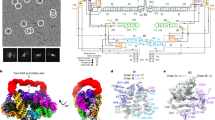
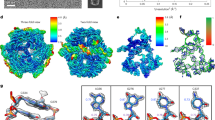
Abstract
Long (>200 nucleotides) non-coding RNAs (lncRNAs) play important roles in diverse aspects of life. Over 20 classes of lncRNAs have been identified in bacteria and bacteriophages through comparative genomics analyses, but their biological functions remain largely unexplored1,2,3. Owing to the large sizes, the structural determinants of most lncRNAs also remain uncharacterized. Here, we report the structures of two natural RNA nanocages formed by the ROOL (rumen-originating, ornate, large) lncRNA found in bacterial and phage genomes. The cryo-electron microscopy (cryo-EM) structures at 2.9-Å resolution reveal that ROOL RNAs form an octameric nanocage with a diameter of 28 nm and an axial length of 20 nm, in which the hollow inside features poorly ordered regions. The octamer is stabilized by numerous tertiary and quaternary interactions, including triple-strand A-minors, for which we propose the term ‘A-minor staples’. The structure of an isolated ROOL monomer at 3.2-Å resolution indicates that nanocage assembly involves a strand-swapping mechanism resulting in quaternary kissing loops. Finally, we show that ROOL RNA fused to an RNA aptamer, transfer RNA or microRNA retains its structure, forming a nanocage with radially displayed cargoes. Our findings, therefore, may enable engineering of novel RNA nanocages as delivery vehicles for research and therapeutic applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The cryo-EM maps and associated atomic coordinate models of ROOLEfa octamer, ROOLFirm octamer and ROOLEfa monomer have been deposited in the wwPDB OneDep System under EMD accession codes EMD-48386, EMD-48389 and EMD-48391 and PDB codes 9MM6, 9MME and 9MMG, respectively. The map of ROOLFirm tetramer has been deposited in the EMDB System under EMD accession code EMD-49873. Source data for mass photometry and electron microscopy micrographs associated with Fig. 4e are available at Figshare (https://doi.org/10.6084/m9.figshare.29142887)35. Source data are provided with this paper.
References
Weinberg, Z., Perreault, J., Meyer, M. M. & Breaker, R. R. Exceptional structured noncoding RNAs revealed by bacterial metagenome analysis. Nature 462, 656–659 (2009).
Weinberg, Z. et al. Detection of 224 candidate structured RNAs by comparative analysis of specific subsets of intergenic regions. Nucleic Acids Res. 45, 10811–10823 (2017).
Harris, K. A. & Breaker, R. R. Large noncoding RNAs in bacteria. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.RWR-0005-2017 (2018).
Pyle, A. M. Group II intron self-splicing. Annu. Rev. Biophys. 45, 183–205 (2016).
Keenan, R. J., Freymann, D. M., Stroud, R. M. & Walter, P. The signal recognition particle. Annu. Rev. Biochem. 70, 755–775 (2001).
Stav, S. et al. Genome-wide discovery of structured noncoding RNAs in bacteria. BMC Microbiol. 19, 66 (2019).
Narunsky, A. et al. The discovery of novel noncoding RNAs in 50 bacterial genomes. Nucleic Acids Res. 52, 5152–5165 (2024).
Cousin, F. J. et al. A long and abundant non-coding RNA in Lactobacillus salivarius. Microb. Genom. 3, e000126 (2017).
Bohdan, D. R., Voronina, V. V., Bujnicki, J. M. & Baulin, E. F. A comprehensive survey of long-range tertiary interactions and motifs in non-coding RNA structures. Nucleic Acids Res. 51, 8367–8382 (2023).
Cate, J. H. et al. Crystal structure of a group I ribozyme domain: principles of RNA packing. Science 273, 1678–1685 (1996).
Doherty, E. A., Batey, R. T., Masquida, B. & Doudna, J. A. A universal mode of helix packing in RNA. Nat. Struct. Mol. Biol. 8, 339–343 (2001).
Nissen, P., Ippolito, J. A., Ban, N., Moore, P. B. & Steitz, T. A. RNA tertiary interactions in the large ribosomal subunit: the A-minor motif. Proc. Natl Acad. Sci. USA 98, 4899–4903 (2001).
Battle, D. J. & Doudna, J. A. Specificity of RNA–RNA helix recognition. Proc. Natl Acad. Sci. USA 99, 11676–11681 (2002).
Ogle, J. M. et al. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 292, 897–902 (2001).
Demeshkina, N., Jenner, L., Westhof, E., Yusupov, M. & Yusupova, G. A new understanding of the decoding principle on the ribosome. Nature 484, 256–259 (2012).
Loveland, A. B., Demo, G., Grigorieff, N. & Korostelev, A. A. Ensemble cryo-EM elucidates the mechanism of translation fidelity. Nature 546, 113–117 (2017).
Teran, D., Zhang, Y. & Korostelev, A. A. Endogenous trans-translation structure visualizes the decoding of the first tmRNA alanine codon. Front. Microbiol. https://doi.org/10.3389/fmicb.2024.1369760 (2024).
Autour, A. et al. Fluorogenic RNA Mango aptamers for imaging small non-coding RNAs in mammalian cells. Nat. Commun. 9, 656 (2018).
Trachman, R. J. et al. Structure and functional reselection of the Mango-III fluorogenic RNA aptamer. Nat. Chem. Biol. 15, 472–479 (2019).
Albers, S. et al. Engineered tRNAs suppress nonsense mutations in cells and in vivo. Nature 618, 842–848 (2023).
Record, M. T. Jr, Zhang, W. & Anderson, C. F. Analysis of effects of salts and uncharged solutes on protein and nucleic acid equilibria and processes: a practical guide to recognizing and interpreting polyelectrolyte effects, Hofmeister effects, and osmotic effects of salts. Adv. Protein Chem. 51, 281–353 (1998).
Shiman, R. & Draper, D. E. Stabilization of RNA tertiary structure by monovalent cations. J. Mol. Biol. 302, 79–91 (2000).
Szatmári, D. et al. Intracellular ion concentrations and cation-dependent remodelling of bacterial MreB assemblies. Sci. Rep. 10, 12002 (2020).
Greening, C. & Lithgow, T. Formation and function of bacterial organelles. Nat. Rev. Microbiol. 18, 677–689 (2020).
Chen, A. G. Functional Investigation of Ribozymes and Ribozyme Candidates in Viruses, Bacteria and Eukaryotes. PhD thesis, Yale Univ. (2015).
Kretsch, R. C. et al. Naturally ornate RNA-only complexes revealed by cryo-EM. Nature https://doi.org/10.1038/s41586-025-09073-0 (2025).
Patel, S. D. et al. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell 124, 1255–1268 (2006).
Häge, F. R. et al. Strand-swapped SH3 domain dimer with superoxide dismutase activity. ACS Cent. Sci. 11, 157–166 (2025).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Jasinski, D., Haque, F., Binzel, D. W. & Guo, P. Advancement of the emerging field of RNA nanotechnology. ACS Nano 11, 1142–1164 (2017).
Shu, Y. et al. Fabrication of 14 different RNA nanoparticles for specific tumor targeting without accumulation in normal organs. RNA 19, 767–777 (2013).
Høiberg, H. C., Sparvath, S. M., Andersen, V. L., Kjems, J. & Andersen, E. S. An RNA origami octahedron with intrinsic siRNAs for potent gene knockdown. Biotechnol. J. 14, e1700634 (2019).
Afonin, K. A., Dobrovolskaia, M. A., Ke, W., Grodzinski, P. & Bathe, M. Critical review of nucleic acid nanotechnology to identify gaps and inform a strategy for accelerated clinical translation. Adv. Drug Deliv. Rev. 181, 114081 (2022).
Ling, X., Golovenko, D., Gan, J., Ma, J., Korostelev, A. A. & Fang, W. Cryo-EM structure of a natural RNA nanocage. Dataset. Figshare https://doi.org/10.6084/m9.figshare.29142887 (2025).
Thompson, R. F., Iadanza, M. G., Hesketh, E. L., Rawson, S. & Ranson, N. A. Collection, pre-processing and on-the-fly analysis of data for high-resolution, single-particle cryo-electron microscopy. Nat. Protoc. 14, 100–118 (2019).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Wu, C., Huang, X., Cheng, J., Zhu, D. & Zhang, X. High-quality, high-throughput cryo-electron microscopy data collection via beam tilt and astigmatism-free beam-image shift. J. Struct. Biol. 208, 107396 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
He, J., Li, T. & Huang, S.-Y. Improvement of cryo-EM maps by simultaneous local and non-local deep learning. Nat. Commun. 14, 3217 (2023).
Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci. 32, e4792 (2023).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Grigorieff, N. Frealign: an exploratory tool for single-particle cryo-EM. Methods Enzymol. 579, 191–226 (2016).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D 74, 519–530 (2018).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
The PyMOL Molecular Graphics System v.3.1.3.1 (Schrödinger, LLC, 2010).
Ontiveros-Palacios, N. et al. Rfam 15: RNA families database in 2025. Nucleic Acids Res. https://doi.org/10.1093/nar/gkae1023 (2024).
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003).
Acknowledgements
Cryo-EM data were collected at the Electron Microscopy Center at Fudan University and the Cryo-EM Core Facility at UMass Chan Medical School. The data were processed at the High Performance Computing Center of the Electron Microscopy Center at Fudan University and the HPC cluster at UMass Chan Medical School. We thank K. Song and C. Ouch at the Cryo-EM Core Facility at UMass Chan Medical School for assistance with collecting 300 kV cryo-EM data; K. Lee at the EM Facility at UMass Chan Medical School for collecting negative-stain EM data; L. Robbins and C. Hull (UMass Chan Medical School) for assistance with HPC usage; D. Zhu (Howard Hughes Medical Institute) and J. Zhang (Harvard University) for assistance with data processing; S. Klinge (Rockefeller University) and T. Li (Huazhong University of Science and Technology) for assistance with modelling; B. Kelch, R. Ahsan, L. Li and Y. A. Nguyen (UMass Chan Medical School) for assistance with ROOL characterization in solution; K. Lowenhaupt at the Biophysical Instrumentation Facility (Massachusetts Institute of Technology) for assistance with mass photometry and dynamic light scattering experiments; Z. Weinberg (Martin Luther University Halle-Wittenberg) for helpful discussions; A. LueCheeLip (UMass Chan Medical School) for technical assistance; and D. Conte Jr (UMass Chan Medical School) for editing the manuscript. This work was supported by National Natural Science Foundation of China (NSFC32471347 to J.M.), Program of Shanghai Academic/Technology Research Leader (to J. M.), National Institutes of Health, National Institute of General Medical Sciences (R35GM127094 to A.A.K. and R35GM150953 to W.F.) and UMass Chan Medical School Startup fund to W.F.
Author information
Authors and Affiliations
Contributions
X.L. conceived the project. J.M., A.A.K. and W.F. supervised the project and participated in experimental design and analysis. X.L. performed experiments and analysed data, prepared cryo-EM samples and collected and processed cryo-EM data. X.L., D.G., J.G., J.M. and A.A.K. built, refined and validated atomic coordinate models. X.L., D.G., A.A.K. and W.F. prepared the manuscript with input from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The University of Massachusetts has filed a provisional patent application based on the innovation disclosed herein.
Peer review
Peer review information
Nature thanks Anna Pyle and Jane Richardson for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Biochemical analyses of ROOL RNAs.
a, Denaturing PAGE analyses demonstrate that the RNAs are intact full-length molecules. RNAs were analysed at least once. b, Mass photometry analysis of ROOLEfa and ROOLFirm at 50–100 nM in 240 mM K+ and 20 mM Mg2+ shows that monomers and octamers are the two most abundant species. The RNA size of each peak was calibrated using the Millennium RNA Marker and labelled. c, Size exclusion chromatography (SEC) analyses of ROOLEfa and ROOLFirm coupled with denaturing PAGE (d), mass photometry (e), and negative-stain EM (f) showed three major states (aggregates, octamer, and monomer) in the cryo-EM samples. Peak 1 (void volume) contained aggregated ROOL of different oligomerization states; peaks 2 and 3 correspond to predominantly octamers and monomers, respectively. Experiments in c and e were repeated at least once with similar results; experiments in d and f were performed once; three EM micrographs were analysed in f. g, Dynamic light scattering shows a diameter range of 10–30 nm for ROOL RNAs, consistent with its oligomerization states. A representative result from two independent technical replicates is shown. For original RNA gel images and negative-stain micrographs, see Supplementary Fig. 1.
Extended Data Fig. 2 Cryo-EM data processing workflow for ROOLEfa.
a, Maximum-likelihood classification of cryo-EM data using cryoSPARC. b, Example of a micrograph with ROOLEfa particles. 6,966 micrographs were obtained and analysed. c, Fourier shell correlation curve as a function of resolution for the final map shown in panels a and e. d, Euler angle distribution of particles contributing to the final reconstruction. e, Cryo-EM maps coloured according to local resolution.
Extended Data Fig. 3 Cryo-EM data processing workflow for ROOLFirm.
a, Maximum-likelihood classification of cryo-EM data using cryoSPARC. b, Example of a micrograph with ROOLFirm particles. 6,390 micrographs were obtained and analysed. c, Fourier shell correlation curves as a function of resolution for the final octamer and tetramer maps shown in panels e and f. d, Euler angle distribution of particles contributing to the final reconstructions. e, f, Cryo-EM maps of the octamer (e) and tetramer (f), coloured according to local resolution.
Extended Data Fig. 4 Cryo-EM data processing workflow for the individual monomer of ROOLEfa.
a, Maximum-likelihood classification of cryo-EM data using cryoSPARC. b, Example of a micrograph with ROOLEfa particles. 5,253 micrographs were obtained and analysed. c, Cryo-EM maps coloured according to local resolution. d, Fourier shell correlation curves as a function of resolution for the final maps shown in panels a and c. e, Euler angle distribution of particles contributing to the final reconstruction.
Extended Data Fig. 5 Tertiary interactions stabilize each ROOLFirm monomer in the nanocage.
a, Molecular model of a ROOLFirm monomer within the octameric structure. Close-up views of examples of tertiary interactions stabilizing the monomer are shown in the corresponding coloured boxes in panels b–g with map rendered as transparent surface (σ = 4.16). Panels b and f show different views for the orange box in panel a. h, Secondary structure annotation of ROOLEfa within the octamer, with tertiary interactions labelled by coloured boxes that match panels a–g. Nucleotides in panels b–h are coloured according to helix colours in a.
Extended Data Fig. 6 Projected secondary structure with tertiary and quaternary interactions of ROOLEfa shown based on the cryo-EM structure.
H1–H16 of monomers 1–4, H7, and H13, H15, H16 of monomers 5–8 are shown to indicate tertiary and quaternary interactions in ROOLEfa. In ROOLFirm, G-A stacking replaces G-C Hoogsteen base pairing.
Extended Data Fig. 7 Quaternary interactions in ROOLFirm and inter-tetramer interactions in ROOLFirm and ROOLEfa.
a, Front view of the ROOLFirm dimer stabilized by four key interactions, the close-up views of which are shown in panels c–f with map rendered as transparent surface (σ = 4.16). b, Side view of the inter-tetramer interface of ROOLFirm, the close-up views of which are shown in panels g and h with map rendered as transparent surface (σ = 4.16). i, Side view of the inter-tetramer interface of ROOLEfa (viewed similarly to ROOLFirm in b), the close-up views of which are shown in panels j and k with map rendered as transparent surface (σ = 2.88).
Extended Data Fig. 8 Mutational analysis of H12 supports the strand-swapping mechanism.
a, Predicted secondary structures (by UNAFold49) of the WT or mutated H12 sequences of ROOLEfa. Nucleotides that participate in new kissing-loop interactions with H2 and H11 in the octamer are circled in red and green boxes, respectively. The purple shade indicates the disorder region in the octamer structure. In mut1, a G is inserted after U356 to pair with C423; in mut2, AAU (417–419) is changed to a C to pair with G360; in mut3, the stem-loop structure in the disordered region was replaced by a more stable hairpin (GC-rich with a GAAA tetra loop); mut4 combines the mutations in mut1 and mut2; mut5 combines mut4 and an additional removal of a bulge shown in dark blue on the WT structure diagram. b, c, Negative-stain EM analysis of the WT and mutant ROOLEfa. Representative micrographs are shown in b and quantifications from three images for each sample are shown in c. Data are presented as mean ± SD. For original negative-stain micrographs, see Supplementary Fig. 1. d, SEC analyses of WT and mutant ROOLEfa constructs. Two independent experiments were performed yielding similar results, and a representative chromatogram is shown for each construct. e, Mass photometry analysis of ROOLEfa H12 mutants. Two technical replicates were performed, yielding similar results.
Extended Data Fig. 9 Analysis of ROOLEfa oligomerization at varying salt concentrations.
a, Negative-stain EM analysis of ROOLEfa at different K+ and Mg2+ concentrations. b, Quantification of ROOLEfa nanocage formation using three different full images from the negative-stain EM analysis of each condition shown in a. Data are presented as mean ± SD. For original negative-stain micrographs, see Supplementary Fig. 1. c, SEC analyses of ROOLEfa show that different K+ and Mg2+ concentrations shift the distributions among three major peaks/states (aggregates, octamer, and monomer). Two independent experiments were performed yielding similar results, and a representative chromatogram is shown for each condition. d, Mass photometry analysis of ROOLEfa at different K+ and Mg2+ concentrations. Two technical replicates were performed, yielding similar results.
Supplementary information
Supplementary Fig. 1
Original gel images and negative-stain micrographs. These include original gel images associated with Extended Data Fig. 1a,d; original negative-stain micrographs associated with Fig. 4c,e and Extended Data Figs. 1f, 8b,c and 9a,b.
Supplementary Table 1
Nucleotide sequences used in the study.
Supplementary Video 1
Structure of ROOLEfa octamer. The overall architecture and important interactions are featured.
Supplementary Video 2
Structure of ROOLEfa monomer and the strand-swapping mechanism. The overall structure of ROOLEfa monomer, its alignment with ROOLEfa monomer within the octamer and the strand-swapping mechanism are featured.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ling, X., Golovenko, D., Gan, J. et al. Cryo-EM structure of a natural RNA nanocage. Nature 644, 1107–1115 (2025). https://doi.org/10.1038/s41586-025-09262-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09262-x