Abstract
The integrity of the plasma membrane is vital for nearly all aspects of cell functioning1. Mechanical forces can cause plasma membrane damage2, but it is unclear whether there are large molecules that regulate the integrity of the plasma membrane under mechanical strain. Here we constructed a 384-well cellular-stretch system that delivers precise, reproducible strain to cultured cells. Using the system, we screened 10,843 small interfering RNAs (siRNAs) targeting 2,726 multipass transmembrane proteins for strain-induced membrane permeability changes. The screen identified NINJ1—a protein that was recently proposed to regulate pyroptosis and other lytic cell death3,4—as the top hit. We demonstrate that NINJ1 is a critical regulator of mechanical-strain-induced plasma membrane rupture (PMR), without the need for stimulating any cell death programs. NINJ1 levels on the plasma membrane are inversely correlated with the amount of force required to rupture the membrane. In the pyroptosis context, NINJ1 on its own is not sufficient to fully rupture the membrane, and additional mechanical force is required for full PMR. Our study establishes that NINJ1 functions as a bona fide determinant of membrane biomechanical properties. Our study also suggests that PMR across tissues of distinct mechanical microenvironments is subjected to fine-tuning by differences in NINJ1 expression and external forces.
Similar content being viewed by others
Main
The plasma membrane is the physical barrier separating the internal contents of the eukaryotic cells from the outside. It also functions as the structural support to house membrane-associated proteins—including sensors, receptors, channels, transporters and cytoskeletal components—that carry out essential cellular functions1. Damage to the plasma membrane compromises its integrity and leads to the ionic imbalance, loss of chemical and electrical gradient across the membrane, leakage of cellular contents and, in severe cases, cell death5,6. Mechanical stress is one of the major contributors to plasma membrane damage2. Physiological processes such as locomotion and resistance exercise cause microscopic tears on the membrane. This light damage activates an array of receptors, including ion channels and G-protein-coupled receptors, to initiate the downstream repair and remodelling pathways7. By contrast, severe membrane damage caused by pathological events such as trauma and exposure to penetrating sound waves may trigger inflammatory responses, leading to the recruitment of immune cells and the release of cytokines and chemokines2,5,8. At the cellular level, external forces, including compression, stretch and fluid shear stress, induce strain on the cellular membrane, cause membrane permeability changes through mechanically activated channels and pore-forming protein complexes, disruption in lipid bilayer and cytoskeleton, damage to membrane-associated proteins and, in extreme cases, membrane rupture8,9,10,11,12. Lytic cell death, which involves a full rupture of the plasma membrane, is the end point of most forms of cell death13. The disintegration of the plasma membrane and the resulting release of cellular contents, known as damage-associated molecular patterns (DAMPs), into the surroundings is instrumental for the recruitment of immune cells, development of inflammation, initiation of repairs or clean-up14. Mechanical strain has been implicated in affecting the plasma-membrane integrity in these processes5. However, how mechanical forces influence the PMR at the cellular level, and how it is regulated at the molecular level, is not fully clear. Here we set out to identify genes that are important for regulating the integrity of the plasma membrane under mechanical strain, using the previously proven approach of high-throughput genetic screening15.
The high-throughput cell stretch system
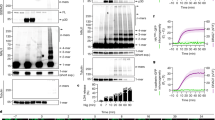
As commercially available mechanical stimulation systems do not provide high enough throughput for performing unbiased genetic screening, we developed a custom setup using optical-quality PDMS stretchable membranes affixed to bottomless 384-well assay plate frames. After sealing the plate, a vacuum is applied from the top, which pulls the PDMS membrane upwards and stretches the cells adhered to its surface (Fig. 1a). The response of the cells can be monitored from the bottom of the plate, either using a microscope in single-well operation mode, or using an image reader in full-plate mode (Fig. 1b and Extended Data Fig. 1a). Finite element analysis (FEA) of the PDMS membrane deformation under various vacuum levels showed that the system generates strain levels higher than 50% with moderate vacuum of −40 kPa (Extended Data Fig. 1b). Particle imagery analysis showed that the system induces 5–60% strain with vacuum varying from −5 to −40 kPa (Extended Data Fig. 1c–e) and, overall, these values are in agreement with the FEA analysis. A video of the system in operation is provided (Supplementary Video 1).
a, Schematic of the high-throughput stretch system. A vacuum applied to sealed wells deforms the PDMS membrane, stretching the adherent cells on top. b, The two operating modes of the system: single-well imaging through a microscope and full-plate imaging through a plate reader. c, Images of HeLa-YFP cells immediately before and 120 s after strain application. Scale bar, 150 µm. d, YFP-intensity traces before and after the strain application. Each trace represents data from a single well; 10 wells were analysed and the red trace is the average. e,f, Trypan Blue and DRAQ7/Hoechst staining of the cells after strain application. Scale bars, 100 µm. g, The workflow of the siRNA screen for regulators of strain-induced membrane damage. h, Overview of the primary screen hitpicking, covering 2,726 genes encoding multipass transmembrane proteins. Each dot represents one of 10,843 siRNAs; the red dots indicate primary hits (z score > 1.5). i, Final validation of the top 20 candidate and 4 control genes showed that NINJ1 was the sole hit. j, YFP quenching in HeLa-YFP cells transfected with pooled siRNAs against NINJ1 or scrambled control. n = 4 trials per group. Statistical analysis was performed using a two-sided unpaired Student’s t-test versus the scrambled control. k, Western blot analysis of endogenous NINJ1 in HeLa cells 48 h after siRNA transfection. l, Trypan Blue staining of the control and NINJ1-knockdown cells after mechanical strain. Scale bar, 100 µm. m,n, LDH release (m) and the percentage of DRAQ7+ cells (n) in the control and NINJ1-knockdown groups, with or without 50% strain for 5 s. n = 4–8 trials per group. Statistical analysis was performed using using two-way analysis of variance (ANOVA) with Bonferroni correction. Unless otherwise indicated, data are mean ± s.e.m. **P < 0.01 versus scrambled. The diagrams in a, b and g were created using BioRender.
Assessing the cellular response to strain
We reasoned that, when the integrity of plasma membrane is compromised and the permeability increases under strain, extracellular anions (such as chloride) will enter the cells, either through ion channels, pores or microscopic tears on the membrane. We therefore chose the highly sensitive YFP-quenching assay as the primary assay for the screen. In brief, a variant of YFP with elevated sensitivity to anions was used to visualize anion level changes inside the cell. The fluorescence intensity of the YFP decreases after binding to chloride due to a decreased quantum yield and shift in the emission spectrum16. We engineered a HeLa cell line stably expressing this anion-sensitive YFP, and monitored the fluorescence while applying a 5 s pulse of strain. At moderate strain levels (40% or lower), this led to a decrease in the YFP fluorescence intensity dependent on the amplitude of the strain applied (Extended Data Fig. 1f; the sharp decrease in the fluorescence during the application of the strain was an artifact resulting from cells going out of focus). The YFP quenching was inhibited by the non-specific chloride channel inhibitor DCPIB in a dose-dependent manner, indicating that, under these conditions, the anions enter the cells through ion channels (Extended Data Fig. 1g). At 50% strain, the quenching was robust and reached half of the original intensity at 120 s after stimulation (Fig. 1c,d and Supplementary Video 2). DCPIB was not able to inhibit the quenching at this strain level (Extended Data Fig. 1h), suggesting that the anions enter the cells through alternative paths, probably through microscopic tears or breaches in the plasma membrane formed under mechanical strain. To confirm this, we stained the cells with Trypan Blue, a dye that does not permeate the intact plasma membrane, and found a substantial percentage of cells with blue signal in the cytoplasm (Fig. 1e). Cells were also positively stained with DRAQ7, a nuclear dye that cannot enter the cell unless there are tears on plasma membrane (Fig. 1f).
To compare the effect of mechanical strain on YFP quenching with that of the commonly used cell death-inducing agents, we tested the following agents: listeriolysin O (LLO), TNF + SMAC mimetic + zVAD.fmk (TSZ) and RAS-selective lethal 3 (RSL3) (Extended Data Fig. 1i). LLO induced slow but significant quenching over time, eventually reaching the same level as the 50% strain treatment at 60 min, consistent with its mechanism of action of forming pores on the plasma membrane as direct paths for anions. TSZ-treated cells displayed baseline quenching kinetics and were not distinguishable from the control. This is because that TSZ mostly impact RIP3 signalling, and HeLa cells do not express RIP3, therefore no MLKL activation, plasma membrane permeabilization or cell swelling takes place17. Notably, RSL3 reduced the YFP intensity very quickly, possibly because it can inhibit GPX4-dependent reduction of peroxidized membrane lipids and affects the YFP signal, in addition to affecting membrane permeability18,19 (Extended Data Fig. 1i). Moreover, nigericin treatment also induced fast YFP quenching, which recovered slowly over time and returned to levels close to those of the groups treated with LLO and 50% strain at 60 min (Extended Data Fig. 1j), probably due to its acute modulation of anion channels and transporters as reported previously20,21. Despite the differences in kinetics, the effect of mechanical strain at rupturing levels is consistent with chemical inducers of cell death. Taken together, these results suggest that HeLa-YFP cells experienced mechanically induced PMR events at 50% strain. We therefore choose it as the optimal stimulus in the following genetic screen.
Screen for plasma-membrane integrity regulators
We reasoned that the proteins that have the most impact on the plasma membrane permeability and integrity are probably the membrane-integrated proteins. Using bioinformatics, we generated a list of 2,726 genes that have two or more predicted transmembrane segments and arrayed the corresponding 10,843 siRNAs against those genes onto 384-well stretch plates, with one siRNA per well. We then seeded the cells with transfection reagents and assayed stretch-induced YFP quenching after incubation for 72 h (Fig. 1g). The primary screen resulted in 399 hit wells, or a primary hit rate of 3.68% (Fig. 1h). Two additional rounds of reconfirmation screen were conducted. In the final round, we obtained a list of the 20 top candidate genes, tested them with siRNAs purchased from a separate source and identified NINJ1 as the sole hit of this screen (Fig. 1i). Note that knocking down the mechanically activated ion channels PIEZO1 and PIEZO2 had no impact on strain-induced YFP quenching. To confirm this, we knocked down PIEZO1 and PIEZO2 and measured the lactate dehydrogenase (LDH) release and the percentage of DRAQ7+ cells after strain application, and found no effect on PMR (Extended Data Fig. 2a,b). This is probably because PIEZO1 and PIEZO2 are highly sensitive mechanosensors functioning at physiological, sub-rupturing-strain levels22, while, under high strain, membrane rupture is mostly physical, and biological signalling has minimal roles.
NINJ1 regulates PMR under strain
To confirm the effect of NINJ1 on the strain-induced PMR, we transfected the HeLa-YFP cells with a pool of four siRNAs against NINJ1 to reduce the off-target effects. Consistent with the results of the high-throughput screen, knocking down NINJ1 significantly reduced YFP quenching after the strain application (Fig. 1j). Western blot confirmed an effective reduction in NINJ1 protein in those cells (Fig. 1k). Trypan Blue staining showed a substantial reduction in cytoplasmic blue signals (Fig. 1l), and both LDH release and the percentage of DRAQ7+ cells were significantly decreased, indicating fewer PMR events (Fig. 1m,n). To examine whether this applies to primary cells, we isolated bone-marrow-derived macrophages (BMDMs) from Ninj1−/− mice (Extended Data Fig. 3a,b). After application of 45% strain for 5 s, the Ninj1−/− BMDMs also exhibited significantly less LDH release and fewer DRAQ7+ cells than the wild type (WT) (Extended Data Fig. 3c,d). These results demonstrate that NINJ1 deficiency enhances the plasma membrane’s resistance to rupture by mechanical strain.
We next examined whether increased NINJ1 expression renders the membrane more susceptible to rupture under strain. Consistent with previous reports, overexpression of NINJ1 using a CMV promoter in HEK293T cells caused a high level of cytotoxicity3, with significantly higher LDH levels detected in the supernatant compared with the cells transfected with the vector control (Fig. 2a). We postulated that this could be due to the excessive NINJ1 production from the strong CMV promoter, making cells highly susceptible to rupture due to minor mechanical stress caused by routine cell culture handling. Indeed, analysis using quantitative PCR (qPCR) showed a 250-fold increase in NINJ1 mRNA at 24 h after transfection (Extended Data Fig. 4a), and western blot analysis showed a considerable increase in NINJ1 protein (Fig. 2b). To visualize the overexpressed NINJ1, we expressed a CMV-driven NINJ1–mCherry fusion protein in HEK293T cells and found that it predominantly localized to the plasma membrane and endoplasmic reticulum, and mCherry puncta were clearly visible, with some cells developing ballooned morphology 24 h after transfection (Extended Data Fig. 4b). These data support the notion that excessive NINJ1 expression could disrupt the integrity of the plasma membrane and cause cytotoxicity.
a, LDH release in HEK293T cells transfected with vector or CMV-driven NINJ1-IRES-mCherry was measured 24 h after transfection. n = 3 trials per group. Statistical analysis was performed using two-sided an unpaired Student’s t-test. b, Western blot analysis of NINJ1 in non-transfected, vector-transfected and CMV-NINJ1-transfected HEK293T cells. c, Western blot analysis of NINJ1 in HEK293T cells stably expressing TRE3G-NINJ1-IRES-mCherry induced with 0–300 ng ml−1 doxycycline (Dox) for 24 h; parental cells were used as controls. d, Confocal images of TRE3G-NINJ1-mCherry stable cells with or without 100 ng ml−1 doxycycline induction for 24 h. The arrowheads indicate fluorescent puncta. Scale bar, 10 µm. e,f, LDH release (e; n = 3–5 wells per group) and the DRAQ7+ cell percentage (f; n = 3–4 trials; 300–500 cells per group) 30 min after strain application in parental or NINJ1-inducible HEK293T cells pretreated with doxycycline. The duration of strain application was 5 s. g, Western blot analysis of endogenous NINJ1 in HeLa and ZOS cells. h,i, LDH release (h; n = 8–12 wells per group) and DRAQ7+ percentage (i; n = 3–6 trials; 300–500 cells per group) in HeLa and ZOS cells 30 min after strain. Statistical analysis was performed using two-way ANOVA with Bonferroni correction. j–l, Relative NINJ1 mRNA levels (j; n = 3 per group), LDH release (k; 50% strain; n = 6 trials per group) and the DRAQ7+ cell percentage (l; 50% strain; n = 3 trials; 300–400 cells per group) across human osteosarcoma cell lines. Statistical analysis was performed using one-way ANOVA with Bonferroni correction versus HeLa. m–o, Single-cell clones of 143B cells (n = 18) were analysed for NINJ1 mRNA using qPCR after treatment with 55% strain. m, Schematic of the experiment. LDH release (n) and DRAQ7+ (o) percentages were measured and correlated with NINJ1 expression (300–400 cells per clone). Unless otherwise indicated, data are mean ± s.e.m. The diagram in m was created using BioRender.
To quantify the relationship between NINJ1 expression level and membrane fragility, we engineered a doxycycline-inducible HEK293T cell line stably expressing NINJ1 (TRE3G-NINJ1). Western blot and qPCR confirmed dose-dependent induction of NINJ1 24 h after treatment (Fig. 2c and Extended Data Fig. 4c), with no apparent cytotoxicity observed at the top doxycycline dose tested (300 ng ml−1). We also generated a TRE3G-NINJ1-mCherry fusion cell line to visualize NINJ1 at lower expression levels. With no doxycycline added, the cells appeared healthy, showing low amounts of NINJ1–mCherry on the plasma membrane and in the endoplasmic reticulum due to leaky expression. With 100 ng ml−1 doxycycline, more mCherry signal was detected, but the cells remained mostly without herniation (Fig. 2d). Assessment of mechanical-strain-induced PMR after doxycycline treatment at various doses revealed that higher NINJ1 expression sensitized cells to rupture at lower strain levels. In cells treated with 300 ng ml−1 of doxycycline, LDH release and the DRAQ7+ cell percentage significantly increased at 45% strain, a level that had minimal effect on either parental cells or TRE3G-NINJ1-mCherry stable cells with no doxycycline treatment (Fig. 2e,f). Notably, overexpression of PIEZO1 or PIEZO2 in HEK293T cells had no impact on the PMR rate under strain (Extended Data Fig. 2c,d), underscoring the important role of NINJ1 in regulating PMR under mechanical strain.
To test whether the effect of NINJ1 on plasma-membrane fragility is a general, cell-type-autonomous phenomenon, we examined ZOS cells, an osteosarcoma cell line with higher endogenous NINJ1 compared with HeLa cells (Fig. 2g and Extended Data Fig. 4d,e). ZOS cells showed a significantly higher PMR rate beginning at 40% strain, with elevated LDH release and DRAQ7+ percentages at 50% strain (Fig. 2h,i), indicating greater susceptibility to rupture. We further extended this analysis to multiple osteosarcoma lines with varying NINJ1 mRNA levels and found that the PMR rate (as indicated by LDH release and DRAQ7+ cells at 50% strain) was correlated with NINJ1 expression, confirming that higher NINJ1 levels predict increased membrane fragility under mechanical stress (Fig. 2j–l).
Genetic background differences among cell lines could result in variations in cytoskeleton organization, extracellular matrix production and extracellular-matrix-binding protein expression, and this could substantially affect the mechanical properties of their plasma membrane in a complex way, potentially confounding the impact of NINJ1 on membrane fragility. To isolate NINJ1’s role, we derived 18 single-cell clones from the same 143B osteosarcoma parental line, measured their endogenous NINJ1 mRNA levels and assessed PMR under 55% strain. We found that the susceptibility to rupture of those clones under strain is correlated with their endogenous NINJ1 expression level (Fig. 2m–o). Taken together, these results show that NINJ1 is a critical, cell-type-autonomous regulator of plasma membrane integrity under mechanical strain.
NINJ1 lowers the mechanical threshold of PMR
At the cellular level, the mechanical integrity of the plasma membrane is influenced by a series of cellular components such as the phospholipid bilayer, membrane-integrated proteins and the cytoskeleton. We first checked the expression, distribution and organization of actin filament and microtubules, and found that they were not affected by different levels of NINJ1 expression (Extended Data Fig. 5a,b). Similarly, fluorescent probes for phosphatidylserine (PS) and phosphatidic acid (PA) detected no changes with varying NINJ1 expression levels (Extended Data Fig. 5c,d). We also evaluated PS flipping by staining the cells with annexin V, and found that no signal was observed with NINJ1 overexpression (Extended Data Fig. 5e,f). These results indicated that NINJ1 does not cause major changes in cytoskeletal or lipid composition.
Given that NINJ1 is a multipass membrane protein, we hypothesized that it may directly alter the biomechanical properties of the membrane. To test this, we used giant plasma membrane vesicles (GPMVs), which are largely free of cytoskeletal components23. In brief, HeLa cells overexpressing NINJ1–mCherry fusion protein were treated with N-ethylmaleimide (NEM) to induce blebbing, the resulting vesicles were picked up by a micropipette, which applied suction with an increasing amplitude until the vesicles ruptured. The lysis tension—the tension within the plasma membrane at the time of rupture—was derived from recorded parameters (Fig. 3a). We found that, with low NINJ1, vesicles resisted rupturing until high pressure; by contrast, with high NINJ1, vesicles ruptured at much lower pressure (Fig. 3b,c and Supplementary Videos 3 and 4). The lysis tension was inversely correlated with NINJ1 protein levels on the vesicle membrane, suggesting that high NINJ1 increases plasma-membrane fragility (Fig. 3d). Consistently, the NINJ1(K45Q) mutant, which has been reported to have reduced activity and cytotoxicity3,24, did not render the cells more susceptible to PMR under strain (Fig. 3d and Extended Data Fig. 6). Moreover, vesicles from cells overexpressing PIEZO1 did not show correlation between lysis tension and the amount of protein expressed either (Extended Data Fig. 2e). These results indicate that NINJ1 uniquely regulates plasma-membrane fragility by directly affecting the biomechanical properties of the lipid bilayer.
a, Schematic of the micropipette aspiration assay. HeLa cells expressing NINJ1–mCherry fusion were treated with 2 mM NEM to generate GPMVs, which were aspirated through a micropipette to determine the lysis tension. Components of the diagram were created by Z. Zhuoyi Xu and using BioRender. b, Representative images of GPMVs with high or low NINJ1–mCherry fluorescence levels collected during the aspiration protocol. GPI–eGFP was used as a membrane marker. Scale bar, 10 µm. c, Traces showing the volume changes in NINJ1high and NINJ1low GPMVs during the pressure ramping. d, Lysis tension of the GPMVs as a function of membrane NINJ1–mCherry fluorescence. GPMVs were generated from HeLa cells overexpressing WT NINJ1–mCherry (n = 30 vesicles), K45Q mutant NINJ1-mCherry (n = 8 vesicles) or GPI–eGFP alone (n = 10 vesicles) across three independent experiments. Unless otherwise indicated, data are mean ± s.e.m. Error bars that were too small, or out of the logarithmic scale, are not displayed.
NINJ1 weakens the membrane in lytic death
Originally identified as an adhesion molecule, NINJ1 has recently been implicated in the membrane rupture of pyroptotic cells, potentially through oligomerization and lipid bilayer disruption3,4,25,26,27,28,29,30. Given that we identified NINJ1 through a mechanical force-centric genetic screen, we tested whether physical force is a previously unidentified regulatory factor of PMR in NINJ1-mediated lytic cell deaths. We first treated THP-1 monocyte-like suspension cells with nigericin, which activates canonical inflammasomes to induce pyroptosis31. As expected, cells showed ballooning and membrane permeabilization, with a steady increase in LDH release and DRAQ7 uptake, reaching the maximum at 6 h (Fig. 4a,b). Native PAGE confirmed NINJ1 oligomerization (Extended Data Fig. 7a). Notably, most of the DRAQ7+ cells maintained a ballooned morphology without full PMR (Fig. 4a,b and Supplementary Video 5), even after 48 h (Extended Data Fig. 7b). This indicates that, in pyroptotic THP-1 cells, the breaches in the plasma membrane are large enough for LDH and DRAQ7 to pass through, yet the plasma membrane still has sufficient structural resilience to prevent a full rupture.
a, Representative images of THP-1 cells treated with 5 μg ml−1 nigericin (Nig). Plasma membranes were labelled with CellBrite (red); DRAQ7 (yellow) marks nuclei of permeabilized cells. b, Time-course of LDH release, the percentage of DRAQ7+ cells and the percentage of ballooned cells. c, Images of parental and NINJ1-KO THP-1 cells 6 h after nigericin treatment. Permeabilized nuclei (DRAQ7+Hoechst+) appeared white; intact nuclei (Hoechst+ only) appeared blue. d, Time-course of LDH release, DRAQ7+ cells and ballooned cells in parental and NINJ1-KO cells. e, The workflow of the experiment testing the effect of flow on PMR. The diagram was created using BioRender. f, Shear rates in various human vascular beds as previously reported31,32. g,h, Images (g) and quantification (h) of pyroptotic THP-1 cells after flow stimulation for 30 min. 5 μg ml−1 nigericin was added to cells 6 h before. The arrows in the left and right panels indicate fully ruptured cells (DRAQ7+ nuclei lacking surrounding CellBrite staining). i, Images of parental and NINJ1-KO THP-1 cells treated with 5 ng ml−1 nigericin for 6 h, then with flow at 2,073 s−1 for 30 min. Static cultures were used as controls. j, LDH release and the full PMR rate in parental and NINJ1-KO cells after nigericin and flow treatment. k, LDH release and the full PMR rate in parental and NINJ1-KO cells after intracellular LPS and flow treatment. l,m, Quantification of dsDNA released from pyroptotic THP-1 cells treated with nigericin (l) and LPS (m) and flow for 30 min. Data are mean ± s.e.m. from three independent trials per group. Image quantification was done with 250–300 cells per trial. Statistical analysis was performed using two-sided unpaired Student’s t-tests versus the control (b) and two-way ANOVA followed by Bonferroni correction (d and j–m) versus the parental + nigericin or parental + LPS group. For a, c, g and i, scale bars, 25 μm. *P < 0.05.
To investigate the role of NINJ1 in this setting, we generated a NINJ1-KO THP-1 cell line (Extended Data Fig. 3e,f). After nigericin treatment, KO cells showed around 50% less LDH release and reduced DRAQ7 positivity at 24 h, but both parental and KO cells maintained intact, ballooned membranes (Fig. 4c,d). This suggests that, although NINJ1 does contribute to membrane permeabilization, an additional factor, possibly mechanical force, is needed for full rupture. We next used a laminar flow system to apply mechanical stimulation to pyroptotic THP-1 cells (Fig. 4e). For suspension cells, the shear rate is the relevant measurement of mechanical force experienced by cells, in contrast to wall shear stress, which pertains to adherent cells. In vivo, shear rate ranges from 10 s−1 in the vena cava to around 2,000 s−1 in arterioles32,33 (Fig. 4f). We treated THP-1 cells with nigericin and, after 6 h, applied flow to them at shear rates from 414 s−1 to 2,073 s−1 for 30 min. At 414 s−1 (equivalent to large arteries, large veins and venules), most THP-1 cells maintained the ballooned morphology (Fig. 4g), and only around 20% of the DRAQ7+ cells had full rupture and showed bare nuclei (Fig. 4g (arrows)). Notably, at 2,073 s−1 (equivalent to arterioles), around 90% of DRAQ7+ cells underwent full rupture, exposing bare nuclei, with no surrounding plasma membrane (Fig. 4g (arrows)). The rupture rate increased with the corresponding shear rate (Fig. 4h), indicating that mechanical force is a crucial driver of full PMR.
We next tested whether NINJ1 affects flow-induced PMR in pyroptotic cells by treating the THP-1 cells with nigericin, then subjected them to flow ranging from 207 s−1 to 2,073 s−1 in shear rate. Parental cells showed an increasing percentage of full PMR events (represented by Hoechst+DRAQ7+ bare nuclei, in white) with shear, whereas NINJ1-KO cells had significantly lower LDH release and fewer fully ruptured nuclei. Notably, more than 60% of the NINJ1-KO cells maintained an intact ballooned plasma membrane even after flow stimulation as intense as 2,073 s−1 (Fig. 4i,j), confirming the role of NINJ1 as a critical determining factor of full PMR under mechanical strain during pyroptosis through canonical inflammasome induced by nigericin.
We also examined pyroptosis induced by intracellular lipopolysaccharide (LPS). Most of the cells swelled and maintained a ballooned morphology within 1 h after treatment with intracellular LPS (Extended Data Fig. 8a). LDH release and the percentage of DRAQ7+ cells also reached the maximum level at 1 h. Notably, there is no difference between NINJ1-KO and parental cells (Extended Data Fig. 8b). However, after flow stimulation, NINJ1-KO cells showed a significantly reduced full PMR rate compared with the parental cells across the shear rate range, especially between 1,244 s−1 and 1,659 s−1 (typical in arterioles and capillaries), suggesting that NINJ1 may regulate pyroptotic cell rupture in small vessels (Fig. 4k and Extended Data Fig. 8c).
We also investigated the role of NINJ1 in cell death induced by direct damage to the plasma membrane, such as those inflicted by pore-forming toxins like LLO. Treating WT THP-1 cells with LLO led to a significant increase in LDH release from 1 h onwards. By contrast, NINJ1-KO cells show lower LDH release after the same LLO treatment (Extended Data Fig. 9a,b). This phenotype was similar to that of the mouse primary BMDMs as previously reported3. After flow at 829 s−1 for 30 min, both genotypes exhibited high LDH release and full rupture rate, indicating that NINJ1 may not be the major determining factor of full PMR in cell death caused by direct membrane damage (Extended Data Fig. 9c,d).
Cell swelling by osmotic stress was recently reported to induce NINJ1 oligomerization and activation in adherent cells like mouse embryonic fibroblasts and mouse BMDMs17,34. In untreated THP-1 cells, hypotonic buffer alone caused minimal PMR in parental and NINJ1-KO cells across the osmotic pressure range (hypotonic, 215, 240 and 260 mOsm; and isotonic, 310 mOsm; Extended Data Fig. 10a,c,d). After nigericin treatment, the PMR rate increased with decreasing osmolarity (Extended Data Fig. 10b–d). NINJ1-KO cells showed slightly but significantly lower LDH release and percentage of DRAQ7+ cells under isotonic conditions. However, as the osmotic pressure decreased, the difference between NINJ1-KO and the parental cells started to diminish and, at 215 mOsm, the NINJ1-KO and the parental cells were indistinguishable (Extended Data Fig. 10c,d). Notably, the full PMR rate under osmotic stress was much lower than that under flow, and the difference between NINJ1-KO and parental cells was visible only at 215 mOsm (Extended Data Fig. 10e). Taken together, these data indicate that, similar to that in adherent cells, osmotic stress plays a role in NINJ1-mediated PMR in suspension cells, especially under mildly hypotonic conditions.
Given NINJ1’s role in regulating full rupture of plasma membrane, we hypothesized that it may be critical for allowing very large DAMPs such as DNA–histone complexes, organelles and intracellular crystals to be released into the extracellular space. To test this, we measured double-stranded DNA (dsDNA) in the supernatants of pyroptotic THP-1 cells after flow stimulation. With no induction of cell death, both NINJ1-KO and parental THP-1 cells released minimal dsDNA across the full range of flow intensities. After nigericin or intracellular LPS treatment, the release of dsDNA by the parental cells was increased as the flow shear rate increased. However, the NINJ1-KO cells showed significantly lower dsDNA release after flow stimulation, consistent with the observation they had significantly fewer DRAQ7+ bare nuclei, suggesting that the intact plasma membrane sphere prevented the majority of dsDNA from being released into extracellular space. These results support the idea that NINJ1 is required for releasing large intracellular contents by enabling full rupture under mechanical strain (Fig. 4l,m).
In summary, our results demonstrate that, in programmed cell death, NINJ1 is critical for modifying the biomechanical properties of the lipid bilayer and, when activated, renders the plasma membrane more fragile. However, NINJ1 alone is not sufficient for the full rupture of the membrane. Additional mechanical force is needed to execute full PMR, facilitating the release of large DAMPs for downstream signalling processes. Our results highlight a mechanosensitive checkpoint in PMR, suggesting that the interplay between NINJ1 and mechanical force provides a complex mechanism to regulate cell-death-associated PMR and DAMP release across tissues with diverse physical microenvironments.
Discussion
Here we designed and constructed a custom high-throughput cellular-stretch system from the ground up. Using a large-scale siRNA screen, we identified NINJ1 as a critical regulator of plasma membrane fragility under mechanical strain. We further demonstrated quantitatively that NINJ1 levels are inversely correlated with the tension required to rupture the membrane. Previous studies showed that NINJ1 could oligomerize into large structures such as filaments, branches or pores, which presumably leads to damage to the plasma membrane3,28,29. We suggest that high levels of NINJ1 protein, possibly in the oligomerized state, could alter the biomechanical properties of the plasma membrane, creating engineered weak points within it. However, this may not necessarily lead to membrane rupture on its own, and mechanical forces are needed for the full execution of PMR. Under strain levels that are normally innocuous to a cell with low or no NINJ1, but damaging to a cell littered with NINJ1 oligomers, larger tears may start to appear and propagate in the plasma membrane, eventually reaching a full rupture, akin to a cellular ‘tear along perforation’ mechanism. Notably, we show in our stretch experimental settings that NINJ1 regulates PMR without the activation of any cell death programs. This suggests that PMR might not be a direct consequence of programmed cell death per se, but an independent post-cell-death or cell-death-accompanying event, and mechanical force has a crucial role in the PMR process.
Exactly what triggers NINJ1 activation or oligomerization remains unclear. During cell death, the changes in the phospholipid composition of the lipid bilayer could cause the conformational change of the transmembrane segments of NINJ1, particularly in the first segment, promoting its oligomerization. The ballooning and stretching of the membrane may bring about curvature and tension changes that could affect the interaction between transmembrane segments of the monomers and favour the aggregated form. Indeed, a recent study showed that NINJ1 exists as dimer at a resting state24. We postulate that tension increases in the membrane may pull apart the NINJ1 dimers and favour the formation of a kink in transmembrane segment 1, therefore starting a chain reaction to push the balance towards NINJ1 oligomerization, forming large oligomer structures and weakening the plasma membrane. However, in our GPMV experiments, the mCherry-tagged NINJ1 displayed a uniform distribution of fluorescence along the membrane, and we did not observe apparent formation of fluorescent puncta while the membrane tension increased, suggesting either that tension may not be a causal factor of the punctum formation that we saw in the other experiments, or that it requires other co-factors that are absent in GPMVs. Owing to the optical limitation of the PDMS substrate used in the stretching experiments, super-resolution imaging was not possible, and we were not able to test whether increasing the membrane strain can induce the formation of the puncta in an intact cell. Future studies, possibly using other techniques such as super-resolution microscopy with new force application methods, or nuclear magnetic resonance, are needed to clarify the mechanism of NINJ1 activation.
The biological function of NINJ1 is relatively understudied. Initially identified as an adhesion molecule induced by nerve injury25, it was recently implicated in PMR and DAMP release in mouse models of infection and liver damage3,4,34,35. Our results show that elevated expression of NINJ1 weakens the plasma membrane and increases PMR probability under mechanical stress. During lytic cell death, stress can arise from osmotic swelling due to pore-forming proteins like GSDMD or lipid peroxidation. Swelling may also stress neighbouring cells through physical contact, promoting PMR. It is also highly likely that the pore-forming proteins themselves affect membrane fragility and work in conjunction with mechanical forces to drive PMR. In vivo, mechanical stress takes many forms. For example, blood flow exerts shear stress on monocytes while circulating in the blood vessels. The beating heart and breathing lungs apply tension to various cardiac and pulmonary cell types. Skeletal muscle cells experience tears during local motion2. These may have marked effects on PMR of the pyroptotic cells and DAMP release in their respective tissue microenvironment. In circulation, high-shear zones like capillaries may exacerbate rupture in dying immune cells, contributing to local inflammation. This may partly explain why the lung, with its fine vasculature and rhythmic stretching, is especially affected in sepsis. NINJ1 was also implicated in disease conditions involving mechanical stress, such as thoracic aortic dissection and traumatic brain injury36,37. Whether NINJ1, in conjunction with physical force, has roles in inflammation modulation in various mechanically active tissue microenvironments warrants further investigation.
Finally, we demonstrated the value of our high-throughput stretch system in revealing previously unreported biology in strain-induced PMR. Our system has the potential for broader impact in mechanobiology. With its vastly improved throughput over traditional means, it could be very useful in identifying the high-threshold mechanosensitive ion channels in DRG neurons that are critical for pain sensation in the absence of PIEZO238. It could also help to reveal the molecular identity of channels that mediate the mechanically activated anion currents. Beyond basic research, it may serve as an effective drug discovery platform, facilitating the identification of specific inhibitors of mechanically activated channels and receptors such as PIEZO1 and PIEZO2 and the TMEM63 family39, contributing to the development of therapies for mechanotransduction-related diseases.
Methods
Engineering of the high-throughput cellular stretch system and the consumables
The high-throughput cellular stretch system consists of two major components, the main unit and the executive terminals. The main unit houses an oil-free vacuum pump (Fujiwara, FUJ-V3) to provide vacuum drive up to −93 kPa. The modulation of vacuum is achieved using an electronic vacuum regulator (SMC, ITV2090-04N2N5) and the vacuum level is monitored by an in-line high-precision digital pressure switch (SMC, ZSE20A-T-C6H-J). A custom user interface was written using C# and enables the user to set the intensity, amplitude and frequency of the stimulation through a touchscreen. The electronic governor performs closed-loop calculations after receiving commanded vacuum parameters from the user interface, and uses the pressure switch’s readings to generate corresponding signals for the vacuum regulator, which functions to achieve stepless control of vacuum in proportion to electrical signals, thereby modulating the levels to match commanded parameters. The real-time vacuum levels are recorded by a pressure switch and are instantaneously displayed on the interface for the user to monitor the operation of the system.
We designed two types of executive terminals for the main unit—one single-well vacuum head intended to use with microscopes and one whole-plate vacuum manifold intended for plate readers (such as Molecular Devices FLIPR). To construct the body of single-well head, a 50 mm diameter brass cylinder stock was cut to 15 mm in heigh using an electric bandsaw, and was manually machined on a lathe to level out the top and bottom surfaces. A 4.0 mm through hole was drilled into the centre and a quick-connect push fitting (SMC, KQ2R04-06) was glued in by epoxy. The brass cylinder then was plasma treated and two silicone rubber rings (25 mm outer diameter, 15 mm inner diameter; and 12 mm outer diameter, 3.5 mm inner diameter) were affixed to the bottom surface (stem-side of the fitting). The head connects to the main unit using a 4.0 mm outer diameter, 2.5 mm inner diameter polyurethane tubing (SMC, TU-0425). The weight of the head assembly (~280 g) helps to seal the wells when resting on top of the assay plate.
The whole plate manifold was manufactured on a CNC bed mill (Kent Industrial USA, KVR-4020A) using aluminium alloy. A 1/8 inch threaded hole was added to the centre of the manifold and an 8-mm-outer-diameter quick-connect push fitting (SMC, KQ2H08-U01A) was fitted. A baffle insert was added to the interior of the manifold to smooth out the air flow for better aerodynamics. Six locator tabs were incorporated to the frame to facilitate the alignment of the manifold to the assay plate. All aluminium parts were anodized to a matte black finish, and fasteners were painted black to reduce light scattering in the imaging chamber of the plate reader. A silicone rubber seal was frictionally fitted to the grove machined in the frame to allow easy disassembly for cleaning and maintenance.
Stretchable PDMS-bottom 384-well assay plates were developed and manufactured in house from bottomless 384-well plate blanks (Greiner Bio-One, 781000-06). The plate blanks were plasma-treated for 5 min, 50% power at a vacuum of 0.2 mBar using a plasma surface treater (Plasma Technology, SmartPlasma 10). Next, a 200-µm-thick optically clear PDMS membrane was affixed to the bottomless 384-well plate using pressure-sensitive adhesive. The plates were stored in dark at room temperature. Before each use, the plates were exposed to ultraviolet irradiation in the biosafety cabinet (NuAire, NU-543) for 30 min.
Estimation of the strain by FEA
To gain a better understanding of the membrane deformation in 3D, we used FEA to model the assay plate with stretchable membrane under vacuum using SimScale CAE cloud analysis platform (SimScale). Only one well was modelled as a representation of the entire plate to assist in computational speed. A second-order finite mesh with 15,657 tetrahedral elements and 28,268 nodes was generated with an automatic mesh generator. The PDMS membrane material was considered incompressible, with a Young’s modulus of E = 1.40 MPa, Poisson’s ratio ν = 0.49 and density ρ = 1120 kg m−3. The bottomless 384-well polystyrene assay plate blank adhered to the PDMS membrane was modelled with a Young’s modulus of E = 1.8 GPa, Poisson’s ratio ν = 0.35 and density ρ = 1200 kg m−3. Fixed support boundary conditions were applied to the exterior walls of the single-well assay plate to represent the connecting, adjacent well walls in a full 384-well assay plate. To simulate the various vacuum levels, negative pressure conditions ranging from −10 to −40 kPa were assigned to the top surface of the PDMS membrane inside the well and the simulation was executed and results saved.
Estimation of the strain by particle imagery analysis
The particle imagery method was used for estimating the strain of PDMS membrane on the bottom of the assay well. In brief, we dispensed a suspension (0.01%, w/v) of green fluorescent polystyrene beads of 5 µm diameter (Tianjin BaseLine, 7-3-0500; excitation, 488 nm; emission, 518 nm) into the wells of the stretch plates and let them settle and adhere to the bottom. On an inverted fluorescence microscope (Olympus, BX63F), we first located the centre of the well and captured still images of beads at resting, non-stretched state, and recorded the z-axis position. We then applied vacuum and held at designated intensities, captured the bead positions and recorded their z-axis positions at stretched state. The strain levels of the PDMS membrane were estimated using the following formula:
where d is the distance of a given bead from the centre of the well at the resting state and d′ is the distance at the stretched state. d and d′ were measured from images take before and during the stretch respectively. h is the height difference between the bead and the top centre of the stretched PDMS dome formed under vacuum, and was derived from z-axis position readings supplied by the microscope stage controller.
Animals
All of the animals used in this study were maintained under specific-pathogen-free conditions and cared for in accordance with National Institutes of Health guidelines. Mice were housed in individually ventilated cages in a room under a 12 h–12 h light–dark cycle with ad libitum access to food and water. Rooms were controlled at 19–23 °C with 40–60% humidity. All experiments involving animals were carried out with experimental protocols and procedures reviewed and approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University (SYSU-IACUC-2023-000575). Ninj1−/− mice were purchased from GemPharmatech (T034357). We obtained homozygous knockout mice (Ninj1−/−) by intercrossing heterozygous mice and compared them with WT littermates in all of the experiments. About 15.8% of the Ninj1−/− mice develop hydrocephalus (large head and low body weight) and die after 4 weeks of age (Extended Data Fig. 3a). Ninj1−/− mice with no hydrocephalus syndrome at 12−16 weeks old were used in the experiments. Knockout of Ninj1 has been confirmed in the spleen by using qPCR with reverse transcription (RT–qPCR; Extended Data Fig. 3b).
Plasmids, chemicals and reagents
Complementary DNA (cDNA) for human NINJ1 and NINJ1K45Q mutant3 were purchased from BGI Genomics. The NINJ1 and NINJ1K45Q mutant cDNAs were inserted into the CMV promoter vector with PVAT-mCherry for transient expression in HEK293T cells and HeLa cells and the pLVX TRE3G vector with PVAT-mCherry for stable expression in HEK293T cells, respectively. The plasmid pCDNA3.1-eYFP-H148Q-I152L was obtained from pCDNA3.1-Ano1-m-T2A-EYFP-H148Q-I152L (P1668) through homologous recombination to construct HeLa-YFP cells16. pcDNA3.1(-)-PIEZO1-IRES-mRuby2-Flag, pcDNA3.1(-)-PIEZO1-mRuby2 fusion, both gifts from B. Xiao, and pFastBac1-PIEZO2-3C-GFP, a gift from Y. Zhang, were used for transient PIEZO1 and PIEZO2 overexpression in HEK293T cells in stretching and GPMV experiments.
Cell culture products were all purchased from Gibco. Poly-d-lysine was used (Thermo Fisher Scientific, A3890401) for 384-well-plate coating. The high-iodine solution for the YFP quenching assay contains 100 mM NaI (Macklin, S817516-100g), 40 mM NaCl (Aladdin, C111535-500g), 5 mM KCl (Aladdin, R100832-250mg), 1 mM CaCl2 (Sigma-Aldrich, C5670-100g) and 20 mM HEPES (Solarbio, H1095-100) with the pH adjusted to 7.4 using NaOH (LEAD-BIO, LD-8013-100ml). The chloride channel inhibitor DCPIB was purchased from Cayman Chemical (34064-5mg). siRNAs were purchased from Genepharma (A10001), and Lipofectamine RNAiMAX (Invitrogen, 13778-150), FuGENE HD Transfection Reagent (Promega, E2311) and Opti-MEM (Thermo Fisher Scientific, 31985070) were used for transfection of cultured cells. Green Lumisphere fluorescent beads (diameter, 5 µm; BaseLin) were used for imagery analysis to derive the strain of PDMS membrane under vacuum. Nigericin (Selleck, S6653) was used to induce cell pyroptosis. Doxycycline (Selleck, S5159) was used for the induction of TRE3G-regulated plasmid expression. CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, G1780) was used for LDH assay. RSL3 (Selleck, S8155) was used for ferroptosis induction. TSZ (Selleck, 187389-52-2) was used for necroptosis induction. LLO (Abcam, ab83345) was used as an exogenous pore-forming toxin. Hoechst (Thermo Fisher Scientific, 62249) was used for cell nucleus staining. Trypan Blue (Gibco, 15250061) and DRAQ7 Dye (Invitrogen, D15106) were used to indicate loss of plasma membrane integrity and cell death. CellBrite Fix 555 (Biotium, 30088) was used for labelling the cell plasma membrane. The PicoGreen dsDNA Quantification Kit (Solarbio, P9740) was used to quantify dsDNA in the supernatant. Histopaque-1077 (Sigma-Aldrich, 10771) was used for isolating peripheral mononuclear blood cells through density-gradient centrifugation. Annexin-V–FITC (MultiSciences, AP101-100) was used to monitor PS on the outer leaflet of the plasma membrane. Stocks of chemicals were reconstituted in DMSO (AAT Bioquest, ST038) and stored at −20 °C unless stated otherwise. Nigericin were dissolved in 100% ethanol and stored at −80 °C. LLO were stored at −80 °C.
DNA and siRNA transfection
Cells were plated into a 24-well plate, reaching a cell confluence of approximately 70–80% before transfection. For transient plasmid transfection, FuGENE HD Transfection Reagent was used according to the manufacturer’s instructions. Plasmids was introduced into the culture system at a concentration of 1 μg ml−1. For stable expression, lentiviral plasmids harbouring the desired gene were first transfected into HEK293T cells together with the packing plasmids pSPAX2 and pMD2G with a ratio of 4:3:1. The supernatants were collected 48 h after transfection and used to infect HEK293T cells for another 48 h. mCherry-positive infected cells were sorted by flow cytometry (BD Biosciences FACSAria II). For siRNA transfection, Lipofectamine RNAiMAX was used at the recommended concentrations according to the manufacturer’s instructions. siRNA was added to the culture system at a concentration of 20 pM.
Cell culture
HeLa (CCL-2) and HEK293T (CRL-3216) cells were obtained from ATCC. HeLa YFP cells were isolated as single-cell-derived clones through flow cytometry sorting after transfection with the halide-sensitive YFP (with H148Q and I152L mutations) plasmid. THP-1 cells were sourced from the Cell Bank of the Chinese Academy of Sciences (SCSP-567). The following osteosarcoma cell lines were from ATCC: U2OS (HTB-96), 143B (CRL-8303), HOS (CRL-1543) and SaoS (HTB-85). ZOS cells were generated in the Zou Lab at First Affiliated Hospital of Sun Yat-sen University. All of the cell lines are frequently checked by morphological features and functionalities, but have not been subjected to authentication by short-tandem-repeat profiling. All of the cell lines have tested negative for the presence of mycoplasma by a detection kit (Vazyme, D101-02). The HeLa, HEK293T and the osteosarcoma cell lines were grown cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, C11330500BT) containing 4.5 mg ml−1 glucose, supplemented with 10% FBS (ExCell Bio, FND500) and 1% penicillin–streptomycin (Gibco, 15140122). THP-1 cells were maintained in RPMI-1640 medium (Gibco, C22400500BT) supplemented with 10% FBS, 1% penicillin–streptomycin and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich, M3148-25). For the experiment, THP-1 cells were cultured in RPMI 1640 supplemented with 1% fetal bovine serum, 1% penicillin–streptomycin and then plated at approximately 2 × 105 cells per ml in 150 µl in 96-well plates. For pyroptosis induction, THP-1 cells were treated with nigericin (5 µg ml−1 for WT THP-1 cells and 20 µg ml−1 for engineered NINJ1-KO and the corresponding parental cell, Selleck, S6653) and analysed at various timepoints. The supernatant was collected for LDH assay. All media were supplemented with 10% (v/v) fetal bovine serum (FBS) and 2 mM l-glutamine. All cells were kept in a humidified incubator set at 37 °C with 5% CO2, with medium refreshment carried out every other day. To obtain single-cell-derived clones of the 143B cell line, parental cells were dissociated from culture vessels and made into a single-cell suspension, then sorted into individual wells of a 96-well plate using a cell sorter (BD FACSAria Fusion) and subsequently cultured for 2 weeks before further study.
YFP-quenching assay
HeLa-YFP cells in a 384-well plate were washed and maintained in 30 μl per well wash solution: 140 mM NaCl, 5 mM KCl, 1 mM CaCl2 and 20 mM HEPES, with the pH adjusted to 7.4 using NaOH. Then, 2 min before the start of the stretch experiment, a 30 μl per well high iodide solution (100 mM NaI, 40 mM NaCl, 5 mM KCl, 1 mM CaCl2 and 20 mM HEPES, with the pH adjusted to 7.4 using NaOH) was added to the wells. A 20–30 s baseline fluorescence was recorded before applying stretch stimulation, with recording continuing for 2 min. The YFP fluorescence 2 s before the stretch stimulation (F0) and at the end of recording (F) was used to quantify the response to the stretch stimulation, termed the quenching score, as follows16:
Assay of mechanically and chemically induced cell death and PMR
For mechanically induced cell death experiments, cells were seeded in a poly-d-lysine-coated PDMS-bottom 384-well plate, achieving approximately 70–80% confluence before experimentation. For cells with genes driven by the TRE3G inducible promoter, doxycycline (Selleck, S5159) at the specified concentration was added to the culture medium to induce gene expression. The cells were then incubated with 1.6 μM Hoechst 33342 (Invitrogen) and 1.5 μM DRAQ7 Dye (Invitrogen, D15106) in 1× Hanks balanced salt solution (HBSS) for 15 min at room temperature. Then, 2 h after stretch stimulation, cells were imaged using an inverted fluorescence microscope (Olympus), and 40 µl of the supernatant was collected for the LDH-release assay. Imaging was performed in the DAPI, Cy5 and bright-field channels. Image analysis was performed using OlyVia software and ImageJ. For chemical-induced cell death experiments, HeLa-YFP cells were seeded into a 384-well plate, washed and maintained in 30 µl of wash solution per well. After 1 h of incubation with cell death agents, a 10-min baseline fluorescence measurement was recorded. Next, 30 µl of iodide solution (40 mM NaI, 100 mM NaCl, 5 mM KCl, 1 mM CaCl2, 20 mM HEPES, pH adjusted to 7.4 with NaOH) was added to each well, and fluorescence recording continued for an additional 50 min.
High throughput siRNA screening
The 384-well PDMS stretch plates were labelled with a microplate labeller (Agilent, G5581AA) to facilitate data processing and management. Then, 24 h before siRNA transfection, plates were coated with poly-d-lysine (Advanced BioMatrix, 5049). Coating was done by dispensing 10 µl 0.1 mg ml−1 poly-d-lysine solution into each well by a high-throughput robotic liquid handler, and then incubating at room temperature for 2 h. The plates were then washed with double-distilled H2O and air-dried in a biosafety cabinet (NuAire, NU-543) for 6 h then kept in a 4 °C cold room overnight. On the day of transfection, the siRNA library was arrayed from master plates to stretch plates using an automated liquid handler (Bravo, Agilent) by transferring 1 µl of 20 µM siRNA stock solution for each well. A master mix of Lipofectamine RNAiMAX/Opti-MEM (ratio, 8 µl:1 ml) was made and dispensed to the wells at 25 µl per well and incubated at room temperature for 20 min. Next, 25 µl of the HeLa-YFP cell suspension (450,000 cells per ml in 20% FBS) was dispensed into each well. The plates were shaken at 6,000 rpm for 10 s on microplate shaker and incubated at 37 °C with 5% CO2. The next day, a medium change was performed by taking out 35 µl old medium and replenishing with 60 µl fresh medium. Then, 72 h after transfection, the cells were washed three times with 1× HBSS on a microplate washer (BioTek, ELx450CW), and moved to assay stretch-induced YFP quenching on a plate reader (Molecular Devices, FLIPR Tetra). A 30 s baseline fluorescence was recorded at 1 frame every second, and a single stretch of 5 s in duration at 50% strain was applied to the cells, while the FLIPR was continuously recording. Then, 60 s later, the recording frequency was reduced to 1 frame every 5 s until the recording ended at 150 s (or manually stopped as required). Raw data were acquired and data sequence files were exported using ScreenWorks v.4.2.1.2 (Molecular Devices) for further analysis. The YFP intensity at end of the recording (150 s) was first normalized to the mean intensity of the whole plate, under the assumption that most siRNAs will not have effects on the quenching response, yielding a value Fnormalized for each well. Well values from all plates were then spliced together to form an overall dataset, and the mean of all datapoints (Foverall_mean) and standard deviation (s.d.overall) were derived. A z score for each well, representing how far away this well is from the bulk of the data, was calculated as follows:
We used the z score of >1.5 as the cut-off line for the primary screen data, yielding a list of 755 primary hit siRNAs. We then collected siRNAs against these genes for a reconfirmation screen, further reducing the size of the hit list by applying more stringent cut-off line. From the second round of screen onwards, genes were required have multi-hits, that is, two or more siRNAs working, to be selected to go forward. In the final round, experiments were performed using siRNAs against 20 genes purchased from a separate source (from a retail catalogue instead of from a library catalogue of Dharmacon) to increase the confidence of the screen.
Western blots
Samples were lysed in RIPA buffer (Beyotime, P0013E) with protease inhibitor PMSF (Beyotime, ST506) at 4 °C for 30 min. The supernatants were collected after centrifuging at 13,800g at 4 °C for 30 min and the protein concentration was measured (Beyotime, P0010). Then, 10 µg protein was mixed with SDS–PAGE sample buffer (Beyotime, P0286) and run in 12.5% SDS–PAGE gel (EpiZyme, PG113). Human NINJ1 antibody (1:200, R&D Systems, AF5105), anti-human GAPDH antibody (1:10,000, Proteintech, 60004-1-LG), HRP-conjugated goat anti-mouse IgG (1:5,000, Proteintech, SA00001-1) and HRP-conjugated rabbit anti-sheep IgG (1:5,000, Abclonal, AS023) were used for blotting. The gel was visualized using the FDbio-Dura ECL chemiluminescence kit (FDbio Science, FD8020). The gel images were acquired using the CCD imager (Cytiva, Amersham ImageQuanta 800). Images were quantified using ImageJ v.1.52p greyscale analysis. For the native gel experiments, sample preparation was performed according to the manufacturer’s recommendations. In brief, the cell samples were digested and centrifuged at 100g for 2 min, and then 1× sample buffer containing 1% digitalin was added. After gentle pipetting five times, the samples were centrifuged at 20,000g at 4 °C for 30 min and the supernatants were collected. 3–12% NativePAGE Bis-Tris Mini Protein Gels (Thermo Fisher Scientific, BN1002BOX) was used. Anti-human NINJ1 antibody (R&D, AF5105) was used at 1 µg ml−1 to visualize the protein.
RT–qPCR
For RT–qPCR analysis, total RNA was extracted using the EZ-press RNA Purification Kit (EZBioscience, B0004D). Subsequently, cDNAs were generated using the PrimeScript RT Reagent Kit (Takara, RRO47A). RT–qPCR was performed using TB Green Premix Ex Taq II (Takara, RB820B) on a real-time PCR system (Thermo Fisher Scientific, QuantStudio). The PCR conditions included an initial denaturation step at 95 °C for 30 s, followed by 40 cycles of amplification at 95 °C for 5 s and 55–60 °C for 30 s. The mRNA expression of target genes was assessed, with 18S serving as the internal reference gene. Relative expression levels were calculated using the \({2}^{\Delta \Delta {C}_{{\rm{t}}}}\) method40.
BMDM isolation and culture
Primary BMDMs were prepared from 8–12-week-old mice (C57BL/6 background, both gender) according to a standard procedure as previously described41,42. For each experiment, four mice were pooled to prepare the BMDMs for assaying the cell death responses. Bone marrow cells from WT or Ninj1−/− mice were differentiated into macrophages in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS, Capricorn Scientific) and macrophage colony stimulating factor (M-CSF, day 0: 10 ng ml−1; day 3: 20 ng ml−1, PeproTech, 315-02) at 37 °C, 5% CO2 for 7 days.
Immunofluorescence of cytoskeleton and phospholipids
HEK293T cells were seeded onto 96-well plates with a glass bottom and transfected with TRE3G-hNINJ1-IRES-mCherry plasmid at 20 ng per well. After 24 h, doxycycline in a series of doses was added to induce NINJ1 expression for 8 h. Cells were then fixed with 4% PFA and stained for F-actin (AAT Bioquest, 22661, 1:500) and β-tubulin (CST, 2128, 1:500), respectively. HRP-conjugated goat anti-rabbit IgG (Proteintech, SA00001-2, 1:5,000) was used as the secondary antibody and images were collected using a confocal microscope (Olympus, FV3000). Quantification of the average F-actin and β-tubulin fluorescence intensity, as well as average branch length of microtubules was done using OlyVia (Olympus) and Image J. Cells with mCherry fluorescence were selected in doxycycline-induced groups for analysis, and cells in the control group were randomly selected. For analysis of phospholipid expression and distribution, HEK293T cells were co-transfected with lipid probes and TRE3G-NINJ1-IRES-mCherry plasmids for 24 h. Cells were then passed to 24-well confocal plates and doxycycline was added for 8 h. After induction, cells were incubated with 1.6 μM Hoechst 33342 (Invitrogen) and 1.5 μM DRAQ7 (Invitrogen, D15106) in 1× HBSS for nuclear and dead cell labelling. For the annexin V experiment, an additional 1× annexin-V–FITC (MultiSciences, AP101-100) was added to the solution, and the mixture was incubated for 15 min at room temperature. Cells were then imaged using a confocal microscope (Olympus, FV3000), configured with the following channels for image collection: DAPI for Hoechst, FITC for PS-probe, PA-probe or annexin-V–FITC, Alexa Fluor 594 for mCherry and Cy5 for DRAQ7. Image analysis was performed using OlyVia (Olympus) and ImageJ.
GPMV preparation and micropipette aspiration on GPMVs
HeLa cells (ATCC, CCL-2) were grown in DMEM high-glucose medium (Gibco, 11965118) supplemented with 10% FBS and 1% penicillin–streptomycin to approximate 50–70% and transfected with plasmids coding hNINJ1–mCherry fusion protein, or hPIEZO1–mRuby using TransIT-X2 (Mirus Bio, MIR 6004). Then, 24 h after the transfection, cells were washed with PBS twice and GPMV buffer (10 mM HEPES, 150 mM NaCl, 2 mM CaCl2, pH 7.4) twice23. The transfected cells were then incubated with 2 mM NEM (Pierce, 23030) in GPMV buffer for 2 h at 37 °C. After 2 h of incubation, the supernatant was transferred to a glass-bottom dish (Cellvis, D35-14-1.5-N) that was coated with 1 mg ml−1 BSA (Gibco, 16170086) for 15 min at room temperature. Micropipettes were prepared by pulling borosilicate glass capillaries (WPI, TW100-4) using a micropipette puller (WPI, PUL-1000). Pulled micropipettes were then cut and bent using a Microforge Controller (WPI, DMF1000). PEG-silane (Gelest, 65994-07-2) was used to modify the micropipette surface to remove possible attachments of the membrane. The pressure in the micropipettes was controlled by a microfluidic system (FLUIGENT, ESORT-PCK01), which was manipulated remotely by OxyGEN software. GPMVs were aspirated and lifted from the bottom of the dish by the micropipettes before starting the experiments. During the experiments, the pressure was increased stepwise at a rate of 10 Pa per 30 s. The volume of each GPMV throughout the aspiration process was tracked continuously. During the application of suction, the vesicle volume decreases very slowly primarily due to buffer evaporation. The baseline evaporation rate was first established by measuring control vesicles. When the vesicle membrane developed large leak or rupture, the vesicles either disappeared completely or abruptly transitioned (within one frame) from a near-spherical shapes to cylindrical ones that fit into the pipette tip, after which they were drawn inside. The lysis tension was defined as the tension value at the point at which the vesicle’s volume decreased beyond what was expected from evaporation alone.
Generation of NINJ1-KO THP-1 cells
Generation of knockout cell lines using CRISPR–Cas9 technology was described previously42. In brief, two gRNAs, 5′-GCTGGGGCCCTGTTCCACGA-3′ and 5′-ATGGAGATGAGGACCACCAG-3′, for NINJ1 were cloned into the gRNA-expression plasmid lentiGuide-Puro (Addgene, 52963). Transient transfection was performed using the JetPRIME kit (Polyplus) according to the manufacturer’s instructions. For stable expression, the lentiGuide-Puro or lentiCas9-Blast (Addgene, 52962) plasmids were first transfected into HEK293T cells together with the packing plasmids psPAX2 (Addgene, 12260) and pMD2.G (Addgene, 12259) at a ratio of 5:3:2. The supernatants were collected 48 h after transfection and used to infect THP-1 through centrifugation at 800g for 90 min in the presence of polybrene. After approximately 18 h of incubation, all infected cells were centrifuged, and the medium was changed to fresh complete medium. The cells were selected by treatment with 2 μg ml−1 puromycin or 30 μg ml−1 blasticidin before flow sorting. Then, 5 days after infection, puromycin- or blasticidin-resistant live cells were sorted into single clones using a cell sorter (BD FACSAria II). The single clones were cultured in 96-well plates for another 10–14 days (or a longer time depending on the cell growth rate). The genotype of the knockout cells was determined by Sanger sequencing.
LDH-release assay
LDH in cell culture supernatant were analysed using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, G1780). The sample absorbance at 490 nm was measured by a microplate reader (Thermo Fisher Scientific, Varioskan LUX). LDH release was normalized to the untreated and 100% lysis control. LDH release was calculated as follows:
dsDNA-release assay
The concentration of dsDNA in the cell culture supernatant was quantified using the PicoGreen dsDNA Quantification Kit (Solarbio, P9740). The fluorescence intensity was recorded using a microplate reader (Thermo Fisher Scientific, Varioskan LUX; excitation, 488 nm; emission, 520 nm). The dsDNA concentration was determined by comparing the fluorescence values to a standard curve generated by dsDNA samples supplied with the kit.
Microscopy imaging of cell membrane morphology and nucleus staining
To examine membrane and nucleus morphology, cells were treated as indicated for static image capture or time-lapse imaging with an inverted fluorescence microscope (Olympus, BX63F). All image data shown are representative of at least three randomly selected fields. Time-lapse imaging was captured using the same microscope with the optional environmental controller and gas mixer to maintain cells at 37 °C and 5% CO2. Video recording started after addition of nigericin and was recorded with 1 min intervals overnight. Manual focal adjustments were performed as needed throughout the imaging process. THP-1 cells were prestained with CellBrite Fix 555 (1:1,000, Biotium, 30088) to visualize cell plasma membrane. After the pyroptosis induction, the THP-1 cells were stained with DRAQ7 dye (1:200) and Hoechst 33342 (1:1,000). Cells were imaged with an inverted fluorescence microscope (Olympus, BX63F). Images were taken in the preset TRITC, DAPI, Cy5 and bright-field channels. Image analysis was performed in OlyVia and ImageJ software.
Estimation of the shear rate
The estimation of the shear rate in the flow experiment using Ibidi system was based on following assumptions: (1) the culture medium is an ideal Newtonian fluid with constant viscosity; (2) the flow is steady and laminar; (3) the elasticity of the plastic syringe and silicone tube is negligible. Under these conditions, the velocity of the fluid in the centre of the tube is highest, where its velocity is zero at the surface of the tube wall (no-slip condition). Thus, the velocity gradient, or shear rate \(\dot{\gamma }\) is:
where u is the fluid velocity and r the radius of the tube43. Poiseuille’s law is applied to determine the shear rate as follows:
where Q is the volumetric flow rate and d is the tube diameter.
Fluid flow stimulation of THP-1 cells
Flow-induced shear stress stimulation of THP-cells was performed using the pneumatic-driven flow system (Ibidi, 10902) with the paired RED tubing set (Ibidi, 10962), connected with a coupler, without a channel slide installed. THP-1 cells were transferred into the flow chamber using a large-orifice transfer pipette and suspended in culture medium to a density of approximately 3 × 105 cells per ml. The dose and treatment time of nigericin for different cell lines were optimized. For experiments with WT THP-1 cells, 5 µg ml−1 nigericin was used to treat the cells for 2 h before the onset of the flow. For NINJ1-KO cells and the corresponding parental cells, 20 µg ml−1 nigericin was used to treat the cells for 6 h before flow stimulation. All flow stimuli were 30 min in duration. Cells were imaged for morphology analysis using various membrane and nuclear stains, and the supernatants were collected for further experiments. The instrument-indicated flow rate, the converted volumetric flow rate and the shear rate calculation used in the current study is listed in Supplementary Table 3.
LPS electroporation of THP-1 cells
To stimulate noncanonical inflammasome activation, LPS was electroporated into THP-1 cells using the Neon Transfection System (Invitrogen) and Neon Transfection System 100 µl Kit (Invitrogen, MPK10096) according to the manufacturer’s instructions. In brief, 2 × 106 cells were transfected with 500 ng LPS (O111:B4, Sigma-Aldrich, 2630) by double pulse (1300 V, 10 ms). After the delivery of the electric pulse, cells were immediately transferred into prepared culture plate or flow chamber containing prewarmed RPMI 1640 with 1% FBS without PS. Cells were imaged and the supernatant was collected for LDH assay at different timepoints. For the mechanical-stimulation assay, 1 h after the LPS electroporation, flow was applied to THP-1 cells at different shear rates in the Ibidi flow chamber for 30 min.
LLO treatment of THP-1 cells
THP-1 cells were treated with 500 ng ml−1 LLO (Abcam, ab83345) and analysed at different timepoints. Cell supernatants were collected for LDH assay. For the mechanical-stimulation assay, 1 h after the LLO treatment, flow was applied to THP-1 at different shear rates for 30 min.
Osmotic stress treatment
Isotonic and hypotonic solutions were made according to Supplementary Table 4. All solutions were adjusted to pH 7.4 by NaOH. The osmolality of the solutions was verified using an osmometer (VOGEL, OM807). THP-1 cells were treated with hypotonic solutions for 2 h. Cells were stained with CellBrite and DRAQ7 then photographed and analysed. The supernatant was collected to detect LDH.
Statistics and data analysis
Unless otherwise stated, data are expressed as mean ± s.e.m. All datasets were analysed using GraphPad Prism (v.8.0.1 and v.10.2.3) and Microsoft Excel Mac (v.16.97). Unpaired Student’s t-tests were used for two-group comparisons. For data with multiple groups, one-way ANOVA or two-way ANOVA was performed followed by Bonferroni corrections. Estimation of association was determined using the Pearson correlation coefficient r value. A two-tailed P < 0.05 was considered to be statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Datasets generated during and/or analysed during the current study are available from J.X. on reasonable request. Source data are provided with this paper.
Change history
06 August 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41586-025-09444-7
References
Alberts, B., Johnson, A. & Lewis, J. Molecular Biology of The Cell (Garland Science, 2002).
Ammendolia, D. A., Bement, W. M. & Brumell, J. H. Plasma membrane integrity: implications for health and disease. BMC Biol. 19, 71 (2021).
Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021).
Kayagaki, N. et al. Inhibiting membrane rupture with NINJ1 antibodies limits tissue injury. Nature 618, 1072–1077 (2023).
Zhang, Y., Chen, X., Gueydan, C. & Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 28, 9–21 (2018).
Suda, K. et al. Plasma membrane damage limits replicative lifespan in yeast and induces premature senescence in human fibroblasts. Nat. Aging 4, 319–335 (2024).
Cooper, S. T. & McNeil, P. L. Membrane repair: mechanisms and pathophysiology. Physiol. Rev. 95, 1205–1240 (2015).
Miyake, K. & McNeil, P. L. Mechanical injury and repair of cells. Crit. Care Med. 31, S496–S501 (2003).
Vahidkhah, K. et al. Flow-induced damage to blood cells in aortic valve stenosis. Ann. Biomed. Eng. 44, 2724–2736 (2016).
Cranmer, S. L. et al. High shear-dependent loss of membrane integrity and defective platelet adhesion following disruption of the GPIbalpha-filamin interaction. Blood 117, 2718–2727 (2011).
Murthy, S. E., Dubin, A. E. & Patapoutian, A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 18, 771–783 (2017).
Wyatt, T., Baum, B. & Charras, G. A question of time: tissue adaptation to mechanical forces. Curr. Opin. Cell Biol. 38, 68–73 (2016).
Vicar, T., Raudenska, M., Gumulec, J. & Balvan, J. The quantitative-phase dynamics of apoptosis and lytic cell death. Sci. Rep. 10, 1566 (2020).
Murao, A., Aziz, M., Wang, H., Brenner, M. & Wang, P. Release mechanisms of major DAMPs. Apoptosis 26, 152–162 (2021).
Xu, J. et al. GPR68 senses flow and is essential for vascular physiology. Cell 173, 762–775 (2018).
Qiu, Z. et al. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157, 447–458 (2014).
Dondelinger, Y. et al. NINJ1 is activated by cell swelling to regulate plasma membrane permeabilization during regulated necrosis. Cell Death Dis. 14, 755 (2023).
Hirata, Y. et al. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis. Curr. Biol 33, 1282–1294 (2023).
Barayeu, U. et al. Hydropersulfides inhibit lipid peroxidation and ferroptosis by scavenging radicals. Nat. Chem. Biol. 19, 28–37 (2023).
Beavis, A. D. & Vercesi, A. E. Anion uniport in plant mitochondria is mediated by a Mg2+-insensitive inner membrane anion channel. J. Biol. Chem. 267, 3079–3087 (1992).
Tang, T. et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 8, 202 (2017).
Lewis, A. H. & Grandl, J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife 4, e12088 (2015).
Sezgin, E. et al. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc. 7, 1042–1051 (2012).
Pourmal, S. et al. Autoinhibition of dimeric NINJ1 prevents plasma membrane rupture. Nature 637, 446–452 (2025).
Araki, T. & Milbrandt, J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron 17, 353–361 (1996).
Araki, T., Zimonjic, D. B., Popescu, N. C. & Milbrandt, J. Mechanism of homophilic binding mediated by ninjurin, a novel widely expressed adhesion molecule. J. Biol. Chem. 272, 21373–21380 (1997).
Ifergan, I. et al. Role of ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann. Neurol. 70, 751–763 (2011).
Degen, M. et al. Structural basis of NINJ1-mediated plasma membrane rupture in cell death. Nature 618, 1065–1071 (2023).
David, L. et al. NINJ1 mediates plasma membrane rupture by cutting and releasing membrane disks. Cell 187, 2224–2235 (2024).
Sahoo, B., Mou, Z., Liu, W., Dubyak, G. & Dai, X. How NINJ1 mediates plasma membrane rupture and why NINJ2 cannot. Cell 188, 292–302.e11 (2025).
Newton, K., Strasser, A., Kayagaki, N. & Dixit, V. M. Cell death. Cell 187, 235–256 (2024).
McDonald, D. A. in Blood Flow in Arteries 356–378 (Williams & Wilkins, 1974).
Whitmore, R. L. Rheology of the Circulation 1st edn (Pergamon, 1968).
Ramos, S., Hartenian, E., Santos, J. C., Walch, P. & Broz, P. NINJ1 induces plasma membrane rupture and release of damage-associated molecular pattern molecules during ferroptosis. EMBO J. 43, 1164–1186 (2024).
Hu, Y. et al. The Ninj1/Dusp1 axis contributes to liver ischemia reperfusion injury by regulating macrophage activation and neutrophil infiltration. Cell. Mol. Gastroenterol. Hepatol. 15, 1071–1084 (2023).
Sheng, Y. et al. Tomo-seq identifies NINJ1 as a potential target for anti-inflammatory strategy in thoracic aortic dissection. BMC Med. 21, 396 (2023).
Zheng, X. B. et al. NINJ1-mediated plasma membrane rupture of pyroptotic endothelial cells exacerbates blood-brain barrier destruction caused by neutrophil extracellular traps in traumatic brain injury. Cell Death Discov. 11, 69 (2025).
Murthy, S. E. et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med. 10, eaat9897 (2018).
Murthy, S. E. et al. OSCA/TMEM63 are an evolutionarily conserved family of mechanically activated ion channels. eLife 7, e41844 (2018).
Xiang, F. L., Fang, M. & Yutzey, K. E. Loss of β-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nat. Commun. 8, 712 (2017).
Zhang, X. et al. EGFR tyrosine kinase activity and Rab GTPases coordinate EGFR trafficking to regulate macrophage activation in sepsis. Cell Death Dis. 13, 934 (2022).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Papaioannou, T. G. & Stefanadis, C. Vascular wall shear stress: basic principles and methods. Hellenic J. Cardiol. 46, 9–15 (2005).
Acknowledgements
We thank A. Patapoutian, B. Xiao, Y. Xiang for discussions, the staff of the scientific core facility the First Affiliated Hospital of Sun Yat-sen University for technical assistance. This study was supported by Natural Science Foundation of China (32371199 and 82070311 to J.X., 82270274 and 82250610229 to F.-l.X.), Guangdong Provincial Science and Technology Department (2023ZT10Y154 and 2021CX02Y025 to F.-l.X., 2019QN01Y210 to J.X.), Chinese National Key Research and Development Program (no. 2023YFC2307004 to F.-l.X.)., National Institutes of Health (R35GM147027 to Z.S.) and Tencent New Cornerstone Science Foundation (to F.S.). Parts of the diagrams in Figs. 1a,b,g, 2m, 3a and 4e were created using BioRender. Some elements of the Fig. 3a were created using original artwork by Z. Zhuoyi Xu, aged 10 years, using Procreate 5.3, and were used with her permission.
Author information
Authors and Affiliations
Contributions
J.X. conceived the project and designed the high-throughput stretch system. Y.Z., Zhirong Wang and L.Z. constructed the stretch system. Y.Z. performed the optimization of the hardware and consumables. Y.Z., F.X., Yufang Wang, J.L. and D.Z. designed and performed the experiments, with assistance from the other authors. Yiling Wang performed the GPMV experiments. Yiling Wang and Z.S. analysed the GPMV data. J.B., C.P. and S.H. performed FEA analysis. Z.H., M.Y., Zhirong Wang, M.Z., Y.S., T.T., R.Z., K.X., Zhongxin Wang, C.C. and X.G. assisted in carrying out experiments. F.-l.X. and J.X. wrote the manuscript with the help from all of the other authors. F.S., Z.S., F.-l.X. and J.X. revised the manuscript. F.-l.X. and J.X. supervised the project. Y.Z., F.X., Yufang Wang, Yiling Wang, J.L. and D.Z. contributed equally to this study. J.X. is the lead contact.
Corresponding authors
Ethics declarations
Competing interests
J.B., C.P. and S.H are employees of Novartis Biomedical Research. F.S. is the scientific founder and chair of the scientific advisory board of Pyrotech Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Ana J. García-Sáez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Characterization of the high-throughput cellular stretch system and the cellular response to mechanical strain.
a, Image of the HT cellular stretch system. b, Finite element analysis of the PDMS membrane under different vacuum levels, showing the strain pattern across the whole well. c, Illustration of the particle imagery assay used to empirically estimate the strain values with the formula shown. d is the distance of the bead from the centre of the well at the resting state, and d’ is the distance of the same bead from the centre in the stretched state. d, Bead images before stretch (grey) and at −30 kPa (magenta) were overlayed. e, Estimated strain values at different vacuum levels from the bead imagery analysis (n = 10 wells from 3 independent experiments). f, YFP intensity traces of HeLa cells subjected to 5 s stretch at a series of strain levels. Each trace is from 150 ~ 200 cells, n = 3 ~ 4 trials per group. g, The inhibition curve of the DCPIB, a non-specific chloride channel inhibitor, on the quenching of YFP induced by 40% strain. Each data point is from ~200 cells of each condition, n = 5 trials per group. h, Inhibition curve of the DCPIB on the quenching induced by 50% strain for 5 s. Each data point is from ~220 cells of each condition, n = 5 trials per group. Half-inhibition concentrations (IC50) are indicated. i, YFP quenching over the course of 60 min. Fluorescence baseline was recorded for 10 min, then the following stimulations were applied: 5 µM RSL3, TSZ, and 500 ng ml−1 LLO. Mechanical stimulation was applied for 5 s at 50% and 55% strain. Control was 0.5% DMSO. Each data point is from ~150 cells of each condition, n = 3 ~ 4 trials per group. j, YFP quenching over the course of 60 min under 20 ng ml−1 nigercin or mechanical strain at 50% or 55% for 5 s. Each data point is from ~150 cells of each condition, n = 3 ~ 4 trials per group. Unless otherwise noted, all data are presented as mean ± s.e.m.
Extended Data Fig. 2 Assessment of the role of PIEZO1 and PIEZO2 in strain-induced plasma membrane rupture.
a, LDH release of PIEZO1, PIEZO2 knock-down and control HeLa cells. n = 4–8 trials per group. b, Percentage of DRAQ7+ cells of PIEZO1, PIEZO2 knock-down and control HeLa cells. n = 4–8 trials per group from ~150 cells of each group. 2-way ANOVA followed by Bonferroni corrections. n.s., not significant versus Scrambled. c, d, LDH release and the percentage of DRAQ7+ cells from the HEK-293T transiently-transfected with human PIEZO1 and PIEZO2. Vector-transfected cells, as well as TRE3G-NINJ1 HEK-293T stable cells under 100 ng ml−1 Dox induction were used as controls. Assay was conducted 24 h after transfection. n = 3 trials for each group, 2-way ANOVA followed by Bonferroni corrections. ** p <0.01 versus Vector at the same strain level. e, The lysis tension of PIEZO1 and NINJ1-expressing vesicles. Owing to the drastically larger size of PIEZO1 (38 transmembrane segments, 287 kDa, compared to 3 transmembrane and 16 kDa for NINJ1), protein levels were derived from fluorescence intensity of mCherry fused to C-terminal of PIEZO1, corrected for number of transmembrane segments to reflect the footprint, and plotted as size-corrected molecular density on the membrane in arbitrary units (A.U.) for a fair comparison with NINJ1. 30 vesicles harbouring WT NINJ1 and 13 vesicles with PIEZO1 were measured. 10 additional vesicles that only overexpress GPI-eGFP were included as the zero intensity points for both NINJ1 and PIEZO1. Unless otherwise noted, all data are presented as mean ± s.e.m.
Extended Data Fig. 3 Validation of NINJ1-deficient animals, primary cells and THP-1 cells.
a, Mortality rate of the Ninj1−/− mice within 2 months from birth. b, Relative Ninj1 mRNA levels from the spleen of WT and Ninj1−/− mice. n = 4 animals per genotype. Two-sided unpaired Student’s t-test. * p < 0.05 versus WT. c, d, The LDH release and the percentage of DRAQ7+ primary BMDMs from Ninj1−/− mice and WT littermates after the application of 5 s stretch at 45% strain. Each data point is from 100 ~ 200 cells in one of the stretched wells. For each genotype, primary BMDMs were isolated and pooled from 4 animals. 2-way ANOVA followed by Bonferroni corrections. * p < 0.05, ** p < 0.01 vs WT. Unless otherwise noted, all data are presented as mean ± s.e.m. e, Sanger sequencing results of the NINJ1 KO THP-1 cells and the parental cells. The KO cells have 38 bp missing starting from base pair 202, causing a frameshift. f, Western blot of endogenous NINJ1 in the NINJ1 KO and the parental cells. GAPDH was used as the control for protein loading. Experiments have been repeated at least 3 times independently with similar results.
Extended Data Fig. 4 Assessment of ectopic and endogenous NINJ1 expression in various cell lines.
a, Relative NINJ1 mRNA level of vector- and CMV-NINJ1-IRES-mCherry-transfected HEK-293T cells 24 h post-transfection. n = 3 for each group. Two-sided unpaired Student’s t-test, ** p < 0.01 vs Vector. b, Representative image of HEK cells overexpressing NINJ1-mCherry fusion under the CMV promoter 24 h post-transfection. Fluorescent puncta were visible (arrowheads) and plasma membrane ballooning were evident in some cells (arrows). NINJ1 was visualized with direct mCherry fluorescence. Nuclei were live stained with Hoechst. Scale bar, 25 µm. c, Relative NINJ1 mRNA levels of HEK-293T parental cells and the cells stably expressing NINJ1 driven by TRE3G promoter. mRNAs were measured 24 h after induction by Doxycycline (Dox) of various doses. n = 4 trials per group. One-way ANOVA followed by Bonferroni corrections. ** p <0.01 versus Parental. d, Western blot of endogenous NINJ1 from HeLa and ZOS cells. 3 replicates were done for each cell line. e, Relative NINJ1 mRNA level of HeLa and ZOS cells. 3 trials were conducted for each group. Two-sided unpaired Student’s t-test, ** p <0.01 versus HeLa. Unless otherwise noted, all data are presented as mean ± s.e.m.
Extended Data Fig. 5 Assessment of cytoskeleton organization and lipid composition in TRE3G-NINJ1-IRES-mCherry stable HEK-293T cells.
a-b, Cells were treated Dox at various doses. Where applicable, mCherry fluorescence intensity were used to assess the relative expression levels of NINJ1. Quantification data are from cells from 3 separate fields for each group using one-way ANOVA followed by Bonferroni corrections. a, Representative images and quantitation of immunofluorescence staining of F-actin in cells express different levels of NINJ1. b, Representative images and quantitation of β-tubulin staining. Average intensity, tubule lengths and tubule count per cells were quantified. c-d, TRE3G-NINJ1-IRES-mCherry stable HEK-293T cells were transfected with Dox-inducible fluorescent probes of phosphatidic acid (PA) and phosphatidylserine (PS), then incubated with 300 ng ml−1 Dox to induce NINJ1 and lipid probe expression. Quantification data are from cells from 3 separate fields for each group and analysed by one-way ANOVA followed by Bonferroni corrections. c, Representative images and quantitation of average intensity of PA-GFP. d, Representative images and average intensity of PS-GFP. e, TRE3G-NINJ1-IRES-mCherry stable HEK-293T cells were incubated with Dox at various doses to induce NINJ1 expression, then stained for Annexin V and quantified for Annexin V intensity (n = 6 ~ 15 wells from 3 independent experiments, one-way ANOVA followed by Bonferroni corrections). f, Validation of Annexin V antibodies was carried out by staining cells treated with nigericin to induce cell death (n = 10 ~ 11 wells from 3 independent experiments, two-sided unpaired Student’s t-test, ** p <0.01 versus control). Unless otherwise noted, all data are presented as mean ± s.e.m. All scale bars are 25 µm.
Extended Data Fig. 6 Evaluation of NINJ1 K45Q mutant’s effect on plasma membrane rupture under mechanical strain.
a, The relative mRNA levels of NINJ1 WT and NINJ1 K45Q in overexpressed in HeLa cells. 3 replicates were done for each group. n = 3 trials per group. One-way ANOVA followed by Bonferroni corrections. ** p <0.01 versus vector. b, c, LDH release and the percentage of DRAQ7+ HeLa cells overexpressing vector control, NINJ1 WT and NINJ1 K45Q, after mechanical stimulation at various strain levels. n = 3 ~ 6 trials each group. 2-way ANOVA followed by Bonferroni corrections. * p < 0.05, ** p <0.01 versus vector at the same strain level. All data are presented as mean ± s.e.m.
Extended Data Fig. 7 The pyroptotic THP-1 cells maintain the ballooned morphology in the absence of mechanical stimulation.
a, Western blot of endogenous NINJ1 of THP-1 cells on a native BN-PAGE gel with or without incubation with 5 µg ml−1 Nigericin for 90 min. b, THP-1 cells were incubated with 5 µg ml−1 Nigericin and stained for plasma membrane (CellBrite, red), nuclei of cells with compromised membrane (DRAQ7, yellow) and all nuclei (Hoechst, blue) 48 h later. Cells with permeabilized plasma membrane showed DRAQ7/Hoechst double staining, represented in white. Scale bar, 50 µm. These experiments have been repeated at least 3 times independently with similar results.
Extended Data Fig. 8 NINJ1 facilitates full PMR under mechanical strain in intracellular LPS induced cell death.
a, NINJ1 KO and parental THP-1 cells were electroporated with LPS and stained with CellBrite (red), DRAQ7 (yellow) and Hoechst (blue) at 1 h, 8 h, 16 h and 24 h after LPS treatment. Almost all cells showed DRAQ7 staining, indicating large breaches on the plasma membrane, yet still displayed intact ballooned shape without full rupture. b, Quantification of LDH release, percentage of DRAQ7+ cells and percentage of ballooned cells at various time points after electroporation of LPS. n = 3 for each group. c, NINJ1 KO and parental THP-1 cells were electroporated with LPS 1 h before being subjected to flow treatment at shear rates ranging from 829 s−1 to 2073 s−1. A significantly lower percentage of NINJ1 KO cells show DRAQ7+/Hoechst+ bare nuclei, indicative of full PMR, after being subjected to flow. In all assays, data are from 3 trials. To quantify DRAQ7 staining and ballooned cell percentage, 250 ~ 300 cells examined for each trial. Data were analysed using 2-way ANOVA followed by Bonferroni corrections and presented as mean ± s.e.m. Scale bars, 25 µm.
Extended Data Fig. 9 NINJ1 does not affect full PMR under mechanical strain in LLO induced cell death.
a, NINJ1 KO and parental THP-1 cells were treated with 500 ng ml−1 LLO and stained with CellBrite (red), DRAQ7 (yellow) and Hoechst (blue) at 1 h, 2 h, 8 h, 12 h and 24 h later. b, Quantification of LDH release, percentage of DRAQ7+ cells and DRAQ7+ cells with bare nuclei at various time points after LLO treatment. n = 3 for each group. Data were analysed using 2-way ANOVA followed by Bonferroni corrections. ** p < 0.01 versus parental+LLO. c, NINJ1 KO and parental THP-1 cells were treated with LLO for 1 h before flow stimulation at the shear rate 829 s−1. Cells were stained after 30 min as previously described. d, Cells were treated with 500 ng ml−1 LLO for 1 h, then subjected to flow at 829 s−1 in shear rate. Measurement of the LDH release, percentage of fully ruptured parental and KO cells were carried after 30 min. Data are from 3 trials. To quantify DRAQ7 staining and ballooned cell percentage, 250 ~ 300 cells examined for each trial, and analysed using two-sided unpaired Student’s t-test, n.s., not significant versus parental. All data are presented as mean ± s.e.m. Scale bars, 25 µm.
Extended Data Fig. 10 NINJ1 facilitates full PMR under osmotic stress in Nigericin-induced pyroptosis.
a, Representative images of NINJ1 KO and parental THP-1 cells subjected to a series of hypotonic (215, 240 and 260 mOsm) and isotonic (310 mOsm) stimulation. Cells were stained with CellBrite (red), DRAQ7 (yellow) and Hoechst (blue). b, Pyroptosis was induced by 20 µg ml−1 nigericin in NINJ1 KO and parental THP-1 cells in hypotonic and isotonic buffers for 2 h. A substantial percentage of cells showed DRAQ7 and Hoechst double staining, indicating large gaps or openings on the plasma membrane, yet most cells still displayed intact ballooned shape without full rupture. Scale bar, 25 µm. c-e, Quantification of LDH release, the percentage of DRAQ7+ cells and the DRAQ7+ bare nuclei in hypotonic and isotonic conditions. n = 3 trials per group. Data were analysed using 2-way ANOVA followed by Bonferroni corrections and presented as mean ± s.e.m. * p < 0.05, ** p < 0.01 vs parental.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Supplementary Tables 3 and 4. Supplementary Fig. 1: uncropped immunoblots for key data presented in the main text and Extended Data figures. Full images of the gels, including markers are presented and areas that were cropped for presentation in the figures are indicated. Supplementary Table 3: the values of the of shear rate generated by the Ibidi flow system. The red tubing set was used with the Ibidi flow controller. The inlet and outlet of the tubing set were directly connected with a coupler, without a channel slide installed. Supplementary Table 4: the composition of the buffers used in the hypotonic stimulation experiment.
Supplementary Table 1
Details of all statistical tests performed with the P values smaller than 0.05.
Supplementary Table 2
The sequences of siRNAs used in the final confirmation screen and all follow-up experiments.
Supplementary Video 1
The running state of the high-throughput stretch system as viewed from bottom of the assay plate. The system was configured for whole-plate stimulation at 30% strain, 200 ms in duration. A total of five stretches was executed.
Supplementary Video 2
HeLa-YFP cells show quenching of YFP in response to a high level of strain. HeLa-YFP stable cells were plated onto a 384-well stretch plate 24 h before the assay. The baseline fluorescence was recorded for 20 s, then a single stretch of 5 s at 50% strain was applied. Scale bar, 200 µm.
Supplementary Video 3
A GPMV from HeLa cell with a low level of NINJ1 expression ruptures at high pressure. Elapsed time and pressure values are indicated.
Supplementary Video 4
A GPMV from HeLa cell with a high level of NINJ1 expression ruptures at low pressure. Elapsed time and pressure values are indicated.
Supplementary Video 5
Time-lapse video of THP-1 cells that underwent pyroptosis. Pyroptosis was induced in THP-1 cells by 5 µg ml−1 nigericin. Images were collected every 60 s for 24 h. The focus was manually adjusted over the course of imaging to compensate for the drift over time. Scale bar, 20 µm.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, Y., Xiao, F., Wang, Y. et al. NINJ1 regulates plasma membrane fragility under mechanical strain. Nature 644, 1088–1096 (2025). https://doi.org/10.1038/s41586-025-09222-5
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09222-5
This article is cited by
-
Ninjurin-1 mediates cell lysis and detrimental inflammation of PANoptosis during influenza A virus infection
Signal Transduction and Targeted Therapy (2025)
-
Breaking point: mechanical stress helps NINJ1 protein to rupture membranes
Nature (2025)