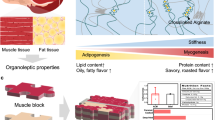
Abstract
Cultured meat needs edible bio-scaffolds that provide not only a growth milieu for muscle and adipose cells, but also biomimetic stiffness and tissue-sculpting topography. Current meat-engineering technologies struggle to achieve scalable cell production, efficient cell differentiation, and tissue maturation in one single culture system. Here we propose an autoclaving strategy to transform common vegetables into muscle- and adipose-engineering scaffolds, without undergoing conventional plant decellularization. We selected vegetables with natural anisotropic and isotropic topology mimicking muscle and adipose microstructures respectively. We further adjusted vegetable stiffness by autoclaving, to emulate the mechanical properties of animal tissues. Autoclaved vegetables preserve rich cell-affinitive moieties, yielding a good cell culture effect with simplified processing. Autoclaved Chinese chive and Shiitake mushroom with anisotropic micro-patterns support the scalable expansion of muscle cells, improved cell alignment and myogenesis. Autoclaved isotropic loofah encourages adipocyte proliferation and lipid accumulation. Our engineered muscle- and fat-on-vegetables can further construct meat stuffing or layered meat chips. Autoclaved vegetables possess tissue-mimicking stiffness and topology, and bring biochemical benefits, operational ease, cost reduction and bioreactor compatibility. Without needing decellularization, these natural biomaterials may see scale-up applications in meat analog bio-fabrication.
Similar content being viewed by others
Introduction
Cultured meat, or ‘cell-based meat’, is ‘meat’ created with cutting-edge biotechnology rather than by traditional farming, to satisfy the ever-increasing demand for meat from the earth’s 8 billion population1,2,3. Scalable production of meat building blocks, mainly muscle and adipose progenitor cells, requires innovative biomaterials supporting efficient cell expansion, differentiation, and tissue morphogenesis4. Commonly used meat-engineering materials include micro-carriers5,6,7 or bulk, porous scaffolds8,9. Though promising for large-scale production, those scaffolds often lack muscle-mimicking geometry to recreate the hierarchical muscle anatomy. Anchored hydrogel strips10, 3D-printed hydrogel lattices11, micro-fibers12,13 and micro-patterned substrates14,15 are alternative muscle-engineering systems offering the desired structural anisotropy. Nevertheless, they either use expensive animal-derived fibrin/collagen hydrogels or demand complex manufacturing such as 3D printing, electrospinning or micro-fabrication. Their throughput is thus limited to lab-scale, and the cost is high for industry. Therefore, cultured meat still awaits biomaterials displaying the following attributes: (1) affordability, which can be achieved by reducing the use of animal-derived matrix; (2) food safety, achieved by selecting edible biomaterials while avoiding hazardous processing; (3) biomimicry, achieved by recreating tissue-mimicking stiffness and micro-architecture2.
Recently, researchers proposed decellularized plants, such as green onion bulbs and Sorghum leaves, as myoblast culture substrates16,17. Using surfactants including sodium dodecyl sulfate (SDS), sodium hypochlorite (NaClO) and Triton X-100, decellularization removes plant cells to expose a highly structured, cellulosic plant skeleton18,19,20, which aids myofiber aligning. However, due to the lack of cell-adhesive motifs in the cellulose backbone, decellularized plants represent a cell-phobic matrix for mammalian cells. Thus, surface modification is obligatory21,22. Decellularized plants can be oxidized using sodium periodate (NaIO4) to increase their binding to cell-affinitive proteins23, or coated with positively charged poly-L-Lysine (PLL) to promote cell adhesion24. Nevertheless, this unwantedly complicates material preparation while introducing potentially hazardous chemicals. Additionally, from a nutritional perspective, decellularization erases beneficial plant proteins, lipids and polysaccharides, causing substantial nutrition loss.
While plants are interesting cell culture materials25,26, we sought a more facile and efficient way to valorize plants for cultured meat bio-fabrication. Here we illustrate common vegetables, in their autoclaved format without decellularization, as meat-engineering scaffolds. Autoclaving is a standard sterilization method for biological research and healthcare practice. Intriguingly, we found that autoclaving imparts tunable, meat-mimicking stiffness to vegetables, while preserving mammalian cell-affinitive molecules. Moreover, we identified a variety of unique, well-ordered topologies on autoclaved vegetables. Their inherent anisotropic or isotropic micro-patterns resemble the anatomical micro-structures of muscle or adipose tissues, and can aid tissue morphogenesis in a way similar to micro-patterned substrates14, but at much lower cost and higher scalability. We therefore propose autoclaved vegetables with tissue-like stiffness and micro-patterns, as widely available, low-cost scaffolds for cultured meat technology.
Results
Screening of autoclaved vegetables with anisotropic and isotropic micro-patterns
Skeletal muscle is highly-aligned. During development, myogenic cells fuse to form multi-nucleated myotubes, which further mature into long, parallel myobundles. Such tissue anisotropy represents a crucial directional cue to be incorporated in muscle engineering. In search of anisotropic muscle scaffolds that are edible and cost-effective, we explored the plant kingdom. Ordered micro-patterns have been reported on decellularized plants to guide the alignment of cultured mammalian cells16,17. However, we observed poor affinity of decellularized plant skeletons for myogenic cells such as C2C12 murine myoblasts, even after soaking materials in protein (Supplementary Fig. 2a–c) or PLL solutions (Supplementary Fig. 2d). NaIO4 oxidation could aid cell adhesion (Supplementary Fig. 2e), but the decellularization plus surface modification was tedious and involved hazardous chemicals. Unexpectedly, when cultured on autoclaved plants, cells showed better adhesion, while still being able to organize themselves along plants’ surface micro-patterns (Figs. 1, 2). This prompted us to use the readily accessible topography on autoclaved vegetables, while bypassing the complex practice of plant decellularization.
a (i), b (i) Representative Live and Dead images (n = three independent experiments) of C2C12 cultured on vegetable slices for 2 days. Vegetables were autoclaved, rinsed with deionized water, and soaked in growth medium containing 1% w/v gelatin overnight. Scale bar = 100 μm. a (ii), b (ii) Alignment angle distribution of C2C12 on respective vegetable slices. The radial axis shows the radial sum intensity and the circumferential axis shows cell orientation angles (0−180 degrees). Detailed quantification method is explained in Supplementary Fig. 1. Panel a (i) and b (i) were, in part, created with BioRender. Lab, D. (2024) https://BioRender.com/x24q862. and with ixintu.com.
a (i), b (i) Representative Live and Dead images (n = three independent experiments) of C2C12 cultured on vegetable slices for 2 days. Vegetables were autoclaved, rinsed with deionized water, and soaked in growth medium containing 1% w/v gelatin overnight. Scale bar = 100 μm. a (ii), b (ii)) Distribution of cell alignment angles on respective vegetable slices. The radial axis shows the radial sum intensity and the circumferential axis shows cell orientation angles (0−180 degrees). Panel a (i) and b (i) were, in part, created with BioRender. Lab, D. (2024) https://BioRender.com/x24q862 and with ixintu.com.
To select plants with good topology and cell affinity, we screened 16 types of vegetables commonly found in our local supermarket. Structurally, we preferred the stem part, as the elongated xylem (water-transporting “highway” in vascular plants) in the stem may mimic the parallel aligned geometry of muscle tissue. Seeding C2C12 myoblasts directly on autoclaved vegetable slices, we observed a wide distribution (>90° in range) of cell alignment angles on half of the screened plants, including banana, apple, Romaine lettuce, cucumber, sweet potato, white radish, eggplant and potato (Fig. 1). This suggests that these plants have isotropic topology proving no directional preference for cultured myoblasts. Interestingly, we discovered several anisotropic candidates including Shiitake mushrooms and lotus sprouts (Fig. 2), on which cells exhibited a narrow distribution (within 30° in range) of alignment angles and meanwhile high confluency. Unexpectedly, we found that leaves of certain plants, such as Chinese chives, also provided alignment cues to myoblasts. Therefore, these vegetables, specifically their stem or leaf parts, qualify as muscle-mimicking scaffolds. On one hand, they possess surface micro-patterns giving clear alignment instructions to cells; on the other, they facilitate cell adhesion and proliferation.
There remain other types of anisotropic plants including green onion, wild celery, bamboo root, and asparagus, which showed a satisfactory degree of cell alignment (within 60° in range) but suboptimal cell coverage (Fig. 2). We also noticed cell death on certain plants such as eggplant (Fig. 1) and bamboo root (Fig. 2). This might be due to material hydrophobicity (Supplementary Fig. 12c) which may discourage cell attachment, or the lack of cell-adhesive motifs, etc. Since the abundance of vegetables is one factor to consider when developing cost-effective scaffolds, we had to forgo lotus sprouts despite their excellent performance (Supplementary Fig. 4), as their harvest is more seasonal (mostly in summer). In the end, this round of vegetable screening pointed us to two most promising muscle-mimicking scaffolds: Chinese chives and Shiitake mushrooms.
Surface chemistry characterization for autoclaved and decellularized vegetable scaffolds
To understand material surface chemistry behind the distinct cell attachment behaviors on autoclaved versus decellularized vegetables, we performed Raman spectroscopy for natural, autoclaved and decellularized vegetable examples, i.e., Chinese chives, Shiitake mushroom and loofah. When preparing decellularized samples, we noticed that decellularized Chinese chive leaves thinned into a membrane, while decellularized loofah lost its structural integrity and became a viscous, gel-like matter (Supplementary Fig. 5a, b). Even worse was the protein- and glucan-rich mushroom, which couldn’t withstand the harsh decellularization and dissolved into tiny particles in NaClO solution (Supplementary Video 1–2).
Raman spectroscopy revealed compositional similarity between natural and autoclaved vegetables, both containing rich functional groups and demonstrating marked differences from decellularized ones (Fig. 3a). Detailed peak information and possible assignments were listed in Supplementary Table 3. For Chinese chives (Fig. 3a (i)), both natural and autoclaved samples showed characteristic Raman peaks of leaf pigments including the green ChlorophyII and yellow carotenoid at 1006, 1156, 1522 and 2160 cm−1. Note that leaves fade in color after autoclaving (from leaf green to olive green, Supplementary Fig. 5a), indicating Mg2+ loss from the center Chlorin ring in ChlorophyII27. Additionally, natural chives displayed minor peaks at 959, 1304, 1440, 1607, 1634, 2850, and 2880 cm−1, indicating the presence of a hydrophobic cuticle wax (cutin) layer that protected the leaf epidermis28. These cutin peaks diminished in autoclaved chives, probably due to our autoclaving and subsequent water-rinsing procedures. This removal of hydrophobic cutin might later aid the attachment of mammalian cells29,30. In contrast to natural and autoclaved samples, decellularized chives exhibited a distinct Raman profile. Instead of characteristic peaks of leaf pigments, decellularized chives showed strong lignin (1638 cm−1) and xylan (2932 cm−1) peaks, suggesting exposure of the ligno-cellulosic xylem and phloem structures after decellularization.
a Raman spectra of Chinese chives, Shiitake mushroom and loofah under different treatments. b, c EDS atomic percentage of major elements (b) and representative EDS mapping images with overlapping nitrogen element (c) for natural, autoclaved and decellularized vegetables. n = three independent experiments. Scale bar = 5 μm. Panel (a, b) were, in part, created with BioRender. Lab, D. (2024) https://BioRender.com/x24q862.
For Shiitake mushroom (Fig. 3a (ii)), both natural and autoclaved ones showed noticeable amide peaks (1257, 1373, 1656 cm−1), suggesting the presence of chitins, proteins and glycoproteins. Both samples showed characteristic peaks associated with α-glucan (476, 540, 941 cm−1), β-glucan (421, 894 cm−1) and polysaccharide (1124, 1064 cm−1). In decellularized mushroom, however, such rich information on amides and polysaccharides largely disappeared, leaving only peaks seemingly representing α-glucan (464, 556, 941 cm−1), β-glucan (800, 1093 cm−1) and chitin (1639 cm−1). For loofah (Fig. 3a (iii)), noticeable Raman peaks for cell wall components lignin (1638, 1528 cm−1) were detected in all the three differently treated samples. Besides, natural and autoclaved loofah revealed rich Raman information between 400 and 1500 cm−1, including possible peaks for cysteine (713 cm−1), tyrosine (823, 853 cm−1), α-glucan (476, 519, 941 cm−1), polysaccharide (1124 cm−1), as well as minor peaks representing cellulose (1062, 1159, 1400 cm−1). Cell wall peaks became noticeable in autoclaved loofah for cellulose (1295 cm−1), and lignin (1437 cm−1), and even more pronounced in decellularized loofah (560, 793, 805, 1092 cm−1). Peaks representing glucan, polysaccharides and amino acids in the 400–1500 cm−1 range disappeared in decellularized loofah, indicating nutrition loss and mere retention of cell wall components that disfavor cell adhesion. Together, Raman data suggests that decellularization exposes plant skeletons containing lignin, cellulose and hemicellulose, while erasing various bioactive molecules, including proteins, chitins, glucans and plant pigments, etc.
FTIR also confirmed compositional differences between autoclaved and decellularized vegetables (Supplementary Fig. 7a). Band information and possible assignments were listed in Supplementary Table 4. For Chinese chives, characteristic β-carotenoid bands at 1008 and 1518 cm−1 were lost in decellularized leaves. In contrast, decellularized leaves revealed more cellulose (1159, 1312 cm−1) and lignin (1035 cm−1) bands. For Shiitake mushroom, though amide (1653, 1558, 1377, 1316, 1259 cm−1) and polysaccharide (1457, 1152, 1030 cm−1) bands were commonly observed for the three treatments, natural and autoclaved mushrooms contained finer band information (1200, 891 cm−1, etc.), suggesting richer and more complex composition than decellularized ones. In natural and autoclaved loofah, a common band near 1397 cm−1 could be assigned to lipids and proteins. Possible amide bands emerged in autoclaved loofah at 1645 and 1540 cm−1. In decellularized loofah, however, more lignin and cellulose bands (1604, 1159, 1107 cm−1) were exposed.
Using EDS, we further examined major elements present on the surface of vegetable slices under different treatments (Fig. 3b, c). While C and O are abundant in all biomolecules, N reflects specifically the presence of proteins, peptides, and nucleic acids. For each of the three tested vegetables, N contents were higher in natural and autoclaved conditions than in decellularized ones. The loss of N-containing biomolecules was especially prominent in decellularized Chinese chives and loofah, whose N content decreased to a negligible amount. This indicates that decellularization erodes vegetable proteins, peptides and nucleic acids, while autoclaving better preserves the N-containing cellular components.
We also detected several classes of substances leaching out from autoclaved vegetables when soaked in water. The majority belongs to flavonoids, small peptides, tryptophan alkaloids, steroids, fatty acids and triterpenoids (Supplementary Fig. 8–10). In case these molecules influence our biomaterial pH, we rinsed autoclaved vegetables thoroughly with deionized water and soaked them in medium before cell culture. This rendered a more neutral and cell-friendly material interface for mammalian cells (Supplementary Fig. 7c).
Protein adsorption, fine micro-structure and stiffness of autoclaved and decellularized scaffolds
Cell-affinitive proteins on biomaterials are a key determinant of mammalian cell attachment and proliferation. Our characterization on scaffold surface chemistry suggests that autoclaved vegetables retained more cell-affinitive functional groups including amides, which not only themselves aid cell adhesion but also increase the adsorption of cell-affinitive proteins31,32. We therefore checked vegetables’ ability to adsorb proteins when soaked in culture medium supplemented with 1% gelatin. We found that autoclaved Chinese chives and loofah tend to adsorb more proteins than their decellularized counterparts (Supplementary Fig. 7b), which helps explain their better cell culture performance (Supplementary Fig. 2–3).
Next, we investigated whether vegetable autoclaving or decellularization bring about microstructural differences. Fine structural details were in general preserved in autoclaved samples (Supplementary Fig. 6), except for some loss of the blade-like trichomes (2–4 μm epicuticular wax crystals33) from autoclaved Chinese chive leaves. This was probably due to plant wax melting during autoclaving, which otherwise can benefit mammalian cell adhesion29,30. Decellularized vegetables, however, displayed a much smoother surface with fewer fine details (<5 μm). Note that as mushroom broke down and dissolved in surfactant solutions, we could only study this decellularized sample by drying the dissolved mushroom solution on glass slides.
As is known that vegetable softens during thermal processing, due to loss of turgor pressure, cell membrane damage, and pectin depolymerization via β-elimination34, we then examined the biomaterial stiffness. Chinese chives, Shiitake mushroom and loofah softened after autoclaving (Fig. 5d, k; Supplementary Fig. 5c, d) without compromising their topology (Fig. 5c, j; Supplementary Fig. 12a). This suggests a convenient way to tune vegetable stiffness by controlling autoclaving time. 1.5 hours of autoclaving is sufficient for Chinese chives (Young’s modulus reduced from average 772.5 kPa to 40.7 kPa, Fig. 5d) to gain a muscle-mimicking stiffness (10–40 kPa)35, and 20 min for mushroom (Young’s modulus reduced from 34.5 kPa to 17.2 kPa, Fig. 5k). 20 min of autoclaving softened loofah from 3.5 kPa to 0.7 kPa (Supplementary Fig. 5d), to match the mechanical range of fat tissue (0.6–2 kPa)36. A further significant drop in stiffness was noticed in decellularized samples. This is owing to surfactants removing cellular contents such as polysaccharides, proteins, nucleic acids, lipids, etc. Decellularized Chinese chives and loofah showed an average Young’s modulus of 8.8 kPa and 0.3 kPa respectively (Supplementary Fig. 5c, d), lower than that of muscle and fat tissues we would like to mimic. Worse still, the mechanically fragile decellularized plants hardly withstand long-term dynamic culture as required in up-scaled bio-fabrication.
Together, our data indicates that autoclaved vegetables bind more proteins and retain structural integrity, while being mechanically more biomimetic and durable for dynamic culture. Vegetable autoclaving also circumvents days of surfactant wash as required in decellularization, yielding a good cell culture effect with much-simplified processing (Fig. 4).
a General procedures to fabricate decellularized plant scaffolds. b General procedures for autoclaved Chinese chives and loofah (b (i)), and for Shiitake mushroom with an optional dopamine coating step (b (ii)). c Summary table comparing key attributes of decellularized and autoclaved vegetable scaffolds.
Transformation of autoclaved anisotropic vegetables into muscle-mimicking scaffolds
After biochemical and biophysical validation of our scaffolds, we moved on to check myoblast performance on autoclaved vegetables. C2C12 cells (average aspect ratio of 2.2 in the proliferating stage, Supplementary Fig. 11a) develop a more elongated morphology (average aspect ratio > 5, Supplementary Fig. 11b, c) upon myogenic differentiation in 2D flasks. Interestingly, on our anisotropic vegetables, proliferating C2C12 cells prior to differentiation already showed such elongated shape (average aspect ratio > 3.7, Fig. 5a, h), in contrast to those on 2D surface lacking directional patterns (Supplementary Fig. 11a). Such cell elongation probably resulted from the contact guidance by vegetable topography. SEM revealed parallel micro-wrinkles on the surface of Chinese chive leaves (average diameter 18.8 μm, Fig. 5b, c); while Shiitake mushroom stem possesses aligned micro-fiber bundles (average diameter 7.6 μm, Fig. 5i, j). The slightly smaller size of mushroom micro-fibers might explain a slightly more elongated cell shape on Shiitake mushrooms than on Chinese chives (Fig. 5a, h).
a, h Distribution of C2C12 cell aspect ratio on autoclaved Chinese chive leaves (a) and Shiitake mushroom stems (h); AR, aspect ratio. b, i Representative SEM images (n = three independent experiments) of fresh Chinese chive leaves (b) and fresh Shiitake mushroom stems (i). Scale bar = 100 μm. c, j Distribution of aligned micro-pattern width on fresh Chinese chive leaves (c) and Shiitake mushroom stem (j). d, k Young’s modulus of fresh and autoclaved Chinese chive leaves (d, Fresh, n = 18; 20 min, n = 49; 1 h, n = 28; 1.5 h, n = 25) and Shiitake mushroom stems (k, Fresh, n = 28; 20 min, n = 53). Magenta lines represent the quartiles. Two-sided Mann-Whitney U test was used, and no multiple testing correction between samples. f, l Water contact angles of Chinese chive leaves (f), and Shiitake mushroom stems before and after dopamine coating (l). e, m Representative SEM images (n = three independent experiments) of autoclaved Chinese chive (e, autoclaved for 1.5 h) and Dopa-mushroom (m, autoclaved for 20 min and Dopa-coated) scaffolds. Scale bar = 100 μm. g, n Representative SEM images (n = three independent experiments) of C2C12 cells growing on autoclaved Chinese chive (g) and Dopa-mushroom (n) scaffolds. Scale bar = 100 μm. Panel (a, h) were, in part, created with BioRender. Lab, D. (2024) https://BioRender.com/x24q862.
We thus obtained two types of muscle-mimicking scaffolds, i.e., autoclaved Chinese chive leaves and Shiitake mushroom slices. Both are anisotropic by nature and within tissue mechanical range. Chinese chives were positively charged (Supplementary Fig. 12b) and hydrophilic (water contact angle of 48°, Supplementary Fig. 12c), while mushroom was negatively charged (Supplementary Fig. 12b) and hydrophobic (water contact angle of 109.4°, Supplementary Fig. 12c). Considering that a moderately hydrophilic surface further enhances cell adhesion30,37,38, we performed an optional dopamine coating for mushroom. This would form a thin layer of adhesive polydopamine on biomaterial surface22,39. Compared with uncoated mushroom, dopamine-coated mushroom (Dopa-mushroom) showed a dark color (Supplementary Fig. 13b), a more neutral charge (Supplementary Fig. 12b), a reduced water contact angle (49.1°, Supplementary Fig. 12c), increased protein adsorption (Supplementary Fig. 7b) and enhanced cell adhesion (Supplementary Fig. 13c). In terms of micro-morphology, dopamine coating did not alter the anisotropy and diameter of mushroom micro-fibers (Fig. 5i, m, j; Supplementary Fig. 12a). Within seven days, C2C12 cells grew into a confluent layer covering the entire surface of Dopa-mushrooms and autoclaved chives (Fig. 5n, g).
Scalable production and differentiation of muscle cells on autoclaved anisotropic vegetables
We next started scalable production of skeletal muscle cells using spinner flask bioreactors (Fig. 6a). To achieve efficient use of culture media and space, we cut the vegetables into thin slices less than 1 mm thick (Supplementary Fig. 13a). This helps to maximize their surface-to-volume ratio, gaining more available culture area within a unit volume. For example, the surface-to-volume ratio of a Chinese chive slice (0.5 mm in thickness) and a mushroom slice (0.6 mm in thickness) is about five times larger than that of a conventional T75 flask (Supplementary Table 1). Although cut thin, vegetable slices maintained their structural integrity for weeks during the spinner flask culture. By proliferation day-5 (Plf D5), C2C12 expanded to 17-fold on Chinese chives (Fig. 6b) and to 11-fold on Dopa-mushroom (Fig. 6g), forming a confluent layer of highly aligned cells on both scaffolds (Fig. 6c, h).
a Schematic representation of scalable muscle engineering using autoclaved vegetable scaffolds with anisotropic micro-patterns. Chinese chives and Shiitake mushroom stems were prepared as aforementioned, and introduced in spinner flask bioreactors along with skeletal muscle cells. Cells were allowed to grow for 5 days into confluent, well-aligned cell colonies before induction of myogenic differentiation. b, g Growth and viability curves of proliferating C2C12 on Chinese chives (b) and Dopa-mushroom (g) scaffolds at a spinning rate of 7 rpm. n = three independent experiments. Data are presented as mean ± s.d. c, h Representative F-actin staining (n = three independent experiments) of Plf Day 5 C2C12 on Chinese chives (c) and Dopa-mushroom (h) scaffolds. Scale bar = 100 μm. d, i Relative mRNA expression (n = three independent experiments) of MYOD, MYOG, MHC-IIx in Dif D6 C2C12 on Chinese chives (d) and Dopa-mushroom (i) scaffolds. Results were normalized to measurements of GAPDH. Data are presented as mean ± s.d. Student’s two-tailed t-test, unpaired and no multiple testing correction between samples. e, j Representative immunofluorescent staining (n = three independent experiments) of terminal differentiation marker MHC in Dif D6 C2C12 on Chinese chives (e) and Dopa-mushroom (j) scaffolds. Scale bar = 100 μm. f, k Representative SEM images (n = three independent experiments) of Dif D6 C2C12 on Chinese chives (f (i)) and Dopa-mushroom (k (i)) scaffolds. Scale bar = 100 μm. f (ii), k (ii) Zoomed-in views of myotube-like structures on respective vegetable scaffolds. Scale bar = 25 μm. Panel (a, b, g) were, in part, created with BioRender. Lab, D. (2024) https://BioRender.com/z27v304.
We then checked C2C12 myogenesis on Chinese chive and Dopa-mushroom scaffolds. C2C12 differentiation was enhanced compared to 2D culture by differentiation day-6 (Dif D6), showing increased expression of myosin heavy chain (MHC) (Fig. 6d, i), a late-stage myogenic marker. Mid-stage myogenic gene MYOG maintained upregulated by Dif D6 on both vegetables; while early-stage gene MYOD returned to a quiescent level. These gene results suggest improved and seemingly earlier myogenesis of C2C12 cells, on anisotropic vegetables compared with 2D culture. SEM and immunofluorescent staining of MHC further validated the formation of highly-aligned myotubes on Chinese chives (Fig. 6f, e) and Dopa-mushrooms (Fig. 6k, j), in contrast to randomly oriented myotubes in 2D flasks (Supplementary Fig. 11c).
We further tested our anisotropic scaffolds with primary porcine myosatellite cells in spinner flasks (Fig. 7a). Porcine myosatellite cells formed a confluent layer on vegetable scaffolds (Supplementary Fig. 15a) and upregulated MHC gene upon myogenic differentiation (Fig. 7b). Differentiated porcine muscle-on-vegetables showed a high water content around 90% (Supplementary Fig. 15c). In terms of nutritional value, engineered Pork-chive and Pork-mush showed a two-fold increase in protein content, compared to autoclaved Chinese chive leaves and mushroom stems (Fig. 7c (i–ii)). This is due to the growth of cultured porcine muscle cells on vegetables. We then wrapped engineered Pork-chive and Pork-mushroom slices into dumplings (Fig. 7d (i–ii)), to create classic Chinese dumplings of pork-mushroom and pork-chive flavors.
a Representative image of porcine myosatellite cells cultured on Chinese chive scaffolds in a 125 mL spinner flask. b Relative mRNA expression (n = three independent experiments) of MYOD, MYOG, MHC-IIA in Dif D6 porcine myosatellite cells cultured on Chinese chives and Dopa-mushroom scaffolds. Results were normalized to measurements of GAPDH. Data are presented as mean ± s.d. Student’s two-tailed t-test, unpaired and no multiple testing correction between samples. c Nutritional evaluation of dried pork-on-chive (i) and pork-on-mushroom tissues (ii). d Representative image of a boiled dumpling (i) stuffed with pork-on-chive and pork-on-mushroom tissues (ii).
Scalable production and differentiation of pre-adipocytes on autoclaved isotropic vegetable
Since fat is another integral part of meat, we next developed appropriate vegetable scaffolds for adipocytes. Adipose tissue exhibits isotropic organization40,41 and a stiffness of 0.6–2 kPa36,42. From our isotropic plant candidates including loofah (Fig. 8b–e) and cucumber (Supplementary Fig. 14c, d), we selected loofah as adipose scaffold, based on its adipose-mimicking stiffness (average 0.7 kPa after 20 minutes’ autoclaving, Supplementary Fig. 14e) and hydrophilicity (water contact angle of 61.7°, Supplementary Fig. 12c). Murine 3T3L1 pre-adipocytes showed random organization on loofah slices (Fig. 8d, e) as guided by the isotropic topology (Fig. 8b), and an average cell aspect ratio of 2.16 (Fig. 8f). Such morphology may reflect a pre-differentiating, intermediate stage between proliferating pre-adipocytes (aspect ratio 2.66, Supplementary Fig. 14a) and differentiated adipocytes (aspect ratio 1.83, Supplementary Fig. 14b). Using spinner flask bioreactors, we expanded 3T3L1 cells to 13-fold on loofah scaffolds within five days (Fig. 8g). Upon induction of adipogenic differentiation, 3T3L1 cells on loofah expressed upregulated adipogenic genes including PPARG, SREBF1 and FAS (Fig. 8h), and accumulated large lipid droplets (Fig. 8i). For illustrative purposes, we made a tarte stuffed with engineered C2C12-chive, C2C12-mushroom and 3T3L1-loofah slices (Supplementary Fig. 15b), which would bring a meaty and veggie savor.
a Schematic representation of scalable adipose engineering using autoclaved vegetable scaffolds with isotropic topography. Autoclaved loofah slices were introduced in spinner flask bioreactors along with pre-adipocytes. Cells were allowed to proliferate for 5 days before induction of adipogenic differentiation. b, c Representative SEM image (b, n = three independent experiments) and pore size characterization of loofah scaffold. Scale bar = 50 μm. d–f Representative Live and Dead image (d, n = three independent experiments), cell alignment angle distribution (e) and aspect ratio (f) of 3T3L1 pre-adipocytes cultured on loofah scaffolds. Scale bar = 100 μm. AR, aspect ratio. g Growth and viability curves of proliferating 3T3L1 cells on loofah scaffolds at a spinning rate of 7 rpm. n = three independent experiments. Data are presented as mean ± s.d. h Relative mRNA expression (n = three independent experiments) of adipogenic genes in Dif D10 3T3L1 cells on loofah scaffolds. Results were normalized to measurements of GAPDH. Data are presented as mean ± s.d. Student’s two-tailed t-test, unpaired and no multiple testing correction between samples. (i) Representative fluorescent staining (n = three independent experiments) of lipid droplets in differentiated 3T3L1 cells on autoclaved loofah scaffolds. Scale bar = 50 μm. Panel (a) was, in part, created with BioRender. Lab, D. (2024) https://BioRender.com/z27v304.
Bio-fabrication of meat-on-vegetable chips
For proof-of-principle demonstration, we finally created fat-on-muscle meat chips, by seeding differentiated 3T3L1 adipocytes atop differentiated C2C12 myofibers cultured on Dopa-mushroom (Fig. 9a). α-Actinin and lipid droplet staining validated the presence of differentiated muscle and fat cells on Dopa-mushroom slices (Fig. 9b). Upon grilling, our engineered meat chips (Fig. 9c) showed around 60% mass loss due to the loss of aqueous and lipid components (Fig. 9d). This is similar to real pork, and is higher than a vegan “minced pork”, a commercial product made of pea protein and vegetable oil by the brand “Beyond Meat”. We further evaluated the sensory attributes of real pork, our engineered meat chip and the vegan Beyond Pork using an electronic tongue. Our meat chip demonstrated a much similar taste profile to real pork (Fig. 9e): it is slightly less sour and less salty, but offers more umami flavor (Fig. 9f).
a Schematic representation of engineered meat on anisotropic vegetable scaffolds. b Representative staining (n = three independent experiments) of myogenic marker α-actinin and lipid droplets in meat-on-mushroom chips. Scale bar = 50 μm. c Representative image of grilled meat-on-mushroom chips. d Cooking loss of real pork, engineered meat chip and Beyond Pork samples upon grilling. n = 3 independent samples. Data are presented as mean ± s.d. e PCA of the taste of real pork, engineered meat chips and Beyond Pork samples. X and Y axis show principal component 1 and principal component 2 that explain 74.6% and 12% of the total variance, respectively. Prediction ellipses are such that with a probability of 0.95, a new observation from the same group will fall inside the ellipse. N = 28 data points. f Heatmap showing the main sensory attributes of real pork, engineered meat chips and Beyond Pork samples. Panel (a) was, in part, created with BioRender. Lab, D. (2024) https://BioRender.com/z27v304.
Discussions
To live up to its promise, cultured meat looks for technological breakthroughs in the following areas: low-cost media, quality muscle and adipose progenitor cells43,44,45, functional scaffolds and large intelligent bioreactors46. In this work, we focused on developing low-cost, tissue-mimicking scaffolds, not by synthesizing them but by borrowing from nature——the largest bank of smart materials. As demonstrated by our engineered meat-on-vegetables, plant autoclaving brings the following advantages: tissue mimicry and biochemical benefits; operational ease and scale-up potential; and nutritional value.
The structural orderliness of biological tissues requires scaffolding materials to not only accommodate cells, but provide anatomically-relevant topology. Plant decellularization is one technique to expose ordered cellulose skeletons from plants using surfactants. Unfortunately, decellularized plants are cell-phobic and nutrition-deprived, and often demand chemical modification to increase cell adhesion47. Here we developed micro-patterned bio-scaffolds via a shortcut of plant autoclaving, while bypassing the removing and regaining of cell-affinitive molecules as in decellularization. This is feasible thanks to the following reasons. Firstly, the surface topography on autoclaved vegetables is readily accessible and can be directly sensed by mammalian cells. Secondly, autoclaved vegetables retained cell-affinitive moieties (such as amides), and further adsorb proteins from medium in which FBS brings in adhesion proteins like fibronectin and vitronectin48,49,50,51. Alternatively, coating vegetables in fibronectin, vitronectin, or RGD before cell seeding was enough to form integrin αvβ3- and paxillin-mediated focal adhesion (Supplementary Fig. 17–18), yielding a similar effect to medium soaking (Supplementary Fig. 16). Considering that serum is less defined in composition, we thus advocate using chemically defined coating agents, such as fibronectin/vitronectin/RGD, in future studies. We also acknowledge other adsorbable protein-coating ingredients, which are worth future exploration with help of advanced chemical characterization tools.
While sterilizing materials, autoclaving concomitantly imparts biomimetic stiffness of animal tissues to vegetables. The adjustable autoclaving time endows vegetables with mechanical tunability while maintaining structural integrity. Murine myoblasts and primary porcine muscle satellite cells expanded rapidly on autoclaved Chinese chives and Shiitake mushrooms in dynamic bioreactors. Meantime, they exhibited a more elongated cell shape and better alignment following vegetable anisotropy, in contrast to 2D culture. Importantly, MHC gene was upregulated and myocytes fused into highly aligned myotubes, circumventing the use of costly animal-derived hydrogels or complex microfabrication. We also achieved scalable expansion and elevated adipogenesis in murine 3T3L1 pre-adipocytes on isotropic loofah.
With standard lab apparatus and minimum pretreatments, autoclaved vegetable scaffolds can be prepared within 8–17 h. In contrast, the existing practice of plant decellularization requires rounds of surfactant wash (3–4 days) to thoroughly remove plant cells (Fig. 4), plus another dozen hours to chemically modify the cellulose matrix21,23,24. Since the vegetables we selected are abundant and the processing setup widely available, biomaterial costs can be substantially reduced. In our experiments, 30 mL media can support the production of around 3 million C2C12 cells within five days in a 125 mL spinner flask, which in theory can be scaled up to 15 L bioreactors to yield one billion cells (Supplementary Table 2), incurring biomaterial cost of only a few dozen dollars. This indicates autoclaved vegetables as cost-effective biomaterials for scalable manufacturing of meat surrogates.
Growing animal cells on vegetables creates a novel class of hybrid food containing a healthy mixture of animal and plant nutrients. The vegetables we selected, ranging from Chinese chives, to Shiitake mushrooms to loofah, are low in calories, rich in beneficial vitamins, minerals and antioxidant carotenoids52, etc. This greatly valorizes the nutritional profile of engineered meat, and can bring health benefits such as lower risk of obesity53, colorectal cancer54, diabetes55, etc. Additionally, mushrooms contain glutamate56, which helps to create umami flavors similar to what is found in real meat57.
Autoclaved vegetables are prepared via a green process and do not involve hazardous chemicals. The only chemical we have mentioned for optional use was dopamine hydrochloride (2 mg/mL, a concentration commonly used for biomaterials22,39), to increase mushroom hydrophilicity. Dopamine is known as a natural neurotransmitter produced by mesencephalic and hypothalamic neurons. It is also substantially synthesized in peripheral organs such as the gut and pancreas58,59,60. Peripheral dopamine participates in diverse roles, such as enhancing intestinal barrier, stimulating mucus secretion, and modulating sodium absorption61,62. Dopamine is rapidly degraded in the gut by metabolic enzymes, such as monoamine oxidase, catechol-O-methyltransferase and aldehyde dehydrogenase63. Several inactive end products are produced, including 3,4-dihydroxyphenylacetic acid, homovanillic acid, methoxytyramine, and sulfate-conjugated dopamine metabolites61. These metabolites are largely excreted in urine and removed from the circulation60,64. Therefore, dopamine ingested within Dopa-mushrooms seems not a foreign molecule to the human digestive system and can be readily metabolized.
Due to a lack of porcine adipogenic cells, we engineered fat-on-loofah using murine 3T3L1 preadipocytes. Also note that the primary porcine myosatellite cells we used for muscle engineering can lose their myogenic capacity during prolonged culture in vitro. Therefore, more stable fat and muscle progenitor cells, derived from consumer-acceptable livestock animals, are desirable. Pasitka et al. have established an immortalized chicken fibroblast cell line that transdifferentiates into adipocyte in serum-free medium45. A long-term expandable myogenic strain has also been developed from porcine pre-gastrulation epiblast stem cells65. Future efforts are needed to develop high-quality myogenic and adipogenic cells from diverse livestock species, which can self-renew for a considerably long period without compromising their differentiation potential.
Vegetables are not only food. Here we illustrated that naturally micro-patterned Chinese chives, Shiitake mushroom and loofah, in their autoclaved form, promoted the proliferation and differentiation of muscle or adipose progenitor cells into aligned myofibers or isotropic fat. This largely relies on their cell-permissive surface chemistry, biomimetic stiffness, as well as tissue-sculpting topology. Autoclaved vegetable scaffolds save processing time and require only standard lab apparatus. Thanks to their abundance, vegetable scaffolds can be readily scaled up to produce meat stuffing and meat-on-vegetable chips. In future studies, we envisage more types of vegetable species being discovered as functional biomaterials, to help create lab-grown meat and even intelligent muscle robots.
Methods
2D cell culture and differentiation
C2C12 murine myoblast cell line (CRL-1772) was purchased from ATCC. C2C12 before passage number 16 was cultured on 2D flasks in proliferation medium: Dulbecco’s Modified Eagle’s Medium (DMEM, Multicell) supplemented with 10% v/v fetal bovine serum (FBS, Multicell) and 1% v/v penicillin-streptomycin (PS, Multicell). Pax 7+ Porcine Satellite Cells (PSC) isolated from one-week-old pig dorsal muscle65 were kindly provided by Prof. Jianyong Han at China Agricultural University. PSCs (before passage number 6) were cultured on 0.05% w/v collagen I (Corning, 354236) coated 2D flasks in proliferation medium: F10 medium (Multicell) supplemented with 15% v/v FBS, 5 ng/mL FGF (Peprotech, 100-18B) and 1% v/v PS. Once C2C12 and PSCs reach 90% confluence, myogenesis was trigged by replacing growth medium with differentiation medium: DMEM supplemented with 5% v/v FBS, 1% v/v PS and 1 μM Erk inhibitor66 (SCH 772984, Cayman). Myogenic differentiation condition was maintained for 7 days.
3T3L1 murine pre-adipocyte cell line (CL-173) was purchased from ATCC. 3T3L1 was cultured on 2D flasks in expansion medium: DMEM supplemented with 10% v/v FBS and 1% v/v PS. To induce adipogenesis, 3T3L1 was cultured to 100% confluence and kept confluent for 48 h. Proliferation medium was switched to adipose differentiation medium: DMEM supplemented with 10% v/v FBS, 1.0 µM Dexamethasone (Beyotime, ST1254-50 mg), 0.5 mM Methylisobutylxanthine (IBMX, Beyotime, SC0195-25 mg), and 1.0 µg/mL Insulin (Macgene, CC122). After 3 days, the differentiation medium was replaced with adipocyte maintenance medium: DMEM supplemented with 10% v/v FBS and 1.0 µg/mL Insulin. Cells were kept in the maintenance medium for 7 days.
Preparation of autoclaved vegetable scaffolds
Fresh plants and mushrooms were purchased in a local supermarket. For the fruit, stem and root parts of a plant, the outmost epidermis was removed and the internal part was used. Vegetables were cut longitudinally (Supplementary Fig. 13a) into 0.5–1.2 mm-thick slices using a Leica vibratome (VT1200S) and sterilized by autoclaving. Sterile slices were rinsed with deionized water three times on an orbital shaker. Plant slices were immersed in cell growth media supplemented with 1% w/v gelatin (Solarbio, G8061) overnight at 37 °C. Optionally, to further enhance cell adhesion, autoclaved mushroom slices were coated with 2 mg/mL Dopamine hydrochloride (Solarbio, D9520) in Tris buffer (10 mM, PH 8.5, Meilun bio, MA0177) for 6 h at 60 °C under constant stirring, and immersed overnight at 37 °C in Tris buffer supplemented with 1% w/v gelatin. For more hydrophilic plants such as Chinese chives and loofah, dopamine coating was not necessary.
Preparation of decellularized vegetable scaffolds
To thoroughly remove living plant cells, plants were decellularized with 1% w/v SDS (Solarbio, S8010) buffer for 3–4 days, with buffer exchange every day. Plants were further decellularized with 2% w/v NaClO (Aladdin, S101639) containing 0.1% v/v Triton (Sigma Aldrich, X-100) for another 24 h. Obtained materials were rinsed thoroughly with deionized water three times on an orbital shaker, sterilized with 70% ethanol for 8 h, and rinsed with deionized water. To improve cell adhesion, decellularized plants could undergo surface modification by oxidation with 0.1% w/v NaIO4 (Aladdin, S104093) at 50 °C for 1 h. Materials were rinsed again with deionized water three times and soaked in 10% w/v gelatin overnight at 37 °C. Figure 4a contains a flowchart showing the whole procedure.
Raman spectroscopy
A LabRAM HR Evolution Raman microscope (HORIBA) with 473 and 532 nm laser excitation source was used to collect the spectra of natural, autoclaved and decellularized vegetable slices. Spectra were measured while samples were immersed in deionized water and imaged with a 50x objective (NA = 0.50, Olympus). The Raman light was detected by an air-cooled front-illuminated spectroscopic charge-coupled device (CCD) behind the spectrometer with a grating of 600 g/mm (Blaze wavelength = 500 nm). Data were processed and analyzed using LabSpec 6 software (HORIBA).
Fourier transform infrared spectroscopy (FTIR) and energy-dispersive spectroscopy (EDS)
Natural, autoclaved and decellularized vegetable slices were prepared as aforementioned and dried at 60 °C. Functional groups were evaluated by an FTIR Spectrometer (Thermo Scientific Nicolet iS50) with an ATR mode. Samples were scanned in the range of 400–4000 cm−1, at a resolution of 4 cm−1. Spectral data is analyzed with OMNIC software (Thermo Scientific).
Dried vegetable samples were coated with platinum for 90 s and examined by a cold-field emission scanning electron microscope (HITACHI, Regulus8230) installed with an EBSD detector (BRUKER, eFlash FS). Samples were scanned for approximately 2 min at an acceleration voltage of 5 kV. Element content was qualitatively analyzed using ESPRIT QUBE software (BRUKER).
Liquid chromatography mass spectrometry (LC-MS)
About 2 g of autoclaved vegetable slices were soaked in 50 mL of deionized water overnight on a shaker. Obtained vegetable extracts were centrifuged at 16,000 × g for 10 min to gather supernatant for LC-MS analysis. A Vanquish UHPLC System (Thermo Scientific) with a 100 mm × 2.1 mm Hypersil GOLD C18 selective column (Thermo Scientific) was used to separate the extracts at 40 °C. The mobile phases consisted of 0.1% (v/v) formic acid in water (solvent A) and 0.1% (v/v) formic acid in acetonitrile (solvent B) at a flow rate of 0.3 mL/min. The gradient program was set as follows: 0–5% B (0–3 min), 5-95% B (3–38 min), 95–5% B (38–38.1 min), and 5% B (40 min). A Q Exactive HF-X mass spectrometer equipped with an electrospray ionization interface in positive and negative ion mode was used for mass spectrometric detection. Mass spectral data (in RAW format provided in reference67) were acquired through Thermo XCalibur program. For MS1 full scan profiling, the mass spectrometer was operated scanning mass range of 80–1200 Da with a mass resolution of 120,000; AGC target 1e6; maximum IT 100 ms. For MS2 profiling, the mass spectrometer was operated with normalized collision energy averaging 30, 40 and 50; resolution 60,000; AGC target 1e5; maximum IT 50 ms; loop count 5; topN 5; isolation window 1.2 Da. Data were analyzed using MS-DIAL 4.70 software68 with the following parameters: accurate mass tolerance 0.01 Da (MS1), 0.05 Da (MS2); identification score cut off, 60%; spectral library, MassBank, Respect and GNPS. One sample extract for each vegetable was analyzed.
Protein adsorption measurement
Autoclaved, decellularized and Dopa-coated biomaterials were weighted and incubated in a protein-rich coating solution (cell growth medium supplemented with 1% w/v gelatin) overnight at 37 °C. Samples were gently rinsed with deionized water for 1 min to remove excessive coating. Adsorbed proteins were extracted by immersing samples in an elution buffer containing 0.5% w/v CHAPS and 0.025% w/v SDS for 1 h at room temperature, followed by centrifugation at 18,000 × g for 12 min. Supernatants were analyzed using a BCA assay kit (Yeasen, 20201ES76). Protein concentration was determined using a microplate spectrophotometer (PerkinElmer EnSight) by measuring absorbance at 562 nm and comparing with a standard curve of bovine serum albumin (0–2000 μg/mL).
pH and contact angle measurement of vegetable scaffolds
Vegetable slices were autoclaved, rinsed with deionized water and soaked in cell culture medium supplemented with 1% w/v gelatin. The pH of as-prepared vegetable slices was measured at room temperature using a pH meter (Smart sensor, PH818M) with a spear-shaped tip to pierce vegetable samples. At least three replicate measurements were performed for each sample. For water contact angle test, as-prepared vegetable slices were placed on a glass slide and dried in an oven (65 °C) for 15 min. Water contact angle was measured using an optical contact angle measuring system (DataPhysics Instruments, OCA 15EC) at room temperature. A 1 μL drop of deionized water was dispensed on the sample surface by a microliter syringe. Images of water droplets were captured within 10 s of delivery. The ellipse fitting method was used to calculate static contact angles on both sides of the droplets. At least three replicate measurements were performed for each slice.
Surface zeta potential measurement of vegetable scaffolds
As-prepared slices were cut into 2 cm long, 1 cm wide sections and loaded on a Surpass 3 electrokinetic analyzer system (Anton Paar) according to the manufacturer’s instructions. Samples were rinsed in 1 mM Potassium Chloride (Aladdin, P433492) solution three times before measurement. The streaming potential method69 was used for the direct analysis of surface zeta potential at the sample/liquid interface.
Atomic force microscopy (AFM) force measurement of vegetable scaffolds
As-prepared vegetable slices were placed on thin coverslips and mounted on an inverted microscope (Zeiss Observer A1 stand). AFM indentation was performed with a Nanowizard AFM system (JPK Instruments). An AFM cantilever with a nominal spring constant of 0.06 N/m was controlled to approach the sample with a speed of 10 μm/s until it touched the sample. When the force between the cantilever and the sample reached 2 pN, the tip was controlled to detach from the sample to finish a single test for local stiffness. During the indentation process, force-displacement curves were recorded, from which the local Young’s modulus of the samples could be calculated.
Static cell culture on vegetable scaffolds
To screen appropriate scaffolds, as-prepared vegetable slices were placed in 24 well plates. C2C12 cell suspension containing about 8 × 106 cells/mL was added dropwise onto vegetable slices. Cells were allowed 2 h to adhere to the materials before culture media was added.
Dynamic cell culture on vegetable scaffolds in spinner flasks
A 3D FloTrix-miniSPIN platform (M1; CytoNiche Biotech, China) was installed inside a cell culture incubator and connected with a spinning rate controller (MP01; CytoNiche Biotech, China). Prepared mushroom and Chinese chives slices with a seeding area of 30 cm2 were placed in sterile 125 mL spinner flask bioreactors (SF125; CytoNiche Biotech, China). Cells were harvested from T75 culture flasks and introduced in the spinner flasks at 1500, 1500, 9000 cells/mL, for C2C12, PSC, and 3T3L1 cells respectively.
Spinner flasks were programmed to perform 12 inoculation cycles. Each cycle started from 35 rpm for 5 min, followed by 25 rpm for 2 min and 1 rpm for 2 h. After the 12 inoculation cycles, the agitation velocity was switched to a constant rate of 7 rpm. Medium was refreshed every 3–4 days.
Cell enumeration and viability assessment
Two cultured tissue-on-vegetable slices were sampled from each spinner flask daily to monitor cell growth. Cells were dissociated from Chinese chives and mushroom slices with Trypsin for 5 minutes at 37 °C. Cell number and viability were evaluated using 0.4% Trypan Blue (Beyotime, ST2780) by Countstar software (ALIT Life Science, China).
Quantitative reverse transcription PCR (qPCR) analysis
Cultured tissue-on-vegetable slices with a total area of around 30 cm2 were sampled from a spinner flask. Cells were dissociated from the scaffolds with Trypsin, while vegetable debris was removed using a nylon mesh with a 22 μm pore size. Trizol reagent (Vazyme, R401-01) was added to the collected cells and total RNA was extracted following the manufacturer’s instructions. cDNA was synthesized from 1 μg total RNA using reverse transcriptase (Vazyme,
R201-01/02). Gene-specific transcription was analyzed by qPCR using AceQ qPCR SYBR Green Master Mix (Vazyme, Q111-02/03) on a CFX96 instrument (Bio-Rad). All genes were normalized to GAPDH and relative expression levels were evaluated using the 2−ΔΔCT method. Primers are listed in Supplementary Table 5.
Live-dead and lipid droplet staining
For live-dead cell staining, cultured tissue-on-vegetable slices were sampled from spinner flasks and incubated with Calcein AM (Wako, 349-07201) and Propidium Iodide dye (Wako, 169-26281) at 1:1000 dilution in PBS at 37 °C for 30 min. Lipid droplets in differentiated 3T3L1 cells were stained with a BODIPY lipid probe (Thermo Fisher, D3922) at 1:1000 dilution in PBS at 37 °C for 20 min. Samples were imaged with a Nikon Eclipse fluorescent microscope.
Immunofluorescent staining
Cultured tissue-on-vegetable slices were sampled from the spinner flasks and fixed with 4% paraformaldehyde (PFA, Solarbio, P1110) for 30 min. Samples were washed three times with PBS and permeabilized in 0.2% v/v Triton X-100 in PBS for 30 min. Samples were blocked with 5% w/v bovine serum albumin (BSA, Multicell) in PBS for 1 h at room temperature. Primary antibodies (1:300, in 5% w/v BSA solution) were added and left overnight at 4 °C, then washed with PBS three times. Secondary antibodies (1:400, in PBS) were incubated for 2 h at room temperature, and then washed off with PBS. To stain the nuclei, DAPI (Beyotime, C1002, 1:1000, in PBS) was incubated for 15 min at room temperature. Antibody information is listed in Supplementary Table 6. Confocal images of Myosin heavy chain and α-actinin were taken by a Leica STED confocal microscope. Focal adhesions were imaged with a Nikon AX R with an NSPARC confocal microscope.
Scanning electron microscope (SEM) imaging
Samples were fixed with 2.5% w/v Glutaraldehyde (Aladdin, G105905) for 2 h, washed with PBS 3 times, and serially dehydrated with 20%, 40%, 60%, 80%, 100% ethanol. Samples were then serially immersed in 50%, 100%, 100% tert-Butyl alcohol (Aladdin, T433627) and freeze-dried. Prepared samples were deposited on a Si wafer and gold-coated for 90 s before SEM (FEI Quanta 200) imaging.
Characterization of vegetable micro-pattern topology and cell morphology on vegetable slices
Topological features of vegetable scaffolds were characterized from SEM images using ImageJ software. Cell aspect ratio was measured from the green channel (representing live cells) of live-dead fluorescent images using ImageJ. Cell alignment angles were statistically quantified using the fast Fourier transformation (FFT) function in ImageJ70. Briefly, the green channel of a live-dead image was converted into a grayscale FFT image, in which the original spatial-domain pixel intensity was transformed into a frequency domain. The Oval Profile plugin was applied to calculate the radial sum intensity (0–360 degree angle) in the FFT image, which was then plotted in polar coordinates to reflect cell alignment angles in the original fluorescent image. Detailed quantification method is explained in Supplementary Fig. 1.
Engineered pork-on-vegetables as dumpling stuffing
Differentiation Day-6 porcine myosatellite cells grown on Dopa-mushroom and Chinese chive scaffolds were collected from spinner flasks and washed with PBS. These tissues were then mixed and enveloped in a dumpling wrapper. The dumpling was cooked in boiled water for 10 min.
Nutritional evaluation of engineered pork-on-vegetables
About 3 g of differentiation Day-6 porcine myosatellite cells growing on vegetable scaffolds were harvested and freeze-dried. Nutrition values were tested by Sci-tech Innovation Quality Testing Co., Ltd (Qingdao, China).
Bio-fabrication of meat-on-vegetable chips
C2C12 cells were inoculated on Dopa-mushroom scaffolds in spinner flasks with a seeding density of 5000 cells/mL. Cultured slices were stained with live-dead dye on Day-4 to check if scaffolds were fully covered with myoblasts. C2C12 growth media was then replaced with myogenic media to allow for myotube development. After 7 days of myogenic differentiation, fully differentiated 3T3L1 adipocytes were inoculated onto C2C12-mushroom slices to generate meat-on-vegetable chips.
Cooking loss and taste analysis of engineered Meat-on-mushroom chips
Fresh pork loin and a commercial plant-based minced pork (Beyond Meat, Z1P22-022, 454 g) were purchased from a local supermarket. Engineered C2C12-3T3L1 tissues on Dopa-mushroom scaffolds were also harvested. Around 0.4 g of each sample was weighed, grilled with sunflower oil, and weighed again. Cooking loss was calculated as follows:
Grilled samples were ground with deionized water in a porcelain mortar, and centrifuged at 9300 × g for 20 min. The supernatant layer containing fat was discarded; the clear liquid in the middle layer was collected, and filtered through 0.45 μm nylon meshes. The filtered liquid was then diluted with deionized water to a final volume of 100 mL for taste analysis using an electronic tongue (Alpha MOS).
Statistics and reproducibility
Quantification data are presented as means ± s.d. Biological replicates are reported in the figure legends. No statistical method was used to predetermine sample size. Statistical analyses were performed in Origin-Lab 2015 and GraphPad Prism software. For normally distributed data sets with equal variances, a two-sample t-test was used. When normal distribution and equal variance were not met, the Mann-Whitney U test was performed. P values less than 0.05 were considered statistically significant. A Principal Component Analysis (PCA) plot was created using the Clustvis web tool.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the article and its supplementary Information. Source data are available via Figshare at https://doi.org/10.6084/m9.figshare.26117344, as well as are provided with this article. Source data are provided with this paper.
References
Post, M. J. et al. Scientific, sustainability and regulatory challenges of cultured meat. Nat. Food 1, 403–415 (2020).
Ng, S. & Kurisawa, M. Integrating biomaterials and food biopolymers for cultured meat production. Acta Biomater. 124, 108–129 (2021).
Rubio, N. R., Xiang, N. & Kaplan, D. L. Plant-based and cell-based approaches to meat production. Nat. Commun. 11, 6276 (2020).
Bomkamp, C. et al. Scaffolding biomaterials for 3D cultivated meat: prospects and challenges. Adv. Sci. 9, 2102908 (2022).
Verbruggen, S., Luining, D., van Essen, A. & Post, M. J. Bovine myoblast cell production in a microcarriers-based system. Cytotechnology 70, 503–512 (2018).
Bodiou, V., Moutsatsou, P. & Post, M. J. Microcarriers for upscaling cultured meat production. Front. Nutr. 7, 10 (2020).
Liu, Y. et al. Engineered meatballs via scalable skeletal muscle cell expansion and modular micro-tissue assembly using porous gelatin micro-carriers. Biomaterials 287, 121615 (2022).
Ben-Arye, T. et al. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 1, 210–220 (2020).
Xiang, N. et al. 3D porous scaffolds from wheat glutenin for cultured meat applications. Biomaterials 285, 121543 (2022).
Pinton, L. et al. 3D human induced pluripotent stem cell–derived bioengineered skeletal muscles for tissue, disease and therapy modeling. Nat. Protoc. https://doi.org/10.1038/s41596-022-00790-8 (2023).
Kang, D.-H. et al. Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting. Nat. Commun. 12, 5059 (2021).
MacQueen, L. A. et al. Muscle tissue engineering in fibrous gelatin: implications for meat analogs. NPJ Sci. Food 3, 20 (2019).
Nakayama, K. H. et al. Rehabilitative exercise and spatially patterned nanofibrillar scaffolds enhance vascularization and innervation following volumetric muscle loss. NPJ Regen. Med. 3, 16 (2018).
Duffy, R. M., Sun, Y. & Feinberg, A. W. Understanding the role of ECM protein composition and geometric micropatterning for engineering human skeletal muscle. Ann. Biomed. Eng. 44, 2076–2089 (2016).
Li, C.-H. et al. The production of fat-containing cultured meat by stacking aligned muscle layers and adipose layers formed from Gelatin-Soymilk Scaffold. Front. Bioeng. Biotechnol. 10, 875069 (2022).
Cheng, Y.-W., Shiwarski, D. J., Ball, R. L., Whitehead, K. A. & Feinberg, A. W. Engineering aligned skeletal muscle tissue using decellularized plant-derived scaffolds. ACS Biomater. Sci. Eng. 6, 3046–3054 (2020).
Yun, J. et al. Aligned skeletal muscle assembly on a biofunctionalized plant leaf scaffold. Acta Biomater. 171, 327–335 (2023).
Hickey, R. J., Modulevsky, D. J., Cuerrier, C. M. & Pelling, A. E. Customizing the shape and microenvironment biochemistry of biocompatible macroscopic plant-derived cellulose scaffolds. ACS Biomater. Sci. Eng. 4, 3726–3736 (2018).
Gershlak, J. R. et al. Crossing kingdoms: using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 125, 13–22 (2017).
Modulevsky, D. J., Lefebvre, C., Haase, K., Al-Rekabi, Z. & Pelling, A. E. Apple derived cellulose scaffolds for 3D mammalian cell culture. PLoS ONE 9, e97835 (2014).
Li, Y. et al. Natural plant tissue with bioinspired nano amyloid and hydroxyapatite as green scaffolds for bone regeneration. Adv. Health. Mater. 11, 2102807 (2022).
Fontana, G. et al. Biofunctionalized plants as diverse biomaterials for human cell culture. Adv. Health. Mater. 6, 1601225 (2017).
S H, A., Mohan, C. C., P S, U., Krishnan, A. G. & Nair, M. B. Decellularization and oxidation process of bamboo stem enhance biodegradation and osteogenic differentiation. Mater. Sci. Eng. C. 119, 111500 (2021).
Contessi Negrini, N., Toffoletto, N., Farè, S. & Altomare, L. Plant tissues as 3D natural scaffolds for adipose, bone and tendon tissue regeneration. Front. Bioeng. Biotechnol. 8, 723 (2020).
Nguyen, M. A. & Camci-Unal, G. Unconventional tissue engineering materials in disguise. Trends Biotechnol. 38, 178–190 (2020).
Toker-Bayraktar, M., Erenay, B., Altun, B., Odabaş, S. & Garipcan, B. Plant-derived biomaterials and scaffolds. Cellulose 30, 2731–2751 (2023).
Giuliani, A., Cerretani, L. & Cichelli, A. Colors: properties and determination of natural pigments. in Encyclopedia of Food and Health (eds. Caballero, B., Finglas, P. M. & Toldrá, F.) 273–283 (Academic Press, Oxford, 2016). https://doi.org/10.1016/B978-0-12-384947-2.00189-6.
Bock, P., Felhofer, M., Mayer, K. & Gierlinger, N. A guide to elucidate the hidden multicomponent layered structure of plant cuticles by Raman imaging. Front. Plant Sci. 12, 793330 (2021).
Kanokpanont, S., Damrongsakkul, S., Ratanavaraporn, J. & Aramwit, P. Physico-chemical properties and efficacy of silk fibroin fabric coated with different waxes as wound dressing. Int J. Biol. Macromol. 55, 88–97 (2013).
Ishizaki, T., Saito, N. & Takai, O. Correlation of cell adhesive behaviors on superhydrophobic, superhydrophilic, and micropatterned superhydrophobic/superhydrophilic surfaces to their surface chemistry. Langmuir 26, 8147–8154 (2010).
Arima, Y. & Iwata, H. Effects of surface functional groups on protein adsorption and subsequent cell adhesion using self-assembled monolayers. J. Mater. Chem. 17, 4079–4087 (2007).
Arima, Y. & Iwata, H. Preferential adsorption of cell adhesive proteins from complex media on self-assembled monolayers and its effect on subsequent cell adhesion. Acta Biomater. 26, 72–81 (2015).
Gorb, E. V. & Gorb, S. N. Anti-adhesive effects of plant wax coverage on insect attachment. J. Exp. Bot. 68, 5323–5337 (2017).
Kunzek, H., Kabbert, R. & Gloyna, D. Aspects of material science in food processing: changes in plant cell walls of fruits and vegetables. Z. Lebensm. Unters. Forsch. A 208, 233–250 (1999).
Kammoun, M. et al. Development of a novel multiphysical approach for the characterization of mechanical properties of musculotendinous tissues. Sci. Rep. 9, 7733 (2019).
Guimarães, C. F., Gasperini, L., Marques, A. P. & Reis, R. L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
Bacakova, L., Filova, E., Parizek, M., Ruml, T. & Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 29, 739–767 (2011).
Ayala, R. et al. Engineering the cell–material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 32, 3700–3711 (2011).
Lee, H., Dellatore, S. M., Miller, W. M. & Messersmith, P. B. Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430 (2007).
Young, D. A., Choi, Y. S., Engler, A. J. & Christman, K. L. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 34, 8581–8588 (2013).
Alkhouli, N. et al. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am. J. Physiol. Endocrinol. Metab. 305, E1427–E1435 (2013).
Comley, K. & Fleck, N. A. A micromechanical model for the Young’s modulus of adipose tissue. Int. J. Solids Struct. 47, 2982–2990 (2010).
Choi, K.-H. et al. Muscle stem cell isolation and in vitro culture for meat production: a methodological review. Compr. Rev. Food Sci. Food Saf. 20, 429–457 (2021).
Stout, A. J. et al. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol. 5, 466 (2022).
Pasitka, L. et al. Spontaneous immortalization of chicken fibroblasts generates stable, high-yield cell lines for serum-free production of cultured meat. Nat. Food https://doi.org/10.1038/s43016-022-00658-w (2022).
Allan, S. J., De Bank, P. A. & Ellis, M. J. Bioprocess design considerations for cultured meat production with a focus on the expansion bioreactor. Front. Sustain. Food Syst. 3, (2019).
Indurkar, A., Pandit, A., Jain, R. & Dandekar, P. Plant-based biomaterials in tissue engineering. Bioprinting 21, e00127 (2021).
Kikuchi, A., Taira, H., Tsuruta, T., Hayashi, M. & Kataoka, K. Adsorbed serum protein mediated adhesion and growth behavior of bovine aortic endothelial cells on polyamine graft copolymer surfaces. J. Biomater. Sci. Polym. Ed. 8, 77–90 (1997).
Sawyer, A. A., Hennessy, K. M. & Bellis, S. L. The effect of adsorbed serum proteins, RGD and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials 28, 383–392 (2007).
Underwood, P. A. & Bennett, F. A. A comparison of the biological activities of the cell-adhesive proteins vitronectin and fibronectin. J. Cell Sci. 93, 641–649 (1989).
Hoshiba, T., Yoshikawa, C. & Sakakibara, K. Characterization of initial cell adhesion on charged polymer substrates in serum-containing and serum-free media. Langmuir 34, 4043–4051 (2018).
Ochoa Becerra, M., Mojica Contreras, L., Hsieh Lo, M., Mateos Díaz, J. & Castillo Herrera, G. Lutein as a functional food ingredient: stability and bioavailability. J. Funct. Foods 66, 103771 (2020).
Kim, D.-H. et al. A type 2 immune circuit in the stomach controls mammalian adaptation to dietary chitin. Science 381, 1092–1098 (2023).
Papadimitriou, N. et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat. Commun. 12, 4579 (2021).
Shikano, A. et al. Effects of Lactobacillus plantarum Uruma-SU4 fermented green loofah on plasma lipid levels and gut microbiome of high-fat diet fed mice. Food Res. Int. 121, 817–824 (2019).
Kobayashi, K. et al. Distinguishing glutamic acid in foodstuffs and monosodium glutamate used as seasoning by stable carbon and nitrogen isotope ratios. Heliyon 4, e00800 (2018).
Fu, Y., Liu, J., Hansen, E. T., Bredie, W. L. P. & Lametsch, R. Structural characteristics of low bitter and high umami protein hydrolysates prepared from bovine muscle and porcine plasma. Food Chem. 257, 163–171 (2018).
Vieira-Coelho, M. A. & Soares-da-Silva, P. Dopamine formation, from its immediate precursor 3,4-dihydroxyphenylalanine, along the rat digestive tract. Fundam. Clin. Pharm. 7, 235–243 (1993).
Mezey, E. et al. A novel nonneuronal catecholaminergic system: exocrine pancreas synthesizes and releases dopamine. Proc. Natl Acad. Sci. 93, 10377–10382 (1996).
Eisenhofer, G. et al. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 82, 3864–3871 (1997).
Liu, C.-Z., Feng, X.-Y., Liu, S., Zhang, X.-L. & Zhu, J.-X. Synthesis and Metabolism of Gut Dopamine. in Dopamine in the Gut (ed. Zhu, J.-X.) 25–51 (Springer Singapore, Singapore, 2021). https://doi.org/10.1007/978-981-33-6586-5_2.
Meiser, J., Weindl, D. & Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 11, 34 (2013).
Elsworth, J. D. & Roth, R. H. Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson’s disease. Exp. Neurol. 144, 4–9 (1997).
Merits, I. Formation and metabolism of [14 C]dopamine 3-O-sulfate in dog, rat and guinea pig. Biochem. Pharm. 25, 829–833 (1976).
Zhu, G. et al. Generation of three-dimensional meat-like tissue from stable pig epiblast stem cells. Nat. Commun. 14, 8163 (2023).
Eigler, T. et al. ERK1/2 inhibition promotes robust myotube growth via CaMKII activation resulting in myoblast-to-myotube fusion. Dev. Cell 56, 3349–3363.e6 (2021).
Liu, Y. et al. Growing meat on vegetables: plants with natural biomimetic micro-patterns as edible meat-engineering scaffolds. Figshare dataset. https://doi.org/10.6084/m9.figshare.26117344 (2024).
Tsugawa, H. et al. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 12, 523–526 (2015).
Szymczyk, A., Fievet, P., Mullet, M., Reggiani, J. C. & Pagetti, J. Comparison of two electrokinetic methods—electroosmosis and streaming potential—to determine the zeta-potential of plane ceramic membranes. J. Memb. Sci. 143, 189–195 (1998).
Taylor, S. E., Cao, T., Talauliker, P. M. & Lifshitz, J. Objective morphological quantification of microscopic images using a fast fourier transform (FFT) analysis. Curr. Protoc. Ess. Lab. Tech. 7, 9.5.1–9.5.12 (2013).
Acknowledgements
We thank Yue Sun and Jingjing Wang from Cell Biology Facility, Center of Biomedical Analysis, Tsinghua University for the technical support on confocal microscopy. We thank Xu Wang and Hao Wang from Beijing Institute of Technology, Shuchen Xin from Chinese National Institute of Biological Sciences for experiment help. This work was supported by Special funding for the Chinese Postdoctoral Science Foundation (043220062, Ye Liu), National Science Foundation for Distinguished Young Scholars (82125018, Yanan Du), Young Scientists Fund of Beijing Natural Science Foundation (7244521, Ye Liu) and the starting grant from Beijing Institute of Technology (Ye Liu). Schematics were created using several icon elements from BioRender.com and ixintu.com.
Author information
Authors and Affiliations
Contributions
Ye Liu and Yanan Du conceived and designed experiments. Ye Liu conducted the 2D and spinner flask culture, biochemical characterization, confocal imaging, SEM imaging, Raman and EDS characterization, and data analysis. A.G. performed the FTIR experiment and band analysis. T.W. performed qPCR and cell enumeration experiments. Y.Y. performed contact angle measurements. Z.W., Y.J. and H.C. performed the AFM test. Yuming Lai prepared decellularized plants. R.Z. draw the schematics. J.H., Yulin Deng, Y.Z., G.Z. and S.L. participated in results discussion. Ye Liu and Yanan Du wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Gao, A., Wang, T. et al. Growing meat on autoclaved vegetables with biomimetic stiffness and micro-patterns. Nat Commun 16, 161 (2025). https://doi.org/10.1038/s41467-024-55048-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55048-6