Abstract
Anaerobic bacteria-mediated tumor therapy faces multiple challenges, including potential toxic side effects, complex manufacturing processes, and impaired hypoxic targeting. Here, based on the excellent biocompatibility and distinctive metabolic ability of natural anaerobic sulfate-reducing bacteria (SRB) to dissimilate sulfate into sulfide, we construct in situ-biosynthesized ferrous sulfide nanoparticle-SRB (FeS@SRB) biohybrid to enhance tumor-targeted therapy. Interestingly, SRB acts as both a biosynthesis factory and active tumor-targeted delivery vehicles. Our systematic studies reveal that FeS@SRB has excellent biosafety and tumor targeting capabilities, achieving over 50% tumor delivery efficiency in female mice post-intravenous injection, which is 17 times higher than that of conventional chemically-synthesized FeS@BSA nanoparticles. Upon near-infrared laser irradiation, FeS@SRB hybrids exhibit synergistic photothermal-chemodynamic effect, amplifying oxidative stress to trigger tumor cells ferroptosis and apoptosis, thereby effectively suppressing both subcutaneous and orthotopic tumor growth. This SRB-based therapeutic strategy expands research into tumor-targeting platforms and the biosynthesis of metal sulfide nanoparticles for enhanced tumor therapy.
Similar content being viewed by others
Introduction
Efficient delivery of therapeutic agents to tumor sites can significantly improve treatment efficacy while minimizing toxic side effects. Although nanotherapeutic agents can accumulate at tumor sites through enhanced permeability and retention effects, etc., various physiological barriers still compromise their delivery efficiency1,2. Due to the hypoxic nature of the tumor microenvironment, anaerobic bacteria can actively target tumor sites through self-propelling3,4. In addition, an immunosuppressive microenvironment and rich nutrients at the tumor tissue provide an ideal habitat for anaerobic bacteria colonization and proliferation. Moreover, these bacteria can inhibit tumor growth through direct destruction of tumor cells, activation of immune responses, and production of antitumor metabolites5,6,7. Consequently, anaerobic bacteria-mediated tumor therapy has emerged as a versatile platform for targeted drug delivery and combination treatments8,9. However, anaerobic bacteria-mediated tumor therapy still suffers toxicity associated with certain pathogenic anaerobic strains and compromised delivery efficiency due to various modifications10,11.
To mitigate the toxicity of pathogenic anaerobic bacteria (e.g., Salmonella typhimurium, Listeria monocytogenes, and Shewanella oneidensis), genetic modifications11 or surface coatings12 have been employed, but these approaches often simultaneously reduce the bacteria’s anaerobic targeting capacity. Probiotics, owing to their intrinsic non-pathogenic, autophagy-inducing properties and immunomodulatory effects13,14, represent an alternative strategy. Nevertheless, both attenuated bacteria and probiotics alone face the challenge of limited therapeutic efficacy. To address these limitations, various chemotherapeutic agents and functionalized nanomaterials have been engineered onto the bacteria to enhance their antitumor effects through various strategies, such as chemical coupling (e.g., amidation, in situ polymerization, or click reaction)9, physical interactions (e.g., electrostatic or hydrophobic interactions)5, and biological orthogonal reactions (e.g., metabolic labeling, ligand-receptor interactions, and genetic engineering)15, etc. However, these engineered bacterial therapeutics are still limited to some extent by complex preparation methods, compromised bacterial viability, diminished hypoxic targeting capability, and safety concerns16,17.
In recent years, in situ biosynthesis, which utilizes organisms as biological factories, has attracted increasing attention due to the advantages of its green, economical, safe, and convenient process18,19,20,21. Especially, this preparation process can protect bacteria from harsh chemical agents and is expected to maintain their high activity and tumor targeting property. If the biosynthesis is mediated by nonpathogenic bacteria, it will offer excellent biosafety and serve as an optimal candidate for tumor-targeted therapy. Sulfate-reducing bacteria (SRB) are anaerobic microorganisms widely distributed in nature22 and specific human ecological niches, including the gastrointestinal tract, oral cavity, and reproductive system23,24. Under normal conditions, these bacteria coexist with human hosts without causing infections or diseases. Notably, SRB possess the unique capability to reduce sulfate to sulfide via cytochrome-mediated electron transport in their outer membrane25. The generated sulfide can subsequently react with metal ions, such as ferrous ions (Fe2+) in the environment, to synthesize metal sulfide nanoparticles (NPs)26,27. However, the anaerobic targeting capability and the potential application of SRB and in situ biosynthesized metal sulfide nanoparticles remain unexplored.
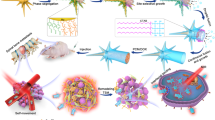
Here, we develop FeS NPs-SRB biohybrids to enhance tumor-targeting capability and integrate multimodal therapies to improve the therapeutic efficacy and safety. As illustrated in Fig. 1A, FeS NPs on the surface of SRB (named as FeS@SRB) are synthesized through a facile one-step biomineralization process using SRB as a biofactory in the presence of Fe2+ ions. The obtained FeS@SRB biohybrids can highly selectively colonize and infiltrate hypoxic tumor tissues after intravenous injection, and enter tumor cells through endocytosis due to the excellent biosafety and anaerobic-targeting property of SRB (Fig. 1B). Upon reaching the tumor, FeS@SRB release Fe2+ in the weakly acidic tumor microenvironment to trigger Fenton reaction, generating highly oxidative hydroxyl radicals (•OH) to destroy tumor cells. Assisted by near-infrared (NIR) laser irradiation, the photothermal effect of FeS NPs induces tumor cell death and further reinforces oxidative stress, ferroptosis, and apoptosis. In a word, FeS@SRB mediates a dual-mode synergistic therapeutic system by activating the Fenton reaction and photothermal effects, significantly inhibiting tumor growth. Our study confirms that SRB and FeS@SRB have robust tumor-targeting ability with delivery efficiency up to 50.5%, 17 times higher than that of conventional chemically-synthesized FeS@BSA NPs after intravenous injection. This work not only proposes a nonpathogenic bacteria-driven hypoxia-targeting strategy but also provides a green biosynthesis platform for nanotherapeutics, which has the potential to realize efficient tumor-targeted delivery, superior biosafety, and enhanced therapeutic effects.
A One-step biosynthesis of FeS@SRB using SRB as a biofactory through self-mineralization. B Mechanism of chemodynamic/photothermal synergistic therapy. FeS@SRB utilized intrinsic anaerobic properties to actively target tumors, which were then partially endocytosed by the tumor cells and released Fe2+ in the acidic tumor microenvironment, which triggered a Fenton reaction to induce a large amount of •OH generation to kill tumor cells. Concurrently, FeS@SRB generated a significant thermal effect under NIR laser irradiation to destroy tumor cells and further enhance oxidative stress and ferroptosis, significantly inhibiting tumor growth. Images were provided by Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/), and created in BioRender. Dong, M. (2025) https://BioRender.com/3s641u7.
Results
FeS nanoparticles were successfully biosynthesized in situ on the SRB surface
SRB (Desulfovibrio) obtained from Shengli oil field in China were used in this work, which was identified as Gram-negative bacteria by Gram staining (Supplementary Fig. 1). Then, SRB were repeatedly domesticated in a modified bacterial medium without iron ions (Supplementary Table 1). The mechanism for preparing FeS@SRB is shown in Fig. 2A. SRB utilize lactate as an electron donor (Supplementary Fig. 2) and SO42- as a terminal electron acceptor to obtain energy through dissimilatory reduction process, generating S2- via cytochrome c (Cyt c)-mediated electron transport21,28,29,30,31. Therefore, H2S will be produced in an acidic medium, and FeS NPs will be formed upon introduction of Fe2+ into the SRB culture medium. The optimal preparation conditions for FeS@SRB were studied by varying the initial concentration of Fe2+ in the culture medium and culture time. The OD600 values of the upper layer suspension initially increased rapidly, reached a maximum on day 2 and stabilized after 3 days (Supplementary Fig. 3A, B). The highest OD600 value, meaning the largest yield of FeS@SRB, was achieved on day 2 with an initial Fe2+ concentration of 200 mg L−1. The oxidation-reduction potential (ORP) of the SRB medium decreased with increasing metabolic activity of SRB32. The lowest ORP was observed at an initial Fe2+ concentration of 200 mg L−1 (Supplementary Fig. 3C), indicating the highest metabolic capacity of FeS@SRB. Although lower Fe2+ concentration and extended culture time resulted in a higher Fe2+ removal rate (Supplementary Fig. 3D), the yield of FeS NPs was low. Therefore, subsequent experiments used an initial Fe2+ concentration of 200 mg L−1 to ensure SRB with high activity and high yield of FeS NPs.
A Schematic illustration of the forming process and mechanism. B SEM-EDS elemental mapping images. Scale bar: 2.5 μm. C Size distribution measured by DLS at room temperature. D Zeta potential. n = 3 independent samples. Data are presented as mean ± SD. E UV-vis-NIR absorption spectra. F FTIR spectra. G XRD patterns. H XPS spectra of Fe 2p, S 2p of FeS@SRB. Source data are provided as a Source Data file. Images in the schematic illustration were provided by Servier Medical Art (https://smart.servier.com/) and licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
The micromorphology of FeS@SRB was characterized using a scanning electron microscope (SEM). After co-incubation for one day, a small amount of FeS NPs was localized on the SRB surface (Supplementary Fig. 4). On day 2, a large number of FeS NPs uniformly dispersed, followed by aggregation at day 2.5 and subsequent detachment from the surface. Therefore, we prepared FeS@SRB with an initial Fe2+ concentration of 200 mg L−1 and an incubation time of 48 h at 37 °C for further use. SEM combined energy dispersive X-ray spectroscopy (SEM-EDS) images showed the distribution of elements including C, N, O, Fe, and S in FeS@SRB, and FeS NPs on SRB surface (Fig. 2B). The average size of FeS NPs was 42 nm measured by software Nano Measure (Supplementary Fig. 5). In addition, dynamic light scattering (DLS) results indicated a size increase in FeS@SRB compared to SRB alone (Fig. 2C). Both SRB and FeS@SRB exhibited negative zeta potentials (Fig. 2D), making them suitable for intravenous injection.
The formation of FeS@SRB was also confirmed by UV-vis-NIR spectrophotometry and Fourier transform infrared spectroscopy (FTIR). The UV-vis-NIR absorbance curve of FeS@SRB suspension showed strong absorption peaks at 260 nm and 410 nm (Fig. 2E), indicating the typical structure in nucleic acids33 and Cyt c3 in SRB34. The higher absorbance in the NIR range was attributed to the generated black FeS NPs, which were expected to produce a photothermal effect to apply in NIR photothermal therapy (PTT). FTIR spectrum of FeS@SRB showed no significant changes compared to SRB (Fig. 2F), indicating that the structure of SRB was not damaged after FeS formation. However, the peak intensity of some groups was reduced, suggesting interactions between extracellular polymeric substances (EPS) components and Fe2+ 35,36.
X-ray diffraction (XRD) patterns confirmed the formation of Mackinawite-dominated iron-sulfur compounds on the SRB surface. The diffraction peaks at 27.12°, 31.55°, 45.22°, and 56.36° were attributed to the (111), (200), (220), and (222) planes of orthorhombic crystal FeS NPs ( JCPDS No. 23-1120), respectively37 (Fig. 2G). X-ray photoelectron spectroscopy (XPS) analysis identified the chemical elements and their valence states in FeS@SRB (Supplementary Fig. 6 and Fig. 2H). The peaks of Fe 2p in FeS@SRB concentrated at 710.9, 712.4, and 724.7 eV, corresponding to Fe(III)-S, Fe(III)-O, and Fe(II)-S, respectively (Fig. 2H). The peaks for S 2p appeared at 161.8, 163.7, 164.8, and 168.1 eV, corresponding to S2-, Sn2-, S0, and SO42-, respectively38. These results indicated that the successful in situ-synthesis of iron-sulfur compounds with hybrid valance states on the SRB surface, potentially endowing the FeS@SRB with a nano-enzymatic catalytic ability.
FeS@SRB exert photothermal and chemodynamic synergistic effects
We investigated the photothermal properties of FeS@SRB under 808 nm laser irradiation. The suspension temperature exhibited a concentration-dependent increase with higher FeS@SRB concentration (Fig. 3A), while also showing positive correlation with laser power density (Supplementary Fig. 7). Five heating-cooling cycles results indicated that FeS@SRB possess excellent photothermal stability (Fig. 3B) with a high photothermal conversion efficiency (η) of 33.9% calculated based on the temperature profile during the first laser irradiation on-off period (Fig. 3C).
A Effect of FeS@SRB concentration on the photothermal effect (808 nm, 1.5 W cm-2). n = 3 independent samples. Data are presented as mean ± SD. B Temperature profiles of FeS@SRB suspension (OD600 = 0.1) under five consecutive on-off cycles of laser irradiation (808 nm, 1.5 W cm−2). C Heating-cooling cycle curve and the corresponding linear fitting curve of the negative natural logarithm of time versus driving force temperature. D−F Detection of •OH generation of FeS@SRB (OD600 = 0.1) using MB probe at (D) pH 7.4, (E) pH 5.5 conditions, and (F) corresponding statistical analysis. n = 3 independent samples. Data are presented as mean ± SD. G UV-vis absorption spectra of different groups reacting with TMB (here heart symbol represents FeS@SRB). The inset showed the color in a different system. H Statistical analysis of absorbance in different groups at 652 nm. n = 3 independent samples. Data are presented as mean ± SD. I ESR profiles of generated •OH in different groups. J Cell viability of HUVECs and L929 cells after co-incubation with SRB for 24 h, respectively. n = 3 biological independent replicates. Data are presented as mean ± SD. K Cell viability of 4T1 cells, and (L) B16F10 cells with different treatments. n = 3 biological independent replicates. Data are presented as mean ± SD. Statistical significance was calculated by one-way ANOVA. **P < 0.05; ***P < 0.001; ****P < 0.0001. Source data are provided as a Source Data file.
Then we assessed the sensitivity of FeS@SRB at acidic environments by measuring the release kinetics of Fe2+ from FeS@SRB. The release amount of Fe2+ was significantly higher at pH 5.5 than that at pH 7.4 (Supplementary Fig. 8). These results suggested that FeS@SRB remained structural stability under normal physiological condition, while selectively releasing Fe2+ in acidic tumor microenvironments, where they would catalyze the conversion of H2O2 into toxic •OH via Fenton reactions. Methylene blue (MB) was used as a probe to verify the generation of •OH. The degradation rate of MB increased in the presence of FeS@SRB, which was further promoted by NIR laser irradiation and acidic conditions (Fig. 3D−F). The catalytic activity of FeS@SRB was further evaluated using tetramethylbenzidine (TMB) as a probe, which could be catalytically oxidized to green oxTMB by •OH (Supplementary Fig. 9). The strongest characteristic absorption at 652 nm was observed in the TMB + H2O2 + FeS@SRB group at pH 5.5 (Fig. 3G, H). Electron spin resonance (ESR) analysis was further used to detect characteristic •OH peaks, which became stronger under pH 5.5 and NIR laser irradiation (Fig. 3I). Besides, 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) probe was used to measure intracellular reactive oxygen species (ROS) generation. As shown in Supplementary Fig. 10, the level of green fluorescence signals generated by FeS@SRB under NIR irradiation was significantly higher than that of the FeS@SRB-treated group, indicating that the photothermal effect could enhance the efficiency of the Fenton reaction through localized warming.
The viability of both human umbilical vein endothelial cells (HUVECs) and L929 cells remained above 90% even at an OD600 of the SRB suspension with 0.2 (Fig. 3J), demonstrating the excellent cytocompatibility of SRB. However, due to the promising photothermal and Fenton reaction properties of FeS@SRB, 4T1 cells and B16F10 cells viabilities were significantly decreased after 24 h co-incubation with FeS@SRB (OD600 = 0.1) at pH 7.4, and was even further reduced at pH 5.5 (Fig. 3K, L), indicating more •OH was produced under acidic conditions. Assisted with NIR laser irradiation (808 nm, 1.5 W cm−2, 10 min), cells damage was enhanced.
Mechanism analysis of the treatment based on FeS@SRB + NIR
In order to deeply explore the mechanism of tumor cell killing by FeS@SRB + NIR, we extracted RNA from 4T1 cells after treated by PBS, FeS@SRB and FeS@SRB + NIR, respectively, and then performed RNA sequencing (RNA-seq) analysis. Compared with the control group, the FeS@SRB-treated group enriched 2129 up-regulated genes and 1805 down-regulated genes (Supplementary Fig. 11A), and FeS@SRB + NIR-treated group induced a total of 1797 up-regulated genes and 1603 down-regulated genes (Fig. 4A). Cluster analysis of differentially expressed genes (DEGs) revealed treatment mechanism of FeS@SRB + NIR primarily associated with cellular metabolic regulations (Hmox1, Srxn1, Nqo1, Fox, Pgd, Ifitm6/3, Ccl5, IL1b, Tnf, Cd274, Irf7, Gstm1/2), heat shock stress response (Hspa1a, Hspb1), and iron ion transport pathways (Fth1, Acot1, Slc40a1, Slc7a11) (Fig. 4B). In other words, FeS@SRB + NIR-induced oxidative stress significantly disrupted cellular metabolic homeostasis and activated intracellular stress responses, including both the heat shock system and ferroptosis-related pathways.
A Volcano plot of DEGs identified at P < 0.05 between the treatments of PBS and FeS@SRB + NIR by RNA-seq analysis. B A cluster analysis of the related genes in upregulated and downregulated DEGs in the FeS@SRB + NIR group. n = 3 biological independent replicates. C GO term enrichment analysis and (D) KEGG enrichment analysis of different expressed genes in PBS and FeS@SRB + NIR-treated 4T1 cells. n = 3 biological independent replicates, one-sided hypergeometric test. (E−K) The relative mRNA expression of (E) HSP70/90 (Hspa1a/Hsp90aa1), (F) GPX4 (Gpx4), (G) CAT (Cat), (H) P53 (Trp53), (I) NF-κB (Nfkb1), (J) ACSL4 (Acsl4), and (K) GADD45 (Gadd45a) treated with PBS, FeS@SRB, and FeS@SRB + NIR, respectively. G1: PBS, G2: FeS@SRB, G3: FeS@SRB + NIR. n = 3 biological independent replicates. Data are presented as mean ± SD. Source data are provided as a Source Data file.
In addition, we employed Gene Ontology (GO) enrichment analysis to reveal the regulation on all biological processes, cellular components and molecular functions. As shown in Fig. 4C, compared with the control group, immune-related functions (e.g., MHC protein complex binding, Toll-like receptor binding, antigen presentation) as well as metabolic pathways (e.g., glycerolipid metabolism, sphingolipid metabolism, glutamine-family amino acid biosynthesis, and glutathione metabolism) were significantly enriched, suggesting FeS@SRB + NIR might be involved in immune regulation and energy metabolism regulation. In addition, the presence of ferroptosis and heat shock protein binding suggests that it may play a role by affecting iron-dependent cell death and stress response pathways (Fig. 4C and Supplementary Fig. 11B). Kyoto Encyclopedia of Genes and Genomes (KEGG)-enriched pathway analyses showed that FeS@SRB + NIR enhanced the enrichment of ferroptosis, glutathione metabolism, and peroxisome proliferator-activated receptors (PPAR) signaling pathway compared to FeS@SRB (Fig. 4D and Supplementary Fig. 11C). Multiple key signaling pathways were also significantly altered, with enrichment of the NF-kappa B (NF-κB) signaling pathway, Toll-like receptor signaling pathway, p53 signaling pathway, and antigen processing and presentation, suggesting that intracellular immune and stress regulatory networks were profoundly activated. The enrichment of NF-κB, a key transcription factor, regulates the expression of many immune-related factors, thus initiating the immune response. The enrichment of Toll-like receptor signaling pathway and p53 signaling pathway further confirmed the activation of the cellular immune defense mechanism, which enhances the body’s abilities of immune surveillance and recognition of tumor cells, thus preventing the further proliferation of tumor cells. These results fully revealed that FeS@SRB effectively exerted biological effects by inducing stronger oxidative stress, ferroptosis and heat stress synergistically under NIR radiation.
To further validate these mechanisms, we evaluated the mRNA expression levels of eight pivotal genes (Fig. 4E–K). In the FeS@SRB-treated group, elevated expression of HSP70/90 (Hspa1a/Hsp90aa1) (Fig. 4E) indicated a cellular stress response, activated with protective mechanisms to maintain proteostasis. NIR irradiation exacerbated this effect by inducing localized hyperthermia, which further amplified HSP70/90 expression. The photothermal effect enhanced Fenton reactions and triggered a massive ROS burst rapidly depleted glutathione (GSH) and inhibited GPX4 activity (Fig. 4F). Moreover, catalase (Cat) expression was significantly reduced in the FeS@SRB + NIR group (Fig. 4G), reflecting severe impairment of cellular antioxidant defenses and heightened oxidative stress. This oxidative microenvironment subsequently activated the p53-mediated apoptosis pathway (Trp53) (Fig. 4H) and NF-κB (Nfkb1) signaling (Fig. 4I), while upregulating ACSL4 (Acsl4) (Fig. 4J) and GADD45 (Gadd45a) expression (Fig. 4K). In summary, FeS@SRB + NIR orchestrates a cascade of tumoricidal effects through photothermal-photodynamic synergy, activating oxidative stress, ferroptosis, apoptosis, and immunogenic cell death pathways.
FeS@SRB exert hypoxic chemotaxis, deep penetration, and tumor targeting property
To confirm the effective hypoxia-targeting synergistic photothermal therapy of FeS@SRB, a certain amount of fluorescein 5-isothiocyanate (FITC)-labeled FeS@SRB were placed in the upper chambers of transwells, and an in vitro hypoxia model was constructed by using glucose/glucose oxidase/hydrogen peroxidase (Fig. 5A). FeS@SRB were initially placed in normoxic upper chambers. With the time extension, the fluorescence intensity (FI) in the upper chambers gradually weakened, while that in the lower chambers greatly enhanced (Fig. 5B). Further semi-quantitative analysis of FI revealed a positive correlation between incubation time and FeS@SRB migration, with the longer durations resulting in increased migration. Particularly, the FI in upper chambers was only 19.2% after 45 min, while that in the lower chambers significantly increased to 80% (Fig. 5C). This phenomenon visualized that a large amount of FeS@SRB migrated to the lower chamber, indicating their excellent hypoxic chemotaxis. In contrast, only few bacterial migrated to the lower chamber under normoxic conditions (Supplementary Fig. 12), which attributed to the suppressed autonomous motility of anaerobic bacteria. Furthermore, we assessed the tumor penetration capabilities of the FeS@SRB by constructing 3D tumor spheroids model. In order to better reflect the heterogeneity and structural complexity of tumors, two kinds of 3D tumor spheroids were constructed using 4T1 cells and B16F10 cells, respectively. After 24 h co-incubation of FITC-labeled FeS@SRB with 4T1 cells-derived 3D tumor spheroids, the FI of FITC initially increased and then decreased with increasing scanning depth (Fig. 5D, E). When the scanning depth was 30 μm, the strongest green fluorescence was clearly observed. Similarly, a stronger green fluorescent signal was internally observed in the 3D tumor spheroids of B16F10 cells (Fig. 5F, G), indicating that FeS@SRB were able to penetrate deeply into the interior of tumor tissue.
A Schematic diagram of the transwell system that simulates the hypoxic chemotaxis of FeS@SRB (OD600 = 0.1) in vitro. B Representative fluorescence images in the upper and lower chambers of FeS@SRB at different time points under hypoxic environment. Scale bar: 50 μm. C Corresponding FI ratio based on fluorescence images. n = 3 independent samples. Data are presented as mean ± SD. Source data are provided as a Source Data file. D Confocal fluorescence images and (E) 3D confocal fluorescence images of 4T1 multicellular 3D spheroids co-incubated with FITC-labeled FeS@SRB for 24 h. Scale bar: 200 μm. F Confocal fluorescence micrographs and (G) 3D confocal fluorescence images of B16F10 multicellular 3D spheroids co-incubated with FITC-labeled FeS@SRB for 24 h. Scale bar: 200 μm. The schematic diagram was created in BioRender. Dong, M. (2025) https://BioRender.com/k7shv52.
As nanoparticles tend to fall off the surface of bacterial carriers after being injected into the organism due to the action of flow, shear force and so on, we assessed the stability of the biosynthesized FeS@SRB by using NaCl solution and simulated body fluid (SBF) to simulate the in vivo physiological environment before animal experiments. Compared to FeS-SRB hybrids prepared via physical electrostatic interaction, both chemically coupled FeS=SRB hybrids and in situ biosynthesized FeS@SRB were stably bound to the SRB surface, with a negligible amount of FeS detached from SRB (Supplementary Fig. 13), suggesting FeS@SRB were stable during system delivery. Despite the FeS=SRB hybrids also possessed excellent stability, the introduction of chemical reagents during the preparation procedure would affect the activity of SRB, which in turn reduces their tumor targeting delivery efficiency. Therefore, the bacterial activity was further evaluated by the plate coating method and the crystalline violet biofilm method. As shown in Supplementary Fig. 14, the FeS=SRB did reduce the activity of SRB, which should be due to the fact that the chemical reagents involved in the chemical binding of FeS nanoparticles to SRB surface affected the survival and activity of bacteria. In conclusion, biosynthesis, as a green, efficient, and biocompatible strategy, can achieve efficient and biosafe nanoparticles-bacteria hybrids for tumor therapy.
To confirm the tumor chemotaxis of SRB in vivo, imaging techniques were used to study the tumor-targeting delivery efficiencies of SRB and FeS@SRB compared to conventional chemically synthesized FeS@BSA39. Subcutaneous 4T1 tumor-bearing mice model was constructed and then IR780-labled SRB and FeS@SRB were injected intravenously, respectively. It’s found that both SRB and FeS@SRB gradually targeted to the tumor sites after intravenous (i.v.) injection (Fig. 6A, B). At 36 h, both groups reached similar delivery efficiency exceeding 50.5%, as calculated from the fluorescence intensity ratio of i.v. injection to intratumoral (i.t.) injection (Supplementary Fig. 15). This result confirmed that the biosynthesized FeS NPs maintain the activity and hypoxic targeting capability of SRB. In contrast, FeS@BSA only achieved a delivery efficiency of 2.9% after i.v. injection, which may be limited by various physiological barriers. The plate colony-counting method revealed dense bacteria colonies in tumor homogenates, small amounts in the liver and kidney, and sporadic colonies in other major organs after 60 h post-i.v. injection of FeS@SRB (Fig. 6C, D). Gram stain experiments revealed similar results with plate colony-counting method (Fig. 6E), confirming FeS@SRB’s excellent tumor-targeting and colonization capabilities.
A Representative fluorescence image of subcutaneous 4T1 tumor-bearing mice at different time after intravenous injection with IR780-labeled SRB, FeS@BSA and FeS@SRB, respectively. B Tumor-targeted delivery efficiency of SRB, FeS@BSA and FeS@SRB via i.v. injection based on FI statistics. n = 3 biological independent replicates. Data are presented as mean ± SD. C, D Biodistribution of FeS@SRB at 60 h post-i.v. injection assessed by plate coating method. C Photos of bacteria colonies after diluted tumor and organ homogenates coated on agar plates and incubated at 37 °C for 24 h (diluted: 12 times), and (D) corresponding bacteria colony counts in major organs and tumor tissues. n = 3 biological independent replicates. Data are presented as mean ± SD. E Gram staining assay for determining the bacterial distribution in individual organs and tumor tissue at 48 h post-i.v. injection (the blue cycles indicated bacteria colony). Scale bar: 100 μm. Statistical significance of (B) was calculated by one-way ANOVA. ****P < 0.0001, and ns, not significant. Source data are provided as a Source Data file.
SRB possess excellent biosafety both in vitro and in vivo
The cytocompatibility of SRB has been confirmed in Fig. 3J. The hemocompatibility of SRB and FeS@SRB in vitro was evaluated using hemolysis and coagulation assay. Their hemolysis ratios were consistently below 5% after incubation with red blood cells (RBC) (Supplementary Fig. 16), indicating no RBC rupture. Besides, both SRB and FeS@SRB did not cause significant blood clot formation (Supplementary Fig. 17). These results suggested their excellent blood compatibility.
To assess the feasibility of SRB for in vivo treatment, SRB was intravenously injected into normal mice. Compared with normal mice without any treatment, there was no significant change in IL-1β level in the sera of mice at 12 h after i.v. injection of SRB, and a slight increase in the concentration of some inflammatory factors including IL-6, TNF-α, and iNOS (retaining at a pg level) (Fig. 7A–D). However, the levels of these factors returned to the normal level after 1 day. The above results indicated that there was no a pathological response induced by SRB. Plate coating results showed that SRB were progressively eliminated from main organs on day 4 (Fig. 7E). Some key physiological indicators such as white blood cell count (WBC), RBC, platelet count (PLT) (Fig. 7F), liver function indicators including alanine transaminase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH) (Fig. 7G), and kidney function indicators including blood urea nitrogen (BUN) and creatinine (CREA) (Fig. 7H) remained in the normal range during the 40-day observation period. The mice gained weight steadily in SRB-treated mice (Fig. 7I). Histopathological analysis on the tissues of major organs of SRB-treated mice at different time points (1, 7, 14, 28 and 40 days) using hematoxylin and eosin (H&E) staining showed no tissue damage and chronic inflammation in major organs (Fig. 7J and Supplementary Fig. 18). These results can strongly argue that SRB have not triggered immune escape, chronic inflammation, and tissue damage, alleviating concerns regarding the biosafety of bacterial therapy based on SRB. Consequently, SRB are promising as nanocarriers for tumor-targeting treatment.
A–D The concentrations of (A) IL-1β, (B) IL-6, (C) TNF-α and (D) iNOS of the mice sera at 12 h and on 1st, 4th, 7th, 14th, 28th, and 40th day after i.v. injection of SRB (1 × 107 CFU mL−1). n = 3 biological independent replicates. Data are presented as mean ± SD. E Photographs of the SRB colony distributed in major organs on the 1st, 4th, and 7th day after i.v. injection of SRB. F Analysis of blood biochemistry of WBC, RBC, and PLT on the 1st, 4th, 7th, 14th, 28th, and 40th day after intravenously injecting SRB. n = 3 biological independent replicates. Data are presented as mean ± SD. G Blood analysis of liver function, including ALT, AST, and LDH, and (H) kidney function, including BUN and CREA on the 1st, 4th, 7th, 14th, 28th, and 40th day after intravenously injecting SRB. n = 3 biological independent replicates. Data are presented as mean ± SD. I Body weight change of SRB-injected mice and healthy mice. n = 3 biological independent replicates. Data are presented as mean ± SD. J Representative H&E staining of major organs of mice harvested on day 1, 7, 40 post-i.v. injection of SRB, respectively. Scale bar: 200 μm. Source data are provided as a Source Data file.
FeS@SRB efficiently target tumor tissues to inhibit subcutaneous 4T1 tumor growth under NIR laser irradiation in vivo
We firstly constructed a subcutaneous 4T1 tumor-bearing mice model to verify the therapeutic efficacy based on the FeS@SRB. Tumors were irradiated with NIR laser (808 nm, 1.5 W cm−2) for 5 min at 48 h post-i.v. injection of various samples (Fig. 8A). There was no significant tumor temperature rise in the PBS and SRB groups under NIR laser irradiation, whereas the tumor temperature rose up to 55 °C in the FeS@SRB group (Fig. 8B, C). These results suggested that FeS@SRB could achieve effective photothermal treatment for tumors. The mice tumors in the PBS and SRB groups increased rapidly during the observation period (Fig. 8D). Tumors in the FeS@SRB-treated group were initially suppressed due to the Fenton reaction. However, they began to grow rapidly after one week due to insufficient treatment via only single-dose injection. In contrast, the tumors in the FeS@SRB + NIR group progressively reduced and some completely disappeared (Fig. 8E, F and Supplementary Fig. 19). Pathologically analysis of tumor tissues showed abundant blue tumor cell nuclei in the PBS, SRB and FeS@SRB groups, while fewer tumor cells were founded in the FeS@SRB + NIR group (Fig. 8G, H). The highest level of green fluorescence (TUNEL) and the lowest level of red fluorescence (Ki67) were observed in the FeS@SRB + NIR group. Notably, photothermal therapy also helped to eliminate SRB within tumors (Supplementary Fig. 20), reducing potential risks. In addition, FeS@SRB-based therapy prolonged mice survival, particularly when combined with NIR irradiation, with the highest survival rate (50%) within 40 days (Supplementary Fig. 21). Therefore, FeS@SRB could efficiently target tumor tissues to inhibit subcutaneous 4T1 tumor growth and prolong the mice survival combined with PTT.
A Schematic illustration of the treatment process. B Representative infrared thermal images of mice and (C) corresponding temperature change curves of tumors in different groups during NIR laser irradiation (808 nm, 1.5 W cm−1). n = 3 biological independent replicates. Data are presented as mean ± SD. D Tumor growth curves. n = 5 biological independent replicates. E Tumor pictures and (F) tumor weight of mice after dissection on the 14th day after various treatments. n = 5 biological independent replicates. Data are presented as mean ± SD. G Representative H&E, TUNEL, and Ki67-stained images of tumor tissues in different treatment groups on the 14th day after various treatments. Scale bar: 200 μm. H Quantitative analysis of the mean fluorescence intensity (MFI) of TUNEL and Ki67 staining of the tumors using software Image J. n = 3 biological independent replicates. Data are presented as mean ± SD. I Representative ROS fluorescence images of the tumor section and ( J) the corresponding MFI analysis at 48 h and 14 days after different treatments, respectively. Scale bar: 500 μm. n = 3 biological independent replicates. Data are presented as mean ± SD. G1, PBS; G2, SRB; G3, FeS@SRB; G4, FeS@SRB + NIR. Statistical significance of (B) was calculated by one-way ANOVA. ***P < 0.001. Source data are provided as a Source Data file. The experimental design diagram was created in BioRender. Dong, M. (2025) https://BioRender.com/imp3wct.
We investigated the potential therapeutic mechanism of FeS@SRB by dissecting tumor tissues at 48 h and on day 14 post-treatment, followed by dihydroethidium (DHE) staining, respectively. No obvious ROS (red fluorescence) was produced in the PBS and SRB groups, while FeS@SRB-treated mice showed a large amount of ROS generated at 48 h post-treatments (Fig. 8I, J). On day 14, a small amount of ROS still remained in the FeS@SRB + NIR group, indicating that •OH production was enhanced by NIR laser irradiation. These results implied that there was a significant chemodynamic/photothermal synergistic effect of FeS@SRB + NIR, which played an important role in antitumor therapy.
In addition, the body weights of mice in all groups remained stable throughout the treatment period (Supplementary Fig. 22). Liver function indicators (Supplementary Fig. 23A) and biomarkers related to kidney function (Supplementary Fig. 23B) of the treated mice showed no significant difference with normal mice after 14 days of treatment. H&E staining of major organs revealed no evident damage (Supplementary Fig. 23C). These results further suggested the potential of FeS@SRB for safe and effective in vivo application in antitumor therapies.
FeS@SRB boost strong anticancer activity against B16F10 tumors in vivo
In order to comprehensively assess FeS@SRB could efficiently target multiple tumor tissues and further induce effective tumor suppression, we constructed subcutaneous B16F10 tumor-bearing mice models (Fig. 9A) and intravenously injected with different material. After 48 h, the mice tumors were irradiated with 808 nm laser (1.5 W cm−2, 5 min) and imaged with infrared thermal imaging to investigate the intratumor photothermal conversion of FeS@SRB. The results revealed that significant temperature increases were achieved in FeS@BSA + NIR and FeS@SRB + NIR treatment groups (Fig. 9B). Especially, the mice treated by FeS@SRB + NIR showed a higher temperature increase reaching over 55 °C (Fig. 9C), similar to the results in the subcutaneous 4T1 tumor model (Fig. 8B, C). These results further confirmed that FeS@SRB had a stronger active tumor-targeting ability than FeS@BSA, thereby allowing more FeS@SRB to migrate in tumor tissues to exert excellent PTT effects.
A Schematic illustration of the treatment process. B Representative infrared thermal images of mice in different groups under irradiation with 808 nm laser (1.5 W cm−2, 5 min) on tumors after 48 h post-i.v. injection with PBS, SRB, FeS@BSA, and FeS@SRB, respectively. C Corresponding temperature change curves of tumor tissues. n = 3 biological independent replicates. Data are presented as mean ± SD. D Tumor growth curves in different groups. n = 5 biological independent replicates. Data are presented as mean ± SD. E Photos of tumor tissues harvested on the 14th day. F Mice weight change curves during the treatment period. n = 5 biological independent replicates. Data are presented as mean ± SD. G Representative H&E staining, TUNEL and Ki67 immunofluorescence staining images of tumor tissues in different groups on the 14th day after various treatments. Scale bar: 200 μm. H Representative H&E staining images of liver tissues in various groups on the 14th day after various treatments (yellow line: vacuolated cytoplasm; green line: inflammatory foci; blue line: portal vein stasis). Scale bar: 200 μm. G1, Control; G2, SRB; G3, FeS@BSA; G4, FeS@BSA + NIR; G5, FeS@SRB; G6, FeS@SRB + NIR. Source data are provided as a Source Data file. The experimental design diagram was created in BioRender. Dong, M. (2025) https://BioRender.com/sjw0c3j.
In addition, B16F10 tumor-bearing mice were randomly divided into six groups with different treatments. After 14 days of treatments, the mice tumors in PBS and SRB explosively grew (Fig. 9D). On the contrary, other groups inhibited tumor growth to varying degrees. Among them, the FeS@SRB + NIR group exhibited superior antitumor efficiency with a tumor growth inhibition value of 88% (Fig. 9D, E), which mainly attributed to the excellent active tumor-targeting colonization and high photothermal conversion ability of the biosynthesized FeS@SRB. During the treatment period, the mice in all treatment groups showed a steady increase in body weight (Fig. 9F). In addition, we further analyzed the tumor tissue necrosis in the different treatment groups by H&E staining. Compared with the other groups, large areas of nuclear condensation and nuclear fragmentation were observed in the FeS@SRB + NIR group, indicating that the tumor cells were severely damaged (Fig. 9G). Besides, immunohistochemical methods (TUNEL and Ki67 staining) further verified the least proliferative activity and the most apoptotic tumor cells in the FeS@SRB + NIR group (Fig. 9G).
In addition, a biochemical analysis of the mice sera was performed to evaluate systemic toxicity. As seen in Supplementary Fig. 24, liver function indicators (ALT and ALP) and kidney function (BUN and CREA) levels in all groups were maintained in a normal range. However, the AST level in the PBS, SRB, FeS@BSA and FeS@SRB treated groups significantly increased. Meanwhile, H&E staining images of livers in these groups further visualized vacuolated cytoplasm (yellow line), inflammatory foci (green line) and portal vein stasis (blue line) (Fig. 9H), which indicated some degree of liver damage. On the contrary, FeS@BSA + NIR and FeS@SRB + NIR could reduce liver injuries occurrence. These results demonstrated that B16F10 tumor growth might cause liver damage without effective intervention40, which positively reflected the safety and efficacy of FeS@SRB + NIR in the treatment of B16F10 tumors. Furthermore, no significant damage was observed in H&E staining images of major organs (heart, spleen, lung, and kidney), confirming their excellent biosafety (Supplementary Fig. 25).
FeS@SRB efficiently inhibit the growth and metastasis of orthotopic breast cancer
To more accurately reflect the anti-tumor efficacy of FeS@SRB combined with NIR laser irradiation, a 4T1 orthotopic breast cancer model was further established (Fig. 10A). Firstly, IR780-labeled SRB, FeS@BSA, and FeS@SRB were administered intravenously into tumor-bearing mice, respectively and then monitored dynamically by an in vivo imaging system. Compared to the weak fluorescence of the FeS@BSA-injected mice, the fluorescence intensity in tumors gradually enhanced and was strongest at 24 h after i.v. injection at the SRB and FeS@SRB groups (Fig. 10B, C). In addition, their fluorescence in tumors could be seen obviously even at 96 h (Supplementary Fig. 26). These results further certified the excellent tumor targeting and colonization properties of SRB and FeS@SRB. Based on the above results, the mice tumors were irradiated using an 808 nm laser (1.5 W cm−2, 5 min) to evaluate the photothermal effect at 48 h after i.v. injection. As expected, there was nearly no change in mice tumor temperature in the groups of PBS and SRB, and the most significant temperature increase was found in the FeS@SRB group (Supplementary Fig. 27).
A Schematic illustration of the treatment procedure. B Representative fluorescence images of orthotopic 4T1 tumor-bearing mice at different time after intravenous injection with IR780-labeled SRB, FeS@BSA and FeS@SRB, respectively, and the corresponding fluorescence images of tumors and organs at 48 h. C Corresponding quantitative statistics of fluorescence intensity at different times post-i.v. injection. n = 3 biological independent replicates. Data are presented as mean ± SD. D The tumor growth curves after various treatments. n = 5 biological independent replicates. Data are presented as mean ± SD. E Tumor images and (F) tumor weight on day 14. n = 5 biological independent replicates. Data are presented as mean ± SD. G Representative spleen images extracted from mice in different treatment groups and (H) the corresponding spleen weight on day 14. n = 3 biological independent replicates. Data are shown as mean ± SD. I Mice weight change profiles during the treatment period. n = 5 biological independent replicates. Data are presented as mean ± SD. J Representative H&E, TUNEL, and Ki67 staining images of tumor tissues receiving various treatments. Scale bar: 200 μm. K Representative H&E staining images of lung and liver in mice on day 14 after different treatments. Scale bar: 400 μm. In the box plot, the box spans the interquartile range (first to third quartile), and the horizontal line indicates the median (second quartile). The lower and upper whiskers indicate the minimum and maximum values within 1.5 times the interquartile range from the first and third quartiles, respectively. G1, Control; G2, SRB; G3, FeS@BSA; G4, FeS@BSA + NIR; G5, FeS@SRB; G6, FeS@SRB + NIR. Source data are provided as a Source Data file. The experimental design diagram was created in BioRender. Dong, M. (2025) https://BioRender.com/38a0yn8.
Based the excellent targeting ability and photothermal effects, the antitumor potential of FeS@SRB was investigated by constructing orthotopic breast cancer tumor models. Compared with other treatment groups, FeS@SRB + NIR significantly inhibited tumor growth (Fig. 10D, E, F). Notably, tumor suppression was also achieved in the FeS@BSA + NIR group during the first 8 days, but the tumor volume increased rapidly at the later stage. It might be due to the limited targeting ability of FeS@BSA. Spleen reduction might indicated a reduction in tumor burden41, and the smallest spleens were observed in the FeS@SRB + NIR group (Fig. 10G, H), which further suggested that FeS@SRB + NIR can effectively inhibit tumor growth. The body weight of mice was normally increased during the treatment period (Fig. 10I).
In addition, H&E, TUNEL and Ki67 staining images of tumor tissues intuitively indicated that FeS@SRB + NIR induced extensive tumor cells apoptosis, while effectively suppressing the tumor proliferation (Fig. 10J). Due to the highly spontaneous metastatic properties of orthotopic breast cancer cells, lung and liver tissues were harvest. H&E staining images indicated that only the PBS and SRB groups had significant lung nodule metastasis (Fig. 10K). However, H&E staining images of liver tissues revealed varying degrees of liver metastases in all treatment groups, with the FeS@SRB + NIR group exhibiting the lowest liver metastases, indicated superior anti-metastatic efficacy of chemodynamic/photothermal synergistic therapy.
At the end of the treatments, mice sera and major organs (heart, liver, spleen, lung, and kidney) were harvested for comprehensive biosafety analysis of FeS@SRB-based therapy. The levels of liver function indicators (ALT, AST, and ALP) and kidney function indicators (BUN and CREA) were maintained in the normal range (Supplementary Fig. 28). There was no significant inflammatory infiltration or pathological damage observed in the organs according to the H&E staining images (Supplementary Fig. 29). All these results suggested that FeS@SRB-based PTT demonstrated good biocompatibility and safety in tumor therapy.
FeS@SRB-based PTT enhances anti-tumor immune responses
Encouraged by the promising treatment efficiency of FeS@SRB-based therapy, we further examined the levels of tumor-associated immune cells and inflammatory factors after various treatments using an orthotopic 4T1 breast cancer-bearing mice model. Dendritic cells (DCs), as the most important antigen-presenting cells, play a key role in tumor-specific immune responses and tumor suppression. By collecting tumor-draining lymph nodes (TDLNs) of mice for flow cytometry analysis, the results showed that most mature DCs (CD80+CD86+) were observed in the FeS@SRB + NIR group, with a relative content of 22.6% (Supplementary Figs. 30, 32A, D). This may be attributed to the strong tumor-targeting and tumor-colonizing abilities of SRB, which allow more FeS@SRB to stay at the tumor site and ultimately induce DCs maturation after NIR laser irradiation. Normally, matured DCs further activate the adaptive immune responses initiated by cytotoxic T cells by presenting antigens. Therefore, the immune activation effect of FeS@SRB + NIR was further assessed by detecting the level of cytotoxic T cells infiltration in tumor tissues. The results indicated that FeS@SRB + NIR significantly promoted the intratumorial infiltration of CD8+ T cells (Supplementary Figs. 31, 32B, E) and CD4+ T cells (Supplementary Figs. 31, 32C, F), which indicated that FeS@SRB + NIR exhibited superior anti-tumor immune responses. Besides, the levels of immunocytokines have been determined, and the highest TNF-α, IL-1β, and IFN-γ were observed in the FeS@SRB + NIR group (Supplementary Fig. 33A−C). In conclusion, these findings revealed that the superior active tumor-targeting of SRB allowed more FeS@SRB to be targeted to tumor tissues, which in turn activated effective anti-tumor immune responses by promoting the maturation of DCs and the infiltration of cytotoxic T cells in tumors after NIR laser irradiation.
Discussion
Anaerobic bacteria-mediated tumor therapy has become a versatile platform for targeted delivery and synergistic treatments. However, current bacterial vectors need to be engineered to reduce their inherent toxicity or enhance the therapeutic efficacy3,4,5,6,7. These engineering approaches reduce bacterial activity and hypoxic targeting capability. Inspired by the advantages of in situ biosynthesis, we screened SRB, a kind of natural nonpathogenic anaerobic bacteria, that are highly compatible with the human body and can dissimilate SO42- into S2- to further synthesis metal sulfides (such as FeS) nanoparticles on their surface (FeS@SRB in this work) for tumor-targeted therapy. FeS@SRB possess excellent biosafety and NIR photothermal effect combined with its enhanced •OH production in tumors, effectively killing tumor cells (Fig. 3).
Benefiting from a large amount of EPS secreted by SRB during their growth42 and lipopolysaccharide (LPS) and phospholipid on the bacteria’s outer membrane, FeS NPs are stable on the surface of SRB. Specifically, organic functional groups in EPS and bacteria’s outer membrane (e.g., carboxyl, hydroxyl, phosphoric acid, and amino groups) would chelate Fe2+ ions, facilitating the formation of FeS NPs via in situ mineralization with metabolically generated S2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36. Furthermore, the negatively charged functional groups in EPS and LPS endow the formed FeS@SRB with negative potentials, improving blood compatibility and facilitating intravenous delivery. The stability experiments demonstrated that the biosynthesized FeS@SRB possess excellent structural stability compared to physically assembled FeS-SRB hybrids, which illustrates that the biosynthesized FeS@SRB will not easily be susceptible to various physical and biological barriers after i.v. injection (Supplementary Fig. 13). In addition, compared to FeS=SRB hybrids obtained through the conventional chemical coupling method, FeS@SRB possess high bacterial activity (Supplementary Fig. 14).
In vivo studies revealed comparable tumor accumulation between SRB and FeS@SRB in 4T1 tumor models (Figs. 6 and 10), as well as no reduction after achieving the maximal value with either i.v. (Fig. 6A, B) or i.t. injection (Supplementary Fig. 15), demonstrating robust FeS@SRB activity and tumor colonization capacity. Notably, we devised a simple method to evaluate delivery efficiency by comparing fluorescence intensity in tumors after i.v. injection and i.t. injection with the same sample amount, which will supply a reference for other similar studies. Comprehensive experiments revealed that the delivery efficiency of self-driven FeS@SRB is about 17 times higher than that of FeS@BSA nanoparticles relying on passive targeting delivery. Importantly, FeS@SRB exerts excellent chemodynamic/photothermal synergistic effects compared to FeS@BSA in vivo, achieving efficient antitumor effects in multiple tumor models, including 4T1 subcutaneous tumor, B16F10 subcutaneous tumor and 4T1 orthotopic tumor (Figs. 8–10). Meanwhile, an effective anti-tumor immune response has been active by FeS@SRB via promoting the maturation of DCs and the infiltration of cytotoxic T cells in tumors, which is further enhanced after NIR laser irradiation.
Though only FeS@SRB were explored in this work, various sulfide nanoparticles-SRB hybrids can be prepared using a similar method, such as MnS@SRB, ZnS@SRB, CuS@SRB, and their heterojunction system. These sulfide nanoparticles-SRB hybrids have their own unique properties and could potentially be used in tumor treatments via diversified mechanisms, such as photodynamic/sonodynamic/chemodynamic therapy, photothermal therapy, gas therapy, and immune regulation, etc.29,43 with good biosafety profiles and tumor-targeting properties.
Consequently, SRB are a promising platform for tumor treatment that combines multiple therapeutic functions with excellent biocompatibility. We believe that with intensive research, SRB-based therapy could enrich research in the field of hypoxic targeting strategies, biological metabolic therapeutic agents, nanocatalytic medicine, and provide additional options to improve the therapeutic efficacy of diseases.
Methods
Ethical statement
All animal experiments were carried out by qualified operators (Certificate number: W23010090 and W23010613) under the protocols approved by the Institutional Animal Care and Use Committee of Huazhong University of Science and Technology (ACUC number: 4186). According to the Institutional Animal Care and Use Committee approved protocol, mice were euthanized when the tumors reached 2000 mm3.
Materials
Sodium lactate, Na2SO4, FeSO4·7H2O, L-ascorbic acid (VC), K2HPO4, and methylene blue were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). TMB, N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and bovine serum albumin (BSA) were acquired from Macklin Biochemical Co., Ltd (Shanghai, China). Yeast extract originated from Thermo Fisher Scientific (Shanghai, China). DMPO was obtained from DOJINDO (Japan). The above reagents were in analytical grade and employed directly. Antifade Mounting Medium with DAPI was provided from bioship brand. FITC was purchased from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). Dulbecco’s modified Eagle medium (DMEM) was bought from Wuhan Pricella Biotechnology Co., Ltd., China. Lactate kit was obtained from Nanjing Jiancheng Bioengineering Institute, China. CCK-8 kits were produced by GlpBio Technology (USA). IR780 was acquired from MedChemExpress (USA). Ultrapure water was used in the trials.
Cell culture and animals
HUVECs (Serial: PCS-100-013) were supplied by the American Type Culture Collection (United States). Mouse fibroblasts L929 cells (Serial: GNM28), mouse breast carcinoma 4T1 cells (Serial: TCM32), and mouse melanoma high metastatic B16F10 cells (Serial: TCM36) were provided from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in complete DMEM with 10% fetal bovine serum and 1% penicillin-streptomycin at 37 °C under a 5% CO2 atmosphere. All the cell lines underwent tested and were confirmed to be free of mycoplasma contamination.
The female BALB/c mice and female C57BL/6 mice aged 6-8 weeks were purchased from Three Gorges University in China. All mice were housed in a sterile facility under a 12-hour light-dark cycle, with temperature controlled at 20 ± 3 °C, relative humidity at 50% ± 10%, and free access to food and water. Sex was considered in our study design. We used female mice for animal operation, primarily based on the reasons including tumor biological characteristics, facilitating comparison with other studies, and complying with animal welfare requirements.
SRB domestication
SRB (Desulfovibrio) isolated from Shengli Oilfield was identified as Gram-negative bacteria by Gram staining (Supplementary Fig. 1). The SRB was repeatedly domesticated using a modified bacterial culture medium to obtain a highly pure strain. The specific procedures were as follows: the medium was prepared according to Supplementary Table 1, and its pH was adjusted to 7.0 using HCl (1 mol L-1). Subsequently, the medium was dispensed into 100 mL injection bottles, each containing 80 mL of medium, and autoclaved at 121 °C for 30 min. After the medium was cooled, 640 µL of UV-sterilized sodium lactate solution was added to each bottle. N2 was then purged into the bottles to further remove oxygen and maintain an anaerobic environment.
2.0 mL of SRB suspension were injected into anaerobic bottles and incubated statically at 37 °C for 48 h. When the supernatant in the anaerobic bottle became visibly turbid with a strong hydrogen sulfide odor44, SRB were collected by centrifugation at 4024 × g for 10 min and then transferred to a new anaerobic bottle for continued cultivation. This subculturing process was repeated ~ 20 times until 90% of the metal ions in the medium were removed. The resulting SRB were subsequently used for further experiments.
Biosynthesis of FeS@SRB
The domesticated SRB with the OD value at 600 nm (OD600) equaling to 0.1 were inoculated into culture bottles containing 80 mL of sterilized anaerobic medium (Supplementary Table 1). Different dose of FeSO4·7H2O were added to give final Fe2+ concentrations of 25, 50, 100, 200, 300, 400, and 500 mg L−1, respectively. In addition, VC (0.1 g L−1) was added to maintain the stability of Fe2+ in the medium. After thorough mixing, these bottles were placed in a microbial incubator at 37 °C for static incubation. The activity of FeS@SRB was assessed by measuring the OD600 and the oxidation-reduction potential of the medium using a UV-visible spectrophotometer (V-1200, Aoyi, Shanghai, China) and a pen-type potentiometer (OPR-BW, YHT, China), respectively. The supernatants were centrifuged at 4024 × g for 10 min to collect FeS@SRB at set time points from 24 h to 120 h. The loading amount of FeS on the SRB surface was determined by measuring free Fe2+ in the medium using an inductively coupled plasma optical emission spectrometer (ICP-OES, ICPA7200, Thermofisher, Germany)45. Ultimately, FeS@SRB with highly active and highly FeS-loaded amounts were prepared at an initial Fe2+ concentration of 200 mg L−1 for 48 h at 37 °C according to the experimental results and were used for subsequent studies.
As a comparison, we prepared FeS@BSA by the traditional chemistry method and two kinds of FeS NPs-SRB hybrids, named FeS-SRB and FeS = SRB, by physical and chemical methods, respectively. The specific preparation processes were as follows.
Preparation of FeS@BSA39
100.0 mg BSA was dissolved in 6.0 mL of deionized water, 2.0 mL of FeCl2 solution (37 mmol L−1) was added under vigorous stirring, followed by the addition of 3 mL of Na2S solution (37 mmol L−1). Then, the mixture was allowed to react at 4 °C for 12 h. FeS@BSA nanoclusters were obtained after dialysis (MW: 8000–14000) against deionized water for 12 h at 4 °C, and freeze-dried for the further use.
Preparation of FeS-SRB
100 mg FeS@BSA was mixed with 10 mL of SRB suspension (1 × 107 CFU mL−1), and then co-incubated with shaking at 120 rpm at 37 °C for 4 h. The mixture was centrifuged at 4024 × g for 10 min to collect FeS-SRB for subsequent use.
Preparation of FeS = SRB
Briefly, 10 mg EDC and 5 mg NHS were added into 10 mL of FeS@BSA suspension (10 mg mL−1), and stirred at 25 °C for 3 h. Then SRB (1 × 107 CFU mL-1) was added and co-incubated for 1 h. The FeS=SRB was centrifugated at 4024 × g for 5 min and then washed for subsequent use.
Characterization of FeS@SRB
SRB lactate metabolic behavior was determined using the lactate kit according to the manufacturer’s instructions. The microscopic morphology of the FeS@SRB was observed using SEM (TESCAN MIRA, Czech). First, FeS@SRB obtained at different incubation time with the initial concentration of Fe2+ of 200 mg L−1 and SRB suspension with OD600 of 0.1 were collected by centrifugation at 4024 × g for 10 min and washed twice with DI water. Subsequently, 50 μL of the FeS@SRB suspension was diluted and added dropwise on a clean silicon wafer. When the surface was half-dry, the wafer was immersed into 2.5% of glutaraldehyde and fixed at 4 °C for 4 h. FeS@SRB on the wafer were sequentially dehydrated using graded ethanol solutions (30, 50, 60, 70, 90, and 100%) for 10 min, respectively. Finally, the samples were dried and sprayed with gold for SEM observation at an accelerating voltage of 10 kV. The size and zeta-potential of FeS@SRB were measured by DLS (BeNano180Zeta, China). In addition, UV-vis-NIR absorption spectra of SRB and the FeS@SRB suspension were measured using a UV-vis-NIR spectrophotometer (UV-2600, Shimadzu, Japan). The chemical groups of the dried FeS@SRB were characterized by FTIR (VERTEX 70, Bruker, Germany). The crystal structure and elemental composition of the FeS@SRB were analyzed using an X-ray powder diffractometer (SmartLab-SE, Rigaku, Japan) and X-ray photoelectron spectroscopy (Axis Ultra DLD-600 W, Shimadzu, Japan), respectively.
Stability of FeS-SRB, FeS = SRB, and FeS@SRB
In order to fully consider the stability of biosynthesized FeS@SRB, we utilized 0.9% saline and SBF to simulate the body fluid microenvironment. Specifically, 20 mL of FeS-SRB, FeS=SRB, and FeS@SRB suspension (1 × 107 CFU mL−1) was added into 0.9% saline and SBF, respectively, and incubated at 90 rpm at 37 °C. 2 mL of the suspension was aspirated at set interval (0, 4, 12, and 20 h) and filtered through a Φ0.22 μm filter membrane. The Fe content in the shedding FeS was determined using ICP-OES after sufficient digestion with 5% nitric acid.
Photothermal effects of the FeS@SRB
The photothermal effects of the FeS@SRB suspension with different OD600 values (OD600 = 0.025, 0.05, 0.1, 0.2) were determined under the irradiation of 808 nm laser with a power density of 1.5 W cm−2. The temperature changes were monitored using a thermal imager. In addition, 1.0 mL of FeS@SRB suspension with OD600 = 0.1 was vertically irradiated with an 808 nm laser with different power density (0.5, 1.0, 1.5, and 2.0 W cm-2) for 10 min. Then FeS@SRB suspension (OD600 = 0.1, 1.0 mL) was irradiated (808 nm, 1.5 W cm-2) for 10 min and then unirradiated for 10 min, which was consecutively repeated for five cycles to study the photothermal stability of the FeS@SRB. Photothermal conversion efficiency (η) of the FeS@SRB was calculated by recording the temperature change during the above first laser on-off irradiation cycle.
Fenton effect of FeS@SRB
In order to determine whether FeS@SRB could release Fe2+ to induce the Fenton reaction, 5 mL of FeS@SRB suspension (OD600 = 0.1) was placed to 30 mL of PBS (pH 7.4, 6.5, and 5.5) for incubation with oscillations at 37 °C, respectively. 5.0 mL of the release medium was collected at the set time, and replenished with PBS with the same pH and volume. The released Fe2+ content was determined using an ICP-OES.
MB was used as a probe to detect the ability of FeS@SRB to catalyze H2O2 to ·OH. 0.5 mL of FeS@SRB (OD600 = 0.1) was mixed with 0.5 mL of MB solution (5 mg L−1) with pH 5.5 and 7.4, respectively, and equilibrated for a few minutes in the dark. Then 0.1 mL of H2O2 (10 mmol L−1) was added to the above mixed solution. After 20 min of homogeneous mixing, the suspensions were centrifuged, and the absorbance of the supernatant at 665 nm was measured to show the degradation profile of MB. The generation of •OH was further determined using the TMB method46. 0.5 mL of TMB (10 mmol L−1), 0.1 mL of H2O2 (4 mmol L−1) and 0.4 mL of FeS@SRB (OD600 = 0.1) were mixed. The mixture was adjusted to pH 5.5 and 7.4 with HCl or NaOH solution, respectively. The absorbance of the mixture at 652 nm was measured immediately after incubation at 37 °C for 15 min. Further, the production of •OH radicals were measured using DMPO as a trapping agent by electron paramagnetic resonance spectroscopy (EPR, EMXmicro-6/1, Germany). Specifically, DPMO, H2O2 (4 mmol L−1), and FeS@SRB (OD600 = 0.1) were mixed with a volume ratio of 1:3:6 for the test.
Intracellular ROS detection
4T1 cells with a density of 1 × 106 cells were inoculated in a 6-well plate, respectively, with three parallels in each group. After the cells were attached to the wall, the culture solutions were replaced by the DMEM solution containing PBS or FeS@SRB (OD600 = 0.1). For the FeS@SRB + NIR group, after 4 hours of co-incubation, cells were irradiated with 808 nm laser for 5 min at 1.5 W cm-2, and the incubation was continued to 12 h. The cell supernatant after co-incubation for 12 h post-treatment was removed, and 10 μmol L−1 of DCFH-DA staining solution was added and further incubated at 37 °C for 20 min in the dark. In the end, the cells were washed several times with PBS, and the ROS levels in the cells were observed under laser scanning confocal microscopy (OLYMPUS, Japan).
Cytocompatibility assessment of SRB and FeS@SRB
HUVECs and L929 cells (1 × 105 cells per well) were inoculated in complete medium in a 24-well plate. After the cells were adhered to the wall, transwell chambers (3 μm of pore size, Corning, USA) loaded with 50 μL of SRB and FeS@SRB with different OD600 were placed into culture dishes and co-incubated for 24 h, respectively. CCK-8 reagent was added to the lower chamber, and then the absorbance at 450 nm was measured. The cell viability in different groups was calculated according to the Eq. (1).
Cytotoxicity assessment of FeS@SRB
4T1 cells and B16F10 cells with a density of 1×105 cells were inoculated in a 24-well plate, respectively. After the cells were adhered to the wall, the transwell chambers (3 μm of pore size, Corning, USA) loaded with 50 μL of FeS@SRB with an OD600 value of 0.1 were placed into culture dishes and irradiated with NIR laser (808 nm, 1.5 W cm−2) for 10 min or not. Subsequently, the transwell chambers were removed, and the cells were incubated for 24 h. Then the viabilities of 4T1 cells and B16F10 cells with different treatments were determined through measuring the absorbance at 450 nm using the CCK-8 method. The cell viability was calculated according to the above Eq. (1).
RNA transcription analyses
To systematically decode the cytotoxic mechanisms of FeS@SRB + NIR against 4T1 cells, we employed a Transwell-based co-culture approach for global transcriptomic analysis. Briefly, 5 × 105 4T1 cells were seeded in 6-well plates. After the cells were attached to the wall, the upper culture chamber was subjected to the following treatments: Control, FeS@SRB (OD600 = 0.1) and FeS@SRB + NIR. For the FeS@SRB + NIR group, after 4 h of co-incubation, an 808 nm laser was introduced to irradiate the cells at 1.5 W cm−2 for 5 min. Then, the cells were further incubated up to 12 h, with three biological replicates in each group. Following treatment, the total RNA was extracted using the Trizol reagent (Servicebio, G3013). RNA integrity was assessed via Agilent 4150 TapeStation, while the concentration and purity were determined by NanoDropTM One/OneC (A260/A280 ≥ 1.8; A260/A230 ≥ 2.0). DNBSEQ-T7RS high-throughput sequencing reagent kit (FCL PE150) V2.0 was used to construct mRNA libraries, which were then sequenced on the DNBSEQ-T7RS platform with paired-end 150 bp reads. After data download, quality control and filtering were performed, followed by alignment to the reference genome using STAR, and then gene expression quantification, differential expression analysis, and enrichment analysis were conducted.
Hypoxic chemotaxis of FeS@SRB in vitro
Hypoxic chemotaxis of the FeS@SRB was studied using a transwell migration experiment with a pore size of 3 μm. First, 500 μL of glucose solution (0.4 mg mL−1), glucose oxidase (0.5 kU), and catalase (0.5 kU) were added to the lower chamber to mimic a hypoxic microenvironment based on glucose oxidase-catalyzed reaction47. For the normoxic control group, the lower chamber was filled with glucose solution only. Then, 200 μL of FeS@SRB (OD600 = 0.1) were added to the upper chamber. The FeS@SRB in the upper and lower chambers were collected at different incubation time and subsequently stained with DAPI. Fluorescence signal of the FeS@SRB was recorded using a confocal laser scanning microscopy (CLSM, FV1200, Japan) to visually observe the hypoxic-targeted migration capacity of the FeS@SRB.
Deep penetration of FeS@SRB in 3D tumor spheroids
FITC-labeled FeS@SRB were obtained by co-incubation of FTIC and FeS@SRB at 37 °C for 15 min. In addition, 4T1 cells and B16F10 with a density of 1×104 cells per well) were seeded in ultra-low adhesive 96-well plates and cultured in a CO2 incubator at 37 °C, respectively48. When the diameter of the 3D tumor spheroids reached 300-400 mm, they were incubated with FITC-labeled FeS@SRB for 24 h. Subsequently, the culture medium was slowly removed, and the tumor spheroids gently washed using PBS to remove the free FeS@SRB. Finally, 4% paraformaldehyde was added to fix tumor spheroids for 30 min. The obtained tumor spheroids were transferred to a confocal dish, which were then scanned layer-by-layer with CLSM Z-stack to acquire images.
Blood compatibility assay of FeS@SRB in vitro
Fresh mouse blood was collected to assess the hemolysis of SRB and FeS@SRB. Briefly, the blood was centrifuged at 4024 × g for 5 min to isolate the red blood cells. The supernatant was washed several times using PBS (pH 7.4) until it became clear and further diluted 10-fold. 0.3 mL of the obtained diluted supernatant was mixed with SRB and FeS@SRB with different OD600 (0.1 and 0.2) and incubated at 37 °C for 4 h, respectively. PBS and DI water were used as the negative and positive control groups, respectively. The absorbance of suspensions at 492 nm was measured. The hemolysis ratio was calculated using Eq. (2).
Where Ap, An and As represented the absorbance of the suspensions in the positive group, negative group and FeS@SRB-treated group, respectively.
In addition, 100 μL of fresh anticoagulated mouse blood were mixed with 5 μL of PBS, SRB (OD600 = 0.1), and FeS@SRB suspension (OD600 = 0.1), respectively, followed by the addition of 5 μL of CaCl2 (0.1 mol L−1) to re-establish the coagulation function of the blood. After storage at 37 °C for 5 min and 10 min, respectively, the blood without thrombus formation was washed away with saline. The absorbance of the washed blood was measured at 540 nm.
Biosafety evaluation of SRB in vivo
Normal mice were used as the control group, and 1 × 107 CFU mL−1 of SRB was injected intravenously into female BALB/c mice. The body weights of mice were recorded every two days. Blood was taken from the eyeballs of mice, followed by routine blood, functions of liver and kidney analysis, inflammatory factors, including IL-1β, IL-6, TNF-α, and iNOS at 12 h and on days 1, 4, 7, 14, 28, and 40 after SRB injection. In addition, major organs (heart, liver, spleen, lungs, and kidneys) were extracted, homogenized to count bacteria colonies on solid media on days 1, 4, and 7. Furthermore, the major organs of mice were collected on days 1, 7, 14, 28, and 40, respectively, and then fixed with 4% paraformaldehyde. After dehydration and deparaffinization, H&E staining was carried out, and the organs were pathologically analyzed.
Evaluation of tumor-targeting ability of FeS@SRB in vivo
The female Balb/C mice were shaved, and 100 μL of 4T1 cells (2 × 106 cells) were injected to construct a subcutaneous 4T1 tumor model. In order to verify the tumor-targeting ability of FeS@SRB in vivo, SRB and FeS@BSA prepared by the traditional chemical method were used as control groups. SRB (1 × 107 CFU mL−1), FeS@BSA (500 μg mL-1), and FeS@SRB (1 × 107 CFU mL−1) were co-incubated with IR780 (0.5 mg mL−1) at 37 °C for 15 min, respectively, followed by washed with PBS for several times to remove unbound IR780 to obtain IR780-labeled SRB, FeS@BSA, and FeS@SRB, respectively. When the tumor volume reached ~ 100 mm3, 100 μL of IR780-labeled SRB (1 × 107 CFU mL−1), FeS@BSA (500 μg mL−1), and FeS@SRB (1 × 107 CFU mL−1) was intravenously injected into mice, respectively. In addition, the above three IR780-labeled samples were intratumorally injected to other 4T1 tumor-bearing mice, respectively. Fluorescence images of the mice were recorded using an in vivo imaging system (Tanon ABL-X5, China) at different time points with λex at 780, λem at 810 nm and an exposure time of 0.5 s. Fluorescence intensity (FI) at the tumor sites were measured using the software ImageJ. The tumor-targeted delivery efficiency of various samples was calculated using Eq. (3).
Where FIi.v. and FIi.t. represented the mean fluorescence intensity (n = 3 biological independent replicates) in tumors via intravenous injection and intratumor injection in each group, respectively.
In addition, when FeS@SRB was intravenously injected into mice for 48 h, major organs and tumor tissues were collected. They were imaged and then homogenized in PBS (pH 7.4). After 12-fold dilution, they were coated on solid medium and placed in a microbial incubator at 37 °C for 24 h. Then bacterial colonies in different plates were counted. Furthermore, the bacteria distribution in the collected major organs and tumor tissues were further observed by Gram staining. In detail, various tissues were fixed by paraformaldehyde and then embedded in paraffin for sectioning. The obtained sections were deparaffinized with xylene and further processed by ethanol gradient dehydration, followed by stained with oxalic acid crystal violet, and then iodine solution. Excess water was rinsed away, and the stain was removed by gentle shaking in 95% alcohol. Finally, the tissues were stained with Safranin O solution, dried and examined microscopically.
Antitumor effects of FeS@SRB-based therapy for subcutaneous 4T1 tumor-bearing mice
Subcutaneous 4T1 tumor-bearing mice were constructed as before. When the tumor volume reached ~100 mm3, the mice were randomly divided into four groups (n = 6): G1, PBS; G2, SRB (1 × 107 CFU mL−1); G3, FeS@SRB (1 × 107 CFU mL−1); G4, FeS@SRB + NIR (1 × 107 CFU mL−1). Namely, mice were injected with 100 μL of different samples through the tail vein, in which the tumors in group G4 were irradiated with NIR laser (808 nm, 1.5 W cm−2) for 5 min at 48 h post-sample injection. Their body temperatures were recorded using an infrared thermal imager during the NIR irradiation. The tumor volume and body weight of mice were recorded every two days. Tumor volume was calculated as follows: tumor volume = length × width2/2.
On day 14, mice were sacrificed. The tumors, major organs (heart, liver, spleen, lungs, and kidneys), and blood were collected for further analysis. The tumor tissues were fixed in 4% paraformaldehyde, followed by embedded in paraffin wax, deparaffinized with xylene, treated by ethanol gradient dehydration, and then sliced and stained with H&E, TUNEL (dilution of 1:2:50, catalog number: GB1502, Servicebio), and Ki67 (dilution of 1:200, catalog number: GB111499, Servicebio), respectively. The final sections were observed and photographed using CLSM to analyze tumor necrosis. The collected blood was undergone blood biochemistry and routine blood tests. Major organs were pretreated similar to tumor tissues, and then stained with H&E. The lesions of each organ were evaluated by pathological analysis. Moreover, the survival rate of tumor-bearing mice after different treatments was observed daily, and mice was died by default when their tumor volumes reached 2000 mm3.
In order to further evaluate the Fenton effect of FeS@SRB occurred in tumor tissues, tumors were collected on day 2 and day 14, respectively to observe ROS levels by fluorescence staining. The specific operation was as follows: frozen tissues were embedded with OCT embedding agent and cut into slices of about 100 μm thickness. The tissue was incubated with tissue anti-fluorescence quenching agent for 5 min. After PBS rinsing, DHE (dilution of 1:200, catalog number: D7008, Sigma-Aldrich) was added and incubated at 37 °C for 30 min. Finally, the nuclei were stained with DAPI and imaged on CLSM.
Effectiveness of FeS@SRB-based therapy in orthotopic 4T1 tumor model and subcutaneous B16F10 tumor model
Orthotopic 4T1 tumor-bearing BALB/c female mice and subcutaneous B16F10 tumor-bearing C57BL/6 female mice were constructed as two distinct models, respectively. After the tumor volume reached ~ 100 mm3, B16F10 tumor and 4T1tumor-bearing mice were randomly divided into four groups, which were injected with PBS, SRB, FeS@BSA and FeS@SRB in the tail vein, respectively. Then the tumors were irradiated with 808 nm laser for 5 min on day 2 post-sample injection (n = 3 biological independent replicates). During NIR irradiation, the mice were imaged, and tumor temperatures were recorded. In addition, IR780-labeled SRB, FeS@BSA, and FeS@SRB were injected intravenously into orthotopic 4T1 tumor-bearing mice, respectively. The fluorescence images of mice were taken dynamically using an in vivo imaging system (GelView6000pro II, Ex/Em: 780 nm/810 nm).
To investigate the antitumor ability of FeS@SRB in vivo, the tumor-bearing mice were randomly divided into six groups (n = 6) and intravenously injected with the following samples: G1, PBS; G2, SRB; G3, FeS@BSA; G4, FeS@BSA + NIR; G5, FeS@SRB; G6, FeS@SRB + NIR. In G4 and G6, the tumors were irradiated with NIR laser (808 nm, 1.5 W cm-2) for 5 min on day 2 post-sample injection. In addition, the NIR laser irradiation was carried out again on the tumors in G4 and G6 for the orthotopic 4T1 tumor-bearing mice on day 4. Tumor volume and body weight were measured every other day until day 14. After treatments, mice were euthanized to excise tumors for H&E, TUNEL (dilution of 1:2:50, catalog number: GB1502, Servicebio), and Ki67 (dilution of 1:200, catalog number: GB111499, Servicebio) staining to analyze the antitumor effect. Liver and lung tissues were collected to observe tumor metastasis. Spleens were also exacted in the orthotopic 4T1 tumor-bearing mice. The isolated blood sera were used to determine the liver and kidney functions.
Tumor immune microenvironment analysis
To assess FeS@SRB-induced immune responses, TDLNs and tumor tissues were harvested at 48 h post-final treatment using an orthotopic 4T1 breast cancer-bearing mice model. Tumors were mechanically dissociated into fragments and enzymatically digested with type I collagenase at 37 °C for 30 min with gentle agitation. Both TDLNs and digested tumor masses were gently pressed through 200 μm nylon mesh filters (triplicate filtration). Cell suspensions were centrifuged at 300 × g for 10 min to collect single cells. Lymphocyte cells were re-suspended in PBS and stained with fluorochrome-conjugated antibodies, CD80 (Elabscience, E-AB-F0992E, Clone: 16-10A1), CD86 (Elabscience, E-AB-F0994D, Clone: GL-1), CD11c (Elabscience, E-AB-F0991C, Clone: N418). Tumor cells were immersed in RBC lysate to remove erythrocytes, washed and centrifuged several times, then dispersed in PBS and stained with CD8 (Elabscience, E-AB-F1104E, Clone: 53-6.7), CD4 (Elabscience, E-AB-F1353C, Clone: RM4-5) and CD3 (Elabscience, E-AB-F1013D, Clone: 17A2) for flow cytometry analysis.
Statistics and reproducibility
All statistical analyses were performed with Origin 2024 or GraphPad Prism 10.2. All quantitative values were presented as mean ± standard deviation (SD). All experiments in this study were repeated independently with similar results at least three times to ensure credible results and statistical validity. The samples/animals were randomly allocated to experimental groups. The investigators were blinded to group allocation during data collection and analysis. The statistical significance among groups was carried out by one-way analysis of variance (ANOVA) with Tukey’s post-hoc test. The statistical significance for the tests was at *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, and ns, not significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The tumor RNA-seq data generated in this study have been deposited in the SRA database under accession code PRJNA1280553.
The authors declare that all data generated or analyzed during this study are available within the Article, Supplementary Information, and the Source Data File. Source data are provided in this paper.
References
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mat. 1, 16014 (2016).
Kwon, S. Y., Thi-Thu Ngo, H., Son, J., Hong, Y. & Min, J. J. Exploiting bacteria for cancer immunotherapy. Nat. Rev. Clin. Oncol. 21, 569–589 (2024).
Gurbatri, C. R., Arpaia, N. & Danino, T. Engineering bacteria as interactive cancer therapies. Science 378, 858–864 (2022).
Huang, X. et al. Bacteria-based cancer immunotherapy. Adv. Sci. 8, 2003572 (2021).
Guo, L., Ding, J. & Zhou, W. Harnessing bacteria for tumor therapy: Current advances and challenges. Chinese Chem. Lett. 35, 108557 (2024).
Zhou, S., Gravekamp, C., Bermudes, D. & Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 18, 727–743 (2018).
He, T. et al. Enhanced immunogenic cell death and antigen presentation via engineered bifidobacterium bifidum to boost chemo-immunotherapy. ACS Nano 17, 9953–9971 (2023).
Guo, H. et al. Integrating bacteria with a ternary combination of photosensitizers for monochromatic irradiation-mediated photoacoustic imaging-guided synergistic photothermal therapy. ACS Nano 17, 5059–5071 (2023).
Lin, S. S. et al. Surface-modified bacteria: Synthesis, functionalization and biomedical applications. Chem. Soc. Rev. 52, 6617–6643 (2023).
Jiang, J., Huang, Y., Zeng, Z. & Zhao, C. Harnessing engineered immune cells and bacteria as drug carriers for cancer immunotherapy. ACS Nano 17, 843–884 (2023).
Suh, S. et al. Nanoscale bacteria-enabled autonomous drug delivery system (NanoBEADS) enhances intratumoral transport of nanomedicine. Adv. Sci. 6, 1801309 (2019).
Vincent, R. L. et al. Probiotic-guided CAR-T cells for solid tumor targeting. Science 382, 211–218 (2023).
Centurion, F. et al. Nanoencapsulation for probiotic delivery. ACS Nano 15, 18653–18660 (2021).
Pan, P. et al. Bio-orthogonal bacterial reactor for remission of heavy metal poisoning and ROS elimination. Adv. Sci. 6, 1902500 (2019).
Park, M., Park, S. & Hyun, J. Use of magnetic nanoparticles to manipulate the metabolic environment of bacteria for controlled biopolymer synthesis. ACS Appl. Mater. Interfaces 4, 5114–5117 (2012).
Van Haasteren, J., Li, J., Scheideler, O. J., Murthy, N. & Schaffer, D. V. The delivery challenge: Fulfilling the promise of therapeutic genome editing. Nat. Biotechnol. 38, 845–855 (2020).
Choi, Y. & Lee, S. Biosynthesis of inorganic nanomaterials using microbial cells and bacteriophages. Nat. Rev. Chem. 4, 638–656 (2020).
Zhou, J., Yang, Y. & Zhang, C. Toward biocompatible semiconductor quantum dots: From biosynthesis and bioconjugation to biomedical application. Chem. Rev. 115, 11669–11717 (2015).
Wang, L. et al. Bacteria‐mediated tumor therapy via photothermally‐programmed cytolysin A expression. Small 17, 2102932 (2021).
Deng, X., Dohmae, N., Kaksonen, A. H. & Okamoto, A. Biogenic iron sulfide nanoparticles to enable extracellular electron uptake in sulfate‐reducing bacteria. Angew. Chem. Int. Edit. 59, 5995–5999 (2020).
Anandkumar, B., George, R. P., Maruthamuthu, S., Parvathavarthini, N. & Mudali, U. K. Corrosion characteristics of sulfate-reducing bacteria (SRB) and the role of molecular biology in SRB studies: an overview. Corros. Rev. 34, 41–63 (2016).
Kushkevych, I. et al. The sulfate-seducing microbial communities and meta-analysis of their occurrence during diseases of sall–large intestine axis. J. Clin. Med. 8, 1656 (2019).
Kushkevych, I. et al. Distribution of sulfate-reducing bacteria in the environment: cryopreservation techniques and their potential storage application. Processes 9, 1843 (2021).
Muyzer, G. & Stams, A. J. M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 6, 441–454 (2008).
Yang, H. et al. Preparation of nickel-iron hydroxides by microorganism corrosion for efficient oxygen evolution. Nat. Commun. 11, 5075 (2020).
Wang, J. et al. The isolation of anaerobic and facultative anaerobic sulfate-reducing bacteria (SRB) and a comparison of related enzymes in their sulfate reduction pathways. Microorganisms 11, 2019 (2023).
Murugan, M., Miran, W., Masuda, T., Lee, D. S. & Okamoto, A. Biosynthesized iron sulfide nanocluster enhanced anodic current generation by sulfate reducing bacteria in microbial fuel cells. ChemElectroChem 5, 4015–4020 (2018).
Fauque, G., Herve, D. & Le Gall, J. Structure-function relationship in hemoproteins: The role of cytochrome c3 in the reduction of colloidal sulfur by sulfate-reducing bacteria. Arch. Microbiol. 121, 261–264 (1979).
Bai, H., Kang, Y., Quan, H., Han, Y. & Feng, Y. Treatment of copper wastewater by sulfate reducing bacteria in the presence of zero valent iron. Int. J. Miner. Process 112-113, 71–76 (2012).
Yang, Y., Cheng, T. & Zhou, G. Effect of sulfate-reducing bacteria on the production of iron sulfides. Geomicrobiol. J. 40, 247–254 (2022).
Dong, Y. et al. Study on the effectiveness of sulfate reducing bacteria to remove heavy metals (Fe, Mn, Cu, Cr) in acid mine drainage. Sustainability 15, 5486 (2023).
Sirajuddin, M., Ali, S. & Badshah, A. Drug–DNA interactions and their study by UV–visible, fluorescence spectroscopies and cyclic voltametry. J. Photoch. Photobio. B 124, 1–19 (2013).
Shi, L. et al. Isolation of a high-affinity functional protein complex between OmcA and MtrC: Two outer membrane decahemec-type cytochromes of Shewanella Oneidensis MR-1. J. Bacteriol. 188, 4705–4714 (2006).
Yang, Y., Cheng, T. & Zhou, G. Structure, composition and interfacial properties of iron sulfides synthesized by sulfate-reducing bacteria (SRB). J. Iran Chem. Soc. 19, 4735–4745 (2022).
Sheng, G., Yu, H. & Wang, C. FTIR-spectral analysis of two photosynthetic H2-producing strains and their extracellular polymeric substances. Appl. Microbiol. Biot. 73, 204–210 (2006).
Liu, F., Lu, T. & Zhang, Y. Performance assessment of constructed wetland-microbial fuel cell for treatment of mariculture wastewater containing heavy metals. Process Saf. Environ. 168, 633–641 (2022).
Yu, Y. et al. Size-controlled biosynthesis of FeS nanoparticles for efficient removal of aqueous Cr(VI). Chem. Eng. J. 379, 122404 (2020).
Xie, C. et al. FeS@BSA nanoclusters to enable H2S-amplified ROS-based therapy with MRI guidance. Adv. Sci. 7, 1903512 (2020).
Papa, S. et al. Tumor induced hepatic myeloid derived suppressor cells can cause moderate liver damage. PLoS ONE 9, e112717 (2014).
Mackay, W. D. Role of splenomegaly in tumour-bearing mice. Nature 205, 918–919 (1965).
Chen, S. & Zhang, D. Study of corrosion behavior of copper in 3.5 wt.% NaCl solution containing extracellular polymeric substances of an aerotolerant sulphate-reducing bacteria. Corros. Sci. 136, 275–284 (2018).
Jamal, F. et al. Review of metal sulfide nanostructures and their applications. ACS Appl. Nano Mater. 6, 7077–7106 (2023).
Kushkevych, I. et al. The sulfate-reducing microbial communities and meta-analysis of their occurrence during diseases of small-large intestine axis. J. Clin. Med. 8, 1656 (2019).
Song, X. et al. Engineering ternary PdMop nanoenzyme for enzyodynamic effect-enhanced ferroptosis and sonocatalysis-enabled tumor immunotherapy. Adv. Funct. Mater. 33, 2312172 (2023).
Zhao, Y. et al. In situ synthesis of Ru/TiO2−x@TiCN ternary heterojunctions for enhanced sonodynamic and nanocatalytic cancer therapy. Adv. Sci. 10, 2307029 (2023).
Chen, F. et al. Nanophotosensitizer-engineered Salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials 214, 119226 (2019).
Chen, J. et al. Sequentially responsive shell-stacked nanoparticles for deep penetration into solid tumors. Adv. Mater. 29, 1701170 (2017).
Acknowledgements
This work was supported by the National Basic Research Program of China (2020YFA07107000 to X.L.Y.) and the Fundamental Research Funds for the Central Universities (No. YCJJ20242410 to M.D.). We thank the Analytical and Testing Center of Huazhong University of Science and Technology (HUST) and the Testing Center in the School of Chemistry and Chemical Engineering, HUST, for the related analysis. We also thank for the support provided by the Laboratory Animal Center of HUST.
Author information
Authors and Affiliations
Contributions
Conceptualized the study: Q.W.; Designed the experiments: Q.W., M.D., H.L.; X.L.Y., and Y.Y.; Conducted the experiments: M.D.; X.H.Y., W. Z. and Y. Q.; Wrote the initial draft of the manuscript: M. D.; Analyzed the data: M.D., Q. W., X.L.Y., and P.S.; Writing-review & editing the manuscript: Q.W. and X.L.Y. Approved the final manuscript: all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dong, M., Yang, X., Zhang, W. et al. Living therapeutics of nonpathogenic bacteria as biosynthesis factory and active carriers for enhancing tumor-targeted therapy. Nat Commun 16, 6532 (2025). https://doi.org/10.1038/s41467-025-61675-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61675-4
This article is cited by
-
Bacteria-powered LA@CaDGP biomotor: a multi-modal weapon integrating calcium overload, chemotherapy, and starvation for breast cancer therapy
Journal of Nanobiotechnology (2026)