Abstract
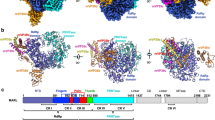
Filoviruses, including Ebola virus, pose an increasing threat to the public health. Although two therapeutic monoclonal antibodies have been approved to treat the Ebola virus disease1,2, there are no approved broadly reactive drugs to control diverse filovirus infection. Filovirus has a large polymerase (L) protein and the cofactor viral protein 35 (VP35), which constitute the basic functional unit responsible for virus genome RNA synthesis3. Owing to its conservation, the L–VP35 polymerase complex is a promising target for broadly reactive antiviral drugs. Here we determined the structure of Ebola virus L protein in complex with tetrameric VP35 using cryo-electron microscopy (state 1). Structural analysis revealed that Ebola virus L possesses a filovirus-specific insertion element that is essential for RNA synthesis, and that VP35 interacts extensively with the N-terminal region of L by three protomers of the VP35 tetramer. Notably, we captured the complex structure in a second conformation with the unambiguous priming loop and supporting helix away from polymerase active site (state 2). Moreover, we demonstrated that the century-old drug suramin could inhibit the activity of the Ebola virus polymerase in an enzymatic assay. The structure of the L–VP35–suramin complex reveals that suramin can bind at the highly conserved NTP entry channel to prevent substrates from entering the active site. These findings reveal the mechanism of Ebola virus replication and may guide the development of more powerful anti-filovirus drugs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The cryo-EM density maps and atomic coordinates have been deposited to the Electron Microscopy Data Bank (EMDB) and the Protein Data Bank (PDB), respectively. The accession numbers are listed as follows: EBOV L–VP35 complex in state 1 (7YER, EMD-33775), EBOV L–VP35 complex in state 2 (7YES, EMD-33776), EBOV L–VP35-suramin complex (7YET, EMD-33777). All other data are available from the authors upon reasonable request.
References
Markham, A. REGN-EB3: first approval. Drugs 81, 175–178 (2021).
Gaudinski, M. R. et al. Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study. Lancet 393, 889–898 (2019).
Muhlberger, E. Filovirus replication and transcription. Future Virol. 2, 205–215 (2007).
Jacob, S. T. et al. Ebola virus disease. Nat. Rev. Dis. Primers 6, 13 (2020).
Languon, S. & Quaye, O. Filovirus disease outbreaks: a chronological overview. Virology 10, 1178122X19849927 (2019).
Languon, S. & Quaye, O. Impacts of the Filoviridae family. Curr. Opin. Pharmacol. 60, 268–274 (2021).
Keita, A. K. et al. Resurgence of Ebola virus in 2021 in Guinea suggests a new paradigm for outbreaks. Nature 597, 539–543 (2021).
Dovih, P. et al. Filovirus-reactive antibodies in humans and bats in Northeast India imply zoonotic spillover. PLoS Negl. Trop. Dis. 13, e0007733 (2019).
Negredo, A. et al. Discovery of an ebolavirus-like filovirus in europe. PLoS Pathog. 7, e1002304 (2011).
Yang, X. L. et al. Characterization of a filovirus (Mengla virus) from Rousettus bats in China. Nat. Microbiol. 4, 390–395 (2019).
Shi, Y. New Virus, New Challenge. Innovation https://doi.org/10.1016/j.xinn.2020.04.005 (2020).
Feldmann, H., Sprecher, A. & Geisbert, T. W. Ebola. N. Engl. J. Med. 382, 1832–1842 (2020).
Stahelin, R. V. Membrane binding and bending in Ebola VP40 assembly and egress. Front. Microbiol. 5, 300 (2014).
Yu, D. S. et al. The lifecycle of the Ebola virus in host cells. Oncotarget 8, 55750–55759 (2017).
Davey, R. T. et al. A randomized, controlled trial of ZMapp for Ebola virus infection. N. Engl. J. Med. 375, 1448–1456 (2016).
Basu, A. et al. Identification of a small-molecule entry inhibitor for filoviruses. J. Virol. 85, 3106–3119 (2011).
Li, H. Y. et al. Chemically modified human serum albumin potently blocks entry of Ebola pseudoviruses and viruslike particles. Antimicrob. Agents Chemother. 61, e02168–16 (2017).
Mulangu, S. et al. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 381, 2293–2303 (2019).
Takashita, E. Influenza polymerase inhibitors: mechanisms of action and resistance. Cold Spring Harb. Perspect. Med. 11, a038687 (2021).
Peng, Q. et al. Structural basis of SARS-CoV-2 polymerase inhibition by favipiravir. Innovation 2, 100080 (2021).
Warren, T. K. et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 508, 402–405 (2014).
Oestereich, L. et al. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir. Res. 105, 17–21 (2014).
Madelain, V. et al. Ebola virus dynamics in mice treated with favipiravir. Antivir. Res. 123, 70–77 (2015).
Warren, T. K. et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 531, 381–385 (2016).
Sissoko, D. et al. Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 13, e1001967 (2016).
Smither, S. J. et al. Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antiviral Res. 104, 153–155 (2014).
Dunning, J. et al. Experimental treatment of Ebola virus disease with TKM-130803: a single-arm phase 2 clinical trial. PLoS Med. 13, e1001997 (2016).
Pan, J. H. et al. Structure of the human metapneumovirus polymerase phosphoprotein complex. Nature 577, 275–279 (2020).
Jenni, S. et al. Structure of the vesicular stomatitis virus L protein in complex with its phosphoprotein cofactor. Cell Rep. 30, 53–60 (2020).
Gilman, M. S. A. et al. Structure of the respiratory syncytial virus polymerase complex. Cell 179, 193–204 (2019).
Horwitz, J. A., Jenni, S., Harrison, S. C. & Whelan, S. P. J. Structure of a rabies virus polymerase complex from electron cryo-microscopy. Proc. Natl Acad. Sci. USA 117, 2099–2107 (2020).
Abdella, R., Aggarwal, M., Okura, T., Lamb, R. A. & He, Y. Structure of a paramyxovirus polymerase complex reveals a unique methyltransferase-CTD conformation. Proc. Natl Acad. Sci. USA 117, 4931–4941 (2020).
Liang, B. Structures of the mononegavirales polymerases. J. Virol. 94, e00175–20 (2020).
Blondot, M. L. et al. Structure and functional analysis of the RNA- and viral phosphoprotein-binding domain of respiratory syncytial virus M2-1 protein. PLoS Pathog. 8, e1002734 (2012).
Richard, C. A. et al. RSV hijacks cellular protein phosphatase 1 to regulate M2-1 phosphorylation and viral transcription. PLoS Pathog. 14, e1006920 (2018).
Leung, D. W. et al. Structural basis for dsRNA recognition and interferon antagonism by Ebola VP35. Nat. Struct. Mol. Biol. 17, 165–U165 (2010).
Liang, B. et al. Structure of the L protein of vesicular stomatitis virus from electron cryomicroscopy. Cell 162, 314–327 (2015).
Zinzula, L. et al. Structures of Ebola and Reston virus VP35 oligomerization domains and comparative biophysical characterization in all ebolavirus species. Structure 27, 39–54 (2019).
Henss, L. et al. Suramin is a potent inhibitor of Chikungunya and Ebola virus cell entry. Virol J. 13, 149 (2016).
Mastrangelo, E. et al. Structure-based inhibition of norovirus RNA-dependent RNA polymerases. J. Mol. Biol. 419, 198–210 (2012).
Yin, W. et al. Structural basis for inhibition of the SARS-CoV-2 RNA polymerase by suramin. Nat. Struct. Mol. Biol. 28, 319–325 (2021).
Kirchdoerfer, R. N., Abelson, D. M., Li, S., Wood, M. R. & Saphire, E. O. Assembly of the Ebola virus nucleoprotein from a chaperoned VP35 complex. Cell Rep. 12, 140–149 (2015).
Leung, D. W. et al. An intrinsically disordered peptide from Ebola virus VP35 controls viral RNA synthesis by modulating nucleoprotein–RNA interactions. Cell Rep. 11, 376–389 (2015).
Wan, W. et al. Structure and assembly of the Ebola virus nucleocapsid. Nature 551, 394–397 (2017).
Luthra, P., Jordan, D. S., Leung, D. W., Amarasinghe, G. K. & Basler, C. F. Ebola virus VP35 interaction with dynein LC8 regulates viral RNA synthesis. J. Virol. 89, 5148–5153 (2015).
Prins, K. C. et al. Basic residues within the ebolavirus VP35 protein are required for its viral polymerase cofactor function. J. Virol. 84, 10581–10591 (2010).
Qiu, S. H., Ogino, M., Luo, M., Ogino, T. & Green, T. J. Structure and function of the N-terminal domain of the vesicular stomatitis virus RNA polymerase. J. Virol. 90, 715–724 (2016).
Wiedemar, N., Hauser, D. A. & Maser, P. 100 years of suramin. Antimicrob. Agents Chemother. 64, e01168–19 (2020).
Nardone, V. et al. Structural basis of inhibition of the pioneer transcription factor NF-Y by suramin. Cells 9, 2370 (2020).
Ono, K., Nakane, H. & Fukushima, M. Differential inhibition of various deoxyribonucleic and ribonucleic acid polymerases by suramin. Eur. J. Biochem. 172, 349–353 (1988).
Peng, R. C. et al. Structural insight into arenavirus replication machinery. Nature 579, 615–619 (2020).
Tchesnokov, E. P., Raeisimakiani, P., Ngure, M., Marchant, D. & Gotte, M. Recombinant RNA-dependent RNA polymerase complex of Ebola virus. Sci Rep. 8, 3970 (2018).
Tchesnokov, E. P., Feng, J. Y., Porter, D. P. & Gotte, M. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses 11, 326 (2019).
Du, X. et al. Combinatorial screening of a panel of FDA-approved drugs identifies several candidates with anti-Ebola activities. Biochem. Biophys. Res. Commun. 522, 862–868 (2020).
Tao, W. Y., Gan, T. Y., Guo, M. Z., Xu, Y. F. & Zhong, J. Novel stable Ebola virus minigenome replicon reveals remarkable stability of the viral genome. J. Virol. 91, e01316–17 (2017).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Acknowledgements
We thank all the staff members at the Center for Biological Imaging (CBI), Institute of Biophysics (IBP), Chinese Academy of Sciences (CAS) for assistance with data collection. We thank the staff of the State Key Laboratory of Membrane Biology, Institute of Zoology (IOZ), CAS for technical support of electron microscope operation. This study was supported by the National Key Research and Development Program of China (2021YFC2300700 to Y.S.), Strategic Priority Research Program of CAS (XDB29010000 to Y.S. and G.F.G.), and National Natural Science Foundation of China (NSFC) (81871658 and 32192452 to Y.S. and 32100119 to Q.P.). Y.S. is also partially supported by the Youth Innovation Promotion Association of CAS (Y201921).
Author information
Authors and Affiliations
Contributions
Y.S. and G.F.G. conceived the study. B.Y., J.C. and M.W. purified the protein samples and conducted biochemical and cellular experiments. B.Y. and Q.P. prepared the cryo-EM specimens and collected data. Q.P. conducted the image processing and reconstruction. J.Q. and Q.P. built the atomic models. Y.S., G.F.G., J.Q., Q.P. and B.Y. analysed the structure. Q.P., B.Y., J.Z., G.F.G. and Y.S. wrote the manuscript. All authors participated in the discussion and manuscript editing. B.Y. and Q.P. contributed equally to this work. Y.S. supervised all the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Ming Luo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The purified EBOV L-VP35 complex has polymerization activity.
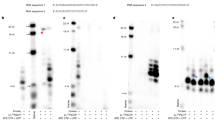
(a, b) Size-exclusion chromatography, SDS-PAGE and western blotting profiles of EBOV L-VP35 WT (a) and D742A mutant (b) proteins. Molecular weights (in kilodaltons, kDa) of ladder makers are shown in the left, and the L and VP35 bands are labeled on the right. (c, d) In vitro primer extension assay of L-VP35 complex. It demonstrates the purified complex protein possesses polymerase activity, and the product bands are inhomogeneous. The active site D742A mutant can abolish the production of RNA. Otherwise, a small percentage of full-length product (indicated by red arrow) can be clearly seen when the product bands are overexposed (d). The data shown above are representative results of at least three independent experiments using different protein preparations.
Extended Data Fig. 2 Structural comparison of EBOV L-VP35 and HPIV5 L-P complex.
Overall structure of EBOV L-VP35 (a) and HPIV5 L-P complex (PDB 6v85) (b). Overlay of the NTD (c), RdRp (d), PRNTase (e) domains and tetrameric P/VP35 protein (f) between EBOV L-VP35 and HPIV5 L-P complex shows a similar architecture, but with local differences.
Extended Data Fig. 3 The paths for RNA synthesis within EBOV L-VP35 structure.
(a) The EBOV L-VP35 structure is shown in surface representation to highlight the entry channel of NTP substrate which was indicated by a red cycle. (b) RNA elongation model of EBOV L-VP35 complex. Template RNA entry and exit channels are indicated as black arrows, and NTP entry and nascent RNA product exit tunnels are indicated by red and purple arrows. (c) Cutoff view of the L protein shown in electrostatic surface representation (blue, positive charge; red, negative charge). The paths are filled by the template and nascent RNA strands which are modeled based on the structure of rotavirus polymerase complex with in situ elongation conformation (PDB 6OGZ).
Extended Data Fig. 4 The critical role of the insertion element in transcription activity of EBOV RNP.
(a) Clear green fluorescence can be visualized with the wild type L protein. However, for the insertion-element-deletion L(196-225)GS construct (residues 196 to 225 consisting of the insertion loop structure were deleted and two ends were linked with GS residues), no green fluorescence was observed. As a negative control, the L gene was not transfected in the replicon system. (b) The expression levels of NP, VP35 and VP30 were measured using Western Blotting assay and the tubulin was used as loading control. The data shown are representative of three independent experiments. (c) The transcription levels of L mRNA were analyzed by RT-PCR. The data represent mean values (histograms) ± s.d. (error bars) from three independent experiments.
Extended Data Fig. 5 The predicted full-length structure of EBOV L by AlphaFold2.
(a) Superimposition of the predicted and solved structures of EBOV L protein, and they could be overlaid well. The predicted structure is colored in grey, while the solved structure is colored as depicted in Fig. 1. (b) The modeled full-length structure of EBOV L-VP35 complex. (c–e) Close-up view of the predicted structures of CD (purple), MTase (magenta) and CTD (black) domains.
Extended Data Fig. 6 Structural analysis of the EBOV L PRNTase domain.
(a) Overlay of the PRNTase domains of L proteins from the EBOV (colored by cyan) and RSV (grey). (b) The same view as in (a) but with catalytic motifs indicated by different colors (motif A’, red; motif B’, purple; motif C’, yellow; motif D’, blue; motif E’, orange). The Cα atoms of the conserved glycines in GxxT motif and motif A’ (Gly1129) are shown in sphere representation.
Extended Data Fig. 7 Conformational change of the priming loop and supporting helix.
The priming loop and supporting helix would clash with RNA duplex formed during the RNA elongation process (a), and would undergo conformational change to release adequate space for RNA elongation (b) with the priming loop retracting into PRNTase domain and the supporting helix moving outward. The RNA was modeled based on the structure of rotavirus polymerase complex with in situ elongation conformation (PDB 6OGZ), and the RdRp active site was indicated by a red star.
Extended Data Fig. 8 Structural comparison of filovirus VP35 ODs.
(a) The structure of tetrameric VP35 OD from EBOV L-VP35 complex. (b–d) The crystal structures of tetrameric REBOV VP35 OD (PDB 6GBQ), trimeric EBOV VP35 OD (PDB 6GBO) and trimeric MARV VP35 OD (PDB 5TOI). (e) The sequence alignment of VP35 OD regions from different filoviruses.
Extended Data Fig. 9 The entangled tetrameric VP35 wraps around L protein.
(a) EBOV L is shown in grey surface representation, and VP35 protomers are shown in cartoon representation with different colors. (b) Structural conformations of different VP35 protomers. (c) The major atomic interactions between VP35 protomers. The key interacting residues are shown in stick representation. Hydrogen bonds are presented in yellow dash lines, while the hydrogen bonds between the anti-parallel β strands are not shown (left side).
Extended Data Fig. 10 Binding interface between VP35 and EBOV L and its comparison with other nsNSV polymerase complexes.
(a) The binding footprints of VP35 protomers on EBOV L are indicated by black dash line. The interacting residues of L are colored and labelled according to the bound VP35 protomers, and the overlapping regions are shown in orange (VP35b/d) and pink (VP35c/d), respectively. (b) Comparison of the binding interfaces of EBOV L-VP35 and RSV L-P polymerase complexes. The L and P of RSV are colored in black and white, respectively. The L and VP35 of EBOV are colored as in Fig. 4. (c) Sequence alignment of critical interactive residues from L proteins among Mononegavirales. The L residues that formed hydrogen bonds with the residues from VP35 of EBOV and P of RSV are indicated by red and blue stars, respectively.
Extended Data Fig. 11 The inhibition mechanism of suramin against EBOV L-VP35.
(a) The chemical structure of suramin. Each benzene ring group is labeled by a unique symbol. (b) Inhibitory activity of suramin against EBOV L-VP35 complex was measured at enzymatic level. The RNA products were shown in urea-PAGE, and a series of concentration of suramin were added in the enzyme reaction system. The data shown are representative of three independent experiments using different protein preparations. (c) The 50% cytotoxicity concentration (CC50) of suramin was determined with the stable replicon cell. Each data point indicates the mean value of three independent experiments and the error bars represent standard deviation. (d–e) The structures of EBOV L-VP35 (d) and L-VP35-suramin (e) complex are shown in surface representation, and the NTP entry channel is indicated by a dashed circle. The suramin is stuck in the NTP entry channel to prevent NTP substrates reaching active site of RdRp. (f-g) The suramin could also hinder the activity of RdRp by occupying the spaces for product RNA strand. Cutoff view of the L-VP35-suramin complex overlapped with the modeled RNA (f). The tail part of suramin molecule would clash with nascent RNA product strand (g). The template (golden) and product (black) RNA strands are modeled based on the structure of rotavirus polymerase with in situ elongation conformation (PDB 6OGZ).
Extended Data Fig. 12 Structural comparison of EBOV L-VP35-suramin complex with SARS-CoV-2 RdRp-suramin and Murine Noroviruses (MNV) RdRp-suramin complex.
(a-b) Superimposition of the EBOV L-VP35-suramin with SARS-CoV-2 RdRp-suramin complex (PDB 7D4F) and MNV RdRp-suramin complex (PDB 3UR0) was performed based on the RdRp domain. A close-up view of suramin within the catalytic chamber in the SARS-CoV-2 RdRp-suramin complex (c), EBOV L-VP35-suramin complex (d) and MNV RdRp-suramin complex (e).
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1-6 and Supplementary Tables 1-3. Supplementary Figs. 1-5 are the cryo-EM analysis processes of structures determined in this paper and Supplementary Fig. 6 shows the uncropped gels, western blotting images and autoradiographs used for preparing Extended Data Fig. 1, 4 and 11. Supplementary Tables 1-2 contain cryo-EM data processing and refinement statistics for L-VP35 complex in two states and L-VP35-suramin complex and Supplementary Table 3 shows interactions between EBOV L protein and VP35.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, B., Peng, Q., Cheng, J. et al. Structure of the Ebola virus polymerase complex. Nature 610, 394–401 (2022). https://doi.org/10.1038/s41586-022-05271-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-022-05271-2
This article is cited by
-
Cryo-EM structures of Nipah virus polymerases and high-throughput RdRp assay development enable anti-NiV drug discovery
Nature Communications (2025)
-
Structural basis for Ebola virus nucleocapsid assembly and function regulated by VP24
Nature Communications (2025)
-
Structure of the measles virus ternary polymerase complex
Nature Communications (2025)
-
Structural basis of Nipah virus RNA synthesis
Nature Communications (2025)
-
Structural insights into the RNA-dependent RNA polymerase complexes from highly pathogenic Marburg and Ebola viruses
Nature Communications (2025)