Abstract
All organisms depend on food digestion for survival, yet the brain-gut signaling mechanisms that regulate this process are not fully understood. Here, using an established C. elegans digestion model, we uncover a pathway in which neuronal ROS (free radicals) signal the intestine to suppress digestion. Genetic screening reveals that reducing genes responsible for maintaining ROS balance increases free radicals and decreases digestion. PRDX-2 knockout in olfactory neurons (AWC) elevates ROS and reduces digestive capacity, mediated by the neuropeptide NLP-1 and activation of the mitochondrial unfolded protein response (UPRmt) in the intestine. Additionally, over-expressing nlp-1 or ablating AWC neurons both trigger UPRmt and inhibit digestion. These findings reveal a brain-gut connection in which neuronal PRDX-2-mediated ROS signaling modulates food digestion, highlighting a critical role of free radicals in shutting down digestion to alleviate stress and reduce food consumption.
Similar content being viewed by others
Introduction
Food is an essential source of nourishment for all living beings, supplying the energy required for growth and vital cellular activities. The digestion of food, a complex and health-critical process, involves the conversion of consumed food into nutrients that the body can utilize for growth, cellular upkeep, and energy production. This process is controlled by both neural and hormonal systems. Sensory neurons of the vagus nerve within the gastrointestinal tract oversee the stomach’s volume and the intestinal contents, thereby managing digestive physiology1. Upon nutrient consumption, enteroendocrine cells are activated, leading to the release of gut hormones such as serotonin, glucagon-like peptide 1 (GLP1), cholecystokinin (CCK), peptide YY, among others2. These gut hormones have diverse roles in digestion, including the secretion of digestive juices, slowing down stomach emptying, regulating energy equilibrium, and maintaining blood glucose levels.
The food digestive system is a key area of interest for researchers and professionals in diverse fields such as nutrition, toxicology, pharmacology, and microbiology. However, the intricate and multi-stage nature of human digestion presents considerable challenges for research. These challenges are amplified by the substantial costs associated with such studies and the ethical dilemmas that emerge, particularly when the research involves potentially harmful entities such as xenobiotics or pathogenic microorganisms3. In our prior research, we utilized C. elegans as an animal model and discovered that C. elegans cannot digest non-edible food, such as Staphylococcus saprophyticus4. Interestingly, this non-edible food can be digested and utilized as a food source if heat-killed Escherichia coli is present, or by inhibiting the innate immune pathway, PMK-15. Consequently, the non-edible bacteria (Staphylococcus saprophyticus)-worm culture system serves as an efficient and cost-effective animal model to investigate the molecular mechanism underlying food digestion in animals6. Using this digestive system, we found that signals such as outer membrane proteins5 and peptidoglycan7 from heat-killed E. coli activate the worm’s food digestive system, enabling them to digest non-edible food SS to support growth. We also discovered that activation of UPRmt inhibits food digestion. However, it remains unclear whether brain-gut signaling regulates food digestion through regulating intestinal UPRmt.
Reactive Oxygen Species (ROS) are recognized to participate in various signaling pathways in several peripheral organs, including the hypothalamus8. In the hypothalamus, ROS regulate food consumption and metabolism by influencing different types of neurons, such as proopiomelanocortin (POMC) and agouti-related protein (AgRP)/neuropeptide Y (NPY) neurons9. When in a fed state, the effects of glucose, lipids, insulin, and leptin lead to the release of ROS in both neuron types. This transient increase in intracellular ROS enhances the activity of POMC neurons and suppresses NPY/AgRP neurons, resulting in decreased food consumption and increased energy expenditure8. The digestive tract can be likened to a bioreactor, with ROS being produced as byproducts of the standard process of food digestion10. Interestingly, there is limited information on the role of ROS in food digestive processes, particularly whether neuronal ROS homeostasis impacts intestinal digestion.
In this study, we revealed an unexpected role of neuronal ROS in controlling food digestion. Through a genome-wide RNAi screen using the established food digestion system in C. elegans, we found that the capacity to digest food is diminished in animals where ctl-1, ctl-2, ctl-3, sod-3, and prdx-2 have been knocked down. These genes influence H2O2 and ROS homeostasis. Interestingly, we observed that PRDX-2 knockout specifically in AWC neurons reduces the ability of animals to digest non-edible food. This happens through the activation of the peripheral UPRmt, which requires the neuropeptide, NLP-1. Moreover, we showed that the ablation of AWC neurons activates intestinal UPRmt and inhibits food digestion. In summary, neuronal “ROS signaling” systematically activates intestinal “eat less signaling” to shut down digestion through UPRmt induction, thereby decreasing food usage to alleviate the cellular stress.
Results
ROS homeostasis as a key factor in regulating food digestion
Previously, we found that the worms arrested development at early larval stages when fed non-edible bacteria Staphylococcus saprophyticus (SS)4,5. SS was accumulated in the gut, and the intestinal lumen of worms fed SS were bloated, suggesting that worms failed to digest the SS5. We have established a food digestion research system where low-quality food (Heat killed-E. coli) activates worm to digest inedible food (SS)5,6.
We discovered that signals such as outer membrane proteins (OMP) and peptidoglycan from heat-killed E. coli activate the food digestive system in worm, thereby enabling them to digest inedible food SS to support growth5,7. In animals, inhibition of the conserved innate immune signaling, PMK-1, stimulates food digestion, demonstrating a prominent role of the innate immune pathway in digestion system5.
To discover other signals involved in digestion regulation, we could perform an RNAi screen and identified the genes that positively regulation food digestion. After knocking down these genes, HK-E. coli failed to promote worms to digest SS, resulting in animals showing a slow growth phenotype when fed HK-E. coli + SS food (Supplementary Fig. 1a). Through a genome-wide RNAi screen, we found that animals growth slowed by RNAi inactivation of 26 genes in HK-E. coli + SS feeding condition (Supplementary Fig. 1b), suggesting that these genes may be involved in promoting animals to digest SS. GO enrichment analysis shows that most enrichment genes are related to H2O2 and ROS homeostasis regulation (Supplementary Fig. 1c), such as ctl-1, ctl-2, ctl-3, sod-3 and prdx-2, suggesting that ROS homeostasis may be a key factor in animals for regulating food digestion.
To confirm this hypothesis, (i) we quantified the oxidative status in animals utilizing the redox-sensitive dye MitoTracker™ Red CMXRos (further details provided in the methods section). This dye is highly sensitive to alterations in mitochondrial membrane potential, which may be impacted by the presence of reactive oxygen species (ROS) during periods of oxidative stress11. We found that ctl-1, ctl-2, ctl-3, sod-3 and prdx-2 mutant animals (Supplementary Fig. 2a) are experiencing oxidative stress, indicating that reactive oxygen species (ROS) homeostasis is disrupted in these mutants; (ii) We assessed the developmental rate of mutant animals fed HK-E. coli + SS and found that all of these mutants grew slower than wild-type animals (Fig. 1a). However, they exhibited a slightly slower growth phenotype under normal E. coli OP50 feeding conditions (Supplementary Fig. 2b). Moreover, this developmental delay phenotype is suppressed by supplementation of antioxidant NAC (Fig. 1b), suggesting that food digestion ability is decreased in animals by disruption of ROS homeostasis; (iii) Moreover, we found that the supplementation of low concentration of H2O2 significantly suppressed development of wild-type animals by feeding with HK-E. coli + SS (Fig. 1c), but not under normal E. coli OP50 feeding conditions (Supplementary Fig. 2c), supporting the idea that oxidative stress inhibit digestion. All of the data indicate that disruption of ROS homeostasis in animals shuts down food digestion.
a Developmental progression of synchronized WT, prdx-2(gk169), ctl-1(ok1242), ctl-2(ok1137), ctl-3(ok2042), sod-3(tm760) L1 animals grown on HK-E. coli + SS bacteria for 96 h at 20°C. Obtained p values (from L4 stage) were as follows: WT vs prdx-2(gk169); p = 0.004. WT vs ctl-1(ok1242); p = 0.0001. WT vs ctl-2(ok1137); p = 0.003. WT vs ctl-3(ok2042); p = 0.003. WT vs sod-3 (tm760); p = 0.01. b Developmental progression of different synchronized WT, prdx-2(gk169), ctl-1(ok1242), ctl-2(ok1137), ctl-3(ok2042), sod-3(tm760) L1 animals grown on HK-E. coli + SS bacteria supplemented with 50 mM NAC for 96 h at 20°C. Obtained p values (from L4 stage) were as follows: WT vs prdx-2(gk169); p = 0.06. WT vs ctl-1(ok1242); p = 0.01. WT vs ctl-2(ok1137); p = 0.29. WT vs ctl-3(ok2042); p = 0.09. WT vs sod-3 (tm760); p = 0.05. c Developmental progression of WT synchronized L1 animals grown on HK-E. coli + SS bacteria supplemented with 0.1 mM, 1 mM H2O2 for 96 h at 20°C. Obtained p values (from L2 stage) were as follows: Control vs 0.1 mM H2O2; p = 0.0001. Control vs 1 mM H2O2; p = 0.04. d Representative microscope images and quantitative analysis of MitoTrackerTM Red CMXRos fluorescent in WT animals after grown on E. coli OP50, HK-E. coli + SS, SS for 24 h. Obtained p-values were as follows: OP50 vs HK-E. coli + SS; p = 0.005. OP50 vs SS; p < 0.0001. See more detail method in the methods section. For all panels, n = the number of worms. Data are represented as mean ± SEM. All statistical analyses were preformed using unpaired two-tailed Student’s t-test. **p < 0.01, ***p < 0.001; n.s., not significant. All experiments were performed independently at least three times. Source data are provided. as a Source Data file. See also Supplementary Figs. 1, 2.
We next investigated whether ROS homeostasis is affected in animals feeding on non-edible or digested food. Our results showed that oxidative stress levels increased in animals fed non-edible food (SS) (Fig. 1d), suggesting that proper ROS homeostasis is crucial for the digestion process.
Peroxiredoxin 2 promotes food digestion through scavenging ROS
Peroxiredoxins (Prxs) are proteins that are highly conserved and found in most organisms. Their primary function is to scavenge reactive oxygen species (ROS)12. To study the role of ROS in food digestion, we began by investigating the function of Peroxiredoxins (Prdx) in food digestion. We found that mitochondrial ROS level is increased in animals with prdx-2 mutation (Fig. 2a) or RNAi (Fig. 2b), which is consistence with PRDX-2’s role in scavenging ROS within the cells. In addition to our study, other laboratories have recently demonstrated increased levels of mitochondrial ROS13 and endogenous H2O214 in prdx-2 mutant animals. Interestingly, we observed that prdx-2 mutants exhibited slower growth than wild-type when fed HK-E. coli + SS (Fig. 2c), suggesting that food digestion ability is decreased in prdx-2 mutant. However, the developmental delay phenotype is suppressed when the antioxidant NAC was supplemented (Fig. 2d). This implies that increased ROS in prdx-2 mutant inhibits animals’ ability to digest SS. Additionally, we observed that NAC treatment also enhanced the growth of wild-type animals (Fig. 2d), suggesting that the antioxidant NAC may promote digestion. Overall, our findings suggest that PRDX-2 enhances the ability of animals to digest SS food by scavenging ROS.
a Representative microscope images and quantitative analysis of MitoTrackerTM Red CMXRos fluorescent showing increased ROS level in WT and prdx-2(gk169) after being grown on E. coli OP50 for 48 h at 20°C. Obtained p value was: WT vs prdx-2(gk169); p = 0.0009. See more detail method in the methods section. b Representative microscope images and quantitative analysis of col-19p::mito::cpYFP fluorescent in WT animals treated with vector control and prdx-2 RNAi bacteria. The col-19p::mito::cpYFP has been used as a sensor for Mitochondrial ROS46. Obtained p value was: Control RNAi vs prdx-2 RNAi; p < 0.0001. c Developmental progression of synchronized WT, prdx-2(gk169) L1 animals grown on HK-E. coli + SS bacteria for 96 h at 20 °C. Obtained p values (from L4 stage) was: WT vs prdx-2(gk169); p = 0.03. d Developmental progression of synchronized WT, prdx-2(gk169) L1 animals grown on HK-E. coli + SS bacteria supplemented with 50 mM NAC for 96 h at 20°C. Obtained p values (from L4 stage) were as follows: WT vs prdx-2(gk169); p < 0.0001. WT + NAC vs prdx-2(gk169)+NAC; p = 0.29. For all panels, n = the number of worms. Data are represented as mean ± SEM. All statistical analyses were preformed using unpaired two-tailed Student’s t-test.**p < 0.01, ***p < 0.001, n.s., not significant. All experiments were performed independently at least three times. Source data are provided as a Source Data file.
Peroxiredoxin 2 (Prdx2) plays a positive role in food digestion within the AWC neuron
To investigate the function of PRDX-2 in food digestion, we first constructed the transgenic animals to analyze the expression and localization of this gene using transgenic strains: prdx-2p::GFP and prdx-2p::PRDX-2::GFP or prdx-2p::GFP::PRDX-2. We observed that prdx-2p::GFP was expressed in head neuron, which co-localized with odr-1p::RFP, an AWC neuronal marker (Supplementary Fig. 3a). This suggests that prdx-2 is expressed in the AWC neuron. In addition to the AWC neuron, prdx-2 was also expressed in other head neuron and tail neuron, which we have not yet identified (Supplementary Fig. 3a).
Using two transgenic animals (prdx-2p::PRDX-2::GFP or prdx-2p::GFP::PRDX-2), we found that PRDX-2 also co-localized with odr-1p::RFP (Fig. 3a and Supplementary Fig. 3b), suggesting its localization in the AWC neuron. We also observed that PRDX-2 was expressed in the intestine and other head neuron (Fig. 3a and Supplementary Fig. 3b).
a Confocal image of expression pattern of PRDX-2. b–e Developmental progression of prdx-2(gk169) carries with (b) prdx-2p::prdx-2::gfp (by its own promoter expression) or (c) rgef-1p::prdx-2::gfp (neuron-specific expression) or (d) vha-6p::prdx-2::gfp (intestine-specific expression) or (e) odr-1p::prdx-2::gfp (AWC neuron-specific expression) animals quantified by relative worm length. Animals carrying transgenes are labeled in yellow. Obtained p values were as follows: (b) Control vs Transgene; p < 0.0001. (c) Control vs Transgene; p < 0.0001. (d) Control vs Transgene; p = 0.14. (e) Control vs Transgene; p = 0.0001. f Representative microscope images and quantitative analysis of MitoTrackerTM Red CMXRos in WT, prdx-2(gk169) or AWC neuron specific knockout prdx-2 animals which grown on E. coli OP50 for 48 h at 20°C. Obtained p values were as follows: WT vs prdx-2(gk169); p < 0.0001. WT vs AWC prdx-2 KO (ylf40); p < 0.0001. See more detail method in the methods section. g Developmental progression of synchronized WT, prdx-2(gk169) or AWC neuron specific knockout prdx-2 animals L1 grown on HK-E. coli + SS bacteria for 96 h at 20°C. Obtained p values (from L4 stage) were as follows: WT vs prdx-2(gk169); p = 0.0004. WT vs AWC prdx-2 KO (ylf40); p = 0.0003. For all panels, n = the number of worms. Data are represented as mean ± SEM. All statistical analyses were preformed using unpaired two-tailed Student’s t-test. **p < 0.01, ***p < 0.001, n.s., not significant. All experiments were performed independently at least three times. Source data are provided as a Source Data file. See also Supplementary Fig. 3.
Considering that PRDX-2 is expressed in both neurons and the intestine, and that neuronal PRDX-2 plays a role in detecting H2O215 and modulating H2O2-induced feeding16 and behavioral responses17, we sought to determine which tissue-specific expression of PRDX-2 is involved in regulating food digestion. We expressed prdx-2 with pan-neuron (rgef-1), AWC neuron (odr-1), intestine (vha-6) and its own promoter into prdx-2(gk169) mutant and fed these animals with HK-E. coli + SS (Fig. 3b–e). We found that pan-neuron and AWC neuron expression of prdx-2 could rescue the development defect of prdx-2(gk169) (Fig. 3c–e), but not in intestine (Fig. 3d). Interestingly, the high level of ROS in prdx-2 mutant was also rescued (reduced) by expression prdx-2 in pan-neuron and AWC neuron, but not in the intestine (Supplementary Fig. 3c). These results suggest that PRDX-2 in AWC neuron promotes food digestion by maintaining ROS homeostasis.
To further confirm the role of PRDX-2 in food digestion in AWC neuron, we specifically knocked out prdx-2 in the AWC neuron using CRISPR-Cas9 (Supplementary Fig. 3d). We observed that (i) the oxidative stress is elevated in animals with prdx-2 knockout specifically in AWC neurons (Fig. 3f); (ii) animals with prdx-2 knockout in AWC neurons exhibited slower growth compared to wild-type animals when fed HK-E. coli + SS (Fig. 3g). To evaluate food digestion capability, we measured the width of the intestinal lumen, a marker that becomes enlarged when digestion is impaired, as our previously reported7. We observed intestinal bloating in animals with a prdx-2 mutation in whole body or in AWC neurons (Supplementary Fig. 3e), indicating a reduced ability to digest food in these mutants. This suggests that PRDX-2 in AWC promotes animals to digest SS food.
A robust activation of UPRmt inhibit food digestion capacity
Mitochondria are both a major source and target of reactive oxygen species (ROS). To combat mtROS and alleviate mitochondrial stress, cellular systems have evolved a quality control mechanism known as the mitochondrial unfolded protein response (UPRmt)18. In previous studies, we found that food digestion ability decreased in bcf-17 or afts-1 (et18) mutant animals (Supplementary Fig. 4a), which exhibited activation of UPRmt. To further validate these results, we assessed food digestion capacity in animals subjected to RNAi targeting genes known to induced by UPRmt based on previous reports19. We observed that food digestion capacity decreased in animals undergoing RNAi of cco-1, spg-7, or gfm-1, which induce robust UPRmt (Supplementary Fig. 4b, c). However, there was not a significant change in food digestion capacity in animals undergoing RNAi of tsfm-1 or nuaf-3, which induce mild UPRmt (Supplementary Fig. 4b, c). This data strongly suggests that robust activation of UPRmt may inhibit food digestion capacity.
We also explored an alternative possibility: mitochondrial dysfunction resulting from the inhibition of the respiratory chain impairs digestion. We knocked down genes associated with the mitochondrial respiratory chain, including atp-2, nuo-1, nuo-6, ndua-5, ndua-2, gas-1, ndub-6, sdbh-1, and sdhd-1. Our findings indicated that all of these RNAi treatments induced the UPRmt, with atp-2, ndua-5, nuo-1, and nuo-6 RNAi leading to a strong UPRmt response (Supplementary Fig. 5a). Interestingly, we observed that the food digestion capacity was not reduced in all RNAi-treated animals due to respiratory chain inhibition, suggesting that the inhibition of the respiratory chain did not directly impair digestion. However, we did find that food digestion capacity decreased in animals subjected to RNAi of atp-2, nuo-1, nuo-6 and ndua-5, which induced a robust UPRmt response (Supplementary Fig. 5b). These data imply that: (i) inhibition of the respiratory chain does not directly hinder digestion, and (ii) a strong activation of UPRmt caused by respiratory chain inhibition suppresses food digestion capacity, whereas a mild activation of UPRmt does not.
To further investigate whether this robust UPRmt activation is indeed responsible for the observed digestion inhibition, we examined the digestive phenotype in cco-1 RNAi animals carrying an atfs-1 mutation. Our results revealed that the digestion defects present in cco-1 RNAi animals were alleviated by the atfs-1 mutation, which effectively abolished UPRmt activation (Supplementary Fig. 5c). These findings suggest that a robust activation of UPRmt plays a crucial role in inhibiting food digestion. This led us to question whether increased ROS in prdx-2 mutant animals also induces UPRmt, thereby inhibiting food digestion.
AWC-specific knockout prdx-2 induces cell non-autonomous UPRmt
UPRmt induction requires the key transcription factor ATFS-1 and its transcriptional co-regulators, including DVE-120,21,22. Our findings showed that prdx-2 knockout induces the mitochondrial unfolded protein response (UPRmt) through activation of hsp-6 expression (Fig. 4a, Supplementary Fig. 6a) and translocation of DVE-1, a regulator of hsp-621, into the nucleus (Fig. 4b) in the intestine. This is dependent on the classical UPRmt pathway, ATFS-1 (Supplementary Fig. 6b). Furthermore, UPRmt was induced in animals with mutations of ctl-2, ctl-3, and sod-3 (Supplementary Fig. 6c), which increase the ROS level in animals. To determine if prdx-2 mutation-induced ROS specifically triggers UPRmt, we also assessed the unfolded protein response in the endoplasmic reticulum (UPRER) and cytosol (UPRCyt). Our findings indicate that neither the hsp-4p::gfp reporter (UPRER) (Supplementary Fig. 6d) nor the hsp-16.2p::gfp reporter (UPRCyt) (Supplementary Fig. 6e) was induced in animals by prdx-2 RNAi. These data suggest that prdx-2 mutation specifically induces UPRmt.
a Representative microscope images and quantitative analysis of hsp-6p::GFP expression in WT and prdx-2(gk169) animals. Obtained p value was: WT vs prdx-2(gk169); p < 0.0001. b Representative microscope images and quantitative analysis of dve-1p::DVE-1::GFP in WT and prdx-2(gk169) animals. Obtained p value was: WT vs prdx-2(gk169); p < 0.0001. c Representative microscope images and quantitative analysis of hsp-6p::GFP expression in WT, prdx-2(gk169), prdx-2(gk169) carries with rgef-1p::prdx-2::mKate2 or odr-1p::prdx-2:: mKate2 animals. Obtained p values were as follows: WT vs prdx-2(gk169); p < 0.0001. prdx-2(gk169) vs ylfEx180 [prdx-2(gk169); rgef-1p::prdx-2::mKate2]; p < 0.0001. prdx-2(gk169) vs ylfEx162 [prdx-2(gk169); odr-1p::prdx-2::mKate2]; p < 0.0001. d Representative microscope images and quantitative analysis of hsp-6p::GFP expression in WT, prdx-2(gk169) or AWC neuron specific knockout prdx-2 animals. Obtained p values were as follows: WT vs prdx-2(gk169); p < 0.0001. WT vs AWC prdx-2 KO (ylf40); p < 0.0001. e Developmental progression of synchronized WT, prdx-2(gk169), atfs-1(tm4525), prdx-2(gk169);atfs-1(tm4525) L1 animals grown on HK-E. coli + SS bacteria for 96 h at 20°C. Obtained p values (from L4 stage) were as follows: WT vs prdx-2(gk169); p < 0.0001. WT vs atfs-1(tm4525); p = 0.03. prdx-2(gk169) vs prdx-2(gk169);atfs-1(tm4525); p = 0.004. For all panels, n = the number of worms. Data are represented as mean ± SEM. All statistical analyses were preformed using unpaired two-tailed Student’s t-test. ***p < 0.001, n.s., not significant. All experiments were performed independently at least three times. Source data are provided as a Source Data file. See also Supplementary Figs. 4–6.
Given that PRDX-2 in neurons promotes food digestion, we wondered if neuronal PRDX-2 regulates intestinal UPRmt. We found that the expression of prdx-2 with pan-neuron (rgef-1) and AWC neuron (odr-1) strongly rescued (inhibited) the activation of hsp-6p::GFP in the intestine of prdx-2 mutants (Fig. 4c). Moreover, prdx-2 knockout in the AWC neuron also induced peripheral UPRmt (Fig. 4d). These data suggest that PRDX-2 in the AWC neuron mediates cell non-autonomous UPRmt.
The UPRmt system consists of chaperones and proteases, which promote protein folding or eliminate mitochondrial proteins damaged by mtROS, respectively. ATFS-1 is known to be a key transcription factor involved in UPRmt activation. We found that UPRmt activation in prdx-2 mutants is abolished by RNAi of atfs-1. Therefore, we wondered whether activated UPRmt also helps or promotes animals to digest food under stress conditions, such as disruption of ROS homeostasis in prdx-2 mutants. Under the food digestion system (HK-E. coli + SS feeding condition), the prdx-2 mutant had a severe synthetic growth defect on an afts-1 (loss-of-function) mutation background, where atfs-1 mutant development was similar to the wild-type (Fig. 4e), suggesting that ATFS-1 is essential for survival under mitochondrial stress induced by prdx-2 inhibition.
prdx-2 knock-out induced cell non-autonomous UPRmt requires neuropeptide (NLP-1)
The mitochondrial unfolded protein response (UPRmt) is triggered non-cell-autonomously through retromer-dependent Wnt signaling23. Specifically, when the Q40 protein is expressed in neurons of C. elegans, it induces UPRmt activation in the intestine, a process that relies on the Wnt signaling pathway mediated by EGL-2023. To investigate whether UPRmt induction due to prdx-2 knockout in AWC neurons also requires EGL-20, we evaluated UPRmt levels in the intestine by knocking down egl-20 in prdx-2 AWC knockout mutants. Surprisingly, we found that UPRmt levels did not decrease in the prdx-2 mutant after egl-20 RNAi treatment (Supplementary Fig. 7a). Instead, UPRmt levels slightly increased in the prdx-2 AWC knockout mutants following egl-20 RNAi (Supplementary Fig. 7a). These results suggest that the activation of intestinal UPRmt in prdx-2 knockout in AWC neurons does not depend on the Wnt signaling pathway mediated by EGL-20.
The AWC neuron does not physically interact with the intestine, yet the AWC-specific knockout of prdx-2 induces intestinal UPRmt. This suggests that a neuroendocrine signal may be required for signaling transduction. Previous studies have shown that NLP-1, released from the AWC, is required for lowering the AWC’s calcium response to odors24. Recent research also indicates that mtROS is necessary for NLP-1 secretion25. Therefore, we asked whether UPRmt induction by prdx-2 knockout also requires neuropeptide, NLP-1.
To test this, we first constructed transgenic animals (nlp-1p::GFP) to observe the expression pattern and found that it was expressed in the AWC neuron (Fig. 5a). We then measured the mRNA level of nlp-1 and found that nlp-1 mRNA is increased in the prdx-2 mutant on OP50 or HK-E. coli + SS feeding conditions (Fig. 5b). Based on western blot analysis using nlp-1p::GFP reporter strain, we observed increased nlp-1 expression in animals subjected to prdx-2 RNAi (Supplementary Fig. 7b). Furthermore, we discovered that the increased UPRmt in prdx-2 mutant animals is suppressed by nlp-1 mutation (Fig. 5c). These data suggest that prdx-2 knockout induces intestinal UPRmt through increasing the expression of NLP-1.
a Confocal image of expression pattern of nlp-1. b qRT-PCR analyses of nlp-1 mRNA level in the WT or prdx-2(gk169) animals feeding OP50 or HK-E. coli + SS. Obtained p values were as follows: WT OP50 vs prdx-2(gk169) OP50; p = 0.0003. WT HK-E. coli + SS vs prdx-2(gk169) HK-E. coli + SS; p = 0.0334. c Representative microscope images and quantitative analysis of hsp-6p::GFP expression in WT, prdx-2(gk169), nlp-1(ok1469), prdx-2(gk169);nlp-1(ok1469) animals. Obtained p values were as follows: WT vs prdx-2(gk169); p < 0.0001. prdx-2(gk169) vs prdx-2(gk169);nlp-1(ok1469); p = 0.0372. d Representative microscope images and quantitative analysis of hsp-6p::GFP expression in WT, WT carries with nlp-1p:: nlp-1::3xFlag, odr-1p:: nlp-1::3xFlag and vha-6p::nlp-1::3xFlag animals. Obtained p values were as follows: WT vs ylfEx216[nlp-1p::nlp-1::3xFlag]; p < 0.0001. ylfEx217[odr-1p::nlp-1:: 3xFlag]; p = 0.001. ylfEx218[vha-6p::nlp-1:: 3xFlag]; p < 0.0001. e, f Developmental progression of synchronized WT animals carries with nlp-1p:: nlp-1::3xflag (e) or odr-1p:: nlp-1::3xflag (f) L1 animals, which was quantified as relative worm length. Transgene worms are indicated by a yellow asterisk. Obtained p values were as follows: (e): Control vs Transgene; p < 0.0001. (f): Control vs Transgene; p < 0.0001. For the panels (c–f) n = the number of worms. For panels (b), n = the number of qPCR experiments performed. Data are represented as mean ± SEM. All statistical analyses were preformed using unpaired two-tailed Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. All experiments were performed independently at least three times. Source data are provided as a Source Data file. See also Supplementary Fig. 7.
Next, we investigated the regulation of nlp-1 expression. In our study, we observed that increasing ROS levels through mutations in ctl or sod genes, or by supplementing with H2O2, inhibits animals’ food digestion. Therefore, we also explored whether ROS could influence nlp-1 expression. We found that nlp-1 expression was elevated in animals supplemented with H2O2 (Supplementary Fig. 7c), suggesting that oxidative stress induces nlp-1 expression to inhibit digestion. Furthermore, we examined nlp-1 expression in animals subjected to ctl-3 and sod-3 RNAi. We observed increased nlp-1 expression in these animals (Supplementary Fig. 7d), indicating that ROS accumulation induces nlp-1 expression. Overall, these findings suggest that elevated ROS levels may induce nlp-1 expression.
To investigate the mechanism by which an increase in neuronal ROS leads to nlp-1 expression, we examined whether this ROS-induced nlp-1 expression relies on neurotransmission. We assessed nlp-1 expression in animals subjected to RNAi targeting unc-31, a gene essential for dense core vesicle neurotransmission26. Our results revealed that the induction of nlp-1 expression by prdx-2 RNAi was inhibited by unc-31 RNAi (Supplementary Fig. 7e), indicating that ROS-induced nlp-1 expression is indeed dependent on neurotransmission.
To determine whether secreted NLP-1 activates UPRmt, thereby regulating food digestion, we overexpressed nlp-1 using its own promoter, the AWC neuron (odr-1p), and the intestinal (vha-6) promoter. We found that UPRmt was activated in all transgenic animals (Fig. 5d), suggesting that secreted NLP-1 activates intestinal UPRmt. Simultaneously, the transgenic animals with over-expressing nlp-1 in native (Fig. 5e) or AWC neuron (Fig. 5f) grew slower than the wild-type when fed HK-E. coli + SS (Fig. 5e, f), suggesting that food digestion ability decreased in animals by overexpressing NLP-1. This implies that NLP-1 secretion activates the intestinal UPRmt, thereby inhibiting food digestion.
Ablation of AWC neurons activate intestinal UPRmt and inhibit digestion
AWC food-sensing neurons release NLP-1, which acts on the NPR-11 receptor in the AIA interneurons to modulate INS-1 insulin release. This in turn feeds back to dampen AWC olfactory responses and alter food-seeking behaviors27. Given that prdx-2 functions in the AWC neuron to regulate UPRmt and digestion, we hypothesized that ablation of AWC neurons could mimic a food depletion condition, potentially shutting down food digestion.
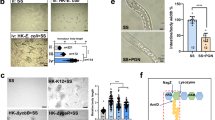
To test this, we first constructed transgenic animals that over-express egl-1, a cell death activator28, in AWC neurons. This was done using the odr-1 promoter in an hsp-6p::GFP background (Fig. 6a). We then crossed a previously validated transgenic strain that expresses cleaved caspase under the AWC-specific promoter, ceh-36 (ceh-36p::caspase)29, into a UPRmt reporter strain (hsp-6p::GFP). We found that UPRmt was activated in both animals with ablation of AWC (Fig. 6a, b), which consistence with recently study showing that olfactory nervous system in C. elegans regulates the UPRmt cell nonautonomously30. Additionally, we observed an elevated oxidative stress in animals where AWC was ablated, as indicated by staining with the redox-sensitive dye MitoTracke™ Red CMXRos (Supplementary Fig. 8).
a Representative microscope images and quantitative analysis of hsp-6p::GFP expression in WT and WT carries with odr-1p:: egl-1::mKate2 animals. Obtained p value was: WT vs ylfEx179[odr-1p::egl-1::mKate2]; p < 0.0001. b Representative microscope images and quantitative analysis hsp-6p::GFP expression in WT, animals with AWC ablation. Animals with AWC ablation (PY7502 strain) contains srtx-1p::GFP, which serves as a co-injection marker expressed in AFD neurons (arrow). Obtained p value was: WT vs AWC ablation; p < 0.0001. c Developmental progression of synchronized L1 animals (WT and WT carries with odr-1p::egl-1::mKate2) grown on HK-E. coli + SS bacteria for 96 h at 20 °C. Obtained p value was: Control vs Transgene; p < 0.0001. d Developmental progression of synchronized L1 animals [WT and AWC (-)] grown on HK-E. coli + SS bacteria for 96 h at 20 °C. Obtained p value (from L4 stage) was: WT vs AWC ablation; p < 0.0001. e A model for the function of neuronal ROS in regulation of food digestion in intestine. Specific knockout of PRDX-2 in olfactory neurons (AWC) leads to increased ROS levels. This triggers the release of the neuropeptide (NLP-1) and induces the intestinal UPRmt, which in turn shuts down food digestion. (Created by the Qian Li using Adobe Illustrator 2020). For all panels, n = the number of worms. Data are represented as mean ± SEM. All statistical analyses were preformed using unpaired two-tailed Student’s t-test. ***p < 0.001 n.s., not significant. All experiments were performed independently at least three times. Source data are provided as a Source Data file. See also Supplementary Fig. 8.
Next, we measured the animals’ development when fed HK-E.coli + SS and found that animals with ablation of AWC grew slower than the wild-type (Fig. 6c, d). This suggests that the ability to digest food decreased when AWC neurons were ablated. In conclusion, these data suggest that the ablation of AWC neurons activates intestinal UPRmt and inhibits food digestion.
PMK-1 contributes to the inhibitory effect of UPRmt activation caused by prdx-2 mutation on food digestion
Our previous study showed that inhibition of the innate immune PMK-1 pathway enhances digestion5. In our recent study, we demonstrated that PMK-1 partially mediates the inhibitory effect of UPRmt activation on food digestion7. Thus, we investigated whether PMK-1 also contributes to the inhibitory effect of UPRmt activation caused by prdx-2 mutation on food digestion. Firstly, we measured PMK-1 levels in prdx-2 mutants and found that p-PMK-1 levels were elevated in prdx-2 mutant animals under E. coli or HK-E. coli + SS feeding conditions, indicating that activation of the PMK-1 pathway may contribute to the digestive defects in prdx-2 mutants (Supplementary Fig. 9a). Secondly, to further explore the role of the PMK-1 pathway in prdx-2 mutant digestive defects, we generated double mutants prdx-2(gk169);pmk-1(km25) and observed that the developmental delay (food digestion defects) in prdx-2 mutants was rescued in the double mutants (Supplementary Fig. 9b). These results suggest that prdx-2 mutation activates innate immunity through the PMK-1 pathway, thereby inhibiting food digestion.
Discussion
Food digestion is a multifaceted process that is crucial for maintaining health. Reactive Oxygen Species (ROS) in the hypothalamus play a role in regulating food intake and metabolism. However, it is still unclear whether neuronal ROS signaling regulates food digestion in the intestine. Here, by using an established food digestion system in C. elegans, we discovered that neuronal ROS (free radicals) shut down the food digestion through signaling the peripheral UPRmt by neuropeptide (Fig. 6e).
Gut hormones and afferent neurons play a crucial role in regulating digestive processes such as gastric emptying and gut motility. It is widely believed that gut-brain signaling may be significant in regulating food-related physiology, including food intake, and preferences. However, our understanding of the neuronal signals that activate intestinal food digestion remains limited. Through a whole-genome RNAi screen, we identified several genes, including ctl-1, ctl-2, ctl-3, sod-3, and prdx-2, which are involved in maintaining ROS homeostasis, positively regulate food digestion. Knocking down of these genes resulted in a decrease in food digestion ability. We discovered that prdx-2 is expressed in AWC neurons, and specifically knocking out this gene in AWC neurons shuts down food digestion, suggesting that free radicals increasing in neurons shuts down digestion.
We found that food digestion ability decreases in animals when AWC neurons are ablated, indicating that AWC neurons also contribute to promoting food digestion. AWC neurons are olfactory neurons critical for chemotaxis to volatile odorants such as benzaldehyde, butanone, isoamyl alcohol31. These neurons also respond to temperature32. Recently study also show that olfactory nervous system in C. elegans regulates the UPRmt cell nonautonomously30.
Pathogen-associated odorants also extend lifespan through TGF-β signaling and UPR activation33. However, whether and how olfactory neurons respond to food and signal to the digestive system has not been reported yet. Our finding that the mutation of prdx-2 in AWC neurons disrupts the food digestive system by inducing intestinal UPRmt is both unexpected and promising. This discovery opens up new possibilities for understanding the complex interplay between the nervous system and digestion.
In mammals, ROS in the hypothalamus regulates food intake and metabolism by acting on different types of neurons, including proopiomelanocortin (POMC) and agouti-related protein (AgRP)/neuropeptide Y (NPY) neurons9. Thus, it is possible that neuronal ROS could potentially shut down the mammalian food digestion system, thereby reducing nutrient utilization. This hypothesis presents an intriguing prospect for future research studies, potentially leading to the development of treatments for digestive diseases or obesity by inhibiting the process of food digestion.
How does free radicals in AWC neurons shut down food digestion? Previously, we found that activation of intestinal UPRmt inhibits food digestion7. Here, we discovered that specifically knocking out prdx-2 in olfactory neurons (AWC) activates the intestinal unfolded protein response (UPRmt), a process that requires the neuropeptide, NLP-1. Over-expressing nlp-1 or ablating AWC neurons also activates UPRmt, suggesting that free radicals in AWC neurons inhibit food digestion by activating this mitochondrial stress signal. Previous studies have demonstrated that the loss of prdx-2 enhances stress resistance34 and decreases insulin secretion, leading to elevated activities of DAF-16 and SKN-135. More recently, research has uncovered a new role for mitochondrial-derived H2O2 in regulating the secretion of the neuropeptide FLP-136, which serves as a neuroendocrine signal during stress, activating oxidative stress responses in distant tissues. Additionally, secretion of FLP-2 is increased in prdx-2 mutant animals, which results in elevated levels of endogenous hydrogen peroxide14. Therefore, it is plausible that mutations in prdx-2 could also affect food digestion through alternative pathways, such as reducing insulin secretion or increasing FLP-2 secretion. Investigating these possibilities will be crucial for future studies in this field.
The mitochondrial unfolded protein response (UPRmt) is activated when mitochondrial integrity and function are compromised. This response promotes cell survival and the recovery of the mitochondrial network to ensure optimal cellular function18. The cell-non-autonomous mitochondrial stress signal between neurons and the intestine in C. elegans has been well studied. Intestinal UPRmt can be induced in animals by disrupting neuronal function, such as by neuronal knockdown of the mitochondrial electron transport chain (ETC) subunit cytochrome c oxidase-1 (cco-1)37, neuronal expression of the Huntington’s disease-causing polyglutamine protein (Q40)38, neuronal depletion of the C. elegans mitofusin (FZO-1)39, or expression of reactive oxygen species (ROS)-generating fluorescent protein KillerRed in neurons40. Shao et al.’s study40 showed that antioxidant NAC or ascorbic acid treatment suppressed KillerRed-induced non-autonomous UPRmt. Thus, mitochondrial ROS generated in the nervous system could potentially initiate the intestinal cell protection process, UPRmt. Consistent with previous studies, we discovered that the knockout of the ROS regulation gene, PRDX-2, in olfactory neurons (AWC), triggers the activation of intestinal UPRmt, thereby inhibiting food digestion. This system may serve as a protective mechanism for organisms, as “eating less” is generally beneficial for animals under stress condition.
Mitochondria, being the primary organelles responsible for energy production, play a crucial role in cellular function. The neural control of visceral organ function is vital for maintaining homeostasis and health. Consequently, we propose that neuronal ROS, indicative of neuronal mitochondrial damage, could be a highly conserved signal across species. This neuronal “damage signal-ROS” systematically activates intestinal UPRmt for initiating the “eat less” response by inhibiting food digestion, thereby protecting the organism under stress condition. By shutting down food digestion, animals potentially mimic the effects of dietary restriction, thereby reducing nutrient utilization. This could lead to a reduction in protein translation, which in turn alleviates the intestinal UPR response.
In our study, we observed elevated mitochondrial ROS (mitROS) in prdx-2 mutant animals, which correlates with impaired food digestion. Previous research has shown that PRDX-2 is essential for normal longevity34. Building on these findings, we asked whether prdx-2 mutants might display heightened vulnerability to illness, resulting in food digestion inhibition. To explore this hypothesis, we present two sets of data: (i) In our recent study, we demonstrated that PMK-1 partly mediates the inhibitory effect of UPRmt activation on food digestion7. We observed increased levels of phosphorylated PMK-1 (p-PMK-1) in prdx-2 mutant animals. Moreover, by generating prdx-2(gk169);pmk-1(km25) double mutants, we observed a rescue of the developmental delay (food digestion defects) observed in prdx-2 mutants alone (Supplementary Fig. 9b). These results indicate that the prdx-2 mutation activates innate immunity through the PMK-1 pathway, thereby inhibiting food digestion. (ii) We also evaluated the lifespan of mutant animals. Consistent with published data34, prdx-2 mutants exhibited a shortened lifespan, similar to pmk-1 mutants. Intriguingly, prdx-2(gk169);pmk-1(km25) double mutant animals showed an even further reduction in lifespan compared to either single mutant (Supplementary Fig. 9c). Based on the lifespan data (Supplementary Fig. 9c), double mutants could be considered more susceptible (“sicker”) animals due to their shortened lifespan phenotype. If prdx-2 mutants were indeed “sicker” and inhibited in food digestion, we would anticipate that prdx-2(gk169);pmk-1(km25) double mutants would exhibit a food digestion defect similar to prdx-2 mutants. However, we found that prdx-2(gk169);pmk-1(km25) double mutants were capable of digesting inedible food (SS), unlike prdx-2 mutants. Therefore, our data suggest that the initial hypothesis—that prdx-2 mutants are “sicker” animals leading to food digestion inhibition—may not be supported.
In summary, our study unveils a mechanism of brain-gut communication in regulating food digestion. In this system, neuronal “ROS signaling” systematically activates intestinal “eat less signaling”, shutting down digestion through UPRmt induction, thereby decreasing food usage. Our findings also suggest that regulating ROS signaling in neurons could potentially serve as a therapeutic strategy for treating digestive diseases or obesity by inhibiting food digestion.
Methods
C. elegans strains and maintenance
Nematode stocks were maintained on nematode growth medium (NGM) plates seeded with bacteria (E. coli OP50) at 20 °C. See all the strains in Supplementary Table 1.
Bacterial strains
coli-OP50 and Staphylococcus saprophyticus were cultured at 37 °C in LB medium. A standard overnight cultured bacteria was then spread onto each Nematode growth media (NGM) plate.
Generation of transgenic strains
To construct the C. elegans plasmid for expression of prdx-2, 1083 bp promoter and genomic DNA of prdx-2 was inserted into the pPD49.26-GFP vector. DNA plasmid mixture containing prdx-2p::prdx-2::GFP (10 ng/µl) and odr-1p::RFP(50 ng/µl) was injected into the gonads of adult N2.
To construct the C. elegans plasmid for expression of prdx-2, 1083 bp promoter and 1296 bp genomic DNA of prdx-2 was inserted into the pPD49.26-GFP vector. DNA plasmid mixture containing prdx-2p::prdx-2::GFP (10 ng/µl) and odr-1p::RFP(50 ng/µl) was injected into the gonads of adult prdx-2(gk169).
To construct the C. elegans plasmid for expression of prdx-2 in neuron, 3057 bp promoter of rgef-1 and 1296 bp genomic DNA of prdx-2 was inserted into the pPD49.26-GFP vector. DNA plasmid mixture containing rgef-1p::prdx-2::GFP (10 ng/µl) and odr-1p::RFP (50 ng/µl) was injected into the gonads of adult prdx-2(gk169).
To construct the C. elegans plasmid for expression of prdx-2 in intestine, 1593 bp promoter of vha-6 and 1296 bp genomic DNA of prdx-2 was inserted into the pPD49.26-GFP vector. DNA plasmid mixture containing vha-6p::prdx-2::GFP (10 ng/µl) and odr-1p::RFP (50 ng/µl) was injected into the gonads of adult prdx-2(gk169).
To evaluate the expression level of hsp-6p::GFP in prdx-2(gk169) after rgef-1p::prdx-2::mKate2 supplementation, 3057 bp promoter of rgef-1 and 1296 bp genomic DNA of prdx-2 was inserted into the pPD49.26-mKate2 vector. DNA plasmid mixture containing rgef-1p::prdx-2::mKate2 (10 ng/µl) and pRF4(rol-6) (50 ng/µl) was injected into the gonads of adult prdx-2(gk169); zcIs13[hsp-6p::GFP + lin-15(+)].
To construct the C. elegans plasmid for expression of prdx-2 in AWC neuron, 1348 bp promoter of odr-1 and 1296 bp genomic DNA of prdx-2 was inserted into the pPD49.26-GFP vector. DNA plasmid mixture containing odr-1p::prdx-2::GFP(10 ng/µl) and odr-1p::RFP(50 ng/µl) was injected into the gonads of adult prdx-2(gk169).
To evaluate the expression level of hsp-6p::GFP in prdx-2(gk169) after odr-1p::prdx-2:: mKate2 supplementation, 1348 bp promoter of odr-1 and 1296 bp genomic DNA of prdx-2 was inserted into the pPD49.26-mKate2 vector. DNA plasmid mixture containing odr-1p::prdx-2::mKate2(10 ng/µl) and pRF4(rol-6) (50 ng/µl) was injected into the gonads of adult prdx-2(gk169);zcIs13[hsp-6p::GFP + lin-15(+)].
To construct the C. elegans plasmid for expression of prdx-2 in GFP C-terminal, 1083 bp promoter and 1296 bp genomic DNA of prdx-2 was inserted into the pPD49.26-GFP vector. DNA plasmid mixture containing prdx-2p::GFP::prdx-2 (10 ng/µl) and odr-1p::RFP (50 ng/µl) was injected into the gonads of adult prdx-2(gk169).
To construct the C. elegans plasmid for tracking original expression of prdx-2, 1083 bp promoter was inserted into the pPD49.26-GFP vector. DNA plasmid mixture containing prdx-2p::GFP(10 ng/µl) and odr-1p::RFP(50 ng/µl) was injected into the gonads of adult N2.
To construct the C. elegans plasmid for killing AWC neuron and evaluating expression level of hsp-6p::GFP, 1348 bp promoter of odr-1 and 875 bp genomic DNA of egl-1 was inserted into the pPD49.26-mKate2 vector, DNA plasmid mixture containing odr-1p::egl-1::mKate2 (10 ng/µl) and pRF4(rol-6) (50 ng/µl) was injected into the gonads of adult N2.
To construct the C. elegans plasmid for tracking original expression of nlp-1, 2019bp promoter was inserted into the pPD49.26-GFP vector. DNA plasmid mixture containing nlp-1p::GFP(10 ng/µl) and odr-1p::RFP(50 ng/µl) was injected into the gonads of adult N2.
To evaluate the expression level of hsp-6p::GFP after nlp-1p::nlp-1::3*Flag supplementation, 2019bp promoter and 691 bp genomic DNA of nlp-1 was inserted into the pPD49.26-3*Flag vector. DNA plasmid mixture containing nlp-1p::nlp-1::3*Flag (10 ng/µl) and odr-1p::RFP(50 ng/µl) was injected into the gonads of adult zcIs13[hsp-6p::GFP + lin-15(+)].
To evaluate the expression level of hsp-6p::GFP after odr-1p::nlp-1::3*Flag supplementation, 1348 bp promoter of odr-1 and 691 bp genomic DNA of nlp-1 was inserted into the pPD49.26-3*Flag vector. DNA plasmid mixture containing odr-1-1p::nlp-1::3*Flag (10 ng/µl) and odr-1p::RFP(50 ng/µl) was injected into the gonads of adult zcIs13[hsp-6p::GFP + lin-15(+)].
To evaluate the expression level of hsp-6p::GFP after vha-6p::nlp-1::3*Flag supplementation, 1593 bp promoter of vha-6 and 691 bp genomic DNA of nlp-1 was inserted into the pPD49.26-3*Flag vector. DNA plasmid mixture containing vha-6p::nlp-1::3*Flag (10 ng/µl) and odr-1p::RFP(50 ng/µl) was injected into the gonads of adult zcIs13[hsp-6p::GFP + lin-15(+)].
See all the primers in Supplementary Table 2.
Generation prdx-2 tissue specific knock out strain and Genotyping
To construct the C. elegans plasmid for knock out of prdx-2 in AWC neuron, 600 bp promoter of eft-3 was replaced by 1348 bp promoter of odr-1 and prdx-2 sgRNA was also inserted into the same CRISPR-Cas9-sgRNA vector pDD16241. The Cas9 target sites were designed via CRISPR design tool (http://crispor.tefor.net/) and the sgRNA sequences was 5’-CGCCTTCTCTGACCGTGCTGAGG-3’. Knockout strains were generated by injecting 25 ng/μl Cas9-sgRNA plasmid, 2 μM repair template, co-injection markers include 20 ng/μl dpy-10 Cas9-sgRNA plasmid and 2 μM dpy-10 repair template.
Worms were picked into 10 μl of worm lysis buffer (50 mM KCl,10 mM Tris-HCl pH 8.0, 2.5 mM MgCl2, 0.45% NP40, 0.45% Tween-20, 0.01% Gelactin, 0.2 mg/mL Proteinase K), quickly freeze-thaw three times using liquid nitrogen, incubated it at 60 °C for 90 min and 95 °C for 20 min. 1 µl supernatant was taken and performed for PCR analysis with the following primers:
prdx-2: forward 5′-catttcgtcctccgattttttttct-3′,reverse 5′-gggcggtctaggaaagtgaca-3′, then digested with NheI endonuclease overnight and identified by DNA agarose electrophoresis.’
Preparation of heat-killed E. coli (HK-E. coli) + S. saprophyticus Food
HK-E. coli was prepared by our established protocol4,42: E. coli OP50 grown in LB with a standard overnight culture condition. Concentrate the cultured bacteria tenfold and then heat-killed at 80 °C for 120 min.
S. saprophyticus was prepared by our established protocol6: S. saprophyticus (SS) grown in LB with a standard overnight culture condition. Inoculate the cultured bacteria into fresh LB (1:100 ratio), 37 °C grew to OD600 = 0.5.
HK-E. coli + S. saprophyticus was prepared by our established protocol5: HK-E. coli and S. saprophyticus (SS) were mixed at a 1:1 ratio, then spread the mixture on the NGM plates.
Analysis of larval growth
C. elegans were grown on E. coli OP50 until to egg-laying adult and then washing in M9 buffer. Eggs were collected by bleaching, then hatched in M9 buffer for 12–16 h. Synchronized L1 larvae were seeded to plates prepared for the specific assay and incubated at 20 °C for 3–4 days.
We employed vulval morphology to determine the stage of L4 and adult animals. However, we used the length of the animals’ gonads to determine stages L1–L3, following the methodology outlined in our published paper4.
In this study, we focused exclusively on worm length for analyzing the growth of transgenic animals (as shown in Figs. 3b–e, 5e, f, 6c). When constructing transgenic animals using extrachromosomal arrays, our populations often included both transgenic and non-transgenic individuals. Due to limitations in obtaining a large number of transgenic animals for certain strains, we conducted measurements of individual worm lengths for both transgenic and control (non-transgenic) animals.
C. elegans RNAi screen
All RNAi by feeding used bacterial clones from the MRC RNAi library43 or the ORF-RNAi Library44. RNAi plates were prepared by adding IPTG to a final concentration of 0.024 mg/mL and Ampicillin to a final concentration of 0.1 mg/mL to NGM agar. Overnight cultured RNAi strains (LB containing 0.1 mg/mL Ampicillin and tetracycline) and the control strain (HT115 strain with empty L4440 vector) were seeded into RNAi feeding plates and cultured at room temperature for 1 days before use.
Synchronized L1 wildtype N2 worms were seed into the RNAi feeding plates for the first generation and grew until to egg-laying adult. Bleached the adult worms and collect the eggs and hatched in M9 buffer for 12–16 h. Synchronized L1 larvae were seeded on the indicated feeding plate (HK-E. coli + SS). The worm development stage was measured after culturing 3–4 days at 20 °C. After knocking down the genes involving in food digestion regulation, HK-E. coli failed to promote worms to digest SS, resulting in animals showing a slow growth phenotype when fed HK-E. coli + SS food (Supplementary Fig. 1a).
Worm total protein extraction
Worms were washed into the tube and the M9 Buffer were washed 3 times to remove the bacteria. Add protein lysis buffer (50Mm Tris-HCL pH 8.0, 50Mm NaCl, 0.5% deoxycholate, 10% glycerol, 1% NP40) containing PMSF (1 mM), cocktail (MCE, HY-K0011), phosphate stop (MCE, HY-K0021). Grind and break worms, centrifuge at 14000 g, 4 °C for 10 min, collected the supernatant. Used the Pierce BCA protein assay kit (Thermo Fisher, 23227) to measure the protein concentration and quantification.
Western blot
Add 5 × SDS loading buffer to the quantified protein sample (1:4), 100 °C, 10 min for denatures proteins. Then run SDS-polyacrylamide gel electrophoresis (SDS-PAGE) to separate the proteins and transfer it to the PVDF membrane. To measure the level of GFP, probed with anti-GFP (dilution = 1:3000; Proteintech, 50430-2-AP) or anti-tubulin (dilution = 1:5000; Sigma T5168) as a loading control. To measure the level of p-PMK-1, probed with anti-p-p38(dilution = 1:3000; Cell Signaling, 4511S) and anti-p38(dilution = 1:3000; Cell Signaling, 9212S) or anti-tubulin (dilution = 1:5000; Sigma T5168) as a loading control.
Oxidative stress detection of C. elegans
MitoTracker™ Red CMXRos is commonly used in oxidative stress studies to assess mitochondrial health11. This dye is sensitive to changes in mitochondrial membrane potential, which can be affected by the presence of reactive oxygen species (ROS) during oxidative stress. A drop in fluorescence intensity indicates a loss of mitochondrial membrane potential, reflecting mitochondrial dysfunction, which is often observed in cells experiencing elevated levels of ROS11. In contrast, healthy mitochondria maintain strong fluorescence under stress conditions, indicating their functionality remains intact. When mitochondrial ROS production is excessive, leading to oxidative damage, the mitochondrial membrane potential diminishes, resulting in reduced dye staining and decreased fluorescence45.
1 mM stock solution of MitoTracker™ Red CMXRos was prepared in DMSO. Next, 0.4 µL of the stock solution was added to 50 µL of M9 buffer and thoroughly mixed. Approximately 20–30 worms were then introduced into the mixture and incubated in the dark for 5–10 min. Following this incubation period, the worms were placed on OP50 agar plates at 20 °C to recover for 30 min. Finally, the fluorescence intensity was assessed and recorded.
N-Acetyl-L-cysteine (NAC) supplementation
500 mM stock solution of N-Acetyl-L-cysteine (SIGMA, A7250-5G) was made in ddH2O. NAC stock solution was added into NGM plates’ medium with a final concentration of 50 mM before pouring of the plates. Spread the HK-E. coli + SS on the plates. Synchronized L1 larvae were seeded to plates and observe its development rates.
Hydrogen peroxide treatment
A 10 mM stock solution of H2O2 was diluted in ddH2O. Dilute the H2O2 stock solution to 0.1 mM and 1Mm in M9 buffer. Synchronized L1 larvae were added into the M9 Buffer (as control)、0.1 mM H2O2、1 mM H2O2 and incubated in a shaking incubator at 20 °C for 30 min. After incubation, wash animals 3 times with M9 Buffer. Then worms were seeded to plates and observe its development rates.
Real-time PCR
Sample preparation: Synchronized L1 worms feed different foods (E. coli OP50 or HK-E. coli + SS) to L4, then worms were collected and wash 3 times with M9 Buffer to remove bacteria.
RNA isolation: The prepared samples (50 µl) were added 500 µl TRIzol (Invitrogen) and preserved at -80 °C until RNA isolation. First, blow the sample 50–100 times with a 1 ml fine needle to break the worms. Then add 100 µl chloroform and shake for 15 s, stood 5 min at room temperature, centrifuged at 14000 g, 4 °C for 15 min. Absorbent supernatant and add isopropanol (1:1), stood 10 min at room temperature, centrifuged at 14000 g, 4 °C for 10 min. Leave the precipitate and wash it with 75% ethanol. Total RNA was obtained by blow-drying precipitation and dissolved with RNA-free water.
cDNA synthesis: 1 µg RNA was reverse transcription with oligo dT primer using the PrimeScript™ II 1st Strand cDNA Synthesis Kit (Takara, 6210 A).
qPCR reaction: Gene expression levels was detected by using PowerUpTMSYBRTM Green (ThermoFisher A25742) on real-time PCR machine (ABI QuantStudio I). Fold changes in gene expression were calculated using the 2-ΔΔCt method. Primer sequences:
nlp-1: fwd 5’-TGTCTTCTTGTGATAGCTGCTG-3’ and rev 5’-GGTCGAGTACGTGAATGATGA -3’,
act-1: fwd 5’-GTTGCCGCTCTTGTTGTAGAC-3’ and rev 5’-GGTGACGATACCGTGCTCAA-3’.
Fluorescence intensity measurement
To measure fluorescence in UPRmt (hsp-6p::GFP), UPRER (hsp-4p::GFP), and UPRCyt (hsp-16.2p::GFP) phenotypes, worms were anesthetized with 10 mM levamisole and imaged under excitation light. Fluorescence intensity across the whole worm was quantified using ImageJ, focusing specifically on intestinal fluorescence.
Microscopy
The fluorescence photographs were taken by Olympus BX53 microscope with a DP80 camera. Development statistics were taken by Olympus MVX10 dissecting microscope with a DP80 camera. The confocal images were taken by inverted Zeiss LSM 880/900 confocal microscope system equipped with an alpha Plan-Apochromat 363 oil-immersion objective lens, and processed and analyzed with ZEN imaging software (v.3.4).
Quantification
Animals were randomly selected for fluorescent photography. The size of transgene worms was photographed by the Nomarski microscope and measured by ImageJ software. ImageJ software was used for quantifying fluorescence intensity of indicated animals, which was then normalized with control group.
Statistical analysis
All experiments were performed independently at least three times with similar results. All statistical analyses were preformed using unpaired two-tailed Student’s t-test, except for Supplementary Fig. 1b which was analyzed by using the Chi-square test. Statistical parameters are presented as mean ± SEM, statistical significance (P < 0.05, *, P < 0.01, **, P < 0.001, ***), and “n” (the number of worms counted). The phenotype observations in our experiments were conducted in a blinded manner to genotype.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data in main Manuscript and Supplementary information are listed in the Source data file. All reagents and strains generated by this study are available through request to the corresponding author with a completed Material Transfer Agreement. Source data are provided with this paper.
References
Brookes, S. J. H., Spencer, N. J., Costa, M. & Zagorodnyuk, V. P. Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastro Hepat. 10, 286–296 (2013).
Chambers, A. P., Sandoval, D. A. & Seeley, R. J. Integration of satiety signals by the central nervous system. Curr. Biol. 23, R379–R388 (2013).
Guerra, A. et al. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol. 30, 591–600 (2012).
Qi, B., Kniazeva, M. & Han, M. A vitamin-B2-sensing mechanism that regulates gut protease activity to impact animal’s food behavior and growth. Elife 6, e26243 (2017).
Geng, S. et al. Gut commensal E. coli outer membrane proteins activate the host food digestive system through neural-immune communication. Cell Host Microbe. 30, 1401–1416.e1408 (2022).
Liu, H. & Qi, B. Protocol for investigating the effect of food digestion in C. elegans on development by feeding the inedible bacteria Staphylococcus saprophyticus. STAR Protoc. 4, 101990 (2023).
Hao, F., Liu, H. & Qi, B. Bacterial peptidoglycan acts as a digestive signal mediating host adaptation to diverse food resources in C. elegans. Nat. Commun. 15, 3286 (2024).
Drougard, A., Fournel, A., Valet, P. & Knauf, C. Impact of hypothalamic reactive oxygen species in the regulation of energy metabolism and food intake. Front Neurosci. 9, 56 (2015).
Andrews, Z. B. et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454, 846–851 (2008).
Vahid, F., Wagener, L., Leners, B. & Bohn, T. Pro- and antioxidant effect of food items and matrices during simulated in vitro digestion. Foods 12, 1719 (2023).
Li, X. et al. High-fat diet promotes experimental colitis by inducing oxidative stress in the colon. Am. J. Physiol.-Gastr L 317, G453–G462 (2019).
Graves, J. A., Metukuri, M., Scott, D., Rothermund, K. & Prochownik, E. V. Regulation of reactive oxygen species homeostasis by peroxiredoxins and c-Myc. J. Biol. Chem. 284, 6520–6529 (2009).
Xia, Q. et al. Peroxiredoxin 2 regulates DAF-16/FOXO mediated mitochondrial remodelling in response to exercise that is disrupted in ageing. Mol. Metab. 88, 102003 (2024).
Jia, Q., Young, D. & Sieburth, D. Endogenous hydrogen peroxide positively regulates secretion of a gut-derived peptide in neuroendocrine potentiation of the oxidative stress response in C. elegans. Elife 13, RP97503 (2024).
Quintin, S., Aspert, T., Ye, T. & Charvin, G. Distinct mechanisms underlie H2O2 sensing in C. elegans head and tail. PLoS One 17, e0274226 (2022).
Bhatla, N. & Horvitz, H. R. Light and hydrogen peroxide inhibit C. elegans feeding through gustatory receptor orthologs and pharyngeal neurons. Neuron 85, 804–818 (2015).
Li, G., Gong, J. K., Lei, H. Y., Liu, J. F. & Xu, X. Z. S. Promotion of behavior and neuronal function by reactive oxygen species in. Nat. Commun. 7, 13234 (2016).
Shpilka, T. & Haynes, C. M. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 19, 109–120 (2018).
Shpilka, T. et al. UPR(mt) scales mitochondrial network expansion with protein synthesis via mitochondrial import in Caenorhabditis elegans. Nat. Commun. 12, 479 (2021).
Nargund, A. M., Pellegrino, M. W., Fiorese, C. J., Baker, B. M. & Haynes, C. M. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337, 587–590 (2012).
Haynes, C. M., Petrova, K., Benedetti, C., Yang, Y. & Ron, D. ClpP mediates activation of a mitochondrial unfolded protein response in C-elegans. Dev. Cell 13, 467–480 (2007).
Tian, Y. et al. Mitochondrial stress induces chromatin reorganization to promote longevity and UPRmt. Cell 165, 1197–1208 (2016).
Zhang, Q. et al. The mitochondrial unfolded protein response is mediated cell-non-autonomously by retromer-dependent Wnt signaling. Cell 174, 870 (2018).
Chalasani, S. H. et al. Neuropeptide feedback modifies odor-evoked dynamics in olfactory neurons. Nat. Neurosci. 13, 615–U130 (2010).
Lee, H. K., Park, K.-S. & Yoon, K.-h. Mitochondrial calcium uniporter regulates odor learning and memory by controlling neuropeptide release. bioRxiv 13, RP102642 (2023).
Speese, S. et al. UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J. Neurosci. 27, 6150–6162 (2007).
Sengupta, L. The belly rules the nose: feeding state-dependent modulation of peripheral chemosensory responses. Curr. Opin. Neurobiol. 23, 68–75 (2013).
Conradt, B. & Horvitz, H. R. The protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell 93, 519–529 (1998).
Beverly, M., Anbil, S. & Sengupta, P. Degeneracy and neuromodulation among thermosensory neurons contribute to robust thermosensory behaviors in. J. Neurosci. 31, 11718–11727 (2011).
Dishart, J. G. et al. Olfaction regulates peripheral mitophagy and mitochondrial function. Sci. Adv. 10, eadn0014 (2024).
L’Etoile, N. D. & Bargmann, C. I. Olfaction and odor discrimination are mediated by the guanylyl cyclase ODR-1. Neuron 25, 575–586 (2000).
Biron, D., Wasserman, S., Thomas, J. H., Samuel, A. D. T. & Sengupta, P. An olfactory neuron responds stochastically to temperature and modulates thermotactic behavior. P Natl Acad. Sci. USA 105, 11002–11007 (2008).
De-Souza, E. A., Thompson, M. A. & Taylor, R. C. Olfactory chemosensation extends lifespan through TGF-β signaling and UPR activation. Nat. Aging 3, 938–947 (2023).
Oláhová, M. et al. A redox-sensitive peroxiredoxin that is important for longevity has tissue- and stress-specific roles in stress resistance. Proc. Natl Acad. Sci. USA 105, 19839–19844 (2008).
Oláhová, M. & Veal, E. A. A peroxiredoxin, PRDX-2, is required for insulin secretion and insulin/IIS-dependent regulation of stress resistance and longevity. Aging Cell 14, 558–568 (2015).
Jia, Q. & Sieburth, D. Mitochondrial hydrogen peroxide positively regulates neuropeptide secretion during diet-induced activation of the oxidative stress response. Nat. Commun. 12, 2304 (2021).
Durieux, J., Wolff, S. & Dillin, A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144, 79–91 (2011).
Berendzen, K. M. et al. Neuroendocrine coordination of mitochondrial stress signaling and proteostasis. Cell 166, 1553 (2016).
Chen, L. T. et al. Neuronal mitochondrial dynamics coordinate systemic mitochondrial morphology and stress response to confer pathogen resistance in C. elegans. Dev. Cell 56, 1770 (2021).
Shao, L. W., Niu, R. & Liu, Y. Neuropeptide signals cell non-autonomous mitochondrial unfolded protein response. Cell Res. 26, 1182–1196 (2016).
Dickinson, D. J., Ward, J. D., Reiner, D. J. & Goldstein, B. Engineering the genome using Cas9-triggered homologous recombination. Nat. Methods 10, 1028 (2013).
Chen, Y., Yang, R., Qi, B. & Shan, Z. Peptidoglycan-Chi3l1 interaction shapes gut microbiota in intestinal mucus layer. Elife 13, RP92994 (2024).
Kamath, R. S. et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 (2003).
Rual, J. F. et al. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14, 2162–2168 (2004).
Nayak, D. et al. Biofilm impeding AgNPs target skin carcinoma by inducing mitochondrial membrane depolarization mediated through ROS production. Acs Appl Mater. Inter 8, 28538–28553 (2016).
Xu, S. H. & Chisholm, A. D. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev. Cell 31, 48–60 (2014).
Acknowledgements
We thank the Caenorhabditis Genetics Center (CGC) (funded by NIH P40OD010440) for strains; We thank Dr. Chongling Yang (Yunnan University), Dr. Shangbang Gao (Huazhong University of Science and Technology), Dr. Xiajing Tong (ShanghaiTech University) for sharing strains. This work was supported by the Ministry of Science and Technology of the People’s Republic of China (2019YFA0803100, 2019YFA0802100 to B.Q.), the National Natural Science Foundation of China (32071129 to Z.S., 32170794 to B.Q.), Yunnan Provincial Science and Technology Project at Southwest United Graduate School (202302AP370005 to B.Q.), Yunnan Applied Basic Research Projects (202201AT070196 to B.Q.), Science and Technological Talent Cultivation Plan of Yunnan Province (C619300A086 to Z.S., K264202230211 to B.Q.).
Author information
Authors and Affiliations
Contributions
Y. L. and Q.L. performed experiments, analyzed data. Y.L., G.T., X.Z., P.C. performed RNAi screen, B.C. performed GO enrichment analysis. Z.S. wrote/modified the manuscript and provided some critical suggestions. B.Q. supervised this study, and wrote the paper with inputs from Y.L. and Q.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Li, Q., Tian, G. et al. Neuronal PRDX-2-Mediated ROS Signaling Regulates Food Digestion via peripheral UPRmt Activation. Nat Commun 15, 10582 (2024). https://doi.org/10.1038/s41467-024-55013-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-55013-3
This article is cited by
-
The shutdown of food digestion due to endoplasmic reticulum homeostasis disruption acts as a protective mechanism in C. elegans
Nature Communications (2025)
-
Overexpression of PRDX2 alleviates chronic thromboembolic pulmonary hypertension by modulating inflammation and mitophagy
Scientific Reports (2025)