Abstract
Excessive fructose consumption is strongly linked to metabolic syndrome, with gut microbiota playing a pivotal role in mediating fructose metabolism and associated metabolic disturbances. In this study, we aimed to characterize Apilactobacillus (A.) kunkeei, a fructophilic lactic acid bacterium from honey, and evaluate its probiotic function in male C57BL/6 J mice fed a high-fructose diet (HFD). Transcriptome analysis was carried out to analyze the activation of pathways under various culture conditions. Pathway inhibitors were used in cell culture and a hepatectomy mouse model to study the function of different pathways in hepatocyte growth and liver regeneration. Our results showed that A. kunkeei FM01 exhibited strong tolerance to simulated gastrointestinal stress in vitro, indicating good probiotic potential. Administration of A. kunkeei FM01 significantly reduced body weight gain, improved glucose tolerance, and attenuated hepatic and visceral (perirenal and epididymal) lipid accumulation in HFD-fed mice. Serum lipid profiling and targeted lipidomic analysis revealed that A. kunkeei FM01 lowered triglycerides, phosphatidylcholine, and lysophosphatidylcholine levels while increasing beneficial phospholipids such as phosphatidylethanolamine. Metagenomic analysis demonstrated that A. kunkeei FM01 modulated gut microbiota composition by reducing pro-inflammatory and fructose-metabolizing taxa, including Alistipes, Oscillibacter, Desulfovibrio, Lawsonibacter, and Enterococcus, while enriching beneficial species, including Kineothrix alysoides and Faecalibaculum rodentium. These microbial shifts were associated with increased abundances in genes encoding carbohydrate-active enzymes and amino acid biosynthesis pathways. Furthermore, A. kunkeei FM01 restored intestinal barrier integrity by upregulating tight junction proteins (Zonula Occludens-1 and occludin) and reduced serum lipopolysaccharide and diamine oxidase levels. Collectively, these findings suggest that A. kunkeei FM01 exerts protective effects against HFD-induced metabolic dysfunction through multi-targeted mechanisms involving lipid metabolism, gut microbiota modulation, and intestinal barrier restoration. This study identifies A. kunkeei FM01 as a promising probiotic candidate for preventing and managing fructose-associated metabolic disorders.

Similar content being viewed by others
Introduction
Since the 1960s, high-fructose corn syrup has become a ubiquitous sweetener in beverages and processed foods, leading to a steady increase in per capita fructose consumption worldwide. The sweetness of fructose is 1.7 times that of sucrose; the metabolism of fructose is not regulated by insulin and does not impose a substantial burden on the pancreas. Therefore, it is added to manufactured food products. However, Excessive dietary fructose intake has been strongly associated with the rising prevalence of metabolic syndrome, including conditions such as non-alcoholic fatty liver disease, obesity, type 2 diabetes, and cardiovascular disease1,2,3.
The liver has been considered the primary site of fructose metabolism. However, a previous study has revealed that fructose metabolism begins at the small intestine, which serves as the main site of dietary fructose clearance. Fructose is transported into intestinal epithelial cells via the GLUT5 transporter and phosphorylated by ketohexokinase to form fructose 1-phosphate, which is then metabolized into glucose, lactate, and other intermediates. At low doses, fructose is almost entirely metabolized within the small intestine; however, when intake exceeds ~1 g/kg body weight, the absorptive and metabolic capacity of the intestine becomes saturated, allowing excess fructose to reach both the liver and colon.
In the liver, fructose mainly enters liver cells through the GLUT2 transporter, catalyzed into fructose-1-phosphate by ketohexokinase-C, that is subsequently cleaved by the rate limiting enzyme aldolase B to glyceraldehyde and dihydroxyacetone phosphate, glyceraldehyde undergoes a series of subsequent metabolic conversions to form pyruvate, from which it can be converted to lactate, undergoes oxidative metabolism via the tricarboxylic acid cycle, or feed de novo lipogenesis after the generation of acetyl and malonyl coenzyme A, finally generating triacylglyceride, contributes to de novo lipogenesis, and elevates triglyceride levels, promoting hepatic steatosis and dyslipidemia4. In the colon, unabsorbed fructose is fermented by gut microbiota into glycerates and organic acids, which can alter microbial composition and function5. Several studies have demonstrated that high-fructose intake significantly impacts gut microbiota diversity and host health. For instance, daily consumption of 100 g of fructose for one week has been shown to reduce gut microbial diversity and decrease the abundance of butyrate-producing genera such as Faecalibacterium and Parabacteroides, while increasing the relative abundance of Veillonella6. Short-term high-fructose diets (HFD) also lead to reduced lactobacilli populations and elevated levels of pro-inflammatory metabolites, including arachidonic acid, stearic acid, and indole-sulfuric acid7. Administration of fructose to piglets resulted in a significant increase in the abundance of Streptococcus, Blautia, and Clostridium, along with reduced expression of tight junction genes. However, no notable alterations were observed in growth performance, antioxidant resistance, or inflammatory responses under these conditions8. Moreover, fructose-induced changes in the gut microbiota are linked to increased intestinal permeability and inflammation. In mice, fructose consumption resulted in altered expression of tight junction proteins and heightened intestinal permeability9. Long-term exposure (12 weeks) to an HFD led to significant increases in plasma cholesterol and total bile acid concentrations, along with shifts in microbial taxa such as Dehalobacterium, Magibacteriaceae, and Akkermansia10. These findings underscore the critical role of gut microbiota in mediating the effects of fructose on intestinal barrier integrity and systemic metabolism.
Recently, a unique group of lactic acid bacteria known as fructophilic lactic acid bacteria (FLAB) has garnered attention due to its preference for fructose over glucose as a growth substrate. This group comprises only a few species from the genus Fructobacillus and certain species formerly classified under Lactobacillus (now reclassified in Apilactobacillus)11. Fructophilic lactic acid bacteria are predominantly found in fructose-rich environments such as wine, fruits, fermented fruit products, honey, and bee-related niches12,13. Unlike typical heterofermentative lactic acid bacteria, FLAB produce mainly lactic acid and mannitol rather than ethanol during fermentation. Additionally, FLAB exhibits strong self-aggregation and hydrophobic properties, which may enhance their survival and colonization potential in the gastrointestinal tract14.
Of particular interest is Apilactobacillus (A.) kunkeei, a prominent member of the FLAB group. A. kunkeei isolates grew poorly on glucose, but grew well on fructose, and displayed higher fructose consumption rate compared to conventional lactic acid bacteria, even Fructophilic Fructobacillus fructosus15. Due to its robust ability to metabolize fructose, making it relevant for managing fructose-associated disorders such as irritable bowel syndrome in grain-based diets16. Additionally, A. kunkeei during simulated gastrointestinal transit, detecting a similar if not a better performance than that showed by Lacticaseibacillus rhamnosus GG17. A. kunkeei displays a broad spectrum of inhibition pathogens, such as Pseudomonas aeruginosa and Enterococcus faecalis16. Owing to these characteristics, A. kunkeei has been proposed as a promising candidate for improving human health. However, most previous research investigating its probiotic potential has been limited to in vitro settings18.
Given this background, this study aimed to evaluate the probiotic properties of A. kunkeei FM01, a strain isolated from honey, and confirm its identity as FLAB. We further sought to determine whether A. kunkeei FM01 exerts protective effects against HFD-induced lipid accumulation in mice and to elucidate the underlying mechanisms. By doing so, we aimed to establish its potential as a novel probiotic candidate tailored for individuals consuming HFD.
Results
Fructophilic characteristics and in vitro probiotic potential of Apilactobacillus kunkeei FM01
Apilactobacillus kunkeei FM01 is a fructose-metabolizing strain originally isolated from honey in China. Its entire genome circle diagram was provided in the supplementary materials (Fig. S1). Following isolation and screening, its fructophilic nature and potential probiotic properties were evaluated in vitro. Results are summarized in Fig. 1. The growth profile of kunkeei FM01 demonstrated a strong preference for fructose. The strain exhibited robust growth in FYP broth but showed minimal growth in glucose-based broth (GYP). However, when supplemented with pyruvate as an external electron acceptor (GYP-P broth), the strain grew efficiently. These findings confirm that A. kunkeei FM01 is an obligate FLAB, relying on exogenous electron acceptors for effective glucose metabolism11.
A Growth characteristics of A. kunkeei FM01 in fructose yeast peptone broth, glucose yeast peptone broth, and glucose yeast peptone broth supplemented with pyruvate. B Survival rates of A. kunkeei FM01 under simulated gastrointestinal conditions exposed to pepsin and pancreatin. C Acid tolerance of A. kunkeei FM01 at different pH levels. D Bile salt resistance of A. kunkeei FM01 in media containing 0.15% and 0.30% bile salts. Data are presented as the mean ± SEM (n = 6). * P < 0.05, ** P < 0.01.
To assess its probiotic potential, several gastrointestinal stress tolerance assays were conducted. Survival in simulated gastric and intestinal conditions is a key attribute of probiotic strains. Viable cell counts revealed that over 85% of A. kunkeei FM01 cells remained viable after incubation with pepsin and pancreatin for 4 h, demonstrating strong resistance to digestive enzymes. Acid tolerance tests showed that A. kunkeei FM01 was able to survive under a range of acidic conditions (pH 2.0, 3.0, and 4.0). Although survival rates decreased with lower pH and longer exposure time, more than 60% of the cells remained viable after 2-h incubation at pH 2.0. At pH 4.0, survival rates were significantly higher than at pH 3.0 (P < 0.05). While no significant differences were observed between pH 2.0 and 3.0 at either 2 or 4 h, these results collectively indicate that the strain possesses notable acid resistance, a relatively rare trait among FLAB, as previously reported18. Bile salt tolerance is another critical factor for probiotic functionality in the gut. Apilactobacillus kunkeei FM01 showed good tolerance to 0.15% bile salts. However, increased bile salt concentration (0.30%) led to a 50% reduction in viability after 4 h, indicating moderate bile sensitivity at higher concentrations. Overall, A. kunkeei FM01 demonstrated a robust survival rate in simulated gastrointestinal conditions, including resistance to low pH, moderate pepsin and pancreatin, and bile salt concentrations, supporting its potential as a probiotic candidate for use in HFD.
Apilactobacillus kunkeei FM01 reduced body weight gain and improved glucose homeostasis in HFD-fed mice
To evaluate the impact of A. kunkeei FM01 on metabolic responses to long-term high-fructose intake, male C57BL/6 J mice were randomly assigned to one of the three experimental groups: NC (standard diet), HFD, and FLAB (HFD supplemented with A. kunkeei FM01) (Fig. 2A). Significant differences in body weight were observed across the groups over the 12-week experimental period (Fig. 2B). Mice fed an HFD exhibited significantly reduced feed intake and fecal output compared to those in the control group throughout the study (P < 0.01). Administration of A. kunkeei FM01 did not restore feed intake or fecal output in HFD-fed mice during any week of the trial (P < 0.01) (Fig. 2C, D). However, despite lower food consumption, HFD-fed mice gained significantly more body weight than control animals (P < 0.001). Notably, supplementation with A. kunkeei FM01 resulted in a substantial reduction in body weight gain compared to the HFD group (Fig. 2E).
A Experimental design schematic. B Representative images of mice from three groups: control, high-fructose diet (HFD), and FLAB (HFD with A. kunkeei FM01 intervention). C Weekly food intake and D fecal output across experimental groups. E Body weight changes over the 12 weeks of dietary intervention. F Fasting blood glucose levels. G Blood glucose levels during the oral glucose tolerance test at week 12th. Data are presented as mean ± SEM (n = 6). * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
In terms of glucose metabolism, mice on the HFD showed elevated fasting blood glucose levels between weeks 4 and 8 of the trial (P < 0.05). In contrast, administration of A. kunkeei FM01 significantly lowered fasting blood glucose levels in HFD-fed mice (Fig. 5F). In addition, during the oral glucose tolerance test, the blood glucose level reached its peak approximately 30 min after intragastric administration of glucose; the FLAB and control groups exhibited significantly lower blood glucose levels than the HFD group after 60 min (P < 0.05; Fig. 5G), indicating A. kunkeei FM01 improved glucose homeostasis. Collectively, these findings suggest that A. kunkeei FM01 helps maintain glucose stability and significantly reduces both fasting blood glucose levels and body weight gain in mice consuming an HFD.
Apilactobacillus kunkeei FM01 reduced lipid accumulation in HFD-fed mice
To evaluate the effect of A. kunkeei FM01 on body fat deposition, visceral adipose tissues were analyzed. Mice fed an HFD exhibited significantly increased masses of both kidney-associated perirenal and epididymal white adipose tissues compared to those on a normal control diet (Fig. 3A, B). Administration of A. kunkeei FM01 led to a significant reduction in the mass of both types of adipose tissue (P < 0.01; Fig. 3C, D). In addition to adipose tissue changes, hepatic lipid metabolism was assessed, as the liver is the central organ for lipid homeostasis and is highly responsive to high-fructose intake19. In this study, HFD-fed mice showed a marked increase in liver weight (P < 0.01), which was significantly attenuated by supplementation with A. kunkeei FM01 (P < 0.05; Fig. 3E, G). Interestingly, splenomegaly was observed in mice consuming the HFD, suggesting systemic metabolic or inflammatory effects. However, treatment with A. kunkeei FM01 effectively restored spleen weight to levels comparable to those in the control group (Fig. 3F). Histological analysis using oil red O staining revealed extensive hepatic lipid accumulation in HFD-fed mice, characterized by abundant lipid droplet formation (Fig. 3G). In contrast, mice supplemented with A. kunkeei FM01 exhibited markedly reduced hepatic steatosis (Fig. 3G). Further biochemical analysis demonstrated that TG and TC levels in the liver were significantly elevated in the HFD group. Notably, A. kunkeei FM01 administration led to a substantial decrease in hepatic TG levels (P < 0.001), nearly restoring them to control group levels.
Representative images of (A) kidneys and (B) epididymis fat pads from mice in the control, high-fructose diet (HFD) and FLAB groups (HFD with A. kunkeei FM01 intervention). Differences in the weight of (C) kidney, (D) epididymis fat, (E) liver, and (F) spleen across experimental groups. G Representative liver images and Oil Red O-stained liver sections showing hepatic lipid accumulation in mice from different groups. H Hepatic triglyceride (TG) levels and (I) total cholesterol (TC) levels. Serum lipid profiles, including (J) TG, (K) TC, (L) low-density lipoprotein cholesterol (LDL-C), and (M) high-density lipoprotein cholesterol (HDL-C). Data are presented as mean ± SEM (n = 6). * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
Serum lipid profiles were also evaluated as key indicators of lipid metabolism. Compared to the control group, mice in the HFD group had significantly higher serum TC, HDL-C, and LDL-C levels (P < 0.001). No significant difference in serum TG levels was observed between the control and HFD groups (P > 0.05). However, A. kunkeei FM01 supplementation significantly lowered serum TC (P < 0.01) and LDL-C levels (P < 0.05) in HFD-fed mice (Fig. 3J–M). Collectively, these findings demonstrate that A. kunkeei FM01 effectively mitigates fructose-induced lipid accumulation in both hepatic and adipose tissues, and improves lipid metabolic profiles in mice.
Apilactobacillus kunkeei FM01 modulated serum lipid metabolism in HFD-fed mice
To further investigate the impact of A. kunkeei FM01 on lipid metabolism, a widely targeted serum lipidomic analysis was conducted using LC-ESI-MS/MS. After excluding two hemolyzed samples, five mice per group were selected to ensure sample homogeneity across experimental conditions. The detected lipid metabolites were classified into various subclasses, with their relative proportions and counts summarized in Fig. 4A, B. Principal component analysis was employed to assess overall differences in serum lipid metabolic profiles across the three groups. The principal component analysis score plot revealed a distinct clustering pattern of the control group from the HDF and FLAB groups, with the latter two displaying partially overlapping yet largely distinct clusters. This suggests that A. kunkeei FM01 modulates the lipidomic alterations induced by high-fructose consumption (Fig. 4C).
A Proportions of major lipid subclasses identified in serum samples. B Detected lipid metabolite counts within each subclass. C Principal component analysis (PCA) of serum lipid metabolic profiles across control, high-fructose diet (HFD), and FLAB groups (HFD with A. kunkeei FM01 intervention). D Volcano plot showing differentially regulated lipid metabolites between the HFD and control groups; red denotes upregulated metabolites, green represents downregulated metabolites, and gray indicates non-significant changes. E Heatmap displaying differential lipid metabolites between the FLAB and HFD groups. F Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of differentially regulated lipid metabolites between the FLAB and HFD groups.
A total of 460 lipid metabolites showed significant differences between the HFD and control groups based on the following criteria: variable importance in projection score > 1, P < 0.05, and fold change > 1 or FC < 0.8. Among these, 124 metabolites increased in the HFD group, mainly including lysophosphatidylcholine, phosphatidylcholine (PC), and TG. In contrast, 336 metabolites decreased, such as phosphatidyl ethanolamine (PE), phosphatidylglycerol, and phosphatidylinositol (Fig. 4D), indicating that the high fructose diet resulted in lipid imbalance. Notably, A. kunkeei FM01 administration significantly reduced several lipid species elevated by the HFD (Fig. 4E). These included specific TGs such as TG (16:0_18:1_22:4) and TG (18:2_20:3_20:4), phospholipids such as PC (18:0_20:4) and PC (19:0_20:4), and free fatty acids (FFAs) including FFA (16:1) and FFA (22:4) (Fig. 4D, E). To explore the functional implications of these changes, KEGG pathway enrichment analysis was conducted on the differentially regulated lipid metabolites. The results indicated that these metabolites were significantly enriched in pathways related to fat digestion and absorption, cholesterol metabolism, glycerophospholipid metabolism, glycerolipid metabolism, and Lipid and atherosclerosis (Fig. 4F). These findings demonstrate that A. kunkeei FM0l influences serum lipid metabolism in HFD-fed mice, potentially contributing to its protective effects against fructose-induced metabolic disturbances.
Apilactobacillus kunkeei FM01 restored intestinal barrier integrity and reduced inflammation in HFD-fed mice
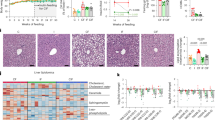
To assess the impact of HFD feeding and the potential protective effects of A. kunkeei FM01, histological and molecular analyses were conducted on intestinal tissues. Histopathological examination via hematoxylin and eosin staining revealed significant structural damage in the intestines of HFD-fed mice, including mucosal erosion or ulceration and submucosal infiltration of inflammatory cells, hallmarks of intestinal inflammation. In contrast, A. kunkeei FM01 supplementation markedly attenuated these pathological changes, with only mild inflammatory cell infiltration observed in the FLAB group (Fig. 5A). Intestinal morphology analysis showed that the HFD significantly increased villus length (P < 0.01), which was attenuated by A. kunkeei FM01 administration. However, no consistent changes were observed in jejunum or colon wall thickness across groups (Fig. 5B). Tight junction proteins, including ZO-1 and occludin, are essential for maintaining intestinal barrier integrity. Both protein and mRNA levels of these markers were significantly reduced in the clone of HFD-fed mice compared to controls. Notably, A. kunkeei FM01 intervention restored their expression in the FLAB group (Fig. 5C, D), indicating its direct role in preserving epithelial barrier function.
A Representative hematoxylin and eosin-stained intestinal tissue sections from control, high-fructose diet (HFD), and FLAB groups (HFD with A. kunkeei FM01 intervention). B Intestinal wall thickness and villus length measurements across experimental groups. C Expression levels of tight junction proteins Zonula Occludens-1 (ZO-1) and occludin in the colon, as determined by western blot. D Relative mRNA expression of ZO-1 and occludin in the colon. E Serum levels of inflammatory cytokines. Data are presented as the mean ± SEM (n = 6). * P < 0.05, ** P < 0.01.
Serum biomarkers further supported this conclusion. Levels of DAO and LPS, biomarkers of intestinal permeability and endotoxemia, were significantly elevated in the HFD group (P < 0.01 for both). These increases were markedly suppressed in the FLAB group following A. kunkeei FM01 administration (P < 0.01; Fig. 5E). In addition, serum concentrations of pro-inflammatory cytokines TNF-α and IL-6 were significantly higher in HFD-fed mice compared to controls (P < 0.01 for both), but both were significantly reduced after A. kunkeei FM01 intervention (Fig. 5E). Collectively, these findings demonstrate that A. kunkeei FM01 protects against HFD-induced intestinal barrier dysfunction and inflammation, supporting its role as a gut barrier-modulating probiotic in the context of high-fructose consumption.
Apilactobacillus kunkeei FM01 modulated gut microbiota in HFD-fed mice
To investigate whether the gut microbiota was involved in the observed metabolic improvements following A. kunkeei FM01 administration, we performed high-throughput metagenomic sequencing of fecal samples collected at week 12 from all experimental groups. Alpha diversity analysis, based on the Shannon and Chao indices (Fig. 6A, B), revealed that the HFD significantly increased microbial diversity compared to the control group (P < 0.05). However, supplementation with A. kunkeei FM01 did not significantly alter α-diversity as measured by the Shannon index (P > 0.05). Similarly, no significant changes in species richness were observed based on the Chao1 index across groups. Non-metric multidimensional scaling analysis showed distinct clustering patterns among the three groups (Fig. 6C). The control group exhibited a clear separation from the HFD and FLAB groups, indicating a shift in gut microbial composition induced by high-fructose feeding. Moreover, the FLAB group formed a separate cluster from the HFD group, suggesting that A. kunkeei FM01 modulated the gut microbiota structure in HFD-fed mice.
A Shannon index and B Chao 1 index of gut microbiota across control, high-fructose diet (HFD), and FLAB groups (HFD with A. kunkeei FM01 intervention). C Non-metric multidimensional scaling (NMDS) plot illustrating differences in gut microbial community structure among the three groups. D Genus-level gut microbiota composition in the three experimental groups. E Heatmap showing the relative abundance of gut microbiota across the three groups. F Differential abundance analysis of specific bacterial species across experimental groups. Data are presented as mean ± SEM (n = 6). * P < 0.05, ** P < 0.01, *** P < 0.001.
At the genus level, high-fructose feeding altered the relative abundances of several key taxa (Fig. 6D). Specifically, the relative abundances of Bacteroides, Prevotella, Duncaniella, and Muribaculum were reduced in both the HFD and FLAB groups compared to the control group. In contrast, the abundances of Faecalibacterium, Desulfovibrio, Oscillibacter, Kineothrix, Eubacterium, Dorea, and Lawsonibacter were elevated in response to high-fructose intake. Notably, A. kunkeei FM01 administration significantly increased the abundance of beneficial genera such as Faecalibacterium and Kineothrix, while decreasing potentially harmful taxa, including Desulfovibrio, Oscillibacter, Alistipes, and Lawsonibacter in HFD-fed mice compared to those without FLAB administration (Fig. 6E). In addition, A. kunkeei FM01 effectively suppressed the fructose-induced increase in Enterococcus to levels comparable to those in the control group (Fig. 6E).
At the species level, A. kunkeei FM01 significantly altered the gut microbiota composition (Fig. 6F). The treatment further enriched Faecalibaculum rodentium (P < 0.05), Kineothrix alysoides (P < 0.01), and Kineothrix sp. (P < 0.05), which had already increased under HFD. Conversely, the HFD led to elevated levels of Alistipes timonensis, Lawsonibacter sp., Desulfovibrio sp., and Oscillibacter sp., all of which were markedly reduced following A. kunkeei FM01 administration (P < 0.05). The relative abundance of Duncaniella muris and Heminiphilus faecis decreased significantly in the HFD group (P < 0.001), but showed a slight increase in the FLAB group (P = 0.075), although not statistically significant. Furthermore, three Enterococcus species, E. hirae (P < 0.05), E. gallinarum (P < 0.001), and Enterococcus sp. (P < 0.05), were significantly enriched in the HFD group compared to controls, but their levels were markedly reduced in the FLAB group after A. kunkeei FM01 intervention (Fig. 6F). Meanwhile, A. kunkeei was only detected in the FLAB group (The average relative abundance was 0.12% ± 0.01), while it was not detected in the control group and the HFD group, which indicated that A. kunkeei FM01 reached the colon (Fig. S2). These findings indicate that A. kunkeei FM01 significantly modulates the gut microbiota composition in HFD-fed mice, promoting a more balanced intestinal microbial profile and potentially contributing to its beneficial metabolic effects.
Correlation analysis reveals key bacterial taxa associated with lipid metabolism
To identify potential bacterial mediators underlying Apilactobacillus kunkeei FM01-induced improvements in lipid metabolism, spearman correlation analysis was performed between differentially regulated lipid metabolites and the top 50 most abundant gut microbial taxa identified in mice. Several bacterial species that were enriched in the HFD group and reduced by A. kunkeei FM01 treatment exhibited strong positive correlations with multiple lipid metabolites associated with metabolic dysregulation (Fig. 7A). These included Alistipes timonensis, Lawsonibacter sp., Desulfovibrio sp., Enterococcus hirae, Enterococcus gallinarum, Oscillibacter sp., and Alistipes sp., which showed significant positive associations with the enriched lipid metabolites in the HFD group, such as FFA (16:1), FFA (22:4), PC(16:0_20:4), PC(18:0_20:4), PC(19:0_20:4), and TG(18:2_20:3_20:4). In contrast, most of the identified differential lipid metabolites were negatively correlated with the relative abundances of beneficial genera such as Ligilactobacillus, Prevotella sp., and Bacteroides sp. Notably, taxa such as Kineothrix alysoides, Kineothrix sp., and Faecalibaculum rodentium, which increased significantly following A. kunkeei FM01 administration showed negative correlations with lipid species elevated in the HFD group, including TG (16:0_18:0_18:2), TG (16:0_16:0_22:6), and 9,10-EpOME (an epoxide fatty acid involved in cellular processes and signaling). These results suggest that A. kunkeei FM01 may exert its lipid-lowering effects primarily by reducing the abundance of pro-inflammatory or dysbiotic taxa (Alistipes timonensis, Lawsonibacter sp., Desulfovibrio sp., Enterococcus hirae, Oscillibacter sp. and Alistipes sp.), while promoting the growth of potentially beneficial species such as Kineothrix alysoides and Faecalibaculum rodentium.
A Spearman correlation network showing significant associations between gut microbial taxa and differentially regulated lipid metabolites. Green circles represent bacterial genera, and purple diamonds represent lipid metabolites. Lines connect significant correlations: black lines denote positive correlations, and red lines represent negative correlations. Line color reflects correlation strength based on Spearman’s rho values, ranging from −0.92 (strongly negative correlation) to 0.94 (strongly positive correlation). B Differences in carbohydrate-active enzyme (CAZyme) families and C Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways among the control, high-fructose diet (HFD), and FLAB groups (HFD with Apilactobacillus kunkeei FM01 intervention), as determined by linear discriminant analysis (LDA) effect size (LEfSe) analysis (LDA score > 3).
Functional profiling of gut microbiota via CAZy and KEGG pathway analysis
To further explore the functional implications of A. kunkeei FM01-mediated microbiota modulation, metagenomic data were analyzed by mapping microbial gene content to the CAZy database and the KEGG pathway database. Twenty-nine CAZyme families with significantly different abundances were identified among the three groups. The control and FLAB groups showed higher abundances of genes encoding glycoside hydrolases, glycosyltransferases, and carbohydrate esterases, indicating enhanced carbohydrate metabolism and mucin degradation capacity in these groups (Fig. 7B).
Additionally, 17 KEGG pathways were found to be differentially represented among the groups. The HFD group was enriched in pathways related to flagellar assembly, quorum sensing, and ribosome, which are often associated with pathogenic or stress-related microbial activity. In contrast, the FLAB group showed enrichment in pathways involved in phosphotransferase system, biosynthesis of amino acids, pentose phosphate pathway, ABC transporters, two-component system, and bacterial chemotaxis, suggesting improved nutrient utilization and microbial homeostasis. The control group was enriched in metabolic pathways, biosynthesis of secondary metabolites, and biosynthesis of cofactors (Fig. 7C). Correlation analysis between A. kunkeei FM01-regulated bacterial taxa and these functional modules revealed significant associations between specific bacteria and both CAZyme profiles and KEGG pathways (Fig. S3), highlighting the contribution of these taxa to the observed functional shifts in the gut microbiome.
Discussion
High-fructose consumption has emerged as a major dietary driver of metabolic diseases, including obesity, insulin resistance, and non-alcoholic fatty liver disease20. Excessive fructose intake not only stimulates hepatic de novo lipogenesis but also disrupts gut microbial homeostasis and intestinal barrier function, promoting systemic inflammation and endotoxemia9. Fructophilic lactic acid bacteria, which preferentially metabolize fructose, represent a promising yet underexplored group of probiotics that may help mitigate these adverse effects. However, most studies to date have been limited to in vitro settings21. In this study, we demonstrated, for the first time in vivo, that A. kunkeei FM01, a FLAB strain isolated from honey, exerts significant protective effects against HFD-induced lipid accumulation and gut dysbiosis in mice.
Apilactobacillus kunkeei FM01 exhibited robust growth in fructose-rich media and showed excellent survival under simulated gastrointestinal conditions ( > 85% viability after exposure to digestive enzymes and low pH). These findings align with previous research showing that A. kunkeei strains outperform established probiotic controls like Lacticaseibacillus rhamnosus GG under similar stressors16, supporting its potential for in vivo application.
Our data revealed that excessive fructose intake significantly increased body weight and fasting blood glucose levels despite reduced food intake, consistent with previous observations9,22. Notably, A. kunkeei FM01 had a significant effect on lowering serum glucose peak value at 60 min compared to the HFD group, indicating that A. kunkeei FM01 supplementation effectively controlled fasting blood glucose levels, improved glucose tolerance, and attenuated weight gain in HFD-fed mice. Given that fructose stimulates hepatic de novo lipogenesis and increases endogenous TG production20, it is not surprising that chronic fructose consumption led to hepatic fat accumulation23. Importantly, our results showed that A. kunkeei FM01 significantly reduced lipid deposition in visceral adipose tissues, including perirenal and epididymal fat, as well as in the liver. Furthermore, the strain significantly decreased hepatic TG levels and serum TC and LDL-C levels in HFD-fed mice. These findings are in line with previous reports showing the lipid-lowering effect of other probiotics such as Levilactobacillus brevis24. It should be noted that Levilactobacillus brevis has also been described as a fructophilic strain25.
To further explore the mechanisms underlying these beneficial effects, we conducted a targeted lipidomic analysis and found that an HFD significantly altered serum lipid profiles. Specifically, TGs, PC, and lysophosphatidylcholine were elevated, whereas PE, phosphatidylglycerol, and phosphatidylinositol were reduced. The increased levels of TG observed in HFD-fed mice corroborated the ELISA results. Lysophosphatidylcholine has been implicated in inflammatory responses, including lymphocyte and macrophage recruitment, pro-inflammatory cytokine release, and induction of oxidative stress and apoptosis26. Moreover, PC and PE are major components of mammalian cell membranes, with the PC/PE ratio as a critical indicator of hepatic health27. The observed imbalance in PC/PE, along with decreases in phosphatidylglycerol and phosphatidylinositol, both important for cellular signaling and membrane integrity28, further suggests that fructose-induced lipid dysregulation contributes to hepatic dysfunction. Notably, A. kunkeei FM01 reversed many of these changes by reducing levels of FFAs, PC, and TGs, thus regulating pathways related to fat digestion and absorption, cholesterol metabolism, glycerophospholipid metabolism, and glycerolipid metabolism. These findings provide mechanistic insights into the protective effects of A. kunkeei FM01 against fructose-induced lipid accumulation in tissues and organs.
We further investigate how A. kunkeei FM01 influences these metabolic pathways by analyzing gut microbiota composition using shotgun metagenomic sequencing. Accumulating evidence supports the role of gut microbiota in mediating fructose metabolism and host metabolic outcomes29,30. While small amounts of fructose are metabolized in the small intestine, excessive intake overwhelms intestinal clearance capacity, allowing fructose to reach the colon, where it is further processed by gut microbes4. Our results found that A. kunkeei FM01 can reach the colon of the mice through intragastric administration, and 12 weeks of A. kunkeei FM01 administration altered the gut microbial community structure without affecting alpha-diversity in HFD-fed mice. Although dominant taxa such as Bacteroidetes and Muribaculum did not recover to control levels, several pathogenic genera, including Desulfovibrio and Enterococcus, were markedly reduced. Desulfovibrio is a sulfate-reducing bacterial genus linked to gut epithelial damage and contributes to gastrointestinal diseases31. Enterococcus species, such as E. hirae and E. gallinarum, are associated with vancomycin resistance and thrive under high-fructose conditions32. Notably, both genera were suppressed by A. kunkeei FM01 treatment. Conversely, beneficial taxa such as Faecalibacterium and Kineothrix, including Faecalibaculum rodentium and Kineothrix alysoides, were enriched. Faecalibaculum rodentium has been shown to negatively correlate with sulfate-reducing bacteria and is enriched in high-fat diet models33. Kineothrix alysoides is linked to improvements in lipid metabolism and liver function34. These shifts suggest that A. kunkeei FM01 exerts its protective effects partly through remodeling lipid-metabolism-related commensals. Additionally, administering A. kunkeei FM01 markedly decreased the abundance of Oscillibacter, Alistipes, and Lawsonibacter, which were enriched in HFD-fed mice. Oscillibacter and Alistipes are known to ferment fructose in the cecum32, while Lawsonibacter is commonly found in western populations35, and their decline may reflect competition with A. kunkeei for fructose utilization.
Correlation analysis showed that administration of A. kunkeei FM01 decreased significantly some bacteria, including Alistipes timonensis, Lawsonibacter sp., Desulfovibrio sp., Enterococcus hirae, Enterococcus gallinarum, Oscillibacter sp., and Alistipes sp., which showed positive associations with the enriched lipid metabolites in the HFD group. Meanwhile, the levels of Kineothrix alysoides, Kineothrix sp., and Faecalibaculum rodentium increased significantly following A. kunkeei FM01 administration, and these bacteria showed negative correlations with lipid species elevated in the HFD group. This further confirmed that A. kunkeei FM01 modulated lipid metabolism primarily by reducing Alistipes, Oscillibacter, Desulfovibrio, and Enterococcus, while increasing Kineothrix alysoides and Faecalibaculum rodentium. These microbial shifts were associated with enrichment in glycoside hydrolases and glycosyltransferases, as well as pathways involved in amino acid biosynthesis, pentose phosphate metabolism, and ABC transporters. Given that the genome of A. kunkeei encodes numerous genes related to amino acid transport and metabolism36 and unique glycosyltransferase genes37, these findings suggest that A. kunkeei FM01 may directly influence intestinal fructose metabolism, though further investigation is warranted.
Finally, we observed that high-fructose intake led to histological signs of intestinal inflammation, including mucosal erosion and submucosal infiltration, along with elevated serum LPS and DAO levels38, markers of increased gut permeability and endotoxemia. However, A. kunkeei FM01 administration significantly ameliorated these pathological changes and restored intestinal barrier integrity, as evidenced by the upregulation of tight junction proteins ZO-1 and occludin. These beneficial effects likely stem from A. kunkeei FM01-mediated modulation of the gut microbiota and its metabolism, along with reduced pro-inflammatory signaling in the context of high-fructose intake.
Based on our findings, we propose a working model in which A. kunkeei FM01 exerts its protective effects through multiple mechanisms: 1) Directly metabolizing fructose in the gut, reducing its availability to pathogenic microbes. 2) Suppressing the growth of harmful taxa such as Desulfovibrio, Enterococcus, and Alistipes. 3) Enriching beneficial commensals like Faecalibacterium and Kineothrix, which support lipid metabolism and gut barrier function. 4) To a certain extent, restoring intestinal tight junction expression and reducing systemic inflammation. These combined actions may contribute to reduced hepatic lipid accumulation and improved metabolic outcomes.
Despite these promising findings, our study has several limitations. First, we did not directly assess colonization dynamics or persistence of A. kunkeei FM01 in the gut, which limits our understanding of its long-term interaction with the host microbiota. Additionally, while we observed significant improvements in metabolic parameters, clinical translation will require validation in human populations consuming HFD. Moreover, although we identified shifts in gut microbiota composition and metabolic pathways following A. kunkeei FM01 administration, the specific host-microbe and microbe-microbe interactions underlying these effects remain unclear. Further mechanistic studies, such as gnotobiotic models or targeted metabolite profiling, are needed to elucidate the functional roles of microbial and host factors in mediating the beneficial effects of A. kunkeei FM01 under high-fructose conditions.
In conclusion, Apilactobacillus kunkeei FM01, a fructophilic lactic acid bacterium isolated from honey, demonstrates significant probiotic potential in mitigating HFD-induced metabolic disturbances in mice. The strain effectively modulates gut microbiota composition by reducing pro-inflammatory and fructose-metabolizing taxa such as Desulfovibrio, Enterococcus, Alistipes, Oscillibacter, and Lawsonibacter, while enriching beneficial genera like Faecalibacterium and Kineothrix. These microbial changes are associated with reduced hepatic and visceral lipid accumulation, improved serum lipid profiles, and favorable shifts in lipidomic signatures, including reductions in harmful phospholipids and triglycerides. Moreover, A. kunkeei FM01 enhances intestinal epithelial barrier integrity by upregulating tight junction proteins ZO-1 and occludin, leading to reduced serum LPS and DAO levels, biomarkers of endotoxemia and gut permeability. These effects contribute to decreased systemic inflammation, as indicated by lower TNF-α and IL-6 levels. Collectively, these findings suggest that A. kunkeei FM01 mitigates fructose-induced metabolic dysfunction through a multi-targeted mechanism involving the gut microbiota-gut barrier-liver axis. This study identifies A. kunkeei FM01 as a promising probiotic candidate for managing fructose-related metabolic disorders. Future studies should evaluate its translational potential in human populations and further elucidate host-microbe interactions underlying its beneficial effects.
Methods
Strain source and culture conditions
Apilactobacillus kunkeei FM01 was isolated from fructose-rich honey collected from a farm located in Beichuan County, Sichuan Province, China, and stored at the Guangdong Microbial Culture Collection Center in China (GDMCC No: 63946). The strain was routinely cultivated at 35 °C in fructose yeast peptone (FYP) broth or on FYP agar as described by Endo et al.11 Each liter of FYP broth composed of 10 g D-fructose, 10 g yeast extract, 5 g polypeptide, 2 g sodium acetate, 0.5 g Tween 80, 0.2 g MgSO4∙7H2O, 0.01 g MnSO4∙4H2O, 0.01 g FeSO4∙7H2O, 0.01 g NaCl, 0.05 g cycloheximide, and 0.05 g sodium azide. The medium was adjusted to pH 6.8 before sterilization.
Evaluation of fructophilic characteristics and probiotic potential
A culture of A. kunkeei FM01 was grown in FYP broth for 24 h at 30 °C under aerobic conditions, after which the cells were harvested by centrifugation and resuspended in 0.1% (w/v) peptone water. The growth characteristics of the strain were evaluated in three different media: FYP broth, glucose yeast peptone (GYP) broth (containing 10 g/L of D-glucose instead of D-fructose), and GYP-P broth (GYP broth supplemented with 0.5% pyruvate as an external electron acceptor). For each condition, 200 μL of medium was dispensed into microplate wells in triplicate and inoculated with 30 µL of the bacterial suspension. Growth was monitored by measuring optical density at 600 nm (OD600 nm) every 2 h over a 24 h period using a spectrophotometer (Genesys 10, Thermo Fisher Scientific, Waltham, MA, USA).
To assess acid tolerance, overnight cultures were centrifuged at 5000 × g for 10 min and washed twice with phosphate-buffered saline (pH 7.4). The cell pellet was resuspended in phosphate-buffered saline (PBS) to a final concentration of 9 × 108 CFU/mL. Subsequently, 0.5 mL of the bacterial suspension was added to FYP broth adjusted to pH 2.0, 3.0, and 4.0, vortexed, and incubated at 35 °C for 2 h or 4 h. Absorbance was measured at OD600 nm, and survival rates were calculated relative to cultures maintained in FYP broth at pH 7.039.
For bile salt tolerance testing, the cell-free supernatant was obtained following the same washing procedure used in the acid tolerance assay. A volume of 0.5 mL of the bacterial suspension was inoculated into FYP broth containing either 0.15%, and 0.30% bile salts, mixed thoroughly, and incubated at 35 °C for 2 h or 4 h. Absorbance was recorded at OD₆₀₀ nm, and survival rates were determined using cultures without bile salts as the control40. To evaluate resistance to digestive enzymes, 0.5 mL of bacterial suspension (pH = 3 for pepsin and pH = 8 for pancreatin) was inoculated into FYP broth containing 1 mg/mL pepsin or pancreatin (Shanghai Yeyuan Biotechnology Co., LTD, Shanghai, China), respectively. The mixtures were incubated at 35°C for 4 h, after which absorbance was measured at OD600 nm. Cultures without pepsin and pancreatin served as controls for calculating survival rates41.
Animal study and sample collection
All animal procedures were approved by the Laboratory Animal Welfare and Ethics Committee of Sichuan Normal University, complied with the ARRIVE guidelines, and were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). Eighteen male C57BL/6 J mice (six weeks old) were purchased from Chengdu Senwell Experimental Animals Co., Ltd. (Chengdu, China). Following a one-week acclimatization period, the animals were randomly assigned to three experimental groups (n = 6 per group): normal control group (NC; standard diet), HFD group (20% fructose diet), and A. kunkeei FM01 intervention group (FLAB; 20% fructose diet with A. kunkeei FM01 supplementation). The FLAB group received daily oral gavage of A. kunkeei FM01 at a dose of 2 × 10⁹ CFU per mouse. The composition of the HFD is detailed in Table S1.
Mice were individually housed in steel micro-isolator cages under controlled environmental conditions (20 °C, 12 h light/dark cycle) and had ad libitum access to tap water throughout the 12-week experimental period. Body weight, food intake, and blood glucose levels were recorded weekly. At the end of week 12, an oral glucose tolerance test was performed after a 12-hour fasting period. The oral glucose tolerance test (OGTT) was chosen over the intraperitoneal glucose tolerance test (ipGTT) because OGTT engages the gut-mediated metabolic pathways, such as incretin secretion (GLP-1, GIP), gut-liver axis signaling, and microbiota-host interactions. OGTT preserves this gut-phase physiology and provides greater physiological relevance in the context of diet-microbiota-host interactions, aligning with our study’s focus, whereas ipGTT bypasses it, limiting the ability to detect microbiota-dependent benefits.
At the end of the experiment, stool and blood samples were collected before sacrifice. The mice were anesthetized with carbon dioxide gas (40% CO2 for 5 min) and killed by decapitation. Tissue samples, including intestinal tissue, liver, kidney, spleen, and epididymal fat, were dissected, weighed, immediately immersed in liquid nitrogen, and stored at -80 °C for subsequent analysis. A section of colon and liver was fixed in 10% formalin to evaluate the pathological activity, and another section of the colon tissue was stored at -80˚C to analyze the gene and protein expression.
Serum and hepatic biochemical analysis
Serum samples were obtained by centrifugation of whole blood at 4500 ×g for 20 min at 4 °C. Serum levels of triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were quantified using an automatic biochemical analyzer (BK-280, Biobase Biodustry Co., Ltd, Shandong, China). Serum concentrations of lipopolysaccharide (LPS), diamine oxidase (DAO), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (CUSABIO, Wuhan, China) according to the manufacturer’s instructions. Hepatic TG and TC levels were also assessed using ELISA kits (Applygen, Beijing, China), following the manufacturer’s protocols.
Histopathological staining
Liver and intestinal tissue samples fixed in 10% formalin were overnight, dehydrated through a graded ethanol series, and embedded in paraffin wax. Tissue sections (4 µm thickness) were deparaffinized in xylene and rehydrated through descending alcohol concentrations. For histological evaluation, liver sections were stained with oil red O to assess lipid accumulation, while intestinal tissues were stained with hematoxylin and eosin to evaluate general morphology and structural integrity. Stained sections were visualized using a DM300 microscope (Leica, Weztlar, Germany), and representative images were captured for analysis.
RNA isolation, cDNA synthesis, and real-time PCR
Total RNA was extracted from colon tissue samples using a Total RNA Extraction Kit (Tiangen Biotech (Beijing) Co., Ltd, Beijing, China) according to the manufacturer’s instructions. RNA concentration and purity were assessed using a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Complementary DNA was synthesized from 1 μg of total RNA using a PrimeScript RT Reagent kit (Takara Bio Inc., Otsu, Japan) at 37 ˚C for 15 min and 85 ˚C for 5 s. Real-time quantitative PCR (RT-qPCR) was performed using SYBR Premix Ex Taq™ (Takara Bio, Shiga, Japan) on an Applied Biosystems QuantStudio™ 3 Real-Time PCR system (Thermo Fisher Scientific Inc., Waltham, MA, USA). Each sample was analyzed in triplicate, and amplification was carried out over 45 cycles. Two sets of gene-specific primers were designed by Primer 5.0 software to target quantify Zonula Occludens-1 (ZO-1) and occludin (Table S2). Relative gene expression levels were calculated using the 2-ΔΔCt method, where Ct is the threshold cycle. The β-actin gene was used as the internal reference gene for normalization.
Western blotting analysis
Total protein was extracted from colon tissue samples using the RIPA buffer (Sigma-Aldrich; Merck KGaA). Protein concentration was measured using the BCA Protein Assay kit (Thermo Fisher Scientific, Waltham, MA, USA). For each sample, 30 µg of total protein was loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and separated under denaturing conditions. Following electrophoresis, proteins were transferred onto either polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.). The membranes were incubated overnight at 4 °C with primary antibodies against ZO-1 (1:1,000; GB111402; Wuhan Seville Biotechnology Co., LTD, Wuhan, China.), occludin (1:1,000; ab216327, Shanghai Abcam Biotechnology Co., Ltd., Shang, China) and and β-actin antibody (1:1,000; 4970; cell signaling Technology, Inc., danvers, MA, USA). The next day, the membranes were washed three times using Tris-buffered saline with Tween-20 (5 min per wash) and incubated with peroxidase-labeled secondary antibodies (1:1,000; cat. no. 7074; Cell Signaling Technology, Inc.) diluted in blocking buffer for 2 h at room temperature. Immunoreactive bands were visualized using an enhanced chemiluminescence detection kit (ABclonal, Wuhan, China) and captured using a chemiluminescent imaging system. Band intensities were quantified using Image J software (National Institutes of Health, Bethesda, MD, USA), and β-actin was used as the internal loading control.
Quantitative lipid profiling
Lipids were extracted from serum samples following a previously described method42. Briefly, samples were ground into a fine powder in liquid nitrogen, and 50 mg of sample powder was transferred to a 2 mL centrifuge tube containing 1 mL of lipid extraction solvent (methyl tertbutyl ether: methanol, 3:1, v/v). The mixture was vortexed for 15 min, and stainless-steel beads were added before homogenization using a ball mill. After removing the steel beads, the samples were sonicated for 5 min to ensure complete emulsification. Then, 200 μL of water was added, followed by vortexing for 1 min. The samples were centrifuged at 12,000 × g for 10 min at 4 °C. Subsequently, 200 µL of the upper organic phase was collected, dried under a stream of nitrogen gas, and redissolved in 200 µL of resuspension solution (acetonitrile: isopropyl alcohol, 1:1, v/v). After vortexing for 3 min, the samples were centrifuged again at 12,000 × g for 3 min at 4 °C. The supernatants were collected for lipidomic analysis. Lipid profiling was performed using LC-MS/MS on an AB Sciex QTRAP 6500 system (AB Sciex, Framingham, MA, USA), through a service provided by MetWare, Co., Ltd. (http://www.metware.cn, Wuhan, China). Data analysis was conducted using a targeted lipidomic platform based on an in-house database (MWLDB, v 3.0).
DNA extraction and high-throughput metagenomics sequencing
Total genomic DNA was extracted from fecal samples using the QIAGEN DNA Stool Mini-Kit (QIAGEN, Hilden, Germany), following the manufacturer’s instructions. The integrity of the extracted DNA was assessed via 1% agarose gel electrophoresis, and the samples were stored at −20 °C prior to further analysis. DNA fragmentation was performed using the Covaris M220 Focused-ultrasonicator™ (Covaris, LLC, Woburn, MA, USA), and fragments of about 400 bp were selected for paired-end library construction. Metagenomic sequencing was performed on an Illumina HiSeq XTEN platform by Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
The raw paired-end reads were subjected to quality control using fastp (version 0.20.0; https://github.com/OpenGene/fastp)43 to filter low-quality sequences and adapter contaminants. High-quality clean reads were retained for downstream analysis. Sequence assembly was performed using MEGAHIT software to generate contigs from the filtered reads44. Taxonomic profiling of the metagenomic data was conducted using MetaPhlAn245, which annotates microbial species based on alignment to a curated non-redundant marker gene database. Functional annotation and pathway analysis were achieved using DIAMOND (version 0.8.35; http://www.diamondsearch.org/index.php)46 against the Carbohydrate-Active enZYmes (CAZy) Database and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases.
Statistical analysis
Data are presented as mean ± standard error of the mean. Statistical comparisons among groups were performed using one-way analysis of variance (ANOVA) followed by Duncan’s post hoc test, using SPSS software (version 21.0, IBM Corp., Chicago, IL, USA). A P-value < 0.05 was considered statistically significant. Additional statistical analyses were performed using R packages (http://www.r-project.org/). Spearman correlation analyses between gut microbiota composition and serum metabolic parameters were conducted to assess potential associations. Correlation results were visualized using Cytoscape (version 3.5.1).
Data availability
The authors declare that all data supporting the findings of this study are presented in the article. All raw data are available on request.
Code availability
No custom code or software was used in this study.
References
Afshin, A. et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377, 13–27 (2017).
Bray, G. A. et al. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 79, 537–543 (2004).
Caliceti, C. et al. Fructose intake, serum uric acid, and cardiometabolic disorders: a critical review. Nutrients 9, 395 (2017).
Jang, C. et al. The small intestine shields the liver from fructose-induced steatosis. Nat. Metab. 2, 586–593 (2020).
Li, J. M. et al. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome 7, 98 (2019).
Beisner, J. et al. Fructose-induced intestinal microbiota shift following two types of short-term high-fructose dietary phases. Nutrients 12, 3444 (2020).
Tan, R. et al. Intestinal microbiota mediates high-fructose and high-fat diets to induce chronic intestinal inflammation. Front. Cell. Infect. Microbiol. 11, 654074 (2021).
P. Guo, et al. Impacts of fructose on intestinal barrier function, inflammation and microbiota in a piglet model, Nutrients, 13, (2021).
Do, M. H. et al. High-glucose or -fructose diet causes changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 10, 761 (2018).
Ahn, I. S. et al. Disparate metabolomic responses to fructose consumption between different mouse strains and the role of gut microbiota. Metabolites 11, 342 (2021).
Endo, A. et al. Isolation and characterization of fructophilic lactic acid bacteria from fructose-rich niches. Syst. Appl. Microbiol. 32, 593–600 (2009).
Endo, A. et al. Fructophilic lactic acid bacteria, a unique group of fructose-fermenting microbes. Appl. Environ. Microbiol. 84, e01290–18 (2018).
Endo, A. & Salminen, S. Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst. Appl. Microbiol. 36, 444–448 (2013).
Ispirli, H. et al. Detection of fructophilic lactic acid bacteria (FLAB) in bee bread and bee pollen samples and determination of their functional roles. J. Food Process. Preserv. 45, 15414 (2021).
Acín Albiac, M. et al. How fructophilic lactic acid bacteria may reduce the fodmaps content in wheat-derived baked goods: a proof of concept. Micro Cell Fact. 19, 182 (2020).
Vergalito, F. et al. Potential application of Apilactobacillus kunkeei for human use: Evaluation of probiotic and functional properties. Foods 9, 1535 (2020).
Filannino, P. et al. Fructose-rich niches traced the evolution of lactic acid bacteria toward fructophilic species. Crit. Rev. Microbiol. 45, 65–81 (2019).
Sakandar, H. A. et al. Isolation and in-vitro probiotic characterization of fructophilic lactic acid bacteria from Chinese fruits and flowers. LWT 104, 70–75 (2019).
DesOrmeaux, G. J. et al. Independent of mitochondrial respiratory function, dietary nitrate attenuates HFD-induced lipid accumulation and mitochondrial ROS emission within the liver. Am. J. Physiol. Endocrinol. Metab. 321, E217–e228 (2021).
Gonzalez, J. T. & Betts, J. A. Dietary fructose metabolism by splanchnic organs: Size matters. Cell Metab. 27, 483–485 (2018).
Dicks, L. M. T. & Endo, A. Are fructophilic lactic acid bacteria (flab) beneficial to humans?. Benef. Microbes 13, 3–11 (2022).
Yu, J. et al. Disruption of the intestinal mucosal barrier induced by high fructose and restraint stress is regulated by the intestinal microbiota and microbiota metabolites. Microbiol. Spectr. 11, e0469822 (2023).
Jang, C. et al. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 27, 351–361.e353 (2018).
Fan, X. et al. The protective effects of Levilactobacillus brevis fzu0713 on lipid metabolism and intestinal microbiota in hyperlipidemic rats. Food Sci. Hum. Wellness 12, 1646–1659 (2023).
Neveling, D. P. et al. Fructophilic Lactobacillus kunkeei and Lactobacillus brevis isolated from fresh flowers, bees and bee-hives. Curr. Microbiol 65, 507–515 (2012).
Liu, P. et al. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 247, 117443 (2020).
van der Veen, J. N. et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembranes 1859, 1558–1572 (2017).
Hara, D. et al. Data-independent acquisition-based lipidomics reveals lipidome alterations associated with growth of Saccharomyces cerevisiae at low temperature. J. Biosci. Bioeng. https://doi.org/10.1016/j.jbiosc.2025.03.004 (2025).
Postic, C. Conversion of a dietary fructose: new clues from the gut microbiome. Nat. Metab. 2, 217–218 (2020).
Zhao, S. et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 579, 586–591 (2020).
Qi, Q. et al. Hydrogen sulfide produced by the gut microbiota impairs host metabolism via reducing GLP-1 levels in male mice. Nat. Metab. 6, 1601–1615 (2024).
Isaac, S. et al. Microbiome-mediated fructose depletion restricts murine gut colonization by vancomycin-resistant Enterococcus. Nat. Commun. 13, 7718 (2022).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Choi, K. J. et al. Gut commensal Kineothrix alysoides mitigates liver dysfunction by restoring lipid metabolism and gut microbial balance. Sci. Rep.13, 14668 (2023).
Manghi, P. et al. Coffee consumption is associated with intestinal Lawsonibacter asaccharolyticus abundance and prevalence across multiple cohorts. Nat. Microbiol. 9, 3120–3134 (2024).
Maeno, S. et al. Genomic characterization of a fructophilic bee symbiont Lactobacillus kunkeei reveals its niche-specific adaptation. Syst. Appl. Microbiol. 39, 516–526 (2016).
Asenjo, F. et al. Genome sequencing and analysis of the first complete genome of Lactobacillus kunkeei strain mp2, an Apis mellifera gut isolate. PeerJ 4, e1950 (2016).
Kawabata, K. et al. A high‑fructose diet induces epithelial barrier dysfunction and exacerbates the severity of dextran sulfate sodium‑induced colitis. Int J. Mol. Med. 43, 1487–1496 (2019).
Jia, X. et al. Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri hmv18. Food Sci. Hum. Wellness 13, 611–620 (2024).
Maragkoudakis, P. A. et al. Probiotic potential of lactobacillus strains isolated from dairy products. Int. Dairy J. 16, 189–199 (2006).
Simsek, D. et al. Investigation of the probiotic and metabolic potential of fructobacillus tropaeoli and Apilactobacillus kunkeei from apiaries. Arch. Microbiol 204, 432 (2022).
Harlina, P. W. et al. Quantification of lipidomics profiling using UPLC-QE-HESI- lipid analysis on the salted duck egg incorporated with clove extract. Eur. J. Lipid Sci. Tech. 123, 2000284 (2021).
Chen, S. et al. Fastp: An ultra-fast all-in-one fastq preprocessor. Bioinformatics 34, i884-i890 (2018).
Li, D. et al. Megahit: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015).
Truong, D. T. et al. Metaphlan2 for enhanced metagenomic taxonomic profiling. Nat. Methods 12, 902–903 (2015).
Buchfink, B. et al. Fast and sensitive protein alignment using diamond. Nat. Methods 12, 59–60 (2015).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 32402039) and the Project of Science and Technology Ministry of Sichuan Province (Grant No. 2025ZNSFSC0227).
Author information
Authors and Affiliations
Contributions
Haiyan Xu: conceptualization, investigation, data curation, formal analysis, writing—original draft. Wenjia Ba, Rongzhuzhu Yu, Zijia He, and Peizhi Wang: formal analysis, methodology. Xun Gou: formal analysis. Xiaoyu Zhang and Fang Wang: writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, H., Ba, W., Yu, R. et al. Fructophilic Apilactobacillus kunkeei alleviates high-fructose diet-induced lipid accumulation by modulating gut microbiota and intestinal barrier function in mice. npj Sci Food 9, 201 (2025). https://doi.org/10.1038/s41538-025-00567-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00567-9