Abstract
Hydrocephalus is one of the most common neurological disorders, but pharmacotherapy options are currently lacking due to the complex pathogenesis. The blood-CSF barrier (BCSFB), consisting of choroid plexus (ChP) epithelial cells, is a crucial gate for the entry of peripheral immune cells and its dysfunction emerges as an important contributor to hydrocephalus pathology. Meanwhile, SPAK-mediated CSF hypersecretion in ChP epithelial cells plays an important role in hydrocephalus. Here, we fabricated a transferrin receptor-targeted nano-drug (siR/RSV@TNP) that can intelligently navigate to the blood-CSF barrier and prepared for combined delivery of resveratrol (RSV) and SPAK siRNA (siSPAK) for synergetic hydrocephalus therapy. As expected, siR/RSV@TNP fulfilled its function of knocking down SPAK expression, relieving inflammation and oxidative stress, retrieving blood-CSF barrier integrity, and ultimately preventing ventriculomegaly and hydrocephalus in male mice. Here, we demonstrate that targeting the choroid plexus blood-CSF barrier and cerebrospinal fluid hypersecretion offers a promising approach for alleviating hydrocephalus.
Similar content being viewed by others
Introduction
Hydrocephalus is one of the most common neurosurgical disorders, imposing a significant financial burden on the global healthcare system1. Annually, an estimated 400,000 new cases of hydrocephalus are diagnosed worldwide2. Elevated intracranial pressure (ICP) in hydrocephalus can lead to visual loss, developmental delay, brain damage, and even death3. Currently, there is no reliable pharmaceutical treatment for hydrocephalus. Surgical cerebrospinal fluid (CSF) shunting, which drains CSF from the brain to another part of the body via a catheter, remains the primary treatment4. However, ~40–50% of patients experience shunt failure due to obstruction or infection. And 60% of surgically treated patients endure lifelong neurological symptoms, such as motor skills impairment, cognitive deficits, or epilepsy. Thus, non-invasive therapies based on targeted nano-delivery systems represent a significant advancement for hydrocephalus patients across all age groups.
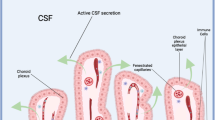
In recent years, increasing attention has been given to the relationship between the structural and functional integrity of the blood-cerebrospinal fluid barrier (BCSFB) and hydrocephalus. The BCSFB, comprised of choroid plexus epithelial cells, serves as a crucial gatekeeper at the brain–immune system interface and is an underestimated gate for entry of immune cells during inflammatory disorders5,6,7. Previous research has indicated that increased immune cell infiltration into the choroid plexus is associated with a loss of BCSFB integrity7,8,9. For example, Kahle et al. have identified that the recruitment of peripheral monocytes to the choroid plexus (ChP), triggered by intraventricular hemorrhage or infection, significantly contributes to hydrocephalus pathogenesis10. In our prior investigations, we observed disrupted BCSFB integrity, evidenced by ZO-1 staining, alongside heightened macrophage accumulation in the choroid plexus during hydrocephalus11. Similarly, Zhang et al. demonstrated that BCSFB dysfunction exacerbates hydrocephalus, attributing mitochondrial reactive oxygen species (ROS) to the destruction of tight junctions in choroid plexus epithelial cells12. Collectively, the evidence supports that dysfunction of BCSFB serves as an important contributor to hydrocephalus pathology, and addressing the comprised BCSFB represents a potential therapeutic strategy for hydrocephalus.
In addition, the ChP secretes over 500 ml/day CSF into the brain’s ventricular spaces, which relies on the vectorial ion transport across the apical and basolateral membranes of the ChP epithelial cells, coupled with water transport—either co-transported or osmotically driven13,14,15,16. Notably, SPAK-NKCC1, a multi-ion transporter complex, prominently expressed on the apical surface of ChP cells, accounts for roughly half of CSF production13. Furthermore, substantial research has established the close relationship between the expression and functional status of the SPAK-NKCC1 complex and CSF secretion10,15. Robert et al. have identified SPAK as a pivotal regulator of a multi-ion transporter complex, including NKCC1, ATP1A1, KCNJ13, and CLIC6, all implicated in CSF hypersecretion in hydrocephalus10. Therefore, targeting SPAK represents a promising approach to mitigate CSF hypersecretion. Considering all the pathological alterations in ChP epithelial cells in hydrocephalus, it is plausible to conclude that the diseased BCSFB-targeted and CSF hypersecretion-targeted modulation strategies can be a promising remedy for hydrocephalus treatment.
To verify our hypothesis, we designed a multifunctional lipid nanoparticle by encapsulating SPAK siRNA (siSPAK) and resveratrol within a T7 peptide-decorated, glutathione (GSH)-responsive lipopolyplex, named as siR/RSV@TNP. In view of the increased expression of SPAK and the subsequent overproduction of hydrocephalus, we employed siSPAK to lower the expression of pathogenic genes and consequently decrease the excessive production of CSF. Nonetheless, the transition of siRNA from the laboratory to clinical use has been hindered by concerns regarding its effective delivery, primarily due to its instability in circulation and vulnerability to degradation by RNases and phosphatases when entrapped in lysosomes. To address the obstacles associated with gene delivery, we utilized a GSH-responsive lipopolyplex featuring disulfide-bonded cationic complexes. This complexes effectively condensed siRNA and responded to intracellular redox environments to facilitate siRNA release. Additionally, a lipid layer was incorporated to conceal the positive charge and extend circulation time. Moreover, T7 peptide, which can specifically bind to TfR was further modified to enhance distribution within ChP epithelial cells17. Resveratrol, a natural polyphenol known for its anti-inflammatory and antioxidant properties, has been harnessed in our system to modulate disrupted BCSFB integrity18. Resveratrol could significantly inhibit the inflammatory response through the TLR4/NF-κB pathway and reduce the inflammatory cytokines18. Besides, it can alter the antioxidant activity by reducing the production of ROS and increasing antioxidant enzymes; and its therapeutic effects have been demonstrated in many clinical trials19. Recent studies have highlighted how inflammatory cytokines and NF-κB pathways can compromise the integrity of the BCSFB barriery20,21,22,23, making resveratrol particularly suitable for modulating BCSFB integrity and ChP inflammation in hydrocephalus.
Here, we showed siR/RSV@TNP not only preserved BCSFB integrity and attenuated macrophage infiltration in the ChP but also suppressed CSF hypersecretion without significant toxicity. This study represents an effective attempt to regulate both BCSFB integrity and CSF hypersecretion using a multifunctional nanomedicine in hydrocephalus treatment.
Results
Construction and characterization of siR/RSV@TNP
The GSH-responsive polymers designed for delivering SPAK siRNA were prepared following established procedures by connecting low molecular weight polyethyleneimine PEI600 with a disulfide bond. The structure of the polymer (named as sPEI) was confirmed by 1H NMR to verify the successful synthesis of sPEI (Supplementary Fig. 1a). Due to the limited gene loading capacity of low molecular weight PEI, we examined whether the complexes could enhance gene loading capacity following the introduction of disulfide bond linkage. The gene loading capacity of sPEI was assessed by agarose gel electrophoresis. As demonstrated in Fig. 1a, LMW PEI600 was unable to fully encapsulate siRNA at a weight ratio of 4, whereas sPEI effectively encapsulated siRNA entirely at a weight ratio of 1, similar to the traditional gene transfection vector polyethyleneimine 25 K (PEI25K). What’s more, the MTT results proved that sPEI showed lower toxicity compared to PEI25K (Supplementary Fig. 1b), indicating its improved biosafety for gene delivery. Considering the instability of siRNA in physiological environments, where they are prone to degradation leading to a loss of their gene silencing efficacy, we conducted tests to confirm the ability of sPEI to shield siRNA from degradation. As demonstrated in Fig. 1b, the naked siRNA experienced considerable degradation after 6 h when exposed to serum. In contrast, sPEI, similar to PEI25K, effectively shielded the siRNA from degradation. Notably, even after 24 h incubation with serum, the siRNA retained its intact structure. Since disulfide bond structures can break in response to intracellular reduction leading to disruption of the nanostructure and facilitate the release of siRNA for gene silence, we examined the reduction responsiveness of the nanoparticle. In Fig. 1c, after incubated with GSH, the spherical structure appeared predominantly disrupted and numerous dispersed fragments were generated. This observation indicates the responsiveness of the polyplexes (siR@sPEI) to GSH. Overall, these results underscore the potential of sPEI for gene delivery. Besides, to optimize the pharmacological effects of siRNA, the sequence of SPAK siRNA was screened using real-time PCR and Western Blot. Among the candidates, SPAK siRNA4 demonstrated the highest inhibitory efficiency, achieving ~80% inhibition in primary ChP epithelial cells and SPAK siRNA4 also demonstrated significant knock-down efficacy on HT22 cells and MC3T3 cells (Supplementary Fig. 2). Consequently, SPAK siRNA4 was chosen for subsequent experiments.
a Gel retardation assay of various siR@polyplexs at different weight ratios. b Evaluation of the protective effect of polyplexs on siRNA against serum. c TEM images showing the morphic changes of siR@sPEI incubated with or without 10 mM GSH. Scale bar, 100 nm. d Hydration particle size and TEM image of siR/RSV@TNP. Scale bar, 50 nm. e Variations in the particle size of siR/RSV@TNP in H2O, PBS, and 10% serum after 24 h of incubation at 37 °C. f, g Lysosome escape test, scale bar 20 μm. h Cellular uptake of free Cy5-siR, Cy5-siR/RSV@NP, Cy5-siR/RSV@TNP, n = 3 biological replicates for each group. i Ex vivo imaging of AD mice brains excised at 2 h post-injection. j Average radiant efficiency of DiD@NP and DiD@TNP group, n = 3 biological replicates for each group. k Cell viability of siR/RSV@TNP, n = 3 biological replicates for each group. All data are presented as mean ± SD. Two-tailed Student’s t test and One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test or Dunnett’s multiple comparisons test were used for the statistical comparison between the two groups and among multiple groups, respectively. Statistical analyses were conducted with a 95% confidence interval, and significance was defined as p < 0.05. Source data are provided as a Source Data file.
To fulfill the need for systematic administration and further improve the treatment effectiveness, a resveratrol-encapsulated, T7-functionalized (confirmed in Supplementary Fig. 3a, b) lipid layer was utilized to shield the positive charge of siR@sPEI polyplexes and to exert combined therapeutical effects. As shown in Supplementary Fig. 3c, the zeta potential of siR/RSV@TNP decreased from +29.7 ± 5.6 to ‒18.9 ± 2.5 mV, suggesting the successful coating of the lipid layer. As shown in Fig. 1e, siR/RSV@TNP with a size of 68.7 ± 29.9 nm was obtained, and transmission electron microscopy (TEM) images demonstrated a typical lipid-vesicle structure (Fig. 1d). Additionally, the size of siR/RSV@TNP showed minimal change within 24 h in PBS and 10% serum (Fig. 1e), indicating its good stability. The DLC and EE of RSV in siR/RSV@TNP were 4.35% and 96.16%, respectively (Supplementary Fig. 3d). We also investigated the release characteristics of RSV and demonstrated that over 80% of the drug could be released from the nano-formulation (siR/RSV@TNP) within 24 h (Supplementary Fig. 4a).
Furthermore, it is crucial for siRNA to escape from the endo/lysosome to induce a pharmacological response. Hence, the lysosomal escape capability of lipid nanoparticles was examined. The findings revealed that following a 1-h incubation of Cy5-siR@TNP with ChP epithelial cells, a significant overlap was observed between red and green fluorescence, indicating the presence of Cy5-siR@TNP within the endosome/lysosome. However, as the incubation time increased, the red fluorescence became distinct from the green fluorescence (Fig. 1f, g). This suggested that our lipid nanoparticles facilitated the successful escape of siRNA from the endosome/lysosome, thereby preventing the degradation of siRNA in the lysosome.
Targeting efficacy of siR/RSV@TNP to blood-CSF barrier
Given the substantial presence of transferrin receptors on the ChP24,25,26,27, our study aimed to investigate the potential enhancement of lipid nanoparticle targeting for the ChP through T7 modification, thereby improving gene delivery efficiency. The in vitro targeting ability of lipid nanoparticles was assessed using primary ChP epithelial cells. Analysis using flow cytometry revealed that the T7-modified Cy5-siR/RSV@TNP group significantly increased cellular endocytosis efficiency compared to both free Cy5-siR and Cy5-siR/RSV@NP groups (Fig. 1h). This finding was further supported by confocal imaging results (Supplementary Fig. 4b). Subsequently, we explored the in vivo targeting efficacy using an IVIS Lumina III Imaging System. The results in Fig. 1i, j demonstrated stronger fluorescent signals in the brain with DiD-loaded DiD@TNP compared to unmodified DiD@NP, with no significant difference observed in other major organs (Supplementary Fig. 4c, d), indicating the benefit of T7 modification for specific targeting of the choroid plexus. Further validation through confocal imaging analysis of brain distribution confirmed that DiD@TNP accumulated in the choroid plexus at a significantly higher level than unmodified DiD@NP (Supplementary Fig. 5a). These results provide evidence that T7 modification can facilitate preferential targeting of the choroid plexus for drug delivery.
siR/RSV@TNP can knockdown SPAK, NKCC1 and KCNJ13 expression
Firstly, in vitro cytotoxicity of lipid nanoparticles in primary ChP epithelial cells and RAW264.7 cells were performed. The lipid nanoparticles exhibited low toxicity in primary ChP epithelial cells within the concentration range of 0.25–7 μg/mL but showed significant cytotoxicity at concentrations above 3 μg/mL in RAW264.7 cells (Fig. 1k and Supplementary Fig. 5b). Hence, a concentration of 3 μg/mL was chosen as the optimal concentration for subsequent cell experiments.
We then investigated the effect of siR/RSV@TNP on SPAK mRNA expression in primary ChP epithelial cells (Fig. 2a). As expected, the lipid nanoparticles effectively silenced the SPAK gene in primary ChP epithelial cells (Fig. 2b). In contrast, the group treated with pure SPAK siRNA exhibited low efficiency, likely due to the negative charge of free siRNA, which impedes cellular entry without a transfection reagent. These results were further corroborated by Western blot analyses (Fig. 2c) and immunofluorescence (Fig. 2d).
a Schematic illustration of the functions of siR/RSV@TNP in ChP epithelial cells. Illustration created using Microsoft PowerPoint, incorporating elements (siR/RSV@TNP and SPAK siRNA) adapted from in BioRender. Gao, H. (2025) https://BioRender.com/i60a962. b The mRNA expression of SPAK after different treatments, n = 4 biological replicates for each group. c Western blot result of SPAK. d Immunofluorescence of SPAK. Scale bar, 20 μm. e Western blot result of SPAK. f, g The mRNA expression of NKCC1 and KCNJ13 after different treatments, n = 4 biological replicates for each group respectively. h Western blot result of p-SPAK, n = 3 biological replicates for each group. i Immunofluorescence of pSPAK. Scale bar, 20 μm. All data are presented as mean ± SD. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test or Dunnett’s multiple comparisons test were used for the statistical comparison among multiple groups, respectively. Statistical analyses were conducted with a 95% confidence interval, and significance was defined as p < 0.05. Source data are provided as a Source Data file.
SPAK has also been identified as a key regulator of a multi-ion transporter complex, including NKCC1, ATP1A1, KCNJ13, and CLIC610. We then examined whether SPAK knockdown could downregulate its downstream ion transporters, such as NKCC1 and KCNJ13. The results demonstrated that siR/RSV@TNP reduced NKCC1 protein expression (Fig. 2e), with downregulation efficiencies of 25% for NKCC1 mRNA (Fig. 2f) and 31% for KCNJ13 mRNA (Fig. 2g). These findings indicated that SPAK siRNA-loaded lipid nanoparticles facilitate siRNA transfection and suggest their potential role in treating hydrocephalus.
We further explored whether the lipid nanoparticle could inhibit pSPAK and pNKCC1 expression in disease conditions. As depicted in Fig. 2h, i, and Supplementary Fig. 6a, siR/RSV@TNP significantly reduced pSPAK and pNKCC1 expression. These results demonstrate that SPAK knockdown via siRNA can inhibit LPS-induced pSPAK and pNKCC1 expression.
siR/RSV@TNP protects the BCSFB integrity
Previous studies have established a link between hydrocephalus and the loss of BCSFB integrity, as well as increased macrophage infiltration into the ChP10,12. To determine whether siR/RSV@TNP could preserve ChP blood-CSF barrier integrity and limit macrophage infiltration, we employed lipopolysaccharide (LPS) to induce BCSFB disruption, as previous reports have shown that LPS can impair BCSFB and increase its permeability28,29,30. Tight junction (TJ) scaffold proteins in ChP epithelial cells are crucial for BCSFB integrity, and their loss leads to barrier impairment9,31. Therefore, we evaluated the expression of tight junction protein ZO-1. As shown in Fig. 3a, b, ZO-1 expression was significantly reduced following LPS incubation. Treatment with various formulations upregulated ZO-1 expression to varying extents, with the most pronounced upregulation observed in the siR/RSV@TNP group. We further investigated whether siR/RSV@TNP could inhibit macrophage recruitment. Using a 2D Transwell co-culture system (Fig. 3c), we cultured RAW264.7 cells in the upper chamber and primary ChP epithelial cells in the lower chamber. After treatment with LPS and different formations, siR/RSV@TNP significantly inhibited macrophage migration from the upper to the lower chamber (Fig. 3d, e). Our previous study underscored the crucial role of the CCL2 signaling molecule in recruiting monocytes to the ChP in hydrocephalus32. We then examined the effect of siR/RSV@TNP on CCL2 expression in LPS-treated ChP epithelial cells. Results from immunofluorescence (Fig. 3f), Western blot (Fig. 3g), and RT-qPCR (Fig. 3h) demonstrated that siR/RSV@TNP significantly inhibited CCL2 signaling. Hence, these findings validated that resveratrol-loaded groups effectively restrained macrophage infiltration by inhibiting CCL2 signaling and protecting the BCSFB barrier integrity (Fig. 3i).
a Expression of ZO-1 mRNA in ChP epithelial cells treated with LPS and different materials, n = 3 biological replicates for each group. b Immunofluorescence of ZO-1(White arrow). Scale bar, 5 μm. c Schematic illustration of transwell experiment. Illustration created using Microsoft PowerPoint by the author. d Images of transwell to test the vertical migrating ability of RAW264.7 cells in the co-culture system at 24 h, Scale bar, 40 μm. e The migration of RAW264.7 was quantitatively analyzed, n = 3 biological replicates for each group. f Immunofluorescence of CCL2. Scale bar, 20 μm. g Western blot result of CCL2. h Expression of CCL2 mRNA in ChP epithelial cells, n = 4 biological replicates for each group. i Schematic illustration of the siR/RSV@TNP in protecting the BCSFB integrity and inhibiting macrophage infiltration. Illustration created using Microsoft PowerPoint by the author, incorporating elements (siR/RSV@TNP) adapted from in BioRender. Gao, H. (2025) https://BioRender.com/i60a962. All data are presented as mean ± SD. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test or Dunnett’s multiple comparisons test were used for the statistical comparison between the two groups and among multiple groups, respectively. Statistical analyses were conducted with a 95% confidence interval, and significance was defined as p < 0.05. Source data are provided as a Source Data file.
siR/RSV@TNP alleviates the ROS and inflammation
Prior studies have demonstrated that reactive oxygen species (ROS) and increased activation of the NF-κB pathway led to the disruption of the BCSFB9,33. Zhang et al. found that mitochondrial dysfunction in ChP epithelial cells contributes to BCSFB impairment by increasing ROS release in hydrocephalus12. Additionally, pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, produced in peripheral tissues and the choroid plexus, can negatively impact BCSFB tight junctions through the NF-κB and MMP9 pathways20,21,22,34.
In this regard, we investigated whether the siR/RSV@TNP lipid nanoparticle could inhibit oxidative stress in primary ChP epithelial cells. The results showed that siR/RSV@TNP significantly decreased the fluorescence intensity of DCFH-DA compared to H2O2-induced positive control cells, indicating its ROS-eliminating properties (Fig. 4a, b). What’s more, we examined the anti-inflammatory activity of the nanoparticle by measuring the expression of pro-inflammatory factors at the mRNA and protein levels in ChP epithelial cells. We first assessed NF-κB signaling after treatment with siR/RSV@TNP. The results demonstrated that siR/RSV@TNP significantly inhibited LPS-induced upregulation of pNF-κB, as shown by Western blot analysis (Fig. 4c) and immunofluorescence (Fig. 4f). Besides, as shown in Fig. 4c–g, TNF-α and IL-1β expression significantly increased in LPS-treated epithelial cells compared to the control group, while siR/RSV@TNP noticeably reduced TNF-α and IL-1β production.
a Flow cytometry to test the ROS in primary choroid plexus epithelial cells after different treatments. b ROS elimination evaluation in primary ChP epithelial cells, n = 3 biological replicates for each group. c Western blot result of NF-κB, pNF-κB and TNF-α. d Immunofluorescence of TNF-α in ChP epithelial cells. e Expression of TNF-α in ChP epithelial cells with different treatments, n = 4 biological replicates for each group. Scale bar, 30 μm. f Immunofluorescence of p-NF-κB. Scale bar, 30 μm. g Western blot result of IL-1β. h Western blot result of p-PI3K, p-mTOR, and p-AKT. i Preparation of macrophage conditional medium. Illustration created using Microsoft PowerPoint by the author. j Expression of TNF-α and IL-1β in ChP epithelial cells treated with conditional medium and different materials, n = 4 biological replicates for each group respectively. k Schematic illustration of the mechanisms of siR/RSV@TNP in protecting BCSF-B integrity. Illustration created using Microsoft PowerPoint by the author, incorporating elements (siR/RSV@TNP) adapted from in BioRender. Gao, H. (2025) https://BioRender.com/i60a962. All data are presented as mean ± SD. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test or Dunnett’s multiple comparisons test were used for the statistical comparison among multiple groups, respectively. Statistical analyses were conducted with a 95% confidence interval, and significance was defined as p < 0.05. Source data are provided as a Source Data file.
In addition, Kahle et al. revealed that a macrophage-derived cytokine storm can act on ChP epithelial cells, activating inflammation and leading to CSF hypersecretion10. They also found that these interactions activate the PI3K-AkT-mTOR signaling pathway. We investigated whether the lipid nanoparticle could inhibit the crosstalk between macrophages and ChP epithelial cells. By treating ChP epithelial cells with the conditional medium of inflammatory macrophages (MCM), we observed that siR/RSV@TNP significantly inhibited cell crosstalk, reduced inflammatory cytokines, and restrained the PI3K-AkT-mTOR signaling pathway in ChP epithelial cells (Fig. 4h–j). Overall, these findings demonstrated that siR/RSV@TNP can alleviate ROS and inflammation in ChP epithelial cells (Fig. 4k).
siR/RSV@TNP inhibited occurrence and progression of hydrocephalus
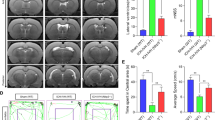
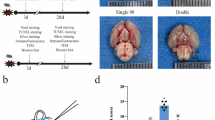
Based on these results, we established a Kaolin-induced mouse hydrocephalus model to investigate the therapeutic effect of siR/RSV@TNP in vivo. Mice received tail injections of various lipid nanoparticles every 24 h, starting 24 h before injecting Kaolin into the lateral ventricles and continuing until 48 h post-injection. The mice were randomly divided into five groups and treated with PBS, RSV@NP, siR/RSV@NP, and siR/RSV@TNP. The therapeutic effect was assessed using 7T cranial magnetic resonance imaging (MRI) 72 h after the Kaolin injection. As expected, the siR/RSV@TNP group showed a significant reduction in lateral ventricle volume compared to the PBS group (Fig. 5a, b). Additionally, hematoxylin-eosin (HE) staining of major organs showed no substantial damage, suggesting the safety of our system (Supplementary Fig. 6b). Compared with RSV@NP and siR/RSV@NP groups, siR/RSV@TNP exhibited the most significant therapeutic effect, highlighting the importance of targeted combination therapy.
a Illustrative T2-weighted images (White arrow) and three-dimensional reconstructions of the lateral ventricles were acquired three days later in Ctrl, hydrocephalus, and hydrocephalus treated with various nanomaterials groups. Scale bar, 250 mm. b Measurement of lateral ventricle volumes using associated T2-weighted images (n = 5 animals per group). c Expression of SPAK in the choroid plexus after treatments, n = 5 biological replicates for each group. d Immunofluorescence SPAK in the choroid plexus. Scale bar, 20 μm. e Immunofluorescence pSPAK in the choroid plexus. Scale bar, 10 μm. f, g Expression of pro-inflammatory cytokines CCL2 and IL-6 in the brain after treatments, n = 3 biological replicates for each group, respectively. All data are presented as mean ± SD. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test or Dunnett’s multiple comparisons test were used for the statistical comparison between the two groups and among multiple groups, respectively. Statistical analyses were conducted with a 95% confidence interval, and significance was defined as p < 0.05. Source data are provided as a Source Data file.
What’s more, RT-qPCR results demonstrated that siR/RSV@TNP significantly decreased the mRNA expression level of SPAK (Fig. 5c). Immunofluorescence showed siR/RSV@TNP significantly decreased the expression of SPAK in vivo (Fig. 5d). The immunofluorescence photographs of p-SPAK and pNKCC1, two major markers of CSF hypersecretion, showed a marked reduction in ChP epithelial cells after siR/RSV@TNP injection (Fig. 5e, Supplementary Fig. 6c). Given that inflammatory factors are also responsible for BCSFB integrity, we measured proinflammatory cytokine levels in the ChP. The level of IL-6 and CCL2 levels significantly decreased across the different treatment groups (Fig. 5f, g). These findings demonstrated that siR/RSV@TNP effectively mitigates hydrocephalus, downregulated SPAK, and alleviated ChP inflammation.
siR/RSV@TNP protects the BCSFB integrity and inhibits inflammation in vivo
To further investigate the impact of siR/RSV@TNP on gene expression in the ChP of the mouse brain, we performed RNA-sequencing on ChP tissues from hydrocephalus and siR/RSV@TNP administrated hydrocephalus mouse. The threshold for selecting differentially expressed genes was |log2foldchange| > 0.6 and an adjusted p-value (p adj) ≤ 0.05. We presented them in the form of heatmap and volcano plots (Fig. 6a, b). Compared to the untreated hydrocephalus group, a total of 3325 genes were significantly altered in expression after being administrated with siR/RSV@TNP, where 1214 genes were downregulated and 2111 genes were upregulated. Gene Ontology (GO) enrichment analysis revealed that the downregulated genes were enriched in cytokine production, leukocyte migration, myeloid leukocyte activation, and regulation of innate immune response (Fig. 6c), while the upregulated genes were associated with cell junctions and cell-substrate adhesion (Fig. 6d). KEGG pathway enrichment analysis indicated that the downregulated differentially expressed genes (DEGs) were involved in several inflammation-related pathways, including the chemokine signaling pathway, NF-κB signaling pathway, TNF signaling pathway, and Toll-like receptor signaling pathway (Fig. 6e). These pathways are known to contribute to the onset and progression of hydrocephalus10. In contrast, the upregulated DEGs were linked to focal adhesion and tight junctions (Fig. 6f).
a Heat map of differentially expressed gene (DEGs) in hydrocephalus and siR/RSV@TNP treated group. b Volcano map of DEGs. GO enrichment analysis of the gene functions of downregulated (c) and upregulated (d) DEGs. e Downregulated and f upregulated DEGs enriched in the KEGG pathway. g The upregulated DEGs are involved in tight junctions, n = 3 biological replicates for each group. h The downregulated DEGs are involved in myeloid leukocyte migration and ChP inflammation, n = 3 biological replicates for each group. i Immunofluorescence staining of ZO-1 in the choroid plexus. Scale bar, 20 μm. j Immunofluorescence staining of IBA-1in the choroid plexus. Scale bar, 40 μm. All data are presented as mean ± SD. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test or Dunnett’s multiple comparisons test were used for the statistical comparison between the two groups and among multiple groups, respectively. Statistical analyses were conducted with a 95% confidence interval, and significance was defined as p < 0.05. Source data are provided as a Source Data file.
Notably, many upregulated DEGs related to tight junctions, such as cldn2, cldn3, ocln, cldn9, and ctnnb1 were identified (Fig. 6g). Tight junctions, formed by occludin, claudins, and the associated cytosolic protein zonula occludens-1, are critical for maintaining BCSFB integrity35. The upregulation of these tight junction-related genes suggests that siR/RSV@TNP helps protect and repair BCSFB integrity in hydrocephalus.
We also observed the downregulation of specific DEGs, including Trem2, CCLs, ILs, and TLRs, which are directly involved in ChP inflammation and macrophage recruitment and activation (Fig. 6h). Both CCL2 and CCR2, key molecules in recruiting peripheral monocytes to the ChP8, were significantly downregulated. To verify the above findings, we labeled the BCSFB with zonula occludens-1 protein (ZO-1) to observe changes in tight junctions after treatment. Figure 6i shows a marked loss of ZO-1 in the hydrocephalus group, while siR/RSV@TNP treatment reversed this destruction. Additionally, we used IBA-1 staining to assess ChP macrophage infiltration (Fig. 6j). The results showed there was a substantial increase in macrophage infiltration in hydrocephalus mice, which significantly decreased after treatment, especially in the siR/RSV@TNP group. Overall, these results demonstrate that siR/RSV@TNP can alleviate hydrocephalus by restoring BCSFB integrity and inhibiting ChP inflammation (Fig. 7).
It can significantly restore blood-cerebrospinal fluid barrier (BCSFB) integrity and inhibit CSF hypersecretion by co-delivery of resveratrol (RSV) and SPAK siRNA. It can effectively attenuate ventriculomegaly and represents a promising therapeutic approach for hydrocephalus. The Scheme was created in BioRender. Gao, H. (2025) https://BioRender.com/i60a962. Agreement number: WL27PVH5O8. Source data are provided as a Source Data file.
Discussion
Hydrocephalus is the most common neurosurgical disorder worldwide. Most patients are treated with VP shunt or endoscopic third ventriculostomy (ETV), both of which have significant long-term morbidity and high failure rates36,37,38,39,40. Currently, drug treatments for hydrocephalus are unavailable, largely due to the poorly understood cellular and molecular mechanisms and the limited efficacy of drug exposure in the brain.
SPAK is highly expressed and predominantly localized to the apical membrane of ChP epithelial cells41, and increased pSPAK is closely linked to CSF hypersecretion and hydrocephalus10. Although inhibitors like STOCK1S-50699 and Closantel, targeting the ATP-binding site of SPAK, have been proposed, their application is limited due to poor in vivo pharmacokinetics and retinal toxicity42,43. Zhang et al. designed a SPAK inhibitor, ZT-1a, which effectively inhibits NKCC1 and decreases SPAK-dependent phosphorylation, however, this drug has not yet been clinically approved44. Small interfering RNAs (siRNAs) offer a promising therapeutic strategy due to their high specificity and low toxicity compared to other targeted drugs45,46. However, siRNAs cannot easily penetrate cell membranes due to their anionic properties and are quickly degraded by serum nucleases, resulting in a short half-life46. To address these challenges, we designed GSH-responsive polymers to deliver SPAK siRNA to the ChP. We utilized sPEI with a proton sponge effect to help siRNA escape from lysosomes and be released into the cytosol. Results confirmed the lysosome escape ability and GSH-sensitivity of our system. After treatment with siR/RSV@TNP, the expressions of SPAK and abnormally upregulated pSPAK-pNKCC1 were reversed both in vitro and in vivo. Additionally, through the incorporation of resveratrol, a naturally occurring bioactive small molecule, the siR/RSV@TNP formulation effectively safeguarded the integrity of the blood-cerebrospinal fluid barrier (BCSFB) by attenuating reactive oxygen species (ROS) production and inflammatory responses in choroid plexus (ChP) epithelial cells. In vivo ChP transcriptomic analysis revealed that the downregulated genes were enriched in leukocyte migration and myeloid leukocyte activation, while the upregulated genes were associated with cell junctions and cell-substrate adhesion. Immunofluorescence demonstrated increased expression of ZO-1 and reduced macrophages in ChP. Previous research has indicated that the recruitment of peripheral macrophages to the choroid plexus coincides with disruptions in its BCSFB integrity8,9. Kahle et al. have highlighted the pivotal role of choroid plexus macrophages in hydrocephalus pathogenesis10. Prolonged inflammation mediated by macrophages in the choroid plexus can lead to scarring in the ependyma and arachnoid, as well as obstruction of brain parenchymal glymphatics and meningeal lymphatics, thereby impairing CSF resorption47. Our present study demonstrated that modulating BCSFB integrity can be a promising therapeutic target to treat hydrocephalus. Furthermore, to enhance the specific distribution of our nano-drug in the ChP epithelial cells, we incorporated a T7 peptide into our nano-system. Transferrin, an 80 kDa serum glycoprotein, facilitates iron transport into proliferating cells via the transferrin receptor (TfR), which is abundantly expressed in ChP epithelial cells24,25,48,49. Our results showed that T7-conjugated siR/RSV@NP accumulates more effectively in the ChP and brain, exhibiting better therapeutic efficacy compared to non-targeted lipid nanoparticles. Hence, this study underscores TfR as a promising target for efficient drug delivery to the BCSFB, offering the potential for treating hydrocephalus.
Despite we have successfully designed an effective nano-modulator to treat hydrocephalus. Several limitations should be reminded. Firstly, the pathogenesis of hydrocephalus is complex and not yet fully understood, our nano-modulator aimed to restrain CSF hypersecretion, a critical mechanism in acute hydrocephalus development. Hence, it may not be broadly applicable to chronic hydrocephalus caused by impaired absorption or obstruction of CSF pathways. Secondly, the primary design of the nanoparticles in this study was to target ChP epithelial cells to suppress CSF hypersecretion. As a result, we did not investigate the long-term effects of treatment on ependymal denudation store, or repair the scarring of intraventricular and parenchymal (glia-lymphatic) CSF pathways. Future studies will address these aspects to provide a more comprehensive understanding. Lastly, our present study targeting the ChP is largely preclinical, and data on long-term safety, tolerability, and efficacy are limited. Translating these findings to clinical practice requires more rigorous investigation in animal models and human trials.
Methods
Ethical statement
All animal experiments were performed under the guidelines, evaluated, and approved by the ethics committee of West China Hospital of Sichuan University (No. 20220304039)
Mice
Male Kunming mice aged 6‒8 weeks and weighing 25‒30 g were purchased from the Chengdu Dossy Experimental Animals Co., Ltd. (Chengdu, China). The animals were housed under SPF condition in groups of 10 mice per cage, and maintained at a temperature of ~25 °C in a humidity-controlled environment with a 12 h light/dark cycle, and with free access to standard food and water.
Reagents and general methods
Low-weight molecular polyethylenimine (PEI) was purchased from RHAWN. Cholesterol and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) were obtained from AVT (shanghai) Pharmaceutical Tech Co., Ltd. (Shanghai, China). 1,2-Dioleoyl-sn-glycero-3-phospho-L-serine (DOPS), was purchased from Bide Pharmatech Co., Ltd. (Shanghai, China). 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethyleneglycol)] (DSPE-PEG-MAL) were obtained from Ponsure Biological Co., Ltd. (Shanghai, China). Sphingomyelin was purchased from Jiuding Chemical Co., Ltd. (Shanghai, China). T7 peptide (Cys-His-Ala-Ile-Tyr-Pro-Arg-His) was synthesized by Taopu Biotech (Nanjing, China). The SPAK siRNA1 (sense: CCAUGUUGGAUAUCAUCAATT, antisense: UUGAUGAUAUCCAACAUGGTT), SPAK siRNA2 (sense: GGAACAGGUGAGAGGCUAUTT, antisense: AUAGCCUCUCACCUGUUCCTT), SPAK siRNA3 (sense: CAGCGCCUUAC CACAAAUATT, antisense: UAUUUGUGG UAAGGCGCUGTT) and SPAK siRNA 4 (sense: GAACUUAAUGACAUACGAUTT, antisense: AUCGUAUGUCAUUAAGUUCTT) were designed and purchased from Genepharma company (Shanghai, China). 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindodicarbocyanine perchlorate (DiD) was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China) Lipopolysaccharide (LPS) was purchased from Solarbio (L8880, Beijing, China). Kaolin was purchased from Sigma-Aldrich (K7375, America). Resveratrol was purchased from MedChemExpress (HY-16561, USA).
Synthesis of sPEI and DSPE-PEG-T7
The synthesis procedure was executed in accordance with the documented protocol, albeit with certain modifications. The pH of a 500 mg aqueous solution of PEI800 was adjusted to 7.2 using 0.5 mol/L HCl through incremental additions. Following the evaporation of water, the resulting yellow solid was dissolved in 30 ml of methanol in a round-bottom flask and purged with nitrogen three times. A calculated excess amount (5-fold molar excess to PEI800) of propylene sulfide was added, and the reaction was carried out at 60 °C for 24 h. The mixture was evaporated to dryness under reduced pressure, dissolved in methanol, and precipitated in cold diethyl ether twice. The resulting precipitate was dissolved in DMSO and stirred at room temperature for 48 h. The product was then purified by dialysis against water (MWCO 3500) and lyophilized. The chemical structure was confirmed using 1H NMR (in D2O, 400 MHz): δ 3.14–2.66 (m, NCH2CH2N, NCH2CHMeS), 1.25–1.05 (m, CH3).
To synthesize DSPE-PEG-T7, DSPE-PEG2000-MAL was dissolved in DMF at a concentration of 10 mg/mL and subsequently a 1.5-fold molar excess of T7 peptide was introduced. The resulting solution was stirred at 40 °C under a nitrogen atmosphere for 24 h. Following the completion of the reaction, the mixture underwent dialysis against deionized water for a duration of 24 h. The ultimate product was acquired through freeze-drying and subjected to time-of-flight mass spectrometry for structural confirmation.
Preparation of polyplexes with different weight ratios
In order to evaluate the encapsulation efficiency of siRNA and the polyplexes’ responsiveness to glutathione (GSH), a gel electrophoresis assay was executed. The siRNA and sPEI were dissolved separately in DEPC water to produce stock solutions with concentrations of 1 mg/mL and 20 μM, correspondingly. Following a predetermined array of weight ratios (w: w = 0, 0.3, 0.5, 1, 2, 3, 4), different volumes of the sPEI solution were combined with 2 uL of siRNA and incubated at room temperature for 10 min. Subsequently, the nucleic acid loading buffer was introduced and samples containing various weight ratios were loaded and subjected to electrophoresis at 100 V for 20 min. To assess the response to glutathione (GSH), the complexes were treated with a 10 mmol/L GSH solution at 37 °C and visualized using TEM imaging.
SPAK knockdown efficiency assessments of polyplexes
To assess the efficiency of siR/RSV@TNP in knockdown SPAK and its downstream ion channels. The primary ChP epithelial cells were seeded in the 24-well and 6-well plates. These cells were treated with polyplexes containing 50 nmol SPAK siRNA for 48 h. RT-qPCR was performed as described previously and the experimental details are described below in Quantitative Real-time PCR section50. The expression of SPAK an NKCC1 protein was detected using the Western Blotting analysis, employing specific antibodies for SPAK (ABclonal, A2275, 1:1000) and NKCC1 (Proteintech, 13884-1-AP, 1:1000).
Preparation and characterization of siR/RSV@TNP
The synthesis of siR/RSV@TNP was conducted as follows: Various lipids and resveratrol respectively were dissolved in a solvent mixture of chloroform, ethanol, or a 1:1 (v/v) methanol-ethanol solution at a concentration of 4 mg/mL. To create the organic phase, cholesterol, sphingomyelin, DOPC, DOPS, DOPE, DSPE-PEG2000, DSPE-PEG2000-T7 and resveratrol were mixed and this lipid-resveratrol solution was incrementally added to an aqueous phase for Preliminary vortices. The resulting mixture underwent ultrasonication (JY92-IIN, SCITNEZ, Ningbo) for 5 min in an ice bath, followed by the evaporation of the organic solvent using a rotary evaporator, producing the lipopolyplex solution. Subsequently, siSPAK was combined with sPEI and allowed to react for 30 min. This mixture was then introduced to the lipopolyplex solution and incubated for 4 h at ambient temperature. To produce Cy5-labeled nanoparticles, all synthesis procedures will remain consistent with those described above, with the exception that Cy5-labeled siNC (scramble siRNA) will replace the SPAK siRNA. For the synthesis of DiD@TNP, all procedures will adhere to the aforementioned steps, except that DiD will be incorporated into the organic phase to label the nanoparticle, and SPAK siRNA will be substituted with scramble siRNA.
Dynamic light scattering (DLS) analysis was performed to characterize the size and zeta potential of the siR/RSV@TNP, while transmission electron microscopy (TEM) was employed to investigate its morphology. For determining the drug loading capacity (DLC) and encapsulation efficiency (EE) of pioglitazone, high-performance liquid chromatography (HPLC) was utilized. The release kinetics of resveratrol were evaluated via the dialysis method, where 1 mL of siR/RSV@TNP solution (containing 1 mg of resveratrol) was placed in a dialysis bag (MWCO = 2000 Da) and dialyzed against 10 mL of PBS with 1% Tween 80 at pH values of 7.4 and 5.0. Samples of the dialysate were collected at specified time intervals for HPLC analysis.
Experimental cells
We cultured primary ChP epithelial cells using a previously described method51 and the experimental details are described below. Initially, the choroid plexus (ChP) was isolated from the brain ventricles of male Sprague-Dawley rats. Prior to dissection, animals were deeply anesthetized with tribromoethanol, TBE. The tissue was immediately placed into sterile dissection media (1× DMEM supplemented with 1× penicillin-streptomycin).” The tissue was finely minced in a 1.5 mL sterile EP tube, digested with pancreatin, and then repeatedly aspirated using a 200 μL pipette. Following digestion, the tissue was centrifuged and resuspended in a specialized ChP epithelial cell medium, containing 10% fetal bovine serum (FBS), 10,000 units/mL penicillin-streptomycin, and 10 ng/mL human epidermal growth factor (EGF) in DMEM. The ChP epithelial cells were cultured for 14 days with media changes every 2–3 days before being utilized for experiments. RAW264.7 cells were obtained from the Haixing Biosciences (TCM-C766, Suzhou, Jiangsu, China). MC3T3 and HT22 cells were obtained from Pricella (CL0710 and CL-0697, Wuhan, Hubei, China). The cells were cultured at 37 °C in DMEM supplemented with 1% penicillin-streptomycin and 10% FBS in a 5% CO2 atmosphere.
Cell uptake and lysosomal escape assay
For the cellular uptake assay, ChP epithelial cells were cultured in 12-well plates to establish a defined density. Cy5-siR/RSV@NP and Cy5-siR/RSV@TNP were subsequently introduced to the cells. After incubation periods of 1 h and 4 h, the cells were rinsed with PBS, and the efficacy of cellular uptake was analyzed using flow cytometry. Additionally, confocal laser scanning microscopy (CLSM) was utilized to visualize the uptake. In this process, cells were treated with Cy5-siR/RSV@NP and Cy5-siR/RSV@TNP for both 1 h and 4 h, followed by a 5-min DAPI staining to label nuclear DNA. The cells were then washed and fixed in 4% PFA prior to imaging.
For the lysosomal escape assay, choroid plexus epithelial cells were seeded onto glass slides. To track siRNA and lysosomes, Cy5 was used to label the siRNA, while Lysotracker Red DND99 was employed for lysosomal visualization.
Cytotoxicity assay
To assess cell viability, a CCK 8 assay was conducted. Specifically, choroid plexus epithelial cells were seeded in 96-well plates and subjected to different treatments. After the treatment period, 10 µL of CCK-8 reagent is added to each well containing 100 µL of culture medium. The absorbance of each well is measured at 450 nm using a microplate reader, with the absorbance values correlating directly to cell viability.
Alleviating intracellular ROS
Primary ChP epithelial cells were seeded in 12-well plates and incubated with various formulations for 48 h, followed by a 2-h incubation with H2O2 (200 μmol/L). After removing the medium, cells were treated with serum-free medium containing 10 μmol/L DCFH-DA (S0033S, Beyotime) for 30 min at 37 °C. Subsequently, cells were collected and washed three times with PBS. Fluorescent intensity was measured using a flow cytometer (Beckman, USA).
Kaolin-induced hydrocephalus models
A recent meta-analysis by Rehman et al. of 31,994 cases found no significant sex-related differences in hydrocephalus incidence52. We thus conducted our study primarily using male mice and a male hydrocephalus mouse model for further investigation. The hydrocephalus mice model was induced via kaolin injection into the right lateral ventricle53,54. After the successful induction of anesthesia using tribromoethanol, TBE, the mice were carefully positioned in a stereotaxic frame. A cranial burr hole was drilled at coordinates 0.3 mm posterior and 1 mm lateral to bregma, ensuring precision. The surgical site was covered with sterile gauze to prevent contamination. A micro-syringe needle was inserted into the lateral ventricle (depth: 2.5 mm) and a 10 μL sterile suspension of 3% kaolin (ultrasonic emulsification about 15 min) in artificial CSF in saline was injected slowly into the lateral ventricle. The needle was kept in place for an additional 10 min to prevent backflow. Finally, the incision site was sutured to ensure proper wound healing.
Western Blot
Primary ChP epithelial cells were collected after different treatments. The lysates were then centrifuged at 14,000 ×g for 15 min at 4 °C, and the supernatant was collected. Protein concentration was determined using a BCA assay. Equal amounts of protein were loaded onto SDS-PAGE gels and separated by electrophoresis. The proteins were transferred onto PVDF membranes, which were subsequently blocked with 5% non-fat milk in TBST for 1 h at room temperature. The membranes were then incubated overnight at 4 °C with primary antibodies: p-NK-κB (CST, 3033, 1:1000), NF-κB (CST,8242,1:1000), TNF-α (Abcam, ab307164,1:1000), p-SPAK (Sigma, 07-2273,1:1000), p-mTOR (Abcam, ab131538,1:2000), p-PI3K (Abcam, ab191606, 1:1000), p-AKT (Abcam, ab38449,1:1000), CCL2 (Abcam, ab7202,1:1000), IL-1β (Abcam,ab283818,1:1000), p-NKCC1 (Sigma,ABS1004, 1:1000). After washing, the membranes were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. Protein bands were visualized using an enhanced chemiluminescence (ECL) detection system. Band intensities were quantified using ImageJ software and normalized to the expression of β-Actin as a loading control.
Quantitative real-time PCR
Total RNA from the cells and ChP samples was extracted using TRIzol reagent from Thermo Fisher Scientific (Waltham, MA, USA). cDNA synthesis was conducted with a reverse transcription kit from Vazyme (Nanjing, China). Subsequently, RT-qPCR was performed using the qPCR SYBR Green Master Mix (Servicebio, China) according to the manufacturer’s protocol. The primer sets for the targeted genes are listed in Supplementary Tables 1 and 2. Gene expression levels were quantified and normalized to GAPDH or β-ACTIN.
In vivo imaging in hydrocephalus mice
Did-encapsulated lipid nanoparticles, with or without T7 modification, were administered to facilitate further tracking of biodistribution. Both the DiD@NP and DiD@TNP lipid nanoparticles were intravenously injected into Kunming mice. The mice were anesthetized using tribromoethanol, TBE and euthanized 2 h after injection to retrieve major organs for ex vivo imaging, followed by quantification of fluorescence intensity using the Lumina III Imaging System from PerkinElmer (Waltham, USA). The brains were fixed in 4% paraformaldehyde for 24 h, and then underwent a structured dehydration process before frozen sectioning. DAPI staining was applied to visualize nuclei on the sections, which were promptly examined under a fluorescence microscope, specifically utilizing the Leica DMi8 (Weztlar, Germany). To further determine the co-localization of different lipid nanoparticles with choroid plexus, immunofluorescence staining was performed on choroid plexus endothelial cells, and an inverted fluorescence microscope captured images.
Cranial MRI scans
The mice were imaged 72 h after Kaolin injection using a Bruker BioSpec 7T MRI (BioSpec 70/30USR, Bruker, Germany) under isoflurane anesthesia. During imaging, breathing, and heart rates were monitored to ensure appropriate anesthesia depth. Axial T2-weighted images were obtained, and the lateral ventricle volumes were measured using 3D-Slicer software55 (4.10.2, NIH, MD, USA).
Immunofluorescence staining
Immunofluorescence staining was performed on brain tissue and cell samples to visualize specific proteins. Before euthanized, the mice were anesthetized using tribromoethanol, TBE. Brain tissues were fixed in 4% paraformaldehyde, cryoprotected in sucrose, and sectioned at 20 μm thickness. Sections were permeabilized with 0.3% Triton X-100 and blocked with 5% bovine serum albumin (BSA). Primary antibodies were incubated overnight at 4 °C, followed by washing and incubation with fluorescently labeled secondary antibodies for 1 h at room temperature. The following antibodies were used: p-NK-κB (CST, 3033, 1:400), TNF-α (Abcam, ab307164, 1:200), p-SPAK (Sigma, 07-2273, 1:400), CCL2(Abcam, ab7202, 1:400), p-NKCC1 (Sigma, ABS1004, 1:200), ZO-1(Proteintech, 21773-1-AP, 1:400), IBA-1(Proteintech, 10904-1-AP, 1:500), SPAK (ABclonal, A2275, 1:200). DAPI was used to stain nuclei. For cell staining, primary ChP epithelial cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 3% BSA. Primary antibodies were applied overnight at 4 °C, followed by secondary antibody incubation and DAPI staining. Samples were mounted with an antifade medium and imaged using a fluorescence microscope.
Statistical analysis and reproducibility
All data were presented as the mean ± SD, with error bars depicted in each graph. Statistical analysis was performed using GraphPad Prism software 10.1.1. Statistical comparisons between two groups were conducted using Two-tailed Student’s t test, while comparisons among three or more groups were analyzed using one-way ANOVA followed by a Tukey post hoc test or Dunnett adjustment. Prior to conducting parametric tests (such as t-tests or ANOVA), all data were assessed for normality. When the assumption of normal distribution was violated, non-parametric tests were employed. Statistical analyses were conducted with a 95% confidence interval, and significance was defined as p < 0.05. Dots overlaid on the bar graphs are the value measured for biological replicates. The micrographs are representative images (n = 3 biological replicates) and data presented are at least included 3 biological replicates. No data were excluded from the analyses. The experiments were randomized. The investigators were blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the results of this study are available within the paper, Supplementary Information or Source data files. Source data are provided with this paper.
References
Boivin, M. J., Kakooza, A. M., Warf, B. C., Davidson, L. L. & Grigorenko, E. L. Reducing neurodevelopmental disorders and disability through research and interventions. Nature 527, S155–S160 (2015).
Dewan, M. C. et al. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J. Neurosurg. 130, 1065–1079 (2018).
Kahle, K. T., Kulkarni, A. V., Limbrick, D. D. Jr. & Warf, B. C. Hydrocephalus in children. Lancet 387, 788–799 (2016).
Oyon, D. E. et al. Ventriculopleural shunt outcomes for pediatric hydrocephalus: a single-institution experience. Childs Nerv. Syst. 39, 2105–2113 (2023).
Ghersi-Egea, J. F. et al. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 135, 337–361 (2018).
Zhu, L. et al. Klotho controls the brain-immune system interface in the choroid plexus. Proc. Natl. Acad. Sci. USA 115, E11388–E11396 (2018).
Demeestere, D., Libert, C. & Vandenbroucke, R. E. Clinical implications of leukocyte infiltration at the choroid plexus in (neuro)inflammatory disorders. Drug Discov. Today 20, 928–941 (2015).
Cui, J. et al. Inflammation of the embryonic choroid plexus barrier following maternal immune activation. Dev. Cell 55, 617–628.e616 (2020).
Wang, J. et al. Role of SPAK-NKCC1 signaling cascade in the choroid plexus blood-CSF barrier damage after stroke. J. Neuroinflamm.19, 91 (2022).
Robert, S. M. et al. The choroid plexus links innate immunity to CSF dysregulation in hydrocephalus. Cell 186, 764–785.e721 (2023).
Wang, Q. et al. Choroid plexus CCL2‒CCR2 signaling orchestrates macrophage recruitment and cerebrospinal fluid hypersecretion in hydrocephalus. Acta Pharm. Sin. B 14, 4544–4559 (2024).
Zhang, Z. et al. NLRP3-dependent lipid droplet formation contributes to posthemorrhagic hydrocephalus by increasing the permeability of the blood-cerebrospinal fluid barrier in the choroid plexus. Exp. Mol. Med. 55, 574–586 (2023).
Steffensen, A. B. et al. Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat. Commun. 9, 2167 (2018).
Castañeyra-Ruiz, L., Hernández-Abad, L. G., Carmona-Calero, E. M., Castañeyra-Perdomo, A. & González-Marrero, I. AQP1 overexpression in the CSF of obstructive hydrocephalus and inversion of its polarity in the choroid plexus of a Chiari malformation type II case. J. Neuropathol. Exp. Neurol. 78, 641–647 (2019).
Karimy, J. K. et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat. Med. 23, 997–1003 (2017).
Krishnamurthy, S., Li, J., Schultz, L. & Jenrow, K. A. Increased CSF osmolarity reversibly induces hydrocephalus in the normal rat brain. Fluids Barriers CNS 9, 13 (2012).
Han, L., Huang, R., Liu, S., Huang, S. & Jiang, C. Peptide-conjugated PAMAM for targeted doxorubicin delivery to transferrin receptor overexpressed tumors. Mol. Pharm. 7, 2156–2165 (2010).
Qureshi, A. A. et al. Inhibition of nitric oxide and inflammatory cytokines in LPS-stimulated murine macrophages by resveratrol, a potent proteasome inhibitor. Lipids Health Dis. 11, 76 (2012).
Ghafouri-Fard, S. et al. Disease-associated regulation of gene expression by resveratrol: Special focus on the PI3K/AKT signaling pathway. Cancer Cell Int. 22, 298 (2022).
Thompson, D., Brissette, C. A. & Watt, J. A. The choroid plexus and its role in the pathogenesis of neurological infections. Fluids Barriers CNS 19, 75 (2022).
Deczkowska, A., Baruch, K. & Schwartz, M. Type I/II interferon balance in the regulation of brain physiology and pathology. Trends Immunol. 37, 181–192 (2016).
Schwerk, C. et al. TNFalpha induces choroid plexus epithelial cell barrier alterations by apoptotic and nonapoptotic mechanisms. J. Biomed. Biotechnol. 2010, 307231 (2010).
Chiu, P. S. & Lai, S. C. Matrix metalloproteinase-9 leads to claudin-5 degradation via the NF-kappaB pathway in BALB/c mice with eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis. PLoS One 8, e53370 (2013).
Moos, T. & Morgan, E. H. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol. Neurobiol. 20, 77–95 (2000).
Moos, T. Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J. Comp. Neurol. 375, 675–692 (1996).
Lu, J., Kaur, C. & Ling, E. A. Expression and upregulation of transferrin receptors and iron uptake in the epiplexus cells of different aged rats injected with lipopolysaccharide and interferon-gamma. J. Anat. 187, 603–611 (1995).
Dabbagh, F., Schroten, H. & Schwerk, C. In vitro models of the blood-cerebrospinal fluid barrier and their applications in the development and research of (neuro)pharmaceuticals. Pharmaceutics 14, 1729 (2022).
Mezzapesa, A. et al. Plasminogen in cerebrospinal fluid originates from circulating blood. J. Neuroinflamm. 11, 154 (2014).
Erickson, M. A. et al. Lipopolysaccharide impairs amyloid beta efflux from brain: altered vascular sequestration, cerebrospinal fluid reabsorption, peripheral clearance and transporter function at the blood-brain barrier. J. Neuroinflamm. 9, 150 (2012).
Boitsova, E. B. et al. The inhibitory effect of LPS on the expression of GPR81 lactate receptor in blood-brain barrier model in vitro. J. Neuroinflamm. 15, 196 (2018).
Wolburg, H., Wolburg-Buchholz, K., Liebner, S. & Engelhardt, B. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci. Lett. 307, 77–80 (2001).
Wang, Q. et al. Choroid plexus CCL2‒CCR2 signaling orchestrates macrophage recruitment and cerebrospinal fluid hypersecretion in hydrocephalus. Acta Pharm. Sin. B, 14, 4544–4559 (2024).
Goldim, M. P. et al. Oxidative stress in the choroid plexus contributes to blood-cerebrospinal fluid barrier disruption during sepsis development. Microvasc. Res. 123, 19–24 (2019).
Tenenbaum, T. et al. Dexamethasone prevents alteration of tight junction-associated proteins and barrier function in porcine choroid plexus epithelial cells after infection with Streptococcus suis in vitro. Brain Res. 1229, 1–17 (2008).
Ueno, M., et al. Transporters, ion channels, and junctional proteins in choroid plexus epithelial cells. Biomedicines 12, 708 (2024).
Rei, K. M., Ghauri, M. S., Uddin, M. B. & Siddiqi, J. Ventriculoperitoneal shunt failure and cerebrospinal fluid protein: a meta-analysis and systematic review. Cureus 16, e54362 (2024).
Paudel, P., Bista, P., Pahari, D. P. & Sharma, G. R. Ventriculoperitoneal shunt complication in pediatric hydrocephalus: risk factor analysis from a single institution in Nepal. Asian J. Neurosurg. 15, 83–87 (2020).
Anderson, I. A. et al. Factors associated with 30-day ventriculoperitoneal shunt failure in pediatric and adult patients. J. Neurosurg. 130, 145–153 (2018).
Wang, Q. et al. Prediction of endoscopic third ventriculostomy (ETV) success with preoperative third ventricle floor bowing (TVFB): a supplement to ETV success score. Neurosurg. Rev. 43, 1575–1581 (2020).
Wang, Q. et al. Third ventricle floor bowing: a useful measurement to predict endoscopic third ventriculostomy success in infantile hydrocephalus. Acta Neurochir. 162, 31–37 (2020).
Piechotta, K., Lu, J. & Delpire, E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J. Biol. Chem. 277, 50812–50819 (2002).
Mori, T. et al. Chemical library screening for WNK signalling inhibitors using fluorescence correlation spectroscopy. Biochem J. 455, 339–345 (2013).
Tabatabaei, S. A. et al. Closantel; a veterinary drug with potential severe morbidity in humans. BMC Ophthalmol. 16, 207 (2016).
Zhang, J. et al. Modulation of brain cation-Cl(-) cotransport via the SPAK kinase inhibitor ZT-1a. Nat. Commun. 11, 78 (2020).
Guo, F. et al. Recent progress of small interfering RNA delivery on the market and clinical stage. Mol. Pharm. 21, 2081–2096 (2024).
Zhang, J. et al. A comprehensive review of small interfering RNAs (siRNAs): mechanism, therapeutic targets, and delivery strategies for cancer therapy. Int. J. Nanomed. 18, 7605–7635 (2023).
Karimy, J. K. et al. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nat. Rev. Neurol. 16, 285–296 (2020).
Mendez-Gomez, H. R. et al. Transcytosis in the blood-cerebrospinal fluid barrier of the mouse brain with an engineered receptor/ligand system. Mol. Ther. Methods Clin. Dev. 2, 15037 (2015).
Singh, M. Transferrin as a targeting ligand for liposomes and anticancer drugs. Curr. Pharm. Des. 5, 443–451 (1999).
Wang, Q. et al. Modulation of cerebrospinal fluid dysregulation via a SPAK and OSR1 targeted framework nucleic acid in hydrocephalus. Adv. Sci. 11, e2306622 (2024).
Lallai, V., Ahmed, A. & Fowler, C. D. Method for primary epithelial cell culture from the rat choroid plexus. Bio Protoc. 10, e3532 (2020).
Rehman, S., Phan, H. T., Chandra, R. V. & Gall, S. Is sex a predictor for delayed cerebral ischaemia (DCI) and hydrocephalus after aneurysmal subarachnoid haemorrhage (aSAH)? A systematic review and meta-analysis. Acta Neurochir. 165, 199–210 (2023).
Shaolin, Z. et al. Hydrocephalus induced via intraventricular kaolin injection in adult rats. Folia Neuropathol. 53, 60–68 (2015).
Shevtsov, M. A. et al. Changes of fractional anisotropy (FA) and apparent diffusion coefficient (ADC) in the model of experimental acute hydrocephalus in rabbits. Acta Neurochir. 157, 689–698 (2015).
Fedorov, A. et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 30, 1323–1341 (2012).
Acknowledgements
We are grateful to the National Natural Science Foundation of China (82201501), the Natural Science Foundation of Sichuan Province (2023NSFC1581), the Fundamental of Research Funds for the Central Universities, and 1·3·5 projects for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2020HXFH013), and Postdoctoral Research Fund of West China Hospital, Sichuan University (No.2024HXBH136), and Sichuan Science and Technology Program (22ZDYF2619, 2022NSFC1380) for financial support.
Author information
Authors and Affiliations
Contributions
Huile Gao and Qiguang Wang designed the research. Qiguang Wang, Xue Xia, and Huan Zhang carried out the experiments and performed data analysis. Lei Zhu performed RNA-seq analysis. Qiguang Wang and Yue Li performed tissue pathological staining and analysis. Huan Zhang and Jian Cheng performed MRI studies. Qiguang Wang, Xuhui Hui, Yulong Shi, and Yuzhao Tang constructed rat PHH models and analyses. Qiguang Wang and Xue Xia wrote the manuscript. Qiguang Wang, Xue Xia, and Huile Gao revised the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kristopher Kahle and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Q., Xia, X., Zhang, H. et al. Targeting modulation of the choroid plexus blood-CSF barrier and CSF hypersecretion via lipid nanoparticle-mediated co-delivery of siRNA and resveratrol. Nat Commun 16, 6389 (2025). https://doi.org/10.1038/s41467-025-61543-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61543-1