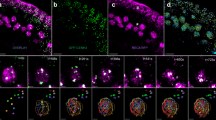
Abstract
Homologous pairing and recombination during meiosis are facilitated by rapid prophase movements (RPMs), which depend on chromosome attachment to the nuclear envelope (NE) and on cytoplasmic forces transmitted to the chromosomes through the NE, mediated by Linker of Nucleoskeleton and Cytoskeleton (LINC) complexes. In plants, only the NE-associated SUN-domain proteins SUN1 and SUN2 have been identified as components of the RPM process. Here we show that, during meiosis, SUN1 and SUN2 form a LINC complex with the KASH-domain protein SINE3, which recruits the meiosis-specific kinesin PSS1 to the NE. These proteins accumulate at telomere-binding sites in the NE, and their loss disrupts telomere attachment and bouquet formation and abolishes RPMs. These defects lead to defective synapsis and clustered crossovers, resulting in chromosome mis-segregation. Our results establish that the mechanism underlying RPMs is conserved in Arabidopsis thaliana, with RPMs primarily facilitating homologous recognition rather than preventing non-homologous interactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The accession codes are provided in Supplementary Table 1. Source data are provided with this paper.
Code availability
The R software scripts used to perform quantitative analyses of centromere dynamics are available at https://doi.org/10.57745/V1NNFI. The scripts used to analyse the inter-MLH1 distance and calculate the CoC are available via GitHub at https://github.com/mapeuch/Arabidopsis_LINC_paper.
References
Zickler, D. & Kleckner, N. Recombination, pairing, and synapsis of homologs during meiosis. Cold Spring Harb. Perspect. Biol. 7, a016626 (2015).
Zickler, D. & Kleckner, N. A few of our favorite things: pairing, the bouquet, crossover interference and evolution of meiosis. Semin. Cell Dev. Biol. 54, 135–148 (2016).
Sybenga, J. Homologous chromosome pairing in meiosis of higher eukaryotes—still an enigma? Genome 63, 469–482 (2020).
Link, J. & Jantsch, V. Meiotic chromosomes in motion: a perspective from Mus musculus and Caenorhabditis elegans. Chromosoma 128, 317–330 (2019).
Fernández-Álvarez, A. Beyond tradition: exploring the non-canonical functions of telomeres in meiosis. Front. Cell Dev. Biol. 11, 1278571 (2023).
King, M. C. Dynamic regulation of LINC complex composition and function across tissues and contexts. FEBS Lett. 597, 2823–2832 (2023).
Zetka, M., Paouneskou, D. & Jantsch, V. The nuclear envelope, a meiotic jack-of-all-trades. Curr. Opin. Cell Biol. 64, 34–42 (2020).
Kim, H. J., Liu, C. & Dernburg, A. F. How and why chromosomes interact with the cytoskeleton during meiosis. Genes 13, 901 (2022).
Alleva, B. & Smolikove, S. Moving and stopping: regulation of chromosome movement to promote meiotic chromosome pairing and synapsis. Nucleus 8, 613–624 (2017).
Burke, B. LINC complexes as regulators of meiosis. Curr. Opin. Cell Biol. 52, 22–29 (2018).
Fan, J., Jin, H., Koch, B. A. & Yu, H.-G. Mps2 links Csm4 and Mps3 to form a telomere-associated LINC complex in budding yeast. Life Sci. Alliance 3, e202000824 (2020).
Trelles-Sticken, E., Adelfalk, C., Loidl, J. & Scherthan, H. Meiotic telomere clustering requires actin for its formation and cohesin for its resolution. J. Cell Biol. 170, 213–223 (2005).
Koszul, R., Kim, K. P., Prentiss, M., Kleckner, N. & Kameoka, S. Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell 133, 1188–1201 (2008).
Lee, C.-Y. et al. Extranuclear structural components that mediate dynamic chromosome movements in yeast meiosis. Curr. Biol. 30, 1207–1216.e4 (2020).
Cromer, L. et al. Rapid meiotic prophase chromosome movements in Arabidopsis thaliana are linked to essential reorganization at the nuclear envelope. Nat. Commun. 15, 5964 (2024).
Varas, J. et al. Absence of SUN1 and SUN2 proteins in Arabidopsis thaliana leads to a delay in meiotic progression and defects in synapsis and recombination. Plant J. 81, 329–346 (2015).
Christophorou, N. et al. Microtubule-driven nuclear rotations promote meiotic chromosome dynamics. Nat. Cell Biol. 17, 1388–1400 (2015).
Lee, C.-Y. et al. Mechanism and regulation of rapid telomere prophase movements in mouse meiotic chromosomes. Cell Rep. 11, 551–563 (2015).
Koszul, R. & Kleckner, N. Dynamic chromosome movements during meiosis: a way to eliminate unwanted connections? Trends Cell Biol. 19, 716–724 (2009).
Mytlis, A., Levy, K. & Elkouby, Y. M. The many faces of the bouquet centrosome MTOC in meiosis and germ cell development. Curr. Opin. Cell Biol. 81, 102158 (2023).
Zhang, F. et al. The SUN domain proteins OsSUN1 and OsSUN2 play critical but partially redundant roles in meiosis. Plant Physiol. 183, 1517–1530 (2020).
Sosa, B. A., Rothballer, A., Kutay, U. & Schwartz, T. U. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell 149, 1035–1047 (2012).
Zhou, X. & Meier, I. Efficient plant male fertility depends on vegetative nuclear movement mediated by two families of plant outer nuclear membrane proteins. Proc. Natl Acad. Sci. USA 111, 11900–11905 (2014).
Graumann, K. et al. Characterization of two distinct subfamilies of SUN-domain proteins in Arabidopsis and their interactions with the novel KASH-domain protein AtTIK. J. Exp. Bot. 65, 6499–6512 (2014).
Zhou, X., Graumann, K., Evans, D. E. & Meier, I. Novel plant SUN–KASH bridges are involved in RanGAP anchoring and nuclear shape determination. J. Cell Biol. 196, 203–211 (2012).
Duroc, Y. et al. The kinesin AtPSS1 promotes synapsis and is required for proper crossover distribution in meiosis. PLoS Genet. 10, e1004674 (2014).
Biel, A., Moser, M. & Meier, I. Arabidopsis KASH proteins SINE1 and SINE2 are involved in microtubule reorganization during ABA-induced stomatal closure. Front. Plant Sci. 11, 575573 (2020).
Biel, A., Moser, M., Groves, N. R. & Meier, I. Distinct roles for KASH proteins SINE1 and SINE2 in guard cell actin reorganization, calcium oscillations, and vacuolar remodeling. Front. Plant Sci. 13, 784342 (2022).
Moser, M., Groves, N. R. & Meier, I. Plant KASH proteins SINE1 and SINE2 have synergistic and antagonistic interactions with actin-branching and actin-bundling factors. J. Exp. Bot. 75, 73–87 (2024).
Gumber, H. K. et al. Identification and characterization of genes encoding the nuclear envelope LINC complex in the monocot species Zea mays. J. Cell Sci. https://doi.org/10.1242/jcs.221390 (2019).
Zhou, X., Graumann, K., Wirthmueller, L., Jones, J. D. G. & Meier, I. Identification of unique SUN-interacting nuclear envelope proteins with diverse functions in plants. J. Cell Biol. 205, 677–692 (2014).
Moser, M., Groves, N. R. & Meier, I. The Arabidopsis KASH protein SINE3 is involved in male and female gametogenesis. Plant Reprod. 37, 521–534 (2024).
Vrielynck, N. et al. SCEP1 and SCEP2 are two new components of the synaptonemal complex central element. Nat. Plants 9, 2016–2030 (2023).
Zhou, X., Graumann, K. & Meier, I. The plant nuclear envelope as a multifunctional platform LINCed by SUN and KASH. J. Exp. Bot. 66, 1649–1659 (2015).
Murphy, S. P., Gumber, H. K., Mao, Y. & Bass, H. W. A dynamic meiotic SUN belt includes the zygotene-stage telomere bouquet and is disrupted in chromosome segregation mutants of maize (Zea mays L.). Front. Plant Sci. 5, 314 (2014).
Zhou, S. et al. Pollen Semi-Sterility1 encodes a Kinesin-1–like protein important for male meiosis, anther dehiscence, and fertility in rice. Plant Cell 23, 111–129 (2011).
Chelysheva, L. et al. The Arabidopsis HEI10 is a new ZMM protein related to Zip3. PLoS Genet. 8, e1002799 (2012).
Hurel, A. et al. A cytological approach to studying meiotic recombination and chromosome dynamics in Arabidopsis thaliana male meiocytes in three dimensions. Plant J. 95, 385–396 (2018).
Prusicki, M. A. et al. Live cell imaging of meiosis in Arabidopsis thaliana. eLife 8, e42834 (2019).
Armstrong, S. J., Franklin, F. C. H. & Jones, G. H. Nucleolus-associated telomere clustering and pairing precede meiotic chromosome synapsis in Arabidopsis thaliana. J. Cell Sci. 114, 4207–4217 (2001).
Mercier, R., Mézard, C., Jenczewski, E., Macaisne, N. & Grelon, M. The molecular biology of meiosis in plants. Annu. Rev. Plant Biol. 66, 297–327 (2015).
Jahns, M. T. et al. Crossover localisation is regulated by the neddylation posttranslational regulatory pathway. PLoS Biol. 12, e1001930 (2014).
Girard, C. et al. AAA-ATPase FIDGETIN-LIKE 1 and helicase FANCM antagonize meiotic crossovers by distinct mechanisms. PLoS Genet. 11, e1005369 (2015).
Zhou, Y. et al. Kinesin-1-like protein PSS1 is essential for full-length homologous pairing and synapsis in rice meiosis. Plant J. 120, 928–940 (2024).
Poulet, A., Probst, A. V., Graumann, K., Tatout, C. & Evans, D. Exploring the evolution of the proteins of the plant nuclear envelope. Nucleus 8, 46–59 (2017).
Penkner, A. et al. The nuclear envelope protein Matefin/SUN-1 is required for homologous pairing in C. elegans meiosis. Dev. Cell 12, 873–885 (2007).
Horn, H. F. et al. A mammalian KASH domain protein coupling meiotic chromosomes to the cytoskeleton. J. Cell Biol. 202, 1023–1039 (2013).
Chikashige, Y. et al. Meiotic proteins Bqt1 and Bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 125, 59–69 (2006).
Rubin, T., Macaisne, N. & Huynh, J.-R. Mixing and matching chromosomes during female meiosis. Cells 9, 696 (2020).
Nebenführ, A. & Dixit, R. Kinesins and myosins: molecular motors that coordinate cellular functions in plants. Annu. Rev. Plant Biol. 69, 329–361 (2018).
Salonen, K., Paranko, J. & Parvinen, M. A colcemid-sensitive mechanism involved in regulation of chromosome movements during meiotic pairing. Chromosoma 85, 611–618 (1982).
Ding, D.-Q., Chikashige, Y., Haraguchi, T. & Hiraoka, Y. Oscillatory nuclear movement in fission yeast meiotic prophase is driven by astral microtubules, as revealed by continuous observation of chromosomes and microtubules in living cells. J. Cell Sci. 111, 701–712 (1998).
Morimoto, A. et al. A conserved KASH domain protein associates with telomeres, SUN1, and dynactin during mammalian meiosis. J. Cell Biol. 198, 165–172 (2012).
Wynne, D. J., Rog, O., Carlton, P. M. & Dernburg, A. F. Dynein-dependent processive chromosome motions promote homologous pairing in C. elegans meiosis. J. Cell Biol. 196, 47–64 (2012).
Yamamoto, T. G., Chikashige, Y., Ozoe, F., Kawamukai, M. & Hiraoka, Y. Activation of the pheromone-responsive MAP kinase drives haploid cells to undergo ectopic meiosis with normal telomere clustering and sister chromatid segregation in fission yeast. J. Cell Sci. 117, 3875–3886 (2004).
Conrad, M. N., Lee, C.-Y., Wilkerson, J. L. & Dresser, M. E. MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 104, 8863–8868 (2007).
Baudrimont, A. et al. Leptotene/zygotene chromosome movement via the SUN/KASH protein bridge in Caenorhabditis elegans. PLoS Genet. 6, e1001219 (2010).
Conrad, M. N. et al. Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3, and CSM4 and is modulated by recombination. Cell 133, 1175–1187 (2008).
Shibuya, H. & Watanabe, Y. The meiosis-specific modification of mammalian telomeres. Cell Cycle 13, 2024–2028 (2014).
Link, J. et al. Transient and partial nuclear lamina disruption promotes chromosome movement in early meiotic prophase. Dev. Cell 45, 212–225.e7 (2018).
Woglar, A. et al. Matefin/SUN-1 phosphorylation is part of a surveillance mechanism to coordinate chromosome synapsis and recombination with meiotic progression and chromosome movement. PLoS Genet. 9, e1003335 (2013).
da Cruz, I., Brochier-Armanet, C. & Benavente, R. The TERB1–TERB2–MAJIN complex of mouse meiotic telomeres dates back to the common ancestor of metazoans. BMC Evol. Biol. 20, 55 (2020).
Su, H. et al. Arabidopsis RAD51, RAD51C and XRCC3 proteins form a complex and facilitate RAD51 localization on chromosomes for meiotic recombination. PLoS Genet. 13, e1006827 (2017).
Sato, A. et al. Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell 139, 907–919 (2009).
Marshall, W. F. & Fung, J. C. Modeling homologous chromosome recognition via nonspecific interactions. Proc. Natl Acad. Sci. USA 121, e2317373121 (2024).
Emmenecker, C., Mézard, C. & Kumar, R. Repair of DNA double-strand breaks in plant meiosis: role of eukaryotic RecA recombinases and their modulators. Plant Reprod. 36, 17–41 (2023).
Xing, H.-L. et al. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 14, 327 (2014).
Zhang, Y., Werling, U. & Edelmann, W. SLiCE: a novel bacterial cell extract-based DNA cloning method. Nucleic Acids Res. 40, e55 (2012).
Ravi, M. & Chan, S. W. L. Haploid plants produced by centromere-mediated genome elimination. Nature 464, 615–618 (2010).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013); http://www.R-project.org/
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Yang, C. et al. The Arabidopsis Cdk1/Cdk2 homolog CDKA;1 controls chromosome axis assembly during plant meiosis. EMBO J. 39, e101625 (2020).
Giraut, L. et al. Genome-wide crossover distribution in Arabidopsis thaliana meiosis reveals sex-specific patterns along chromosomes. PLoS Genet. 7, e1002354 (2011).
Fernandes, J. B., Séguéla-Arnaud, M., Larchevêque, C., Lloyd, A. H. & Mercier, R. Unleashing meiotic crossovers in hybrid plants. Proc. Natl Acad. Sci. USA 115, 2431–2436 (2018).
Fischer, D. Fluidigm: An R package for converting SNP genotype data from Fluidigm to PLINK and performing basic QC v.0.2 (2024).
Lorieux, M. MapDisto: fast and efficient computation of genetic linkage maps. Mol. Breed. 30, 1231–1235 (2012).
Christophorou, N. et al. AXR1 affects DNA methylation independently of its role in regulating meiotic crossover localization. PLoS Genet. 16, e1008894 (2020).
Acknowledgements
This work has benefited from the support of the Institut Jean-Pierre Bourgin’s Plant Observatory technological platforms PO-Plants and PO-Cyto. This research was funded by the National Natural Science Foundation of China (grant nos 32300296 to B.C. and 32370360 and 32170354 to C.Y.) and the ANR (COPATT ANR-20-CE12-0006 to M.G. and M.T.-A. and MeioMove ANR-21CE12-0042 to M.G., P.A., L.C. and S.L.). The Institute Jean-Pierre Bourgin benefits from the support of Saclay Plant Sciences (ANR-17-EUR0007).
Author information
Authors and Affiliations
Contributions
M.G., C.M., P.A., L.C. and C.Y. conceived and designed the experiments. B.C., M.T.-A., Y.L., S.L., F.C., A.C., X.Y., M.P., Y.Z., A.H., J.G., N.V., C.M. and L.C. performed the experiments. M.G., B.C., M.T.-A., S.L., A.C., M.P., C.M., P.A., L.C. and C.Y. analysed the data. M.G., B.C., M.T.-A., S.L., M.P., C.M., P.A. and C.Y. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Mónica Pradillo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Phenotypes and Epistatic Analyses of the sine3 Mutants.
(a) Generation and mutation identification of CRISPR-Cas9 sine3 and sine4 mutants. (b) Vegetative growth of Wt, sine3-1, sine3-4, sine3-5, sine4-1, sine4-2, sine3-1 sine4-1, and sine3-1 sine4-2 plants. Bar: 10 cm. (c) Seed viability quantification. Horizontal magenta lines indicate mean \(\pm \,\)SD. Horizontal black lines indicate the results of an ordinary one-way ANOVA, followed by Tukey’s multiple comparison test (comparing all mean values), ns= non significant, **** indicates p values < 0.0001. (d-f) Pollen viability after Alexander staining in single and multiple mutants. Pollen grains from at least five different plants of each genotype were analyzed. Horizontal magenta lines indicate mean \(\pm \,\)SD. Asterisks indicate differences that were found significant after ordinary one-way ANOVA, followed by Tukey’s multiple comparison test (comparing all mean values) (d, e), or unpaired t test (f). ns= non significant, **** indicates p values < 0.0001. (g) Box plot that shows the quantification of the minimum chiasma number at metaphase/anaphase transition. The boxes extend from the 25th to 75th percentiles, whiskers extend from minimum to maximum values. Asterisks indicate the results of an ordinary one-way ANOVA, followed by Dunnett’s multiple comparison test (comparing each mean value to the Wt mean values). ns= non significant, **** indicates p values < 0.0001.
Extended Data Fig. 2 Functional validation of SINE3 reporter lines.
(a-b) Expression pattern of SINE3:GFP (a) and GFP:SINE3 (b) in male meiosis of sine3-1 mutant at early and late prophase. Bars: 10 µm. (c) Siliques of Wt, sine3-1, GFP:SINE3 (in sine3-1), SINE3:GFP (in sine3-1), SINE3:interGFP (in sine3-1) plants. Bar: 1 cm. (d) Pollen viability of Wt, sine3-1, GFP:SINE3 (sine3-1), SINE3:GFP (sine3-1), SINE3:interGFP (sine3-1) plants. Asterisks indicate significant difference (one-way ANOVA followed by Tukey’s multiple comparaison test, **** indicate p values < 0.0001, ns= non significant). Magenta lines indicate mean \(\pm \,\)SD. (e) Expression pattern of SINE3:interGFP during male meiosis of sine3-1 mutants at early, mid, and late prophase. Bars: 10 µm. White arrowheads indicate the expression of SINE3:interGFP in some tapetal cells.
Extended Data Fig. 3 Functional validation of PSS1 reporter lines.
(a) Siliques of Wt, pss1-3, PSS1:GFP (in pss1-3) line1, and PSS1:GFP (in pss1-3) line2 plants. Bar: 1 cm. (b) Pollen viability of Wt, pss1-3, PSS1:GFP (in pss1-3) line1, and PSS1:GFP (in pss1-3) line2 plants. Asterisks indicate significant difference (one-way ANOVA followed by Tukey’s multiple comparaison test, **** indicate p values < 0.0001, ns= non significant). Magenta lines show mean \(\pm \,\)SD. (c) Expression pattern of PSS1:GFP in male meiosis of pss1-3 mutants at early, mid, and late prophase. Bars: 10 µm.
Extended Data Fig. 4 PSS1 is associated to the meiocyte NE from late leptotene to diplotene.
Co-immunostaining of REC8 (magenta), with HEI10 (yellow) and PSS1 (green) on 3D-preserved male meiocytes from wild-type plants. For each cell from Fig. 2d (main text), the maximum intensity projection of each channel is shown, as well as the DAPI signal (grey). Bars: 2 µm. EL= Early Leptotene, LL= Late Leptotene, Z=Zygotene, P= Pachytene, D= Diplotene. The negative control for anti-PSS1 staining is also shown (pss1-1 mutant).
Extended Data Fig. 5 Interactions between the components of the cytoplasmic motor-LINC chain.
(a-b) Experimental controls for in planta BiFC. (a) The BiFC control testing the interaction of SINE3:interVenusNter with ASY1:VENUSCter. (b) The BiFC control testing the interaction of PSS1:VenusCter with ASY1:VENUSNter. All images were captured from A. thaliana male meiocytes of plants transformed by the relevant combinations of constructs. All bars: 10 µm. (c) The C-terminal region of PSS1 interacts with SINE3 in yeast two-hybrid assay. Monomeric GFP (mGFP) fused with AD or BD domains was used as a negative control. The synthetic dropout media in the absence of Leu, Trp, and His (-L/W/H) or Leu, Trp, His, and Ade (-L/W/H/A) were used for interaction test.
Extended Data Fig. 6 Localization dependency between SINE3, PSS1, and SUN1-Live imaging.
(a, b) Localization of PSS1:GFP in male meiocytes of wild type (a) and sine3-1 (b) during prophase. (c, d) Localization of SUN1:GFP in male meiocytes of wild type (c) and sine3-1 (d) during prophase. (e) Localization of SINE3:GFP in male meiocytes of sun1 sun2. (f) Localization of PSS1:GFP in male meiocytes of sun1 sun2 during prophase. (g) Localization of SINE3:GFP in male meiocytes of pss1. (h) Localization of SUN1:GFP in male meiocytes of pss1. The RFP:TUA5 labels the microtubule and helps to determine the meiotic stages together with bright field (BF) images. All bars: 10 µm.
Extended Data Fig. 7 Localization dependency between SINE3, PSS1, and Cter-SUN proteins – Immunocytology.
Co-immunostaining of REC8 (magenta), with Cter-SUNs or PSS1 or SINE3:interGFP on 3D-preserved male meiocytes from wild-type or mutants. For each cell from Fig. 4d (main text), the maximum intensity projection of each signal is shown. Bars: 2 µm, except for pss1 meiocyte: 3 µm. For the 3D movie stacks, see the following videos:. Movie11_4D_Wt: Wt with anti REC8, and PSS1. Movie12_4D_Wt: Wt with anti REC8, and Cter-SUNs. Movie13_4D_sine3: sine3-1 with anti REC8, and PSS1. Movie14_4D_sine3: sine3-1 with anti REC8, and Cter-SUNs. Movie15_4D_pss1_SINE3interGFP: pss1-1_SINE3:interGFP with anti REC8, GFP, and Cter-SUNs. Movie16_4D_sun1sun2_SINE3interGFP: sun1sun2_SINE3:interGFP with anti REC8, GFP, and PSS1.
Extended Data Fig. 8 Localization dependency between SINE3, PSS1, and Cter-SUNs.
(a) Analysis of the protein intensity of SUN1:GFP on the NE of wild type and sine3-1 mutant plants. Black horizontal lines indicate mean, magenta lines +/− SD. Asterisks indicate significant difference (Students’ t-test, P < 0.01). (b) Examples for the occasionally observed polarization of SINE3:interGFP and SUN1:GFP in pss1-3 mutants. Bars: 10 µm.
Extended Data Fig. 9 SINE3 and PSS1 are enriched at telomere anchorage sites on the NE and are required for telomere attachment to the NE and bouquet formation.
(a) Maximum intensity projections of each channel for the cells shown in Fig. 5a (main text), Bars: 2 µm. Corresponding 3D movie stacks are: Movie17_5A_Wt_SINE3interGFP and Movie18_5A_Wt_SINE3interGFP. (b) Maximum intensity projections of each channel for the cells shown in Fig. 5b (main text), Bars: 3 µm. Corresponding 3D movie stacks are: Movie19_5B_Wt, Movie20_5B_sine3, and Movie21_5B_pss1. (c) Example of a mutant meiocyte where telomere cluster is observed in the nucleoplasm. Bars: 3 µm. Corresponding 3D movie stacks are Movie22_sine3 and Movie23_pss1. For B and C, and on 3D movie stacks, nucleolus and NE segmentations are shown, with the telomere locations (coloured spheres). The colour code is based on the proximity to the nuclear periphery (the telomeres at the periphery are the pinker).
Extended Data Fig. 10 The cytoskeletal motor-LINC complex chain (PSS1-LINC) is required for class I CO distribution.
(a) Representative example of late diplotene cells on which the MLH1 foci quantification has been performed (data shown in Fig. 7b, main text). Bars: 3 µm. (b) HEI10 immunostaining in male meiocytes of Wt, sine3-1, pss1-3, sine3-1 pss1-3, and sun1 sun2 mutant plants at late pachytene or late pachytene-like stages. Bars: 10 µm. The red rectangles highlight the chromosome regions with closely localized HEI10 foci. The corresponding quantification of the number of HEI10 foci is shown below. Error bars indicate the mean ± SD and asterisks indicate significant difference (Tukey’s multiple comparison test, P < 0.01).
Supplementary information
Supplementary Information
Supplementary Tables 1–5.
Supplementary Video 1
SINE3–interGFP expression patterns in an early leptotene cell.
Supplementary Video 2
SINE3–interGFP expression patterns in a late leptotene cell.
Supplementary Video 3
SINE3–interGFP expression patterns in a zygotene cell.
Supplementary Video 4
SINE3–interGFP expression patterns in a pachytene cell.
Supplementary Video 5
SINE3–interGFP expression patterns in a diplotene cell.
Supplementary Video 6
PSS1 expression patterns in an early leptotene cell.
Supplementary Video 7
PSS1 expression patterns in a late leptotene cell.
Supplementary Video 8
PSS1 expression patterns in a zygotene cell.
Supplementary Video 9
PSS1 expression patterns in a pachytene cell.
Supplementary Video 10
PSS1 expression patterns in a diplotene cell.
Supplementary Video 11
Localization dependency between SINE3, PSS1 and Cter-SUNs in the WT with anti-REC8 and anti-PSS1.
Supplementary Video 12
Localization dependency between SINE3, PSS1 and Cter-SUNs in the WT with anti-REC8 and anti-Cter-SUNs.
Supplementary Video 13
Localization dependency between SINE3, PSS1 and Cter-SUNs in sine3 with anti-REC8 and anti-PSS1.
Supplementary Video 14
Localization dependency between SINE3, PSS1 and Cter-SUNs in sine3 with anti-REC8 and anti-Cter-SUNs.
Supplementary Video 15
Localization dependency between SINE3, PSS1 and Cter-SUNs in pss1-SINE3-interGFP with anti-REC8, anti-GFP and anti-SUNs.
Supplementary Video 16
Localization dependency between SINE3, PSS1 and Cter-SUNs in sun1 sun2-SINE3-interGFP with anti-REC8, anti-GFP and anti-PSS1.
Supplementary Video 17
The PSS1–LINC chain is required for telomere attachment to the NE. SINE3–interGFP with anti-REC8, anti-GFP and anti-Cter-SUNs.
Supplementary Video 18
The PSS1–LINC chain is required for telomere attachment to the NE. SINE3–interGFP with anti-REC8, anti-GFP and anti-PSS1.
Supplementary Video 19
The PSS1–LINC chain is required for telomere attachment to the NE. WT with anti-REC8, anti-ASY1 and anti-ZYP1.
Supplementary Video 20
The PSS1–LINC chain is required for telomere attachment to the NE. sine3 with anti-REC8, anti-ASY1 and anti-ZYP1.
Supplementary Video 21
The PSS1–LINC chain is required for telomere attachment to the NE. pss1 with anti-REC8, anti-ASY1 and anti-ZYP1.
Supplementary Video 22
The PSS1–LINC chain is required for telomere attachment to the NE. sine3 with anti-REC8, anti-ASY1 and anti-ZYP1.
Supplementary Video 23
The PSS1–LINC chain is required for telomere attachment to the NE. pss1 with anti-REC8, anti-ASY1 and anti-ZYP1.
Supplementary Video 24
The PSS1–LINC chain is required for prophase centromere movements. WT_GFP-CENH3_REC8–RFP.
Supplementary Video 25
The PSS1–LINC chain is required for prophase centromere movements. sine3-1_GFP-CENH3_REC8–RFP.
Supplementary Video 26
The PSS1–LINC chain is required for prophase centromere movements. pss1-1_GFP-CENH3_REC8–RFP.
Supplementary Video 27
The PSS1–LINC chain is required for prophase centromere movements. sine4-1_GFP-CENH3_REC8–RFP.
Supplementary Video 28
The PSS1–LINC chain is required for prophase centromere movements. wip123_GFP-CENH3_REC8–RFP.
Source data
Source Data Figs. 1 and 5–8 and Extended Data Figs. 1–3, 8 and 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, B., Tiscareno-Andrade, M., Luo, Y. et al. Identification of the cytoplasmic motor–LINC complex involved in rapid chromosome movements during meiotic prophase in Arabidopsis thaliana. Nat. Plants 11, 1608–1627 (2025). https://doi.org/10.1038/s41477-025-02043-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41477-025-02043-4