Abstract
Pesticides affect a diverse range of non-target species and may be linked to global biodiversity loss. The magnitude of this hazard remains only partially understood. We present a synthesis of pesticide (insecticide, herbicide and fungicide) impacts on multiple non-target organisms across trophic levels based on 20,212 effect sizes from 1,705 studies. For non-target plants, animals (invertebrate and vertebrates) and microorganisms (bacteria and fungi), we show negative responses of the growth, reproduction, behaviour and other physiological biomarkers within terrestrial and aquatic systems. Pesticides formulated for specific taxa negatively affected non-target groups, e.g. insecticidal neonicotinoids affecting amphibians. Negative effects were more pronounced in temperate than tropical regions but were consistent between aquatic and terrestrial environments, even after correcting for field-realistic terrestrial and environmentally relevant exposure scenarios. Our results question the sustainability of current pesticide use and support the need for enhanced risk assessments to reduce risks to biodiversity and ecosystems.
Similar content being viewed by others
Introduction
Pesticides are used globally to support agricultural production and protect human and animal health in both domestic and commercial situations1,2,3,4,5,6,7,8. At sufficient environmental concentrations, insecticide, fungicide and herbicide exposure can affect non-target organisms by disrupting their survival, growth, reproduction and behaviour (e.g. detection of stimuli), as well as effects on other metabolic and physiological processes (e.g. biomarkers of neural function or immunity, cellular respiration, photosynthesis)9. Some of the negative impacts of pesticides are known. For example, fungicides may decrease the biomass of arbuscular mycorrhizal fungi, affecting their symbioses with higher plants10; herbicides may reduce plant pollen viability11 and carbohydrate metabolism12; insecticides (targeting pest herbivores) may cause long-term declines in non-target insect pollinators associated with mass-flowering crops13. Such impacts appear widespread across taxa, with individual studies reporting effects on microorganisms14, plants15, invertebrates5,16,17, amphibians18, birds6,19 and mammals20.
In addition to effects on individual species, the influence of pesticides can propagate across trophic levels, impacting ecosystem-scale species interactions that may lead to secondary effects21. Often due to practical constraints, pesticide regulatory risk assessments remain focused on a limited number of easily cultured model species, e.g. rats, zebrafish, Xenopus, Daphnia, chironomids, algae, honeybees, and/or earthworms. As such, they are unlikely to capture the variety of responses to pesticide exposure seen across the diversity of species and communities found in both managed and natural systems13. At the same time, there remains significant debate among the agricultural industry, governmental bodies and conservation organizations concerning the real-world hazards posed by pesticides. A major factor affecting the resolution of this issue is the lack of a cross-taxa synthesis of pesticide effects. Such an analysis is needed to support a robust evidence base for non-target effects that extend beyond scenario-specific case studies and model species.
Previous meta-analyses of pesticide impacts on non-target species have either considered only particular taxonomic groups, such as fish22 or bees23, or have considered specific habitats such as aquatic ecosystems24. To our knowledge, there has been no systematic and overarching synthesis of how different types of pesticides affect the diversity of multiple non-target eukaryotic and prokaryotic organisms across all trophic levels. Furthermore, current syntheses have not considered how the impacts of pesticides differ globally across climatic zones or for major mechanisms of exposure, such as those acting in aquatic or terrestrial environments. Without a unified synthesis applying common data capture and analytical standards, the global consequences of pesticide use across diverse ecosystems and taxa remain poorly understood. Such knowledge derived from a careful quantitative synthesis is vital to inform national and international policy goals to provide a framework for targeted mitigation to identify the role of pesticide use in biodiversity declines.
Here, we address these limitations by integrating 20,212 estimates of pesticide effects reported from 1705 experimental studies across the globe. These studies collectively measured the effects of insecticides, fungicides and herbicides on animals (invertebrates and vertebrates), plants (dicotyledonous, monocotyledonous and spore-producing) and microorganisms (bacteria and fungi). The included studies encompass laboratory and field experiments from temperate and tropical climatic zones, both in aquatic and terrestrial systems (Fig. 1 and Supplementary Data 1). We classified the effects on multicellular animals (vertebrates and invertebrates), plants and microorganisms based on changes in growth, reproduction, and behaviour (animals only) as well as other relevant biomarkers (e.g. enzymatic activities, metabolic reaction and plant photosynthesis). For all meta-analyses, we explicitly accounted for phylogenetic effects for species where published phylogenies were available (Supplementary Methods). As well as considering the consequences of taxa-specific pesticides (e.g. effect of herbicides on plants), we also address potential pathways by which pesticides can affect non-target organisms. This includes impacts on taxonomic groups for which a particular class of pesticide was not developed to control, e.g. insecticides affecting fungi or plants. Further, we assess whether ‘new’ or ‘old’ pesticides, based on the rigorous European Union (EU) regulatory framework, differ in their effects. To do this, we applied meta-regression of log response ratios (lnRR) to test our hypotheses that: (i) pesticides (insecticides, fungicides and herbicides) suppress growth, reproduction, and behavioural traits; (ii) metabolic biomarkers of non-target organisms may be up- or down-regulated in response to pesticides, thus signalling a physiological perturbation; (iii) identified pesticide effects are consistent between taxonomic groups, experimental types, climatic zones, modes of exposure (terrestrial or aquatic), and broad pesticide classes (insecticides, fungicides or herbicides); and (iv) the effects also hold under realistic terrestrial and aquatic exposure scenarios.
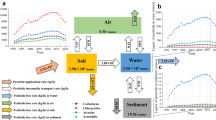
Study locations across the globe are shown (world map in World Robinson projection). A literature search identified study locations for insecticide impact on animals (820 locations), plants (112 locations) and microorganisms (56 locations), for fungicide impact on animals (148 locations), plants (74 locations) and microorganisms (52 locations), and for herbicide impact on animals (286 locations), plants (161 locations) and microorganisms (69 locations), respectively, from a total of 1705 published articles from 1497 locations. Nineteen articles included more than one study location (range 2–5). Grey and non-English literature were not included in this meta-analysis. Map © ARCGIS Software. Source data are provided as a Source Data file.
Results and discussion
Responses of non-target organisms to pesticide use
We derived responses for 830 different species (560 animals, 192 plants, 78 microorganisms) and 129 non-species-level groups (i.e. 79 non-species-level animals, 8 non-species-level plants and 42 non-species-level microorganisms) to 471 different pesticide active ingredients (243 insecticides, 104 fungicides and 124 herbicides) (Supplementary Tables 1–24; Supplementary Data 1–23). The median value of the sample size is 4 for both the control and treatment. Below we report responses for overall combined pesticides, as well as separately for insecticide, herbicide and fungicide pesticide classes.
For animals, pesticides overall decreased growth [CI (95 percent confidence interval) = −0.1277 to −0.055, df = 1504, ES (effect size) = −0.091, P < 0.001] and reproduction (CI = − 0.464 to −0.325, df = 5475, ES = − 0.395, P < 0.001) while also modifying behaviour (CI = − 0.415 to −0.210, df = 1328, ES = − 0.313, P < 0.001) (Fig. 2a). Considering pesticide classes separately, we show that insecticides had negative effects on animal growth (CI = − 0.176 to −0.070, df = 793, ES = − 0.123, P < 0.001), reproduction (CI = − 0.550 to −0.382, df = 4225, ES = − 0.466, P < 0.001) and behaviour (CI = − 0.421 to −0.202, df = 934, ES = − 0.311, P < 0.001) (Fig. 2b). Such broad-spectrum effects can be linked to insecticide impacts on, among other factors, nervous system functioning affecting longevity, fecundity and survival across a range of animal taxa25. Fungicides reduced animal growth (CI = − 0.153 to −0.005, df = 171, ES = −2.091, P = 0.037), reproduction (CI = − 0.521 to 0.001, df = 448, ES = − 0.260, P = 0.051) and expression of measured behaviours (CI = − 0.693 to −0.145, df = 164, ES = − 0.419, P = 0.003) (Fig. 2c). Fungicide exposure can lead to changes in endogenous metabolism, such as intracellular glutathione depletion and decreased cellular respiration, that alter physiological functioning potentially explaining these responses26. Our analysis also detected impacts of herbicides on animal reproduction (CI = − 0.362 to −0.145, df = 800, ES = − 0.253, P < 0.001) and behaviour (CI = − 0.475 to −0.126, df = 228, ES = − 0.301, P < 0.001) but not animal growth (CI = − 0.061 to 0.058, df = 538, ES = − 0.001, P = 0.969), (Fig. 2d). This may have been caused by narcosis effects reported for herbicides on animals resulting in negative effects on physiological pathway link to neurotoxic effects and impacts on metabolism27. Pesticides overall (CI = 0.338 to 0.430, df = 4469, ES = 0.384, P < 0.001), as well as separately for insecticides (CI = 0.325 to 0.440, df = 2708, ES = 12.972, P < 0.001), fungicides (CI = 0.321 to 0.564, df = 683, ES = 7.149, P < 0.001) and herbicides (CI = 0.255 to 0.405, df = 1076, ES = 0.330, P < 0.001) perturbed animal biomarkers, including indicators of neurophysiological response and cellular processing (n.b., as biomarkers include processes that may be both up and down-regulated, like gene regulation, we consider only absolute deviations relative to the reference control).
Horizontal lines indicate the 95% confidence intervals around the means; numbers in brackets indicate the numbers of observations and studies. Blue lines represent the growth of animals, plants or microorganisms, green lines represent the reproduction of animals, plants or microorganisms, red lines represent the biomarker of animals, plants or microorganisms, and black lines represent the animal behaviour, respectively. For biomarkers, absolute values were taken. Z test is used for tests of individual coefficients. Each test is two-sided, and the original P value is reported with no multiple comparisons. a Responses of animals, plants and microorganisms to pesticides. b Responses of animals, plants and microorganisms to insecticides. c Responses of animals, plants and microorganisms to fungicides. d Responses of animals, plants and microorganisms to herbicides. Source data are provided as a Source Data file.
Pesticides decreased plant growth (all, CI = − 0.422 to −0.255, df = 2576, ES = − 0.338, P < 0.001; insecticides, CI = − 0.307 to −0.113, df = 624, ES = − 0.210, P < 0.001; fungicides, CI = − 0.433 to −0.045, df = 346, ES = − 0.239, P = 0.016; herbicides, CI = − 0.602 to −0.362, df = 1604, ES = − 0.482, P < 0.001) and plant reproduction (all, CI = − 0.538 to −0.155, df = 668, ES = − 0.346, P < 0.001; insecticides, CI = − 0.448 to −0.167, df = 293, ES = − 0.308, P < 0.001; fungicides, CI = − 1.173 to 0.430, df = 119, ES = − 0.372, P = 0.363; herbicides, CI = − 0.619 to −0.242, df = 254, ES = − 0.431, P < 0.001). Pesticides also perturbed plant biomarkers, including effects on metabolism, photosynthesis and transpiration. This was the case when considering pesticides together (CI = 0.292 to 0.501, df = 1810, ES = 0.396, P < 0.001), as well as separately by class (insecticides, CI = 0.133 to 0.686, df = 421, ES = 0.410, P = 0.004; fungicides, CI = 0.190 to 0.454, df = 260, ES = 0.322, P < 0.001; herbicides, CI = 0.369 to 0.554, df = 1127, ES = 0.462, P < 0.001). The decreased growth and reproduction of plants may be linked to reductions in photosynthesis through known modes of action of herbicides28, as well as by off-target effects for selected insecticides29 and fungicides30. These include impacts on the cell cycle (e.g. abnormal cytoskeletal distribution, tube morphology and microtubule organization)31, direct or indirect interactions leading DNA genotoxicity32 and via non-specific cellular reactivity33.
For microorganisms, pesticide exposure decreased growth (all, CI = − 0.726 to −0.376, df = 422, ES = − 0.551, P < 0.001; insecticides, CI = − 0.712 to −0.258, df = 132, ES = − 4.185, P < 0.001; fungicides, CI = − 1.659 to −0.060, df = 59, ES = − 2.107, P = 0.035; herbicides, CI = − 0.694 to −0.327, df = 229, ES = − 0.510, P < 0.001) and reproduction (all, CI = − 1.025 to −0.548, df = 764, ES = − 0.787, P < 0.001; insecticides, CI = − 0.741 to −0.252, df = 264, ES = − 0.496, P < 0.001; fungicides, CI = − 1.079 to −0.418, df = 235, ES = − 0.748, P < 0.001; herbicides, CI = − 1.570 to −0.469, df = 263, ES = − 1.019, P < 0.001) (Fig. 2). Microorganism biomarkers (i.e. indicators of enzymatic reaction system) were also affected by exposure to pesticides overall (CI = 0.411 to 0.619, df = 1186, all ES = 0.515, P < 0.001), as well as separately for insecticides (CI = 0.327 to 0.562, df = 531, ES = 0.444, P < 0.001) and herbicides (CI = 0.403 to 0.729, df = 548, ES = 0.566, P < 0.001), but not fungicides (CI = − 0.202 to 1.377, df = 105, ES = 0.588, P = 0.145) (Supplementary Table 3; Fig. 2). The negative responses of microorganism growth and reproduction to fungicides can be linked to impacts on spore germination, germ tube elongation, sporulation, and root colonization34, as well as through effects on electron transport and energy metabolism35. The impacts of insecticides and herbicides can be linked to cellular chemical reactivity leading to intracellular damage of microorganisms, as well as denaturing of key macromolecules and/or changing cell membrane permeability36,37. When animals, plants and microorganisms were subdivided (e.g. animals subdivided into invertebrates and vertebrates) (Fig. 3), we found similarly negative responses.
Horizontal lines indicate the 95% confidence intervals around the means; numbers in brackets indicate the numbers of observations and studies. Blue lines represent the growth of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; green lines represent the reproduction of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; red lines represent the biomarker of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; and black lines represent animal behaviour. Absolute values of ‘biomarker’ values were taken. a Responses of animals, plants and microorganisms to pesticides. Z test is used for tests of individual coefficients. Each test is two-sided, and the original P value is reported with no multiple comparisons. b Responses of animals, plants and microorganisms to insecticides. c Responses of animals, plants and microorganisms to fungicides. d Responses of animals, plants and microorganisms to herbicides. Source data are provided as a Source Data file.
Across the 1705 papers, the effect of new vs. old pesticides was similar independent of which taxonomic groups (animals, plants and microorganisms) or which of their trait responses (growth, reproduction, behaviour and biomarker) were considered (Supplementary Data 2; Supplementary Table 15). There was, therefore, limited evidence that the negative environmental impact of pesticides on wider biodiversity has been reduced by the development and authorisation of novel active ingredients and formulations. When non-target organisms were classified as either model species for hazard assessment (e.g. honeybees or Daphnia) or non-model species (e.g. solitary bees), there was also no evidence of a difference in average negative effects (Supplementary Data 3; Supplementary Table 16).
We also tested whether the predominantly negative effects of pesticides on major taxonomic groups differed among pesticide types, experiment types, exposure media (aquatic or terrestrial), climatic zones, conflict-of-interest status and publication year. In the case of synthetic pesticides, which included insecticides, fungicides, and herbicides, the same negative trend described above for all pesticide classes was found (Supplementary Table 5). This was observed in both mineral-based and biogenic pesticides, which, despite having smaller sample sizes, exhibited responses of similar magnitude compared to the overall effects of pesticides (Fig. 4). However, there was no effect on plant reproduction (Supplementary Table 5; Fig. 4). Across experiment types, we found that reported effects were larger under laboratory than field experimental conditions (Fig. 5), and that there was no significant interaction between pesticide application rates and experiment types (CI = − 4.699 × 10−5 to 1.187 × 10−5, df = 20,181, ES = − 1.756 × 10−5, P = 0.242). This was likely due to larger sample sizes in laboratory experiments, lower variability associated with more controlled conditions, and generally lower exposure levels in field settings where environmentally realistic exposure rates are more likely to be considered (Supplementary Table 6). Across climatic zones, we found a stronger response for studies in temperate than in tropical zones (Supplementary Table 7; Fig. 6), perhaps due to faster pesticide detoxification and dissipation in tropical ecosystems, e.g. caused by increased UV exposure and higher temperatures38. There was no evidence of effect size differences resulting from exposure in aquatic (e.g. through immersion in contaminated water) vs. terrestrial (e.g. through direct contact or oral exposure) environments (Fig. 7). This suggests similar sensitivity of species to pesticides, independent of the medium through which exposure occurred (Supplementary Table 8). Of the 1705 papers included in the meta-analysis, 26 reported a conflict of interest statement, with 241 papers lacking clear conflict of interest statements or information on funding sources. In cases where no conflict of interest had been reported (N = 1,438), the same negative trends described above were found for all pesticide classes (Supplementary Table 23). However, where a conflict of interest was self-declared, pesticides were in contrast found to have no effect on the growth of animals (CI = − 0.312 to 0.243, df = 16, ES = − 0.035, P = 0.807) and behaviour (CI = − 0.305 to 0.094, df = 35, ES = − 0.106, P = 0.299), or the growth (CI = − 0.724 to 0.411, df = 35, ES = − 0.156, P = 0.589) and reproduction (CI = − 0.706 to 1.208, df = 8, ES = 0.251, P = 0.607) of plants (Supplementary Table 23). The responses of the growth, reproduction and biomarkers of microorganisms to pesticides (with conflict of interest) lacked sufficient sample sizes for analysis. Similarly, sample sizes were insufficient to test the effects of conflict-of-interest statements on insecticides, fungicides and herbicides on animals, plants and microorganisms (Supplementary Table 23). Additional information on the responses of different taxonomic groups (e.g. at order level) is provided in Supplementary Data 5–23. The overall negative impact of pesticides on non-target organisms was consistently observed in the separate impacts of insecticides, fungicides, and herbicides on finer-resolution taxonomic groups, e.g. vertebrates and invertebrates were equally affected by pesticides (Supplementary Table 4).
No observations were found to study the responses of the animals, plants and microorganisms to mineral-based herbicides. Horizontal lines indicate the 95% confidence intervals around the means; numbers in brackets indicate the numbers of observations and studies. Blue lines represent the growth of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; green lines represent the reproduction of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; red lines represent the biomarker of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; and black lines represent animal behaviour. Absolute values of ‘biomarker’ values were taken. Z test is used for tests of individual coefficients. Each test is two-sided and the original P value is reported with no multiple comparisons. a Chemical pesticides. b Mineral-based pesticides. c Biogenic pesticides. d Chemical insecticides. e Mineral-based insectides. f Biogenic insectides. g Chemical fungicides. h Mineral-based fungicides. i Biogenic fungicides. j Chemical herbicides. k Biogenic herbicides. Source data are provided as a Source Data file.
Horizontal lines indicate the 95% confidence intervals around the means; numbers in brackets indicate the numbers of observations and studies. Blue lines represent the growth of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; green lines represent the reproduction of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; red lines represent the biomarker of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; and black lines represent animal behaviour. Absolute values of ‘biomarker’ values were taken. Z test is used for tests of individual coefficients. Each test is two-sided and the original P value is reported with no multiple comparisons. a Laboratory experiments of pesticides. b Laboratory experiments of insecticides. c Laboratory experiments of fungicides. d Laboratory experiments of herbicides. e Field experiments of pesticides. f Field experiments of insecticides. g Field experiments of fungicides. h Field experiments of herbicides. Source data are provided as a Source Data file.
No observations were found to study the responses of the animals, plants and microorganisms to fungicides in tropical zones. Data from laboratory conditions were removed from the models with climatic predictors. Horizontal lines indicate the 95% confidence intervals around the means; numbers in brackets indicate the numbers of observations and studies. Blue lines represent the growth of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; green lines represent the reproduction of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; red lines represent the biomarker of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; and black lines represent animal behaviour. Absolute values of ‘biomarker’ values were taken. Z test is used for tests of individual coefficients. Each test is two-sided and the original P value is reported with no multiple comparisons. a Temperate zone experiments of pesticides. b Temperate zone experiments of insecticides. c Temperate zone experiments of fungicides. d Temperate zone experiments of herbicides. e Tropical zone experiments of pesticides. f Tropical zone experiments of insecticides. g Tropical zone experiments of herbicides. Source data are provided as a Source Data file.
Horizontal lines indicate the 95% confidence intervals around the means; numbers in brackets indicate the numbers of observations and studies. Blue lines represent the growth of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; green lines represent the reproduction of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; red lines represent the biomarker of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; and black lines represent animal behaviour. Absolute values of ‘biomarker’ values were taken. Z test is used for tests of individual coefficients. Each test is two-sided and the original P value is reported with no multiple comparisons. a Terrestrial experiments of pesticides. b Terrestrial experiments of insecticides. c Terrestrial experiments of fungicides. d Terrestrial experiments of herbicides. e Aquatic experiments of pesticides. f Aquatic experiments of insecticides. g Aquatic experiments of fungicides. h Aquatic experiments of herbicides. Source data are provided as a Source Data file.
Our main overall findings are based on an analysis of effect sizes for all reported pesticide exposure rates, including some levels unlikely to occur during authorised use. To ensure that our results reflect real-world exposure hazards in terrestrial and aquatic systems (hypothesis iv), we checked for the consistency of responses by restricting the dataset to exposure rates representative of field-realistic concentrations. To do this, we repeated our analysis using a subset of effect sizes, where: (1) pesticide exposure was within real-world application rates in terrestrial systems, based on information from recommended application rates from pesticide product labels; or (2) concentrations of pesticides within waterbodies were below those reported from real-world situations in governmental monitoring or targeted scientific monitoring studies (Supplementary Data 4). This analysis, thus, excluded effect sizes from studies that may have used higher application rates and environmental concentrations, for example, those used in the derivation of exposure-response curves for hazard-focused studies.
Our analysis of field-realistic application rates confirmed the strong evidence for overall (insecticides, fungicides and herbicides together) significant negative effects on animal reproduction (Fig. 8; CI = − 0.549 to −0.230, df = 470, ES = − 0.389, P < 0.001) and animal behaviour (CI = − 0.919 to −0.224, df = 53, ES = − 0.571, P = 0.001), although effects on animal growth (CI = − 0.527 to 0.256, df = 23, ES = − 0.136, P = 0.497), while negative, were non-significant. This may be due to insufficient replication, with only 7 studies included on the response of animal growth to pesticides. Realistic pesticide application rates had non-significant negative effects on plant growth (CI = − 0.418 to 0.127, df = 176, ES = − 0.146, P = 0.295), plant reproduction (CI = − 0.408 to 0.124, df = 60, ES = − 0.142, P = 0.295), and microorganism reproduction (CI = − 0.562 to 0.082, df = 68, ES = − 0.240, P = 0.145), as well as non-significant positive effects on microorganism growth (CI = − 0.688 to 0.835, df = 14, ES = 0.074, P = 0.850). Pesticides did not significantly perturb metabolic biomarkers of plants (CI = − 0.225 to 0.550, df = 93, ES = 0.162, P = 0.411) and microorganisms (CI = − 1.160 to 1.717, df = 27, ES = 0.279, P = 0.704). Insecticides, fungicides and herbicides affected different taxonomic groups through decreased growth, reproduction or behavioural responses, and biomarkers being perturbed from baseline conditions (see Supplementary Tables 10–14). For exposures to pesticides at environmentally relevant concentrations in aquatic ecosystems, qualitatively similar patterns emerged for the separate taxonomic groups (animals, plants and microorganisms) in terms of growth, reproduction, behaviour and biomarkers, as seen in the main analysis reported above (Supplementary Tables 17−22).
Horizontal lines indicate the 95% confidence intervals around the means; numbers in brackets indicate the numbers of observations and studies. Blue lines represent the growth of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; green lines represent the reproduction of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; red lines represent the biomarker of invertebrate animals, vertebrate animals, seed plants, spore-producing plants, bacteria and fungicides; and black lines represent animal behaviour. Absolute values of ‘biomarker’ values were taken. Z test is used for tests of individual coefficients. Each test is two-sided and the original P value is reported with no multiple comparisons. a Field realistic application rate of pesticides. b Field realistic application rate of insecticides. c Field realistic application rate of fungicides. d Field realistic application rate of herbicides. e Environmentally relevant application rate of pesticides. f Environmentally relevant application rate of insecticides. g Environmentally relevant application rate of fungicides. h Environmentally relevant application rate of herbicides. Source data are provided as a Source Data file.
Effects of increasing pesticide application rates on non-target organisms
We tested for application rate-dependent effects on non-target organisms, i.e. how responses increased with increasing rate of application (defined below), by using a meta-regression to relate effect sizes to increased application rates (Fig. 9a–i; Supplementary Figs. 1−21; Supplementary Data 24–26). Because different studies defined pesticide exposure in different units, we defined pesticide application rates as multiples of the lowest non-control treatment which we then log2-transformed (see “Methods”). For example, dose rates of 2.5, 5, 10 and 15 ng a.i. bee−1 would have been given the relative dose rates of 1 (lowest non-control dose of 2.5 ng a.i. bee−1), 2 (e.g. 5 ng a.i. bee−1 dose/2.5 ng a.i. bee−1 lowest non-control), 4 and 6 before log-transforming. We found negative relationships between increased application rates of all pesticides and growth (CI = − 4.39 × 10−5 to −6.29 × 10−6, t1141 = −2.619, R2 = 0.026, P = 0.009), reproduction (CI = − 1.08 × 10−4 to −8.33 × 10−5, t3811 = −14.949, R2 = 0.274, P < 0.001) and behaviour (CI = − 1.62 × 10−5 to −6.10 × 10−6, t955 = −4.335, R2 = 0.154, P < 0.001) of animals (Fig. 9a). Similar negative relationships were detected for plant growth (CI = − 4.65 × 10−5 to −1.67 × 10−5, t2211 = −4.17, R2 = 0.128, P < 0.001) and reproduction (CI = − 8.42 × 10−5 to 3.53 × 10−5, t476 = −0.804, R2 = 0.392, P = 0.422) (Fig. 9b) and microorganism growth (CI = − 0.0024 to −0.0016, t334 = −9.12, R2 = 0.231, P < 0.001) and reproduction (CI = − 3.89 × 10−4 to −1.24 × 10−4, t587 = −3.794, R2 = 0.341, P < 0.001) (Fig. 9c). Positive relationships between increased pesticide application rates and the magnitude of perturbations of biomarkers of animals (CI = − 1.18 × 10−7 to 2.66 × 10−5, t4009 = 1.94, R2 = 0.067, P = 0.052), plants (CI = 3.38 × 10−4 to 6.16 × 10−4, t1623 = 6.733, R2 = 0.185, P < 0.001) and microorganisms (CI = 5.55 × 10−4 to 0.012, t1083 = 5.246, R2 = 0.113, P < 0.001) were found, indicating physiological responses to increasing exposure. Application rate-dependent effects on different major taxonomic groups were also qualitatively equivalent to the broader findings (see Supplementary Data 8 & Supplementary Note 1).
The absolute values of pesticide application rates were transformed into relative values (i.e. the lowest non-zero pesticide application rate among multiple pesticide application rates with the same unit was considered to be 1, and the value of the other pesticide application rates with the same unit were considered to be multiples of 1). This was log2-transformed. Scatter plots show the response to application rates for the growth, reproduction, behaviour (animals only) and other biomarkers of animals, plants and microorganisms. The medium purple, blue, black and red dots and lines represent the effects of growth, reproduction, behaviour and biomarker, respectively. The shaded region indicates the 95% confidence interval for the predicted average lnRR. Absolute values of ‘biomarker’ values were taken. Effect sizes >4 are not shown for clarity of visualisation. Regression lines are presented only when the slope is significant. The regression model intercepts, slopes and the P-values for the slopes are presented in Supplementary Data 8. T-test is used for tests of individual coefficients. Each test is two-sided and the original P value is reported with no multiple comparisons. a Relationships between pesticide application rate and animals. b Relationships between pesticide application rate and plants. c Relationships between pesticide application rate and microorganisms. d Relationships between insecticide application rate and animals. e Relationships between insecticide application rate and plants. f Relationships between insecticide application rate and microorganisms. g Relationships between fungicide application rate and animals. h Relationships between fungicide application rate and plants. i Relationships between fungicide application rate and microorganisms. j Relationships between herbicide application rate and animals. k Relationships between herbicide application rate and plants. l Relationships between herbicide application rate and microorganisms. Source data are provided as a Source Data file.
Overall, our synthesis comprehensively shows that insecticides, fungicides and herbicides have broad-scale detrimental effects on all groups of non-target organisms tested. Pesticides consistently decreased growth and reproduction across all taxonomic groups, while also eliciting behavioural responses in animals and perturbing multiple endpoints linked to metabolic or physiological status. Given the ubiquitous use of pesticides worldwide, estimated at about 4.1 million tons of active ingredient applied in 201539, these findings emphasize the potential for prevalent non-target impacts relating to widespread pesticide use. Specifically, these results suggest that pesticide use has the potential to result in wider perturbation of ecological communities and functions. While we identified responses for individual compounds, it is likely that these effects may be exacerbated through additive or potentially synergistic effects where compounds are applied in combination or to areas with existing residues present4. We acknowledge that the reduction of pesticide use, while a superficially simple solution, is, in practice, likely to have widespread and significant consequences for food production and farmer livelihoods. Nevertheless, our findings highlight the need for widespread policy drivers to adopt solutions to reduce pesticide use and/or maximize the efficiency of inputs. Integrated Pest Management (IPM) programmes that emphasize non-chemical control systems (e.g. timing of sowing or resistant varieties) as well as optimizing threshold-based application decisions for chemical control are clearly part of this solution. Advancing the development of green pesticides and wider agroecosystem diversification strategies could also play a role, including ecological intensification, spatio-temporal crop diversification, including intercropping, as well as other cultural controls of pest pressure, like traditional rice-fish co-culture management systems40. Such approaches would likely need to consider landscape-scale management strategies that would require wide-scale adoption to reduce impacts on non-target organisms at national and international scales. Our findings indicate that the low cost of pesticides fails to account for hidden costs to wildlife and ecosystems. This makes sustainable practices less attractive financially for farmers, who might keep using pesticides as a preventative measure. Our findings also show the limitations of regulatory assessments in predicting real-world hazards like long-term low-level exposure, cumulative effects at the landscape level, and synergistic interactions between active ingredients. The implementation of post-licensing biodiversity monitoring could help address this problem41. In conclusion, while pesticides are likely to remain part of the toolbox of pest management options, their universal cross-taxa impact means that current usage is likely unsustainable for modern agriculture. Unless changes occur, the hazard of severe, unexpected and long-term impacts on biodiversity and ecosystem functioning will remain unacceptably high.
Methods
Study selection
We searched the Web of Science Core Collection, MEDLINE, SciELO Citation Index and KCI-Korean Journal Database (1900 to present) using a Boolean search string querying the “Topic” field, as follows: [“pesticide” OR “insecticide” OR “fungicide” OR “herbicide”] AND [“non-target*” OR “carnivor*” OR “saprophag*” OR “saprovor*” OR “omnivor*” OR “bee” OR “pollinat*” OR “pollen*” OR “predat*” OR “parasitoid” OR “wasp” OR “mammal” OR “amphibi*” OR “reptil*” OR “bird” OR “fish” OR “mollus*” OR “invertebrate” OR “vertebrate” OR “herbivore” OR “arthropod” OR “plant” OR “dicot*” OR “monocot*” OR “alga*” OR “microorganism” OR “bacteria” OR “fung*”]. We set up the search string based on panel discussions and consulting with associated experts majoring in ecology and pesticides. This literature search was initiated in November 2012 and was finalized in May 2022. In total, the search yielded 887,482 papers (Fig. 10). Grey and non-English literature were not included in this meta-analysis. While this may have introduced some bias, the meta-analysis included studies from all continents and biomes within the finalized data set. Given the breadth of this data set, we do not consider there to be any a priori reason to assume that the directionality of detected trends would have been qualitatively different with the addition of non-English language papers. Of this large number of initially identified papers, we undertook a rapid screening process that focused on the titles and abstracts to determine whether the study had measured a response variable (growth, reproduction, behaviour or biomarker) for non-target organisms in response to exposure to insecticides, fungicides or herbicides. In the process of screening both the titles and abstracts, we abandoned 10,971 papers related to acaricides, bactericides, molluscicides and nematicides. Among these 10,971 papers, we found a subset of just 100 papers that had measured the effects of acaricides, bactericides, molluscicides and nematicides on the growth, reproduction, behaviour or biomarkers of non-target organisms (i.e. 59, 5, 16 and 20 papers for acaricides, bactericides, molluscicides and nematicides, respectively). Due to the very low sample sizes associated with these pesticide groups, we decided to focus only on insecticides, fungicides and herbicides (Fig. 10).
Our preliminary screening removed 878,772 papers. The remaining 8710 (i.e. 8710 = 887,482–878,772) papers were then screened in full to assess if they met the following criteria: (a) the study included at least one comparison between a randomly allocated control group (without pesticide) and one or more treatment groups exposed to insecticides, fungicides, or herbicides; (b) similarly to reduce within study heterogeneity for field experiments, control and treatment areas were required to have comparable non-pesticide agricultural practices, e.g. fertilizer, irrigation, soil tillage etc; (c) treatment groups are needed to include single insecticide, fungicide, or herbicide active ingredient at a time, so that multiple pesticide exposure experiments were discarded (this was done to avoid the risk of bias associated with study heterogeneity resulting from unexpected synergistic interactions between compounds42); (d) control and pesticide-treated groups were required to include the same non-target organisms for lab or field experiments; and finally (e) the measurements of control and pesticide-treated groups had to have been undertaken at the same spatiotemporal scale43. These screening criteria resulted in 1705 papers from which it was possible to extract effect sizes for inclusion in the meta-analysis (see Fig. 10 for details on data selection process). Data were extracted from the papers by N.F.W. and L.F. during which regular cross-checking was performed to ensure consistency in extracted effect sizes. A second complete recheck of data extraction was undertaken to ensure consistent recording of values and to identify any differences in interpretation of the inclusion criteria for studies or transcribing errors that may have occurred. Where records were rejected in this validation process an attempt was made to identify papers by the same author(s) or their affiliations using the same data set that may have been missed in the first ‘rapid’ screening. If found these were included. In total 312 substitute articles were included in the second recheck from late January 2024. Thus, 1705 articles were finally included in this paper. Data was re-extracted and cross-checked by S.S. and N.F.W.
When studies reported mean values across multiple sampling dates or years these were included as separate data points. Where this was not the case, we focused on the latest sampling period to be presented43. For studies that included more than one location, we considered these experimental observations separately (see locations in Fig. 1). When numeric values were not provided directly, we extracted them from figures using the “GetData Graph Digitizer” software43. However, where linear or non-linear relationships between pesticide application rates and one of these response indicators were presented in a figure, we extracted the values via fitted regression equations44.
When the pesticide-treated group was paired with the control group, we excluded multiple comparisons within a single study and selected different comparison data. As such, observations without pesticide use were considered as control groups, while those with different pesticide application rates were considered as the treatment groups. When a study included different levels of pesticide application rates, measurements for the control groups without pesticides versus different pesticide application rates were considered independent paired observations. For laboratory-based studies that considered pesticides that were water-soluble, we selected pure distilled water as the control, even when multiple control solvents had been present (e.g. ethanol). If pesticides were not water soluble, we ensured that the control and treatment groups had a treatment with the same solvent added as a control treatment. Note this is only relevant for laboratory studies, where control mortality for the pesticide carrier (e.g. water) is a standard approach. Field studies rarely treat control areas with such a matched unspiked solvent carrier.
Definition of pesticide use
In the main meta-analysis, “pesticide use” refers to any activity that applies insecticides, fungicides or herbicides relative to a control treatment without any pesticide input. Thus, this is essentially a binary variable (zero or one), irrespective of the pesticide application rate. Examples of this are provided in Fig. 2–8. When working with the actual concentrations or doses, we specifically term this “pesticide application rate”.
Predictor variables
The analysis considered six categorical explanatory variables, one binary variable and two continuous variables (see detailed description in the Supplementary Methods). These were (1) Non-target organism group: divided into (i) animals (distinguishing between invertebrates and vertebrates), plants (distinguishing between seed-producing plants and spore-producing plants), and microorganisms (distinguishing between bacteria and fungi); and (ii) model and non-model species. Model species represent a set of widely used cultured animals, plants and micro-organisms that are directly referred to in ecotoxicological test protocols or associated scientific publications. These species are routinely used for regulatory or scientific studies conducted for risk assessment (Supplementary Data 3). Of these, the most important regulatory documents used to identify model species were those of the Organisation for Economic Cooperation and Development (OECD - widely adopted by the EU, USA and other countries), International Organization for Standardization (ISO) and the US-Environmental Protection Agency (EPA; see Supplementary Data 3). Species identified in this documentation were classified as model species. (2) Response variable: growth, reproduction, measures of behaviour (animals only) and other biomarkers. The category of ‘animal behaviour’ was evaluated in the context of the expected effect of pesticides on the animal fitness or fitness correlates (i.e. locomotor activity, feeding rate and attack rate instead of response time of animals to pesticides). The category of physiological ‘biomarker’ included a wide range of sub-individual level processes (e.g. photosynthesis, transpiration, neurophysiological response and cell processing) that could not be categorised under more general terms such as general growth or reproduction rates. From the perspective of interpretation, a decrease in growth, reproduction or behavioural responses was considered to denote a negative effect of pesticides. In the case of biomarkers, both up- and down-regulation in response to pesticide exposure have been reported. To avoid such processes cancelling each other out in the meta-analysis, we here used the absolute values of biomarkers to evaluate perturbations (either positive or negative) in response to pesticides. This was done following approaches defined by Swart et al.45. (3) Experiment type: laboratory (including glasshouse) and field experiments44. (4) Pesticide variables: divided into (i) insecticide, herbicide and fungicide classes (there was insufficient literature on effects of acaricides, bactericides, molluscicides and nematicides on non-target organisms); (ii) pesticide types according to whether they were mineral-based, biogenic or chemical-synthetic pesticides; and (iii) ‘old’ and ‘new’ pesticides. This is an arguably hard-to-define category as there is considerable international variability in what constitutes an older no longer approved pesticide. However, we consider ‘new’ pesticides to be those that are currently approved under European Union regulation. This regulatory framework is considered one of the most precautionary systems in the world. Old pesticides are those currently not authorised for widespread agricultural use in the EU (principally because authorization has been withdrawn, although this category potentially included a small subset of yet-to-be-approved products; Supplementary Data 2). The responses of animals, plants and microorganisms to pesticide types are in Supplementary Methods and Supplementary Data 5−7, and the effects of pesticide application rate-dependent increase are listed in Supplementary Data 8. (5) Climatic zone: temperate or tropical (temperate zones ranged from 23.5°N to 66.5°N and from 23.5°S to 66.5°S, and the tropical zones ranged from 0 − 23.5° N and from 0 − 23.5° S). (6) Exposure medium: aquatic (exposed to pesticides in a water medium) or terrestrial (oral consumption, soil or contact exposure in terrestrial systems). (7) Conflict of interest status: for articles that have a conflict-of-interest statement we include a binary variable that denotes whether a conflict was present (“1” for a conflict, i.e. where funding was from the agrichemical industry) or “0” where there was no self-declared conflict. Note that many studies have no conflict-of-interest statement. (8) Publication year: a continuous metric according to the year when the articles were published intended to identify systematic bias in reporting trends over time. (9) Pesticide application rate: a continuous metric based on the lowest application rate (not zero) compared to the lowest baseline reference exposure value (not the control) in each study. To standardize these differences, we used multiples of the lowest application rate used within a study, defining that lowest application rate as 1. For example, a baseline application rate of 2 mg·mL−1 was given a relative application rate of 1, the next two highest application rates in the study of 4 and 8 mg·mL−1 were respectively given the application rate of 2 and 4. These values were then log2-transformed. This approach was necessary as different units (e.g. mg·mL−1, mg·cm−2, mg·(kg soil)−1, mg·(kg body weight)−1, mg·pot−1, mg·plant−1, mg·seed−1, µg·individual−1, kg·ha−1, mL·ha−1, mL·kg−1, spores·ha−1 and conidia·g−1) were used to describe pesticide exposure46. The use of the log transformation of dose or application data is common practice in ecotoxicology for interpreting biological responses (e.g. Wigger et al., 2020)46. In terms of the choice of base transformation, there was no mathematical reason to select one or the other (e.g. Loge or Log10). However, Log2-transformed data based on a doubling of concentrations were likely to be close to biologically detectable response and, from an interpretation perspective, are sufficiently “fine-grained” to be easily visually interpreted. There may have been other potential moderator factors that could have explained differential responses to pesticides but that were insufficiently defined in papers to be considered in subsequent analytical models. For example, only 10 papers consider the sex of the tested animal while the study funding source (public or industry) may have affected the interpretation of results but was not consistently recorded between papers or journals over the time considered to be useful.
Effect size (ES) measure
The log response ratio effect size (lnRR) was calculated to examine the pesticide use effects. Log response ratios and their associated sampling variances are normally calculated according to:
where m1 and m2 are the observed value of the treatment and control group, v represents the sampling variance, sd1 and sd2 the standard deviation per group, CV´s are the corresponding coefficients of variation for treatment and control groups, and n1 and n2 the sample sizes per group. However, the data set used in the study has studies missing measures of variance. To account for this, we applied the approach proposed by Nakagawa et al.47 to weight the average coefficient of variation estimated from studies that fail to report measures of variance using the approach:
where CV = sd/m is the coefficient of variation, with sd and n representing the corresponding standard deviations and sample sizes; \({{{{\rm{CV}}}}}_{1{{{\rm{i}}}}}\) and \({{{{\rm{CV}}}}}_{2{{{\rm{i}}}}}\) are the CVs from the ith study (study: i = 1,2,…,K). Equations 3 and 5 can improve the accuracy and precision of the overall mean estimate. Equations 5 and 6 can be used to calculate the effect sizes and sample variances when SDs are missing, and Eqs. 3 and 4 can be used to calculate the effect size and sample variances when SDs are not missing. In the present paper, we use the “All Cases” method, defined in Eq. 5, to calculate effect sizes, regardless of whether SDs were missing47. When combining lnRR of observations in each group, we used the combined lnRR average effect size for each group.
Meta-regression models for pesticide use
Meta-regression48 was performed to evaluate whether the effects of pesticide use on various taxonomic groups could be explained by the different moderator variables and their interactions. Statistical analyses were done using R (version 4.1.0)49 package “metafor” (version 3.4-0) to fit multilevel mixed-effects, meta-regression models. The function “lnrr_laj()” in the supplementary R function “func.R”, developed by Nakagawa et al.47, was used to calculate the effect size metric lnRR for each observation. We used Eq. 6 to compute the sampling variances (using the function “v_lnrr_laj()” in R.file “func.R”). The lnRRs were employed as the response variable in our models. Taxonomic groups (i.e. animals, plants and microorganisms) were treated as fixed effects, along with the moderator variables (i.e. pesticide class, study type, climatic zone, exposure medium, pesticide type, experimental organism type, conflict of interest status, publication year, and number of added pesticide application rates). As measures from the same control individual were sometimes used repeatedly in comparisons of the effects of different doses of the same pesticides, this could have resulted in a form of “double-counting”. To account for this potential form of pseudoreplication, we assigned the argument “V” in the “rma.mv()” function, with the sampling variance-covariance matrix estimated by function “vcalc()” in R package metafor50. Thus, V is adjusted to account for the repeated measures on the same control individual by fixing the between-subject correlation within a study to values of 0.3, 0.5 or 0.6 (argument “rho” in the vcalc() function in R).
To account for between-pesticide differences, we used the active ingredient pesticide identity as a random effect in the models. Although not considered in the main results, the included studies were also analysed in subsets (e.g. as higher resolution taxonomic groups) to better understand the effects within these various strata (see Supplementary Methods). We used a mixed-effects model meta-analysis. In such analyses, the underlying phylogeny may additionally have affected our variance estimates. To account for potential phylogenetic non-independence, we performed a phylogenetic correction for animals, plants and microorganisms. In these analyses, “animal species”, “plant species” or “microorganism species” was included as a random effect, with phylogenetic relatedness as part of the correlation structure, in which 600 animal species (excluding 12 animal species), 212 plant species (excluding 32 algae species) or 68 microorganism species (excluding 28 microorganism species) defined the correlation structure based on the animal phylogeny (see Supplementary Table 1.4 and 2.4)51,52, plant phylogeny (see Supplementary Table 1.3 and 2.3)51 and microorganism phylogeny (see Supplementary Table 1.5 and 2.5; Supplementary Methods)51,52, respectively.
To construct each mixed-effects meta-analysis model, we first fitted a base model integrating the taxonomic group as the only fixed effect. Second, we fitted a model integrating the interactions between the taxonomic group and other moderator variables to allow for a sequential test of whether they improved model fit based on likelihood-ratio tests (LRT). Third, for model comparisons, we employed LRT of the model including the taxonomic group response category (growth, reproduction, behaviour (animals only) and biomarker of animals, plants and microorganisms) and the interactions between the response category and any evaluated moderator (Supplementary Table 1). Specifically, a model with a single predictor (e.g. taxonomic group) was compared with a model with two moderators (e.g. taxonomic group plus climatic zone types) and their interaction (e.g. taxonomic group × climatic zone types) (see Supplementary Tables 1 and 2 for detailed explanation). The t-values and their 95% confidence intervals were derived from the corresponding fitted meta-regression models to evaluate whether the mean effect sizes were significant in the various categories.
An important aspect of the analysis was to determine whether the observed effects of pesticides would be seen under real-world exposure conditions, as opposed to unusually high application rates imposed as part of an experimental protocol (e.g. to create an application rate response curve). Here we focused on studies where treatment levels were a subset of the main data based on information on normal field or environmentally relevant exposure rates. For terrestrial systems, this required us to focus on systems where at least one of the treatment levels was commensurate with field-recommended pesticide application rates (i.e. those provided as maximum legal application rates on product labels). We deleted the data when the pesticide application rates in field experiments exceeded the maximum recommended rate. In the case of aquatic systems, we reviewed governmental monitoring and published scientific studies to determine the maximum reported environmental concentrations of active ingredients in water bodies (rivers, streams, lakes and ponds). For aquatic exposure systems we then filtered treatment levels effect sizes for those that fell at or below the reported maximum concentrations in water bodies. This produced two new data sets, one was for field realistic terrestrial exposure effect sizes, and the other for environmentally realistic exposure for aquatic systems. Within these, the impacts of pesticides (insecticides, fungicides and herbicides) on the non-target organisms (i.e. animals, plants and microorganisms) were assessed using the same meta-analytical framework described above. The responses of different taxonomic groups and their growth, reproduction, behaviour and biomarkers to application rates under recommended terrestrial field application rates are presented in Supplementary Tables 10–14, and these responses to application rates under maximum environmentally relevant concentrations are presented in Supplementary Tables 17–22.
Publication bias test
We employed regression tests to estimate the publication bias53 (based on the raw effect size) for the 20,212 observations of the 1705 cited articles in this paper (value of regression test = −24.879, P < 0.001) (see Supplementary Table 2). To undertake this, a regression coefficient between the effect size and the sample variance was evaluated. We assessed the impact of publication bias using the trim-and-fill method54 as suggested by Egger et al.53 was performed which supported the robustness of our results (e.g. Supplementary Table 9 showed the results of sensitivity analysis for the different taxonomic groups—animals, invertebrates, vertebrates, plants, seed plants, spore-producing plants, microorganisms, bacteria and fungi). Nakagawa and Santos48 suggest that residuals from various established models should be used to assess publication bias in mixed-effects meta-regression analysis. We employed this approach by using sampling variances as an additional moderator within the mixed-effect model to examine publication bias. Finally, we applied the Rosenthal fail-safe method to the full dataset55 (Detailed publication bias description was presented in Supplementary Methods).
Regression analysis of pesticide application rate
A further analysis was undertaken to test whether the change of effect size was correlated with the number of added pesticide application rates (as defined above). We investigated the response of lnRR to the predictor of pesticide application rate using linear mixed-effects models in the “nlme” package56. In these linear mixed-effect models, we treated the amount of added pesticide application rates as the fixed effect. Pesticide and study identity were accounted for in the model by: (1) including random intercepts for pesticide identity; (2) adding study identity as a random intercept to incorporate the hierarchical error structure of multiple effects coming from the same study. To account for heteroscedasticity among studies, the term “vi” calculated from the “metafor” package for each observation was added as a fixed weight in the “lme” function, using a varFixed() variance function, as each observation has one certain pesticide type in a study, and distinct “vi” may reflect the heteroscedasticity. Using the “effects” package version 4.2.057 we derived predicted values with a 95% confidence interval, assuming unequal variances among observations. The data and code used in this study are publicly available in Zenodo (https://doi.org/10.5281/zenodo.14683219) (ref. 58).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw and processed data used in this study is available and is deposited to Zenodo (https://doi.org/10.5281/zenodo.14683219) (ref. 58). Source data are provided with this paper.
Code availability
The code that supports the findings of this study has been deposited in Zenodo (https://doi.org/10.5281/zenodo.14683219) (ref. 58).
References
Woodcock, B. A. et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 356, 1393–1395 (2017).
Crall, J. D. et al. Neonicotinoid exposure disrupts bumblebee nest behaviour, social networks, and thermoregulation. Science 362, 683–686 (2018).
van Klink, R. et al. Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368, 417–420 (2020).
Siviter, H. et al. Agrochemicals interact synergistically to increase bee mortality. Nature 596, 389–392 (2021).
Weidenmüller, A., Meltzer, A., Neupert, S., Schwarz, A. & Kleineidam, C. Glyphosate impairs collective thermoregulation in bumblebees. Science 376, 1122–1126 (2022).
Hallmann, C. A., Foppen, R. P. B., van Turnhout, C. A. M., de Kroon, H. & Jongejans, E. Declines in insectivorous birds are associated with high neonicotinoid doses. Nature 511, 341–344 (2014).
Yamamuro, M. et al. Neonicotinoids disrupt aquatic food webs and decrease fishery yields. Science 366, 620–623 (2019).
Köhler, H. R. & Triebskorn, R. Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science 341, 759–765 (2013).
Fishel, F. M. Pesticide effects on nontarget organisms. EDIS PI-85. (Gainesville: University of Florida Institute of Food and Agricultural Sciences, 2005).
Buysens, C., de Boulois, H. D. & Declerck, S. Do fungicides used to control Rhizoctonia solani impact the non-target arbuscular mycorrhizal fungus Rhizophagus irregularis? Mycorrhiza 25, 277–288 (2015).
Thomas, W. E. et al. Glyphosate negatively affects pollen viability but not pollination and seed set in glyphosate-resistant corn. Weed Sci. 52, 725–734 (2004).
Magné, C., Saladin, G. & Clément, C. Transient effect of the herbicide flazasulfuron on carbohydrate physiology in Vitis vinifera L. Chemosphere 62, 650–657 (2006).
Woodcock, B. A. et al. Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat. Commun. 7, 12459 (2016).
Druille, M., García-Parisi, P. A., Golluscio, R. A., Cavagnaro, F. P. & Omacini, M. Repeated annual glyphosate applications may impair beneficial soil microorganisms in temperate grassland. Agri. Ecosyst. Environ. 230, 184–190 (2016).
Triques, M. C. et al. Assessing single effects of sugarcane pesticides fipronil and 2, 4-D on plants and soil organisms. Ecotox. Environ. Saf. 208, 111622 (2021).
Stanley, D. A. et al. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 528, 538–550 (2015).
Tsvetkov, T. et al. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 356, 1395–1397 (2017).
Baker, N. J., Bancroft, B. A. & Garcia, T. S. A meta-analysis of the effects of pesticides and fertilizers on survival and growth of amphibians. Sci. Total Environ. 449, 150–156 (2013).
Eng, M. L., Stutchbury, B. & Morrissey, C. A. A neonicotinoid insecticide reduces fueling and delays migration in songbirds. Science 365, 1177–1180 (2019).
Prahl, M. et al. Exposure to pesticides in utero impacts the fetal immune system and response to vaccination in infancy. Nat. Commun. 12, 132 (2021).
Tooker, J. F. & Pearsons, K. A. Newer characters, same story: neonicotinoid insecticides disrupt food webs through direct and indirect effects. Curr. Opin. Insect Sci. 46, 50–56 (2021).
Santana, M. S., Sandrini-Neto, L., Domenico, M. D. & Prodocimo, M. M. Pesticide effects on fish cholinesterase variability and mean activity: a meta-analytic review. Sci. Total Environ. 757, 143829 (2021).
Siviter, H., Koricheva, J., Brown, M. J. F. & Leadbeater, E. Quantifying the impact of pesticides on learning and memory in bees. J. Appl. Ecol. 55, 2812–2821 (2018).
Posthuma, L., van Gils, J., Zijp, M. C., van de Meent, D. & de Zwart, D. Species sensitivity distributions for use in environmental protection, assessment, and management of aquatic ecosystems for 12386 chemicals. Environ. Toxicol. Chem. 38, 905–917 (2019).
Desneux, N., Decourtye, A. & Delpuech, J. M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106 (2007).
Cereser, C., Boget, S., Parvaz, P. & Revol, A. Thiram-induced cytotoxicity is accompanied by a rapid and drastic oxidation of reduced glutathione with consecutive lipid peroxidation and cell death. Toxicol 163, 153–162 (2001).
Wagner, N., Lotters, S., Veith, M. & Viertel, B. Effects of an environmentally relevant temporal application scheme of low herbicide concentrations on larvae of two anuran species. Chemosphere 135, 175–181 (2015).
Mateos-Naranjo, E. & Perez-Martin, A. Effects of sub-lethal glyphosate concentrations on growth and photosynthetic performance of non-target species Bolboschoenus maritimus. Chemosphere 93, 2631–2638 (2013).
Bertrand, L., Marino, D. J., Monferrán, M. V. & Amé, M. V. Can a low concentration of an organophosphate insecticide cause negative effects on an aquatic macrophyte? Exposure of Potamogeton pusillus at environmentally relevant chlorpyrifos concentrations. Environm. Exp. Bot. 138, 139–147 (2017).
Garanzini, D. S., Medici, S., Moreyra, L. D. & Menone, M. L. Acute exposure to a commercial formulation of Azoxystrobin alters antioxidant enzymes and elicit damage in the aquatic macrophyte Myriophyllum quitense. Physiol. Mol. Biol. Plants 25, 135–143 (2019).
Prado, R., Rioboo, C., Herrero, C. & Cid, Á. Characterization of cell response in Chlamydomonas moewusii cultures exposed to the herbicide paraquat: Induction of chlorosis. Aquat. Toxicol. 102, 10–17 (2011).
Fatma, F., Verma, S., Kamal, A. & Srivastava, A. Monitoring of morphotoxic, cytotoxic and genotoxic potential of mancozeb using Allium assay. Chemosphere 195, 864–870 (2018).
Mahapatra, K., De, S., Banerjee, S. & Roy, S. Pesticide mediated oxidative stress induces genotoxicity and disrupts chromatin structure in fenugreek (Trigonella foenum - graecum L.) seedlings. J. Hazard. Mater. 369, 362–374 (2019).
Shang, A. H. et al. Physiological effects of tetracycline antibiotic pollutants on non-target aquatic Microcystis aeruginosa. J. Environ. Sci. Health B 50, 809–818 (2015).
Ziogas, B. N., Baldwin, B. C. & Young, J. E. Alternative respiration: a biochemical mechanism of resistance to Azoxystrobin (ICIA 5504) in Septoria tritici. Pest Manage. Sci. 50, 28–34 (1997).
Bernat, P. et al. 2,4-dichlorophenoxyacetic acid-induced oxidative stress: metabolome and membrane modifications in Umbelopsis isabellina, a herbicide degrader. PLoS ONE 13, e0199677 (2018).
Magnoli, K. et al. Effects of chlorpyrifos on growth and aflatoxin B1 production by Aspergillus section Flavi strains on maize-based medium and maize grains. Mycotoxin Res. 37, 51–61 (2021).
Daam, M. A. et al. Environmental risk assessment of pesticides in tropical terrestrial ecosystems: test procedures, current status and future perspectives. Ecotox. Environ. Saf. 181, 534–547 (2019).
Food and Agriculture Organization of the United Nations. Database Collection of the Food and Agriculture Organization of the United Nations, http://www.fao.org/faostat/en/#data.
Wan, N. F., Dainese, M., Wang, Y. Q. & Loreau, M. Cascading social-ecological benefits of biodiversity for agriculture. Curr. Biol. 34, R587–R603 (2024).
Milner, A. M. & Boyd, I. L. Toward pesticidovigilance. Science 357, 1232–1234 (2017).
Cedergreen, N. Quantifying synergy: a systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE 9, e96580 (2014).
Wan, N. F. et al. Global synthesis of effects of plant species diversity on trophic groups and interactions. Nat. Plants 6, 503–510 (2020).
Wan, N. F. et al. Plant genetic diversity affects multiple trophic levels and trophic interactions. Nat. Commun. 13, 7312 (2022).
Swart, E., Martell, E., Svendsen, C. & Spurgeon, D. J. Soil ecotoxicology needs robust biomarkers: a meta-analysis approach to test the robustness of gene expression-based biomarkers for measuring chemical exposure effects in soil invertebrates. Environ. Toxicol. Chem. 41, 2124–2138 (2022).
Wigger et al. Systematic consideration of parameter uncertainty and variability in probabilistic species sensitivity distributions. Integr. Environ. Asses. Manage. 16, 211–222 (2020).
Nakagawa, S. et al. A robust and readily implementable method for the meta-analysis of response ratios with and without missing standard deviations. Ecol. Lett. 26, 232–244 (2023).
Nakagawa, S. & Santos, E. S. Methodological issues and advances in biological meta-analysis. Evol. Ecol. 26, 1253–1274 (2012).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria (2020).
Nakagawa, S., Yang, Y., Macartney, E. L., Spake, R. & Lagisz, M. Quantitative evidence synthesis: a practical guide on meta-analysis, meta-regression, and publication bias tests for environmental sciences. Environ. Evid. 12, 8 (2023).
Michonneau, F., Brown, J. W. & Winter, D. J. rotl: an R package to interact with the Open Tree of Life data. Methods Ecol. Evol. 7, 1476–1481 (2016).
Hinchliff, C. E. et al. Synthesis of phylogeny and taxonomy into a comprehensive tree of life. Proc. Nat. Acad. Sci. USA 112, 12764–12769 (2015).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Brit. Med. J. 315, 629–634 (1997).
Duval, S. & Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463 (2000).
Rosenberg, M. S. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 59, 464–468 (2005).
Pinheiro, J. C. & Bates, D. M. Mixed-effects models in S and S-PLUS. (Springer, 2000).
Fox, J. & Weisberg, S. Visualizing fit and lack of fit in complex regression models: effect plots with partial residuals. J. Stat. Softw. 87, 1–27 (2018).
Wan, N. F. Data and code for “Pesticides have negative effects on non-target organisms”. Zenodo https://doi.org/10.5281/zenodo.14683219 (2025).
Acknowledgements
We thank all researchers whose data and work have been included in this meta-analysis. We thank Professor Shinichi Nakagawa for providing us with constructive suggestions. N.F.W. was supported by the Shanghai Agriculture Applied Technology Development Programme, China (Grant No. 2023-02-08-00−12-F04586), Natural Science Foundation of Shanghai (22ZR1417200), National Natural Science Foundation of China (32172484), Fundamental Research Funds for the Central Universities (JKY01231718), Shanghai Science and Technology Innovation Action Plan from Shanghai Municipal Science and Technology Commission of China (22015821000) and National Ten Thousand Plan-Young Top Talents of China. L.F. was supported by the National Natural Science Foundation of China (82204063), Y.Q.H. was supported by the National Key R&D Programme of China (2023YFF1205101). D.J.S. and B.A.W. were supported by Natural Environment Research Council of UK (NE/S00135/1, NE/W005050/1, NE/S000100/1, NE/V007548/1), and C.S. was supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement 727284, the European Union’s Horizon Europe programme under grant agreement 101081964 and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy (EXC 2070-390732324).
Author information
Authors and Affiliations
Contributions
N.F.W. conceived the idea. N.F.W. and L.F. collected and analysed the data and drafted the article. C.S. contributed to statistical analyses. S.S. contributed to phylogenetic analysis. N.F.W., L.F., M.D., L.P.K., Y.Q.H., F.X., D.G., B.A.W., A.J.V., D.J.S., S.S. and C.S wrote the manuscript. All authors prepared and edited the final drafts.
Corresponding author
Ethics declarations
Competing interests
B.A.W. has been on projects as a researcher which have received funding from Syngenta and Bayer, including those looking at the role of pollinators in oilseed rape yield and investigating the impacts of Neonicotinoid insecticides on bees - this includes project funding as well as covering of expenses and travel to meetings. As part of this work, B.A.W. has worked with Eurofins, who undertake regulatory assessments. B.A.W. has also received funding from the UK Health and Safety Executive to look at pollinator pesticide regulatory assessments. C.S. has received funding from Syngenta for a project on enhancing biodiversity in farmland (www.naturpositiv.de). All other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Heinz Köhler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wan, NF., Fu, L., Dainese, M. et al. Pesticides have negative effects on non-target organisms. Nat Commun 16, 1360 (2025). https://doi.org/10.1038/s41467-025-56732-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56732-x
This article is cited by
-
Isolation and production of newly oligosporogenic polyvalent Bacillus thuringiensis mutants for agricultural pest control
BMC Microbiology (2026)
-
MIND: a proposed strategic and collaborative platform for advancing chemical environmental risk assessment
Environmental Sciences Europe (2026)
-
The engineered Immunoprotein enhance broad-spectrum disease resistance in solanaceous crops
BMC Plant Biology (2026)
-
Behavioral and lethal effects of yeast based bioformulations on Bactrocera dorsalis
Scientific Reports (2026)
-
Exploring gene expression as a sublethal endpoint in gammarids exposed to pesticides: insights from next-generation sequencing
Scientific Reports (2026)