Abstract
Flexible biosensors enable real-time, point-of-care health monitoring through wearable systems and must withstand complex mechanical deformation during use. Their performance is governed by coupled material properties, structural design, and fabrication strategies. This review integrates mechanistic insights, bio-interface design, data handling, fabrication methods, and hybrid material strategies, and outlines pathways toward advanced biosensors capable of maintaining structural integrity and high performance in real-world applications.
Similar content being viewed by others
Introduction
The recent advancement of flexible biosensing technologies is driving transformative progress in personalized healthcare, enabling real-time continuous monitoring and acquisition of physiological and biochemical biomarkers. These systems facilitate seamless integration of clinical-grade diagnostics with dynamic, point-of-care monitoring and enhance precision medication frameworks. This progress addresses the growing global prevalence of chronic diseases such as diabetes, cough, and cardiovascular disorders1,2,3,4,5 and the demand for decentralized, patient-centric care, especially accelerated by the COVID-19 pandemic’s emphasis on remote health monitoring6,7. Recent advances have transitioned these devices from bulky, rigid systems to conformable and wearable platforms capable of simultaneous detection of metabolites in sweat, muscle swelling, temperature, electrolytes in interstitial fluid, and even neurotransmitters in organoid cultures8,9,10,11,12,13,14,15,16. Such multimodal sensing aligns with the “lab-on-skin” vision, where real-time health profiling enables early disease detection and tailored therapeutic modulations17.
Mechanics, as a foundational branch of physics, provides the theoretical framework for analyzing system behavior under external stimuli, including mechanical forces, kinematic constraints, and structural deformations. During functioning, flexible and wearable biosensors (active layer/substrate) undergo mechanical deformations, which in turn induce strains and stresses18. By analyzing stress distribution and material responses, mechanics enables the prediction of mechanical reliability and efficacy of the devices. Likewise, these deformations change when the feature size and structural design of the biosensors differ. If these unwanted strains increase beyond a threshold value, the material can experience mechanical failure, i.e., fracture, delamination, or both. Likewise, cyclic mechanical loading during repeated usage can lead to material fatigue, microfractures, or delamination, ultimately degrading sensor performance. In addition to these factors, the choice of fabrication methods also contributes to evolved stresses, i.e., residual stresses, during the process. Moreover, similar conditions may arise during the installation, wireless transmission, and handling of data. Engineers must choose better materials, adopt a suitable cost-effective fabrication method, and optimize structural design under mechanistic guidelines, ensuring long-term functionality and high mechanical resilience. Thus, the widespread adoption of wearable biosensing technologies depends on overcoming persistent challenges: (1) mechanical durability under cyclic strain (e.g., joint movement), (2) bio-interface stability against delamination, (3) wireless signal integrity in dynamic environments, and (4) the trade-off between high efficacy and scalable, cost-effective manufacturing.
Although in the field of biosensors, the recent reviews have explored fabrication, design, and material innovations19,20,21,22,23,24,25, or discussed the specific applications, e.g., sweat sensing, electrochemical-activity sensing, nervous system diseases prediction, living cells detection, and microneedle-based sensing26,27,28,29,30, a complete culmination of how structural designs & mechanics of biosensors, interplay of mechanics at bio-interfaces, associated challenges during wireless signal operations, and the type of fabrication strategies, affect biosensor performance, remains lacking. In the literature, there are a few studies that reviewed the insight of mechanics (computational and experimental-based) and design optimizations for similar applications31,32,33,34. Nevertheless, the studies have not focused on continuous monitoring through particular flexible biosensing technologies and their impact on wireless operation. For instance, Rogers et al.35, reviewed the interplay of mechanics and materials, but the study mainly covered the effect when devices stretch only, i.e., the study did not cover the flexible (bendable) and twistable structures. Also, the authors mainly discussed a broader range of stretchable devices, including optoelectronics, electrodes, and displays. Similarly, another review discussed the analytical mechanics of stretchable electronics enabled by wavy structures and fractal designs, lacking a broader spectrum in this area32. Another great work has been done by Zhu et al., analyzing the detailed mechanics and designs for stretchable and flexible electronics. However, the study did not discuss the biosensors in-depth, and the focus was on hydrogel, i.e., hydrogel biosensing technologies36. Additionally, prior works often overlook critical interdependencies—for instance, how a sensor’s geometry impacts both strain tolerance and antenna performance, or how covalent vs. non-covalent hybrid materials dictate long-term bio-interface stability.
This review provides practical guidance associated with structural designs and underlying mechanics for developing next-generation continuous monitoring flexible biosensors. For instance, epidermal electronics must mimic the mechanical properties of human skin (elastic modulus ~10–500 kPa, stretchability >30%) to ensure conformal contact without signal distortion during its functioning, while preserving high performance and mechanical reliability37. Likewise, design optimization and conformability of the whole packaging allow devices to withstand repetitive motion (e.g., joint movement, muscle contraction), while fracture resistance and fatigue durability ensure long-term functionality. Recent advances evolving around bioinspired designs further enhance conformability. As these biosensors transmit the data after detecting bioactivity, smooth data acquisition and transmission without losing components’ mechanical reliability and operations is also crucial, which has not been discussed before. Here, we systematically establish the interplay between mechanics, structural design, bio-interface engineering, and fabrication strategies to achieve high-performance flexible biosensors, as shown in Fig. 1. This review analyzes fundamental mechanical properties and associated mechanics impacting performance and how researchers have attained state-of-the-art biosensing applications, followed by bioinspired structural designs and their encapsulations. It then outlines the challenges during wireless data communication, a paramount feature essential for continuous monitoring. Finally, it assesses manufacturing methods, including hybrid materials that retain biocompatibility and electronic functionality, and concludes with perspectives on future prospects. By advancing mechanical resilience, bio-interface stability, and scalable fabrication, researchers can unlock the full potential of biosensing technologies for global impact.
Mechanics of biosensors for continuous health monitoring: fundamental principles and structural design considerations
In recent years, electronic biosensors have transitioned from traditional rigid structures to highly flexible and multifunctional systems, reforming their role in state-of-the-art applications such as human-machine interfaces, biomedical platforms, artificial skin, epidermal electronics, personalized healthcare devices, and next-generation functional biosensors. The mechanical properties of biosensors are critical, particularly for wearable, implantable, and ingestible applications, where flexibility, stretchability, and mechanical durability are key features. These attributes allow biosensors to withstand continuous deformation that occurs during their functioning. A well-engineered structural design and associated mechanics ensure seamless adaptation to complex biological surfaces, reducing discomfort and structural failure while enhancing mechanical reliability and long-term functionality. In this section, we discuss the design strategies, key mechanical parameters, and mechanistic foundations shaping high-performance biosensors.
Fundamental mechanistic insights
Mechanical properties are crucial to the design and proper functioning of flexible biosensors. These devices operate in soft, dynamic, and mechanically complex environments, such as skin, joints, muscles, and internal organs, where they are continuously subjected to deformations and different stresses. A deep understanding of the mechanical behavior of biosensors is thus essential for designing systems that retain high sensing performance over time. Key mechanical attributes, including flexibility, stretchability, elasticity, tensile strength, fracture resistance, fatigue durability, and tissue adhesion, must first be carefully understood to meet application-specific demands.
Flexibility is a foundational property for biosensors that must conform to non-flat, irregular, or moving biological surfaces. A highly flexible biosensor should wrap around skin, adapt to muscle curvature, or follow organ motion without delamination or signal degradation38,39. Stretchability refers to a sensor’s ability to elongate under mechanical strain without structural failure. This is especially crucial for biosensors located on highly dynamic body regions, such as joints and muscles, which undergo large strains40. Elasticity, in parallel, ensures that the sensor returns to its original shape after deformation. Materials such as hydrogels, shape-memory polymers, and elastomers like poly(trimethylenecarbonate), poly(glycerolsebacate) (PGS), and poly(diolcitrate) (PDC) are useful to achieve sensor performance over repeated cycles41. Together, stretchability and elasticity prevent cracking, delamination, and signal drift during motion42.
The bending of biosensors induces large mechanical stresses and strains. Mechanistic insight into bending is essential for researchers, which will help them to optimize their devices. Fundamental bending theory helps us to calculate the induced bending strain. For instance, to avert fracture, the maximum induced bending strain should always be less than the intrinsic maximum allowable strain, also called the threshold strain43. If the curvature radius (\(R\)), and thickness of the biosensor (\(t\)) is known, the bending strain (\({\varepsilon }_{b})\) can be calculated as follows:
Flexible substrates such as Ecoflex, polydimethylsiloxane (PDMS), PET, and hydrogels are often employed to achieve low bending stiffness and high mechanical compliance33,44,45.
Similarly, when a structure experiences mechanical deformations owing to bending, the stress (\(\sigma )\) is evolved in the structure, leading to mechanical failure. Using Hook’s law46, and given the intrinsic value of elastic modulus (\(E)\) of the chosen material, we can estimate the failure criteria in terms of \(\sigma\), as given below:
To get the fracture-free structural design of the flexible biosensors, the above Eqs. (1) and (2) can provide the guidelines. In addition to resistance to deformations; tensile strength and fracture resistance are essential to withstand mechanical loading and prevent structural failure47. For example, metallic materials like titanium alloys or SUS316L stainless steel offer high strength (~110 GPa Young’s modulus) but may present stiffness mismatches with biological tissues such as bone (10–30 GPa), necessitating trade-offs between robustness and biocompatibility48,49. Likewise, fatigue resistance plays a pivotal role for skin-mounted electronics, neural interfaces, and e-skins that undergo thousands of bending or stretching cycles. For cyclic bending/stretching under stress-controlled conditions, Basquin’s Law (Stress-Life approach) governs the structure’s response, as given below:
whereas, \({\sigma }_{a},\) \({\sigma }_{f}\), \({N}_{f}\), and \(b,\) are stress amplitude, fatigue strength coefficient (for most metals corresponds to intrinsic fracture strength), number of cycles at failure, and fatigue strength exponent (typically −0.12 to −0.05 for polymers/metals), respectively.
Likewise, for plain soft elastomeric substrates (PDMS, Ecoflex), another governing equation- Coffin–Manson law (Strain-Life approach) can be employed, as given below:
Where, \(C\), \(\alpha\) are empirical constants (i.e., \(\alpha \,\approx 2\) for PDMS).
Low fatigue resistance not only leads to crack initiation, growth, and delamination within biosensors, but also causes signal drift, reducing sensing fidelity and lifespan50. Based on the guidelines provided by the above mechanics, the researchers used the strategies to improve fatigue performance, including the utilization of the nanostructured reinforcements, self-healing polymers, and hybrid material systems that absorb stress and self-repair microdamage51,52. Another important parameter is reversible adhesion between the biosensor and the biological substrate, which is critical for accurate detection and continuous signal acquisition. Inadequate adhesion can result in poor skin contact, motion-induced noise, and device detachment15. Solutions range from bioadhesive hydrogels and microstructured surfaces for skin-mounted devices to biocompatible coatings that encourage tissue integration in implantable devices. Strong but reversible adhesion maintains performance while ensuring user comfort and preventing irritation53,54. In the case of pressure sensing technologies, biosensors are exposed to lateral (shear) and normal (compressive) forces. High shear strength prevents sliding or delamination during lateral movement, while compressive strength enables the sensor to endure physical loading without collapse55,56. These attributes are essential for maintaining mechanical integrity under unpredictable or repeated contact forces, such as joint motions or load-bearing tissues. The development of flexible systems with tailored mechanical properties is critical for enabling next-generation health monitoring biosensors. Figure 2 highlights distinguishing platforms that resolve the mechanical integrity issues by following the associated mechanics. Transparent touch sensor arrays (Fig. 2a) demonstrate exceptional mechanical resilience, maintaining functionality under repeated deformation32. Silicone-Ecoflex microsheets (Fig. 2b) combine stretchability with robust adhesion, enabling conformal attachment57. For neural interfaces, polyimide-based substrates (Fig. 2c) exhibit tissue-like elasticity, facilitating stable integration with the cerebral cortex without inducing inflammatory responses58. Hydrogel platforms further expand these capabilities, offering exceptional toughness for wound monitoring (Fig. 2d)59, while magnetically responsive films (Fig. 2e) enable precise cortical stimulation60. Finally, modified hydrogels (Fig. 2f) achieve reversible, tissue-specific adhesion through supramolecular interactions61. Collectively, these applications exemplify the convergence of mechanics and bioengineering, where tailored mechanical and interfacial properties enable seamless communication between electronic devices and living tissues.
a Schematic illustration of transparent and wearable touch sensor array and its mechanical flexibility32. b Mechanical flexibility, stretchability, compressive strength, and suture-free adhesiveness of silicone microchannels embedded in an Ecoflex microsheet57. c Flexible substrates composed of methyl-2-pyrrolidinone embedded with polyimide for brain–computer interface (BCI) applications, demonstrating elasticity and photographic evidence of device implementation on a rat’s brain58. d Mechanical robustness of P(AM-APBA)XLG/CNTs hydrogels demonstrated through multiple mechanical tests underscoring their suitability for continuous wound monitoring applications59. e Magnetically Driven Film (MDF) synthesized using magnetic nanomaterials for magnetically induced contact stimulation on the cerebral cortex, demonstrating biocompatibility with neuronal cell tissues60. f Cell adhesion and adhesive strength of β-CD-g-(pAAm/pAETAc) hydrogels, including their adhesion performance61.
Role of mechanics and structural design at bio-interface
Wearable bioelectronics are transforming healthcare and personalized medicine by enabling continuous monitoring of physiological and biochemical signals62. A critical aspect of their performance lies at the bio-interface, where flexible biosensors interact with soft and dynamic biological tissues. At this bio-interface, the integration of structural design principles or the interplay of mechanics is essential for ensuring high efficacy, stability, and user comfort63. Figure 3 exhibits a range of structural design considerations and materials employed in the advancements of these biosensors. As mentioned above, the selection of materials and structural design plays a pivotal role in achieving mechanical durability guided by underlying mechanics, Fig. 3a summarizes a set of crucial mechanical properties (e.g., stretchability, modulus matching, and self-healing), which must retain reliability when subject to complex biological environments64,65,66.
a Mechanical properties considered in the design and fabrication of wearable and implantable biosensors for continuous health monitoring65. b Young’s modulus of organs and materials for biosensors. c Modulus tunable adhesive skin medical patches using PDMS71. d Dissolvable silk fibroin films engineered into mesh-like neural electrodes for brain interfaces, demonstrating strong tissue adhesion and biocompatibility77. e Ultrathin, highly flexible, and biodegradable organic electrochemical transistor (OECT) designed for brain–machine interface applications78. f, g Ultrastretchable Kirigami-inspired bioprobe for high-fidelity heart and brain monitoring82. h Serpentine- inspired biosensors for heart and ECG monitoring83.
To meet the complex mechanical and functional demands at the bio-interface, biosensors must operate on non-planar, soft, and continuously moving areas such as skin, joints, and internal organs. A key design consideration is modulus matching, where the biosensor’s Young’s modulus must align with human tissue (Fig. 3b). For instance, soft organs (e.g., brain, ~5 kPa) require substrates like hydrogels, while stiffer tissues (e.g., muscle, ~500 MPa) may tolerate elastomers, plastics, and thin metals37. Mismatched stiffness can cause mechanical irritation, delamination, or wireless signal issues during motion or prolonged use. Thus, achieving conformal contact is essential not only for comfort but also for preserving sensing accuracy and mechanical integration. To address these challenges, a wide range of soft substrates have been developed, offering tailored combinations of the abovementioned characteristics67. Materials such as elastomers, hydrogels, and tissue-mimetic polymers provide low modulus and high compliance, enabling seamless contact with biological surfaces. Two major design strategies have emerged to enhance mechanical adaptability: (1) employing intrinsically flexible and conductive materials, and (2) engineering rigid or semi-rigid materials with structural designs such as serpentine patterns, kirigami cuts, or porous frameworks to introduce stretchability68. Common examples of flexible substrates include polyurethane (PU), polyvinyl alcohol (PVA), polycaprolactone (PCL), polyethylene glycol, and polyethylene terephthalate (PET). These non-toxic materials can be easily shaped into complex, conformable structures, making them suitable for both in vitro and in vivo bioelectronic applications69. For instance, polyimides (e.g., Kapton®) are thermally stable and can be functionalized for use in neural implants due to their excellent dielectric properties and compatibility with metal deposition70. Similarly, PDMS’s tunable modulus (Fig. 3c) enables its use in adhesive skin patches that conform to dynamic surfaces while maintaining robust interfacial adhesion71. In addition to polymers, thin metallic foils (e.g., Au, Cu, Ag, Al, Pt, <200 µm thick) provide excellent conductivity, mechanical robustness, and deformation tolerance, making them ideal for skin-mounted or implantable devices72,73. Semi-metallic films, such as indium tin oxide (ITO) and doped graphene, provide a balance of transparency and conductivity, suitable for transparent biosensors, stretchable displays, and electronic skin74,75. However, their high processing temperatures and lack of biodegradability limit their suitability for eco-friendly or bio-compatible applications.
Another important feature is adhesion, which plays a foundational role in securing implantable sensors within the body and supporting their functionality. Adhesion strategies are broadly categorized into physical and chemical mechanisms. Physical bonding, including hydrogen bonding, hydrophobic interactions, ionic coordination, and interfacial diffusion, offers reversible and dynamic anchoring suitable for the body’s constantly changing internal environment. In contrast, covalent chemical bonds enabled through biocompatible reactions such as Schiff base or Michael addition provide more robust and permanent adhesion, often triggered by external stimuli like light or redox conditions. The mechanically engineered skin-mounted biosensors must maintain adequate adhesion under dynamic conditions. The associated mechanics governs the strength of adhesion at bio-interfaces, providing guidelines for achieving high-performance biosensors. Depending on the boundary conditions, there are various theories that could be utilized to calculate the adhesion strength. For instance, in the case of soft biosensors attached to the skin or placed over the compliant substrate in dry conditions, the Johnson–Kendall–Roberts (JKR) theory is employed, assuming the presence of short-range adhesion or no viscoelasticity exists, which is given as below:
Where, \({F}_{{adh}}\), \(R\), \({W}_{{adh},}\) are pull-off force to detach the biosensor, radius of the contact area, and work of adhesion or sum of relevant surface energies, i.e., \({\gamma }_{1}+\,{\gamma }_{2}+{\gamma }_{12}\), respectively.
Likewise, a most relevant model for e-skins and epidermal electronics could be regarded as the Peel Test model. The analytical relation can be given as below:
Where, \(P\), \(b\), \({W}_{{adh}}\), and \(\theta\) are peel force/width, width of adhesive strip, strain in peeling arm, and peel angle, respectively. The peel angle is generally taken as 90֯ for wearable biosensors.
As mentioned above, the fundamental mechanics depends on the boundary conditions and nature of the application. If the biosensor detects the activity while experiencing shear stress, i.e., governed by interfacial mechanics, the Shear Lag model will map the more accurate adhesion in terms of shear stress or delamination at the interface, as given below:
Where, \(\tau\), \(G\), \(t,h\), and \(E\), are shear stress at the interface, shear modulus of adhesive layer, thickness of adhesive, thickness of substrate, and elastic modulus of the biosensor, respectively.
Alternatively, to get the required adhesion, there are other approaches, such as utilizing novel structural designs, such as microstructured adhesives, suction-inspired motifs, and interlocking surfaces to promote breathability and minimize skin irritation76. For implantable biosensors, mechanical compliance is essential to avoid immune reactions and inflammation. For example, dissolvable silk fibroin mesh electrodes (Fig. 3d) combine transient functionality with tissue-like modulus, enabling minimally invasive neural interfaces77. Similarly, ultrathin biodegradable organic electrochemical transistors (Fig. 3e) achieve long-term biocompatibility in brain-machine interfaces via mechanical and chemical harmony with neural tissues78. On the other side, latest solutions like softening electronics, i.e., transition from rigid to soft states after implantation (via hydration or thermal triggers), offer a promising path to reduce mechanical mismatch and enhance long-term biocompatibility79. Overall, the ideal adhesive at the bio-interface must be bio-compatible and non-immunogenic, align with the mechanical properties of the tissue, establish strong adhesion over a specified timeframe, and, in some cases, provide reversible bonding capabilities80.
From the perspective of structural designs, researchers have adopted novel approaches, such as serpentine traces, cellular meshes, and wrinkled or kirigami films that effectively distribute strain and minimize stress concentration, reducing mechanical failure during motion81. Kirigami-inspired ultrastretchable bioprobes (Fig. 3f, g) exemplify this approach, maintaining high-fidelity electrophysiological signals even under extreme deformation (e.g., cardiac or brain monitoring)82. Similarly, serpentine-interconnects (Fig. 3h) mitigate delamination in electrocardiogram (ECG) sensors by localizing strain away from active components83. Likewise, techniques like metallic buckling, where metal films wrinkle on pre-strained elastomers, allow stretchability without compromising conductivity, as demonstrated with Gallium arsenide (GaAs) nanoribbons on PDMS53,84. Serpentine configurations, often made with materials like Au or Pt, offer conformal contact with soft tissues, enabling stable real-time monitoring of physiological signals81. Kirigami-inspired structures further enhance deformation tolerance, as shown in PI-based supercapacitors that retain electrochemical performance under strains exceeding 282.5%68,85. This highlights the potential of structural designs to enhance the mechanical flexibility of bioelectronics while retaining functionality, making them ideal for use in wearable sensors and other bio-integrated devices. At the nanoscale, strategies such as nano-patterning, fractal layouts, and the use of Ag or Au nanomaterials provide additional flexibility without sacrificing electrical performance86,87. These advancements in structural design not only support mechanical compliance and durability but also pave the way for integrating biorecognition elements, enabling the creation of high-performance biosensors.
Bioinspired innovations: transforming mechanical architectures
The bio-inspired approach has become a driving force in designing novel materials and structures that support sustainable innovation and multifunctional integration. Figure 4 highlights representative bioinspired strategies for physiological signal monitoring in wearable bioelectronics. Examples such as gecko feet, tree frog toes, spider silk, butterfly wings, shark skin, cuttlebone, cephalopods, lotus leaves, and pitcher plants have provided blueprints for next-generation devices. Stable adhesion and comfort are critical challenges in continuous health monitoring. Inspired by the hierarchical adhesive setae and hooked claws of geckos, Yuan et al.88 developed a bioelectrode that adheres strongly to both dry and moist skin, maintaining high-fidelity ECG signals during motion. Figure 4a shows an example where a novel structure inspired by the starfish was used to monitor ECG along with other outputs1. Similarly, shark skin-inspired textures (Fig. 4b)89 can mitigate biofouling in implantable sensors, leveraging microgrooves to repel contaminants. In another study, drawing inspiration from the refractive index–responsive architecture of butterfly wings, Narasimhan et al.90 designed a micro-optical implant for intraocular pressure (IOP) sensing. Inspired by the hierarchical, mucus-secreting structures of tree frog toes (Fig. 4c)91, recent bioelectrodes achieve strong adhesion to moist skin. Integration of these nanostructures significantly expanded the implant’s detection range, reduced the mean in vivo IOP error threefold, and suppressed biofouling and inflammation by 12-fold, making it a highly practical platform for long-term glaucoma management. Furthermore, Choi et al20. developed a cephalopod-inspired medical skin (Fig. 4d) featuring miniaturized suction cups (mSCs) that provided glue-free adhesion through strong van der Waals forces and negative pressure. The mSCs, exhibiting an ultralow system modulus (~108 kPa), ensured skin contact and improved signal sensitivity for continuous monitoring of vital signs and physical activity without irritation or slippage. Extending this bioinspired approach, electronic tongue (e-tongue) systems developed by Pettinelli et al.92 mimic the human gustatory system to enable selective biochemical sensing. Likewise, bioinspired e-tongues (Fig. 4e) mimic gustatory systems, using soft polymeric membranes and enzymes to discriminate analytes like phenols and sugars93. Their high-performance e-tongue, based on carboxylated polyvinyl chloride membranes embedded with specific enzymes and electron mediators, exhibited enhanced sensitivity and selectivity. Similarly, bioinspired electronic nose (e-nose) and electronic eye (e-eye) systems replicate olfactory and visual perception, enabling selective detection and optical signal processing for real-time biosensing94. In another work, e-eye systems (Fig. 4f) mimic optical perception, such as glucose-sensing contact lenses with nanostructured transparency for real-time diagnostics95. Looking ahead, bio-inspired electronic sensors are poised to transform healthcare through intelligent, real-time, and personalized diagnostics.
a Star fish1. b Shark skin89. c Tree frog inspired adhesive electrode91. d Cephalopod-Inspired miniaturized suction cups for wearable bioelectronic systems as smart medical skin for physiological signal monitoring20. e Electronic tongues taste sensing systems93. f Electronic eye inspired contact lens for glucose monitoring95.
Encapsulation strategies for long-term mechanical and interfacial stability
The preceding sections have established the critical importance of modulus matching, adhesion mechanics, and bioinspired structural designs for ensuring the conformability and initial functionality of flexible biosensors. However, long-term operational stability in the dynamic, hydrated, and protein-rich environment of the human body requires robust encapsulation. Encapsulation layers serve not merely as impermeable barriers to moisture and ions but as integral mechanical stabilizers that mitigate cyclic strain, suppress crack propagation in active layers and interconnects, and preserve interfacial integrity under prolonged physiological deformation96,97. PDMS and Ecoflex are the most commonly known encapsulation materials for flexible biosensors due to their compliant nature and low elastic moduli (PDMS: ~0.36–3 MPa, Ecoflex: ~20–69 kPa). This mechanical compatibility prevents stress relaxation and fracture in the active biosensing layer, ensuring consistent function in wearable applications. However, these encapsulations are not completely effective against biofouling, i.e., their hydrophobic surfaces can promote protein and microbial adhesion. Therefore, they are often modified using common antifouling strategies such as surface grafting, plasma treatment, and antimicrobial coatings. These modifications make biosensors suitable for continuous monitoring in biological environments while retaining their mechanical integrity.
We will discuss here the latest encapsulation strategies that utilize the hybrid architectures for enhanced functionality. Figure 5 exhibits some interesting applications of such strategies. Parylene–PDMS multilayers have demonstrated remarkable efficacy. Parylene (e.g., Parylene C) offers excellent conformality and barrier properties but can be relatively stiff and prone to pinhole formation under strain. Conversely, PDMS is highly compliant and stretchable but is permeable to water vapor. By combining them in a multilayer stack, for instance, a thin Parylene layer sandwiched between two PDMS layers, the system leverages Parylene’s barrier quality while using PDMS as a strain-relaxing intermediary that prevents crack propagation into the critical barrier film98,99 (Fig. 5a). This hybrid architecture significantly enhances the biosensor’s performance under cyclic mechanical loading due to the abovementioned characteristics. Another architecture includes the fluorinated elastomers, such as perfluoropolyethers (PFPE) and Viton, which offer excellent hybrid interface-based encapsulation. These settings inherently possess better chemical inertness, low surface energy, and high resistance to biofouling. Their strong hydrophobic nature provides an exceptional barrier against moisture. Furthermore, certain fluorinated elastomers can be engineered to exhibit high elasticity and toughness, making them suitable for encapsulating stretchable bioelectronics subjected to cyclic bending100. The synthesis of fluorinated elastomers’ architecture and its application showing the benefits has been described in Fig. 5b. The non-sticky surface minimizes the protein adsorption, thereby preserving the sensor’s bio-interface and signal fidelity over time.
a The cross-section of a typical parylene-PDMS encapsulated sensor via SEM (left) and representative electrical signal stability under cyclic mechanical loading enabled by strain-isolated barrier layers (right)99. b Schematic preparation steps of a fluorinated elastomer network (left), with representative sensor output signals demonstrating mechanical robustness and environmental stability (right)100. c Schematic of mechanically interlocked hydrogel–elastomer hybrid structure, alongside on-skin application images demonstrating conformal contact and deformation-tolerant operation (right)169. d Preparation of zwitterionic hydrogel coatings through sequential initiator diffusion and UV-triggered polymerization (top) combined with a conceptual illustration of protein-resistant, hydrated interfaces enabling stable long-term signal acquisition at the interface (bottom)102.
For ultrastretchable biosensors requiring uniform integration with wet biological tissues, hydrogel–elastomer hybrids have emerged. The mechanical mismatch between a typical elastomer (e.g., PDMS) and hydrated biological tissue can lead to interfacial slippage and delamination. By designing a hybrid engineered interface where a hydrogel is covalently bonded to a silicone elastomer, referred as mechanically interlocked hydrogel–elastomer, researchers have developed encapsulations that provide a gradual modulus transition101 (Fig. 5c). The hydrogel surface offers tissue-like mechanical properties, promoting stable adhesion and reducing inflammatory responses, while the elastomer layer ensures robust encapsulation and structural support (shown in Fig. 5c). This approach is particularly beneficial for implantable sensors, where chronic foreign body reactions are a major concern. Finally, molecular-level surface engineering through zwitterionic polymer coatings (e.g., poly(sulfobetaine methacrylate) or poly(carboxybetaine)) offers another excellent way to enhance interfacial stability by preventing biofouling. These coatings form a dense, electrostatically induced hydration layer via strong ionic solvation, which effectively resists the adsorption of proteins, cells, and bacteria102 (Fig. 5d). By preventing the formation of a biological fouling layer, zwitterionic coatings maintain consistent interfacial contact and transport properties, which are crucial for the long-term accuracy of electrochemical and affinity-based biosensors. When grafted onto the surface of a mechanically robust encapsulation layer like PDMS or a hydrogel-elastomer hybrid, they provide a dual benefit of biofouling resistance and mechanical stability. In summary, moving from single-material barriers to multi-material hybrid systems, such as Parylene-PDMS multilayers for strain-isolated barrier performance, fluorinated elastomers for inertness and moisture resistance, hydrogel-elastomer hybrids for tissue-integrated interfaces, and zwitterionic coatings for effective biofouling are essential for achieving the long-term mechanical resilience and interfacial stability needed for real-world continuous monitoring flexible biosensors.
Performance limitations in wireless flexible biosensor operations
The incorporation of wireless technologies has fundamentally transformed conventional biosensors by eliminating complicated wire connections and reducing dependence on onboard batteries, thereby enabling seamless integration into wearable and implantable platforms for continuous health monitoring. Wireless communication strategies in biosensors are broadly classified into near-field and far-field regions, depending on the operating distance between the transmitter and receiver relative to the electromagnetic (EM) wavelength, a distinction that critically determines power transfer efficiency, communication range, and signal attenuation in biological tissues103.
Near-field wireless communication relies on non-radiative EM coupling mechanisms, primarily inductive and capacitive coupling, and is typically employed over short distances (<10 cm) with high energy efficiency. Among these, near-field communication (NFC) and radio-frequency identification (RFID) are widely adopted in wearable and implantable biosensors due to their low power consumption, short operational range, and compatibility with passive, battery-free operation. RFID enables wireless data transmission and unique identification through EM coupling between a reader and a sensor-integrated antenna and can be readily interfaced with biochemical or physical sensing elements for applications including physiological monitoring, environmental sensing, and smart healthcare. RFID systems operate across multiple frequency bands, spanning low frequency (LF, 30–300 kHz), high frequency (HF, 3–30 MHz), and ultra-high frequency (UHF, 300 MHz–3 GHz), with communication distances ranging from centimetre-scale near-field coupling at lower frequencies to metre-scale far-field transmission at UHF, albeit with increased tissue absorption at higher frequencies. NFC represents a standardized subset of HF RFID operating at 13.56 MHz and is specifically optimized for short-range, secure, and bidirectional communication. Unlike conventional reader-centric RFID systems, NFC supports peer-to-peer data exchange and incorporates built-in encryption protocols, enhancing data security and privacy in wearable biosensing applications. These attributes combined with favorable magnetic-field penetration through soft tissues, minimal electromagnetic interference, and direct compatibility with consumer electronics make NFC particularly attractive for flexible biosensors requiring battery-free operation, rapid contactless data transfer, and reliable physiological signal acquisition104,105,106. Despite substantial innovations in functional materials and fabrication processes, NFC-based biosensors remain constrained by their limited working distance and discontinuous data transmission, which hinder reliable long-term and continuous health monitoring. Although the magnetic fields used in NFC are strongly confined and rapidly decay with distance, their localized energy absorption is considered advantageous for biomedical applications, as it enables low-power operation with minimal tissue heating and reduced exposure to surrounding biological structures, consistent with established safety guidelines107.
In contrast, radiative wireless communication strategies rely on propagating electromagnetic waves and underpin active transmission technologies such as Bluetooth Low Energy (BLE), ultra-wideband (UWB), and Wi-Fi. While BLE, UWB, and Wi-Fi fundamentally operate via radiative EM wave propagation, their practical implementation in wearable and on-body biosensors often occurs within the radiating near-field or Fresnel region rather than the strict electromagnetic far-field, owing to small antenna dimensions and short communication distances. BLE operates in the industrial, scientific, and medical band at 2.4 GHz and is specifically optimized for low-power, intermittent data transmission, enabling meter-scale wireless communication with consumer electronics such as smartphones, tablets, and smartwatches. Owing to its ease of integration, low energy consumption, and standardized protocol stack, BLE is widely used in daily-life applications including fitness tracking, healthcare monitoring, smart homes, and Internet-of-Things systems. However, the requirement for onboard batteries, rigid printed circuit boards, and discrete RF components often increases system bulk and mechanical mismatch with soft tissues, while limited transmission power and imperfect skin–device compatibility remain obstacles for long-term wearable biosensing.
UWB communication employs short-duration pulses distributed over a broad frequency spectrum, typically spanning 3.1–10.6 GHz, offering high temporal resolution, precise localization accuracy, and robustness against multipath interference. These features make UWB attractive for applications such as high-resolution motion tracking and spatial positioning; however, its shallow tissue penetration and relatively low energy efficiency limit its applicability in continuous wearable or implantable biosensing108,109. Wi-Fi-based wireless biosensing, operating primarily in the 2.4 and 5 GHz bands, supports high data throughput and long communication distances and has recently attracted attention for contactless physiological monitoring. Advanced signal modeling and machine learning algorithms enable Wi-Fi sensing for applications such as human localization, activity recognition, gesture detection, and respiration and heart-rate monitoring. Nevertheless, Wi-Fi incurs substantial power consumption and pronounced signal attenuation in soft tissues when applied to wearable or implantable sensors, restricting its practical use primarily to non-invasive or ambient sensing scenarios. Compared with Wi-Fi, Zigbee is a relatively low-power and low-cost wireless technology suitable for large-scale sensor networks; however, its slow transmission speed and increased performance loss at reduced power levels limit its suitability for high-bandwidth or real-time biosensing applications110. LoRa (long-range radio) employs spread-spectrum modulation techniques that provide robust, ultra-low-power wireless communication over long distances, typically ranging from 5 to 15 km. This capability makes LoRa particularly attractive for remote health monitoring and distributed biosensing systems, where sensor data can be transmitted to cloud servers and accessed by remote healthcare facilities for real-time diagnosis and monitoring. Despite these advantages, LoRa suffers from inherently low data rates compared with other wireless technologies, and its performance can be constrained by complex network deployment requirements and susceptibility to interference in densely populated communication environments111. Table 1 summarizes the comparative overview of different wireless modalities used in flexible wearable and implantable biosensors.
Taken together, the wide range of wireless communication strategies highlights that improvements at the materials and device level alone are not sufficient to guarantee reliable long-term biosensing. Each wireless sensing system introduces its own constraints on data continuity, power consumption, and system integration, which ultimately affect how physiological signals are collected, transmitted, and processed in real-world settings. As a result, the performance of wireless biosensors must be evaluated not only in terms of communication efficiency, but also from a system-level perspective that considers data handling, mechanical reliability, and long-term operation.
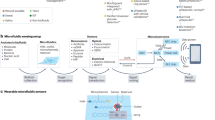
For the continuous functioning of health monitoring biosensors, data collection and handling operations are very crucial. The dataset is gathered and sent to the designated system for further analysis or storage. The widely used wired medical systems are functionally mature; however, they often suffer from mechanical incompatibility with soft tissues, limiting structural adaptability and long-term reliability. For instance, pacemakers frequently face complications due to lead breakage, infection, thrombosis, and MRI-induced interference. In contrast, wireless systems mitigate these issues by enabling minimally invasive continuous monitoring with reduced risk and enhanced data fidelity. Moreover, wireless data transmission facilitates integration with consumer electronics, improving patient comfort and reducing disruption to daily life. Figure 6 depicts a range of existing key innovations and challenges in wireless biosensor operations. Figure 6a illustrates their basic architecture, highlighting the interplay of sensing, power, and communication modules112. However, despite their advantages, they pose a new set of challenges, particularly in balancing mechanical adaptability with electronic reliability, which are discussed below.
a Schematic illustration of the basic architecture of biosensor networks112. b Limited bandwidth in wireless biomedical systems and mitigation via Event-Triggered Learning115, c NFC-based system for efficient power management and secure data transmission106. d, e Self-powered wireless bioelectronics systems integrating BFC and TENG for power management and data transmission106,107,116. f, g Antenna design for efficient wireless signal processing106,122. h Wireless power transfer (WPT) methods for battery-free alternatives107.
Mechanical-electronic co-design challenges
From the perspective of mechanical challenges, every time the biosensor and/or other components flex, they face unique mechanical-electronic trade-offs, where flexibility and deformability often conflict with signal integrity and energy efficiency. Stretchable antennas, for instance, must maintain stable resonant frequencies under strain or when subjected to mechanical deformations, even as the body moves, which can degrade transmitted data, increase power loss, and distort radiation patterns. Likewise, mechanical deformations (axial stretching, torsional twisting, or biaxial strain) induce not only substrate-level stresses but also electron transport perturbations, manifesting as phase noise, harmonic distortion, and intermodulation effects that degrade wireless signal fidelity in flexible RF components. Although materials with high dielectric stability (e.g., liquid metal composites) and optimized structural designs (e.g., serpentine or fractal designs) are emerging to address these challenges, issues like power loss during deformation, miniaturization limits, and tissue-interference-induced signal drift are still present. Additionally, these components experience oxidation, unpredictable permittivity of hydrated skin, interfacial cracks, and the slow creep of material fatigue. To address energy needs, self-powered systems or wireless power transfer (WPT) devices are needed, which also face challenges under mechanical deformation (misalignment between transmitter and receiver components). Overcoming these hurdles requires co-designing materials, structural designs, mechanics, and electronic properties to ensure robust wireless signal processing or operation in dynamic biological environments. In this context, Table 2 provides a comparative overview of representative wireless flexible biosensors, highlighting how mechanical compliance, electrical response, and wireless readout are intrinsically coupled at the device level. The summary illustrates that designs enabling large deformation and conformal tissue integration frequently require compromises in wireless range, signal continuity, or power efficiency, whereas architectures supporting robust wireless communication often introduce increased stiffness, higher energy demand, or reduced mechanical tolerance. These observations emphasize that mechanical and electronic performance cannot be treated independently in flexible biosensors and instead must be jointly optimized to maintain reliable wireless operation under continuous and complex physiological deformation.
Beyond mechanics: wireless data and energy challenges
Beyond physical constraints, wireless biosensors experience non-mechanical barriers, particularly in data management, transmission, and security. Among these challenges, the first issue is data scalability and storage, which remains critical. Wireless signal processing systems generate vast amounts of high-frequency, heterogeneous data due to continuous signal transmission and reception across dynamic environments. This influx of time-sensitive data strains traditional storage and real-time analysis frameworks. Machine learning (ML) offers solutions by enabling noise filtering113,114. The second constraint is related to bandwidth, i.e., when multiple signals from different body segments are tracked simultaneously, bandwidth limitations impose significant constraints. Researchers use different techniques to tackle the situation. Figure 6b demonstrates how Event-Triggered Learning dynamically optimizes bandwidth usage115. Real-time feedback systems further demand immediate data processing and response to physiological changes. Integrating Cognitive Radio (CR) technology enables sensors to access underutilized frequency bands, thereby enhancing spectrum efficiency, minimizing interference, and ensuring reliable, high-quality communication106.
The third issue belongs to power usage during the transmission of the data. The biosensor signals are transmitted via interfaces such as Bluetooth, RFID, and NFC, shown in Fig. 6c106, UWB, or Wi-Fi. These components require continuous power, making efficient on-board energy management critical, especially for real-time and long-term health monitoring applications. Recent research has focused on self-powered systems such as biofuel cells (BFCs) and energy harvesting technologies like Triboelectric Nanogenerator (TENG), solar, piezoelectric, and thermoelectric modules that convert biomechanical or environmental stimuli into usable energy (Fig. 6d,e)107,116. However, they face challenges in long-term operational stability and biofouling117,118,119. In contrast, WPT methods (Fig. 6h), such as inductive, capacitive, or radiofrequency coupling, have gained attention as battery-free alternatives107. Still, misalignment between transmitter and receiver components can lead to substantial power losses and reliability issues120. To address these issues, recent innovations have introduced multi-coil geometries, self-aligning magnetic interfaces, and distributed micro-storage elements such as flexible micro-supercapacitors, enabling more stable and efficient power delivery121.
The fourth issue is related to the path loss, which stems from transmission distance, medium properties, antenna design, and environmental interference. Additional impairments such as scattering, reflection, and multipath fading further degrade signal quality. Advanced antenna design (Fig. 6f, g)106,122 and novel materials can help to reduce the path loss and enhance data integrity123,124. Last but not least, biosensor systems transmit sensitive health data over wireless channels prone to jamming, spoofing, and eavesdropping. To ensure data confidentiality and system reliability, strong encryption, authentication, and secure protocol design are essential. Recent strategies employ lightweight machine learning algorithms for real-time intrusion detection, although protecting training data and maintaining computational efficiency remain ongoing challenges. Ensuring secure and reliable signal processing is vital for the safe integration of biosensors in clinical and personal health monitoring systems125,126. The future of high-performance wireless biosensors depends on co-optimizing mechanics and electronics. Innovations like self-aligning WPT coils, distributed micro-supercapacitors, and adaptive ML-driven signal processing are steps in the right direction. Yet, as these systems evolve, engineers must continually balance flexibility with functionality, ensuring that wireless biosensors not only adapt to the human body but also deliver uncompromising performance in real-world settings. In the following section, we will present the importance of fabrication methods used to manufacture these biosensors, which dictate the cost and efficacy of the flexible biosensors.
Fabrication methods and hybrid materials for flexible biosensors
The fabrication of next-generation flexible biosensors with the capability of continuous monitoring demands an integrated approach combining manufacturing innovation with material science breakthroughs. The development of flexible biosensors is closely linked to the growth and progress of various state-of-the-art printing techniques, which include inkjet printing, screen printing, aerosol jet printing, roll-to-roll printing, flexographic printing, and electrohydrodynamic printing. We will cover inkjet printing and screen printing due to their high throughput, low setup and operational costs, high to moderate resolution, and a vast range of printed materials for flexible biosensing technologies. In this context, we methodically assesses four fundamental technological frameworks (1) printing methods (focusing on screen and inkjet printing) enabling scalable production of wearable sensors; (2) high-resolution microfabrication techniques; (3) beyond conventional approaches i.e., 3D printing’s transformative potential; and (4) hybrid organic-inorganic materials that synergize soft organic components facilitating bioelectronic integration while maintaining tissue compatibility.
Printed bioelectronics
Inkjet printing is a digital printing technique characterized by the deposition of liquid ink droplets through a micrometric nozzle onto a surface. This method does not require direct contact with the substrate and allows for customization and on-demand printing127. For instance, Baek et al. focused on the development of soft sensors based on organic thin-film transistors, which were capable of mimicking the flexibility and elastic modulus of human skin for spatiotemporal measurement of arterial signals (Fig. 7a)128. The printing process is based on the layout-to-bitmap (L2B) conversion, which optimizes the final circuit quality, ensuring the creation of a grid of sensitive pixels that enhances the device’s sensitivity and supports low power operation129. Further exploring the use of inkjet printing in the fabrication of biosensors, both Bihar et al. and Lo et al. investigated PEDOT: PSS as the base material for forming electrodes; the former developed an all polymer disposable biosensor for glucose detection in human saliva, while the latter formulated a PEDOT: PSS/PEO composite ink to develop a soft sensory patch for real-time ECG and photoplethysmogram (PPG) recording (Fig. 7b,c). Bihar et al.'s130 device is printed on a paper substrate. Although it has a fast response time (≈60 s) and 1 month of shelf life, it is suited for intermittent rather than real-time monitoring. On the other hand, Lo et al.131 achieved thin films with low surface resistivity (84 Ω/□), capable of withstanding up to 50% stretching for thousands of cycles. While the films exhibit excellent mechanical robustness, their electrical performance still reflects the intrinsic conductivity limits of the PEDOT: PSS-based system. Nevertheless, this material is also used in screen printing, as in the case of the flexible biosensor for heart rate monitoring based on pressure detection (Fig. 7d)132.
a Schematic of pulse wave measurement over the radial artery and 3D schematic of the wearable active-matrix pressure sensor arrays. (ip)128. b Exploded schematic of the working electrode showing all separately printed layers: PEDOT:PSS electrode, dielectric layer, enzyme/mediator coating (GOx and ferrocene), and Nafion encapsulation (ip)130. c Schematic and photograph of a PDMS patch integrating stretchable ECG and PPG sensors for simultaneous physiological signal monitoring. (ip)131. d Schematic of the fabrication process of fully printed vital sensors on PEN substrate, including planarization, PEDOT:PSS electrode printing, and P(VDF-TrFE) layer patterning.(sp)132. e MoS₂-based sensing pads with laser-patterned Au electrodes on PI substrate, shown in schematic and applied on forearms under flat and bent conditions for ECG acquisition. (sp)134. f Photographs of the implanted PPG/GOx/PU CGM system alongside the FreeStyle Libre CGM reference on a rabbit back. (sp)4. g On-body test of the wearable sweat sensor during stationary cycling (sp)135.
On the other hand, screen printing deposits ink through a mesh screen onto a substrate using a squeegee. This contact-based method enables high-throughput patterning of conductive inks and allows for the formation of a predefined pattern133. Li et al. leveraged this technique for the fabrication of wearable biosensors based on MoS₂, designed for real-time monitoring of parameters such as electromyogram (EMG) (Fig. 6e)134. The process involves integrated printing, i.e., inkjet printing of a precursor ink onto a UV/O₃-treated PI substrate to improve adhesion, followed by annealing to form MoS₂. The electrodes, then printed with silver paste using screen printing, are thermally fixed, while gold electrodes ensure resistance to high temperatures. The devices showed a sensitivity of −0.98 ± 0.03%°C⁻¹ and a rapid response time of about 1.4 s. To complete this analysis, two sensors capable of detecting specific parameters are examined, i.e., glucose in interstitial fluid and sodium in human sweat (Fig. 7f, g)4,135. The first one is a flexible biosensor consisting of three electrodes printed on a flexible PET film, with an outer polyurethane membrane to improve the sensor’s response. The sensor showed a sensitivity of 12.69 μA mM⁻¹⋅cm² and long-term stability of 30 days when stored in PBS solution at 4 °C. The second sensor, on the other hand, is an extensible electrochemical type, based on conductive graphene-based inks. The fabrication of the sodium sensor is carried out using screen printing with a stainless-steel mask to define the geometry of the electrodes; the TPU/EGF ink is deposited onto substrates such as thermoplastic polyurethane (TPU), PET, and stretchable fabrics. To ensure the stable functioning of the sensor, a reference electrode in TPU with electroconductive graphene flakes (TPU/EGF) is printed. The final sensor showed a conductivity of 433 S m⁻¹, a high stretching capability (51.5%), and excellent electrochemical stability even under high deformation levels (300%) for over 10,000 test cycles. Although printing techniques have brought significant improvements to the fabrication of flexible biosensors that detect the signals continuously, there are still challenges that need to be resolved, particularly in terms of resolution (for nano sized features) and functional complexity (as shown in Table 3). To address this, microfabrication offers an effective method, providing high precision and the possibility of integration at the micrometer scale.
Microfabrication
The development of flexible biosensors for personalized medicine and real-time monitoring of biological parameters can occur through device miniaturization. The main fabrication techniques discussed below include etching, deposition, and photolithography, as well as integration with electronics to create increasingly sophisticated devices. These biosensors are often made using polymers such as PDMS, owing to their favorable mechanical properties and biocompatibility136,137. One of the methodologies used for the precise detection of biomarkers is represented by microneedles, when placed on a miniature patch, making biosensors for real-time monitoring138. Table 4 provides an analytical overview of the selected studies, highlighting the materials used, targeted applications, respective benefits and drawbacks associated with each approach. An example based on this solution is the biosensor developed by Goud et al., designed for continuous and minimally invasive monitoring of Levodopa, the most effective drug for the treatment of Parkinson’s disease, to optimize its dosage in patients (Fig. 8a)139. The biosensor consists of an array of three hollow microneedles filled with carbon paste: one electrode is enzyme-modified (with tyrosinase) and the other unmodified, while the third serves as a reference140. This device has demonstrated a non-enzymatic sensitivity of 0.037 μA/μM and an enzymatic sensitivity of 0.048 nA/μM, based on dual detection mechanisms. However, since the method has only been tested on a biomimetic gel and not yet in vivo, the long-term stability of the electrode and the fragility of the microneedles are uncertain.
a Schematic of the microneedle sensor for L-Dopa monitoring in interstitial fluid (ISF)139. b The structure of the Nafion/GOx/AuNPs/OPPy/AuMNs glucose sensor, layer by layer141. c Schematic of microneedle surface coating and mechanism of glucose detection using GOx and PB142. d Top view of the integrated stent and sensor showing the low-profile cross-section143. e Schematic of drug evaluation using PD midbrain organoids interfaced with a mesoporous Au nanodot-patterned 3D concave electrode145. f Exploded view of the miniaturized i-WPS (right) and images of its implantation in a mouse body in dorsal and ventral positions (left)149.
Microneedle-based devices are known to be used for glucose monitoring, as validated by both Zhang et al. and Kim et al. (Fig. 8b, c)141,142. While the former obtains the biosensor through hot pressing, annealing, and sputtering, the latter uses ICP-RIE (Inductively Coupled Plasma - Reactive Ion Etching) along with electrochemical coating with PEDOT: PSS to improve conductivity. The device established by Zhang et al. has only been tested in vitro, which may account for its lower limit of detection; in contrast, the latter was validated in vivo in mice, although it involves a SU-8/SWCNT composite that may reduce the mechanical robustness of the microneedles. Likewise, Rigo et al. developed an implantable bioelectronic device for wireless, minimally invasive monitoring of in-stent restenosis (Fig. 8d)143. Their research led to the fabrication of a miniaturized capacitive strain-based biosensor, made via a high-resolution microneedle-based printing method, capable of being integrated directly on the inner face of a vascular stent. The used fabrication technique enables the creation of thin, high-resolution layers of conductive and dielectric materials. The system operates as an Inductor-Capacitor resonant circuit (LC resonant circuit), allowing for wireless detection of arterial strain variations. The biosensor showed a maximum capacitance variation of 26% under 5% strain and exhibited wireless sensitivity three times higher than its counterparts.
Microfabrication plays a key role in the study of diseases and drug effects, as well as in real-time monitoring of biological status, enabling the design of biosensors capable of monitoring organoids144. For instance, An et al. conducted a study on brain organoids wherein the development of mesoporous gold nanodot-patterned 3D concave electrodes for measuring dopamine in neurodegenerative diseases (Fig. 8e)145. The device consists of a single electrode obtained through laser interference lithography to generate a gold nanodot structure and is characterized by high signal selectivity. Also, in the fabrication of electrochemical electrodes for monitoring neurotransmitters such as dopamine, Chang et al.146 developed an innovative microfabrication process by combining lithography, micropillar structuring, and carbon nanotube (CNT) infiltration. The problem of CNT coating detachment is solved by this configuration, increasing stability and sensitivity (0.453 nA/nM). Despite promising results obtained on both rigid and flexible substrates, its in vivo applicability remains uncertain due to the limited biocompatibility of SU-8 and the limited scalability of the fabrication process.
Another demonstration of microfabrication is the manufacturing of devices monitoring cardiovascular events, developed by Wang et al.147,148. The first one, composed of PDMS and Ecoflex, enables continuous monitoring of deeply embedded vessels such as carotid and jugular arteries through a stretchable circuit design, transfer printing, and soft elastomer encapsulation. In a separate work, another Wang et al. group developed a Parylene-based device for pressure monitoring, based on an electrolytic bubble generated and maintained in a microfluidic channel with platinum electrodes.
Photolithography, an essential part of microfabrication, was employed to test and improve protective coatings of implantable biosensors. For instance, Mariello et al. developed a wireless, battery-free sensor to monitor the permeability of protective capsules in biosensors. The goal was to detect the infiltration of water and ions, which could damage the internal electronics (Fig. 8f)149. The sensor showed an excellent sensitivity (3.3 × 10⁻⁸ g/m²/day at 25 °C), along with robust performance. In another work, Dion et al. introduced a novel wearable device based on MEMS integrating sensing and neuromorphic computing (processing within the sensor) for real-time monitoring and processing of human movements without the need for external data transmission150.
Beyond conventional fabrication methods
While the previous section addresses scalable printing methods, the potential utilization of 3D printing in flexible biosensors demands a focused analysis. The merits of 3D printing include a variety of biosensing solutions, i.e., hydrogels, composites, polymers, metals, controlled and precise structural designs, along with multilayer capability, embedded electronics, creation of controlled microstructures, miniaturized devices, and customized solutions. Among the most commonly used processes of 3D printing are extrusion-based processes such as Fused Deposition Modeling and Direct Ink Writing (DIW), laser-based processes such as stereolithography, and material deposition-based processes such as material jetting151,152,153 (a comparison is shown in Table 5). Regarding the printing of flexible biosensors, one of the main types of biosensors is the pressure sensor. For instance, a similar biosensor was developed, enabling the recording of arterial pulse, monitoring of respiratory rate, and detection of vocal cord vibrations (Fig. 9a)154. These biosensors were made through extrusion-based 3D printing with PDMS and CNT-based inks, allowing the direct printing of conductive layers and a microstructured dielectric layer. The device showed high durability (1600 compression cycles) and high-pressure sensitivity (0.512 kPa−1). In another work, Yi et al. developed a device that was not only flexible, wearable, and 3D-printed, but also self-powered, using a TENG made of MXene/SEBS, utilizing human motions (Fig. 9b)155. This printed sensor continuously monitored the pulsation of the radial artery with a sensitivity of 6.03 kPa⁻¹ and a response time of 80 ms. Although it demonstrated excellent flexibility and biocompatibility, its dependence on human motion for power generation may restrict continuous monitoring.
a Scheme of the M2A3DNC pressure sensor for multiple physiological signals monitoring154. b Optical image of the wearable MSP2S3. Inset shows LED ‘on’ and ‘off’ states corresponding to the pulse signal peak and valley detected by the device in mode one155. c Example of a fabricated Bioimpedance (Bio-Z) ring sensor custom-fitted to the user’s finger size, ensuring electrode-skin contact157. d Schematic illustration demonstrating respiratory monitoring159. e Attachment of sensing electrodes to the skin near the upper arm and heart. The signals were transmitted via Bluetooth through a laptop interface160.
In another representative work, Mandal et al. developed piezoelectric sensors using nanocomposites of polyvinylidene fluoride (PVDF) and barium titanate (BaTiO₃) fabricated via DIW 3D printing156. The sensor showed high sensitivity (0.024 V/kPa) and excellent linearity (R² = 0.99), indicating high precision in detecting blood pressure variations. Among the different pressure sensor configurations, a notable example is the ring-shaped 3D-printed sensor based on bioimpedance, designed for continuous blood pressure monitoring (Fig. 9c)157. Other approaches include flexible multimodal tactile sensors developed via DIW using carbon-filled conductive inks, enabling the detection of pressure, strain, and temperature158. 3D printing has also been used to print a flexible humidity sensor for continuous respiratory monitoring (Fig. 9d)159, demonstrating high sensitivity (1.4 RH⁻¹) and fast response time (0.35 s). To manufacture a biosensor for the acquisition of physiological signals such as ECG and electromyography (EMG), this method provides flexibility and mechanical reliability along with adequate adhesion owing to its homogeneous photopolymerizable resin (Fig. 9e)160. The nanocomposite material, which exhibited a sheet resistance of ~3 × 10³ Ω/□ and good flexibility, was then attained as microneedle structures through 3D printing. While the microneedle structure enhances adhesion to the skin surface, prolonged use could cause skin irritation.
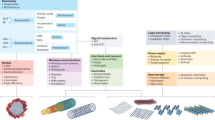
Hybrid materials
Successful implementation of flexible biosensing platforms requires concurrent advancement in two domains: (i) scalable and cost-effective manufacturing ensuring mechanical compliance, and (ii) multifunctional material systems maintaining electronic stability under deformation. While earlier discussions explored fabrication methods (e.g., inkjet and screen printing, microfabrication, and 3D printing), this section focuses on hybrid materials, strategically engineered combinations of organic and inorganic components that address critical challenges in multifunctional integration. The term “hybrid material” refers to a material composed of at least one organic and one inorganic component, resulting in enhanced properties such as optical, magnetic, and electronic characteristics, or a combination of these. Hybrid materials can be classified by the nature of their components (organic-in-inorganic and inorganic-in-organic), or by the type of interaction between them, ranging from weak physical forces to strong covalent bonding. More recent classifications categorize hybrid materials based on their functionalities, distinguishing structural, functional, and chemically hybridized systems161,162.
At the nanometric level, hybrid nanomaterials exhibit great potential for advanced applications, particularly in the biomedical field163. One example is the flexible fiber-based sensor developed by Ryu et al. for continuous body temperature monitoring164. The used hybrid material is a polymer-nanocomposite system, combining polylactic acid (PLA) and Linear Low-Density Polyethylene (LLDPE), where PLA provides the structural matrix and LLDPE ensures flexibility, while the conductive nanomaterial rGO enhances thermal stabilization. The sensor was fabricated using the thermal drawing process (Fig. 10a). This device demonstrates a fair sensitivity (−0.285%/°C) with fast response and recovery times of 11.6 s and 14.8 s, respectively. However, the sensor’s durability requires further improvement through mechanical reinforcement and moisture resistance. Likewise, Li et al. developed a flexible biosensor for the simultaneous monitoring of ECG signals and glucose levels in sweat, based on a hybrid material composed of an inorganic component—platinum nanoparticles and conductive silver and carbon pastes—and organic components such as PEDOT: PSS, glucose oxidase, and paper165. The sensor utilizes Whatman filter paper as a substrate, and through a screen-printing process, the ECG electrodes and electrical circuits are fabricated using silver paste, Ag/AgCl, and carbon paste (Fig. 10b). PEDOT: PSS is used to create circular electrodes for ECG and rectangular electrodes for the glucose sensor with a sensitivity of 325.99 ± 0.8 μA mM⁻¹ ⋅ cm⁻² (in the range of 0–2.5 Mm). Although the paper substrate provides flexibility, its limited mechanical robustness and the finite operational lifespan of glucose oxidase remain constraints; however, the PEDOT: PSS hydrogel improves signal transduction and sweat uptake due to its intrinsic conductivity and hydrophilicity. In another work, Zhang et al. developed a flexible multifunctional sensor using a hybrid material for the precise monitoring of physiological parameters such as strain deformation, ECG signals, and body temperature166. The composite material consists of MXene-Ti₃C₂Tₓ, laser-induced graphene (LIG) coated with the organic polymer EDOT, transferred onto an elastomeric SEBS substrate. EDOT is first polymerized on the surface of MXene-Ti₃C₂Tₓ, followed by the electrophoretic deposition onto the LIG structure and then transferred onto SEBS. The sensor’s structure includes ECG electrodes and the temperature sensor in the lower layer, the strain sensor in the middle, and a second ECG electrode in the upper layer (Fig. 10c). The device demonstrated a high strain sensitivity (Gauge factor) of 2075 and a skin-contact impedance (51.08 kΩ at 10 Hz) lower than the Ag/AgCl electrode impedance.
a Schematic representation of the fiber drawing process and the temperature-sensing mechanism of the thermally drawn polymer–nanocomposite fiber sensor164. b Schematic illustration of the complete HPP system165. c Layered view of the LIG/MXene-Ti₃C₂Tx@EDOT-based multifunctional sensors166. d Photographs of the fingertip-sized stretchable and flexible wearable patch alongside a schematic of its layer assembly, including the soft and stretchable poly[styrene-b-(ethylene-co-butylene)-b-styrene] (SEBS) substrate, a custom-engineered electrochemical biosensor array, a pair of voltage-modulated electrodes for controlled drug release and electrical stimulation, and an anti-inflammatory and antimicrobial drug-loaded electroactive hydrogel layer167. e Images showing: conformal wrapping of the device around curved vasculature (large image, left); encapsulated device application on the skin (top left); exposed femoral artery in a swine before device application (center); and after device mounting on the femoral artery (right)168.
In a different approach, Sani et al. presented one of the most advanced integrated systems for chronic wound management: a wearable multimodal patch capable of real-time monitoring combined with non-invasive therapy167. Using e-beam evaporation, photolithography, and laser patterning, they fabricated a multimodal electrochemical biosensor array for biomarker analysis in wound exudate, combining it with a drug-releasing hydrogel and electrodes to enhance tissue regeneration (Fig. 10d). The device exhibits high sensitivity for glucose (16.34 nA/mM), lactate (41.44 nA/mM), and uric acid (189.60 nA/mM). However, its performance is limited by the lack of continuous wound-fluid sampling and by potential interferences from the wound exudate, which affect long-term stability.
Deng et al. introduced a soft, wearable device for continuous blood flow monitoring in patients with chronic kidney disease undergoing hemodialysis168. Using thermal anemometry, the device non-invasively measures flow variations, enabling real-time thrombosis detection with a thermal response of ΔT = 7.87 °C. To achieve a functional and biocompatible structure, the device consists of electronic components and flexible materials, forming a hybrid material: conductive copper layers are integrated with flexible polymers (polyimide, silicone, PMMA), ensuring adaptability to the skin (Fig. 10e). The fabrication process combines laser lithography, polymer encapsulation and medical-grade adhesives, enabling wireless flow monitoring with fair sensitivity. However, at high blood flow rates, the device becomes less responsive, increasing the risk of errors due to environmental noise and potentially reducing thrombosis-detection accuracy.
Conclusions and future prospects
Flexible biosensors have emerged as one of the state-of-the-art sensing technologies, leveraging advanced mechanics, innovative structural designs, advanced fabrication techniques, and hybrid materials to facilitate high-performance continuous monitoring of physical and biological health conditions. The fundamental understanding of mechanical properties of these biosensors and associated underlying mechanics, i.e., bending, twisting, fatigue, stretchability, flexibility, bending, and others, helps to resolve the critical issues such as fracture and delamination. Furthermore, innovations in structural designs, such as serpentine interconnects, kirigami architectures, strain-isolation designs, and bioinspired architectures, help to mitigate stress concentrations during operation, ensuring long-term reliability. Another important factor is adhesion and modulus-matching at the interface of the biosensors and human skin or soft substrates. These devices exhibit excellent mechanical resilience, enabling conformal integration with skin interfaces while maintaining robust performance under cyclic strain and multi-axial deformation. To operate these devices seamlessly and wirelessly, the mechanical (i.e., deformations in antenna) and non-mechanical challenges (i.e., storage, power, security) need to be understood and tackled accordingly. By employing hybrid organic-inorganic composites, they achieve synergistic optimization of electrical conductivity, fracture resistance, and tissue-modulus matching. Likewise, the type of fabrication method dictates the performance and controls the cost of the biosensing technologies. This review has systematically examined these critical aspects, highlighting how mechanistic insights, novel material composites, and advanced manufacturing approaches address key challenges like signal drift, mechanical failure, and wireless data transmission.
An emerging perspective reframes the transition from merely identifying problems to implementing integrated, intelligent solutions. A primary challenge lies in the complex, often inverse, relationship between mechanical compliance and electronic performance. Emerging multi-objective optimization frameworks powered by machine learning (ML) present a transformative solution. By training and integrating deep learning models with finite element analysis and electromagnetic simulation data, it is possible to rapidly generate and evaluate millions of structural designs, from serpentine interconnects to kirigami patterns, to identify evolutionary optimization that maximizes both strain tolerance and electrical stability. Similar data-driven co-design approaches will be crucial for developing devices that maintain signal integrity even under extreme and dynamic biomechanical loading, resolving a key limitation. Consequently, the next generation of flexible biosensors will evolve beyond passive monitoring devices into autonomous, adaptive systems. These systems will leverage advanced computational design, novel materials, and closed-loop functionality to overcome current limitations.
An additional perspective recognizes that the advancement of bioelectronics continues to confront enduring engineering challenges, primarily system-level miniaturization, high-precision signal acquisition, and the design of materials with inherent anti-inflammatory properties. A critical limitation is that persistent issues such as biofouling and interfacial instability cannot be fully resolved through static material strategies alone. Consequently, the future of mechanically robust bioelectronics is likely to shift toward dynamic, “smart” bio-interfaces capable of actively sensing and adapting to their physiological environment. Promising directions in this domain could include the development of stimuli-responsive hydrogels and polymers that undergo reversible swelling/contraction or modulate surface energy in response to local biochemical signals. Complementing these material-level strategies, hybrid encapsulation architectures, some of which have been surveyed in preceding sections, offer another crucial pathway toward enhanced interfacial stability.
From the perspective of functional materials for bioelectronic sensors, a key parameter to ensure long-term operational stability is bioabsorption, i.e., the ability of the used material to safely degrade without causing cytotoxic effects or negative environmental impacts. Therefore, another futuristic direction could be to develop programmable feedback-controlled components that optimize themselves to the best of their abilities by comparing their instant performance with a reference database and adapt changes in shape, heal themselves to avert microcracks, harness the power through self-powered components, and coordinate with each other as a human body does. These programmable electronic components should align with the United Nations Sustainable Development Goals (SDGs), particularly SDG 12, which promotes environmental impact reduction through minimizing CO₂ emissions and sustainable management of electronic waste (e-waste). Additionally, another critical issue is related to the questionable standards of the used off-the-shelf functional materials (conductive polymers, bioactive nanocomposites, bioresorbable materials), which pose significant challenges in terms of foreign body reactions after prolonged usage, reproducibility, chemical-physical stability, and long-term biocompatibility.
Regarding the effect of mechanical deformations on wireless systems, overcoming the limitations requires moving beyond conventional approaches. Futuristic approaches should incorporate real-time adaptability to maintain link quality. This can be achieved through the use of reconfigurable metamaterial antennas whose resonant frequency can be electronically tuned to compensate for detuning caused by stretching or bending. On the data side, lightweight, on-sensor embedded ML models can perform feature extraction and anomaly detection (e.g., identifying motion artifacts), transmitting only clinically relevant, compressed data. This “edge intelligence” drastically reduces bandwidth requirements and power consumption. For power delivery, adaptive WPT systems using beamforming and receiver feedback can dynamically optimize power transmission efficiency, mitigating losses due to misalignment or changing tissue properties, thereby enabling truly perpetual operation for deep-tissue implants.
Moving forward, a pathway for next-generation biosensing should involve bidirectional neural integration, where implantable biosensor-actuator systems establish AI-driven closed-loop feedback with the central nervous system. The system should harness the required power from body temperature or body movements, track mechanical stress levels of components, compare with benchmarks, and if needed, initiate an action to avert any micro-cracks to retain mechanical and electronic reliability. Such systems would leverage real-time sensing coupled with adaptive AI algorithms. In summary, the integration of AI-driven analytics, self-healing materials, and programmable bioelectronics will further enhance biosensor performance, enabling adaptive and long-term monitoring. Meanwhile, the development of fully biodegradable components and adherence to regulatory standards will ensure sustainability and safe clinical adoption. By converging the proposed solutions, i.e., AI/ML-based intelligent co-design, adaptive interfaces, and smart wireless systems, researchers can transcend the current state-of-the-art, creating a new paradigm of robust, low-cost, reliable, and clinically transformative flexible biosensors.
Data availability
No datasets were generated or analysed during the current study.
References
Chen, S. et al. Starfish-inspired wearable bioelectronic systems for physiological signal monitoring during motion and real-time heart disease diagnosis. Sci. Adv. 11, eadv2406 (2025).
Fullenkamp, D. E. et al. Simultaneous electromechanical monitoring in engineered heart tissues using a mesoscale framework. Sci. Adv. 10, eado7089 (2024).
Imani, S. et al. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 7, 11650 (2016).
Jin, X. et al. Fully integrated flexible biosensor for wearable continuous glucose monitoring. Biosens. Bioelectron. 196, 113760 (2022).
Tzavelis, A. et al. Development of a miniaturized mechanoacoustic sensor for continuous, objective cough detection, characterization and physiologic monitoring in children with cystic fibrosis. IEEE J. Biomed. Health Inf. 28, 5941–5952 (2024).
Botonis, P. G., Arsoniadis, G. G., Smilios, I. & Toubekis, A. G. In-season training load variation - heart rate recovery, perceived recovery status, and performance in elite male water polo players: a pilot study. Sports Health 17, 144–149 (2024).
Gadaleta, M. et al. Passive detection of COVID-19 with wearable sensors and explainable machine learning algorithms. NPJ Digit Med. 4, 166 (2021).
Cho, S. I. et al. Engineering TALE-linked deaminases to facilitate precision adenine base editing in mitochondrial DNA. Cell 187, 95–109.e126 (2024).
Krishnan, S. K., Prokhorov, E., Bahena, D., Esparza, R. & Meyyappan, M. Chitosan-covered Pd@Pt core-shell nanocubes for direct electron transfer in electrochemical enzymatic glucose biosensor. ACS Omega 2, 1896–1904 (2017).
Ling, W., Shang, X., Liu, J. & Tang, T. A skin-mountable flexible biosensor based on Cu-MOF/PEDOT composites for sweat ascorbic acid monitoring. Biosens. Bioelectron. 267, 116852 (2025).
Madhvapathy, S. R. et al. Implantable bioelectronics and wearable sensors for kidney health and disease. Nat. Rev. Nephrol. 21, 443–463 (2025).
Nyein, H. Y. Y. et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 5, eaaw9906 (2019).
Qaiser, N. et al. A robust wearable point-of-care CNT-based strain sensor for wirelessly monitoring throat-related illnesses. Adv. Funct. Mater. 31, 2103375 (2021).
Trung, T. Q. & Lee, N. E. Flexible and stretchable physical sensor integrated platforms for wearable human-activity monitoringand personal healthcare. Adv. Mater. 28, 4338–4372 (2016).
Wu, S. J. & Zhao, X. Bioadhesive technology platforms. Chem. Rev. 123, 14084–14118 (2023).
Min, J. et al. Skin-interfaced wearable sweat sensors for precision medicine. Chem. Rev. 123, 5049–5138 (2023).
O’Brien, M. K., Hohl, K., Lieber, R. L. & Jayaraman, A. Automate, illuminate, predict: a universal framework for integrating wearable sensors in healthcare. Digit Biomark. 8, 149–158 (2024).
Qaiser, N., Khan, S. M. & Hussain, M. M. In-plane and out-of-plane structural response of spiral interconnects for highly stretchable electronics. J. Appl Phys. 124, 034905 (2018).
Bandodkar, A. J. et al. Tattoo-based noninvasive glucose monitoring: a proof-of-concept study. Anal. Chem. 87, 394–398 (2015).
Choi, M. K. et al. cephalopod-inspired miniaturized suction cups for smart medical skin. Adv. Health. Mater. 5, 80–87 (2016).
Derkus, B. Applying the miniaturization technologies for biosensor design. Biosens. Bioelectron. 79, 901–913 (2016).
Kim, J. Y. et al. Continuous glucose monitoring with structured education in adults with type 2 diabetes managed by multiple daily insulin injections: a multicentre randomised controlled trial. Diabetology 67, 1223–1234 (2024).
Vora, L. K. et al. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 15, 1916 (2023).
Sharma, A., Badea, M., Tiwari, S. & Marty, J. L. Wearable biosensors: an alternative and practical approach in healthcare and disease monitoring. Molecules 26, 748 (2021).
Yoon, J. et al. Nanotechnology-based wearable electrochemical biosensor for disease diagnosis. ACS Sens. 10, 1675–1689 (2025).
Bandodkar, A. J. et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 5, eaav3294 (2019).
Bandodkar, A. J. & Wang, J. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 32, 363–371 (2014).
Meng, J. et al. A tonically active master neuron modulates mutually exclusive motor states at two timescales. Sci. Adv. 10, eadk0002 (2024).
Ayçiçek, S., Cevher, S. u. C. k. & Acar, S. Recent trends in hydrogel-based biosensor technology for the diagnosis of neurodegenerative diseases. ACS Appl. Bio Mater. 8, 5424–5444 (2025).
Keles, G., Derici, U. S., Altunay, B. B., Yilgor, P. & Kurbanoglu, S. Applications of 3D-printed electrochemical sensors in medical diagnostics. in Additively Manufactured Electrochemical Sensors: Design, Performance, and Applications, 177–250 (Wiley, 2025).
Atabay, M., Inci, F. & Saylan, Y. Computational studies for the development of extracellular vesicle-based biosensors. Biosens. Bioelectron. 277, 117275 (2025).
Song, J. K. et al. Wearable force touch sensor array using a flexible and transparent electrode. Adv. Funct. Mater. 27, 1605286 (2017).
Wang, S., Chinnasamy, T., Lifson, M. A., Inci, F. & Demirci, U. Flexible substrate-based devices for point-of-care diagnostics. Trends Biotechnol. 34, 909–921 (2016).
Kumar, R. & Parashar, A. Atomistic simulations of pristine and nanoparticle reinforced hydrogels: a review. Wiley Interdiscip. Rev. Comput. Mol. Sci. 13, e1655 (2023).
Yan, Z. et al. Mechanical assembly of complex, 3D mesostructures from releasable multilayers of advanced materials. Sci. Adv. 2, e1601014 (2016).
Zhu, Y. & Lu, N. Mechanics of Flexible and Stretchable Electronics (Wiley-VCH, 2025).
Cui, X., Wu, L., Zhang, C. & Li, Z. Implantable self-powered systems for electrical stimulation medical devices. Adv. Sci. 12, e2412044 (2025).
Yang, Y. et al. Ultrafine graphene nanomesh with large on/off ratio for high-performance flexible biosensors. Adv. Funct. Mater. 27, 1604096 (2016).
Bocchetta, P. et al. Soft materials for wearable/flexible electrochemical energy conversion, storage, and biosensor devices. Materials 13, 2733 (2020).
Shetti, N. P. et al. Skin-patchable electrodes for biosensor applications: a review. ACS Biomater. Sci. Eng. 6, 1823–1835 (2020).
Vedadghavami, A. et al. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 62, 42–63 (2017).
Ates, H. C. et al. End-to-end design of wearable sensors. Nat. Rev. Mater. 7, 887–907 (2022).
Carpinteri, A. Decrease of apparent tensile and bending strength with specimen size: two different explanations based on fracture mechanics. Int. J. Solid Struct. 25, 407–429 (1989).
Kenry, Yeo, J. C. & Lim, C. T. Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst. Nanoeng. 2, 16043 (2016).
Vaghasiya, J. V., Mayorga-Martinez, C. C. & Pumera, M. Wearable sensors for telehealth based on emerging materials and nanoarchitectonics. npj Flex. Electron. 7, 26 (2023).
Himmelhaus, M. & Francois, A. In-vitro sensing of biomechanical forces in live cells by a whispering gallery mode biosensor. Biosens. Bioelectron. 25, 418–427 (2009).
Prasad, S. et al. 3D nanorhombus nickel nitride as stable and cost-effective counter electrodes for dye-sensitized solar cells and supercapacitor applications. RSC Adv. 8, 8828–8835 (2018).
Ge, Z., Yang, F., Goh, J. C., Ramakrishna, S. & Lee, E. H. Biomaterials and scaffolds for ligament tissue engineering. J. Biomed. Mater. Res. A 77, 639–652 (2006).
Niinomi, M. & Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 836587 (2011).
Pena, A. E. et al. Mechanical fatigue resistance of an implantable branched lead system for a distributed set of longitudinal intrafascicular electrodes. J. Neural Eng. 14, 066014 (2017).
Mousavi, A., Rahimnejad, M., Azimzadeh, M., Akbari, M. & Savoji, H. Recent advances in smart wearable sensors as electronic skin. J. Mater. Chem. B 11, 10332–10354 (2023).
Zeng, Q. & Huang, Z. Challenges and opportunities of implantable neural interfaces: from material, electrochemical and biological perspectives. Adv. Funct. Mater. 33, 2301223 (2023).
Park, B., Jeong, C., Ok, J. & Kim, T.-i. Materials and structural designs toward motion artifact-free bioelectronics. Chem. Rev. 124, 6148-6197 (2024).
Gong, S., Lu, Y., Yin, J., Levin, A. & Cheng, W. Materials-driven soft wearable bioelectronics for connected healthcare. Chem. Rev. 124, 455–553 (2024).
Tan, E. L. et al. Implantable biosensors for real-time strain and pressure monitoring. Sensors 8, 6396–6406 (2008).
Wang, L. & Beebe, D. J. Characterization of a silicon-based shear-force sensor on human subjects. IEEE Trans. Biomed. Eng. 49, 1340–1347 (2002).
Yamagishi, K., Zhou, W., Ching, T., Huang, S. Y. & Hashimoto, M. Ultra-deformable and tissue-adhesive liquid metal antennas with high wireless powering efficiency. Adv. Mater. 33, e2008062 (2021).
Guo, Z. et al. A flexible neural implant with ultrathin substrate for low-invasive brain-computer interface applications. Microsyst. Nanoeng. 8, 133 (2022).
Shen, K. et al. Nanocomposite conductive hydrogels with Robust elasticity and multifunctional responsiveness for flexible sensing and wound monitoring. Mater. Horiz. 10, 2096–2108 (2023).
Zhao, L. et al. On-demand contact-mode switchable cerebral cortex biosensors enhanced by magnetic actuation. ACS Appl. Mater. Interfaces 17, 20671–20684 (2025).
Roy, A. et al. A highly stretchable, conductive, and transparent bioadhesive hydrogel as a flexible sensor for enhanced real-time human health monitoring. Adv. Mater. 36, e2404225 (2024).
Zhai, Q. & Cheng, W. Soft and stretchable electrochemical biosensors. Mater. Today Nano 7, 100041 (2019).
Hu, B. et al. Ultrathin crystalline silicon–based omnidirectional strain gauges for implantable/wearable characterization of soft tissue biomechanics. Sci. Adv. 10, eadp8804 (2024).
Deo, K. A. et al. Nanoengineered ink for designing 3 d printable flexible bioelectronics. ACS Nano 16, 8798–8811 (2022).
Wang, C., Sani, E. S. & Gao, W. Wearable bioelectronics for chronic wound management. Adv. Funct. Mater. 32 https://doi.org/10.1002/adfm.202111022 (2022).
Shao, Y. et al. Multi-functional, conformal systems with ultrathin crystalline-silicon-based bioelectronics for characterization of intraocular pressure and ocular surface temperature. Biosens. Bioelectron. 267, 116786 (2025).
Su, X. et al. Integrated wearable sensors with bending/stretching selectivity and extremely enhanced sensitivity derived from agarose-based ionic conductor and its 3D-shaping. Chem. Eng. J. 389, 124503 (2020).
Brooks, A. K., Chakravarty, S., Ali, M. & Yadavalli, V. K. Kirigami-inspired biodesign for applications in healthcare. Adv. Mater. 34, e2109550 (2022).
Gideon, O., Samuel, H. S. & Okino, I. A. Biocompatible materials for next-generation biosensors. Discov. Chem. 1, 34 (2024).
Ashok, A. et al. Flexible nanoarchitectonics for biosensing and physiological monitoring applications. Small 19, e2204946 (2023).
Bae, W. G. et al. Enhanced skin adhesive patch with modulus-tunable composite micropillars. Adv. Health. Mater. 2, 109–113 (2013).
Choi, S., Lee, H., Ghaffari, R., Hyeon, T. & Kim, D. H. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 28, 4203–4218 (2016).
Ling, H., Liu, S., Zheng, Z. & Yan, F. Organic flexible electronics. Small Methods 2, 1800070 (2018).
Choi, C. K., English, A. E., Jun, S. I., Kihm, K. D. & Rack, P. D. An endothelial cell compatible biosensor fabricated using optically thin indium tin oxide silicon nitride electrodes. Biosens. Bioelectron. 22, 2585–2590 (2007).
Miao, J. & Fan, T. Flexible and stretchable transparent conductive graphene-based electrodes for emerging wearable electronics. Carbon 202, 495–527 (2023).
Liu, X. et al. Bioinspired, microstructured silk fibroin adhesives for flexible skin sensors. ACS Appl Mater. Interfaces 12, 5601–5609 (2020).
Kim, D. H. et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 9, 511–517 (2010).
Shi, Z. et al. Silk-enabled conformal multifunctional bioelectronics for investigation of spatiotemporal epileptiform activities and multimodal neural encoding/decoding. Adv. Sci. 6, 1801617 (2019).
Sang, M., Kim, K., Shin, J. & Yu, K. J. Ultra-thin flexible encapsulating materials for soft bio-integrated electronics. Adv. Sci. 9, e2202980 (2022).
Bovone, G., Dudaryeva, O. Y., Marco-Dufort, B. & Tibbitt, M. W. Engineering hydrogel adhesion for biomedical applications via chemical design of the junction. ACS Biomater. Sci. Eng. 7, 4048–4076 (2021).
Li, Y., Veronica, A., Ma, J. & Nyein, H. Y. Y. Materials, structure, and interface of stretchable interconnects for wearable bioelectronics. Adv. Mater. 37, e2408456 (2025).
Morikawa, Y. et al. Ultrastretchable kirigami bioprobes. Adv. Health. Mater. 7, 1701100 (2018).
Han, W. B. et al. Ultra-stretchable and biodegradable elastomers for soft, transient electronics. Nat. Commun. 14, 2263 (2023).
Wu, H., Huang, Y., Xu, F., Duan, Y. & Yin, Z. Energy harvesters for wearable and stretchable electronics: from flexibility to stretchability. Adv. Mater. 28, 9881–9919 (2016).
Xu, R. et al. Kirigami-inspired, highly stretchable micro-supercapacitor patches fabricated by laser conversion and cutting. Microsyst. Nanoeng. 4, 36 (2018).
Xiao, Y. et al. High-adhesive flexible electrodes and their manufacture: a review. Micromachines 12, 1505 (2021).
Zhang, Y. Stretchable Bioelectronics for Medical Devices and Systems (eds John A. R., Roozbeh, G. & Kim, D. H.) 53–68 (Springer International Publishing, 2016).
Yuan, C. et al. Bionic design and performance of electrode for bioelectrical signal monitoring. Adv. Mater. Interfaces 9, 2200532 (2022).
Yeh, C. et al. Bioinspired shark skin-based liquid metal triboelectric nanogenerator for self-powered gait analysis and long-term rehabilitation monitoring. Nano Energy 104, 107852 (2022).
Narasimhan, V. et al. Multifunctional biophotonic nanostructures inspired by the longtail glasswing butterfly for medical devices. Nat. Nanotechnol. 13, 512–519 (2018).
Lan, T. et al. Treefrog-inspired flexible electrode with high permeability, stable adhesion, and robust durability. Adv. Mater. 36, e2404761 (2024).
Pettinelli, S. et al. High-performance bioelectronic tongue for the simultaneous analysis of phenols, sugars and organic acids in wines. J. Sci. Food Agric. 105, 1430–1438 (2025).
Khan, M. R., Khalilian, A. & Kang, S. W. A high sensitivity idc-electronic tongue using dielectric/sensing membranes with solvatochromic dyes. Sensors 16, 668 (2016).
Pal, S., Kumar, D., Ulucan-Karnak, F., Narang, J. & Shukla, S. K. Bio-inspired electronic sensors for healthcare applications. Chem. Eng. J. 499, 155894 (2024).
Alam, F. et al. Prospects for additive manufacturing in contact lens devices. Adv. Eng. Mater. 23. https://doi.org/10.1002/adem.202000941 (2020).
Chen, Y. et al. A biochemical sensor with continuous extended stability in vivo. Nat. Biomed. Eng. 9, 1517–1530 (2025).
Liu, Y. et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 3, 58–68 (2019).
Zhao, K., Yuan, Y., Wang, S. & Cui, L. Effect of parylene coating on the performance of implantable pressure sensor. IEEE Sens. J. 24, 24593–24599 (2024).
Hashemi, S. et al. Ultra-sensitive wireless capacitive nanocomposite-based pressure sensors for health monitoring. Adv. Mater. Technol. 10, e01316 (2025).
Li, Z. et al. An all-solid-state fluorinated ion-conductive elastomer with outstanding mechanical properties and high environmental stability for flexible electronics. Chem. Eng. J. 505, 159437 (2025).
Yuk, H. et al. Hydraulic hydrogel actuators and robots optically and sonically camouflaged in water. Nat. Commun. 8, 14230 (2017).
Zhang, J. et al. “Self-Defensive” antifouling zwitterionic hydrogel coatings on polymeric substrates. ACS Appl. Mater. Interfaces 14, 56097–56109 (2022).
Kim, H.-J. et al. Review of near-field wireless power and communication for biomedical applications. IEEE Access 5, 21264–21285 (2017).
Zou, H. et al. NFC/RFID-enabled wearables and implants for biomedical applications. Microsyst. Nanoeng. 11, 191 (2025).
Lin, R. et al. Wireless battery-free body sensor networks using near-field-enabled clothing. Nat. Commun. 11, 444 (2020).
Xu, Z., Hao, Y., Luo, A. & Jiang, Y. Technologies and applications in wireless biosensors for real-time health monitoring. Med-X 2, 24 (2024).
Kim, H., Rigo, B., Wong, G., Lee, Y. J. & Yeo, W. H. Advances in wireless, batteryless, implantable electronics for real-time, continuous physiological monitoring. NanoMicro Lett. 16, 52 (2023).
Liu, Y. & Bao, Y. Real-time remote measurement of distance using ultra-wideband (UWB) sensors. AUTCON 150, 104849 (2023).
Mustaqim, M. et al. Ultra-wideband antenna for wearable Internet of Things devices and wireless body area network applications. Int. J. Numer Model Electron Netw. Device Field 32, e2590 (2019).
Kim, C. Y. et al. Wireless technologies for wearable electronics: a review. Adv. Electron Mater. 11, 2400884 (2025).
Kong, L. et al. Wireless technologies in flexible and wearable sensing: from materials design, system integration to applications. Adv. Mater. 36, e2400333 (2024).
Jin, X., Liu, C., Xu, T., Su, L. & Zhang, X. Artificial intelligence biosensors: challenges and prospects. Biosens. Bioelectron. 165, 112412 (2020).
Hadi, M. S., Lawey, A. Q., El-Gorashi, T. E. H. & Elmirghani, J. M. H. Big data analytics for wireless and wired network design: a survey. Comput. Netw. 132, 180–199 (2018).
L’Heureux, A., Grolinger, K., Elyamany, H. F. & Capretz, M. A. M. Machine learning with big data: challenges and approaches. IEEE Access 5, 7776–7797 (2017).
Beuchert, J., Solowjow, F., Trimpe, S. & Seel, T. Overcoming bandwidth limitations in wireless sensor networks by exploitation of cyclic signal patterns: an event-triggered learning approach. Sensors 20, 260 (2020).
Yin, L. et al. A self-sustainable wearable multi-modular E-textile bioenergy microgrid system. Nat. Commun. 12, 1542 (2021).
Jegan, R. & Nimi, W. S. On the development of low power wearable devices for assessment of physiological vital parameters: a systematic review. J. Public Health 32, 1093–1108 (2024).
Smith, A. A., Li, R. & Tse, Z. T. H. Reshaping healthcare with wearable biosensors. Sci. Rep. 13, 4998 (2023).
Tiwari, N. et al. Recent advancements in sampling, power management strategies and development in applications for non-invasive wearable electrochemical sensors. J. Electroanal. Chem. 907, 116064 (2022).
Abduljaleel, H. K., Gharghan, S. K. & Al-Gburi, A. J. A. Multi-layer square coil-based wireless power transfer for biomedical implants. Prog. Electromagn. Res. B 111, 83–98 (2025).
Nguyen, T. H., Lee, J., Lee, D., Nguyen, M. C. & Kim, J. Omni-directionally flexible, high performance all-solid-state micro-supercapacitor array-based energy storage system for wearable electronics. Chem. Eng. J. 505, 159375 (2025).
He, Z. et al. Highly stretchable, deformation-stable wireless powering antenna for wearable electronics. Nano Energy 112, 108461 (2023).
Liu, Q., Mkongwa, K. G. & Zhang, C. Performance issues in wireless body area networks for the healthcare application: a survey and future prospects. SN Appl Sci. 3, 155 (2021).
Lopez-Linares Roman, K., Vermeeren, G., Thielens, A., Joseph, W. & Martens, L. Characterization of path loss and absorption for a wireless radio frequency link between an in-body endoscopy capsule and a receiver outside the body. EURASIP J. Wirel. Commun. Netw. 2014, 21 (2014).
Kumar, M., Yadav, V. & Yadav, S. P. Advance comprehensive analysis for Zigbee network-based IoT system security. Discov. Comput. 27, 22 (2024).
Mohan, A. & Kumar, N. Implantable antennas for biomedical applications: a systematic review. Biomed. Eng. Online 23, 87 (2024).
Hussain, A., Abbas, N. & Ali, A. Inkjet printing: a viable technology for biosensor fabrication. Chemosensors 10, 103 (2022).
Baek, S., Jo, Y., Lee, Y., Kwon, J. & Jung, S. Design and integration of organic printed thin-film transistor-based soft biosensors for wearable applications. ACS Appl. Electron. Mater. 6, 7657–7678 (2024).
Kwon, J., Baek, S., Lee, Y., Tokito, S. & Jung, S. Layout-to-bitmap conversion and design rules for inkjet-printed large-scale integrated circuits. Langmuir 37, 10692–10701 (2021).
Bihar, E. et al. A fully inkjet-printed disposable glucose sensor on paper. npj Flex. Electron 2, 30 (2018).
Lo, L. W. et al. An inkjet-printed PEDOT:PSS-based stretchable conductor for wearable health monitoring device applications. ACS Appl. Mater. Interfaces 13, 21693–21702 (2021).
Sekine, T. et al. Fully printed wearable vital sensor for human pulse rate monitoring using ferroelectric polymer. Sci. Rep. 8, 4442 (2018).
Zavanelli, N. & Yeo, W. H. Advances in screen printing of conductive nanomaterials for stretchable electronics. ACS Omega 6, 9344–9351 (2021).
Li, W. et al. Large-scale ultra-robust MoS2 patterns directly synthesized on polymer substrate for flexible sensing electronics. Adv. Mater. 35, e2207447 (2023).
Park, H. J. et al. Fluid-dynamics-processed highly stretchable, conductive, and printable graphene inks for real-time monitoring sweat during stretching exercise. Adv. Funct. Mater. 31, 2011059 (2021).
Becker, H. & Gärtner, C. Polymer microfabrication methods for microfluidic analytical applications. Electrophoresis 21, 12–26 (2000).
Voldman, J., Gray, M. L. & Schmidt, M. A. Microfabrication in biology and medicine. Annu. Rev. Biomed. Eng. 1, 401–425 (1999).
Erdem, O., Es, I., Akceoglu, G. A., Saylan, Y. & Inci, F. Recent advances in microneedle-based sensors for sampling, diagnosis and monitoring of chronic diseases. Biosensors 11, 296 (2021).
Goud, K. Y. et al. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: toward parkinson management. ACS Sens. 4, 2196–2204 (2019).
Windmiller, J. R. et al. Microneedle array-based carbon paste amperometric sensors and biosensors. Analyst 136, 1846–1851 (2011).
Zhang, B. L., Yang, Y., Zhao, Z. Q. & Guo, X. D. A gold nanoparticles deposited polymer microneedle enzymatic biosensor for glucose sensing. Electrochim. Acta 358, 136917 (2020).
Kim, J. et al. Individually-addressable composite microneedle electrode array by mold-and-place method for glucose detection. Sens. Actuator B Chem. 401, 134884 (2024).
Rigo, B. et al. Soft implantable printed bioelectronic system for wireless continuous monitoring of restenosis. Biosens. Bioelectron. 241, 115650 (2023).
Kim, Y., Chica-Carrillo, E. C. & Lee, H. J. Microfabricated sensors for non-invasive, real-time monitoring of organoids. Micro Nano Syst. Lett. 12, 26 (2024).
An, J. et al. Drug evaluation of parkinson’s disease patient-derived midbrain organoids using mesoporous Au nanodot-patterned 3D concave electrode. ACS Sens. 9, 3573–3580 (2024).
Chang, A.-Y. et al. Dopamine sensing with robust carbon nanotube implanted polymer micropillar array electrodes fabricated by coupling micromolding and infiltration coating processes. Electrochim. Acta 368, 137632 (2021).
Wang, C. et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2, 687–695 (2018).
Wang, X., Yoon, E. & Meng, E. A microfabricated nanobubble-based sensor for physiological pressure monitoring. J. Microelectromech. Syst. 32, 542–551 (2023).
Mariello, M. et al. Wireless, battery-free, and real-time monitoring of water permeation across thin-film encapsulation. Nat. Commun. 15, 7443 (2024).
Dion, G. et al. In-sensor human gait analysis with machine learning in a wearable microfabricated accelerometer. Commun. Eng. 3, 48 (2024).
Ali, M. A., Hu, C., Yttri, E. A. & Panat, R. Recent advances in 3D printing of biomedical sensing devices. Adv. Funct. Mater. 32, 2107671 (2022).
Parupelli, S. K. & Desai, S. The 3D printing of nanocomposites for wearable biosensors: recent advances, challenges, and prospects. Bioengineering 11, 32 (2023).
Silva, L. R. G. et al. Electrochemical Biosensors 3D printed by fused deposition modeling: actualities, trends, and challenges. Biosensors 15, 57 (2025).
Yi, Q. et al. All-3D-printed, flexible, and hybrid wearable bioelectronic tactile sensors using biocompatible nanocomposites for health monitoring. Adv. Mater. Technol. 7, 2101034 (2021).
Yi, Q. et al. A self-powered triboelectric MXene-based 3D-printed wearable physiological biosignal sensing system for on-demand, wireless, and real-time health monitoring. Nano Energy 101, 107511 (2022).
Mandal, A., Morali, A., Skorobogatiy, M. & Bodkhe, S. 3D printing of polyvinylidene fluoride-based piezoelectric sensors for noninvasive continuous blood pressure monitoring. Adv. Eng. Mater. 26. https://doi.org/10.1002/adem.202301292 (2023).
Sel, K. et al. Continuous cuffless blood pressure monitoring with a wearable ring bioimpedance device. npj Digit. Med. 6, 59 (2023).
Ma, C. et al. 3D-printing of conductive inks based flexible tactile sensor for monitoring of temperature, strain and pressure. J. Manuf. Process 87, 1–10 (2023).
Chen, X. et al. Fast-response non-contact flexible humidity sensor based on direct-writing printing for respiration monitoring. Biosensors 13, 792 (2023).
Li, J.-W., Lee, J. C.-M., Chuang, K.-C. & Chiu, C.-W. Photocured, highly flexible, and stretchable 3D-printed graphene/polymer nanocomposites for electrocardiography and electromyography smart clothing. Prog. Org. Coat. 176, 107378 (2023).
Gopinath, S. C. & Ramli, M. M. Hybrid-Nanomaterials: Fabrication, Characterization and Applications (Springer Nature, 2024).
Vargas-Bernal, R. in Hybrid Nanomaterials - Flexible Electronics Materials (eds Vargas-Bernal, R., He, P., & Zhang, S.) (IntechOpen, 2020).
Mahato, K. et al. Hybrid multimodal wearable sensors for comprehensive health monitoring. Nat. Electron. 7, 735–750 (2024).
Ryu, W. M., Lee, Y., Son, Y., Park, G. & Park, S. Thermally drawn multi-material fibers based on polymer nanocomposite for continuous temperature sensing. Adv. Fiber Mater. 5, 1712–1724 (2023).
Li, T. et al. An integrated and conductive hydrogel-paper patch for simultaneous sensing of chemical-electrophysiological signals. Biosens. Bioelectron. 198, 113855 (2022).
Zhang, S. et al. On-skin ultrathin and stretchable multifunctional sensor for smart healthcare wearables. npj Flex. Electron. 6, 11 (2022).
Shirzaei Sani, E. et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 9, eadf7388 (2023).
Deng, Y. et al. A soft thermal sensor for the continuous assessment of flow in vascular access. Nat. Commun. 16, 38 (2025).
Pan, S. et al. Mechanically interlocked hydrogel–elastomer hybrids for on-skin electronics. Adv. Funct. Mater. 30, 1909540 (2020).
Chaudhary, S., Agarwal, A., Mishra, D. & Shah, S. A review on green communication for wearable and implantable wireless body area networks. Comput. Netw. 252, 110693 (2024).
Waly, M. I. et al. Optimization of a compact wearable LoRa patch antenna for vital sign monitoring in wban medical applications using machine learning. IEEE Access 12, 103860–103879 (2024).
Cong, C. et al. Self-powered strain sensing devices with wireless transmission: DIW-printed conductive hydrogel electrodes featuring stretchable and self-healing properties. J. Colloid Interface Sci. 678, 588–598 (2025).
Ma, X. et al. A monolithically integrated in-textile wristband for wireless epidermal biosensing. Sci. Adv. 9, eadj2763 (2023).
Kim, T. et al. Spider-inspired tunable mechanosensor for biomedical applications. npj Flex. Electron. 7, 12 (2023).
Rauf, S. et al. Fully screen-printed and gentle-to-skin wet ECG electrodes with compact wireless readout for cardiac diagnosis and remote monitoring. ACS Nano 18, 10074–10087 (2024).
Zhang, Z. et al. Thermoresponsive dynamic wet-adhesive epidermal interface for motion-robust multiplexed sweat biosensing. Biosens. Bioelectron. 290, 117949 (2025).
del Bosque, A. et al. Highly flexible strain sensors based on CNT-reinforced ecoflex silicone rubber for wireless facemask breathing monitoring via bluetooth. ACS Appl. Polym. Mater. 5, 8589–8599 (2023).
Xiao, G. et al. Weavable yarn-shaped supercapacitor in sweat-activated self-charging power textile for wireless sweat biosensing. Biosens. Bioelectron. 235, 115389 (2023).
Herbert, R., Lim, H.-R., Rigo, B. & Yeo, W.-H. Fully implantable wireless batteryless vascular electronics with printed soft sensors for multiplex sensing of hemodynamics. Sci. Adv. 8, eabm1175 (2022).
Yeh, C.-C., Lo, S.-H., Xu, M.-X. & Yang, Y.-J. Fabrication of a flexible wireless pressure sensor for intravascular blood pressure monitoring. Microelectron. Eng. 213, 55–61 (2019).
Stauffer, F. et al. Soft electronic strain sensor with chipless wireless readout: toward real-time monitoring of bladder volume. Adv. Mater. Technol. 3, 1800031 (2018).
Acknowledgements
This research was supported by the Global Research Development Center (GRDC) Cooperative Hub Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (MSIT) (RS-2023-00257595) and baseline fund by King Abdullah University of Science and Technology (KAUST).
Author information
Authors and Affiliations
Contributions
N.Q.: Writing – review & editing, Writing – original draft, Conceptualization. T.S.: Writing – review & editing, Writing – original draft, Conceptualization. F.V.: Writing – Original draft, Writing – review & editing, Conceptualization. B.H.: Writing – review & editing, Conceptualization, Funding acquisition. N.E.: Writing – review & editing, Funding acquisition, Conceptualization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qaiser, N., Suryaprabha, T., Vetrano, F. et al. Mechanics and bio-interface engineering in flexible biosensors for continuous health monitoring. npj Flex Electron 10, 23 (2026). https://doi.org/10.1038/s41528-025-00524-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41528-025-00524-2