Abstract
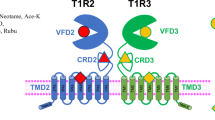
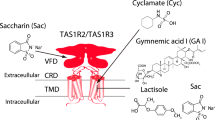
Sweet taste perception influences dietary choices and metabolic health. The human sweet taste receptor, a class C G-protein-coupled receptor (GPCR) heterodimer composed of TAS1R2 and TAS1R3 (refs. 1,2), senses a wide range of sweet compounds—including natural sugars, artificial sweeteners and sweet proteins—and affects metabolic regulation beyond taste. However, the lack of three-dimensional structures hinders our understanding of its precise working mechanism. Here we present cryo-electron microscopy structures of the full-length human sweet taste receptor in apo and sucralose-bound states. These structures reveal a distinct asymmetric heterodimer architecture, with sucralose binding exclusively to the Venus flytrap domain of TAS1R2. Combining mutagenesis and molecular dynamics simulations, this work delineates the sweetener-recognition modes in TAS1R2. Structural comparisons further uncover conformational changes upon ligand binding and a unique activation mechanism. These findings illuminate the signal transduction mechanisms of chemosensory receptors in the class C GPCR family and provide the molecular basis for the design of a new generation of sweeteners.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The atomic coordinates and cryo-EM density maps for apo-TAS1R2–TAS1R3 heterodimer and sucralose-bound TAS1R2–TAS1R3 heterodimer have been deposited in the Protein Data Bank and Electron Microscopy Data Bank, respectively. The accession codes for TAS1R2–TAS1R3 heterodimer reported in this paper are 9UT8 and EMDB-64484 (apo full-length TAS1R2–TAS1R3); 9UT9 and EMDB-64485 (apo-TAS1R2-VFTD–TAS1R3); 9UTA and EMDB-64486 (apo TMD of TAS1R2–TAS1R3); 9UTB and EMDB-64487 (sucralose-bound full-length TAS1R2–TAS1R3); 9UTC and EMDB-64488 (sucralose-bound TAS1R2-VFTD–TAS1R3). Source data are provided with this paper.
References
Nelson, G. et al. Mammalian sweet taste receptors. Cell 106, 381–390 (2001).
Zhao, G. Q. et al. The receptors for mammalian sweet and umami taste. Cell 115, 255–266 (2003).
Lindemann, B. Taste reception. Physiol. Rev. 76, 719–766 (1996).
Lindemann, B. Receptors and transduction in taste. Nature 413, 219–225 (2001).
Lee, H., Macpherson, L. J., Parada, C. A., Zuker, C. S. & Ryba, N. J. P. Rewiring the taste system. Nature 548, 330–333 (2017).
Beauchamp, G. K. Basic taste: a perceptual concept. J. Agric. Food Chem. 67, 13860–13869 (2019).
Chandrashekar, J., Hoon, M. A., Ryba, N. J. & Zuker, C. S. The receptors and cells for mammalian taste. Nature 444, 288–294 (2006).
Max, M. et al. encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus. Nat. Genet. 28, 58–63 (2001).
Montmayeur, J. P., Liberles, S. D., Matsunami, H. & Buck, L. B. A candidate taste receptor gene near a sweet taste locus. Nat. Neurosci. 4, 492–498 (2001).
Adler, E. et al. A novel family of mammalian taste receptors. Cell 100, 693–702 (2000).
Chandrashekar, J. et al. T2Rs function as bitter taste receptors. Cell 100, 703–711 (2000).
Matsunami, H., Montmayeur, J. P. & Buck, L. B. A family of candidate taste receptors in human and mouse. Nature 404, 601–604 (2000).
Tomonari, H. et al. Gα-gustducin is extensively coexpressed with sweet and bitter taste receptors in both the soft palate and fungiform papillae but has a different functional significance. Chem. Senses 37, 241–251 (2012).
Yarmolinsky, D. A., Zuker, C. S. & Ryba, N. J. Common sense about taste: from mammals to insects. Cell 139, 234–244 (2009).
Damak, S. et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301, 850–853 (2003).
Li, X. D. et al. Human receptors for sweet and umami taste. Proc. Natl Acad. Sci. USA 99, 4692–4696 (2002).
Reiner, A. & Levitz, J. Glutamatergic signaling in the central nervous system: ionotropic and metabotropic receptors in concert. Neuron 98, 1080–1098 (2018).
Heaney, C. F. & Kinney, J. W. Role of GABAB receptors in learning and memory and neurological disorders. Neurosci. Biobehav. Rev. 63, 1–28 (2016).
Hannan, F. M., Kallay, E., Chang, W., Brandi, M. L. & Thakker, R. V. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat. Rev. Endocrinol. 15, 33–51 (2018).
Liu, H., Li, Y. & Gao, Y. in G Protein-Coupled Receptors—Part B 1st edn, Vol. 195 (ed. Shukla, A. K.) Ch. 4 (Academic Press, 2023).
Nie, Y., Vigues, S., Hobbs, J. R., Conn, G. L. & Munger, S. D. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr. Biol. 15, 1948–1952 (2005).
Jiang, P. H. et al. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J. Biol. Chem. 279, 45068–45075 (2004).
Walters, D. E. & Hellekant, G. Interactions of the sweet protein brazzein with the sweet taste receptor. J. Agr. Food Chem. 54, 10129–10133 (2006).
Servant, G. et al. Positive allosteric modulators of the human sweet taste receptor enhance sweet taste. Proc. Natl Acad. Sci. USA 107, 4746–4751 (2010).
Belloir, C., Jeannin, M., Karolkowski, A., Scott, C. & Briand, L. A receptor-based assay to study the sweet and bitter tastes of sweeteners and binary sweet blends: the SWEET project. Chem. Senses 49, bjae041 (2024).
Zuker, C. S. Food for the brain. Cell 161, 9–11 (2015).
Nieh, E. H. et al. Decoding neural circuits that control compulsive sucrose seeking. Cell 160, 528–541 (2015).
Margolskee, R. F. et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na-glucose cotransporter 1. Proc. Natl Acad. Sci. USA 104, 15075–15080 (2007).
Kyriazis, G. A., Soundarapandian, M. M. & Tyrberg, B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc. Natl Acad. Sci. USA 109, E524–E532 (2012).
Ren, X., Zhou, L., Terwilliger, R., Newton, S. S. & de Araujo, I. E. Sweet taste signaling functions as a hypothalamic glucose sensor. Front. Integr. Neurosci. https://doi.org/10.3389/neuro.07.012.2009 (2009).
Janssen, S. et al. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl Acad. Sci. USA 108, 2094–2099 (2011).
Lee, S. J., Depoortere, I. & Hatt, H. Therapeutic potential of ectopic olfactory and taste receptors. Nat. Rev. Drug Discov. 18, 116–138 (2019).
Nuemket, N. et al. Structural basis for perception of diverse chemical substances by T1r taste receptors. Nat. Commun. 8, 15530 (2017).
Gadella, T. W. J. Jr et al. mScarlet3: a brilliant and fast-maturing red fluorescent protein. Nat. Methods 20, 541–545 (2023).
Shaner, N. C. et al. A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat. Methods 10, 407–409 (2013).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Juen, Z. et al. The structure of human sweetness. Cell https://doi.org/10.1016/j.cell.2025.04.021 (2025).
Masuda, K. et al. Characterization of the modes of binding between human sweet taste receptor and low-molecular-weight sweet compounds. PLoS ONE 7, e35380 (2012).
Zhang, F. et al. Molecular mechanism of the sweet taste enhancers. Proc. Natl Acad. Sci. USA 107, 4752–4757 (2010).
Assadi-Porter, F. M. et al. Key amino acid residues involved in multi-point binding interactions between brazzein, a sweet protein, and the T1R2–T1R3 human sweet receptor. J. Mol. Biol. 398, 584–599 (2010).
Liu, B. et al. Molecular mechanism of species-dependent sweet taste toward artificial sweeteners. J. Neurosci. 31, 11070–11076 (2011).
Servant, G., Kenakin, T., Zhang, L., Williams, M. & Servant, N. The function and allosteric control of the human sweet taste receptor. Adv. Pharmacol. 88, 59–82 (2020).
Hu, X. et al. Bitter taste TAS2R14 activation by intracellular tastants and cholesterol. Nature 631, 459–466 (2024).
Xu, W. et al. Structural basis for strychnine activation of human bitter taste receptor TAS2R46. Science 377, 1298–1304 (2022).
Koehl, A. et al. Structural insights into the activation of metabotropic glutamate receptors. Nature 566, 79–84 (2019).
Shaye, H. et al. Structural basis of the activation of a metabotropic GABA receptor. Nature 584, 298–303 (2020).
DuBois, G. E. & Prakash, I. Non-caloric sweeteners, sweetness modulators, and sweetener enhancers. Annu. Rev. Food Sci. Technol. 3, 353–380 (2012).
Valente, C. et al. Genes from the TAS1R and TAS2R families of taste receptors: looking for signatures of their adaptive role in human evolution. Genome Biol. Evol. 10, 1139–1152 (2018).
Dias, A. G. et al. Variation in the TAS1R2 gene, sweet taste perception and intake of sugars. J. Nutrigenet. Nutrigenomics 8, 81–90 (2015).
Melis, M., Mastinu, M., Naciri, L. C., Muroni, P. & Tomassini Barbarossa, I. Associations between sweet taste sensitivity and polymorphisms (SNPs) in the TAS1R2 and TAS1R3 genes, gender, PROP taster status, and density of fungiform papillae in a genetically homogeneous Sardinian cohort. Nutrients 14, 4903 (2022).
Serrano, J. et al. The Ile191Val is a partial loss-of-function variant of the TAS1R2 sweet-taste receptor and is associated with reduced glucose excursions in humans. Mol. Metab. 54, 101339 (2021).
Dubovski, N., Ben-Shoshan Galeczki, Y., Malach, E. & Niv, M. Y. Sensitivity of human sweet taste receptor subunits T1R2 and T1R3 to activation by glucose enantiomers. Chem. Senses 48, bjad005 (2023).
Yoshimura, A. et al. Taste receptor type 1 member 3 in osteoclasts regulates osteoclastogenesis via detection of glucose. J. Biol. Chem. 301, 108273 (2025).
Schmidt, P. et al. Tas1R3 dependent and independent recognition of sugars in the urethra and the role of tuft cells in this process. Adv. Biol. 8, e2400117 (2024).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Tunyasuvunakool, K. et al. Highly accurate protein structure prediction for the human proteome. Nature 596, 590–596 (2021).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. Recent developments in the PHENIX software for automated crystallographic structure determination. J. Synchrotron Radiat. 11, 53–55 (2004).
Robertson, M. J., van Zundert, G. C. P., Borrelli, K. & Skiniotis, G. GemSpot: a pipeline for robust modeling of ligands into cryo-EM maps. Structure 28, 707–716.e703 (2020).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Varadi, M. et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2022).
Yang, Y. et al. Efficient exploration of chemical space with docking and deep learning. J. Chem. Theory Comput. 17, 7106–7119 (2021).
Jacobson, M. P. et al. A hierarchical approach to all-atom protein loop prediction. Proteins 55, 351–367 (2004).
Lu, C. et al. OPLS4: improving force field accuracy on challenging regimes of chemical space. J. Chem. Theory Comput. 17, 4291–4300 (2021).
Farid, R., Day, T., Friesner, R. A. & Pearlstein, R. A. New insights about HERG blockade obtained from protein modeling, potential energy mapping, and docking studies. Bioorg. Med. Chem. 14, 3160–3173 (2006).
Bowers, K. J. et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proc. 2006 ACM/IEEE Conference on Supercomputing (ACM, IEEE, 2006).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Acknowledgements
This work was supported by the National Natural Science Foundation of China grants 32271262 and 32471267 (T.H.), and 32230026 (Z.-J.L.), ShanghaiTech University School of Life Science Development Fund Project (Z.-J.L.), National Key Research and Development Program of China grant 2022YFA1302903 (T.H.) and 2023YFC2509100 (T.H.), Shanghai’s Top Priority Research Center 2022ZZ01017 (Z.-J.L.), AI4S Program at ShanghaiTech University (Z.-J.L.), Shanghai Frontiers Science Center for Biomacromolecules and Precision Medicine at ShanghaiTech University. We thank the Shanghai Municipal Government and ShanghaiTech University for financial support. We thank S.-W. Zhao, Y.-R. Wu and F. Zhao for discussions and suggestions for sucralose binding pose modelling; N. Chen, S.-W. Hu and P. Si at cell expression core; and Q.-Y. Shi at protein purification core of iHuman Institute for their support. The cryo-EM data were collected at the Bio-Electron Microscopy Facility of ShanghaiTech University, with the assistance of L. Wang and Q.-Q. Sun.
Author information
Authors and Affiliations
Contributions
Z.-J.L. and T.H. initiated the project. Z.S., W.X. and X.Y. designed the expression constructs, purified the receptor complexes, prepared the final samples for cryo-EM data collection, participated in the electron microscopy data processing, and participated in the figure and manuscript preparation. L.W. performed the electron microscopy data processing and structure determination. W.X. performed the functional assays. S.L. performed the molecular docking and molecular dynamics simulations. W.D. assisted with the electron microscopy data processing. J.Z. and B.M. performed the initial constructs and sample optimization. L.Z. assisted with the construct design with fluorescent protein. X.L. performed the mammalian cell expression. J.L. performed the insect cell expression. Z.-J.L. and T.H. conceived and supervised the research, analysed the structures and wrote the manuscript with the input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Loïc Briand and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Construct design and protein property analysis of human TAS1R2-TAS1R3.
a, Schematic diagram of the modified constructs for human TAS1R2 and TAS1R3 used for structure determination. b, The representative SDS-PAGE and western blotting of the TAS1R2-TAS1R3 heterodimer sample (≥ 4 independent experiments). The presence of TAS1R2 and TAS1R3 was confirmed by western blotting with anti-TAS1R2 and anti-TAS1R3 antibody, respectively. c, Analytical size-exclusion chromatograph (aSEC) analysis of the TAS1R2-TAS1R3 heterodimer sample, using UV280 absorbance and fluorescence detection. Left vertical axis: UV280 absorbance values (protein elution profile); Right vertical axis: normalized fluorescence values. Fluorescence detection parameters: TAS1R2-mScarlet3: Excitation 569 nm/ Emission 592 nm; for TAS1R3-mNeonGreen: Excitation 506 nm/ Emission 517 nm. d, Dose-response curves of glycerol on WT human sweet taste receptor measured in calcium mobilization assay. Data are mean ± s.e.m. of three independent experiments performed in triplicate.
Extended Data Fig. 2 Structure determination of human TAS1R2-TAS1R3 heterodimer in apo state.
a, Size exclusion chromatogram profile of the apo TAS1R2-TAS1R3 heterodimer. b, Representative cryo-EM micrograph (30,495 micrographs) (scale bars, 50 nm). c, Two-dimensional (2D) class averages of apo TAS1R2-TAS1R3 particles, showing different particle view. d, Data processing pipeline for the apo TAS1R2-TAS1R3 complex, including 2D classification and 3D reconstruction workflow and final 3D density map colored according to local resolution (Å). e, Fourier shell correlation (FSC) curves for the final 3D density maps of the apo TAS1R2-TAS1R3 complex. Also shown are the Euler Angle Distributions of particles used for the final 3D reconstruction.
Extended Data Fig. 3 Structure determination of human TAS1R2-TAS1R3 heterodimer in sucralose bound state.
a, Size exclusion chromatogram profile of the TAS1R2-TAS1R3 in complex with sucralose. b, Representative cryo-EM micrograph (scale bars, 50 nm) (120,009 micrographs). c, Two-dimensional (2D) class averages of TAS1R2-TAS1R3 in complex with sucralose, showing different particle views. d, Data processing pipeline for the sucralose bound TAS1R2-TAS1R3 complex, including 2D classification and 3D reconstruction workflow and final 3D density map colored according to local resolution (Å). e, Fourier shell correlation (FSC) curves for the final 3D density maps of the TAS1R2-TAS1R3 in complex with sucralose. Euler Angle Distributions of the particles used for the final 3D reconstruction are also shown.
Extended Data Fig. 4 Representative density maps for apo- and sucralose-bound TAS1R2-TAS1R3 structures.
a, Cryo-EM density maps of representative regions in VFTD and CRD of apo TAS1R2-TAS1R3 heterodimer structure. b, Glycosylation sites in VFTD and CRD of the apo TAS1R2-TAS1R3 heterodimer, shown in ball sticks representation. Extra EM density around the side chains of these glycosylation sites is displayed. c, Cryo-EM density maps of representative regions in VFTD, CRD, TMD and ECL2 of sucralose-bound TAS1R2-TAS1R3 heterodimer structure. d, Cryo-EM density maps of loops in CRDs that insert into the VFTD dimerization interface in apo TAS1R2-TAS1R3 heterodimer structure. e, Cryo-EM density maps of extracellular regions of TMDs including the conserved disulfide bonds between ECL2 and TM3 in TAS1R2 and TAS1R3, respectively, in apo-state structure. f, Cryo-EM density maps for helices TM1-TM7 of transmembrane domain, ICL2 and ECL2 of TAS1R2 and TAS1R3, respectively, in apo-state structure. g, Structural comparison of TAS1R2 and TAS1R3 in apo state aligned with TMD.
Extended Data Fig. 5 Structure determination of human TAS1R2-TAS1R3 heterodimer in glycerol-free condition and structural comparisons with reported sucralose-bound sweet taste receptor.
a, Size exclusion chromatogram profile of the apo TAS1R2-TAS1R3 heterodimer in the glycerol-free buffer condition. b, Representative cryo-EM micrograph (scale bars, 50 nm) (6,412 micrographs). c, Two-dimensional (2D) class averages of apo TAS1R2-TAS1R3 particles in the glycerol-free buffer condition, showing different particle view. d, The raw map for apo TAS1R2-TAS1R3 in the glycerol-free buffer condition. e, The raw map for apo TAS1R2-TAS1R3 in the glycerol-free buffer condition aligns well with that of apo TAS1R2-TAS1R3 map. f, The apo TAS1R2-TAS1R3 structure fits well into the two raw maps. g, Structural comparisons of reported full-length TAS1R2-TAS1R3 (PDB: 9NOR) with apo TAS1R2-TAS1R3 (left) (RMSD: 2.07 Å) and sucralose-bound TAS1R2-TAS1R3 (right) (RMSD: 0.99 Å), respectively.
Extended Data Fig. 6 Structural features of full-length TAS1R2-TAS1R3 heterodimers.
a, Molecular surface of the apo-state TAS1R2-TAS1R3 complex, showing the plane of heterodimer interfaces for the VFTD and TMD. Structural elements involved in heterodimer formation are highlighted in cartoon representation. b, Structural comparison of TAS1R2 and TAS1R3 in apo- and sucralose-bound states. TAS1R3 is shown as cartoon and TAS1R2 is shown as transparent surface (left). TAS1R2 is shown as cartoon and TAS1R3 is shown as transparent surface (right). c, Conformational changes of VFTDs in apo- and sucralose-bound states viewed from the extracellular top. d, The bending of CRDs in TAS1R2 and TAS1R3 from apo- to sucralose-bound states.
Extended Data Fig. 7 Sequence alignment of human TAS1R2 and TAS1R3.
The secondary structure is shown.
Extended Data Fig. 8 Molecular dynamics simulations of sucralose binding pose in TAS1R2 and the docking poses of sucrose and neotame in sweet taste receptor.
a, Cryo-EM density maps for sucralose and surrounding residues in its binding pocket. b, RMSD of sucralose during 500 ns simulations of sucralose-TAS1R2-TAS1R3 (VFTDs). The colors denote for 3 independent runs. c, Structural comparisons with reported sucralose-bound TAS1R2-TAS1R3 VFTDs (PDB: 9NOV) and sucralose adopts a similar binding pose in TAS1R2-VFTD. d, Comparison of docking poses of sucrose and neotame with the experimental determined sucralose binding pose in TAS1R2-VFTD. e-f, The docking pose of neotame (e) and sucrose (f) in TAS1R2-VFTD. g-h, Dose-response curves of key residues mutations in LB1 and LB2 of TAS1R2-VFTD in response to stimulation with neotame (g) and sucrose (h) in calcium mobilization assay. Data are mean ± s.e.m. of three independent experiments performed in triplicate.
Extended Data Fig. 9 Structural comparison of VFTD and TMD in TAS1R2-TAS1R3 structure with other class C GPCRs and AlphaFold 3 predicted model of TAS1R2-TAS1R3 in complex with G protein.
a, Electrostatic potential mapped onto the cleft formed by LB1 and LB2 in the VFTDs of TAS1R2 and TAS1R3. b, The structural comparison of TMD between agonist-bound mGlu5 homodimer and sucralose bound TAS1R2-TAS1R3 heterodimer. c, ICL2 in TAS1R2 and TAS1R3 forms a similar short helix conformation with that in bitter taste receptors TAS2R14 and TAS2R46. d, Conformational changes of VFTDs from apo- to agonist-bound states in TAS1R2, GABAB1, mGlu5 and CaSR. e-f, Structural comparison of VFTDs between L-Gln bound medaka fish TAS1R2-TAS1R3 (PDB: 5X2M) and sucralose bound human TAS1R2-TAS1R3 (e), the ligands binding modes in TAS1R2 (f). g, The overall structure model of TAS1R2-TAS1R3 in complex with Gustducin (Gαgustducin β1γ2) predicted by AlphaFold3. h, AlphaFold3 predicted TAS1R2-Gαgustducin complex model is similar with that of TAS1R2TMD-miniGαs/gust (PDB: 9NOX).
Supplementary information
Supplementary Figure
Uncropped SDS–PAGE and western blotting for Extended Data Fig. 1.
Supplementary Video 1
The conformational changes between full-length apo-TAS1R2–TAS1R3 and sucralose-bound TAS1R2–TAS1R3 structures.
Supplementary Video 2
The binding mode of sucralose in TAS1R2-VFTD and the conformational changes of key residues from apo to sucralose-bound states.
Supplementary Video 3
The conformational changes of VFTDs from apo to sucralose-bound states in sweet taste receptor.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Z., Xu, W., Wu, L. et al. Structural and functional characterization of human sweet taste receptor. Nature 645, 801–808 (2025). https://doi.org/10.1038/s41586-025-09302-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09302-6
This article is cited by
-
Structure and activation mechanism of human sweet taste receptor
Cell Research (2025)