receptors in Lymnaea, underscoring the model’s validity for studying the effects of alprazolam [24, 68]. Thus, we determined the optimal dose and treatment duration of alprazolam in snails and assessed its sedative and anxiolytic effects as well as its potential to induce anterograde amnesia —defined as the inability to form new memories following a specific event, injury, or drug exposure, while previously established memories remain intact [69,70,71]. Since temporary anterograde amnesia is a well-documented side effect of benzodiazepines [71], testing this effect in a model organism with well-characterized learning and memory processes [38, 39] provides valuable insight into how alprazolam influences memory retention. By evaluating whether snails can learn and form memory after alprazolam exposure, we can further investigate the mechanisms underlying anterograde amnesia and the broader effects of benzodiazepines across species [72].
To our knowledge, this is the first study to investigate anxiety-like behavior in a molluscan model. These findings pave the way for future research aimed at dissecting the components of anxiety-like behavior and evaluating anxiolytic drugs in non-vertebrate organisms. Additionally, the evolutionary perspective of inclusivity of different model systems to understand the primary underlying principles of processes like fear and anxiety opens the doors to investigating more questions on other complex cognitive functions that till now were only being researched on in vertebrates. This study brings together theories from evolutionary biology, neurobiology, and pharmacology to investigate anxiety which is the most prevalent psychiatric condition in humans and predictively in other animal systems in recent times.
Material and methods
Snails
L. stagnalis used in this study were bred and raised in the snail facility at the University of Modena and Reggio Emilia (Italy) from the strain from Vrije Universiteit in Amsterdam. The ancestors of these snails were obtained from ditches in a polder located near Utrecht in the early 1950s and since then they have not been exposed to naturally occurring predators. In this study, we used adult (6 months old) snails, with a ~2.5 cm shell length. Snails were housed in home aquaria filled with artificial pond water (PW) (0.26 g L⁻¹ Instant Ocean; Spectrum Brands Inc., Madison, WI, USA) with calcium sulfate dihydrate added to maintain a Ca²⁺ concentration of 80 mg L⁻¹. They were kept at room temperature (20–22 °C) under a 12 h:12 h light-dark cycle (L:D) and fed lettuce ad libitum until experimental treatment [40, 59, 73, 74].
Predator scent
Snails innately detect and respond to crayfish predators with anti-predator behaviors [42, 50, 51, 54, 75, 76]. In this study, we expanded on this by using fish predators and pseudo-predators (Cyprinus carpio and Carassius auratus) to investigate whether similar responses occur across different predator types. Carp and goldfish were housed in 50 L and 20 L aquariums, respectively, maintained at a temperature of 22 °C, and fed fish pellets and snails twice a week. The tanks were equipped with a filtration system and aeration to ensure optimal water quality. Each aquarium contained 3–5 fish. The water in the fish tank, referred to as fish water (i.e., FW), was collected after the fish had inhabited the tanks for 7 days. Thus, in this study, snails were never directly exposed to fish but only to their waters.
Alprazolam treatment
To prepare a stock solution of Alprazolam 10 mM [77], we dissolved 3 mg of alprazolam (MW: 308,765 g/mol) in 1 mL of Dimethyl sulfoxide (DMSO) [65, 67, 78]. This stock solution was defined based on previous studies from rodent models. From this stock, we made the following dilutions in 500 mL of artificial pond water (PW): 0.1, 1, and 10 μM. Adult animals (25–30 mm in shell length) were exposed to these doses for 15, 30, 45, or 60 min. Based on our previous studies, we recorded snails’ feeding behavior in lettuce slurry (i.e., a familiar taste, made by blending and straining a mixture of two leaves of romaine lettuce along with 500 mL of artificial PW) three hours before and at different times points after treatment [52, 60]. As feeding is a homeostatic and robust behavior in L. stagnalis [38, 60], any reduction in the number of rasps (i.e., repeated movements of the radulae scraping the surface of a substrate, leading to the ingestion of food) elicited by lettuce slurry was considered indicative of an aversive/sedative effect of the treatment [40]. To observe and record snails’ feeding behavior, animals were placed in a 14 cm Petri dish with enough slurry for them to be partially submerged [58].
Anxiety-like behaviors: aerial respiration, righting time, and escape
Anxiety triggers a range of adaptive or defensive behaviors aimed at escaping from potential threats or resolving motivational conflicts, with specific behaviors varying according to context and species [79]. To assess whether exposure to FW induces anxiety-like behaviors in our model organism, we focused on three key indicators of prey vigilance: (1) aerial respiration [80], (2) righting time after dislodgement from the surface [75], and (3) exploratory (escape) behavior [81]. These behaviors have been strongly validated and characterized in our model organism [75]. Notably, an increase in aerial respiration, alongside reductions in righting time and escape behavior following FW exposure, were considered predator-induced fear responses [6, 14, 22]. If these behaviors persisted 18 h after FW exposure in a different context (i.e., PW), they were considered indicative of anxiety-like behavior, pointing to a stress-induced response rather than an innate behavioral response.
Aerial respiration
L. stagnalis is a bimodal breather, obtaining oxygen either through their skin or, in a hypoxic environment, via aerial respiration. In such conditions, they move to the water surface and open their pneumostome (i.e., respiratory orifice) [80]. To assess snails’ aerial respiration, we measured the duration of pneumostome opening (i.e., total breathing time – TBT) in hypoxic (PO2 < 931 Pa) artificial PW for 30 min. Hypoxia was induced by bubbling N2 gas through the water for 20 min. Three hours later, we recorded the TBT in hypoxic FW, and again in artificial hypoxic PW after another 18 h. A significant increase in TBT in hypoxic FW compared to artificial PW indicated antipredator behavior. The TBT of control snails was recorded in hypoxic artificial PW throughout the experiment.
Righting behavior
To assess righting behavior, snails were first exposed to soaked filter paper with artificial PW for 2 min, then flipped onto their dorsal surface using a wooden stick. Each snail was dislodged 5 times, and the average righting time was recorded for each treatment [75]. After a 3-h interval, snails were exposed to soaked filter paper with FW for 2 min, and their righting time was recorded again. The procedure was repeated 18 h later in artificial PW. Control snails were exposed to artificial PW instead of FW throughout the experiment.
Exploratory behavior
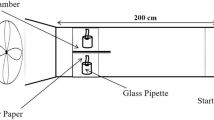
Finally, we tested exploratory behavior, an indicator of vigilance that manifests itself in snails’ natural tendency to explore their surroundings (i.e., escaping from a lid). Each snail was placed in the lid of a 35 mm plastic Petri dish filled with artificial PW [81, 82]. The lids were positioned on soaked filter paper with artificial PW and, 3 h later, with FW.
The number of escape attempts was recorded over 20 min, with subsequent recordings 18 h later in artificial PW. An “escape” was defined when the snail extended its head out of the lid and contacted the water. Control snails were exposed to artificial PW instead of FW throughout the experiment.
Learning and memory paradigms: operant conditioning of aerial respiration and configural learning
To test whether exposure to FW would enhance long-term memory (LTM) formation (i.e., lasting for at least 24 h) for the operant conditioning of aerial respiration, snails were removed from home aquaria and exposed to FW for 1 h [83, 84]. Three hours later, snails were put into a 1 L beaker containing 500 mL of hypoxic PW and given a 10-min acclimatization before a 30-min training session (TS). During the TS, snails were operantly conditioned by applying a gentle tactile stimulus with a sharpened wooden stick to their pneumostome as it began to open (i.e., an attempted pneumostome opening). The stimulus was strong enough to cause the snails to close the pneumostome yet gentle enough that the snails did not perform the full body withdrawal response [80]. Every time the snail opened its pneumostome, it received a poke. The snails were then tested for memory formation (MT) using a procedure similar to that of the training. Memory was defined as a significant reduction in the number of pneumostome openings after TS. This behavioral protocol typically results in an intermediate-term memory (ITM) lasting for at least 3 but not 24 h [85]. Thus, if following FW exposure LTM is formed (i.e., a significant reduction in the number of attempted pneumostome openings is found when snails are tested at 24 h post-TS), this suggests a FW-induced memory enhancement. Control snails were exposed to artificial PW for 1 h instead of FW.
Operant conditioning of aerial respiration was also adopted to study the effects of alprazolam treatment on learning and memory formation. As this learning paradigm is extremely robust [85], any memory impairment observed would suggest a memory block (i.e., anterograde amnesia) induced by the treatment.
In this study, we also investigated whether the simultaneous exposure to two contrasting stimuli together, such as FW and food taste, resulted in configural learning, a higher form of learning [42, 86]. In particular, we investigated whether snails can assign a new ‘meaning’ to the food stimulus as a result of experiencing it simultaneously in the presence of FW, which evokes anti-predator responses. If snails form configural learning, the food stimulus becomes a risk signal and evokes a significant reduction in feeding, which is part of a suite of anti-predator behaviors, phenotypically similar to those elicited by exposure to FW. In our validated configural learning procedure [86], snails were first exposed to an appetitive stimulus, carrot slurry (prepared by mixing and blending two medium-sized organic carrots along with 500 mL of artificial PW), and the number of rasps was recorded for 2 min [29]. To observe and record the snails’ feeding behavior, the animals were placed in a 14 cm Petri dish with enough carrot slurry for them to be partially submerged. The snails were given a 3-min acclimation period in each session. Animals were then returned to their home aquaria for 18 h, before being exposed simultaneously to carrot slurry + FW for 45 min.
Three hours later, the number of rasps elicited by the carrot slurry alone was calculated. A significant reduction in feeding response elicited by the carrot slurry alone following its simultaneous presentation with FW suggests that snails have the ability to undergo configural learning.
2D PCA
To compare all experimental groups, we performed principal component analysis (PCA). We first classified the data into four distinct groups: (1) Control snails not exposed to FW, not treated with alprazolam, and tested in PW, (2) Predator-exposed snails, not treated with alprazolam, and tested in FW, (3) Predator-exposed snails, not treated with alprazolam, and re-tested in PW 18 h after being exposed to FW, and (4) alprazolam-treated snails before being exposed to FW. These groups were represented in purple, blue, green, and yellow, respectively. To analyze the behavioral data—TBT, righting time, and exploratory behavior—we performed a 2D Principal Component Analysis. The PCA plot revealed the distribution of data across two principal components, PC1 and PC2, which represent the most significant variance within the dataset. Data points are further distinguished by shape—circles and triangles—to indicate two experimental conditions: “PW” and “FW.” PC1 and PC2 serve as the primary axes of data distribution, with PC1 capturing the highest variance and PC2 the second highest. Black arrows with red labels (vectors) indicate the direction and influence of specific behavioral metrics on the principal components. Colored ellipses surrounding each group represent 95% confidence intervals, showing where the majority of data points are concentrated within each category. These ellipses illustrate the variance within and overlap among the groups, providing insights into how different experimental conditions cluster and separate along the principal components.
Experimental design and statistical analysis
The sample size for each experiment was determined through a power analysis conducted using G*Power, ensuring adequate statistical power to detect a predefined effect size and minimizing the risk of Type II errors while accounting for variability in behavioral responses. Animals were randomly selected from their aquaria and assigned to experimental groups through a randomized allocation process, ensuring unbiased group distribution. Exclusion criteria were not predefined, data were screened for outliers using the ‘outlier’ function in SPSS (version 23.0, IBM Inc., Armonk, NY, USA), though no outliers were identified. The researchers conducting the behavioral experiments were not blinded to group allocation, whereas those performing the data analysis were blinded to group assignment throughout the study. Statistical analyses were performed using GraphPad Prism (version 10.2, GraphPad Software Inc., La Jolla, CA, USA) and SPSS (version 23.0, IBM Inc., Armonk, NY, USA). Data are presented as mean ± SEM. Prior to statistical analysis, the normality of the data was evaluated using the Shapiro-Wilk test, and homogeneity of variances was assessed using Levene’s test. For normally distributed data with equal variances, differences between two groups were evaluated using a paired Student’s t-test. Differences among three or more groups were analyzed using one-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons. For non-normally distributed data, the Kruskal-Wallis H test was employed, followed by Dunn’s post-hoc test for pairwise comparisons among groups. In cases involving non-parametric data with repeated measures, the Friedman test was used to assess differences between groups, with Dunn’s post-hoc test used to identify specific group differences. Statistical significance was set at p < 0.05 for all tests.
Results
The exposure to the fish effluent increases snails’ aerial respiration and reduces snails’ righting time and exploratory behavior
Our study first revealed that exposure to hypoxic FW significantly increased snails’ aerial respiratory behavior (Friedman test: F = 27.70; P < 0.0001, Fig. 1A). Specifically, there was a significant increase in aerial respiration in FW compared to PW (Dunn’s post hoc: p < 0.0001). Notably, this heightened respiration persisted 18 h after FW exposure, even in a different context (PW) (Dunn’s post hoc: p = 0.0001), indicating a long-lasting, context-independent effect. Control snails, tested in hypoxic PW three times without FW exposure, showed no significant changes in aerial respiration (RM ANOVA: F 10, 20 = 82.62; P < 0.0001, Fig. 1B), confirming that the increased respiration in the experimental group was specifically linked to FW exposure and not due to repeated testing or hypoxic conditions alone. Next, we observed that FW exposure significantly reduced the time it took for snails to right themselves from a vulnerable position (i.e., with the ventral part of the foot exposed and away from the substrate) and regain their foothold, minimizing the time spent upside-down (RM ANOVA: F 19,38 = 2.33; P = 0.017 - Fig. 1C). In particular, the righting time was significantly shorter after FW exposure compared to PW (Tukey’s post hoc test: p < 0.0001, q = 12.88), and this heightened responsiveness persisted 18 h later in PW (Tukey’s post hoc test: p = 0.005, q = 6.61), suggesting that the snails remained on high alert, while a significant increase - albeit slight - in the righting time was observed when animals were re-tested in PW after being exposed to FW (p = 0.011 and q = 4.57).
A timeline of the experiment is presented above the data. A-B Aerial Respiration. A Naïve snails (N = 20) were placed in hypoxic artificial pond water (PW) for 30 min (beige bars), and the total breathing time was recorded. Three hours later, the total breathing time was recorded in hypoxic fish water (FW) (green bar). A significant increase in aerial respiration was observed in FW compared to artificial PW. This anti-predatory behavior persisted 18 h later when aerial respiration was recorded again in artificial PW (beige bars). B Control snails (N = 11) exposed only to artificial PW (beige bars) showed no significant differences in total breathing time. C-D Righting Time. C Naïve snails (N = 20) were placed in a vulnerable position with their ventral part exposed, and the righting time was recorded after exposure to PW (beige bar) and 3 h later to FW (green bar). Snails significantly decreased their righting time following FW exposure. This reduced righting time persisted when tested 18 h later in artificial PW. D Control snails (N = 10) exposed only to artificial PW (beige bars) showed no significant differences in righting time. E-F Escape Behavior. E Naïve snails (N = 20) were placed in a lid containing artificial PW. The lids were placed on filter paper soaked with artificial PW, and the number of escapes was recorded for 30 min (beige bar). Three hours later, the lids were placed on filter paper soaked with FW, and the number of escapes was recorded again (green bar). A significant reduction in the number of escapes was found following FW exposure. This reduced escape behavior persisted when tested 18 h later in artificial PW. F Control snails (N = 10) exposed only to artificial PW (beige bars) showed no significant differences in escape behavior. The data demonstrate that exposure to FW induces significant anti-predatory behaviors in snails, evidenced by increased aerial respiration, decreased righting time, and reduced escape behavior. These responses persisted even after the initial FW exposure, indicating a lasting behavioral change. Control snails that were not exposed to FW did not show significant changes in any of the measured behaviors, confirming that the observed effects were specific to the FW exposure. Data shown in Fig. 1A were analyzed using the Friedman test followed by Dunn’s post-hoc test, whereas data shown in Fig. 1B-F were analyzed using repeated measures ANOVA (RM ANOVA) followed by Tukey post hoc tests B-F. **** p < 0.0001, *** p < 0.001, * p < 0.05, ns = not significant (p > 0.05). The solid line represents the mean, and the error bars represent the standard error of the mean (SEM.).
Control snails not exposed to FW showed no significant differences in righting time across trials (RM ANOVA: F9,18 = 1.036; P = 0.45, Fig. 1D), indicating that the observed behavioral changes in the experimental group were specifically due to FW exposure and not repeated testing or handling. Finally, we found that FW exposure significantly reduced snails’ exploratory behavior (RM ANOVA: F 19,38 = 2,16; P = 0.0213, Fig. 1E). The number of escape attempts was significantly lower in FW compared to PW (Tukey’s post hoc test: p < 0.0001, q = 11.33), and this reduction persisted 18 h later when tested in PW (Tukey’s post hoc test: p < 0.0001, q = 13.07). Control snails not exposed to FW showed no significant differences in exploratory behavior across trials (RM ANOVA: F 9,18 = 2.26; P = 0.067, Fig. 1F), further supporting that the behavioral changes observed in the experimental group were specifically due to FW exposure and not repeated testing or handling.
These findings collectively demonstrate that FW exposure induced long-lasting anxiety-like behaviors in snails, as evidenced by increased aerial respiration, reduced righting time, and decreased exploratory behavior. Having shown that these anti-predatory behaviors persisted for at least 18 h after FW exposure in a different context (i.e., PW), they were considered indicative of anxiety-like behavior, pointing to a stress-induced response rather than an innate behavioral response. In other words, they reflected a stress-induced response rather than simple conditioned reflexes, providing a robust model for studying anxiety-like states and their modulation by pharmacological interventions.
Exposure to fish water enhances long-term memory for the operant conditioning of aerial respiration and results in configural learning
Next, we investigated whether exposing snails to FW before the 30-min TS would enhance LTM formation for the operant conditioning of aerial respiration (Fig. 2). Thus, snails (N = 10) were exposed to FW for 1 and, 3 h later, were trained with a single 30-min TS in PW. Twenty-four hours later, LTM was tested, and a significant reduction in the number of attempted pneumostome openings was found (paired t-test: t = 8.94, df = 19, p < 0.0001), indicating that LTM was enhanced (Fig. 2A). Control snails were exposed to artificial PW for 1 h instead of FW and consistent with previous studies [85]; LTM was not formed as no significant differences between TS and MT performed 24 h post-TS were found (paired t-test: t = 1.74, df = 19, p = 0.96) (Fig. 2B). The enhanced LTM formation following FW exposure suggests that FW may induce a state of heightened alertness or stress, potentially facilitating memory consolidation processes.
A timeline of the experiment is presented above the data. A Ten naïve snails were exposed to fish water (FW) for 1 h. Three h later, snails were trained with a single 30-min TS (grey circles), and long-term memory (LTM) was tested 24 h post-TS (i.e., MT – black circles). LTM was formed as a significant reduction in the number of attempted pneumostome openings was found. B Ten naïve snails were exposed to artificial PW for 1 h before being trained for 30 min 3 h later (TS - grey circles). Twenty-four hours after training, LTM was tested (MT – black circles). No significant reductions in attempted pneumostome openings were found between TS and MT, suggesting that LTM was not formed. Data are represented as means ± SEM and analyzed with a paired t-test. ****p < 0.0001, ns = not significant as p > 0.05.
We then tested whether snails possess the capacity to form LTM after the configural learning training following exposure to two conditions simultaneously: an appetitive taste (carrot slurry) and FW as the stressful stimulus. Forming LTM for configural learning requires snails to make a higher-order association between two opposing stimuli which in our study are an appetitive food stimulus and predator odour [86] (Fig. 3). Snails (N = 16) were first exposed to the carrot slurry (prepared using PW – C pre) and the number of rasps was counted for 2 min; 18 h later, animals were exposed to the carrot slurry in FW for 45 min and their response to the carrot slurry (in PW) was tested 3, 24, and 48 h later. Friedman test (F = 32.63; P < 0.0001 - Fig. 3A) revealed a significant reduction in the number of rasps at 3 and 24 h following the configural learning procedure (p < 0.0001 and p = 0.0047, respectively - Dunn’s post hoc). When tested 48 h post-configural learning procedure, the response to carrot slurry was not significantly different from C pre (p > 0.99), suggesting that configural learning LTM lasted for at least 24 but not 48 h. Control snails (N = 10), exposed only to carrot slurry (without simultaneous FW exposure), showed no significant differences in rasping behavior when tested at 3, 24, and 48 h (RM ANOVA: F9, 27 = 9.06; P < 0.0001 – Fig. 3B).
A timeline of the experiment is presented above the data. A Sixteen naïve snails were exposed to an appetitive taste (carrot slurry – C), and the number of rasps was recorded for 2 min. Eighteen hours later, snails were simultaneously exposed to fish water (FW) and C for 45 min (CL), and the number of rasps elicited by C alone was recorded 3, 24, and 48 h later. LTM for configural learning was formed as a significant reduction in the number of rasps was found at 3 and 24 h post-CL. At 48 h post-CL, no significant differences in the number of rasps compared to C pre were found, suggesting that LTM for CL lasted for at least 24 h but not 48 h. B Control snails (N = 10) were exposed to PW + C instead of FW + C. No significant differences in the number of rasps elicited by C were found, suggesting that CL was not formed, and the handling procedure did not affect snails’ feeding behavior. Data are represented as means ± SEM and analyzed. Data shown in Fig. 3A were analyzed using the Friedman test followed by Dunn’s post-hoc test, whereas data shown in Fig. 3B were analyzed with RM-ANOVA followed by Tukey post hoc. ****p < 0.0001, **p < 0.01, ns = not significant as p > 0.05.
To ensure that repeated exposure to carrot slurry did not influence snails’ feeding behavior and to confirm that the observed reduction was due to memory formation rather than handling procedures, we conducted control experiments (Supplementary Material). Two cohorts of naïve snails (N = 10 per group) were exposed to carrot slurry (prepared with PW) for 2 min, followed by exposure for 45 min to carrot slurry prepared with FW 18 h later. Memory formation for configural learning was assessed using carrot slurry (prepared with PW) after 24 h (Supplementary Fig. 1A) or 48 h (Supplementary Fig. 1B). Consistent with Fig. 3A, we observed a significant reduction in the number of rasps at 24 h but not at 48 h post-configural learning (paired t-test: t = 8.35, df = 9, p < 0.0001; t = 0.31, df=9, p = 0.76, respectively). Thus, our study shows for the first time FW can lead to LTM formation for the operant conditioning of aerial respiration and configural learning task in L. stagnalis.
Defining the optimal dose and exposure duration of alprazolam
We next examined whether a well-characterized anxiolytic drug, alprazolam, could prevent the behavioral changes induced by FW. First, we performed experiments aimed at defining the optimal dose and treatment duration, avoiding the sedative/hypnotic effects typically induced by benzodiazepine. Based on data from rodents [77, 78], we selected 3 doses: 0.1, 1, and 10 µM. For each dose, 4 groups of snails (N = 13 each group) were used, and the snails’ feeding behavior was tested 3 h after being exposed to alprazolam for 15, 30, and 60 min (Fig. 4). Control snails were not exposed to alprazolam.
A The timeline of the experiment is presented above the data. Snails were exposed to alprazolam 0.1 μM (B), 1 μM (C), or 10 μM (D) for 15, 30, and 60 min, and the feeding behavior was tested 3 h later in lettuce slurry. Control snails (CTRL) were not treated. We found that alprazolam 1 μM and 10 μM treatment led to a significant reduction in feeding behavior across all treatment durations (15, 30, and 60 min), with the sole exception of 0.1 μM for 15 min. N = 13-14. Data are represented as means ± SEM. Data shown in Figs. 4B and D were analyzed using the Kruskal-Wallis H test, followed by Dunn’s post-hoc, whereas data shown in Fig. 4C were analyzed with one-way ANOVA followed by Tukey post hoc. ****p < 0.0001, ** p < 0.01, ns = not significant as p > 0.05.
As feeding behavior is a robust homeostatic behavior [52, 57, 87], any significant reduction in the number of rasps in lettuce slurry (i.e., a familiar taste) post-treatment was considered indicative of a sedative effect induced by the treatment. We found that treating snails with alprazolam 1 or 10 µM induced a significant reduction in feeding behavior, regardless of the treatment duration (One-way ANOVA: F3, 48 = 43.75; P < 0.0001 - Fig. 4C and Kruskal-Wallis test: H = 30.39; P < 0.0001 - Fig. 4D). Similar results were observed when snails were treated with alprazolam 0.1 µM for 30 or 60 min, but not 15 min (Kruskal-Wallis test: H = 38.48; P < 0.0001, Fig. 4B). Thus, the dose of 0.1 µM for 15 min was identified as optimal because it did not significantly reduce feeding behavior, suggesting it avoided the sedative effects of alprazolam observed at higher doses and longer durations, making it a suitable condition for studying the anxiolytic effects of alprazolam without sedation. To ensure that the observed effects were due to alprazolam treatment rather than the potential toxicity of its vehicle, DMSO [64], we conducted additional control experiments. Three cohorts of naïve snails (N = 8, each group) were exposed to DMSO at 0.1 µM (Supplementary Fig. 2A), 1 µM (Supplementary Fig. 2B), or 10 µM (Supplementary Fig. 2C) for 15, 30, or 60 min. Their feeding behavior was then assessed in lettuce slurry 3 h later. Regardless of concentration or exposure duration, no significant differences in feeding behavior were observed compared to untreated control snails (One-way ANOVA: F3,28 = 0.49, P = 0.68, F3,28 = 1.28, P = 0.30, and F3,28 = 1.6, P = 0.21).
Exposing snails with alprazolam 0.1 µM for 15 min induces anti-anxiety effects
We next investigated whether treating snails with alprazolam 0.1 µM for 15 min could counteract the anti-predatory behaviors induced by FW (i.e., increased TBT and reduced righting time and exploratory behavior), indicating an anti-anxiety effect. Thus, 4 groups of snails (N = 20, each) were used for each behavioral procedure: (1) snails exposed to PW for 15 min and, 3 h later, tested in PW; (2) snails exposed to alprazolam 0.1 μM for 15 min and, 3 h later, tested in PW; (3) snails exposed to PW for 15 min and, 3 h later, tested in FW; (4) snails exposed to alprazolam 0.1 μM for 15 min and, 3 h later, tested in FW.
Consistent with the data shown in Fig. 1, FW exposure significantly increased aerial respiration (Kruskal-Wallis: H = 39.45, p < 0.0001; Fig. 5B) and reduced both righting time (Kruskal-Wallis: H = 37.48, p < 0.0001; Fig. 5C) and exploratory behavior (Kruskal-Wallis: H = 44.46, p < 0.0001; Fig. 5D). However, these FW-induced behavioral changes were prevented by alprazolam treatment for 15 min. Compared to untreated snails tested in FW, those treated with alprazolam and tested in FW showed a significant increase in TBT and a reduction in both righting time and escape behaviors (p < 0.0001, Dunn’s post-hoc for all).
A Study design: 4 distinct experimental conditions were used in this study: animals treated with PW for 15 min and tested in PW 3 h later (beige bars); animals treated with alprazolam 0.1 μM for 15 min and tested in PW 3 h later (diagonal beige bars); animals treated with PW for 15 min and tested in FW 3 h later (green bars), and animals treated with aprazolam 0.1 μM for 15 min and tested in FW 3 h later (diagonal green bars). B Aerial Respiration: alprazolam prevented the FW-induced increase in breathing time. C Righting Behavior: alprazolam prevented the FW-induced reduction in righting time. D Escape Behavior: alprazolam prevented the FW-induced reduction in escape behavior. Data are represented as means ± SEM and analyzed with the Kruskal-Wallis H test, followed by Dunn’s post-hoc. ****p < 0.0001, ns = not significant as p > 0.05.
No significant differences were observed between control snails (i.e., untreated snails tested in PW), those treated with alprazolam and tested in FW, and those treated with alprazolam and tested in PW. These findings confirm that alprazolam effectively prevents the behavioral changes induced by FW.
The effects induced by alprazolam 0.1 µM for 15 min last for at least 24 h but not 48 h
To test the duration of the effects induced by alprazolam 0.1 µM for 15 min, we focused on the exploratory behavior in FW (Fig. 6). We selected this behavior as it is largely used to test anxiety in mammals [88]. Five cohort of snails (N = 20, each) were used in this study: (1) snails exposed to PW for 15 min and tested in PW 18 h later (PW group); (2) snails exposed to PW for 15 min and tested in FW 18 h later (FW group); (3) snails treated with alprazolam 0.1 μM for 15 min and tested in FW 18 h later; (4) snails treated with alprazolam 0.1 μM for 15 min and tested in FW 24 h later; and (5) snails treated with alprazolam 0.1 μM for 15 min and tested in FW 48 h later (Fig. 6B). A one-way ANOVA revealed a significant main effect of the procedure (F₄,₉₅ = 22.38, P < 0.0001). Consistent with Fig. 1, the number of escapes in FW was significantly lower than in PW (Tukey’s post hoc: p < 0.0001; q = 10.23). However, snails treated with alprazolam and tested in FW at 18 and 24 h post-treatment showed no significant differences in escape behavior compared to the PW group. In contrast, snails tested 48 h post-alprazolam treatment exhibited a significantly lower number of escapes compared to both the PW group (Tukey’s post hoc: p < 0.0001, q = 8.93) and those tested at 18 (Tukey’s post hoc: p < 0.0001, q = 8.09) and 24 h post alprazolam treatment (Tukey’s post hoc: p = 0.0002, q = 6.39). Thus, the anti-anxiety effects of alprazolam persist for at least 24 but not 48 h.
A Study design: five cohorts of snails were used in this study: (1) snails exposed to PW for 15 min and tested in PW 18 h later (beige bar); (2) snails exposed to PW for 15 min and tested in FW 18 h later (green bar); (3) snails treated with alprazolam 0.1 μM for 15 min and tested in FW 18 h later (diagonal green bars); (4) snails treated with alprazolam 0.1 μM for 15 min and tested in FW 24 h later (diagonal green bars); and (5) snails treated with alprazolam 0.1 μM for 15 min and tested in FW 48 h later (diagonal green bars). N = 20 each group. B The number of escapes was recorded for 20 min. Alprazolam treatment prevents FW-induced reduction in the number of escapes and this effect lasts for at least 24 h but not 48 h. No effects induced by Alprazolam alone were found. Data are represented as mean ± SEM and analyzed using one-way ANOVA followed by Tukey post hoc tests. ****p < 0.0001; ***p < 0.001; ns = not significant (p > 0.05).
Treating snails with alprazolam 0.1 µM for 15 min prevents the effects of predator exposure on learning and memory abilities
Having shown that treating snails with alprazolam 0.1 µM for 15 min prevents anxiety-like behaviors induced by predator cues, we investigated whether the same treatment could prevent predator-induced LTM formation for the operant conditioning (Fig. 7A) and configural learning (Fig. 7B). Thus, snails (N = 10) were exposed to alprazolam 0.1 µM for 15 min and immediately later to FW for 1 h, before being trained with a single training session (30 min). LTM was tested 24 h later. As no significant differences were found in the number of pneumostome openings between TS and MT (paired t-test: t = 0.001, df = 9, p = 0.99) (Fig. 7A), we concluded that alprazolam prevented the FW-induced LTM enhancement for the operant conditioning of aerial respiration. Then, a naïve cohort of snails was first exposed to carrot slurry for 2 min and, 18 h later, was treated with alprazolam for 15 min, followed by a 45-min exposure to carrot slurry mixed with FW. Three hours later, their feeding behavior (i.e., number of rasps) in the carrot slurry alone was recorded for 2 min.
A timeline of the experiment is presented above the data. A Ten naïve snails were exposed to alprazolam 0.1 μM for 15 min and immediately later to FW for 1 h. Three hours later, snails were trained with a single training session (TS), and the LTM for the operant conditioning of aerial respiration was tested 24 h later (MT). No significant reduction in the number of attempted pneumostome openings was found, suggesting that LTM was not enhanced. B Ten naïve snails were exposed to carrot slurry (C), and the feeding behavior (i.e., number of rasps) was recorded for 2 min. 18 h later, snails were exposed to alprazolam 0.1 μM for 15 min and, immediately later, were simultaneously exposed to fish water (FW) and carrot. When the number of rasps elicited by the carrot was recorded 3 h later, no significant reduction in the number of rasps was found, suggesting that configural learning was not formed. Data are represented as single data points and were analyzed with paired t-tests. ns = not significant as p > 0.05.
The number of rasps did not significantly decrease (paired t-test: t = 0.57, df = 9, p = 0.57), indicating that configural learning was not formed (Fig. 7B). These results support the hypothesis that reducing anxiety can prevent memories associated with stress or danger.
Treating snails with alprazolam 0.1 µM for 15 min blocks memory
To test if alprazolam affects snails’ ability to form memory resulting in an anterograde amnesia-like effect (i.e., a loss of memory for events occurring forward in time) [71] similar to what observed in mammals (including humans), we treated snails (N = 15) with alprazolam for 15 min before undergoing a 30-min TS for operant conditioning of aerial respiration. This procedure typically results in a 3 h-lasting ITM [85], however, the alprazolam treatment blocked memory formation (RM ANOVA: F14,42 = 1.77; P = 0.07- Fig. 8A) as memory testing (MT1) conducted 3 h post-training showed no significant reduction in memory performance. In a follow-up experiment 24 h later, the same snails underwent a new training session (TS2) after a 15-min exposure to artificial PW instead of alprazolam. This time, ITM was successfully formed, as evidenced by a significant reduction in pneumostome openings (Tukey’s post hoc: p < 0.0001, q = 14.55). These results suggest that alprazolam blocks learning and/or ITM, preventing the snails from remembering the association between the pneumostome opening and poking. The effects of alprazolam suggest that the drug’s memory-blocking effects are shorter-lived than its anti-anxiety effects. Control snails (N = 15) exposed to PW instead of alprazolam (RM ANOVA: F14, 42 = 37.49; P < 0.0001 - Fig. 8B) formed ITM after both training sessions (Tukey’s post hoc: TS1 vs MT1: p < 0.0001 and q = 10.79; TS2 vs MT2: p < 0.0001 and q = 15.11), confirming that the repeated operant conditioning procedure did not affect the ability of snails to form ITM. Overall, these findings suggest that alprazolam can block learning or the new protein synthesis necessary for ITM, resulting in somewhat similar to anterograde amnesia.
A timeline of the experiment is presented above the data. A Fifteen naïve snails were exposed to alprazolam for 15 min and then were trained with a 30-min training session (TS1). Three hours later, ITM was tested (MT1). No significant reduction in the number of attempted pneumostome openings was found (i.e., ITM was not formed). Twenty-four hours later, these snails were exposed to artificial PW for 15 min and then re-trained (TS2) for 30 min. ITM was tested 3 h later (MT2). As a significant reduction in the number of attempted pneumostome openings was found, ITM was formed. B Fifteen control snails were exposed to artificial PW for 15 min and were trained with a 30-min training session (TS1). Three hours later, ITM was formed (MT1) as a significant reduction in the number of attempted pneumostome openings was found. Twenty-four hours later, these snails were re-exposed to artificial PW for 15 min and then re-trained (TS2) for 30 min. ITM was tested 3 h later (MT2), and a significant reduction in the number of attempted pneumostome openings was found, suggesting that ITM was formed. Data are represented as means ± SEM and analyzed with RM-way ANOVA followed by Tukey’s post hoc ****p < 0.0001; ns = not significant as p > 0.05.
PCA to further explore alprazolam’s modulation of anxiety-like behaviors
While t-tests and ANOVA identify significant differences between groups for individual behavioral variables, they assume these variables are independent. However, in behavioral pharmacology, multiple measures often reflect interrelated aspects of a broader behavioral construct, such as anxiety-like responses. PCA complements these analyses by identifying correlations between variables, allowing us to visualize how behavioral patterns emerge across experimental groups in a multidimensional space (Fig. 9). This provides a more holistic view of how anxiety-like behaviors manifest and how pharmacological interventions modulate them. Additionally, when PCA results align with univariate tests, they reinforce the robustness of the findings by demonstrating that group differences are not limited to single variables but rather reflect a broader shift in behavioral profiles. The PCA plot (PC1 vs. PC2) in Fig. 9 captures 85% of the variance in the dataset and provides clear evidence of distinct behavioral clustering between experimental conditions.
The PCA plot presents the behavioral data of snails classified into four groups: Control (not exposed to fish water (FW) and not treated with alprazolam – purple), Predator-exposed snails (green), Predator-exposed snails tested in artificial pond water (PW - blue), and alprazolam-treated snails before being exposed to FW (yellow). The data are plotted along two principal components, PC1 and PC2, which capture the most significant variance in the dataset. Shapes: Circles refer to PW, and Triangles to FW. PC1 and PC2 axes represent the most substantial variance in the behavioral data. Vectors: Black arrows with red labels indicate the influence and direction of specific behavioral metrics on the principal components.
The exploratory behavior aligns with negative PC1 and positive PC2 values, righting time with negative values for both PC1 and PC2, and TBT with positive PC1 and negative PC2 values. The control group (purple) is centrally positioned, showing a balanced behavioral profile without strong alignment to any specific variable. This suggests a baseline state where snails exhibit natural exploratory tendencies, normal righting times, and a stable breathing pattern. The predator-exposed groups (blue and green) show a marked shift towards TBT, indicating an anxiety-like state characterized by increased aerial respiration—a key indicator of heightened stress in L. stagnalis. This effect is particularly pronounced in snails tested directly in FW (green), suggesting that the presence of predator cues leads to a stronger and more persistent physiological response compared to those re-tested in PW (blue), where the behavioral shift is still present but less extreme. The alprazolam-treated group (yellow) clusters near exploratory behavior and righting time, demonstrating a restoration of normal behavioral responses. Unlike predator-exposed snails, this group does not strongly correlate with TBT, reinforcing the anxiolytic effect of alprazolam in preventing predator-induced behavioral alterations. The fact that the PCA positions this group close to controls suggests that alprazolam effectively counteracts the physiological and behavioral impact of stress, allowing the snails to behave as if they had never been exposed to predator cues. This multivariate approach strengthens our interpretation of the data by showing that anxiety-like behavior is not limited to changes in individual measures but rather represents a coordinated shift across multiple behavioral dimensions. PCA highlights the strong relationship between predator exposure and altered respiratory behavior, confirming that snails exposed to FW exhibit a stress response even when tested in a different environment later. Moreover, it demonstrates that alprazolam effectively mitigates this response, normalizing exploratory and righting behaviors while reducing excessive aerial respiration. By providing a comprehensive, integrated view of behavioral changes, PCA proves to be a powerful tool for a clearer differentiation between experimental groups and enhances the translational relevance of our findings by confirming that fundamental mechanisms of anxiety and its treatment are preserved across species.
Discussion
The behavioral consequences of predator odor exposure have long been studied as an anxiety-like paradigm in rodents [89, 90]. To our knowledge, this is the first study where a molluscan model—L. stagnalis— shows anxiety-like behaviors, which can be prevented by a well-established anxiolytic drug. Our findings contribute to a deeper understanding of anxiety-like behavior in invertebrates and offer insights into the conserved mechanisms underlying stress responses. We found that exposure to FW, a proxy for predator presence, induces a marked increase in aerial respiration and a decrease in righting behavior from a vulnerable position and exploratory behavior among snails lasting for at least 18 h. These behavioral shifts suggest a severe stress response characterized by heightened vigilance and a reduction in risk-taking behaviors [43, 91].
Notably, these changes persisted even after the removal of the stressor, indicating that the snails exhibited a sustained anxiety-like state rather than a mere reflexive response [92]. Interestingly, we observed a “faster recovery” in righting time compared to TBT and escape behavior. Specifically, when snails were re-tested in PW after exposure to FW, we found that the reduction in righting time induced by FW was less intense and less reinforced, and significantly longer than the response recorded in FW. A possible explanation for this could be the energy demands of the righting process, which requires considerable muscular effort and coordination. As a result, the increase in righting time when snails were re-exposed to PW following FW may be attributed to the greater complexity of the rotational process. Nevertheless, both FW and PW post-FW recordings exhibited significantly shorter righting times compared to the initial PW recordings, suggesting that after FW exposure, snails remain in a heightened state of alertness when re-tested in PW.
The persistence of FW-induced response in a different and ‘safe’ context is consistent with the criteria for anxiety observed in vertebrates, reflecting a deeper, potentially evolutionary conserved mechanism. In particular, studies from rodents show that exposure to predator odors or other threats can trigger behavioral changes similar to those observed in snails, such as increased vigilance, reduced exploration, and avoidance of risky situations [56, 89]. These behaviors are critical for survival, helping animals avoid potential dangers in their environment. Similarly, in humans, exposure to stressful or threatening situations can result in increased anxiety, hypervigilance, and avoidance behaviors [2]. The implications of these findings are significant, suggesting that the mechanisms underlying anxiety and stress responses are deeply rooted in evolutionary history and may have been conserved across diverse species as a critical survival strategy [93]. This could provide valuable insights into the evolutionary origins of anxiety and stress-related behaviors and offer a more comprehensive understanding of how stress and anxiety are regulated at the molecular and neural levels [92, 94, 95]. We also found that exposure to FW can enhance LTM formation for the operant conditioning of aerial respiration and induce configural learning [76, 86]. These results allow us to uncover the basic principles of stress-related memory modulation, offering a more comprehensive view of how stress and environmental cues influence cognitive functions.
In particular, our results on configural learning directly demonstrate that lab-bred snails have the capacity and ability to form a relationship between two stimuli (i.e., carrot slurry and FW) and can treat them as different from the simple sum of the stimuli alone (i.e., are capable of configural learning) [14, 20, 58]. As the ability to form associations between predator risk factors and the surrounding environment is necessary for predator avoidance strategies, configural learning should be seen as a key adaptation enabling animals to assign new meaning to stimuli in their environment [96]. These data are all consistent with previous studies from Lukowiak’s lab, further remarking on the solidity of this behavioral paradigm and its validity for pharmacological studies [49, 53, 62, 64, 75, 83], and aligns with the snail’s ability in other learning paradigms in Koene’s lab [97, 98] and Kemenes’s lab [91, 99]. Moreover, data from operant conditioning strongly suggest that the detection of predators primes the molecular mechanisms responsible for LTM formation, consistent with previous studies from L. stagnalis [51, 84]. Similar to snails, rodents have been shown to experience enhanced memory formation when exposed to stressors or predator-related cues [100]. For instance, the presence of a predator or predator odor can enhance the rodent’s ability to remember and avoid locations associated with danger, akin to the way fish kairomones or crayfish exposure primes memory formation in snails [101]. Overall, the findings in snails, when considered alongside research in rodents and humans, highlight the conserved nature of memory modulation mechanisms across species [37]. These parallels suggest that studying simpler organisms like snails can offer valuable insights into the basic principles of cognitive function, which can be applied to understanding more complex brains in rodents and humans [102].
We also assessed the effectiveness of alprazolam in modulating the anxiety-like behavior induced by FW. At higher doses (1 and 10 µM), snails exhibited a significant reduction in feeding behavior regardless of the treatment duration [65].
This reduction in feeding, a solid homeostatic behavior, indicates a sedative effect, consistent with the known properties of benzodiazepines like alprazolam [67, 103, 104]. Such sedation is characterized by a diminished ability to engage in normal feeding activities, reflecting a hypnotic state rather than simply an anxiety reduction. Conversely, alprazolam at 0.1 µM for 15 min did not significantly alter feeding behavior, suggesting it did not induce noticeable sedation. This dosage, in fact, prevented predator-induced increased aerial respiration, restored normal exploratory behavior, and adjusted righting times to baseline levels. This outcome is particularly significant as it mirrors the anxiolytic effects of alprazolam observed in rodents, where similar doses are used to reduce anxiety-like behaviors across various tests, such as the elevated plus maze and open field test [22]. The duration of alprazolam’s anxiolytic effects was evaluated by measuring exploratory behavior over time. Our data indicate that the anxiolytic effects lasted for up to 24 h, aligning with the drug’s pharmacokinetic profile and offering a window into its practical application in managing acute anxiety-like behavior [66, 67, 105].
Overall, our behavioral data offer valuable insights into how alprazolam influences snails exposed to predator cues and helps to clarify how these behaviors change under different conditions, particularly in the context of stress induced by predator scent [106]. This may be particularly relevant in a more ecological context, given that many pharmaceuticals (including alprazolam) can also be found in the aquatic environment in which these species normally live (https://www.norman-network.com/). Such pollutants, especially if their effects are persistent and/or if they are not easily broken down [107, 108], may affect snail survival, given that they interfere with natural predator avoidance responses and predicting opportunities based on learning and memory. For this purpose, we plan to conduct a detailed investigation into the potential behavioral differences between freshly collected snails and lab-inbred ones. Understanding whether the behavior of freshly collected snails aligns with that of lab-grown snails would shed light on the ecological relevance of our experimental models [60, 109,110,111].
Lab-inbred snails are typically used for their controlled genetic backgrounds, whereas freshly collected snails might display different behavioral patterns influenced by factors such as predator exposure, environmental stressors, and genetic variability. This comparison will enhance the ecological validity of our findings. Thus, by expanding our investigations to include freshly collected snails and assessing environmental contaminants in natural habitats, we aim to deepen our understanding of how our experimental results may translate to ecological and environmental contexts.
Alprazolam’s effects on learning and memory were also assessed, revealing the impaired formation of new memories for up to 3 h post-treatment [69, 105]. This temporary memory block aligns with the known effects of benzodiazepines in rodents, where benzodiazepines can disrupt the encoding of new memories.
The memory-blocking effects of alprazolam observed in L. stagnalis raise intriguing parallels with its well-documented impact on anterograde amnesia in rodents [69]. Future studies will assess memory formation at different time intervals post-treatment, comparing short- and long-term memory effects and investigating specific neural correlates of memory disruption. The results of these studies not only deepen our understanding of how benzodiazepines affect memory across species but also establish L. stagnalis as a potential model for studying pharmacologically induced amnesia and its underlying mechanisms.
The conserved nature of anxiolytic responses across species suggests that fundamental mechanisms of anxiety and its modulation by different benzodiazepine drugs are broadly applicable [112, 113], providing insights into the evolution of stress responses. In particular, our study demonstrates that invertebrates offer a simpler yet effective model for studying the conserved mechanism of anxiety-like behaviors and pharmacological interventions, complementing the more complex rodent models typically used in this field.
These results paved the way for future studies currently undergoing in our lab to investigate the molecular mechanisms underlying the effects of FW and the alprazolam treatment. We hypothesized that the GABAergic system plays a key role in regulating anxiety-like behavior in snails as in mammals [114]. As mentioned in the Introduction, pharmacological and scant molecular evidence support the presence of GABA-A receptors in mollusks as well [115]. Moreover, just like mammalian GABA-A receptors, invertebrate GABA-A receptors seem to be activated by anxiolytic drugs [22]. However, in contrast to Drosophila melanogaster [116] or vertebrate models, much less information is available on molluscan GABA-A receptors (e.g., number of homolog subunits, structure). Investigations are already in progress in our lab to unequivocally clear the function and evolution of the GABAergic system in L. stagnalis (and mollusks in general). Based on our unpublished data, we propose that functional GABA-A receptors are present in L. stagnalis, which can potentially be activated by alprazolam, contributing to the prevention of anxiety-like responses demonstrated in the present study. Based on the findings of a previous study, the RPeD1 neuron of L. stagnalis, one of the CPG neurons of the aerial respiration behaviour and a key neuron in operant conditioning [28], has a functional GABA-A receptor [117]. Future experiments will allow us to investigate whether alprazolam would exert its anxiolytic effect via this neuron in L. stagnalis.
With their relatively simple nervous systems, open circulatory system, and easily measurable but solid behaviors, Lymnaea provide a straightforward platform for evaluating drug effects, including optimal dosing and duration for alprazolam without significant sedation. This simplicity, alongside the conserved nature of anxiety mechanisms across species, supports the use of invertebrate models like snails for translational research. Despite the study’s novelty, there are limitations. The simplicity of the behavioral assays may not fully capture the complexity of anxiety symptoms or drug effects seen in higher organisms. Additionally, while snails exhibit anxiety-like behaviors and pharmacological responses similar to those in rodents, the study’s focus on a single drug (alprazolam) and the short-term nature of assessments limit the scope of insights. Future research should expand to include a broader range of anxiolytic compounds, explore long-term effects, and delve into the neurobiological mechanisms underlying these behaviors. By refining this model and incorporating more complex behavioral assays, as well as molecular analysis, we can further enhance the validity and applicability of findings from invertebrates to more complex systems.
Conclusions
Historically, research into anxiety has predominantly focused on vertebrate models, particularly rodents and humans, due to their more complex nervous systems and behavioral repertoires. By extending this research to pond snails, a simpler invertebrate model, the study provides novel insights into the fundamental mechanisms of anxiety and its modulation. First, our study introduces a new model organism for studying anxiety-like behavior. Pond snails, with their relatively straightforward nervous system, offer a unique perspective on anxiety research, allowing for the investigation of basic neural and behavioral processes without the confounding complexity of more advanced organisms. This innovative approach helps elucidate the evolutionary conservation of anxiety mechanisms and the basic principles underlying stress responses across different phyla. Second, the application of a benzodiazepine like alprazolam in an invertebrate model is pioneering. While the effects of such compounds have been well-documented in vertebrates, their impact on simpler nervous systems has been less explored. Finally, we emphasize the ethical significance of this study, which adheres to the ‘3Rs principles’ for more responsible preclinical research [118]. Rather than considering Lymnaea as an ‘alternative’ model, we define it as a complementary organism that enhances, rather than replaces, mammalian models in preclinical studies. By integrating snail models, we can significantly reduce the reliance on mammals, reserving their use for validating findings from invertebrate studies. This approach not only upholds ethical research standards but also dramatically reduces costs by several orders of magnitude. Moreover, research in mammalian models can inspire targeted studies in Lymnaea (like we did here), while findings from Lymnaea can, in turn, simplify and guide mammalian research. Given the complexity of psychiatric disorders, certain processes are often difficult to dissect in mammals, whereas the simpler nervous system of L. stagnalis allows for a more precise and mechanistic understanding. Overall, our findings underscore the value of L. stagnalis as a robust and versatile model system, providing a powerful experimental tool with direct translational relevance. By bridging the gap between simple and complex systems, our findings contribute to a more comprehensive understanding of anxiety and drug action, ultimately guiding the development of targeted therapeutic strategies for anxiety disorders in humans.
Data availability
The data will be made available upon reasonable request from Veronica Rivi and Cristina Benatti.
References
Steimer T. The biology of fear- and anxiety-related behaviors. Dialogues Clin Neurosci. 2002;4:231–49.
Mobbs D, Hagan CC, Dalgleish T, Silston B, Prévost C. The ecology of human fear: survival optimization and the nervous system. Front Neurosci. 2015;9:55.
Belzung C, Philippot P. Anxiety from a phylogenetic perspective: is there a qualitative difference between human and animal anxiety? Neural Plast. 2007;2007:59676.
Ydenberg RC, Dill LM The economics of fleeing from predators. In: Rosenblatt JS, Beer C, Busnel M-C, Slater PJB (eds). Adv stud behav. Academic Press: New York 1986, pp 229-49.
Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: the role of threat appraisal and fear learning. Depress Anxiety. 2011;28:5.
Adolphs R. The biology of fear. Curr Biol. 2013;23:R79–93.
Zanette LY, Hobbs EC, Witterick LE, MacDougall-Shackleton SA, Clinchy M. Predator-induced fear causes PTSD-like changes in the brains and behaviour of wild animals. Sci Rep. 2019;9:11474.
Nyberg J, Henriksson M, Wall A, Vestberg T, Westerlund M, Walser M, et al. Anxiety severity and cognitive function in primary care patients with anxiety disorder: a cross-sectional study. BMC Psychiatry. 2021;21:617.
Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety. Nat Rev Neurosci. 2013;14:488–501.
Yang Z, Li Z, Guo Z, Ren Y, Zhou T, Xiao Z, et al. Antitumor effect of fluoxetine on chronic stress-promoted lung cancer growth via suppressing kynurenine pathway and enhancing cellular immunity. Front Pharmacol. 2021;12:685898.
Zhao C. Cell culture: in vitro model system and a promising path to in vivo applications. J Histotech. 2023;46:1–4.
Cloninger CR, Cloninger KM, Zwir I, Keltikangas-Järvinen L. The complex genetics and biology of human temperament: a review of traditional concepts in relation to new molecular findings. Transl Psychiatry. 2019;9:290.
Lowe R, Ziemke T. The feeling of action tendencies: on the emotional regulation of goal-directed behavior. Front Psychol. 2011;2:346.
Pribadi AK, Chalasani SH. Fear conditioning in invertebrates. Front Behav Neurosci. 2022;16:1008818.
Shanazz K, Dixon-Melvin R, Bunting KM, Nalloor R, Vazdarjanova AI. Light-Dark Open Field (LDOF): a novel task for sensitive assessment of anxiety. J Neurosci Methods. 2021;363:109325.
Campos-Cardoso R, Godoy LD, Lazarini-Lopes W, Novaes LS, dos Santos NB, Perfetti JG, et al. Exploring the light/dark box test: protocols and implications for neuroscience research. J Neurosci Methods. 2023;384:109748.
Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44.
Kandel ER. From metapsychology to molecular biology: explorations into the nature of anxiety. Am J Psychiatry. 1983;140:1277–93.
Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–21.
Perry CJ, Baciadonna L. Studying emotion in invertebrates: what has been done, what can be measured and what they can provide. J Exp Biol. 2017;220:3856–68.
Bridges AD, Royka A, Wilson T, Lockwood C, Richter J, Juusola M, et al. Bumblebees socially learn behaviour too complex to innovate alone. Nature. 2024;627:572–8.
Fossat P, Bacqué-Cazenave J, De Deurwaerdère P, Delbecque J-P, Cattaert D. Comparative behavior. Anxiety-like behavior in crayfish is controlled by serotonin. Science. 2014;344:1293–7.
Roth T, Roehrs T, Wittig R, Zorick F. Benzodiazepines and memory. Br J Clin Pharmacol. 1984;18:45S–49S.
Arora I, Mal P, Arora P, Paul A, Kumar M. GABAergic implications in anxiety and related disorders. BBRC. 2024;724:150218.
Shymansky T, Hughes E, Rothwell CM, Lukowiak K. Propranolol disrupts consolidation of emotional memory in Lymnaea. Neurobiol Learn Mem. 2018;149:1–9.
Everitt JI. The future of preclinical animal models in pharmaceutical discovery and development: a need to bring in cerebro to the in vivo discussions. Toxicol Pathol. 2015;43:70–77.
Rivi V, Benatti C, Colliva C, Radighieri G, Brunello N, Tascedda F, et al. Lymnaea stagnalis as model for translational neuroscience research: from pond to bench. Neurosc Biobehav Rev. 2020;108:602–16.
Rivi V, Benatti C, Lukowiak K, Colliva C, Alboni S, Tascedda F, et al. What can we teach Lymnaea and what can Lymnaea teach us? Biol Rev Camb Philos Soc. 2021;96:1590–602.
Rivi V, Benatti C, Actis P, Tascedda F, Blom JMC. Behavioral and transcriptional effects of short or prolonged fasting on the memory performances of Lymnaea stagnalis. Neuroendo. 2022;113:406–22. https://doi.org/10.1159/000527489
O’Reilly LP, Luke CJ, Perlmutter DH, Silverman GA, Pak SC. C. elegans in high-throughput drug discovery. Adv Drug Deliv Rev. 2014;0:247–53.
Weinkove D, Zavagno G. Applying C. elegans to the industrial drug discovery process to slow aging. Front Aging. 2021;2:740582. https://doi.org/10.3389/fragi.2021.740582
Tickoo S, Russell S. Drosophila melanogaster as a model system for drug discovery and pathway screening. Curr Opin Pharmacol. 2002;2:555–60.
Tascedda F, Malagoli D, Accorsi A, Rigillo G, Blom JMC, Ottaviani E. Molluscs as models for translational medicine. Med Sci Monit Basic Res. 2015;21:96–99.
Rivi V, Batabyal A, Lukowiak K. The multifaceted effects of flavonoids on neuroplasticity. Restor Neurol Neurosci. 2024;42:93–111. https://doi.org/10.3233/RNN-230150
Maleszka R. The social honey bee in biomedical research: realities and expectations. Drug Dis Tod Dis Mod. 2014;12:7–13.
Huber R, Panksepp JB, Nathaniel T, Alcaro A, Panksepp J. Drug–sensitive reward in crayfish: an invertebrate model system for the study of SEEKING, reward, addiction, and withdrawal. Neurosci Biobehav Rev. 2011;35:1847–53.
Fodor I, Hussein AA, Benjamin PR, Koene JM, Pirger Z. The unlimited potential of the great pond snail, Lymnaea stagnalis. eLife. 2020;9:e56962.
Rivi V, Benatti C, Rigillo G, Blom JMC. Invertebrates as models of learning and memory: investigating neural and molecular mechanisms. J Exp Biol. 2023;226:jeb244844.
Benjamin PR, Kemenes G Invertebrate models to study learning and memory: Lymnaea. In: Squire LR (ed). Encyclopedia of neuroscience. Academic Press: Oxford, 2009, pp 197–204.
Rivi V, Batabyal A, Juego K, Kakadiya M, Benatti C, Blom JMC, et al. To eat or not to eat: a Garcia effect in pond snails (Lymnaea stagnalis). J Comp Physiol A. 2021;207:479–95.
Rivi V, Batabyal A, Benatti C, Sarti P, Blom JMC, Tascedda F, et al. A translational and multidisciplinary approach to studying the Garcia effect, a higher form of learning with deep evolutionary roots. J Exp Biol. 2024;227:jeb247325.
Batabyal A, Rivi V, Benatti C, Blom JMC, Lukowiak K. Long-term memory of configural learning is enhanced via CREB upregulation by the flavonoid quercetin in Lymnaea stagnalis. J. Exp. Biol. 2021;224:jeb242761.
Batabyal A, Chau D, Rivi V, Lukowiak K. Risk in one is not risk in all: snails show differential decision making under high- and low-risk environments. Anim Behav. 2022;190:53–60.
Ito E, Yamagishi M, Hatakeyama D, Watanabe T, Fujito Y, Dyakonova V, et al. Memory block: a consequence of conflict resolution. J. Exp. Biol. 2015;218:1699–704.
Aonuma H, Kaneda M, Hatakeyama D, Watanabe T, Lukowiak K, Ito E. Relationship between the grades of a learned aversive-feeding response and the dopamine contents in Lymnaea. Biol Open. 2016;5:1869–73.
Kagan D, Rivi V, Benatti C, Tascedda F, Blom JMC, Lukowiak K. No food for thought: an intermediate level of food deprivation enhances memory in Lymnaea stagnalis. J. Exp. Biol. 2023;226:jeb245566.
Kagan D, Batabyal A, Rivi V, Lukowiak K. A change in taste: the role of microRNAs in altering hedonic value. J. Exp. Biol. 2022;225:jeb243840.
Rivi V, Batabyal A, Benatti C, Tascedda F, Blom JMC, Lukowiak K. Quercetin, the new stress buster: investigating the transcriptional and behavioral effects of this flavonoid on multiple stressors using Lymnaea stagnalis. Comp Biochem Physiol C Toxicol Pharmacol. 2024;287:110053.
Rivi V, Batabyal A, Benatti C, Tascedda F, Blom JMC, Lukowiak K. Aspirin reverts lipopolysaccharide-induced learning and memory impairment: first evidence from an invertebrate model system. N-S Arch Pharmacol. 2022;395:1573–85. https://doi.org/10.1007/s00210-022-02286-4
Rivi V, Batabyal A, Benatti C, Tascedda F, Blom JMC, Lukowiak K. Prey populations with different predation histories show differences in behavioral and transcriptional effects under acute predation threat. Neurobiol Learn Mem. 2023;203:107775.
Orr M, Lukowiak K. Sympatric predator detection alters cutaneous respiration in Lymnaea. Commun Integr Biol. 2010;3:42–45.
Rivi V, Batabyal A, Benatti C, Tascedda F, Blom JM, Lukowiak K. Too hot to eat: wild and lab-bred lymnaea stagnalis differ in feeding response following repeated heat exposure. Bioll Bull. 2022;243:38–43.
Rivi V, Batabyal A, Benatti C, Tascedda F, Blom JMC, Lukowiak K. Hot and cold exposure triggers distinct transcriptional and behavioral responses in laboratory-inbred pond snails. WatBS. 2024;4:100315.
Rivi V, Rigillo G, Batabyal A, Lukowiak K, Pani L, Tascedda F, et al. Different stressors uniquely affect the expression of endocannabinoid-metabolizing enzymes in the central ring ganglia of Lymnaea stagnalis. Jf Neurochem. 2024;168:2848–67. https://doi.org/10.1111/jnc.16147
Lukowiak K, Sunada H, Teskey M, Lukowiak K, Dalesman S. Environmentally relevant stressors alter memory formation in the pond snail Lymnaea. J Exp Biol. 2014;217:76–83.
Natsi A, Valkanou M, Anousi E, Labrakakis C. Differential behavioral response to predator odor in neuropathic pain in mice. Front Pain Res. 2024;4:1283550. https://doi.org/10.3389/fpain.2023.1283550
Batabyal A, Rivi V, Benatti C, Blom JMC, Tascedda F, Lukowiak K. Snails go on a fast when acetylsalicylic acid comes along with heat stress: a possible effect of HSPs and serotonergic system on the feeding response. Comp Biochem Physiol C Toxicol Pharmacol. 2024;276:109805.
Rivi V, Batabyal A, Benatti C, Blom JMC, Tascedda F, Lukowiak K. Investigating the interactions between multiple memory stores in the pond snail Lymnaea stagnalis. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2023;210:91–102. https://doi.org/10.1007/s00359-023-01649-3
Rivi V, Batabyal A, Wiley B, Benatti C, Tascedda F, Blom JMC, et al. Fluoride affects memory by altering the transcriptional activity in the central nervous system of Lymnaea stagnalis. NeuroTox. 2022;92:61–66.
Rivi V, Batabyal A, Benatti C, Blom JM, Tascedda F, Lukowiak K. Novel taste, sickness, and memory: lipopolysaccharide to induce a Garcia-like effect in inbred and wild strains of Lymnaea stagnalis. Physiol Behav. 2023;263:114137.
Hollings J, Kagan D, Batabyal A, Lukowiak K. How to reduce fear in a snail: take an aspirin, call me in the morning. Comp Biochem Physiol C Toxicol Pharmacol. 2024;284:109978.
Rivi V, Caruso G, Caraci F, Alboni S, Pani L, Tascedda F, et al. Behavioral and transcriptional effects of carnosine in the central ring ganglia of the pond snail Lymnaea stagnalis. J Neurosci Res. 2024;102:e25371.
Rivi V, Batabyal A, Benatti C, Tascedda F, Blom JMC, Lukowiak K. A novel behavioral display in Lymnaea induced by quercetin and hypoxia. Biol Bull. 2023;244:115–27.
Rivi V, Batabyal A, Benatti C, Blom JM, Tascedda F, Lukowiak K. A flavonoid, quercetin, is capable of enhancing long-term memory formation if encountered at different times in the learning, memory formation, and memory recall continuum. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2021;208:253–65. https://doi.org/10.1007/s00359-021-01522-1
Pieróg M, Guz L, Doboszewska U, Poleszak E, Wlaź P. Effects of alprazolam treatment on anxiety-like behavior induced by color stimulation in adult zebrafish. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:297–306.
Nishimura H, Tanaka M. Effects of alprazolam on anxiety-related behavior of rats in a modified forced-swim test employing straw suspension. Pharmacol Biochem Behav. 1992;41:425–7.
Steinglass JE, Kaplan SC, Liu Y, Wang Y, Walsh BT. The (lack of) effect of alprazolam on eating behavior in anorexia nervosa: a preliminary report. Int J Eat Disord. 2014;47:901–4.
Holmes A, Chen A. GABA receptors in a state of fear. Nat Neurosci. 2015;18:1194–6.
Mejo SL. Anterograde amnesia linked to benzodiazepines. Nurse Pract. 1992;17:49–50. 44.
King DJ. Benzodiazepines, amnesia and sedation: theoretical and clinical issues and controversies. Hum Physchopharmacol. 1992;7:79–87.
Kaplan K, Hunsberger HC. Benzodiazepine-induced anterograde amnesia: detrimental side effect to novel study tool. Front Pharmacol. 2023;14:1257030.
Aggleton JP. Looking beyond the hippocampus: old and new neurological targets for understanding memory disorders. Proc Biol Sci. 2014;281:20140565.
Rivi V, Batabyal A, Benatti C, Tascedda F, Blom JMC, Lukowiak K. Comparison of behavioural and transcriptional responses to a heat stressor between freshly collected and an inbred strain of Lymnaea. Can J Zool. 2023;101:904–12.
Dalesman S, Lukowiak K. Effect of acute exposure to low environmental calcium on respiration and locomotion in Lymnaea stagnalis (L.). J Exp Biol. 2010;213:1471–6.
Orr MV, El-Bekai M, Lui M, Watson K, Lukowiak K. Predator detection in Lymnaea stagnalis. J Exp Biol. 2007;210:4150–8.
Orr MV, Lukowiak K. Electrophysiological and behavioral evidence demonstrating that predator detection alters adaptive behaviors in the snail Lymnaea. J Neurosci. 2008;28:2726–34.
Battisti UM, Citti C, Rastelli G, Pinzi L, Puja G, Ravazzini F, et al. An unexpected reversal in the pharmacological stereoselectivity of benzothiadiazine AMPA positive allosteric modulators. Med Chem Commun. 2016;7:2410–7.
Freire-Garabal M, Núñez MJ, Fernández-Rial JC, Couceiro J, García-Vallejo L, Rey-Méndez M. Phagocytic activity in stressed mice: effects of alprazolam. Res Immunol. 1993;144:311–6.
Drzewiecki CM, Fox AS. Understanding the heterogeneity of anxiety using a translational neuroscience approach. Cogn Affect Behav Neurosci. 2024;24:228–45.
Lukowiak K, Martens K, Orr M, Parvez K, Rosenegger D, Sangha S. Modulation of aerial respiratory behaviour in a pond snail. Respir Physiol Neurobiol. 2006;154:61–72.
Benatti C, Rivi V, Colliva C, Radighieri G, Tascedda F, Blom JMC. Redefining operant conditioning of escape behaviour in Lymnaea stagnalis. Inv Surv J. 2020;17:129–37.
Kobayashi S, Kojima S, Yamanaka M, Sadamoto H, Nakamura H, Fujito Y, et al. Operant conditioning of escape behavior in the pond snail, Lymnaea stagnalis. jzoo. 1998;15:683–90.
Lukowiak K, Ringseis E, Spencer G, Wildering W, Syed N. Operant conditioning of aerial respiratory behaviour in Lymnaea stagnalis. J Exp Biol. 1996;199:683–91.
Braun MH, Lukowiak K. Intermediate and long-term memory are different at the neuronal level in Lymnaea stagnalis (L.). Neurobiol Learn Mem. 2011;96:403–16.
Lukowiak K, Adatia N, Krygier D, Syed N. Operant conditioning in Lymnaea: evidence for intermediate- and long-term memory. Learn Mem. 2000;7:140–50.
Swinton C, Swinton E, Shymansky T, Hughes E, Zhang J, Rothwell C, et al. Configural learning: a higher form of learning in Lymnaea. J Exp Biol. 2019;222:jeb190405.
Benjamin PR. Gastropod feeding: behavioural and neural analysis of a complex multicomponent system. Symp Soc Exp Biol. 1983;37:159–93.
Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44.
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–44.
Atkins R, Blumstein DT, Moseby KE, West R, Hyatt M, Letnic M. Deep evolutionary experience explains mammalian responses to predators. Behav Ecol Sociobiol. 2016;70:1755–63.
Kemenes G, Benjamin PR. Training in a novel environment improves the appetitive learning performance of the snail, Lymnaea stagnalis. Behav Neural Biol. 1994;61:139–49.
Daviu N, Bruchas MR, Moghaddam B, Sandi C, Beyeler A. Neurobiological links between stress and anxiety. Neurobiol Stress. 2019;11:100191.
Nesse RM. Evolutionary psychiatry: foundations, progress and challenges. World Psychiatry. 2023;22:177–202.
Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci. 2018;12:127. https://doi.org/10.3389/fnbeh.2018.00127
Schiele MA, Gottschalk MG, Domschke K. The applied implications of epigenetics in anxiety, affective and stress-related disorders - a review and synthesis on psychosocial stress, psychotherapy and prevention. Clin Psychol Rev. 2020;77:101830.
Herberholz J, Marquart GD. Decision making and behavioral choice during predator avoidance. Front Neurosci. 2012;6:125.
Koene JM, Cosijn J. Twisted sex in an hermaphrodite: mirror-image mating behaviour is not learned. J Moll Stud. 2012;78:308–11.
Álvarez B, Koene JM, Hollis KL, Loy I. Learning to anticipate mate presence shapes individual sex roles in the hermaphroditic pond snail, Lymnaea stagnalis. Anim Cogn. 2022;25:1417–25.
Crossley M, Lorenzetti FD, Naskar S, O’Shea M, Kemenes G, Benjamin PR, et al. Proactive and retroactive interference with associative memory consolidation in the snail Lymnaea is time and circuit dependent. Commun Biol. 2019;2:1–11.
Dalesman S, Lukowiak K. How stress alters memory in ‘Smart’ snails. PLoS ONE. 2012;7:e32334.
Ferrero DM, Lemon JK, Fluegge D, Pashkovski SL, Korzan WJ, Datta SR, et al. Detection and avoidance of a carnivore odor by prey. Proc Natl Acad Sci USA. 2011;108:11235–40.
Kambona CM, Koua PA, Léon J, Ballvora A. Stress memory and its regulation in plants experiencing recurrent drought conditions. Theor Appl Genet. 2023;136:26.
Garakani A, Murrough JW, Freire RC, Thom RP, Larkin K, Buono FD, et al. Pharmacotherapy of anxiety disorders: current and emerging treatment options. Front Psychiatry. 2020;11:595584.
Cheung H, Yu T-Z, Yi X, Wu Y-J, Wang Q, Gu X, et al. An ultra-short-acting benzodiazepine in thalamic nucleus reuniens undermines fear extinction via intermediation of hippocamposeptal circuits. Commun Biol. 2024;7:1–17.
Chowdhury ZS, Morshed MM, Shahriar M, Bhuiyan MA, Islam SM, Bin Sayeed MS. The effect of chronic alprazolam intake on memory, attention, and psychomotor performance in healthy human male volunteers. Behav Neurol. 2016;2016:3730940.
Tariel J, Plénet S, Luquet E. How do developmental and parental exposures to predation affect personality and immediate behavioural plasticity in the snail Physa acuta? Proc Biol Sci. 2020;287:20201761.
Fogliano C, Motta CM, Acloque H, Avallone B, Carotenuto R. Water contamination by delorazepam induces epigenetic defects in the embryos of the clawed frog Xenopus laevis. Sci Total Environ. 2023;896:165300.
Lebreton M, Malgouyres J-M, Carayon J-L, Bonnafé E, Géret F. Effects of the anxiolytic benzodiazepine oxazepam on freshwater gastropod reproduction: a prospective study. Ecotox. 2021;30:1880–92.
Rivi V, Batabyal A, Benatti C, Blom JMC, Lukowiak K. Nature versus nurture in heat stress induced learning between inbred and outbred populations of Lymnaea stagnalis. J Therm Biol. 2022;103:103170.
Rivi V, Batabyal A, Lukowiak K, Benatti C, Rigillo G, Tascedda F, et al. LPS-induced garcia effect and its pharmacological regulation mediated by acetylsalicylic acid: behavioral and transcriptional evidence. Biology. 2023;12:1100.
Orr MV, Hittel K, Lukowiak K. ‘Different strokes for different folks’: geographically isolated strains of Lymnaea stagnalis only respond to sympatric predators and have different memory forming capabilities. J Exp Biol. 2009;212:2237–47.
da Silva AF, Cordeiro LM, Soares MV, Zamberlan DC, Baptista FBO, da Silveira TL, et al. JM-20 affects GABA neurotransmission in Caenorhabditis elegans. Neurotox. 2022;93:37–44.
Mohammad F, Aryal S, Ho J, Stewart JC, Norman NA, Tan TL, et al. Ancient anxiety pathways influence drosophila defense behaviors. Curr Biol. 2016;26:981–6.
Gravitz L. The forgotten part of memory. Nature. 2019;571:S12–S14.
Miller MW. GABA as a neurotransmitter in gastropod molluscs. Biol Bull. 2019;236:144–56.
Bailey JE, Nutt DJ. GABA-A receptors and the response to CO2 inhalation — A translational trans-species model of anxiety? Pharmacol Biochem Behav. 2008;90:51–57.
Rubakhin SS, Szücs A, Rózsa KS. Characterization of the GABA response on identified dialysed Lymnaea neurons. Gen Pharmacol. 1996;27:731–9.
Hubrecht RC, Carter E. The 3Rs and humane experimental technique: implementing change. Animals. 2019;9:754.
Acknowledgements
We extend our heartfelt gratitude to the following undergraduate students for their invaluable assistance with the experiments: Christian Ambla, Giulia Bulgarelli, Elia Simoni, and Federico Imbeni. We are also deeply appreciative of Gin, Tonic, and Abramo for generously providing water. A special thanks to Simone and Iris Bussetti for their remarkable efforts in transporting snails all the way from Amsterdam to Modena. Your support made all the difference!
Funding
This research was supported by FAR2023_Ricerca diffusa and FAR2024_Ricerca diffusa, University of Modena and Reggio Emilia.
Author information
Authors and Affiliations
Contributions
VR designed the study, performed behavioral experiments, data analysis, and wrote the original draft. PS carried out formal analysis and contributed to data curation and manuscript editing. IF and ZP contributed to data visualization, validation, and manuscript revision. JK provided experimental resources, supported data validation, and assisted in manuscript editing. AB helped with visualization, validation, and critical review of the manuscript. KL supervised experimental design, validated findings, and contributed to manuscript editing. LP, JMCB and FT provided supervision, resources, and participated in critical manuscript revision. CB contributed to study supervision, methodology, data validation, and manuscript editing. All authors (VR, PS, IF, ZP, JK, LP, AB, KL, JMCB, FT, CB) reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
We declare we have no competing interests.
Ethics approval
Ethical approval was not required for experiments involving Lymnaea stagnalis, as this species is an invertebrate and not subject to regulations under current animal welfare legislation (e.g., EU Directive 2010/63/EU). Nonetheless, all procedures were conducted in accordance with the principles of ethical research and animal welfare. The study adhered to the 3Rs (Replacement, Reduction, and Refinement) to ensure responsible use of animals in research. The number of animals used was minimized without compromising scientific validity, and all efforts were made to ensure animal well-being. Snails were maintained under optimal conditions, including clean, oxygenated water, appropriate nutrition, and low-density housing, to reduce stress and promote natural behavior. Importantly, the experimental procedures used in this study were non-invasive; snails were not sacrificed and displayed normal behavior following testing, indicating minimal impact on their well-being.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rivi, V., Sarti, P., Fodor, I. et al. First evidence of an anxiety-like behavior and its pharmacological modulation in a molluscan model organism, Lymnaea stagnalis. Transl Psychiatry 15, 177 (2025). https://doi.org/10.1038/s41398-025-03399-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03399-z