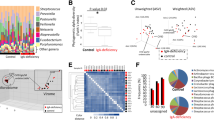
Abstract
IgA, the primary human antibody secreted from the gut mucosa, shapes the intestinal microbiota. Methodological limitations have hindered defining which microbial strains are targeted by IgA and the implications of binding. Here we develop a technique, metagenomic immunoglobulin sequencing (MIg-seq), that provides strain-level resolution of microbes coated by IgA and use it to determine IgA coating levels for 3,520 gut microbiome strains in healthy human faeces. We find that both health and disease-associated bacteria are targeted by IgA. Microbial genes are highly predictive of IgA binding levels; in particular, mucus degradation genes are correlated with high binding, and replication rates are significantly reduced for microbes bound by IgA. We demonstrate that IgA binding is more correlated with host immune status than traditional relative abundance measures of microbial community composition. This study introduces a powerful technique for assessing strain-level IgA binding in human stool, paving the way for deeper understanding of IgA-based host–microbe interactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Metagenomic reads are available on the NCBI Short Read Archive via BioProject accession PRJNA1049470. The protocol is available at https://doi.org/10.17504/protocols.io.5jyl8pk6dg2w/v1. Additional data, including genomes, genes and additional tables of processed data are available on Zenodo at https://doi.org/10.5281/zenodo.11154974 ref. 66. This manuscript uses the following public databases: Pfam v.32.0, KOfam v.103, CARD v.3.2.5 and CAzy dbCAN v.11.
Code availability
Custom code, including code used to develop machine learning models, is available at https://github.com/MrOlm/MIGSeq_code ref. 67.
References
Catanzaro, J. R. et al. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory IgM. Sci. Rep. 9, 13574 (2019).
Macpherson, A. J., McCoy, K. D., Johansen, F.-E. & Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008).
Sender, R. et al. The total mass, number, and distribution of immune cells in the human body. Proc. Natl Acad. Sci. USA 120, e2308511120 (2023).
Conrey, P. E. et al. IgA deficiency destabilizes homeostasis toward intestinal microbes and increases systemic immune dysregulation. Sci. Immunol. 8, eade2335 (2023).
Fadlallah, J. et al. Microbial ecology perturbation in human IgA deficiency. Sci. Transl. Med. 10, eaan1217 (2018).
Yel, L. Selective IgA deficiency. J. Clin. Immunol. 30, 10–16 (2010).
Palm, N. W. et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010 (2014).
Huus, K. E. et al. Immunoglobulin recognition of fecal bacteria in stunted and non-stunted children: findings from the Afribiota study. Microbiome 8, 113 (2020).
Shapiro, J. M. et al. Immunoglobulin A targets a unique subset of the microbiota in inflammatory bowel disease. Cell Host Microbe 29, 83–93.e3 (2021).
Yang, C. et al. Immunoglobulin A antibody composition is sculpted to bind the self gut microbiome. Sci. Immunol. 7, eabg3208 (2022).
Weis, A. M. & Round, J. L. Microbiota–antibody interactions that regulate gut homeostasis. Cell Host Microbe 29, 334–346 (2021).
Moor, K. et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature 544, 498–502 (2017).
Macpherson, A. J. & Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 (2004).
Rollenske, T. et al. Parallelism of intestinal secretory IgA shapes functional microbial fitness. Nature 598, 657–661 (2021).
Huus, K. E. et al. Commensal bacteria modulate immunoglobulin A binding in response to host nutrition. Cell Host Microbe 27, 909–921.e5 (2020).
Joglekar, P. et al. Intestinal IgA regulates expression of a fructan polysaccharide utilization locus in colonizing gut commensal Bacteroides thetaiotaomicron. MBio 10, e02324-19 (2019).
Donaldson, G. P. et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 360, 795–800 (2018).
McLoughlin, K., Schluter, J., Rakoff-Nahoum, S., Smith, A. L. & Foster, K. R. Host selection of microbiota via differential adhesion. Cell Host Microbe 19, 550–559 (2016).
Bansept, F. et al. Enchained growth and cluster dislocation: a possible mechanism for microbiota homeostasis. PLoS Comput. Biol. 15, e1006986 (2019).
Bunker, J. J. & Bendelac, A. IgA responses to microbiota. Immunity 49, 211–224 (2018).
Gopalakrishna, K. P. et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 25, 1110–1115 (2019).
Korem, T. et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 349, 1101–1106 (2015).
Brown, C. T., Olm, M. R., Thomas, B. C. & Banfield, J. F. Measurement of bacterial replication rates in microbial communities. Nat. Biotechnol. 34, 1256–1263 (2016).
Jackson, M. A. et al. Accurate identification and quantification of commensal microbiota bound by host immunoglobulins. Microbiome 9, 33 (2021).
Bunker, J. J. et al. B cell superantigens in the human intestinal microbiota. Sci. Transl. Med. 11, eaau9356 (2019).
Wastyk, H. C. et al. Gut-microbiota-targeted diets modulate human immune status. Cell 184, 4137–4153.e14 (2021).
Bunker, J. J. et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science 358, eaan6619 (2017).
Planer, J. D. et al. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature 534, 263–266 (2016).
Wilmore, J. R. et al. Commensal microbes induce serum IgA responses that protect against polymicrobial sepsis. Cell Host Microbe 23, 302–311.e3 (2018).
Almeida, D. et al. Evolving trends in next-generation probiotics: a 5W1H perspective. Crit. Rev. Food Sci. Nutr. 60, 1783–1796 (2020).
Henrick, B. M. et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898.e11 (2021).
Yu, L. et al. Molecular cloning and functional characterization of an ATP-binding cassette transporter OtrC from Streptomyces rimosus. BMC Biotechnol. 12, 52 (2012).
Declerck, N., Vincent, F., Hoh, F., Aymerich, S. & van Tilbeurgh, H. RNA recognition by transcriptional antiterminators of the BglG/SacY family: functional and structural comparison of the CAT domain from SacY and LicT. J. Mol. Biol. 294, 389–402 (1999).
Tailford, L. E., Crost, E. H., Kavanaugh, D. & Juge, N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 6, 81 (2015).
Saez-Lara, M. J., Gomez-Llorente, C., Plaza-Diaz, J. & Gil, A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. BioMed. Res. Int. 2015, 505878 (2015).
Takeuchi, T. et al. Acetate differentially regulates IgA reactivity to commensal bacteria. Nature 595, 560–564 (2021).
Morita, N. et al. GPR31-dependent dendrite protrusion of intestinal CX3CR1+ cells by bacterial metabolites. Nature 566, 110–114 (2019).
Kubinak, J. L. et al. MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17, 153–163 (2015).
Nakajima, A. et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J. Exp. Med. 215, 2019–2034 (2018).
Huang, J. Y., Lee, S. M. & Mazmanian, S. K. The human commensal Bacteroides fragilis binds intestinal mucin. Anaerobe 17, 137–141 (2011).
An, D. et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156, 123–133 (2014).
Bae, M. et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 608, 168–173 (2022).
Singh, V. et al. Butyrate producers, ‘The Sentinel of Gut’: their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 13, 1103836 (2022).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Luk, A. W., Beckmann, S. & Manefield, M. Dependency of DNA extraction efficiency on cell concentration confounds molecular quantification of microorganisms in groundwater. FEMS Microbiol. Ecol. 94, fiy146 (2018).
Nurk, S., Meleshko, D., Korobeynikov, A. & Pevzner, P. A. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 27, 824–834 (2017).
Gurevich, A., Saveliev, V., Vyahhi, N. & Tesler, G. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075 (2013).
Kang, D. D. et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7, e7359 (2019).
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. & Tyson, G. W. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015).
Olm, M. R., Brown, C. T., Brooks, B. & Banfield, J. F. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 11, 2864–2868 (2017).
Almeida, A. et al. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat. Biotechnol. 39, 105–114 (2020).
Chaumeil, P.-A., Mussig, A. J., Hugenholtz, P. & Parks, D. H. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics https://doi.org/10.1093/bioinformatics/btz848 (2019).
Olm, M. R. et al. inStrain profiles population microdiversity from metagenomic data and sensitively detects shared microbial strains. Nat. Biotechnol. https://doi.org/10.1038/s41587-020-00797-0 (2021).
Hunter, J. D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Ondov, B. D. et al. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol. 17, 132 (2016).
Jones, E., Oliphant, T. & Peterson, P. SciPy: open source scientific tools for Python. http://scipy.org (2001).
Lee, M. D. GToTree: a user-friendly workflow for phylogenomics. Bioinformatics 35, 4162–4164 (2019).
Moshiri, N. TreeSwift: a massively scalable Python tree package. SoftwareX 11, 100436 (2020).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23, 127–128 (2007).
El-Gebali, S. et al. The Pfam protein families database in 2019. Nucleic Acids Res. https://doi.org/10.1093/nar/gky995 (2018).
Aramaki, T. et al. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 36, 2251–2252 (2020).
Jia, B. et al. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 45, D566–D573 (2017).
Drula, E. et al. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. 50, D571–D577 (2022).
Gellman, R. H. et al. Hadza Prevotella require diet-derived microbiota-accessible carbohydrates to persist in mice. Cell Rep. 42, 113233 (2023).
Olm, M. Data supporting publication ‘Metagenomic immunoglobulin sequencing (MIG-Seq) exposes patterns of IgA antibody binding in the healthy human gut microbiome’. Zenodo https://doi.org/10.5281/ZENODO.11154974 (2023).
Olm, M. MrOlm/MIGSeq_code: Version 1.1 (1.1). Zenodo https://doi.org/10.5281/zenodo.14269815 (2024).
Acknowledgements
We thank L. H. Lam, H. Maeker, J. Fessler, M. Carter and K. C. Huang for helpful discussions during the preparation of this manuscript.
Support for the project was provided by National Institutes of Health grant F32DK128865 (M.R.O.), National Institutes of Health training grant T32 AI007328-30 (M.R.O.), the Colleen and Robert D. Hass fund (S.P.S.), and National Institutes of Health grants T32DK007056, K08DK134856 (S.P.S.), R01DK085025 (J.L.S.) and DP1AT009892 (J.L.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.L.S. is a Chan Zuckerberg Biohub Investigator.
This publication includes data generated at the UC San Diego IGM Genomics Center utilizing an Illumina NovaSeq 6000 that was purchased with funding from a National Institutes of Health SIG grant (#S10 OD026929). This publication includes data generated at the Stanford Shared FACS Facility (NIH S10 Shared Instrument Grant 1S10OD026831-01).
Author information
Authors and Affiliations
Contributions
M.R.O., S.P.S. and J.L.S. designed the study. S.P.S. led the development of the MIg-seq protocol. M.R.O. performed bioinformatic analysis. M.R.O., S.P.S., T.T. and E.L.S. performed wet lab experiments. M.R.O. wrote the manuscript and all authors contributed to manuscript revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Gregory Donaldson, Oliver Pabst, Emma Slack and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
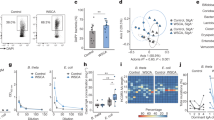
Extended Data Fig. 1 Effectiveness of magnetic cell sorting for enriching IgA+ cells.
Aggregated (A) and sample-level (B) fraction of bacterial cells bound by IgA as assessed by bacterial flow cytometry. IgA+ (blue) is the result of magnetic cell sorting, unsorted (orange) is the native unsorted sample, and unstained (green) is the unsorted fraction without the Anti-Human IgA APC antibody added. Boxplots show the minimum and maximum (whiskers), median (center line), and interquartile range (box bounds); whiskers extend up to 1.5 times the interquartile range. n = 38 biological replicates.
Extended Data Fig. 2 Gating strategy for MIg-Seq protocol.
The preliminary gate to identify IgA-positive cells was SSC and SYBR Green, with SYBR-positive gate drawn based on preliminary experiments using a sample without SYBR green (SYBR stains all bacteria). SYBR-positive cells were then analyzed for IgA staining, with an IgA-positive gate drawn based on a sample without IgA staining. Representative flow plots are shown for a preliminary experiment of a single sample (#1037) that is unstained and without SYBR (A), staining and unsorted (B), and stained and sorted (C).
Extended Data Fig. 3 MIg-Seq assay antibody binding validation.
A) Schematic of assay design for multistep magnet cell sorting, highlighting the motivation of using irrelevant magnetic beads to assess for non-specific bacterial binding to the magnetic beads. B) Stool was stained with Anti-Human IgA APC antibody and then with either anti-APC + magnetic beads as an experimental condition or anti-PE + magnetic beads as an irrelevant control prior to magnetic enrichment and analysis of the positive fraction via flow cytometry. C) DNA concentration in the positive fractions was obtained using Qubit™ dsDNA Quantification Assay Kit.
Extended Data Fig. 4 Phylum-Level Relative Abundance of Native and IgA+ Metagenomes Across 30 Samples.
Phylum-level relative abundance of native metagenomes (left) and IgA+ fraction metagenomes (right) for the 30 samples used in this study.
Extended Data Fig. 5 Comparative Analysis of IgA-Bound Bacteria and Microbiome Shifts Post-Dietary Intervention.
A) Boxplots comparing the overall fraction of bacteria bound by IgA in native samples (as assessed by bacterial flow cytometry) in baseline samples versus after dietary intervention of increased consumption of fermented foods (left) or fibre (right). Lines connect samples from the same subjects over time. P-value from Wilcoxon signed-rank test. For fermented and fibre groups, n = 4, 7; p = 0.25, 0.38, respectively. B) Violin plot of weighted UniFrac distance between the same subjects in baseline vs. intervention samples (top) and weighted UniFrac distance between different subjects (bottom; this represents the UniFrac distances between samples taken from unrelated subjects at either timepoint). P-values from comparing the distribution of IgA+ vs native weighted UniFrac distances using two-sided Wilcoxon rank-sum test; n = the number of comparisons considered in each the IgA+ and native fractions. C) Principal component analysis of weighted UniFrac distance between all metagenomes in this study. Lines connect native and IgA+ metagenomes from the same stool sample. D) Violin plot comparing the weighted UniFrac distance between samples from the same subjects (top) and the absolute abundance of the difference in the total fraction of bacteria bound by IgA (bottom). Normalization of differences performed using min-max scaling prior to comparison. P-value from two-sided Wilcoxon signed-rank test. Boxplots show the minimum and maximum (whiskers), median (center line), and interquartile range (box bounds); whiskers extend up to 1.5 times the interquartile range.
Extended Data Fig. 6 Concatenated Gene Phylogeny of Microbial Strains with Relative IgA Binding Abundance.
A concatenated gene phylogenetic tree of all microbial strains detected in at least 5 samples. Stacked bar plots on the right display the relative abundance of IgA binding categories for each detection. The total bar length represents the total number of detections. Black stars mark taxa that appear to have substantially different IgA coating than their phylogenetic neighbors based on manual inspection.
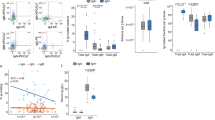
Extended Data Fig. 7 Association of Microbial Functions with IgA+ Probability Scores and Statistical Significance.
For each microbial function (dot), the association of that function with IgA+ probability score (x-axis; median IgA+ probability score of microbes encoding function - median IgA+ probability score of microbes not encoding function) versus the p-value of the association of the function with IgA+ probability score (two-sided Wilcoxon rank-sum test with FDR correction). Horizontal dotted line at p = 0.05. Each phylum is treated independently.
Extended Data Fig. 8 Comparison of iRep in IgA+ vs. Native Fractions Across Phyla and Genera.
For each phylum (A) and genus (B) with at least 5 measurements, the difference in iRep in IgA+ vs. native fractions of all species detected within that phylum or genus. Negative values indicate IgA is associated with reduced replication rates. P-values from post hoc two-sided pairwise test for multiple comparisons of mean rank sums (Dunn’s test). For Firmucutes_A, Bacteroidota, Firmicutes, and Actinobacteria, n = 144, 32, 10, and 8, respectively. All exact p-values > 0.05 are shown in the figure. For genera, in order from top to bottom, n = 5, 6, 19, 8, 6, 5, 5, 6, 5, 7, and 6. Boxplots show the minimum and maximum (whiskers), median (center line), and interquartile range (box bounds); whiskers extend up to 1.5 times the interquartile range.
Extended Data Fig. 9 Significant Correlations Between IgA-Associated Microbial Genes and Human Health/Microbiome Function Metrics.
A correlation analysis between the 986 microbial genes significantly associated with IgA binding and 9 measurements of human health and microbiome function. The x-axis indicated the number of significant (p < 0.05) correlations identified after false discovery rate correction.
Supplementary information
Supplementary Information
Supplementary Notes 1 and 2.
Supplementary Table 1
Information on the DNA extraction concentration, sequencing depth, IgA sorting information and metadata for all 38 samples.
Supplementary Table 2
Microbial relative abundance and IgA+ probabilities for all samples.
Supplementary Table 3
Statistics for IgA binding enrichment among phyla and genus-level taxonomic groups.
Supplementary Table 4
Statistics for association of microbial annotations with IgA binding.
Supplementary Table 5
Paired iRep values for all species detected in both IgA+ and native samples.
Supplementary Table 6
Statistical associations between each phylum and each immune metric.
Supplementary Table 7
Statistical associations between each gene that is significantly positively associated with IgA+ probability score and each immune metric.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olm, M.R., Spencer, S.P., Takeuchi, T. et al. Metagenomic immunoglobulin sequencing reveals IgA coating of microbial strains in the healthy human gut. Nat Microbiol 10, 112–125 (2025). https://doi.org/10.1038/s41564-024-01887-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-024-01887-4
This article is cited by
-
Identification of immunogenic commensal antigens using phage display
Nature Protocols (2026)