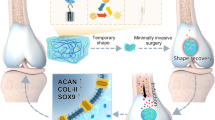
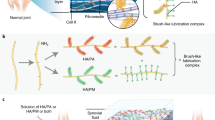
Abstract
Rheumatoid arthritis (RA) is a common chronic autoimmune condition accompanied by lubrication dysfunction, inflammatory infiltration, and cartilage wear. Long-term improvements in joint lubrication, inflammation elimination, and worn cartilage repair are crucial for effective RA treatment. Herein, we present an injectable bioadhesive and lubricating hydrogel containing a dopamine-modified hyaluronic acid (DA-HA) network, sulfonated hyaluronic acid (SO3−-HA) network, and kartogenin (KGN)-grafted dopamine-hybridized graphene quantum dot-supported Cu single-atom nanozyme (DAGQD@Cu@KGN SAN) designed to restore cartilage lubrication and repair worn cartilage in RA. DA within the hydrogel networks provides bioadhesion, allowing it to persist in the joint cavity for extended periods. The hydrogel with SO3− group offer lubricity, reducing friction coefficient and alleviating cartilage wear. The DAGQD@Cu@KGN SAN exhibits excellent superoxide dismutase, catalase, and •OH scavenging activities, effectively inhibiting inflammation. KGN is sustainably released from the hydrogel, recruiting bone marrow mesenchymal stem cells to repair damaged cartilage by promoting their differentiation into chondrocytes. In vivo experimental results demonstrate that this injectable bioadhesive and lubricating hydrogel not only prevents cartilage wear and tear, providing long-term anti-oxidation and anti-inflammatory effects in early RA, but also repaired damaged cartilage in late-stage RA. This bio-adhesive and lubricating hydrogel presents a potential full-cycle strategy for RA therapy.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) stands as a prevalent chronic autoimmune disease affecting 1% of the global population, imposing substantial socioeconomic burdens1,2. Synovial joints in early stage of RA exhibit heightened levels of reactive oxygen species (ROS) and inflammatory cytokines3, triggering a cascade that activates metalloproteinases, hampers cartilage proteoglycan synthesis, promotes chondrocyte apoptosis, and culminates in cartilage wearing. Late-stage of RA is characterized by cartilage and bone tissue degradation, ultimately lead to joint destruction, joint deformity and dysfunction4,5. Moreover, RA correlates with a diminished lubricant presence, heightening friction between joints and perpetuating irreversible cartilage damage6. Present treatments involve intra-articular injections of anti-inflammatory drugs (such as dexamethasone, corticosteroids, and methotrexate)7, along with cartilage lubricants (hyaluronic acid, aggrecans, and phospholipids)8. However, these treatments pose challenges. First, anti-inflammatory drugs injected into the joint cavity easily cleared through the synovial capillaries and lymphatic drainage, leading to diminished drug utilization efficiency. Although repeated administrations enhance anti-inflammatory effectiveness, they heighten the risk of joint infections and systemic side effects. Second, supplemental lubricants lack targeted efficacy and provide limited lubrication due to their brief residence time on joint surfaces. Therefore, the imperative lies in conferring tissue adhesion to lubricants while endowing them with local, sustained, efficient antioxidant, and anti-inflammatory properties for the effective treatment of RA.
Injectable hydrogels, comprising hydrophilic polymer networks characterized by soft elasticity, low friction coefficients, biocompatibility, and drug delivery capabilities, stand as promising candidates for RA treatment9,10. Currently, hydrogels used in RA therapy fall into two primary categories. The first category involves bioadhesive hydrogels that target the pathological microenvironment of RA, addressing joint inflammation through drug release11. However, they often overlook long-term joint friction resulting from reduced lubrication, leading to irreversible cartilage damage. The second category includes lubricating hydrogels primarily addressing joint friction and wear caused by reduced joint lubrication12,13. Although considerable efforts have been dedicated to developing injectable hydrogels mimicking synovial fluid components to enhance lubrication, they often fail to mitigate RA-induced inflammatory joint microenvironments14,15. These hydrogels lack tissue adhesion and robust mechanical properties, hindering their prolonged retention in joint cavities16. Frequent joint injections may heighten infection risk and cause discomfort. Therefore, developing an innovative strategy that enhances both hydrogel lubrication and bioadhesion, while enabling local drug delivery. Such an approach holds significant promise in reducing articular cartilage wear and alleviating inflammatory symptoms in RA.
Graphene quantum dot (GQD) nanozymes have found wide application in inflammatory disease therapy due to their exceptional water dispersibility, ultrasmall size, biocompatibility, and intrinsic enzyme-like activities17,18,19,20. Moreover, these nanozymes exhibit stable and broad-spectrum ROS scavenging activities, overcoming drawbacks associated with conventional antioxidants or natural enzymes, such as poor stability, high cost, and limited ROS scavenging capacity21,22. However, the current limitations in ROS scavenging hinder the application of GQD in RA treatment. Recently, single atom nanozyme (SAN) with atomically dispersed metal centers emerged, maximizing intrinsic enzyme-like activities23. Our recent research identified polyphenol chemistry as a potent approach to synthesize SAN on a GQD template. Catechols on GQD act as surface anchoring sites, effectively confining isolated metal atoms via strong chelation effects24. Moreover, these polyphenol-mediated SANs, with a high surface area, serve as excellent carriers for loading small molecule drugs, enhancing the hydrogel with robust mechanical, bioadhesive, and injectable properties25. Therefore, combining SAN into a bioadhesive and lubricating hydrogel emerges a potential strategy to modulate the inflammation microenvironment in RA therapy.
In this work, we develop an injectable bioadhesive and lubricating hydrogel incorporating kartogenin (KGN)-grafted dopamine-hybridized graphene quantum dots (DAGQD)-supported Cu SAN (DAGQD@Cu SAN) as a targeted modulation niche to restore cartilage lubrication and repair cartilage in RA. The injectable hydrogel comprises a bioadhesive dopamine-modified hyaluronic acid (DA-HA) network and a lubricating sulfonated hyaluronic acid (SO3−-HA) network crosslinked with DAGQD@Cu SAN. The injectable hydrogel can be gelatinized in situ in the joint cavity using the DAGQD@Cu SAN, which also exhibits robust bioadhesion and lubrication properties. The DAGQD@Cu SAN also displays excellent superoxide dismutase (SOD), catalase (CAT), and •OH scavenging capabilities. The findings show that the hydrogel modulates the local immune microenvironment by inhibiting oxidative stress in the joint cavity. The long-term adhesion of the hydrogel offers local lubrication, effectively mitigating cartilage wear induced by RA. Furthermore, the sustained release of KGN facilitates the repair of damaged articular cartilage.
Results
Design strategy
The DAGQD@Cu SAN was synthesized using a DAGQD template. Subsequently, KGN, a small molecule that induces mesenchymal stem cell differentiation into chondrocytes, was grafted onto the surface of the DAGQD@Cu SAN (DAGQD@Cu@KGN SAN) using the amide reaction (Fig. 1A). In this process, catechols of DA act as anchoring sites that enhance the interaction between DAGQD and Cu2+, allowing Cu single atoms to uniformly disperse on the surface of DAGQD. Moreover, catechols in DA can improve the ROS-scavenging ability of SAN.
A Synthesis of DAGQD@Cu SAN and DAGQD@Cu@KGN SAN. B Synthesis of DAGQD@Cu@KGN-SO3−/DA-HA hydrogel. C DAGQD@Cu@KGN-SO3−/DA-HA hydrogel for RA therapy. (I) Hydrogel self-cures in situ within the joint cavity; (II, III) DAGQD@Cu@KGN-SO3−/DA-HA hydrogel displays lubrication and adhesion properties; (IV) Regulation of inflammatory microenvironment facilitated by SAN with remarkable ROS-scavenging capabilities; (V) KGN released from the hydrogel promotes the recruitment of BMSCs and induces their differentiation into chondrocytes, facilitating the repair of cartilage damaged by RA.
The sol to gel transition of the injectable hydrogel was triggered by DAGQD@Cu SAN, acting as the crosslinking points via metal coordination between -SH in SO3−-HA and catechol in DA-HA networks, along with Cu2+ in DAGQD@Cu SAN (Fig. 1B). This unique property allowed the hydrogel to be injected into the joint cavity and undergo self-curing in situ (Fig. 1CI). Simultaneously, bioadhesion and lubrication were achieved through the enhanced complementary hydrogel network enabled by DAGQD@Cu SAN. This network integrated the adhesive properties of the DA-HA network for cartilage surface attachment with the wear reduction and lubrication enhancement capabilities of the SO3−-HA network (Fig. 1CII and III). Consequently, this mechanism helped mitigate cartilage wear caused by elevated friction coefficients in RA models.
The bioadhesiveness of the hydrogel originates from DAGQD@Cu SAN and DA-HA, drawing inspiration from mussel adhesion mechanisms. Meanwhile, the lubricity of the SAN-SO3−/DA-HA hydrogel stems from SO3−-HA, inspired by the composition of synovial fluid. Synovial fluid within joints comprises HA, lubricating proteins, lipids, globulin, albumin, and glycosaminoglycans. Among these components, HA, lubricating proteins, and lipids amalgamate into lubricating complexes that form a stable hydration layer, termed the lubrication layer, on cartilage surface, effectively preventing cartilage wear. Furthermore, the GAGQD@Cu SAN embedded in the hydrogel mitigated the inflammatory microenvironment within the RA-affected joint cavity due to its outstanding ROS-scavenging activity (Fig. 1CIV). Moreover, the release of KGN from the hydrogel facilitated the recruitment of endogenous host bone marrow mesenchymal stromal cells (BMSCs), promoting homing and inducing BMSCs differentiation into chondrocytes for the repair of cartilage damaged by RA (Fig. 1CV).
Characterization of SAN
The DAGQD appeared as monodisperse black dots measuring ~5 nm in size. Analysis revealed a lattice spacing of 0.21 nm, corresponding to the (001) crystal plane of graphite carbon (Supplementary Fig. 1). Upon integration with Cu, the DAGQD@Cu SAN exhibited a monodisperse spherical shape with a size ranging ~5–10 nm (Fig. 2A). The slightly increased size could be attributed to Cu chelation with catechol on the DAGQD. HRTEM imaging indicated a lattice spacing of ~0.25 nm, aligning with the (100) crystal plane of graphite carbon (Supplementary Fig. 2A). This suggested that the introduction of a small amount of Cu minimally affected the structure of the DAGQD. Comparatively, the size of DAGQD@Cu@KGN SAN remained largely unchanged from DAGQD@Cu SAN (Fig. 2B), maintaining a lattice spacing of ~0.25 nm (Supplementary Fig. 2B). KGN grafting did not alter the size or structure of the DAGQD@Cu SAN. Notably, HAADF-STEM imaging depicted atomically dispersed Cu single atoms within the DAGQD@Cu SAN (Fig. 2C). Moreover, XRD analysis did not reveal significant diffraction peaks of crystalline Cu NPs, further corroborating the presence of single Cu atoms (Supplementary Fig. 3).
TEM images of (A) DAGQD@Cu SAN and (B) DAGQD@Cu@KGN SAN. C High-Angle Annular Dark-Field Scanning Transmission Electron Microscopy (HAADF-STEM) image of DAGQD@Cu SAN. D The UV-Vis spectrum of DAGQD, DAGQD@Cu SAN, and DAGQD@Cu@KGN SAN. E The C1s spectrum and (F) Cu2p spectrum of DAGQD@Cu SAN. G Hydroxyl radical scavenging ability, (H) SOD activity, and (I) CAT activity of GQD, DAGQD, DAGQD+Cu2+, DAGQD@Cu SAN, and DAGQD@Cu@KGN SAN (n = 3 samples). J The mechanism of ROS elimination of DAGQD@Cu SAN. (Data are presented as the mean ± SD. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test. *, **, and **** indicate significance at p < 0. 05, p < 0.01, and p < 0.0001, respectively).
The UV–Vis absorption spectrum of DAGQD displayed a shoulder peak at 280 nm and a notably robust characteristic absorption peak at 315 nm. This peak was attributed to the π-π* electronic transition of conjugated C = C units within the carbon core and n-π* transition of the C = O bond, respectively26. The absorbance of DAGQD@Cu SAN at 280 nm and 315 nm proved lower than that of DAGQD, illustrating efficient chelating interactions between Cu(II) and surface functional groups such as catechol and carboxylic groups on the DAGQD27. Moreover, the absorbance of DAGQD@Cu@KGN SAN at 280 nm surpassed that of DAGQD@Cu SAN, attributed to the distinctive absorption peak of KGN (Fig. 2D).
X-ray photoelectron spectroscopy (XPS) results showed characteristic peaks at 285 and 288 eV, corresponding to the C-O and C = O bonds representing the catechol and quinone groups in the DAGQD@Cu SAN, respectively (Fig. 2E, Supplementary Fig. 4, 5). The Cu2p spectrum of DAGQD@Cu SAN exhibited multiple peaks, with the peaks at 932.2 and 952.0 eV were attributed to Cu0/Cu(I)2p. Furthermore, two peaks at 933.9 and 953.5, together with satellite peaks around 940.8, 943.9, and 962.2 eV were indicative of Cu(II)2p (Fig. 2F). Notably, DAGQD@Cu@KGN SAN displayed the same copper valence state, indicating that the structure of DAGQD@Cu remained unchanged after the introduction of KGN (Supplementary Fig. 6). The grafting degree of KGN in DAGQD@Cu SAN was 58.6%, and the load rate of KGN in DAGQD@Cu SAN was 1.1%. These findings confirmed the successful introduction of KGN into the DAGQD@Cu SAN.
ROS-scavenging activities of SAN
The DAGQD@Cu@KGN SAN exhibited high enzyme-like activity in scavenging ROS (Fig. 2G–I). DAGQD displayed higher SOD and CAT activities along with enhanced clearance rates for •O2− and •OH than that of GQD, owing to the present of catechol groups in DAGQD. To establish the enhanced ROS-scavenging potential of DAGQD@Cu SAN with multivalent copper over DAGQD with single-valent copper, we combined DAGQD and CuCl2 solutions, recorded as DAGQD+Cu2+. Results showed that DAGQD@Cu SAN displayed superior ROS-scavenging and CAT activities compared with DAGQD and DAGQD+Cu2+. The exceptional catalytic activity can be ascribed to the variable valence of copper single atoms and the abundant catechol structure. Furthermore, no significant difference was observed in ROS scavenging and CAT activities between DAGQD@Cu SAN and DAGQD@Cu@KGN SAN, suggesting that the introduction of KGN did not disrupt the structure of DAGQD@Cu SAN. In addition, DAGQD@Cu SAN has excellent stability (Supplementary Fig. 7).
The high catalytic activity of the DAGQD@Cu SAN was attributed to a dual catalytic mechanism. First, single Cu atoms exhibit SOD- and CAT-like activities, catalyzing •O2−, •OH, and H2O2 to produce nontoxic water and O2. Second, electrons were transferred from the single Cu atom to the quinone groups of DA, forming a catechol-quinone redox pair, endowing the system with sufficient catechol groups for ROS elimination (Fig. 2J).
SAN-induced hydrogel gelation
DAGQD@Cu@KGN SAN rapidly initiated the gelation of the SO3−/DA-HA hydrogel due to the metal coordination bond between -SH and Cu (Figs. 3A, Supplementary Fig. 8, 9). Mixing DAGQD@Cu@KGN SAN and SO3−/DA-HA precursor solutions and continuously injecting them into a culture dish using a 1 mL syringe and 0.5 mm needle, filamentous strips can be observed suspending in the air and the formation of the two letters “HY” in the culture dish (Fig. 3B). Rheological measurements indicated that the gelation time of the hydrogel could be adjusted by changing the SAN concentration (Supplementary Fig. 10). The gelation time decreased with increasing SAN content, reaching 21 s at SAN concentration of 1 mg mL−1 (Fig. 3C). Moreover, the hydrogel was gelatinized in situ within the defective cartilage area (Fig. 3D).
A Photographs depicting the self-curing process of SO3−/DA-HA hydrogel precursor initiated by DAGQD@Cu@KGN SAN. B The injectable property of DAGQD@Cu@KGN-SO3−/DA-HA hydrogel. C Rheological properties of DAGQD@Cu@KGN-SO3−/DA-HA hydrogel. D Images illustrating the self-curing process of DAGQD@Cu@KGN-SO3−/DA-HA hydrogel in the cartilage defect of a rabbit knee joint. E Hydrogel self-healing ability. F Schematic depiction of the self-healing mechanism. G Coefficient (COF) comparison between Cu2+-SH-HA hydrogel, SAN-SH-HA hydrogel, and SAN-SO3−-HA hydrogel (n = 3 samples). H COF variation of SAN-SO3−/DA-HA hydrogel with different ratios of SO3−-HA and DA-HA (n = 3 samples). I Adhesion strength of SAN-SO3−/DA-HA hydrogel with different ratios of SO3−-HA and DA-HA on cartilage samples (n = 3 samples). J Adhesion strength of the hydrogel with different concentrations of SAN on cartilage samples (n = 3 samples). K Anatomic picture of hydrogel adhesion in joint. L The FESEM and (M) metallographic microscopy image of hydrogel-cartilage cross-section (n = 3 independent experiments). N Illustration of the bioadhesion mechanism of DAGQD@Cu@KGN SAN hydrogel on cartilage tissue, involving (1) hydrogen bonds, (2) cation-π interaction, and (3) covalent linking. O Compressive strength and (P) compression modulus analysis of the hydrogel with varying concentrations of DAGQD@Cu@KGN SAN (n = 3 samples). (Data are presented as the mean ± SD. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test, with a value of *P < 0.05, **P < 0.01, and ****P < 0.001).
The DAGQD@Cu@KGN-SO3−/DA-HA hydrogel also exhibited self-healing effects (Fig. 3E). The hydrogels were dyed blue and red, then combined. The two hydrogels fused into one after incubation at 37 °C for 2 min. The red hydrogel did not fall down when lift the blue one with tweezer. Placing the four hydrogels in order and incubating them at 37 °C for 2 min resulted in a round, well-fused structure. These results indicate the favorable self-healing properties of the hydrogel. The self-healing properties of the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel were analyzed using rheological measurements. The G’ of the hydrogel was significantly higher than the G” under low strain, indicating hydrogel formation. With increases strain, the hydrogel transitioned to a fluid state, with a critical strain value of ~550% (Supplementary Fig. 11A). Dynamic step strain measurement revealed that the hydrogel behaved colloidal (G’ > G”) at a low shear rate 10%. However, under a strain of 600%, the hydrogel network was swiftly disrupted (G’ < G”). Upon cessation of the high shear rate, the G’ and G” of the hydrogel recovered to 100% within seconds (Supplementary Fig. 11B). The robust self-healing ability of the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel stemmed from the dynamic and reversible bonds of-SH/DA and Cu2+ in SAN (Fig. 3F). Under shear force, the metal coordination bond broke, rendering the hydrogel flowable. Upon removal of the shear force, the metal coordination bond restored, reverting the hydrogel to a solid state. The self-healing performance of hydrogels proves essential for frequently manipulated joints.
In vitro lubrication and adhesion of the hydrogels
The hydrogel exhibited commendable lubricity due to its SO3−-HA network. Friction coefficients (COF, frictional load was at 1 N, the setting amplitude at 5 mm, sliding frequency at 1 Hz) of the Cu2+-SH-HA, DAGQD@Cu@KGN-SH-HA, and DAGQD@Cu@KGN-SO3−-HA hydrogels were measured (Fig. 3G). Results showed COF values of 0.157 for Cu2+-SH-HA hydrogels, 0.154 for DAGQD@Cu@KGN-SH-HA, and 0.028 for DAGQD@Cu@KGN-SO3−-HA hydrogels. The COF of DAGQD@Cu@KGN-SO3−-HA was significantly lower than that of the other non-sulfate modified hydrogels. Furthermore, the SO3−-HA solution also demonstrated a lower COF in comparison to PBS and SH-HA solution (Supplementary Fig. 12). This suggests that introducing -SO3− significantly enhances hydrogel lubricity.
The hydrogel displayed both lubrication and bioadhesion after the incorporation of DA-HA. Investigating hydrogel lubrication and bioadhesion involved varying the ratio of SO3−-HA and DA-HA. Results revealed an inverse correlation with an increase in the DA-HA ratio. The hydrogel lubrication performance decreased as the proportion of DA-HA increased (Fig. 3H). Conversely, the adhesion of the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel significantly improved with an increased ratio of DA-HA to SO3−-HA, and the adhesion strength was optimal when SO3−-HA: DA-HA = 1:1 (Fig. 3I, Supplementary Fig. 13). However, at a 1:1 ratio of SO3−-HA: DA-HA, the precursor solution displayed extremely poor flowability, rendering it to be injected through the needle (Supplementary Fig. 14). Therefore, the optimal ratio for preparing bioadhesive and lubricating hydrogels appears to be SO3−-HA: DA-HA = 3:1. The adhesion strength (Figs. 3J, Supplementary Fig. 15) of the hydrogel increased with raised SAN content from 0.2 to 1.0 mg mL−1. However, hydrogel adhesion strength decreased when the SAN concentration was further increased to 2.0 mg mL−1. Thus, hydrogels with optimal adhesion properties were obtained at a SAN concentration of 1.0 mg mL−1.
The hydrogel can well adhere to the surface of the rat’s joints (Fig. 3K). A precursor solution of hydrogel with blue dye was injected into the joint cavity of healthy rats, which were dissected the rats five minutes later. Anatomical photographs showed that the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel and Cu2+-SO3−-HA hydrogel spread across the cartilage surface. After cartilage with DAGQD@Cu@KGN-SO3−-/DA-HA hydrogel was immersed in PBS for 5 min, the hydrogel’s blue color and original appearance remained unchanged. In contrast, the blue color faded, and the outline of the hydrogel disappeared for cartilage with Cu2+-SO3−-HA hydrogel under the same conditions. This indicates that DAGQD@Cu@KGN-SO3−-/DA-HA hydrogel adheres tightly to the cartilage surface, which can be attributed to the abundant catechol structures provided by DA-HA and DAGQD@Cu@KGN SAN. The interface between the DAGQD@Cu@KGN-SO3−-/DA-HA hydrogel and the articular cartilage surface was further observed by a microscope (Fig. 3L, M). The results indicated that there was no gap between the hydrogel and the cartilage surface, demonstrating that the hydrogel adhered well to the cartilage.
The lubrication and adhesion mechanisms of the hydrogels can be attributed to specific factors. The inclusion of SO3−-HA in the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel mimics the natural brush-like lubricating composite nanofibers. SO3− inherently reduces friction by featuring a sulfonate group (akin to lubricin), naturally binding water to hydrate surfaces6. The hydrogel bioadhesiveness stems from DA, drawing inspiration from mussel adhesion (Fig. 3N). The mussels exhibit strong adhesion to diverse substrates in seawater through their secreted mussel foot proteins (Mfps), which are rich in catechol groups that effectively bind to the substrate surfaces28,29. However, catechol groups are prone to oxidation into quinone groups, resulting in the loss of adhesion. To overcome this problem, an antioxidant protein containing cysteine thiol is secreted to reduce the quinone back to the catechol, facilitating long-term adhesion. In essence, the redox reaction between catechol and quinone is an electron transfer mechanism. In this study, DAGQD@Cu@KGN-SO3−/DA-HA hydrogel mimicked the complex adhesion mechanism of mussels. Electrons were transferred from the Cu SAN to the quinone group in DAGQD@Cu SAN, preserving the redox balance between quinone and catechol groups within the hydrogel network. Consequently, the hydrogel retains sufficient catechol groups that interact with the tissue through hydrogen bonds, cation-π interactions, and covalent links, ensuring lasting adhesion.
The mechanical properties of the hydrogels
The mechanical properties of the hydrogel were enhanced by the nanoenhancement effect of the DAGQD@Cu@KGN SAN. The compressive strength (Fig. 3O, P), and anti-swelling properties (Supplementary Fig. 16) of the hydrogel increased with raised SAN content from 0.2 to 1.0 mg mL−1. The increase indicated that elevated SAN content can endow hydrogel with excellent mechanical properties and improved anti-swelling rate by augmenting crosslinking density. However, hydrogel compressive strength, and anti-swelling properties decreased when the SAN concentration was further increased to 2.0 mg mL−1. This declined may be attributed to excess SAN agglomerated within the hydrogel. Thus, hydrogels with optimal compressive performance and anti-swelling properties were obtained at a SAN concentration of 1.0 mg mL−1. SEM images and EDS analysis confirmed that morphology of SAN-SO3−/DA-HA hydrogel consisted of a porous network structure, with well-dispersed SAN and absence of agglomeration (Supplementary Fig. 17). The degradation rate of DAGQD@Cu@KGN-SO3−/DA-HA hydrogel after subcutaneous storage for 28 days was only 7.3%, indicating that the hydrogel had robust stability in vivo (Supplementary Fig. 18). Moreover, sustained release of KGN from the hydrogel network was observed (Supplementary Figs. 19, 20).
In vitro CAT-like activity
DAGQD@Cu-SO3−/DA-HA hydrogel exhibited high CAT-like activity. The CAT-like activity of hydrogels was demonstrated by measuring intracellular oxygen using tris (4,7-diphenyl-1,10-phenanthroline) ruthenium (II) chloride complex ([Ru(dpp)3]Cl2, RDPP). The results demonstrated a decrease in fluorescence intensity following hydrogel treatment (Fig. 4A, B). The fluorescence intensity, ranked from highest to lowest, was as follows: Cu2+-SO3−-HA hydrogel (72.8%), Cu2+-SO3−/DA-HA hydrogel (69.8%), and DAGQD@Cu-SO3−/DA-HA hydrogel (24.2%). The DAGQD@Cu-SO3−/DA-HA hydrogel showed superior CAT-like activity compared with the Cu2+-SO3−/DA-HA hydrogel, due to the efficient CAT-like activity of the DAGQD@Cu SAN. Relative to H2O2 group (85.1%), the fluorescence intensity of the Cu2+-SO3−-HA hydrogel and Cu2+-SO3−/DA-HA hydrogel groups was slightly reduced, which may be attributed to the intrinsic CAT-like activity of Cu2+. Additionally, no significant difference in CAT-like activity was observed between the DAGQD@Cu-SO3−/DA-HA (24.2%) and DAGQD@Cu@KGN-SO3−/DA-HA (24.5%) hydrogel groups, indicating that the KGN had minimal impact on the CAT-like ability of DAGQD@Cu SAN.
A Intracellular oxygen level of RAW264.7 cells subjected to different treatments was tested using RDPP (n = 3 independent experiments). Scale bar = 100 μm (B) Flow cytometry analysis of RDPP-labeled cells for different groups. C Intracellular ROS staining images of RAW264.7 cells subjected to different treatments (n = 3 independent experiments). Scale bar = 100 μm. D Flow cytometry analysis of ROS-labeled cells for different groups. E–G ELIZA results showed extracellular expression of (E) TNF-α, (F) IL−1β, and (G) IL−10 (n = 3 samples). H BMSCs proliferation on various hydrogels was evaluated using CCK8 after 3 or 7 days of cultivation (n = 3 samples). I Spreading morphology of BMSCs on hydrogel after being cultured for 12 h (n = 3 independent experiments). Scale bar = 200 μm. J–M The expression of cartilage-related markers Sox9, aggregatan (Acan), type II collagen (Col II), and collagen type X (Col X) (n = 3 samples). (Data are presented as the mean ± SD. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test, with a value of *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.001).
In vitro ROS scavenging activity
The DAGQD@Cu@KGN SAN endowed the hydrogel with a high ROS scavenging capacity, not only due to the abundance of catechol groups on the hydrogel but also because of the single-atom Cu, which exhibited multiple ROS scavenging and enzyme catalytic activities. Lipopolysaccharide (LPS)-induced macrophages (LPS group) displayed the highest intense fluorescence (70.46%) (Fig. 4C, D). Following hydrogel treatment, the fluorescence intensity decreased. The fluorescence intensity ranked from highest to lowest was Cu2+-SO3−-HA hydrogel (64.07%), Cu2+-SO3−/DA-HA hydrogel (50.54%), and DAGQD@Cu-SO3−/DA-HA hydrogel (38.18%). The slight ROS scavenging capacity of the Cu2+-SO3−-HA hydrogel stemmed from the exposure of a small number of sulfhydryl groups on hydrogel surface. The Cu2+-SO3−/DA-HA hydrogel exhibited better ROS clearance ability than the Cu2+-SO3−-HA hydrogel due to the abundance of catechol groups within it. The DAGQD@Cu-SO3−/DA-HA hydrogel showed an excellent ROS-scavenging effect compared with the Cu2+-SO3−/DA-HA hydrogel, attributed to the efficient SOD and •OH scavenging activities of the DAGQD@Cu SAN. Additionally, nearly no difference was observed in ROS clearance between the DAGQD@Cu-SO3−/DA-HA (38.18%) and DAGQD@Cu@KGN-SO3−/DA-HA (34.30%) hydrogel groups. This indicates that the KGN had minimal impact on the ROS scavenging ability of DAGQD@Cu SAN. In addition, The DAGQD@Cu@KGN-SO3−/DA-HA hydrogel has enhanced cell uptake efficiency attributed to the catechol groups on the surfaces of SAN (Supplementary Fig. 21) and it is non-cytotoxicity even with an increase in SAN concentration from 0.4 to 2.0 mg mL−1 (Supplementary Fig. 22).
In vitro anti-inflammatory activity
The DAGQD@Cu-SO3−/DA-HA hydrogel exhibited excellent anti-inflammatory effects by suppressing M1 macrophage polarization and promoting M2 macrophage activation. The relative expression level of the M1 macrophage-related gene tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) was evaluated (Fig. 4E, F). The strength of inhibition of TNF-α and IL-6 ranked from high to low was DAGQD@Cu-SO3−/DA-HA hydrogel, Cu2+-SO3−/DA-HA hydrogel, and Cu2+-SO3−-HA hydrogel. The DAGQD@Cu-SO3−/DA-HA hydrogel displayed the highest anti-inflammatory activity, attributed to the collaborative ROS clearance capabilities of the DAGQD@Cu, DA-HA, and SO3−-HA networks. Additionally, the relative expression level of the M2 macrophage-related gene interleukin-10 (IL-10) was evaluated. The results indicated that the DAGQD@Cu-SO3−/DA-HA hydrogel group significantly upregulated IL-10 expression (Fig. 4G), suggesting that the DAGQD@Cu-SO3−/DA-HA hydrogel exerted excellent anti-inflammatory effects by suppressing M1 macrophage polarization and promoting M2 macrophage activation.
In vitro cell proliferation, adhesion and differentiation
The DAGQD@Cu-SO3−/DA-HA hydrogel exhibited favorable properties for BMSCs proliferation, adhesion, and differentiation. Groups 2 (Cu2+-SO3−/DA-HA hydrogel), 3 (DAGQD@Cu-SO3−/DA-HA hydrogel), and 4 (DAGQD@Cu@KGN-SO3−/DA-HA hydrogel groups) demonstrated exceptional proliferation after 3 days of incubation, surpassing that of Group 1 (Cu2+-SO3−-HA hydrogel) (Fig. 4H). This enhanced proliferation was attributed to the excellent cell affinity of catechol in the DA-HA network. After 7 days, the cell numbers in Groups 3 and 4 were significantly higher than those in Group 1, potentially due to the proliferation-promoting effects of GQD and Cu in the DAGQD@Cu SAN30,31.
BMSCs on the surface of Group 1 displayed a circular morphology (Fig. 4I). In Group 2, cell tentacles were observed, indicating improved cell affinity with DA. BMSCs exhibited well-spread morphology on the surfaces of Groups 3 and 4, with larger adhesive spots than on the Cu2+-SO3−/DA-HA and Cu2+-SO3−-HA hydrogels. The spread skeleton and large adhesive spots were attributed to the cell affinity and adhesion of the catechol groups on the surfaces of the DA-HA and DAGQD@Cu SAN. These findings highlight the favorable biocompatibility, cell adhesion, and proliferation activities of DAGQD@Cu-SO3−/DA-HA hydrogels. In addition, DAGQD@Cu@KGN-SO3−/DA-HA hydrogel showed efficient recruitment of BMSCs due to the cell recruitment effect of catechol and KGN on DAGQD@Cu@KGN SAN (Supplementary Fig. 23), suggesting that DAGQD@Cu@KGN-SO3−/DA-HA hydrogel can mobilize BMSCs to migrate into the cartilage defect site.
Furthermore, the expression of Sox9, aggrecan (Acan), type II collagen (Col II), and collagen type X (Col X) in Group 4 (DAGQD@Cu@KGN-SO3−/DA-HA hydrogel) was significantly higher than that in the other groups, illustrating the efficiency of KGN in promoting BMSCs differentiation into chondrocytes (Fig. 4J–M).
RNA sequencing analysis
The results of differential gene analysis showed that there were 785 differential genes between Group 2 and Group 1 (Fig. 5A), 1259 differential genes between Group 3 and Group 1 (Fig. 5B), and 365 genes were the same between the two groups of differential genes (Fig. 5C). Further, the differential genes between Group 3 and Group 1 (894 in total) were analyzed to further explore the mechanism of action of DAGQD@Cu-SO3−/DA-HA hydrogel. GO enrichment analysis found that these genes were enriched in oxidative stress response, autophagy, tissue repair, etc. (Fig. 5D), indicating that the effect of DAGQD@Cu-SO3−/DA-HA hydrogel was significantly correlated with these aspects. GSEA analysis results showed that DAGQD@Cu-SO3−/DA-HA hydrogel can promote the FOXO pathway, inhibit lysosomes, osteoclast differentiation, and TNF pathways, among which the FOXO pathway is related to anti-oxidation, while lysosomes are related to autophagy, osteoclast differentiation is related to cartilage damage, and TNF pathway is related to inflammation (Fig. 5E). Genes related to anti-oxidation, autophagy, repair, and inflammation were selected for further analysis. It can be seen intuitively from the heat map that DAGQD@Cu-SO3−/DA-HA hydrogel has a good effect in promoting the expression of antioxidant-related genes (Sod1, Sod2, Cat, Foxo1, Foxo3, Foxo4), autophagy-related genes (Atg2, Atg3, Atg12), and repair-promoting genes (IL-4 and IL-10). At the same time, it inhibits the expression of pro-inflammatory factors (IL-1a, IL-1b, IL-6) (Fig. 5F). Therefore, DAGQD@Cu-SO3−/DA-HA hydrogel likely reduces the level of cellular oxidative stress and inhibits inflammation through antioxidant effects, while promoting tissue regeneration and removing harmful factors through upregulation of autophagy, ultimately aiding the repair of cartilage defects (Fig. 5G).
A The different changes of the expression of genes (Group 2 v.s. Group 1). B The different changes of the expression of genes (Group 3 v.s. Group 1) (C) Venn diagram of differentially expressed genes among the three groups. D GO enrichment of sole differentially expressed genes in Group 3. BP, biological processes. CC, cellular component. MF, molecular functions. E GSEA analysis of Foxo signaling pathway, Lysosome, osteoclast differentiation, TNF signaling pathway of DAGQD@Cu-SO3−/DA-HA hydrogel. F Heatmap analysis of differentially expressed genes involved in anti-oxidative stress, autophagy, repairment, inflammation. G Illustrating the potential mechanism by which DAGQD@Cu-SO3−/DA-HA hydrogel enhances cartilage repair and suppresses inflammation.
Hydrogel prevents cartilage wear and tear in RA rats
The cartilage was well protected by the bioadhesive and lubricating DAGQD@Cu@KGN-SO3−/DA-HA hydrogel in early RA within the CIA rat model. The CIA rat model was established through secondary immunization (Fig. 6A). Micro-CT 3D reconstruction results indicated an evident inflammatory reaction in the knee joint of the PBS group, coupled with subchondral bone destruction and hyperostosis (Fig. 6B, Supplementary Fig. 24). Joint injury was partially inhibited by SO3−/DA-HA solution treatment (Group 1), through significant subchondral bone damage persisted. The Cu2+-SO3−/DA-HA hydrogel group (Group 2) exhibited reduced subchondral bone damage compared with the PBS and SO3−/DA-HA solution groups (Group 1), suggesting the ability of the hydrogel to resist cartilage friction to some extent due to its long-term adhesion and lubrication. The destruction of the subchondral bone on the articular surface was effectively suppressed in the DAGQD@Cu-SO3−/DA-HA hydrogel group (Group 3). This outcome stems from the fact that the DAGQD@Cu-SO3−/DA-HA hydrogel not only exhibited local, long-term bioadhesion and lubrication but also exhibited exceptional antioxidant and anti-inflammatory effects for RA treatment. Importantly, no signs of cartilage collapse or damaged boundaries were observed in the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel group (Group 4). The joint surface of rats in Group 4 closely resembled that of healthy rats, illustrating the excellent bioadhesion, lubrication, and cartilage repair capabilities of the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel. Moreover, the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel does not affect the joint movement (Supplementary Fig. 25).
A Schematic illustration of the experimental procedure. B Micro-CT images of rat knee joints after 4 weeks of treatment. The yellow dotted line represents the location of the histological section of the rat. C, H, E and (D) Safranine-O stain images of rat knee joints after 4 weeks of treatment (n = 3 independent experiments). E–G Immunohistochemical staining of cartilage markers (Sox9、Col II and Acan). The black symbol represents the positive expression. Scale bar = 200 μm. H–J The quantitative analysis of cartilage markers (Sox9、Col II and Acan) (n = 3 samples). K–M Levels of IL-6, IL−1β, and IFN- γ in rat serum after 4 weeks of treatment (n = 3 samples). (Data are presented as the mean ± SD. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test, with a value of *P < 0.05, **P < 0.01, and ***P < 0.005).
Inflammation was effectively alleviated by bioadhesive and lubricating hydrogels in RA. The results showed that the SO3−/DA-HA solution (Group 1), Cu2+-SO3−/DA-HA hydrogel (Group 2), DAGQD@Cu-SO3−/DA-HA hydrogel (Group 3), and DAGQD@Cu@KGN-SO3−/DA-HA hydrogel (Group 4) all exhibited an RA-suppressing effect, accompanied by the reduction in swelling of hind paws, as compared with PBS-treated CIA rats (PBS group) (Supplementary Fig. 26A, B). The clinical arthritis scores were consistent with the severity of arthritis in the paws (Supplementary Fig. 26C). The DAGQD@Cu@KGN-SO3−/DA-HA hydrogel group (Group 4) demonstrated the highest efficacy in suppressing paw swelling and clinical arthritis scores, indicating that the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel effectively alleviated inflammation in RA.
In addition to its anti-inflammatory activity, the hydrogel exhibited excellent cartilage repair ability. The H&E staining results showed that the PBS-treated CIA rat joints had a large amount of inflammatory cell infiltration accompanied by severe erosion and thinning of the cartilage layer (Fig. 6C). Safranin O staining of PBS-treated CIA rat joints showed cartilage destruction and stratification, with a significant decrease in cartilage layer thickness (Fig. 6D). These results indicated that the joint cavity of CIA rats can form an inflammatory storm in the short term, leading to significant degradation of the joint cartilage. Groups 3 (DAGQD@Cu-SO3−/DA-HA hydrogel) and 4 (DAGQD@Cu@KGN-SO3−/DA-HA hydrogel groups) elicited excellent effects against RA-induced inflammatory infiltration compared with Groups 2 (Cu2+-SO3−/DA-HA hydrogel) and 1 (SO3−/DA-HA solution group), attributed to the excellent ROS clearance ability of the DAGQD@Cu@KGN SAN. Safranin-O staining images showed that the protein polysaccharides in the cartilage matrix of Group 4 were significantly increased and were higher than those in Groups 3, 2, and 1 with fewer protein polysaccharides. Moreover, the cartilage thickness and structure in Group 4 were closer to those of normal rats (control group).
The immunohistochemical staining analysis revealed a significant reduction in the expression of cartilage proliferation and differentiation-related proteins Sox9, Col II, and Acan in the PBS group compared with normal rats. This observation suggests that PBS-treated CIA rats exhibited significant cartilage wear characteristics (Fig. 6E–J). The expression levels of Sox9, Col II, and Acan in the SO3−/DA-HA solution group (Group 1) were lower than those in the Cu2+-SO3−/DA-HA hydrogel group (Group 2), indicating that the long-term bioadhesion and lubrication properties of the hydrogel play a crucial role in cartilage protection. The expression levels of Sox9, Col II, and Acan were higher in the DAGQD@Cu-SO3−/DA-HA hydrogel group (Group 3) than in the Cu2+-SO3−/DA-HA hydrogel group (Group 2). This difference can be attributed to the excellent antioxidant activity of DAGQD@Cu SAN, effectively alleviating inflammation in RA and mitigating cartilage damage. Remarkably, the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel group (Group 4) showed the highest expression levels of Sox9, Col II, and Acan. This enhancement is attributed to the promotion of cartilage differentiation by KGN in the SAN.
The cartilage repair ability of the hydrogel was attributed to its immunoregulatory activity (Supplementary Fig. 27). The expression of M1 related inflammatory factors (TNF-α and IL-6) was highest in PBS-treated CIA rat joints. The expression of TNF-α and IL-6 were slightly inhibited after treatment with SO3−/DA-HA solution (Group 1) and Cu2+-SO3−/DA-HA hydrogel (Group 2). This is due to the antioxidant capacity of the catechol structure on the surface of the DA-HA network. Moreover, the expression of TNF-α and IL-6 was significantly reduced in SAN-contained groups (DAGQD@Cu-SO3−/DA-HA hydrogel and DAGQD@Cu@KGN-SO3−/DA-HA hydrogel), whereas the expression of M2 related anti-inflammatory factor IL-10 was significantly increased, illustrating that SAN has excellent ROS scavenging ability, and can regulate the RA-induced inflammatory microenvironment by promoting M1 to M2 transformation. Moreover, the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel not only inhibited systemic inflammation in CIA rats (Fig. 6K–M), but also did not cause systemic toxicity (Supplementary Fig. 28, 29).
Hydrogel promotes cartilage reconstruction in RA rabbit
The DAGQD@Cu@KGN-SO3−/DA-HA hydrogel demonstrates a significant capacity for reconstructing severe cartilage defects in rabbits with RA. First, a complex model, combining cartilage and subchondral bone defects with RA, was established in rabbits (Fig. 7A). After 8 weeks of induction, the osteochondral defect in the PBS group was severely corroded, with inflammation spreading to the surroundings and bottom of the defect (Fig. 7B). Following treatment, inflammation was alleviated, and the defect underwent repair. Micro-CT scanning (Fig. 7C) and histopathological (Fig. 7D, E) analyzes confirmed the expansion of the defect area and inflammation surrounding the defect in the PBS group. In contrast, the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel treatment demonstrated the most efficient defect repair and inflammation inhibition, followed by the DAGQD@Cu-SO3−/DA-HA hydrogel, Cu2+-SO3−/DA-HA hydrogel, and SO3−/DA-HA solution. The defect showed partial repair, and inflammation was slightly alleviated after the SO3−/DA-HA solution treatment (Group 1). This can be attributed to the antioxidant capacity of catechol in the DA-HA network.
A Schematic illustration of the experimental procedure. B Macroscopic observation of repaired cartilages at 8 weeks (black arrow: defect cartilage site). C Micro-CT images of rabbit’s knee joints after 8 weeks of treatment. The blue dotted line represents the location of the histological section of the rabbit. D, H, E Safranine-O stain images of the rat knee joints after 8 weeks of treatment (n = 3 independent experiments). Scale bar = 2 mm. F Quantitative analysis of bone volume over total volume ratio (BV/TV) and (G) trabecular number (Tb.N) of CIA rats in different treated groups (n = 3 samples). H Histological synovitis scoring (HSS) and (I) modified Osteoarthritis Research Society International (OARSI) score of rabbit knee joints after 8 weeks of treatment (n = 5 samples). J Indentation test results and (K) corresponding reduced modulus of the newly formed tissues in different groups (n = 6 samples). (Data are presented as the mean ± SD. Ordinal data such as HSS score and OARSI score was analyzed by Kruskal-Wallis test and continuous data was analyzed by one-way ANOVA followed by Tukey’s post-hoc test, with a value of **P < 0.01, ***P < 0.005, and ****P < 0.001).
After the hydrogel treatment, the defect diameter and depth decreased, and the degree decreased from high to low in Groups 4 (DAGQD@Cu@KGN-SO3−/DA-HA), 3 (DAGQD@Cu-SO3−/DA-HA), and 2 (Cu2+-SO3−/DA-HA hydrogels). The slight recovery of the cartilage and subchondral bone in Group 2 was attributed to the ability of Cu to promote cartilage differentiation32. Group 3 showed better cartilage repair ability than the Group 2 because of the exceptional antioxidant capacity of DAGQD@Cu SAN, which promoted self-repair of the matrix by regulating the inflammatory microenvironment. Group 4 displayed the best cartilage repair ability. This is because the released KGN promotes cartilage reconstruction by recruiting endogenous BMSCs, except for the excellent anti-inflammatory activity of DAGQD@Cu SAN in the DAGQD@Cu-SO3−/DA-HA hydrogel. Quantitative analysis of the micro-CT images also revealed progressive improvements in both the bone volume to total volume ratio (BV/TV) at the defect site (Fig. 7F) and the trabecular number (Tb.N) (Fig. 7G) within the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel group (Group 4). Moreover, the histological synovitis scoring (HSS) score and Osteoarthritis Research Society International (OARSI) score exhibited a significant decrease following treatment with the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel in Group 4 (Fig. 7H, I). The mechanical test results of the newly formed organization showed that the deformation resistance and reduced modulus of the newly formed tissues in the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel group (Group 4) were significantly higher than those in the other treatment groups and close to those in the control group (normal rabbit without hydrogel treatment) (Fig. 7J, K). DAGQD@Cu@KGN-SO3−/DA-HA hydrogel group (Group 4) exhibited superior cartilage repair ability, which can be attributed to the KGN-mediated promotion of cartilage reconstruction, coupled with the excellent anti-inflammatory activity of DAGQD@Cu SAN in the hydrogel.
The cartilage reconstruction mechanism of the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel in rabbits with RA can be summarized as follows: (1) The abundant catechol groups in DA provided the hydrogel with long-term adhesion to the cartilage surface. (2) The SO3− endowed the hydrogel with lubricity, preventing further cartilage wear and tear in RA. (3) The GAGQD@Cu SAN alleviated the inflammatory microenvironment in RA joint cavity due to its excellent ROS-scavenging activity. Moreover, Cu2+ in the SAN demonstrated a certain ability to promote cartilage differentiation. (4) KGN triggered the differentiation of BMSCs into chondrocytes by recruiting endogenous host BMSCs guiding them to mitigate and target the damaged cartilage affected by RA, thus contributing to its repair.
Discussion
We have developed an injectable bioadhesive and lubricating DAGQD@Cu@KGN-SO3−/DA-HA hydrogel with anti-inflammatory and cartilage-repair capabilities for full-cycle therapy of RA. The abundant catechol groups in the DA-HA network provide the hydrogel with firm adhesion to the cartilage layer, simultaneously alleviating the inflammatory microenvironment through ROS clearance by DA. The SO3−-HA network in contact with cartilage surfaces enhanced lubrication, effectively preventing cartilage wear and tear by reducing the friction coefficient between cartilages in the RA models. The DAGQD@Cu -SO3−/DA-HA hydrogel alleviated cellular oxidative stress and inflammation through antioxidant effects, while promoting tissue regeneration and removing harmful factors through upregulating autophagy. KGN released from the hydrogel played a crucial role in recruiting endogenous host MSCs and inducing their differentiation into chondrocytes, thereby repairing RA-induced cartilage and subchondral bone defects.
The DAGQD@Cu@KGN SAN in the hydrogel alleviated the inflammatory microenvironment and within the joint cavity of RA owing to its effective enzyme-like ROS-scavenging activity. The DAGQD@Cu@KGN SAN was created by in situ reduction of Cu single atoms on a DAGQD support. Subsequently, KGN was grafted onto DAGQD@Cu SAN via an amide reaction. The SAN has the following advantages: (1) The ultrasmall DAGQD@Cu SAN features numerous catechols as surface anchoring sites to stabilize isolated Cu atoms, providing excellent water dispersibility. This DAGQD support strategy prevents the common aggregation seen in carbon-supported single atom nanomaterials within hydrogel matrices. (2) DAGQD@Cu SAN exhibits high catalytic activity due to a dual catalytic mechanism. The catechol groups enhance ROS elimination, while Cu single atoms mimic SOD and CAT activities, converting •O2−, •OH, and H2O2 into H2O and O2. This effective ROS scavenging reduces the inflammatory microenvironment in RA. (3) The introduction of Cu SAN serves as a cross-linking point for designing injectable hydrogels via metal coordination bonds.
In conclusion, with a single administration, the DAGQD@Cu@KGN-SO3−/DA-HA hydrogel, offering both bioadhesion and lubrication as a localized modulation niche, demonstrates the potential to restore cartilage lubrication, repair cartilage in RA, and serves as a promising full-cycle clinical therapeutic strategy for RA. However, to facilitate the successful translation of this promising hydrogel from the laboratory to clinical practice, scalable and stable manufacturing processes, detailed cost analyses, ease of use and patient education should be considered in further study.
Methods
All animal experiments were performed according to protocols approved by the local ethical committee and the laboratory animal administration rules of the Soochow University (ethical code: SUDA20221124A21).
Materials
Dopamine hydrochloride (DA, 98%) was procured from Sigma-Aldrich St. Louis, MO, USA, while l-arginine (98%) originated from Solarbio, Beijing, China. Other reagents including l-ascorbic acid (99%), hyaluronic acid (HA, Molecular weight: 400000 to 800000), 3,3’-Dithiobis-(propanoic dihydrazide) (95%), dimethoxy-1,3,5-triazin-2-yl-4-methyl morpholinium chloride (DMTMM, 97%), 2-(N-morpholino)-ethanesulfonic buffer (MES, 99%), NaOH (98%), and NaCl (99.5%) were sourced from Macklin, Shanghai, China. Further chemicals, namely, tris(2-carboxyethyl)phosphine hydrochloride (98%), 3-Sulfopropyl methacrylate potassium salt (98%), 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC, 98%), and N-hydroxysuccinimide (NHS, 98%), were procured from Rhawn, Shanghai, China. Triethanolamine buffer (99%), 2,2,-Dimethoxy-2-phenylacetophenone (DMPA, 99%), and Dimethylsulfoxide (DMSO, AR, 99%) were secured from Aladdin, Shanghai, China. Additionally, CuCl2·2H2O (AR, 99%) was sourced from Kermel, Tianjin, China. Unless otherwise specified, all the aforementioned substances were utilized in their received state without further modification.
The preparation of DAGQD@Cu SAN
Initially, CuCl2·2H2O (0.08 g) was dissolved in 50 mL of deionized water and agitated at 80 °C for 10 minutes using an oil bath combined with a magnetic stirrer. Subsequently, an aqueous solution of l-ascorbic acid (0.05 g in 50 mL of 2 mg mL-1 DAGQD solution) was introduced dropwise into the CuCl2 solution. The pH of the reaction mixture was adjusted to 8.0 via the addition of a 1 M NaOH solution. This mixture was sustained at 80 °C for 12 h with continuous stirring. Post-reaction, any larger aggregates were eliminated via centrifugation (11,180 × g, 10 min) and the supernatant was subjected to dialysis against water for 2 days (utilizing dialysis tubing with a molecular weight cutoff of 1000 Da), this step served to remove smaller molecules present. The purified DAGQD@Cu powder was then obtained by subsequent concentration and freeze-drying.
The preparation of DAGQD@Cu@KGN
In the preparation process of DAGQD@Cu@KGN SANs, KGN (100 μg), EDC (6.04 mg), and NHS (1.21 mg) were dissolved in 2 mL of DMSO. This solution was stirred at 25 °C for a duration of 30 min. Concurrently, DAGQD@Cu SANs (5 mg) were dispersed in a 1 mL solvent mixture (comprising of water and DMSO in a 1:9 volume ratio) through a sonication process of 10 min. This dispersion was introduced into the aforementioned mixed solution and the resultant mixture was stirred in darkness for a period of 12 h. Upon culmination of the reaction, larger aggregates were eradicated through centrifugation at 11,180 × g for 10 min. The product was then shifted into 27 mL of deionized water and underwent a second round of centrifugation (at 11,180 × g for 10 minutes) to eliminate excess KGN that was water-insoluble. Subsequently, the supernatant was dialyzed against water for 24 h using a dialysis tubing with a molecular weight cut-off of 1000 Da. This step served to remove smaller molecules. Finally, purified DAGQD@Cu@KGN SANs powder can be obtained by concentration and freeze-drying.
The preparation of hydrogels
The preparation of DAGQD@Cu-SO3−/DA-HA hydrogel and DAGQD@Cu@KGN-SO3−/DA-HA hydrogel were as follows. A certain proportion of SO3−-HA and DA-HA were dissolved in 1 mL of deionized water, to prepare SO3−/DA-HA hydrogel precursor. In order to fully dissolve the SO3−/DA-HA, the hydrogel presolution was stirred overnight in an ice bath. Then, add DAGQD@Cu SANs or DAGQD@Cu@KGN SANs into the precursor solution and shake immediately to form a gel. The specific concentrations and dosages required for the synthesis of hydrogel were delineated in Supplementary Table 1.
Superoxide dismutase (SOD) activity of NPs
The nanozyme’s superoxide dismutase (SOD) functionality was evaluated using a SOD activity detection kit as per the manufacturer’s (Solarbio) guidelines. In this process, the superoxide anion (•O2−), produced from the xanthine-xanthine oxidase system, results in the reduction of nitroblue tetrazolium to yield the blue formazan pigment. This pigment undergoes light absorption at 560 nm. By scavenging •O2−, SOD inhibits formazan formation. In conformity to the manufacturer’s instructions, GQD, DAGQD, DAGQD+CuCl2, DAGQD@Cu SANs, DAGQD@Cu@KGN SANs, and the kit’s reagents were added to a 96-well plate, reaching a cumulative volume of 200 µL per well. Afterwards, the plate was placed in a water bath at 37 °C for a duration of 30 min; resulting in absorbance measurements being taken at 560 nm using a microplate reader. The rate of •O2− elimination was quantified by observing the decline in color development. The final concentrations of GQD, DAGQD, DAGQD@Cu SANs, and DAGQD@Cu@KGN SANs were maintained at 100 µg mL−1. Furthermore, in the DAGQD+CuCl2 group, the concentration of DAGQD was maintained at 100 µg mL−1, and CuCl2 at 10 µg mL−1.
Hydroxyl radical scavenging activity of NPs
The hydroxyl radical (•OH) scavenging activities of GQD, DAGQD, DAGQD+CuCl2, DAGQD@Cu SANs, and DAGQD@Cu@KGN SANs were scrutinized via electron paramagnetic resonance (EPR) assessments using a Bruker EMXPlus spectrometer (Bruker EMXPlus-10/12). In this assessment, •OH was trapped through 5,5-dimethyl-1-pyrroline N-oxide (DMPO) in an aqueous medium, forming a spin coupling product, DMPO/OH. The hydroxyl radicals were instigated via the Fenton reaction with the corresponding electron spin resonance (ESR) signals derived from a concoction of 25 mM BMPO, 1.0 mM FeSO4, and 1.0 mM H2O2 in water. In the assessments related to •OH scavenging activity, EPR spectra of DMPO/OH were chronicled at both 0- and 90-seconds post-incubation of the newly spawn •OH, DMPO, and the samples. The assigned concentrations of GQD, DAGQD, DAGQD+CuCl2, DAGQD@Cu SANs, and DAGQD@Cu@KGN SANs were all regulated at 20 µg mL−1. Typically, the EPR spectrum of DMPO/OH exhibits four lines with a relative intensity sequence of 1:2:2:1. Therefore, the intensity of the second line was leveraged to determine the elimination rate of the sample.
Friction coefficient test of hydrogel
The frictional coefficient of the hydrogel was determined using a high-speed friction and wear testing machine (MFT-R4000). We adjusted the mass ratio of SO3−-HA and DA-HA, as documented in Supplementary Table 2, in addition to making alterations in the concentration of SANs, outlined in Supplementary Table 3. The fabricated Cu2+-SH/DA-HA hydrogel, DAGQD@Cu@KGN-SH/DA-HA hydrogel, and DAGQD@Cu@KGN-SO3−/DA-HA hydrogel were all affixed to a polyethylene surface owing to the inherent self-adhesion trait of the hydrogel. In this scenario, we employed the hydrogel and steel ball as the frictional pair, and we tracked the correlation between the coefficient of friction (COF) and time throughout our experiment. The operational parameters were set as follows: frictional load was at 1 N, the setting amplitude at 5 mm, sliding frequency at 1 Hz, and the duration was 20 minutes. The friction coefficient test parameters for PBS, SH-HA solutions (10 mg mL−1), and SO3−-HA solution (10 mg mL−1), matched those of the hydrogel. However, the solution was submerged in the polyethylene block without hydrogels.
ROS clearance ability in vitro
RAW264.7 cells were seeded in 24-well plates with hydrogel (Cu2+-SO3−-HA hydrogel, Cu2+-SO3−/DA-HA hydrogel, DAGQD@Cu-SO3−/DA-HA hydrogel, and DAGQD@Cu@KGN-SO3−/DA-HA hydrogel) at a density of 1.5 × 104 cells per well. After cell adherence, the medium was removed and the high-glucose DMEM medium containing lipopolysaccharide (LPS) (L4391, Sigma, USA) (100 ng mL−1) was added and incubated with the cells for 24 h. Thereafter, the cells were washed with PBS and stained with the ROS-sensitive probe 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA, 500 µL, 10 μM). Finally, the characteristic fluorescence of ROS in the cells was observed using a confocal laser scanning microscope (CLSM, LSM880, Zeiss, Germany) after washing the cells three times with PBS. Flow cytometry was used to quantify the intracellular ROS levels. After DCFH-DA staining, the cells were washed three times with PBS, scraped off the well, and dispersed in 1 mL PBS for detection on a flow cytometer (Cytoflex, Beckman, USA).
Anti-inflammatory activity in vitro
RAW264.7 cells were seeded in 12-well culture plates with hydrogel (Cu2+-SO3−-HA hydrogel, Cu2+-SO3−/DA-HA hydrogel, DAGQD@Cu-SO3−/DA-HA hydrogel, and DAGQD@Cu@KGN-SO3−/DA-HA hydrogel) at a density of 1 × 107 cells per well. Upon successful attachment of cells, we proceeded to replace the existing medium with a high-glucose DMEM supplemented with LPS (100 ng mL−1). Following a 24-hour treatment phase, we collected the supernatant for further examination. The specific inflammatory mediators, namely tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-10 (IL-10), were investigated using an enzyme-linked immunosorbent assay (ELIZA), all whilst adhering to the manufacturer’s explicit protocol outlined by eBioscience.
The chondrogenic differentiation ability
BMSCs cells were seeded in 24-well culture plates with hydrogel (Cu2+-SO3−-HA hydrogel, Cu2+-SO3−/DA-HA hydrogel, DAGQD@Cu-SO3−/DA-HA hydrogel, and DAGQD@Cu@KGN-SO3−/DA-HA hydrogel) at a density of 1 × 105 cells per well. Upon complete adherence of the cells, the original culture medium was substituted by cartilage-induced differentiated complete culture medium (OriCell®). After seven days of chondrogenesis induction, Real-Time Polymerase Chain Reaction (RT-PCR) was employed to scrutinize the expression levels of distinctive chondrogenic marker genes of BMSCs on different hydrogels. These marker genes included Sox9, aggrecan (Acan), collagen II (Col II), and collagen type X (ColX). The respective primer sequences implemented for this analysis purchased from Sangon biotech are diligently outlined in Supplementary Table 4 for further reference
In vivo therapeutic for CIA rat
36 Sprague-Dawley (SD) rats (male, 8 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. All animal experiments were performed according to protocols approved by the local ethical committee and the laboratory animal administration rules of the Soochow University (ethical code: SUDA20221124A21). Here, 36 SD rats were randomly divided into six groups (n = 6 rats per group), healthy group, PBS group, and treatment groups including group1-group4. PBS group and treatment groups are CIA rat model groups. On the day of secondary immunity, PBS group was joint cavity injected of 36 μL PBS, Group1 was joint cavity injected of 30 μL SO3−/DA-HA solution (SO3−/DA-HA = 60 mg mL−1) and 6 μL H2O, Group 2 was joint cavity injected of 36 μL SO3−/DA-HA precursor solution (SO3−/DA-HA = 60 mg mL−1) and 6 μL CuCl2 solution (6 mg mL−1), Group 3 was joint cavity injected of 36 μL SO3−/DA-HA precursor solution (SO3−/DA-HA = 60 mg mL−1) and 6 μL DAGQD@Cu solution (6 mg mL−1), Group 4 was joint cavity injected of 36 μL SO3−/DA-HA precursor solution (SO3−/DA-HA = 60 mg mL−1) and 6 μL DAGQD@Cu@KGN solution (6 mg mL−1). Upon completion of a 28-day treatment period, evaluations are performed inclusive of scoring the severity of inflammation in the rear foot, with the thickness of the foot palm precisely quantified via a Vernier caliper. Blood samples are duly collected for assessing the levels of systemic inflammatory markers, specifically Tumor Necrosis Factor alpha (TNF-a), Interleukin-6 (IL-6), Interleukin-1 beta (IL-1β), and Interferon-gamma (IFN-γ). Parallel to these procedures, knee joints from the rats are harvested to facilitate subsequent Micro-Computed Tomography (Micro-CT) and elaborate pathological assessments.
In vivo therapeutic for OIA rabbit with cartilage defect
Collagen-induced arthritis (CIA) rat model was used to mimic early RA symptoms. However, due to the small size of rat joints, ovalbumin induced arthritis (OIA) rabbit (male) model co-operated with standardized articular cartilage destruction was used to simulate late-stage RA and evaluate the cartilage repair capability of the hydrogels.
To evaluate the cartilage reconstruction of hydrogel on the cartilage defect of OIA rabbits, a series of hydrogel precursor were injected after full-thickness cartilage defects. Here, 24 rabbits were randomly divided into six groups (n = 6 rats per group), healthy group, PBS group, and treatment groups including Group1-Group4. PBS group and treatment groups are OIA rabbits model groups. PBS group was defect injected of 36 μL PBS, Group1 was defect injected of 30 μL SO3−/DA-HA solution (SO3−/DA-HA = 60 mg mL−1) and 6 μL H2O, Group 2 was defect injected of 36 μL SO3−/DA-HA precursor solution (SO3−/DA-HA = 60 mg mL−1) and 6 μL CuCl2 solution (6 mg mL−1), Group 3 was defect injected of 36 μL SO3−/DA-HA precursor solution (SO3−/DA-HA = 60 mg mL−1) and 6 μL DAGQD@Cu solution (6 mg mL−1), Group 4 was defect injected of 36 μL SO3−/DA-HA precursor solution (SO3−/DA-HA = 60 mg mL−1) and 6 μL DAGQD@Cu@KGN solution (6 mg mL−1). 8 weeks later, the rabbits were euthanized, and the knee joint was collected for further analysis.
Statistics and reproducibility
The sample size in each experiment is indicated in the figure or corresponding figure legends. The experiments were performed at least in triplicate and with similar results. The data of the experiments were presented as mean ± Standard Deviation (SD). Discrete metrics such as arthritis scores, osteoarthritis research society international (OARSI) and histological synovitis scoring (HSS) scores were analyzed by Kruskal-Wallis test with a value of *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.001. Statistical significance among different groups with continuous variables was analyzed by the One-way analysis of variance (ANOVA) test with a value of *p < 0. 05, **p < 0. 01, ***p < 0. 005 and ****p < 0. 001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are available from the corresponding authors upon request. The RNA-seq data generated in this study are deposited in the NCBI Sequence Read Archive under accession number PRJNA1146715. Source data are provided with this paper.
References
Alivernini, S., Firestein, G. S. & McInnes, I. B. The pathogenesis of rheumatoid arthritis. Immunity 55, 2255–2270 (2022).
Zhang, F. et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nature 623, 616–624 (2023).
Chen, J. et al. Photoacoustic image-guided biomimetic nanoparticles targeting rheumatoid arthritis. Proc. Natl Acad. Sci. USA 119, e2213373119 (2022).
Ji, X. et al. Injectable immunomodulation-based porous chitosan microspheres/HPCH hydrogel composites as a controlled drug delivery system for osteochondral regeneration. Biomaterials 285, 121530 (2022).
Kim, T., Suh, J. & Kim, W. J. Polymeric aggregate-embodied hybrid nitric-oxide-scavenging and sequential drug-releasing hydrogel for combinatorial treatment of rheumatoid arthritis. Adv. Mater. 33, 2008793 (2021).
Xie, R. et al. Biomimetic cartilage-lubricating polymers regenerate cartilage in rats with early osteoarthritis. Nat. Biomed. Eng. 5, 1189–1201 (2021).
Qindeel, M., Khan, D., Ahmed, N., Khan, S. & Rehman, A. U. Surfactant-free, self-assembled nanomicelles-based transdermal hydrogel for safe and targeted delivery of methotrexate against rheumatoid arthritis. ACS Nano 14, 4662–4681 (2020).
Lin, W. & Klein, J. Recent progress in cartilage lubrication. Adv. Mater. 33, 2005513 (2021).
Schmidt, T. A. Lubricating lipids in hydrogels. Science 370, 288–289 (2020).
Xue, X., Hu, Y., Deng, Y. & Su, J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 31, 2009432 (2021).
Bruno, M. C. et al. Injectable drug delivery systems for osteoarthritis and rheumatoid arthritis. ACS Nano 16, 19665–19690 (2022).
Zhang, Y. et al. Modulus adaptive lubricating prototype inspired by instant muscle hardening mechanism of catfish skin. Nat. Commun. 13, 377 (2022).
Qiu, W. et al. A solid-liquid composite lubricating “nano-snowboard” for long-acting treatment of osteoarthritis. Adv. Funct. Mater. 32, 2208189 (2022).
Xiang, L. et al. Motion lubrication suppressed mechanical activation via hydrated fibrous gene patch for tendon healing. Sci. Adv. 9, eadc9375 (2023).
Yao, Y., Wei, G., Deng, L. & Cui, W. Visualizable and lubricating hydrogel microspheres via nanoPOSS for cartilage regeneration. Adv. Sci. 10, 2207438 (2023).
Zhang, F. et al. Injectable mussel-inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 278, 121169 (2021).
Wang, H., Wan, K. & Shi, X. Recent advances in nanozyme research. Adv. Mater. 31, 1805368 (2019).
Wei, H. & Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 42, 6060–6093 (2013).
Xi, J. et al. Copper/carbon hybrid nanozyme: Tuning catalytic activity by the copper state for antibacterial therapy. Nano Lett. 19, 7645–7654 (2019).
Cai, X. et al. Nanozyme-involved biomimetic cascade catalysis for biomedical applications. Mater. Today 44, 211–228 (2021).
Gao, F., Liu, J., Gong, P., Yang, Y. & Jiang, Y. Carbon dots as potential antioxidants for the scavenging of multi-reactive oxygen and nitrogen species. Chem. Eng. J. 462, 142338 (2023).
Zhao, H., Zhang, R., Yan, X. & Fan, K. Superoxide dismutase nanozymes: an emerging star for anti-oxidation. J. Mater. Chem. B 9, 6939–6957 (2021).
He, W., Wu, J., Liu, J. & Li, J. Single-atom nanozymes for catalytic therapy: Recent advances and challenges. Adv. Funct. Mater. 34, 2312116 (2024).
He, H. et al. Bioadhesive injectable hydrogel with phenolic carbon quantum dot supported Pd single atom nanozymes as a localized immunomodulation niche for cancer catalytic immunotherapy. Biomaterials 280, 121272 (2022).
Jiang, Y. et al. Infant skin friendly adhesive hydrogel patch activated at body temperature for bioelectronics securing and diabetic wound healing. ACS Nano 16, 8662–8676 (2022).
Ghasemlou, M. et al. Silicon-doped graphene oxide quantum dots as efficient nanoconjugates for multifunctional nanocomposites. ACS Appl. Mater. Inter. 14, 7161–7174 (2022).
Liu, X. et al. One-pot synthesis of graphene quantum dots using humic acid and its application for copper (II) ion detection. J. Mater. Sci. 56, 4991–5005 (2021).
Guo, Q. et al. Hydrogen-bonds mediate liquid-liquid phase separation of mussel derived adhesive peptides. Nat. Commun. 13, 5771 (2022).
Xie, C. et al. Mussel‐inspired hydrogels for self‐adhesive bioelectronics. Adv. Funct. Mater. 30, 1909954 (2020).
Zhu, Z. et al. 3D printing drug-free scaffold with triple-effect combination induced by copper-doped layered double hydroxides for the treatment of bone defects. ACS Appl. Mater. Inter. 15, 58196–58211 (2023).
Geng, B. et al. Surface charge-dependent osteogenic behaviors of edge-functionalized graphene quantum dots. Chem. Eng. J. 417, 128125 (2021).
Yu, B. et al. Using Cu-based metal-organic framework as a comprehensive and powerful antioxidant nanozyme for efficient osteoarthritis treatment. Adv. Sci. 11, 2307798 (2024).
Acknowledgements
The authors wish to acknowledge the assistance on materials characterization received from Analytical & Testing Center of the Southwest Jiaotong University. This work was supported by grants from National Key Research and Development Program of China (2023YFC2411300), National Natural Science Foundation of China (32471392, 82172387, 82372371, 52035012, 82402763), Sichuan Science and Technology Program (2025NSFJQ0025), New Interdisciplinary Cultivation Fund of SWJTU (2682023JX005), Fundamental Research Funds for the Central Universities (2682023ZTPY056, 2682024ZTPY003), Gusu Health Talents Program (GSWS2020023), the Natural Science Foundation of Jiangsu Province (BK20230002), the Suzhou Science and Technology Development Plan (SKY2023035, SZM2023008), the National Postdoctoral Researcher Program (GZC20241187), and the China Postdoctoral Science Foundation (2023T00235), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
B.L., J. L., X.L., and C.X. led this protect. H.H., B.L., J. L., X.L., and C.X. designed the experiments and analyzed the data. H.H. conducted the experiments. Q.Z. contributed to the design of CIA and OIA model. Y.Z. participated in the synthesis of hydrogels. S.Q. provided the technical supports. H.H., B.L., J.L., X.L., and C.X. discussed the results and wrote the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Peer review
Peer review information
Nature Communications thanks Bing Han, Dangsheng Xiong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, H., Zhang, Q., Zhang, Y. et al. Injectable bioadhesive and lubricating hydrogel with polyphenol mediated single atom nanozyme for rheumatoid arthritis therapy. Nat Commun 16, 2768 (2025). https://doi.org/10.1038/s41467-025-58059-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58059-z