Abstract
The medial prefrontal cortex (MPF) regulates autonomic and neuroendocrine responses to stress1,2 and coordinates goal-directed behaviours such as attention, decision-making and social interactions3,4,5,6,7,8. However, the underlying mechanisms remain unclear due to incomplete circuit-level MPF characterization7. Here, using integrated neuroanatomical, physiological and behavioural approaches, we construct a comprehensive wiring diagram of the MPF, focused on the dorsal peduncular area (DP)—a poorly understood prefrontal area. We identify its deep (DPd) and superficial (DPs) layers, along with the infralimbic area, as major components of the visceromotor cortex that directly project to hypothalamic and brainstem structures to govern neuroendocrine, sympathetic and parasympathetic output. Notably, the DP functions as a network hub integrating diverse cortical inputs and modulating goal-directed behaviour through a largely unidirectional cortical information flow. On the basis of the mesoscale MPF connectome, we propose a unified network model in which distinct MPF areas orchestrate physiological and behavioural responses to internal and external stimuli.
Similar content being viewed by others
Main
Traditionally, identification of the MPF (or mPFC) relied on its connections to brain structures involved in autonomic and emotional responses, such as the hypothalamus and periaqueductal gray (PAG)4,9. Accordingly, the MPF arguably includes the anterior cingulate (ACA), prelimbic (PL) and infralimbic (ILA) cortices7,10. Yet, ambiguity persists in the precise definition of the rodent MPF, particularly concerning the DP, a unique medial prefrontal region that is situated between the six-layered neocortex and the three-layered olfactory cortex11. Studies suggest that the DP, together with the ventrally adjacent dorsal tenia tecta (TTd), regulates autonomic and behavioural responses to psychological stress12,13, anxiety and depression14, fear conditioning15, threat16 and opioid reward17, which aligns with MPF function; however, a thorough understanding of its neural circuits and structural–functional position within the MPF are obscure. Without the DP, proposed unified MPF network models would be incomplete and inaccurate.
DP is a unique MPF area
The DP, initially included as part of the ILA18, was defined as part of the olfactory cortex19. In the mouse brain, the anatomical borders of the DP are inconsistent across mouse brain atlases7,11,20. To address this, we refined the borders of the DP (Allen Reference Atlas (ARA) levels 37–41; Supplementary Fig. 1) on the basis of its cytoarchitecture, neuronal connectivity and gene expression (Fig. 1a–d and Extended Data Figs. 1 and 2).
a, DP delineation based on Nissl cytoarchitecture. Two DP sublayers were identified: the DPs and DPd. Connectivity confirms DPs and DPd distinction: the BLAal (PHAL, magenta) targets the DPs, while the BLAam (AAV-GFP) innervates the ILA and DPd. b, Inputs from the AUDv and RE terminate in DP layer 1, defining the DP boundaries. c, Connectivity-based characterization of DP sublayers. FG-labelled DP to ACAd neurons are confined to DPs, whereas MPO- and thalamus-projecting neurons localize in the DPd. LHA-projecting neurons span the DPd and deep DPs. ProSUB axons densely innervate the DPs. d, Cell-type-specific gene expression supports regional and laminar DP organization. The RNAscope analysis shows that Cux2 is absent in the DP, Etv1 is enriched in the DPs and Tle4 is expressed in the DPd. Cacna1h is expressed in the neocortex and DPs. DPd is distinguished with Vglut2 expression in VGLUT2 Cre × Ai14 mice and via in situ hybridization. Bright-field in situ hybridization images from the ABA (https://mouse.brain-map.org/). e, Schematic of neuron types in the DPs and DPd. BS, brainstem; d, deep; s, superficial. f, Dendritic morphology of reconstructed DP neurons (left). Morphological clustering (right, top) based on the size (x axis) and complexity (y axis) of dendritic arbours (n = 47) identifies two morphological groups: one with smaller, simpler dendrites (n = 32; dendritic length, 3,024 ± 833 µm; number of branches, 38 ± 12), and another with larger, more complex dendrites (n = 15; dendritic length, 5,821 ± 958 µm; number of branches, 76 ± 13). The 47 DP neurons were pooled from four MORF3 mice. On the basis of the somatic distance from the midline, neurons also were clustered as superficial-layer (n = 26; distance from the midline, 288 ± 81 µm) or deep-layer (n = 21; distance from the midline, 605 ± 106 µm) neurons and are plotted in the size and complexity dimensions (middle right). Examples of superficial and deep neurons are presented from a single brain (bottom left). The box plot (bottom right) demonstrates that superficial neurons have a greater dendritic length (superficial, 4,275 ± 1,717 µm; deep, 3,472 ± 1285 µm; one-tailed Wilcoxon signed-rank test, FDR-corrected P = 0.0465), a higher number of branches (superficial, 56 ± 24; deep, 42 ± 16; FDR-corrected P = 0.0317) and contain a greater proportion of dendrites between soma and midline (superficial, 65 ± 17%; deep, 53 ± 17%; FDR-corrected P = 0.026). The smaller–simpler neurons were more distant from the midline compared with the larger–complex neurons (small–simple distance, 472 ± 184 µm; large–complex distance, 340 ± 153 µm; FDR-corrected P = 0.026). The line inside the box plot shows the median, the top and bottom edges are the upper (0.75) and lower (0.25) quartiles, the top whisker connects the upper quartile to the non-outlier maximum, and the bottom whisker connects the lower quartile to the non-outlier minimum. Scale bars, 200 μm (a (middle), b, c and d (left)), 300 μm (d (right) and f) and 500 μm (a (left and right)). A full list of abbreviations is provided in Supplementary Table 1.
Cytoarchitecturally, the DP has a notably thicker layer 1 compared with the dorsally adjacent ILA and ventrally adjacent TTd (Fig. 1a, Supplementary Fig. 1a,b and Supplementary Methods). The DP also exhibits a unique laminar organization that distinguishes it from other six-layered neocortical areas. On the basis of Nissl staining (Fig. 1a), we identified two primary cellular DP layers: a superficial layer (DPs) characterized by loosely arranged, relatively larger cell bodied neurons, and a deep layer (DPd) in which neurons with smaller cell bodies are densely packed. This cytoarchitectonic feature is also distinct from the densely packed, darkly stained cellular layer 2 and cell-sparse layer 3 of the TTd.
Our connectivity-based anatomic parcellation validated the laminar-specific DP features. Axon projections from the ventral auditory cortex (AUDv), lateral entorhinal cortex (ENTl) and reuniens thalamic nucleus (RE) generate dense terminals specifically in DP layer 1 (Fig. 1b and Extended Data Fig. 1a–c). Projections originating from the piriform cortex (PIR), anterior basolateral amygdala (BLAa; lateral part, BLAal)21 and posterior BLA (BLAp) densely distribute in DPs, while the BLAa medial (BLAam) and caudal (BLAac) parts generate axons in the DPd and ILA (Fig. 1a and Extended Data Fig. 1d–f). The cortical amygdala posterior lateral part (COApl) generates dense axons in DP layer 1 and the DPd but avoids DPs, while the COA posterior medial part (COApm) generates dense projections in the DPd (Extended Data Fig. 1g), illustrating the clearly distinguishable DP laminar organization.
Connectionally defined IT, CPT and CT DP neurons
Using multifluorescence retrograde tracing, we found that intratelencephalic (IT) cortical projection neurons like those that target the ACAd and anterior COA (COAa) primarily distribute in the DPs (Fig. 1c and Extended Data Fig. 1h,i). By contrast, corticothalamic (CT) neurons projecting to the RE and parataenial thalamic (PT) nuclei are exclusively in the DPd (Fig. 1c and Extended Data Fig. 1j (injection sites))22. Hypothalamic and brainstem projecting corticofugal pyramidal tract (CPT) neurons display target-specific distribution patterns. Neurons that project to the medial preoptic area (MPO) are located primarily in the DPd, whereas those projecting to the lateral hypothalamic area (LHA) distribute in both the DPd and deeper portions of the DPs (Fig. 1c and Extended Data Fig. 1k–l).
Molecularly defined DP cell types
We investigated the expression patterns of over 100 genes from the Allen Brain Atlas (ABA) database, to examine their regional and laminar DP patterns. RNAscope analysis of the select marker genes Cux2, Etv1 and Tle4 confirmed specificities of DP neuron types (Fig. 1d and Extended Data Fig. 2a). Our analysis revealed several key findings (Fig. 1d and Extended Data Fig. 2b–g): (1) marker genes for cortical layer 5 (L5) IT cells, Cacna1h and Plxnd1 (ref. 23), are expressed in the DPs but not in the DPd, suggesting that DPs neurons share similar cell-type-specific molecular features of L5 IT neurons; (2) the L6 CT or L6 CT/IT marker genes Tle4 and Pamr1 are expressed in the DPd; (3) the L5 CPT marker genes Etv1 (also known as Er81) and Fezf2 (also for L5 IT cells)23 are expressed in both the DPd and the DPs. Cortical L2/3 marker genes such as Cux2 and TTd marker genes such as Lrmp2 do not extend into the DP. Additional genes such as Htr2c and Fxyd6, which are uniquely expressed in the DPs and DPd, respectively, lend further evidence for their distinction. Combining retrograde tracing with RNAscope demonstrated that a large population of CPT cells in the DPd expressing Fezf2 project to hypothalamic structures such as the MPN, LHA and dorsomedial hypothalamic nucleus (DMH), and hypothalamic paraventricular nucleus (PVH) (Extended Data Fig. 3). Finally, we identified a unique population of neurons that expresses Vglut2 in the DPd, but not in the DPs or other MPF areas (Fig. 1d).
In summary, the DPs is characterized by L5 IT-type neurons that project to cortical areas and to the amygdala, and CPT-like neurons that project to the LHA (Fig. 1e). However, the DPd predominantly contains CT- and CPT-type neurons, with the latter projecting to hypothalamic periventricular structures such as the MPO, as well as brainstem structures such as the parabrachial nucleus (PB)17.
Morphological DP cell types
Studies examining the morphological features of MPF neurons24 have excluded the DP. We used the genetic sparse labelling MORF3 (ref. 25) reporter line, brain clearing and 3D microscopy imaging to acquire and systematically categorize morphological features of sparsely labelled excitatory DP projection neurons (Fig. 1f, Extended Data Fig. 4 and Methods). In total, 47 reconstructed DP neurons were categorized into two morphological clusters on the basis of dendritic size (quantified by dendritic length) and complexity (quantified by number of branches). Cluster 1 included smaller, less-complex neurons (32 neurons, average total dendritic length, 3,024 ± 833 µm; average number of branches, 38 ± 12). Cluster 2 contained larger, more-complex dendritic arbours (15 neurons; average total dendritic length, 5,821 ± 958 µm; average number of branches, 76 ± 13). Next, DP neurons were classified into superficial- versus deep-layer clusters on the basis of the distances of their cell bodies from the midline. Comparing deep (21 neurons; distance from the midline, 605 ± 106 µm) and superficial (26 neurons; distance from the midline, 288 ± 81 µm) clusters revealed that deep neurons were smaller and less complex (average length, 3,472 ± 1,285 µm; average number of branches, 42 ± 16), whereas the arbours of superficial neurons were larger and more complex (average length, 4,275 ± 1,717 µm; average number of branches, 56 ± 24). In total, 86% of the deep neurons (18 out of 21) fell within the smaller and less complex morphological class. By contrast, superficial DP neurons fell across both (larger–complex and smaller–simpler) morphological clusters. Overall, superficial layer neurons were larger (one-tailed Wilcoxon signed-rank test, false-discovery rate (FDR)-corrected P = 0.0465) and more complex (one-tailed Wilcoxon signed-rank test, FDR-adjusted P = 0.0317) compared with deep cluster neurons. They also had a greater proportion of the dendritic arbour between the cell body and the midline (one-tailed Wilcoxon signed-rank test, FDR-adjusted P = 0.0261). These results align with the cytoarchitectonic data suggesting distinct cell-type-specific morphological features of DPs and DPd neurons.
Distinct neural network features of DPs and DPd
Extensive research has been conducted on MPF connectivity in rats and primates4,26,27. In mice, investigations have focused on cortical and thalamic MPF connectivity at regional, cell-type-specific and single-neuron resolutions24,28,29,30,31, although the global neural network of individual MPF regions, including the DP, has not been systematically generated.
Our data production and annotation pipelines were implemented to investigate the brain-wide input/output connectivity of the DPd, DPs, ILA, PL, ACAv and ACAd. Anterograde (AAV, PHAL, BDA) and retrograde (CTB, Fluorogold (FG)) tracer injections were placed into each MPF area (Methods, Extended Data Fig. 5 and Supplementary Methods). Image data were registered, and tracer labels were thresholded and annotated using our custom software, Outspector21,32,33,34 (Extended Data Fig. 6a–e, Supplementary Figs. 2–4 and Supplementary Methods; the specificity and accuracy of the Outspector pipeline is shown in Supplementary Fig. 5).
To ensure consistent labelling, 2D hierarchical clustering was performed on data from multiple injections into individual regions of interest (ROIs; Extended Data Fig. 6f). Annotated data were normalized and analysed, with visualizations of anterograde, retrograde and reciprocal connections presented in connectivity matrices. Aggregated anterograde and retrograde connectivity data from select cases with injections in each MPF area—DPs (n = 4 (anterograde) and n = 3 (retrograde)), DPd (n = 2 and n = 1), ILA (n = 3 and n = 2), PL (n = 3 and n = 3), ACAd (n = 3 and n = 3) and ACAv (n = 3 and n = 3)—were quantitatively analysed to construct 2D hierarchical clustering for the projection fraction matrix, input fraction matrix and reciprocity fraction matrix for each MPF area (Supplementary Methods). Accordingly, we constructed comprehensive global input/output MPF connectivity matrices (Extended Data Fig. 7) and MPF connectivity flatmaps (Extended Data Fig. 8) that illustrate the distinct global input/output connectivity patterns of each MPF area. An online tool for viewing all reconstructed connectivity maps is also available (https://brain.neurobio.ucla.edu/mpf/). An additional 96 tracer-injection experiments targeting the cortex, hippocampus, amygdala, thalamus, hypothalamus and brainstem were performed to ensure comprehensiveness, cross-validate anterograde and retrograde datasets, and to address potential tracer tropism issues (the specificity and reliability of the tracing data is shown in Extended Data Fig. 9 and Supplementary Fig. 6). Moreover, AAV1-Cre-based trans-synaptic tracing and TVA-mediated rabies tracing were conducted to delineate multisynaptic and cell-type-specific MPF networks.
DPs and DPd receive distinct cortical inputs
Retrograde tracer injections made into either the DPs or DPd revealed their distinct cortical inputs (Fig. 2a–c, Extended Data Figs. 9 and 10, Supplementary Fig. 7a–f and Supplementary Results), which were all validated with anterograde tracer injections made into their upstream source structures (Extended Data Fig. 11a). Cortical inputs to the DPs and DPd can be categorized as follows: (1) both the DPs and DPd receive dense inputs from olfactory cortical areas and the COA. The DPs receives much denser inputs from the PIR and dorsal endopiriform nucleus (EPd), which processes main olfactory information, while the DPd, but not the DPs, receives dense input from the COApm and moderate input from the posterior amygdalar nucleus (PA). The COApm and PA receive pheromonal information directly from the accessory olfactory bulb (AOB) and are involved in sexual and social behaviours35,36. (2) Inputs from the ENTl: the DPs receives inputs primarily from ENTl L2/3, while ENTl L5 neurons (not L2/3) robustly project to the DPd and ILA28 (Fig. 2a–c, Extended Data Fig. 10a,b and Supplementary Fig. 7d,e). (3) Inputs from the temporal association (TEa), ectorhinal (ECT) and perirhinal (PERI) cortical areas, together termed the rhinal cortex37: these areas receive multimodal information from somatosensory and motor cortical areas28 and generate direct projections to both the DPs and DPd (Fig. 2a,b, Extended Data Fig. 10a,b and Supplementary Fig. 7d). (4) Input from the AUDv (Figs. 1b and 2a, Extended Data Fig. 10a,b and Supplementary Fig. 7c), which is responsible for processing auditory information associated with fear conditioning38, is primarily to the DPs. (5) The claustrum (CLA), which projects to the DPd, but not the DPs, shares massive bidirectional connections with the ILA and PL28 (Extended Data Figs. 9g,h and 10a,b and Supplementary Fig. 6g,h and 7b). (6) Inputs from the subiculum and CA1: consistent with our previous report33, anterograde tracer injections into the prosubiculum (ProSUB) resulted in dense axonal terminals in the DPs (Fig. 1c), while anterograde tracer injections into the ventral subiculum (SUBv) and CA1 resulted in axonal terminals in the DPd and ILA (Fig. 2a and Extended Data Fig. 10a,b). (7) Inputs from the BLAa, which is integrally involved in fear conditioning39: the BLAal and BLAp, which integrates olfactory and visceral information, project to the DPs, while the BLAac and BLAam project to the DPd and ILA21 (Figs. 1a and 2a,b and Extended Data Figs. 10a,b and 11b). (8) The dorsal, ventral and posterior agranular insular areas (AId, AIv and AIp), which process gustatory and visceral sensory information40, project to the DPd, DPs and ILA (Fig. 2a,b, Extended Data Fig. 10a,b and Supplementary Fig. 7b). (9) In contrast to the DPs, the DPd also receives input from subcortical structures including, but not limited to, the lateral septum (LS) (Extended Data Fig. 10c).
a, Distribution map of cortical neurons projecting to the DPs, DPd and other MPF areas from retrograde tracing experiments, revealing diverse cortical inputs (left). Right, quantified cortical inputs to the DPs and DPd (representative CTB-labelled neurons are shown; the full datasets and connectivity map are shown in Extended Data Figs. 9 and 10 and Supplementary Fig. 7; all MPF cases are available at https://brain.neurobio.ucla.edu/mpf/; validation of the connections is provided in Extended Data Fig. 11a,b). b, Summary of inputs to the DPs and DPd from cortical, amygdalar and hippocampal regions. c, Anterograde tracing confirmed distinct ENTl inputs: Cux2-positive L2/3 neurons project to the DPs, while L5/6 neurons target the DPd. d, Quantitative comparison of DPs outputs to, and inputs from, other MPF (ILA, PL, ACAv, ACAd) and cortical (medial orbitofrontal area (ORBm), MOp, MOs) areas (top). Middle, PHAL-labelled axons from the DPs travel through layer 1 and deep layer 5, terminating densely in the MPF, MOp, MOs and association cortices (for example, PTLp, RSPd, visual areas; Extended Data Fig. 11e). Bottom, by contrast, retrograde CTB labelling reveals sparse DPs input from these regions (see Extended Data Figs. 5a,b and 10a for PHAL and CTB injections). These data highlight the primarily unidirectional DPs to cortex connections. e, Schematic of the DP as a network hub relaying inputs from cortical areas within the lateral cortical network28, olfactory cortex (OLF), amygdala (AMY) and hippocampus (HPF) to other MPF and cortical areas within the medial cortical network28 and somatic sensorimotor cortices. f, 2D hierarchical clustering of the MPF to cortex projection fraction matrix reveals distinct clusters of cortical targets for the DPs, DPd and other MPF areas (based on anterograde tracer injections made into each MPF area). Prefrontal (PFC) regions are grouped to the left of the grey line; additional clusters are boxed in colour. Further details are provided in the Supplementary Results. Scale bars, 200 μm (d (right)), 300 μm (c (top)) and 500 μm (a, c (bottom) and d (left and middle)). A full list of abbreviations is provided in Supplementary Table 1.
DP projections to the MPF and cortical areas
The DPs and DPd generate minor projections back to regions that heavily innervate them, such as olfactory cortex (Supplementary Fig. 6a–f). Instead, the DPs projects densely to other MPF areas, including the ILA, PL, ACAv and ACAd, which, except for the ILA, send only light projections back to the DP (Fig. 2d–f and Supplementary Fig. 7a). This is atypical for MPF structures, which are predominantly reciprocally interconnected (Extended Data Fig. 11c,d). Moreover, the DPs projects substantially to the secondary motor (MOs) and primary motor (MOp) domains involved in controlling whisker, upper limb and trunk movements, as well as to the dorsal retrosplenial (RSPd), posterior parietal (PTLp) and anteromedial visual (VISam) cortical areas (Fig. 2e,f, Extended Data Fig. 11e and Supplementary Fig. 7f). Although these cortical structures share reciprocal connections with the ACAd, ACAv, PL and ILA28, they send little to no projections to the DP (Supplementary Fig. 8). Further details are provided in the Supplementary Results.
Together, the data show that the DP mediates a predominantly unidirectional flow of cortical information—a distinguishing DP feature. It receives convergent inputs from olfactory areas, amygdala, hippocampus, ENTl and other areas along the lateral cerebral mantle such as the AUDv and AIp and, in turn, densely innervates other MPF and cortical areas along the medial neocortex (Fig. 2e and Supplementary Fig. 8). Supporting this notion, TVA-receptor-mediated cell-type-specific rabies viral tracing demonstrated that DPs to ACA-projecting neurons receive monosynaptic inputs from the AON, PIR, SUB, CA1 and ENTl (Extended Data Fig. 11f). Further details are provided in the Supplementary Results.
DP connections with midline thalamic nuclei
The MPF is known for its reciprocal connections with the mediodorsal thalamic nucleus (MD) as well as other medial and midline thalamic nuclei4, although the thalamic connectivity of the DP has not been investigated. Comparative distributions of thalamocortical projection neurons and corticothalamic projection terminals for MPF regions are displayed in connectivity maps (Extended Data Fig. 12a; https://brain.neurobio.ucla.edu/mpf/). Quantitative analyses of all thalamic connectivity are visualized in matrices (Extended Data Fig. 12e and Supplementary Fig. 9; additional details are provided in the Supplementary Results).
The DPs (layer 1) receives extensive input from the RE (Extended Data Fig. 12b; validation of RE to DP projections is shown in Fig. 1b and Extended Data Fig. 1c), which accounts for 76.79% of its total thalamic input. The DPs does not provide significant thalamic projections given its lack of CT neurons. By contrast, the DPd bidirectionally connects with several midline and medial thalamic nuclei, including the RE, paraventricular (PVT), parataenial, medial part of the mediodorsal (MDm), rhomboid (RH) and intermediodorsal (IMD) thalamic nuclei (Extended Data Fig. 12c and Supplementary Fig. 9b). Quantitative analysis revealed that the DPd and ILA share similar thalamic connections specifically with the RE, PT, PVT and MDm, while the PL, ACAd and ACAv demonstrate distinct connections with different clusters of thalamic nuclei (Extended Data Fig. 12e, Supplementary Fig. 9a,b and Supplementary Results).
Anterograde/retrograde tracer co-injections were made into the PVT, PT or RE (Extended Data Figs. 12f and 13a,b), which validated thalamocortical and corticothalamic connections, but also identified information relayed to the DP and ILA through these thalamic nuclei. The PVT bidirectionally connects with structures in a network that regulates visceral sensorimotor activities (Extended Data Fig. 12f,g). These include (1) the PB and nucleus of the solitary tract (NTS), the primary structures that process visceral sensory inputs. Notably, the PB also generates direct projections to the ILA41 (Supplementary Fig. 10); (2) the median preoptic nucleus (MEPO), which processes information related to fluid balance, thirst and cardiovascular function42; (3) the agranular insular areas (AId, AIv, AIp), central nucleus of the amygdala (CEA), bed nuclei of the stria terminalis (BST) and PAG, all of which are involved in autonomic function control43.
By contrast, the RE and PT bridge the DP and ILA with hypothalamic networks that govern two basic classes of social behaviour (namely, reproductive and defensive) as well as with structures implicated in spatial navigation and conditioned memory (Extended Data Figs. 12f,g and 13a,b). Given that the RE is a primary source of thalamic input to the DPs, we used a TVA-receptor-mediated rabies tracing technique to further examine which structures provide monosynaptic inputs to the RE to DP neurons (Extended Data Fig. 13c,d). Additional details are provided in the Supplementary Results.
DP projects to structures regulating behaviour
DP projections to cerebral nuclei that control visceral motor activities
All neocortical areas, including MPF areas, except for the DP, project heavily to the caudoputamen (CP)32. Instead, the DPs exhibits highly dense projections to the CEA and substantia innominata (SI), and moderate projections to the anterolateral division of the BST (BSTal) (Extended Data Fig. 14a–e), all of which are extensively interconnected in a core network that regulates autonomic function44. By contrast, the DPd densely projects to the anteromedial BST (BSTam) (Extended Data Fig. 14a,d,e), which regulates neuroendocrine activities of the hypothalamic–pituitary–adrenal axis in response to stress1,45,46. The DPd and ILA provide significantly weaker projections to the CEA and, instead, topographically project to the medial amygdalar nucleus (MEA), posterior division of the BST (BSTp) and hypothalamic medial nuclei (Extended Data Fig. 14a,c,e), which form two parallel subnetworks that govern reproductive and defensive behaviours36,43. Like the ILA, the DPs and DPd also topographically project to the olfactory tubercle (OT) and nucleus accumbens (ACB) (Extended Data Fig. 14b–d), known for their roles in reward and social interactions47.
DPs projections to preautonomic hypothalamic structures
The MPF coordinates autonomic, neuroendocrine and behavioural responses that are important for maintaining emotional equilibrium1,2,3. The DPs exhibits extensive projections that innervate the descending parts of the PVH (PVHd), namely the lateral parvicellular (PVHlp) and forniceal (PVHf) (Fig. 3a (i and iii) and Extended Data Fig. 15a,b). These regions contain VGLUT2-positive preautonomic neurons that send descending projections to the spinal cord’s intermediolateral (IML) column (Fig. 3a (ii and iv)) for sympathetic output control43,48. Moreover, the DPs generates dense projections to the LHA (Fig. 3a (v) and Extended Data Figs. 15a,c and 16a), which also contains preautonomic neurons that project to the spinal cord (Fig. 3a (v and vi)), suggesting that DPs neurons probably innervate spinal-projecting LHA neurons.
a, The DPs innervates two parts of the PVHd (1–3), marked by VGLUT2 expression (2; Bright-field in situ hybridization images from the ABA (https://Brain-map.org)): the PVHlp and PVHf, which contain spinal-projecting neurons labelled by CTB spinal cord injection (4; see Extended Data Fig. 15a for PHAL injection). DPs axons also densely innervate the LHA (5), which contains CTB-labelled spinal-projecting neurons (6). fx, fornix; mtt, mammilothalamic tract; v3, third ventricle. b, In MORF3 mice, AAV-RFP was injected into the DP (1 and 2) and AAVretro-Cre was injected into the spinal cord to identify potential synaptic contacts between DP axons and hypothalamic spinal-projecting neurons. Cre-induced MORF3 expression revealed the morphology of spinal-projecting neurons (3). High-resolution light-sheet (1–4 and 7) and confocal (5, 6 and 8–12) images show close appositions between DP terminals and MORF3-labelled somas and dendrites in PVHd (6) and LHA (7–12). The blue arrowheads mark corresponding neurons across magnifications; the white arrowheads indicate putative axodendritic contacts (Supplementary Video 1). c, AAV1-Cre trans-synaptic tagging and Cre-dependent anterograde tracing confirmed the DP to LHA to IML column (sympathetic preganglionic neurons) pathway. AAV1-Cre DP injection (top left) transported Cre to postsynaptic LHA neurons, which were labelled through Cre-dependent AAV-GFP (bottom left). Middle, GFP-labelled axons in the IML (thoracic levels T1–T2). Right, magnified images showing terminal boutons apposed to neurons in the IML (1–3) and near the central canal (4). d, Pathways through which the DPs regulates vagal parasympathetic output. AAV1-Cre DPs injection (1) anterogradely transported Cre to the CEA, where Cre-dependent AAV-GFP was injected (2) to label axons from Cre-positive postsynaptic neurons (3–5). CEA starter cells co-expressed Cre and GFP (2). GFP-labelled axons in the DMX and NTS (5), confirming the DPs to CEA to DMX pathway. Projections targeted other structures that regulate autonomic function, for example, BST and SI (3), PB (4), PARN and IRN (5). Image 6 shows DMX/NTS labelling from non-selective CEA neurons. V, trigeminal motor nucleus. e, Schematic of the DPs, DPd and ILA in a cerebral network regulating autonomic outputs. f, Direct projections to Barrington’s nucleus (B) from the ILA (left) and DPd (right; green), indicating disynaptic MPF connections controlling lumbosacral parasympathetic outputs (DPd/ILA to Barrington’s nucleus to spinal cord). Note that PL also projects to Barrington’s nucleus (right; red). Scale bars, 5 μm (b (10 and 11)), 7 μm (b (12)), 10 μm (b (9)), 20 μm (c (right)), 30 μm (b (6)), 100 μm (c (top left, inset)), 200 μm (a and c (bottom left and middle)), 300 μm (b (2–5, 7 and 8)), 500 µm (d and f) and 1 mm (c (top left, main image)). A full list of abbreviations is provided in Supplementary Table 1.
To test this, in MORF3 mice25, AAV-RFP was injected into the DP to label its axonal projections, while AAVretro-Cre was injected into the spinal cord (Fig. 3b (i and ii)). Cre was retrogradely transported to spinal projecting neurons and stochastically unlocked MORF3 expression. This revealed the dendritic morphology of MORF3-labelled PVHd and LHA neurons, which heavily intermingled with axonal terminals arising from the DP (Fig. 3b (iii–v, vii and viii)). Despite MORF3-labelled neurons accounting for only a small fraction (3–5%) of total spinal projecting neurons owing to the sparse labelling MORF3 properties25, significant numbers of close appositions were observed between DP axon terminals and MORF3-labelled somas and dendrites (Fig. 3b (vi and ix–xii) and Supplementary Video 1), indicating putative synaptic connectivity through which the DP innervates LHA spinal projecting neurons.
Next, using AAV1-Cre anterograde trans-synaptic tagging alongside Cre-dependent AAV anterograde tract tracing, we confirmed that the postsynaptic DP-input-recipient neurons in the LHA project directly to IML preautonomic neurons in the spinal cord (Fig. 3c, Extended Data Fig. 16b1–b3; see Supplementary Fig. 11a for the unidirectional DP to LHA connection). Meanwhile, those DP-input-recipient LHA neurons also generate dense projections to the SI, lateral habenular nucleus and brainstem structures such as PAG, PB and nucleus raphe magnus (RM) (Extended Data Fig. 16b4–b19), all of which directly regulate autonomic function. Additional details are provided in the Supplementary Results.
DP projections to autonomic brainstem structures
In rats26,27, the ILA sends direct excitatory projections to the dorsal motor nucleus of the vagus nerve (DMX), which controls vagal parasympathetic output. Our findings in mice confirmed that the ILA, but not the DP, projects directly to the DMX (Extended Data Fig. 16c). Instead, the DPs projects to the CEA (Extended Data Fig. 14a,c,d), which in turn projects to the DMX. Using AAV1-Cre trans-synaptic tagging combined with Cre-dependent AAV-GFP anterograde tracing, we verified that CEA neurons receiving direct inputs from the DP display dense axonal terminals in the DMX (Fig. 3d; DP to CEA projections are unidirectional; Supplementary Fig. 11b). Considering the CEA’s GABAergic nature, these data establish a disynaptic pathway, DPs to CEA to DMX, through which the DPs can inhibit vagal parasympathetic outputs. Notably, DPs-input-recipient CEA neurons generate extensive projections to the BSTal, PB and NTS (Fig. 3d). The PB and NTS are primary structures that process visceral sensory information. While the ILA generates dense projections to both structures, the DPd also projects to the PB41 (Supplementary Fig. 10). Together, the ILA, DPs, CEA, BSTal, PB and NTS form a network that regulates vagal parasympathetic output (Fig. 3e and Extended Data Fig. 16d).
Furthermore, the ILA and DPd project to Barrington’s nucleus (Fig. 3f), which directly innervates parasympathetic motor neurons in the lumbosacral spinal cord that generate the pelvic nerves to regulate micturition, defecation and penile erection49. Finally, like other MPF components4, the ILA, DPs and DPd generate topographic projections to the PAG (Supplementary Fig. 12) through which they can regulate autonomic and behavioural outputs. Further details are provided in the Supplementary Results.
DPd projections to the hypothalamic neuroendocrine zone
The prevailing understanding is that the MPF (ILA and PL) does not directly innervate hypothalamic neuroendocrine cells, but instead regulates neuroendocrine activities indirectly through the BST and other structures46,50. Our data confirm the lack of direct projections from ILA and PL to the hypothalamic neuroendocrine zone. However, we show that the DPd sends direct and extensive projections to the entire hypothalamic neuroendocrine zone along the third ventricle (Fig. 4a,b and Extended Data Fig. 17a–d). Hypothalamic nuclei within this zone, such as the PVH, supraoptic nucleus (SO) and arcuate nucleus (ARH), contain various parvo- and magnocellular neurosecretory neurons43,48 (Extended Data Fig. 17d).
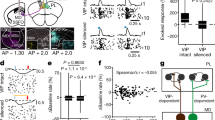
a, The DPd generates dense terminals throughout the hypothalamic periventricular zone, which houses neuroendocrine neurons. BSTif, bed nuclei of the stria terminalis, interfascicular nucleus. See Supplementary Table 1 for a full list of abbreviations. b, MPF projections to the PVH (neuroendocrine, preautonomic) and PV (neuroendocrine). The Pareto chart shows the DPd as main input source to both nuclei. The curved line represents cumulative projections. DPs projections to the PVH target the PVHd. c, DPd labelled neurons after PVH CTB injection confirms the direct DPd to PVH connection. d, AAV-FLEX-RFP DP injection in VGLUT2 Cre mice shows projections to hypothalamic neuroendocrine cells (labelled through tail-vein (intravenously, iv) FG injection; middle and right). DPd axon terminals formed close appositions with neuroendocrine somatostatin (SS)-positive intermediate periventricular nucleus (PVi) and PVHpv neurons and CRH PVHmpd neurons. e, AAV-synaptophysin-GFP DP injection in CRH-Cre Ai14 mice. High-resolution ×60 images show that GFP labelled DP terminals (green) form close appositions with CRH PVH neurons (red) across planes. f, Schematic showing how the DP, MEA and BST regulate hypothalamic neuroendocrine outputs directly through the periventricular zone or indirectly through the AVPV, MEPO, MPNm and DMH. g, In CRH-Cre mice (n = 5), ChR2 was injected into the DP, while Cre-dependent AAV-RFP was injected into PVH (left). Middle, DP ChR2 injection and CRH-positive PVH neurons. The boxed region shows a representative biocytin-labelled recorded CRH neuron. The magnified images show co-localized biocytin (left) and CRH (right). Right, the distribution of peak amplitude responses evoked by optogenetic stimulation of DP inputs (12 out of 14 neurons responded). The box boundaries mark the 25th and 75th percentiles, the centre line shows the median and the whiskers (error bars) indicate the 90th and 10th percentiles. The outlying points (dots) are shown. The trace (right) shows a representative CRH neuron response. oEPSC, optically evoked excitatory postsynaptic current; vh, holding potential. h, AAV1-Cre was injected into the SUBv and BLAa to trans-synaptically deliver Cre to DPd neurons, subsequently targeted with Cre-dependent AAV-FLEX-ChR2 (AAV-mCherry for the control). Optogenetic DPd neuron stimulation induced FOS in the PVHmpd. The top micrographs show the fibre tip and Cre, ChR2 and FOS expression. Bottom, ChR2+ axons and FOS induction in the PVHmpd across ARA levels. i, Plasma CORT levels were significantly elevated in the ChR2 group (n = 6) compared with the controls (n = 9). Statistical analysis was performed using a two-sided unpaired t-test (t13 = 3.379, P = 0.0049) (left) and a Mann–Whitney U-test (U = 4, P = 0.0048) (right). Data are mean ± s.e.m. Scale bars, 10 μm (d (right), e (top right) and g (bottom)), 20 μm (e (middle right and bottom) and g (top right)), 30 μm (e (middle left)), 50 μm (e (top left)), 100 μm (h (bottom)), 150 μm (g (top middle)), 200 μm (c, d (left and middle) and h (top right)), 500 μm (a and h (top left)) and 700 μm (g (top left)).
Retrograde tracer injections in the PVH (Fig. 4c) and ARH (Extended Data Fig. 17e) confirmed these connections and showed retrogradely labelled neurons only in DPd and none in the ILA or other MPF areas. Using VGLUT2-Cre mice, we showed that DPd VGLUT2-positive neurons directly innervate hypothalamic parvocellular and magnocellular neuroendocrine cells, which were labelled through an intravenous FG injection48. Axon terminals originating from VGLUT2 DPd neurons intermingled with FG-labelled cells in the PVH, SO and periventricular nucleus (PV) (Fig. 4d and Extended Data Fig. 18a–c), suggesting putative synaptic connections between DPd axonal terminals and neuroendocrine cells. Using RNAscope in the same animals, we confirmed that the preoptic (PVpo) and anterior (PVa) PV and the periventricular part of the PVH (PVHpv), of which the neurons synthesize SS, receive dense input from the DPd (Fig. 4d). Importantly, the dorsal medial parvicellular part of the PVH (PVHmpd), which contains CRH- and TRH-synthesizing neurons, receives substantial input from DPd VGLUT2-expressing neurons (Fig. 4d).
To confirm direct innervation of CRH neurons by DP axons, we injected an AAV1-synaptophysin-GFP anterograde tracer into the DPd, labelling its axonal terminals in the PVH of CRH-Cre × Ai14 mice in which CRH neurons express tdTomato (Fig. 4e and Methods). High-resolution ×60 confocal imaging of the PVH revealed numerous GFP-labelled axonal boutons in close apposition to CRH neurons (Fig. 4e). Furthermore, cell-type-specific monosynaptic rabies tracing in CRH-Cre mice identified back-labelled neurons in the DPd to confirm direct monosynaptic input from the DPd to PVH CRH neurons (Extended Data Fig. 18e).
Within the ARH, a highly dense DPd axonal terminal plexus was concentrated dorsomedially (Fig. 4a), where dopamine neuroendocrine motoneurons are located. Comparatively, lighter inputs from the DPd were observed in ventrolateral ARH regions, where neurons synthesizing growth-hormone-releasing hormone predominate. Moreover, immunostaining confirmed that DPd axons form putative synaptic connections with magnocellular oxytocin-positive neurons in the PVH and SO (Extended Data Fig. 18d).
The DPd densely innervates the BSTam (Fig. 4a and Extended Data Fig. 14a,e). In turn, the BSTam provides extensive projections to the entire hypothalamic neuroendocrine zone45 (Extended Data Fig. 18f). Both the DPd and BSTam send dense projections to other hypothalamic structures, including the anteroventral periventricular (AVPV), MEPO, medial preoptic (MPN) and dorsomedial (DMH) hypothalamic nuclei and MPO (Fig. 4a and Extended Data Fig. 17a,f,g). These structures, which are components of the hypothalamic visceral motor pattern generator43, densely project to the hypothalamic neuroendocrine zone (Extended Data Fig. 19a) and other structures involved in autonomic function (for example, the PVHlp, LHA, PAG, PB and Barrington’s nucleus) and social behavioural activities (for example, hypothalamic ventromedial, ventral and dorsal premammillary nuclei)43. Together, the DPd, BSTam and these hypothalamic structures form a network that regulates neuroendocrine and motor responses underlying social behaviour (Fig. 4f and Extended Data Fig. 19b). Additional details are provided in the Supplementary Results.
DPd neurons regulate neuroendocrine responses
We validated functional monosynaptic connections from the DPd onto CRH neuroendocrine cells by performing single-cell electrophysiological recordings using channelrhodopsin-assisted circuit mapping (CRACM) combined with anterograde trans-synaptic tracing (Fig. 4g and Methods). Cre-dependent AAV1-expressing tdTomato was injected into CRH-Cre mice to label PVH CRH neurons, while AAV-ChR2-YFP was injected into the DPd to label its axon projections. After 4 weeks, 350-µm brain slices containing the PVH were prepared to patch and record from tdTomato-labelled CRH neurons, with optical stimulation of ChR2-labelled axonal terminals originating from the DPd. The data showed that 12 out of 14 tdTomato-labelled PVH neurons responded to channelrhodopsin stimulation, with an average response amplitude of 83 ± 21 pA (Fig. 4g), confirming monosynaptic inputs from the DPd onto PVH CRH neurons.
We next examined which neural inputs might drive the DPd to regulate motor outputs of CRH neuroendocrine cells as indicated by changes in corticosterone (CORT) levels. We focused on two upstream structures: the SUBv, which is associated with psychological stress and emotional regulation1, and the BLAa, which is implicated in fear conditioning. Using a combination of AAV1-Cre anterograde trans-synaptic tagging and multifluorescence anterograde tract tracing, we found that postsynaptic DPd neurons receiving inputs from the SUBv and BLAa project extensively to the PVH (Extended Data Fig. 20a–h; unidirectional BLA/SUBv to DP connections are shown in Supplementary Fig. 11d,e). Our findings also suggest that inputs from the SUBv and BLAa potentially converge onto the same DPd projection neurons (Extended Data Fig. 20i–n). Together, these data validate a disynaptic SUBv/BLAa to DPd to PVH circuit.
To functionally confirm this hypothesis, we administered AAV1-Cre into the SUBv and the BLAa, and Cre-dependent AAV-ChR2 into the DP. As a result, postsynaptic DP neurons that receive inputs from the SUBv and BLAa would express ChR2 and send downstream projections to the PVH (Fig. 4h). Optogenetic activation of these specific DPd postsynaptic neurons led to a significant increase in plasma CORT levels compared with the control group (P = 0.0049, unpaired t-test; P = 0.0048, Mann–Whitney U-test) (Fig. 4i). This evidence of a functional circuit suggests that DPd neurons can directly modulate plasma CORT levels (and potentially other neuroendocrine activities) in response to signals from the SUBv and BLAa. Consistent with our results, a recent study also showed that chemogenetic stimulation of DP/TTd results in increased plasma CORT14.
Defining the functional relevance of DP neurons
We showed that the DP receives an array of cortical and thalamic inputs and, in turn, regulates neuroendocrine, autonomic and behavioural responses to environmental stimuli. To further validate this hypothesis and explore its functional relevance, we conducted several different experiments.
DP neurons receive convergent sensory and contextual memory synaptic inputs
The DP is the only prefrontal cortical area that receives converging input from olfactory (PIR and EPd), auditory (AUDv) and lateral entorhinal (ENTl) cortices (Fig. 1b and Extended Data Fig. 1a,b,d). The integration of inputs from these cortical areas is important because olfactory and auditory signals are important for social cues and for responding to predators, while the ENTl provides spatial and contextual memory information36,38.
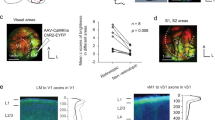
To functionally validate the synaptic convergence of these neural inputs onto DP neurons, we conducted single-cell electrophysiological recording experiments using CRACM combined with anterograde trans-synaptic tracing (Methods). We injected AAV.ChR2 into the ENTl and AAV1-Cre into the AUDv (Fig. 5a; see Supplementary Fig. 11f for the unidirectional AUDv to DP connection). The AAV1-Cre anterogradely transported from the AUDv to DP to trans-synaptically infect postsynaptic DP neurons, which expressed tdTomato. Meanwhile, ChR2-labelled axons arising from the ENTl distributed in DP layer 1. Patch-clamp recordings of tdTomato-labelled DP neurons showed that nearly all of the neurons (n = 10 out of 11) responded to stimulation of ChR2 axons (Fig. 5b), suggesting that ENTl and AUDv inputs converge onto the same DP neurons. Using the same strategy, we demonstrated that DP neurons (n = 12 out of 17) receive convergent auditory (from the AUDv) and olfactory (from the PIR) inputs (Fig. 5b,c).
a,c, AAV1-Cre was injected into the AUDv, AAV-ChR2 into the ENTl (a; n = 6) or PIR (c; n = 5), and Cre-dependent AAV-RFP into the DP to reveal postsynaptic neurons receiving direct input from the AUDv. The magnified images show biocytin-labelled neurons recorded by patch clamp. b, The distribution of peak amplitude (amp) responses evoked by optogenetic stimulation of ENTl (top; 12 out of 17 neurons responded) or PIR (bottom; 10 out of 11 neurons responded) inputs (left). Right, representative responses from RFP-tagged DP neurons (see Extended Data Fig. 20o for isolated monosynaptic responses). The median peak current amplitudes evoked by optogenetic stimulation were not significantly different (ENTl, 218.3 pA, n = 12; PIR, 137.4 pA, n = 11; Mann–Whitney U-test, U = 61, P = 0.782). Whisker plot definitions are as described in Fig. 4g. d, Miniscope calcium imaging was performed during non-social (open-field, object exploration) and social (same-sex and opposite-sex) interactions synchronized with behavioural camera recording. e, Calcium imaging field of view and representative extracted traces. f,g, The percentage of time that mice (under miniscope analysis) spent interacting with non-social versus social targets. DP activity (f); ILA activity (g). The interaction time significantly differed across interaction type. Statistical analysis was performed using one-way ANOVA: DP: F(1.473, 5.154) = 13.47, *P = 0.0108; ILA: F(1.646, 8.230) = 6.883, *P = 0.0205; Tukey’s multiple-comparison test (family-wise error rate controlled) for DP: **P = 0.0051. Dots represent the n per group. Data are mean ± s.e.m. h,i, The percentage of behaviourally responsive cells during interactions with non-social (object) or social targets. DP activity (h); ILA activity (i). No significant differences for the DP or ILA were detected. j, The percentage of all behaviourally responsive cells (excited (E) + inhibited (I)). DP contained significantly more opposite-sex interaction responsive cells than object exploration responsive cells (Tukey’s multiple-comparison test: *P = 0.0168). Significantly more opposite-sex interaction-responsive cells were identified in the DP than in the ILA (Šidák’s multiple-comparison test with adjusted P values: **P = 0.0027). k, The frequency of calcium events during non-social (open-field test) and social (same-sex, opposite-sex) trials. In the DP, the calcium event frequency is significantly higher during same-sex interaction compared with during the open-field test (Tukey’s multiple-comparison test, *P = 0.0303). In the ILA, the frequencies of calcium events during both same-sex (Tukey’s multiple-comparison test, **P = 0.0086) and opposite-sex (Tukey’s multiple-comparison test, **P = 0.0094) interactions are significantly higher than that during the open-field test. Between the DP and ILA, the frequencies of calcium events during the open-field test (Šidák’s multiple-comparison test, *P = 0.0416) and same-sex interaction (Šidák’s multiple-comparison test, *P = 0.0199) are higher in the DP than in the ILA. Scale bars, 250 μm (a (top right) and e), 500 μm (a (bottom right)) and 1 mm (a (left)). A full list of abbreviations is provided in Supplementary Table 1.
DP neurons’ role in perceiving social and environmental stimuli
We explored the potential functional relevance of DP neurons in social cognition and interaction—an extensively studied MPF function3,5,6,51. Social interactions necessitate the perception and integration of diverse neural inputs, including olfactory, pheromonal, auditory, visual and somatosensory cues. These inputs undergo instantaneous modifications through dynamic, mutual feedback between participants, as well as past social experiences36. The DP receives and integrates information relayed from cortical and thalamic inputs and, in turn, projects to the MEA, BST, hypothalamic and other structures implicated in social behaviour. These connectivity data lead us to hypothesize that DP neurons have a role in perceiving and regulating social behaviour.
We performed calcium imaging using miniaturized microscopy in DP during both social and non-social behavioural contexts (Fig. 5d and Methods). We expressed GCAMP6f in VGLUT2-positive DP neurons in VGLUT2 Cre mice (Fig. 5e). In social trials, we monitored DP activity in mice engaged in interactions including chasing, grooming or mounting either with a same-sex or opposite-sex partner (Fig. 5f). In non-social trials, we recorded DP activity in mice within an open-field arena or during exploration of an object (Fig. 5f). We used receiver operating characteristic (ROC) analysis to identify DP neurons that were either significantly inhibited or excited during interactions with same-sex partners, opposite-sex partners or objects. Among the recorded DP neurons (6 male mice, 63.3 ± 9.9 per animal), a substantial proportion responded during either same-sex (6 male mice, 30.3 ± 4.0%) or opposite-sex (3 male mice, 42.2 ± 1.5%) social interactions (Fig. 5h–j). Notably, socially excited neurons outnumbered socially inhibited neurons (Fig. 5h and Extended Data Fig. 20p).
While we also observed that DP neurons respond to interactions with a non-social object (23.9 ± 2.8%), the frequency of calcium events during non-social behaviour trials was significantly lower compared with during social behaviour trials (same-sex interaction) (Fig. 5k; DP, object versus same-sex, *P = 0.0303; Tukey’s multiple-comparison test).
As a control, we examined ILA neuronal activity in response to the same social and non-social cues (6 male mice; average number of ILA neurons recorded, 133.5 ± 14.4). As the ILA lacks VGLUT2-positive neurons, we expressed GCaMP6f in VGLUT1-positive glutamatergic ILA neurons (Fig. 5e). The ILA displays distinct input/output patterns compared with the DP, notably lacking significant inputs from the COApm or PA, and its projections to the hypothalamus primarily target the anterior hypothalamic nucleus (AHN), which is involved in defensive behaviour43. Notably, compared with the DP, the ILA contains significantly fewer opposite-sex interaction-responsive cells (Šidák’s multiple-comparison test, **P = 0.0027; Fig. 5g,i,j) and a lower frequency of calcium events during non-social open-field test (Šidák’s multiple-comparison test, *P = 0.0416) and same-sex social interaction (Šidák’s multiple-comparison test, *P = 0.0199) (Fig. 5g,k and Extended Data Fig. 20q).
Consistent with these findings, using FOS as a marker of neuronal activity in male mice, we show that DP neurons respond to the presence of male (intruders) and female mice, as well as pups (Supplementary Fig. 13a). Furthermore, DP neurons are activated by a variety of stressors and perceived threats, including acute-restraint stress, sudden looming sounds and foot-shock-based fear conditioning (Supplementary Fig. 13b,c). These findings, coupled with the broad range of neural inputs received by the DP, support its role in processing environmental cues that are important for social interactions and evaluation of threat. This ability is essential for assessing risks, making decisions and preparing for appropriate behavioural responses to safeguard well-being. Consistent with this hypothesis, recent research suggests that neurons in the DP/TTd are implicated in psychological stress resulting from social defeat12,13, top-down control of defensive behaviour16, depression, anxiety and encoding of fear memories related to auditory cues14,15, as well as opioid reward17.
Discussion
The concept of the primary visceromotor cortex
The distinct input/output connectivity pattern of the DP compared with other MPF areas and the TTd is striking. Furthermore, the DPs and DPd also exhibit distinct connectivity patterns. On the basis of these findings, we propose the concept of the primary visceromotor cortex (Fig. 6a). Although the notion of the visceromotor cortex has been speculated4,9, our comprehensive MPF mapping determined its main components and their network organization. We propose that the DPd, DPs and ILA compose the core components of the primary visceral motor cortex, respectively regulating neuroendocrine, sympathetic and parasympathetic functions, thereby governing different aspects of visceral motor activities. This network model follows a similar organizational logic to that of the primary motor cortex (MOp) in its control of somatic movements of the head, neck and limbs through its direct innervations of motor neurons in the cranial motor nuclei and ventral horn of the spinal cord43.
a, Schematic showing that the DPs, DPd and ILA form the primary visceral motor cortex, regulating neuroendocrine, sympathetic and parasympathetic outputs. Right, their network pathways across the whole mouse brain. The 3D brain outline is from the Allen Institute Common Coordinate Framework (CCF: https://alleninstitute.github.io/abc_atlas_access/descriptions/Allen-CCF-2020.html). b, 2D anatomic location of the DPs and DPd (left). The ARA digital atlas is available online (https://atlas.brain-map.org/). Middle, schematic and wiring diagram illustrating that the DP is a key node mediating a predominantly unidirectional cortical information flow from caudal to rostral and lateral to medial. The DP integrates convergent inputs from lateral cortical subnetworks, the olfactory cortex, amygdala and hippocampus, and relays this information to the MPF and other cortical areas within the medial cortical network and somatic sensorimotor areas. Right, 3D anatomical views of the DP, ILA and other MPF regions. The 3D brain outline is from the Allen Institute CCF as described in a. c, A proposed unified MPF model based on network analysis. The DP serves as an integrative centre, receiving comprehensive information from both external and internal environments. It transmits this information to other cortical areas including the ILA, PL, ACAv, ACAd, MOs and MOp. Each of these MPF areas, along with the MOp/MOs, carries out specific physiological and motor responses to various stress stimuli and has a role in regulating different goal-directed behaviours to ensure long-term homeostasis through their projections to different brain structures (see the main text for details). For example, the ACAd (and its adjacent MOs frontal eye field (MOs-fef)) and ACAv send dense projections to the dorsomedial striatum or the CP and SC, which are involved in coordinating eye and head movement during navigation32,34 and attention3. The ACA and its subcortical targets, such as the SC, are also important for escape or prey-capture behaviours34. These downstream effectors send projections to the thalamus, creating a feedback loop that allows for the regulation of MPF activities. This network model aligns well with the classic perception–evaluation–action model3,5. Additional details are provided in the Supplementary Discussion. A full list of abbreviations is provided in Supplementary Table 1.
The DPd, DPs and ILA send direct projections to motor or premotor neurons that regulate neuroendocrine and autonomic functions. Notably, we identify monosynaptic projections from the DPd to neuroendocrine cells in the PVH and to the broader hypothalamic neuroendocrine zone (Fig. 6a), enabling direct cortical control of CORT release. The DPs densely innervates preautonomic neurons in PVHd and LHA, presumably regulating sympathetic output through a feedforward DPs to PVHd/LHA to IML pathway. The DPs also inhibits parasympathetic output through a disynaptic DPs to CEA to DMX/NTS pathway. By contrast, the ILA directly excites DMX neurons (ILA to DMX), thereby promoting vagal parasympathetic outputs. Together, the DPs and ILA generate direct cortical projections to brainstem and spinal preautonomic structures that regulate digestive, respiratory and cardiovascular functions. Notably, the ILA and DPd, but not the DPs, also directly innervate Barrington’s nucleus, which governs lumbosacral parasympathetic activities49.
Similar to the way MOp regulates motor functions through its projections to the basal ganglia, the DPd, DPs and ILA generate dense projections in a topographic manner to several cerebral nuclei (striatal or pallidal-like structures with GABAergic projection neurons43), such as the CEA, MEA, LS, ACB and BST, through which they regulate visceral motor activities, innate behaviour and reward. Among them, the BSTam, which receives dense inputs from the DPd, is known for its role in regulating neuroendocrine activities43,45,46,50. The CEA and BSTal, which receive dense inputs from the DPs, have essential roles in regulating autonomic activities through their projections to the DMX and other brainstem structures43,44. Moreover, the DPd and ILA generate dense projections to the MEA and BST posterior division, which in turn project to the hypothalamic medial behavioural column to regulate social behaviours35,36,43. Like direct projections of the MOp to the subthalamic nucleus (STN) and superior colliculus (SC), the DPd, DPs and ILA also project densely to the LHA (in the vicinity of the STN) and PAG (in the vicinity of the SC), which contain preautonomic neurons that regulate cardiovascular, respiratory and other autonomic functions.
The DPd, DPs and ILA maintain bidirectional connections with several thalamic nuclei, including the RE, PVT and PT, which relay hypothalamic and brainstem information related to homeostasis (for example, visceral sensory input, fluid balance) and social behaviours (for example, defensive, reproductive)35,36,43. The PVT and PT receive dense inputs from the PB and NTS—key regions that process visceral sensory information. Moreover, the PB exhibits connectivity with both the DP and ILA, as shown here and elsewhere41. Further details on DPs, DPd and ILA involvement in neural circuits regulating social behaviour are provided in the Supplementary Discussion.
A proposed unified MPF model for motivated behaviour
Our understanding of the prefrontal cortex has been hindered by the lack of a unified working model3,5,7. On the basis of our newly constructed, comprehensive whole-brain MPF wiring diagram, we propose a testable network model to understand how different MPF components coordinate and synchronize into a unified system to regulate physiological and motor actions in response to environmental and social challenges.
The DP mediates a predominantly unidirectional cortical information flow (Fig. 6b). It receives and integrates extensive inputs from cortical areas along the lateral cortical mantle, both dorsal and ventral to the rhinal fissure. These areas include the olfactory cortex, ENTl, amygdala, subiculum and neocortical areas within the lateral cortical subnetworks28, such as the PERI, ECT, TEa, AUDv, AIp, AId and AIv. Consequently, the DP processes a vast range of information from both external and internal environments, including olfactory, pheromonal, visceral sensory, auditory, fear conditioning and spatial orientation signals. Integrating these diverse modalities is crucial for perception, risk evaluation, decision-making and memory formation3,5.
The DP then projects densely to other MPF and cortical areas involved in motor control (Fig. 6c): (1) the DPd, DPs and ILA comprise the primary visceral motor cortex, regulating neuroendocrine and autonomic functions. (2) The ILA and PL target the ACB, which is linked to reward processing through the VTA. (3) The ACAv, ACAd and MOs-fef, along with other medial network areas (for example, PTLp, RSPv), project to the dorsomedial striatum32 and SC34, coordinating eye, head and neck movements for spatial orientation and navigation3,43. (4) The DPs and other MPF areas also send direct inputs to the MOs and MOp, supporting control of trunk and limb movements. These circuits form the structural basis for integrating visceral and somatic motor actions in goal-directed behaviours3,5. See the Supplementary Discussion for further discussion topics.
Related to the primates and human
On the basis of connectivity, the DPd, DPs, ILA and PL probably correspond to components of the ventromedial prefrontal cortex (vmPFC) in primates and humans4,7,9,10,51. Subgenual vmPFC regions, including Brodmann area 25, are part of the central autonomic network7,10, but their role in neuroendocrine regulation is unclear. Studies showing vmPFC involvement in mood disorders have yielded mixed results51,52. Our findings suggest that functional divergence between the anatomically adjacent two areas may underlie these inconsistencies: the DPd enhances plasma CORT levels14, whereas the ILA suppress neuroendocrine and cardiovascular stress responses1.
Recent studies show that the DP and TTd are activated by social interactions and stressors like restraint, looming sounds, fear memory, predatory threats and opioid exposure12,13,14,16,17. We found that DP neurons respond to both social (sex dependent) and non-social stimuli, whereas the ILA does not exhibit sex-specific social responses. Defining the homologous regions of the DPs, DPd and ILA in primates and humans remains essential, alongside validating their roles in physiological and social responses critical for homeostasis and social behaviour3,5,6,36. The DP functions as a hub for integrating global cortical information flow (Fig. 6b). Lesions affecting the DP or ILA (or the vmPFC in humans) presumably impair integration of sensory and interoceptive signals, disrupt MPF connectivity, and lead to disorganized emotional or behavioural responses (Fig. 6c and Supplementary Discussion). This is often observed in various psychiatric disorders, including classic Phineas Gage-like personality disorders, major depressive disorder as well as post-traumatic stress disorder3,6,39.
Methods
Animals
For neuroanatomical tracing experiments, most of the animals used were C57BL/6J male mice aged 2 months (n = 95; Jackson Laboratories). Mice were housed in a temperature-controlled (21–22 °C), humidity-controlled (51%) and light-controlled (12 h–12 h light–dark cycle; lights on, 06:00; lights off, at 18:00) room. Food and water were given ad libitum. After arrival at the facility, the mice were allowed a minimum of 1 week to adapt to the housing environment before surgeries were performed. All of the experiments were conducted according to the standards set by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the institutional guidelines of the University of Southern California (USC) and University of California Los Angeles (UCLA).
Male MORF3 mice (n = 20; aged 2 months), were also used for neuroanatomical tract tracing. The MORF3 mouse line (C57BL/6-Gt(ROSA)26Sortm3(CAG-sfGFP*)Xwy/J), generated by X. W. Yang’s laboratory at UCLA25, is a Cre reporter mouse line that uses a mononucleotide repeat frameshift (MORF) as a translation switch for cell labelling in vivo. MORF3 mice express a Cre-dependent tandem ‘spaghetti monster’ fluorescent protein with 20 V5 epitopes (smFP-V5) preceded by a polycytosine repeat (C22) MORF switch under the control of a CAG promoter. Cre recombination combined with a spontaneous frameshift results in sparse and stochastic labelling of neural cells.
Two other transgenic mouse lines, including VGLUT2 Cre (B6J.129S6(FVB)-Slc17a6tm2(cre)Lowl/MwarJ) and CRH-Cre (B6(Cg)-Crhtm1(cre)Zjh/J) were purchased from JAX and breeding colonies for these mouse lines were established at UCLA. CRH-Cre mice were crossed with Ai14 mice to generate CRH-Cre Ai14 mice for some of the experiments. A total of 5 male VGLUT2 Cre, 5 CRH-Cre and 4 CRH-Cre Ai14 mice aged 2 months were used for the neuroanatomic tracing experiments. Animal information for non-tracing studies is included in their corresponding subsections below.
Sample sizes for each of the tracing and morphology experiments were chosen based on previous experience. Randomization was not performed for the neural tracing experiments as animals were not assigned to different groups. Randomization is necessary for reducing bias and controlling variability. Instead, the data were validated in different ways (see the ‘Data reproducibility’ section). Randomization was also not necessary for morphology experiments. Owing to the characteristics of MORF3, we achieve random labelling of neurons and then reconstruct the traceable neurons. For neuroanatomic tracing and morphology experiments, blinding also was not necessary because the animals were not assigned to different groups.
Ethics statement
We are committed to promoting ethical research practices and ensuring the welfare of all animals involved in our studies. All procedures adhered to regulatory standards as delineated in the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as well as institutional guidelines set forth by the Institutional Animal Care and Use Committees at the University of Southern California (USC) and the University of California, Los Angeles (UCLA).
Tracers
Phaseolus vulgaris leucoagglutinin (PHAL; 2.5%; Vector Laboratories) was used as the main chemical anterograde tracer. Chemical retrograde tracers included cholera toxin subunit b (CTB Alexa Fluor 647 conjugate, 0.25%; Invitrogen) and FG (1%; Fluorochrome). FG was also injected through the tail vein (2%, 30 μl) to label neuroendocrine cells. Each anterograde and retrograde tracer has distinct characteristics and exhibits varied neurotropism that can meaningfully affect connectivity results. For example, anterograde AAVs label fibres of passage, while PHAL does not. These differences underscore the importance of data validation. In this work, the connectivity data were validated in multiple ways to ensure the reliability of the results. Retrograde tracers were placed in regions of anterograde terminations, while anterograde tracers were placed in regions of retrogradely labelled cells. For example, Extended Data Fig. 10 shows retrograde tracer injection in the DPs and DPd that reveal their distinct brain-wide inputs (brain-wide ROIs to the DPs or DPd). To validate these results, Extended Data Fig. 11 shows anterograde tracer injections delivered to those brain-wide ROIs to validate their projections to either the DPs or DPd. Furthermore, we have previously demonstrated that PHAL and AAV produce similar brain-wide anterograde labelling patterns21 (Supplementary Fig. 6).
AAVretro-hSyn-Cre-WPRE (AAV retro Cre; 1.6 × 1013 genome copies (GC) per ml; Addgene, 105553), AAV1-Syn-Flex-GCaMP6f-WPRE-SV40, AAV1-hSyn-SIO-stGtACR2-FusionRed and AAV-Ef1a-mCherry-IRES-Cre (retrograde)53 were produced by Addgene.
Viral anterograde tracers AAV encoding enhanced green fluorescent protein (AAV-GFP; AAV2/1.hSynapsin.EGFP.WPRE.bGH) and tdTomato (AAV1.CAG.tdtomato.WPRE.SV40) and AAV1-hSyn-Cre-WPRE-hGh (anterograde trans-synaptic) were packaged by UPenn Vector Core. AAV5-Ef1a-DIO-hChR2(H134R)-eYFP, AAV9-CAG-FLEX-GFP (Cre-dependent channelrhodopsin expressing AAV) was produced by the University of North Carolina vector core facility.
For experiments investigating the synaptic contacts of DP axon terminals onto PVH CRH neurons, an AAV-synaptophysin-GFP injection was made into the DP of CRH-Cre Ai14 male mice (n = 4, aged 2 months). A 1:1 mixture of AAV Cre and AAV synaptophysin-GFP was generated and a 100 nl pressure injection was made into the desired ROI. The viruses used for the mixture were AAV1-hSyn-FLEX-tdTomato-T2A-SypGFP-WPRE (Addgene, 51509, 1.3 × 1013 GC per ml) and AAV1-hSyn-Cre-WPRE (Addgene, 105553, 1.8 × 1013 GC per ml).
The viral vectors used for the genetically engineered rabies tracing system were produced by Wickersham Lab at MIT and included AAV2/9-CAG-FlexmKate-T2A-TVA, AAV2/9-CAG-Flex-mKate-T2A-N2c-G and Rbv-CVS-N2c-ΔG-GFP (the modified rabies virus). All viral vectors were aliquoted and stored at 80 °C until use. Importantly, rabies virus has been widely used as a tool for reliably mapping presynaptic inputs to a specific brain region or starter cell population54,55,56. While its tropism appears broad, it remains possible that specific cell types in different pathways may be over- or under-represented relative to the performance of other retrograde tracers and viruses (for example, AAVretro, CAV2, retrobeads and so on)57,58. Moreover, the potential for cytotoxicity within the starter cell population may provide an opportunity for rabies to non-specifically spread to nearby synapses rather than through those specifically contacting starter cells. To control for these possibilities, retrograde tracing results must be validated using complementary anterograde approaches, such as by tracing axonal connections from the upstream region, or by optogenetically stimulating upstream axons and recording synaptic responses in the target cell population. As such, the data that we present were validated in several different ways.
Stereotaxic surgeries for neuroanatomical tract tracing
Surgical and microscopy imaging procedures for neuroanatomical tract tracing experiments have been described previously28,32,59. Stereotaxic coordinates of targeted injection centres were determined through the ARA11 and empirically adjusted when needed. On the day of the experiment, mice were deeply anaesthetized and mounted into a Kopf stereotaxic apparatus where they were maintained under isoflurane gas anaesthesia (Datex-Ohmeda vaporizer). Before the surgery, mice were given one subcutaneous injection of Ketoprofen (4 mg per kg) and a protective ophthalmic ointment was applied to their eyes. For single anterograde tracer injection experiments (PHAL or AAV), tracers were iontophoretically delivered through glass micropipettes (inner tip diameter, 24–32 μm) using alternating 7 s on–7 s off pulsed positive electrical current (Stoelting, current source) for 10 min, and AAVs were delivered through the same method for 2 min (inner tip diameter, 8–12 μm). For anterograde/retrograde co-injection experiments (PHAL/CTB-647 and AAV/FG), tracer cocktail iontophoretic injections were made through glass micropipettes (inner tip diameter, 28–32 μm) for 10 min (PHAL/CTB-647) or 5 min (AAV/FG).
For multiple retrograde tracing experiments, at each injection site, 50 nl of the retrograde tracer was individually pressure-injected through glass micropipettes at a rate of 10 nl min−1 (Drummond Nanoject III). All injections were placed into the right hemisphere. After injections, incisions were sutured, and mice were returned to their home cages for recovery.
TRIO tracing (Cre-dependent TVA receptor mediated rabies tracing)
To reveal monosynaptic inputs to projection-defined neuronal populations in the DP (for example, DP neurons projecting to the ACA), we used a modified TRIO (tracing the relationship between input and output) strategy60. In brief, AAVretro-Cre was injected into a downstream projection target of DP (for example, ACA), and Cre-dependent TVA- and RG-expressing helper virus (AAV8-hSyn-FLEX-TVA-P2A-GFP-2A-oG) and mCherry-expressing G-deleted rabies virus (produced by the laboratory of I. Wickersham) were injected into the DP to label the DP projection neurons population (first order) and their brain-wide monosynaptic inputs (second order). The same strategy also was applied to trace brain structures that generate monosynaptic inputs to the DP-projecting neurons in the RE.
We used a modified rabies tracing strategy to identify monosynaptic inputs from the DPd to CRH-expressing neuroendocrine neurons in the PVH. In CRH-Cre transgenic mice, a Cre-dependent helper virus (AAV8-hSyn-FLEX-TVA-P2A-GFP-2A-oG) was injected into the PVH to drive expression of TVA and rabies glycoprotein (RG), followed by injection of mCherry-expressing G-deleted rabies virus (RbV-ΔG-mCherry). This approach selectively labels neurons providing direct monosynaptic input to CRH neurons. All viral vectors were produced by the Wickersham laboratory at MIT.
AAV1-Cre-based anterograde trans-synaptic tracing
This technique leverages the fact that when AAV1 is injected at sufficiently high concentration into a neuronal population, viral particles will travel down the axons and be released from the synaptic terminals where they can infect postsynaptic neurons. Detailed methodology was described previously61. In brief, anaesthetized mice were iontophoretically injected with Cre-dependent AAV-FLEX-RFP or GFP in the target structures (for example, the LHA), and pressure injected (20–80 nl) with AAV1-Cre in an upstream structure (for example, the DP). The AAV1-Cre is transported anterogradely down the axons and is released from the terminals, where it transfects postsynaptic cells that have been infected with high concentrations of Cre-dependent AAV-FLEX-RFP. The scant Cre expression is sufficient to unlock strong fluorophore expression in the downstream neurons, therefore revealing their axonal projections and terminals. After a 3-week post-operative recovery, the mice were anaesthetized with pentobarbital and perfused. The Cre injection site was verified by staining with mouse anti-Cre recombinase monoclonal primary antibody (see the ‘Tissue preparation and immunohistochemistry’ section for details). One caveat to using AAV1-Cre is that the virus can also travel retrogradely and should be used in situations in which the connection is known to be primarily unidirectional. In all of the experiments in which AAV1-Cre was used in the current paper, the connections were shown to be predominantly unidirectional (BLA/SUBv/AUDv to DP and DP to CEA/LHA/PAG; Supplementary Fig. 11). As an example, we injected AAV1-Cre into the DP and a Cre-dependent AAV into the LHA to show that LHA neurons that receive input from the DP project to the spinal cord (DP to LHA to spinal cord). If LHA projected back to the DP, we would possibly be showing that LHA neurons that project to DP also project to the spinal cord. However, we know that the DP to LHA connection is unidirectional (Supplementary Fig. 11b), which lends reasonable confidence to our conclusion of DP to LHA to spinal cord.
Details regarding how injection site locations were accurately determined are provided in the ‘Injection Site Analysis’ section of the Supplementary Information.
Data reproducibility
Reported connections underwent validation through at least one of the following methods. Injections targeting different regions were repeated to evaluate consistency of labels. The Cre-dependent anterograde and TVA receptor mediated rabies tracing also validated the connections. Furthermore, retrograde tracers were introduced into regions displaying anterograde terminal labelling to confirm anterograde connections, whereas anterograde tracers were administered into the sites of retrogradely-labelled projection cells to validate retrograde injection data. In some instances, channelrhodopsin assisted circuit mapping also validated connections.
Tissue preparation and immunohistochemistry
Animals were euthanized with an overdose injection of sodium pentobarbital (6 mg per kg) 7 days (chemical tracers) or 14 days (viral tracers) after surgeries. Each animal was transcardially perfused with approximately 50 ml of 0.9% NaCl followed by 50 ml of 4% paraformaldehyde solution (PFA; pH 9.5). The brains were post-fixed in 4% PFA for 24–48 h at 4 °C, after which they were embedded in 3% type I-B agarose (Sigma-Aldrich) before sectioning. Four series of coronal sections were sliced at a thickness of 50 µm using a Compresstome (VF-700, Precisionary Instruments) and prepared for processing. One of the four-section series was immunostained for imaging. The series that contained coronal level 53 of the Allen Reference Atlas was selected to maintain similar section-level distributions across experiments.
The following primary antibodies were used across the studies: rabbit anti-PHAL (1:1,000; Vector Laboratories, AS-2300), rabbit anti-fluorogold antibody (1:5,000; Millipore-Sigma, AB153-I), mouse anti-Cre recombinase (1:4,000; EMD Millipore, MAB3120), rat monoclonal FOS antibody (1:1,000; Synaptic Systems, 226017), mouse anti-oxytocin antibody (4G11, 1:5,000; Millipore-Sigma, MAB5296), rabbit anti-vasopressin antibody (1:5,000, Millipore-Sigma, AB1565), rabbit-anti somatostatin antibody (1:500, Millipore-Sigma, SAB4502861) and streptavidin for biocytin-labelled recorded neurons (1:1.000; Thermo Fisher Scientific, S21374).
In brief, sections were transferred to a blocking solution containing normal donkey serum (Vector Laboratories) and Triton X-100 (VWR) for 1 h. After three 5-min rinses, the sections were incubated in a KPBS solution comprising donkey serum, Triton X-100 and a primary antibody at the appropriate dilution (the concentration for each primary antibody is described above) for 48–72 h at 4 °C (Vector Laboratories, AS-2300). The sections were rinsed three times in KPBS and then soaked for 3 h in the secondary antibody solution, which contained donkey serum, Triton X-100 and the corresponding secondary antibody solution at the appropriate dilution (1:500 for anti-rabbit IgG conjugated with Alexa Fluor 488, 555 or 647; Invitrogen, A-21206 (488), A-31573 (647); anti-mouse IgG conjugated with Alexa Fluor 488 or 647: Life Technology: A-21202 (488) and A-31571 (647); anti-mouse IgG conjugated with Alexa Fluor 647: Jackson ImmunoResearch, 715-605-150). The sections were again rinsed with KPBS three times. All selected section series were counterstained with a fluorescent Nissl stain, NeuroTrace 435/455 (NT, 1:500, Invitrogen, N21479). The sections were then mounted and coverslipped using 65% glycerol.
RNAscope experiments
Deeply anaesthetized animals were perfused and brains were post-fixed in 4% PFA overnight followed by sequential immersion in 15% and 30% sucrose for cryoprotection. The brains were then sectioned (20 µm) and the RNAscope Multiplex Fluorescent v2 assay was performed according to the manual provided by Advanced Cell Diagnostics. In brief, slides containing tissue sections were post-fixed in the prechilled 4% PFA for 15 min at 4 °C, and then dehydrated in a series of ethanol solutions 50%, 70% and 100% for 5 min at room temperature. Hydrogen peroxide was applied to each section for 10 min at room temperature, and target retrieval was then performed at 99 °C for 5 min. Protease III was then added to the samples and incubated in a HybEZ oven for 30 min at 40 °C. The subsequent RNAscope assay was composed by multiple steps of hybridization (probe and AMP binding) and signal development (HRP channel binding), each followed by washing the slides in 1× wash buffer as described in the manufacturer’s protocol (Multiplex fluorescent v2 kit). Opal fluorophores (Akoya Biosciences) were diluted at 1:1,500 to visualize RNA signals. After the RNAscope assay, the sections were counterstained with DAPI (1:500, Thermo Fisher Scientific, D1306) and mounted. Images were acquired using a Dragonfly High Speed Confocal Microscope System (Andor). The following probes were used in this study: Cux2, Etv1, Tle4, SS, CRH, and TRH.
Image acquisition and 2D processing
All 50-µm sections were scanned as high-resolution virtual slide image (VSI) files using the Olympus VS120 high-throughput microscope. Post-acquisition processing methods, including image registration, tracer segmentation and quantification, are described in detail below. In brief, all images were flipped to ensure that the injection site and its corresponding ipsilateral labelling were in the correct right hemisphere. This was performed using the VS-Desktop software that accompanies the microscope. Images were then imported into Outspector—our custom software for image processing (see the ‘Outspector: our proprietary 2D image processing pipeline’ section of the Supplementary Methods) and processed on a high-performance computing cluster. A first-pass fully automatic registration was executed and subsequently its results were inspected and fine-tuned when needed. Tracer segmentation was performed on registered images with the default parameters. Segmentation results were also inspected, followed by interactive parameter adjustments if needed. Segmented pixel counts and cell counts in each brain region at all section levels were generated and used for analysis.
Outspector
Outspector is our informatics workflow specifically designed to reliably warp, reconstruct, annotate and analyse the labelled pathways in a high-throughput manner. Tissue sections from each analysed case were assigned and registered to standard templates of the corresponding ARA levels. All images shown in this Article are from the raw data of unwarped, unregistered VSI images. Threshold parameters were individually adjusted for each case and tracer. Adobe Photoshop was used to correct conspicuous artifacts in the threshold output files. Each colour channel was brightness/contrast adjusted to maximize labelling visibility (Nissl Neurotrace 435/455 is converted to bright field), and TIFF images are then converted to JPEG file format. The detailed calculations used for all Outspector processing steps and analysis are provided in the ‘Outspector: our proprietary 2D image processing pipeline’ section of the Supplementary Methods.
Quantifying colocalization
Using spots function on Imaris, thresholds were set to select most positive cells for each channel (405, 488, 561 and 642). After automatic detection, manual quality control was necessary. The spatial relationship between DAPI in 405 and RNA puncta for Fezf2 was used as a visual aid to correct false negatives and remove false-positive spots from the automatic detection. Each channel was quality controlled multiple times to ensure accuracy.
Tissue processing and imaging for 3D single-neuron labelling
To elucidate the finely detailed morphology of DP neurons, we injected with either AAV-retro Cre or AAV1-Cre into MORF3 mice. Then, 3–4 weeks after the injections, the mice were perfused. Immunostaining with the V5 antibody allows the visualization of the finely detailed neuronal morphology of these MORF3 neurons. This approach enabled us to image, reconstruct and analyse the cell-type-specific morphological features of DP cortical projection neurons. Following the same strategy, we also revealed sparsely labelled neurons with finely detailed dendritic morphology in the LHA.
The SHIELD clearing protocol was used for the 3D tissue processing62. Mice were transcardially perfused with cold saline and SHIELD perfusion solution. Brains were dissected, subjected to a SHIELD-based brain clearing method and immunostained for V5 (to reveal MORF3 with secondary antibody conjugated with Alexa Fluor 647) using eFLASH25,62,63. Brains were processed using the SmartClear and SmartLabel machines (LifeCavas SmartBatch). Whole brains were imaged using the Life Canvas SmartSPIM lightsheet microscope at ×15 (0.42 μm × 0.42μm × 0.42 μm). Three lasers (488, 555, 647 nm) were used, respectively, to image Syp–eGFP axonal terminals, tdTomato-positive cortical neurons and their axons and MORF3-positive postsynaptic neurons.
For acquiring images on thick sections, the whole brain was cut into 500 µm sections and were cleared in the SDS buffer at 37 °C for 72 h. The sections were then washed three times with KPBS and incubated in KPBS at 4 °C for 24 h. The sections were mounted and cover-slipped onto 25 × 75 × 1 mm glass slides with an index matching solution (100%, EasyIndex, LifeCanvas Technologies, EI-Z1001). The sections were imaged with a high-speed spinning-disk confocal microscope (Andor Dragonfly 202 Imaging System, Andor, Oxford Instruments Company, CR-DFLY-202-2540). ×10 magnification (NA 0.40, Olympus, UPLXAPO10X) was used to acquire an overview, after which ×30 magnification (Olympus, UPLSAPO30xSIR, NA 1.05, W.D.: 0.80 mm) at 1 µm z steps or ×60 magnification (Olympus, UPLASAPO60XS2, NA 1.30, W.D.: 0.30 mm) were used to acquire high-resolution images for fine-detailed dendritic morphology and putative synaptic connections. The sections were imaged with four excitation wavelengths: 405 nm (blue Nissl background), 488 nm (for AAV-GFP) and 647 nm (far red for MORF3) with respective emission detection wavelengths of 450 nm, 525 nm and 600 nm.
3D reconstructions, visualizations and analysis of neuronal morphology
Manual reconstruction of neurons was performed using Vaa3D64,65 or Fast Neurite Tracer66, followed by morphological repairing, sorting and typing of the dendritic arbours. Reconstructions were finalized by loading them in Imaris and neuTube67 and finalized into the widely used swc file format68. In total, 47 reconstructed DP neurons were morphologically analysed in MATLAB, leveraging functions from the Trees Toolbox environment69. The 47 DP neurons were pooled from four MORF3 mice. Specifically, analysis scripts measured all neurons across a series of parameters including total dendritic wiring length, number of branches, average branch angle, average root angle, branch asymmetry, average branch order, average Strahler order and the distance from the midline. The coefficients of variances across the parameters were then measured, followed by classification using k-means clustering. The size and complexity of dendritic arbours, defined by total dendritic length and the total number of branches, emerged as the most varied. We then classified all of the neurons, first based on dendritic arbour size and complexity, followed by superficial versus deep-layer classification based on the distance of all cell bodies from the midline. Both classifications created two neuronal clusters each. The superficial versus deep-layer neurons were then compared for size, complexity and dendritic orientation. Similarly, the two morphological clusters (small-simple and large-complex) were compared for their distance from midline.
Statistical analyses
These comparisons were made using one-tailed nonparametric Wilcoxon signed rank tests. The P values from the tests were corrected using FDR correction to account for multiple comparisons.
In vitro patch-clamp slice recordings
In wild-type mice, AAV1-Cre (anterograde trans-synaptic Cre) was injected into the AUDv, while AAV-hSyn-ChR2-YFP (UPenn Vector Core) was injected into either the ENTI (n = 5; Fig. 5a) or PIR (n = 6 Fig. 5c) of different animals. In separate experiments, the AAV-hSyn-ChR2-YFP was injected into the DP of CRH-Cre Ai14 mice (n = 7; Fig. 4g). Sample sizes were chosen on the basis of previous experience. Randomization and blinding were not performed as the mice were not assigned to different groups.
Two weeks after channelrhodopsin and tracer injections, acute brain slices were prepared for electrophysiological recordings. After anaesthesia with isoflurane, the mouse was decapitated and the brain was rapidly removed and immersed in ice-cold high sucrose slice solution: 208 mM sucrose, 2.5 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO3, 1.3 mM MgCl2, 8 mM MgSO4 and 10 mM glucose; saturated with 95% O2 and 5% CO2; pH 7.4. Brain slices (thickness, 350 μm) were cut in the coronal plane using a vibrating microtome (Leica, VT1000S). Slices were allowed to recover for at least 30 min in a submersion chamber filled with warmed (32–34 °C) ACSF containing 130 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO3, 2 mM CaCl2, 2 mM MgCl2 and 10 mM glucose, oxygenated with 95% O2 and 5% CO2, pH 7.2–7.4, 290–310 mOsm, and then cooled gradually to room temperature until recording. The presence of RFP and ChR2 (YFP) labelling was determined with green and blue fluorescence excitation, respectively, in the slices before recording. Patch pipettes with about 3–4 MΩ impedance were used for whole-cell patch clamp recordings. Glass pipettes contained a Cs-methanesulfonate-based internal solution with the following salt concentrations: 130 mM Cs-methanesulfonate, 10 mM CsCl, 4 mM NaCl, 1 mM MgCl2, 5 mM mM 150 mM MgATP, 5 mM EGTA, 10 mM HEPES, 5 mM GTP, 10 mM phosphocreatine and 0.1 mM leupeptin (pH 7.2 with 151 CsOH, 270 mOsm) and biocytin (0.2%). For isolation and recording of monosynaptic responses to blue-light stimulation, tetrodotoxin (Na+ channel blocker, 1 μM) and 4-aminopyridine (K+ channel blocker, 100 μM) were added to the external solution. Signals were recorded from red-labelled neurons with a MultiClamp 700B amplifier (Molecular Devices) running pClamp software (v.10.5) under voltage clamp mode at a holding voltage of −70 mV for excitatory currents, filtered at 1 kHz and sampled at 10 kHz. Blue light (470 nm) stimulation was delivered using a 0.5 ms pulse at ~1 mW power for 5 trials, delivered through a LED (CoolLED). Signals were analysed using Clampfit (v.10.5). For responding neurons, peak responses were averaged across trials (technical replicate). The averaged value of each cell was then combined with the other cells for each experiment to calculate the mean, median, s.d. and so on (biological replicate). To demonstrate that light-evoked synaptic responses were indeed excitatory, the selective glutamate receptor antagonists CNQX (20 µM) for AMPA receptors and APV (50 µM) for NMDA receptors were added to the bathing solution.
After recording, the slices were fixed with PFA and then transferred to PBS for biocytin staining to reveal and validate the location of the recorded neurons. The biocytin staining also revealed the morphology of the recorded DP or PVH neurons (see the ‘Tissue preparation and immunohistochemistry’ section for details).
Statistical analyses
To compare the median peak amplitude of currents evoked by optogenetic stimulation of AUDv to DP neurons receiving either ENTl or PIR input, nonparametric Mann–Whitney U-tests were used and P value adjustments were not necessary.
Optogenetic stimulation and measurements of plasma CORT hormone
A total of 15 mice (male C57BL/6, aged 2 months) were randomly assigned to either the experimental or control group. Sample sizes for each of the experiments were chosen based on our previous experience and, based on the results, sufficient power was achieved to detect significant differences. Each of the animals received bilateral injections of AAV1-Cre into the SUBv and the BLAa, allowing Cre to anterogradely transport to and trans-synaptically label DP neurons. The animals also received a bilateral injection of Cre-dependent AAV-FLEX-ChR2 (30 nl) into the DP. Consequently, only DP neurons postsynaptic to the SUBv and BLAa express ChR2 and generate projections to the hypothalamic neuroendocrine zone, including the PVH. Optic fibres (200 μm core diameter, 0.37 numerical aperture; Inper) were implanted above the injection site at a depth of −1.6 mm ventral to the bregma skull surface and secured to the skull using super glue and dental cement.
The optogenetic stimulation was performed 7–8 weeks after the surgery. Before testing, the mice were individually habituated to the testing cage (new mouse cage) and optic fibres over 4 consecutive days, for 30 min daily. On the test day, the mice were habituated to the testing cage alone for 2 h and attached to optic fibres for 15 min before optogenetic stimulation. A 473 nm laser, pulsing at 20 Hz with 15 ms pulses, was applied for 30 s on and 30 s off cycles, for a total of 20 min. The light irradiance was around 4–5 mW mm−2 in the target region. After laser stimulation, the optic fibres were removed, and the mice remained undisturbed in the testing cage for 25 min before euthanasia. To minimize circadian rhythm effects on CORT levels, animals were tested between 1.5 to 5 h after lights turned off in the dark phase, with control and ChR2 groups counterbalanced during the test.
Serum collection and CORT assaying
After rapid cervical dislocation, terminal trunk blood was collected with heparinized microcapillary tubes (Thermo Fisher Scientific) and transferred into microfuge tubes, which were left on ice for at least 15–20 min. The blood samples were centrifuged at 2,500 rpm for 15 min at 4 °C. The supernatant (serum) was collected and transferred into new tubes and the serum samples were frozen at −80 °C. The samples were packed and shipped on dry ice to the Center for Research in Reproduction Ligand Assay and Analysis Core at the University of Virginia School of Medicine, where they were processed (in duplicate) using a radioimmunoassay kit for CORT measurements. The brains were sectioned at 50 μm thickness and Nissl stained to assess the location of the implanted optic fibres. The researcher assessing the accuracy of the bilateral implant location was blinded to the treatment condition of the animal. Only animals with accurate bilateral implants in the DP were included in the analysis.
Statistical analyses
To compare the CORT levels between the experimental and control groups, Student’s t-test and nonparametric Mann–Whitney U-tests were used. Owing to the two-group comparisons, P value adjustment was not necessary.
Social interaction experiments
Surgery
Six VGLUT2-Cre (for DP) and six VGLUT1-Cre (for ILA) male mice (aged 6–8 weeks) were anaesthetized using a 1–2% isoflurane–oxygen mixture and injected unilaterally with 350 nl (1 nl s−1) pAAV.Syn.Flex.GCaMP6f.WPRE.SV40 (Addgene, 100833-AAV1) into the DP. Then, 30 min after virus injection, a 6.1-mm-long (0.5 mm diameter) relay lens (Inscopix) was implanted over the DP: anteroposterior, +2.1 mm, mediolateral, 0.6 mm; dorsoventral, −3.2 mm. The lens was securely affixed to the skull using cyanoacrylate glue and dental cement and then covered with Kwik-Sil (WPI). Sample sizes for each of the experiments were chosen based on previous experience. Randomization was not necessary as VGLUT2-Cre animals received injection into the DP and the VGLUT1-Cre mice received injection in the ILA. Also, each of the animals underwent both social and non-social interaction behaviour tests (see below).
Three weeks later, the mice were anaesthetized as described previously and a UCLA V4 miniscope with an attached baseplate was positioned over the lens to visualize GCaMP6f expression. The focal plane was adjusted to optimize cell visibility, and the baseplate was secured in place with dental cement. A plastic cap was used to cover and protect the lens over the baseplate.
Behavioural tests
Mice with lens implantation in the DP or ILA were habituated to handling and the miniscope for 4–5 days. On the day of the open-field test, the miniscope was attached to the baseplate and the mice freely ran in an open arena (45 cm × 45 cm × 30 cm) for 20 min. On the day of same-sex social interaction, mice performed social interaction counterbalanced with object exploration in the same arena as the open-field test for 8 min per session. The next day, mice performed social interaction with opposite sex social targets for 8 min. Each social trial included an 8-min recording session, beginning with a 1-min period of the subject mouse alone, followed by a 7-min interaction with the social partner. On the day of same-sex social interaction counterbalanced with object exploration, after the first trial, the subject mouse rested with the LED off for approximately 5 min before starting the second trial. Calcium imaging and behaviour (miniCam) were recorded simultaneously at 30 Hz.
Pre-processing and extraction of calcium signals
Calcium imaging videos were motion corrected (NoRMCorre: https://github.com/flatironinstitute/NoRMCorre; MiniAn: https://github.com/denisecailab/minian) and analysed with extended constrained non-negative matrix factorization (CNMF-E)-algorithm-based packages (MiniscopeAnalysis: https://github.com/etterguillaume/MiniscopeAnalysis; MiniAn: https://github.com/denisecailab/minian) to identify cells and extract cell activity. Denoised and demixed trace data were used for further analysis.
Identification of behaviour responsive cell
To determine whether an individual neuron’s activity was significantly modulated by social interaction, we used ROC analysis to calculate an area under the curve (AUC) value for denoised calcium trace correspondence to binarized manually scored interaction bouts. The interaction bouts were defined when the imaged mouse actively investigated the social target/object within a distance of one-quarter to one-third of mouse body length. To determine whether the AUC values indicated that the neuron was significantly excited or inhibited by the interaction, we compared observed AUC values to a null distribution derived from circularly shuffled calcium signals (1,000 shuffles). Significance was determined using percentile thresholds, classifying neurons as significantly responsive if their auROC values surpassed the 97.5th percentile for excited neurons or the 2.5th percentile for inhibited neurons. Neurons that were neither excited nor inhibited were deemed non-responsive.
Calcium event calculation
Calcium transients were identified using automatic detection algorithms in MATLAB (peakfinder) (https://github.com/GradinaruLab/striatum2P).
Statistical analyses
To examine whether there were differences in DP-miniscoped or ILA-miniscoped animals in the time spent interacting during non-social (object) versus social (same- or opposite-sex partner) testing, an omnibus one-way ANOVA was performed followed by post hoc Tukey’s multiple-comparison test, which controls for the family-wise error rate. To assess the percentage of behaviourally responsive cells and frequency of calcium events during non-social (open-field) and social (same- or opposite-sex partner) testing, an omnibus two-way ANOVA was performed followed by post hoc analyses using either Tukey’s tests (for within DP or ILA comparisons) or Šidák’s tests with adjusted P values (for across DP and ILA comparisons) for multiple comparisons.
DP neuronal activities in response to social and environmental stimuli
Male mice in response to social stimuli
Twenty-four male mice, aged between 12 and 14 weeks, were used for the study. To reduce potential stress by the testing environment, each mouse was habituated to a clean cage for four consecutive days, spending 2 to 4 h in the cage and with approximately 30 min in the recording position daily. On the testing day, the mice were habituated in the clean cage for about 2 h before the experiment. Unfamiliar male mice, female mice and postnatal day 7 pups were used as the social stimuli. Each mouse was exposed to three instances of the same type of social stimulus, lasting about 6 min each, for a total duration of 20 min, with six mice in each group. In the control group, the cages were opened three times as well, but without introducing any intruders. The mice remained undisturbed in their individual cages for 80 min before perfusion.
Acute-restraint stress
Animals (n = 4, male) subjected to acute-restraint stress were removed from the colony room, transferred to a behavioural testing room for 1 h and then placed into a Tailveiner restrainer for mice (Braintree Scientific). This device consists of a clear tube, within which a plug was adjusted to limit voluntary movement. Acute restraint stress was applied for 30 min and terminated 30 min before the animals were euthanized. Throughout all studies, control mice (n = 4, male) underwent numerous sensory manipulations that were carried out in parallel with those for the mice in the restraint stress group.
Acute looming sound stimuli in comparison with white noise and foot shock
Subjects
TRAP2-CreER70 and Ai14 mice were acquired from the Jackson Laboratory (www.jax.org/strain/030323; www.jax.org/strain/007908). Homozygous TRAP2-CreER and Ai14 mice were bred to generate TRAP2-CreER/Ai14 offspring.
Drug preparation
4-Hydroxytamoxifen (4-OHT; Sigma-Aldrich, H6278) was dissolved at 20 mg ml−1 in ethanol by shaking at 37 °C for 15 min, then corn oil was added to give a final concentration of 10 mg ml−1, and the ethanol was evaporated by vacuum under centrifugation. 4-OHT (intraperitoneally (i.p.), 50 mg per kg) was injected in all TRAP2-CreER experiments70,71.
Stimulation for TRAP
Four TRAP-Cre mice were randomly assigned into two groups: control (n = 2) and foot-shock-based fear conditioning (n = 2). For auditory stimulation (noise group), on day 1 and 2, the TRAP2/Ai14 mice were adapted to custom-made boxes in a sound-proofed room for 1 h per day. On day 3, the mice were placed into their home cage for 12 h in a sound-proofed room before white-noise exposure. The mice were then placed in the custom-made boxes and exposed to 1 h broadband white-noise stimulation (70 dB SPL).
For the foot-shock group, on day 1, the mice were acclimatized in custom-made boxes in a sound-proofed room for 1 h. On day 2 (conditioning), the mice were put into custom-built boxes with a metal grid floor. After 10 min habituation to the conditioning chamber, the animals were exposed to the 20 s noise (70 dB SPL) co-terminated with a 0.75 mA foot shock (5 Hz for 1 s with the duration of each pulse = 100 ms) five times72. On day 3, the mice were subjected only to 1 h broadband white-noise exposure. Thereafter, mice received an injection of 4-OHT (i.p., 50 mg per kg) and were returned to their home cage. The cage was moved back to our animal facility 12 h after injection of 4-OHT. Sound stimulation and injection of 4-OHT were conducted in a sound-proofed room. On day 12, the mice were further presented with looming sound stimuli. A train of ten consecutive crescendo sound stimuli (with intensity increased linearly from 20 to 70 dB SPL in 0.4 s and followed by 0.6 s interval with intensity at 20 dB SPL) was repeated for 10 min with an interval of 10-s between the train stimuli. The mouse was euthanized after a 1.5 h rest.
Control mice were treated the same procedures as the auditory stimulation group but without any sound stimuli.
Images used from open-access resources
Images from the mouse ARA used in this paper are available online (https://mouse.brain-map.org/)11. Images of the flatmaps are freely downloadable from the cited article73. Bright-field in situ hybridization images from the ABA are available online (https://mouse.brain-map.org/)74. The Common Coordinate Framework (CCF) was used to generate the background 3D images of the brain, and are available online (https://alleninstitute.github.io/abc_atlas_access/descriptions/Allen-CCF-2020.html)75.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Code availability
All code used for this study is available at GitHub (https://github.com/ucla-brain/mpf).
Data availability
Source data for the Supplementary Figures are provided in the Supplementary Data. An online application is available for viewing the reconstructed pathways (https://brain.neurobio.ucla.edu/mpf/). The annotated data for each of the processed MPF cases used in this work is also available at GitHub (https://github.com/ucla-brain/mpf). Source data are provided with this paper.
References
McKlveen, J. M., Myers, B. & Herman, J. P. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J. Neuroendocrinol. 27, 446–456 (2015).
Hansel, A. & von Kanel, R. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosoc. Med. 2, 21 (2008).
Fuster, J. The Prefrontal Cortex 5th edn (Elsevier, 2015).
Ongur, D. & Price, J. L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 10, 206–219 (2000).
Etkin, A., Buchel, C. & Gross, J. J. The neural bases of emotion regulation. Nat. Rev. Neurosci. 16, 693–700 (2015).
Roberts, A. C. Prefrontal regulation of threat-elicited behaviors: a pathway to translation. Annu. Rev. Psychol. 71, 357–387 (2020).
Le Merre, P., Ahrlund-Richter, S. & Carlen, M. The mouse prefrontal cortex: unity in diversity. Neuron 109, 1925–1944 (2021).
Anastasiades, P. G. & Carter, A. G. Circuit organization of the rodent medial prefrontal cortex. Trends Neurosci. 44, 550–563 (2021).
Ongur, D., Ferry, A. T. & Price, J. L. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 460, 425–449 (2003).
Carlen, M. What constitutes the prefrontal cortex? Science 358, 478–482 (2017).
Dong, H. W. The Allen Reference Atlas (Book + CD-ROM): A Digital Color Brain Atlas of the C57BL/6J Male Mouse (Wiley, 2007); atlas.brain-map.org/.
Kataoka, N., Shima, Y., Nakajima, K. & Nakamura, K. A central master driver of psychosocial stress responses in the rat. Science 367, 1105–1112 (2020).
Nakamura, K., Nakamura, Y. & Kataoka, N. A hypothalamomedullary network for physiological responses to environmental stresses. Nat. Rev. Neurosci. 23, 35–52 (2022).
Botterill, J. J. et al. Dorsal peduncular cortex activity modulates affective behavior and fear extinction in mice. Neuropsychopharmacology https://doi.org/10.1038/s41386-024-01795-5 (2024).
Campos-Cardoso, R. et al. The mouse dorsal peduncular cortex encodes fear memory. Cell Rep. 43, 114097 (2024).
Borkar, C. D. et al. Top-down control of flight by a non-canonical cortico-amygdala pathway. Nature https://doi.org/10.1038/s41586-023-06912-w (2024).
Smith, A. C. W. et al. A master regulator of opioid reward in the ventral prefrontal cortex. Science 384, eadn0886 (2024).
Swanson, L. W. Brain maps 4.0—structure of the rat brain: an open access atlas with global nervous system nomenclature ontology and flatmaps. J. Comp. Neurol. 526, 935–943 (2018).
Haberly, L. B. & Price, J. L. Association and commissural fiber systems of the olfactory cortex of the rat. J. Comp. Neurol. 178, 711–740 (1978).
Paxinos, G. & Franklin, K. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates 5th edn (Elsevier, 2019).
Hintiryan, H. et al. Connectivity characterization of the mouse basolateral amygdalar complex. Nat. Commun. 12, 2859 (2021).
Mathiasen, M. L., Nelson, A. J. D., Amin, E., O’Mara, S. M. & Aggleton, J. P. A direct comparison of afferents to the rat anterior thalamic nuclei and nucleus reuniens: overlapping but different. eNeuro https://doi.org/10.1523/ENEURO.0103-20.2021 (2021).
Munoz-Castaneda, R. et al. Cellular anatomy of the mouse primary motor cortex. Nature 598, 159–166 (2021).
Gao, L. et al. Single-neuron analysis of dendrites and axons reveals the network organization in mouse prefrontal cortex. Nat. Neurosci. 26, 1111–1126 (2023).
Veldman, M. B. et al. Brainwide genetic sparse cell labeling to illuminate the morphology of neurons and glia with Cre-dependent MORF mice. Neuron 108, 111–127 (2020).
Hurley, K. M., Herbert, H., Moga, M. M. & Saper, C. B. Efferent projections of the infralimbic cortex of the rat. J. Comp. Neurol. 308, 249–276 (1991).
Vertes, R. P. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58 (2004).
Zingg, B. et al. Neural networks of the mouse neocortex. Cell 156, 1096–1111 (2014).
Hunnicutt, B. J. et al. A comprehensive thalamocortical projection map at the mesoscopic level. Nat. Neurosci. 17, 1276–1285 (2014).
Harris, J. A. et al. Hierarchical organization of cortical and thalamic connectivity. Nature 575, 195–202 (2019).
Zhang, S. et al. Organization of long-range inputs and outputs of frontal cortex for top-down control. Nat. Neurosci. 19, 1733–1742 (2016).
Hintiryan, H. et al. The mouse cortico-striatal projectome. Nat. Neurosci. 19, 1100–1114 (2016).
Bienkowski, M. S. et al. Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nat. Neurosci. 21, 1628–1643 (2018).
Benavidez, N. L. et al. Organization of the inputs and outputs of the mouse superior colliculus. Nat. Commun. 12, 4004 (2021).
Simerly, R. B. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 25, 507–536 (2002).
Chen, P. & Hong, W. Neural circuit mechanisms of social behavior. Neuron 98, 16–30 (2018).
Gloor, P. The Temporal Lobe and Limbic System (Oxford Univ. Press, 1997).
Li, W. G. et al. Input associativity underlies fear memory renewal. Natl Sci. Rev. 8, nwab004 (2021).
Andrewes, D. G. & Jenkins, L. M. The role of the amygdala and the ventromedial prefrontal cortex in emotional regulation: implications for post-traumatic stress disorder. Neuropsychol Rev. 29, 220–243 (2019).
Evrard, H. C. The organization of the primate insular cortex. Front. Neuroanat. 13, 43 (2019).
Grady, F., Peltekian, L., Iverson, G. & Geerling, J. C. Direct parabrachial-cortical connectivity. Cereb Cortex 30, 4811–4833 (2020).
Allen, W. E. et al. Thirst-associated preoptic neurons encode an aversive motivational drive. Science 357, 1149–1155 (2017).
Swanson, L. W. Cerebral hemisphere regulation of motivated behavior. Brain Res. 886, 113–164 (2000).
Dong, H. W. & Swanson, L. W. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J. Comp. Neurol. 468, 277–298 (2004).
Dong, H. W. & Swanson, L. W. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J. Comp. Neurol. 494, 142–178 (2006).
Radley, J. J., Gosselink, K. L. & Sawchenko, P. E. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J. Neurosci. 29, 7330–7340 (2009).
Lind, E. B. et al. A quadruple dissociation of reward-related behaviour in mice across excitatory inputs to the nucleus accumbens shell. Commun. Biol. 6, 119 (2023).
Biag, J. et al. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J. Comp. Neurol. 520, 6–33 (2012).
Verstegen, A. M. J., Vanderhorst, V., Gray, P. A., Zeidel, M. L. & Geerling, J. C. Barrington’s nucleus: Neuroanatomic landscape of the mouse “pontine micturition center”. J. Comp. Neurol. 525, 2287–2309 (2017).
Herman, J. P. Neural control of chronic stress adaptation. Front. Behav. Neurosci. 7, 61 (2013).
Alexander, L., Wood, C. M. & Roberts, A. C. The ventromedial prefrontal cortex and emotion regulation: lost in translation? J. Physiol. 601, 37–50 (2023).
Wallace, T. et al. Sexually divergent cortical control of affective-autonomic integration. Psychoneuroendocrinology 129, 105238 (2021).
Fenno, L. E. et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772 (2014).
Wickersham, I. R. et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647 (2007).
Wall, N. R., Wickersham, I. R., Cetin, A., De La Parra, M. & Callaway, E. M. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl Acad. Sci. USA 107, 21848–21853 (2010).
Yao, S. et al. A whole-brain monosynaptic input connectome to neuron classes in mouse visual cortex. Nat. Neurosci. 26, 350–364 (2023).
Sun, L. et al. Differences in neurotropism and neurotoxicity among retrograde viral tracers. Mol. Neurodegener. 14, 8 (2019).
Chatterjee, S. et al. Nontoxic, double-deletion-mutant rabies viral vectors for retrograde targeting of projection neurons. Nat. Neurosci. 21, 638–646 (2018).
Foster, N. N. et al. The mouse cortico-basal ganglia-thalamic network. Nature 598, 188–194 (2021).
Schwarz, L. A. et al. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524, 88–92 (2015).
Zingg, B., Dong, H. W., Tao, H. W. & Zhang, L. I. Application of AAV1 for anterograde transsynaptic circuit mapping and input-dependent neuronal cataloging. Curr. Protoc. 2, e339 (2022).
Park, Y. G. et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat. Biotechnol. https://doi.org/10.1038/nbt.4281 (2018).
Choi, S. W., Guan, W. & Chung, K. Basic principles of hydrogel-based tissue transformation technologies and their applications. Cell 184, 4115–4136 (2021).
Peng, H., Bria, A., Zhou, Z., Iannello, G. & Long, F. Extensible visualization and analysis for multidimensional images using Vaa3D. Nat. Protoc. 9, 193–208 (2014).
Chen, H. et al. Fast assembling of neuron fragments in serial 3D sections. Brain Inform. 4, 183–186 (2017).
Gao, L. et al. Single-neuron projectome of mouse prefrontal cortex. Nat. Neurosci. 25, 515–529 (2022).
Feng, L., Zhao, T. & Kim, J. neuTube 1.0: a new design for efficient neuron reconstruction software based on the SWC format. eNeuro https://doi.org/10.1523/ENEURO.0049-14.2014 (2015).
Nanda, S. et al. Design and implementation of multi-signal and time-varying neural reconstructions. Sci. Data 5, 170207 (2018).
Cuntz, H., Forstner, F., Borst, A. & Hausser, M. One rule to grow them all: a general theory of neuronal branching and its practical application. PLoS Comput. Biol. https://doi.org/10.1371/journal.pcbi.1000877 (2010).
DeNardo, L. A. et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci. 22, 460–469 (2019).
Tasaka, G. I. et al. The temporal association cortex plays a key role in auditory-driven maternal plasticity. Neuron 107, 566–579 (2020).
Zhang, G. W. et al. A non-canonical reticular-limbic central auditory pathway via medial septum contributes to fear conditioning. Neuron 97, 406–417 (2018).
Hahn, J. D. et al. An open access mouse brain flatmap and upgraded rat and human brain flatmaps based on current reference atlases. J. Comp. Neurol. 529, 576–594 (2021).
Lein, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Wang, S. L. et al. The Allen mouse brain common coordinate framework: a 3D reference atlas. Cell 181, 936–953 (2020).
Acknowledgements
Funding for this research is provided by NIH grants U01MH114829 (H.-W.D.), R01 MH094360-06 (H.-W.D.), 1R01NS133744-01 (H.-W.D.), 1R01MH132736 (P.G.), U01NS122124 (P.G.), 1P50HD103557 (P.G.), R01 MH130941 (W.H.), R01 NS113124 (W.H.), RF1 NS132912 (W.H.), R01 MH132736 (W.H.), UF1 NS122124 (P.G. and W.H.); The Cells, Circuits and Systems Core is supported by NIH grant P50HD103557 (C. Cepeda and M.S.L.), RF1MH114112 (L.I.Z. and H.-W.D.), R01DC008983 (L.I.Z.) and RF1MH128888 (X.W.Y. and H.-W.D.).
Author information
Authors and Affiliations
Contributions
H.-W.D., H.H. and M. Zhu conceived, designed and managed the project, wrote the manuscript, performed data analysis, and prepared all of the figures for publication. M. Zhu developed methods and performed quantitative analysis and hierarchical clustering of connectivity data. P.Z., S.M. and P.G. designed and performed social interaction and calcium imaging experiments, performed data analysis, prepared the figure and wrote the corresponding portion of the manuscript. M. Zhang, H.H., A.W., W.H. and P.M. designed and performed optogenetic experiments for measuring plasma CORT. M. Zhang and W.H. designed and performed social interaction experiments. J.B., C. Cepeda, H.H. and M.S.L. conducted electrophysiology experiments, analysed the data, generated graphs and prepared the corresponding written portion of the manuscript. S.N. performed single-neuron morphological analysis of DP neurons, prepared the figure and wrote the corresponding section. J.W., Q.Z. and L.I.Z. designed and performed the acute looming sound and foot-shock experiments. J.S. performed RNAscope and acute restraint stress experiments. L. Gou, C. Cao, M. Zhu, B.Z. and N.N.F. performed stereotaxic surgeries, immunohistology, and image acquisition to produce and collect connectivity data. M.R. performed brain clearing experiments and 3D image acquisition. K.M., C.E. and A.D. performed 3D image processing. I.B. and L. Garcia developed Outspector as part of our informatics pipeline that was used to process all 2D sections. D.L. and T.B. performed image registration and assisted with connectivity data analysis. A.G. and H.-S.M. reconstructed single neurons for morphometric analysis. S.Y. and C.E. developed our online connectivity viewer. N.N.F., C.S.P. and X.W.Y. generated MORF3 labelling data for neuron reconstructions. Y.E.H. helped with the breeding of MORF3 and Cre mouse lines. H.X., J.G. and G.Y. assisted with histology.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Connectivity based-parcellation of DP.
a-d, Anterograde tracer injections into AUDv, ENTl, RE, and PIR revealed dense axon terminals in DP layer 1, delineating DP boundaries from the adjacent ILA (dorsal) and TTd (ventral). PIR projections also extended into DPs. e, PHAL injection into BLAp and BLAal resulted in dense terminals in the DPs, while AAV-GFP injection into adjacent BLAam labelled terminals in ILA and DPd, but not DPs. These patterns help define the ILA–DP boundary and highlight laminar organization within the DP. For subdivisions of the BLAa, see our previous work21. f, Dual AAV-RFP and AAV-GFP injections into BLAam confirm consistent projection patterns to ILA, DPd, and other MPF areas, highlighting the repeatability and accuracy of our injections. g, AAV-RFP injection into COApl labels axons in ILA, DP layer 1, and DPd but avoid DPs (top); PHAL from COApm densely labels DPd (bottom). h, FG injection into ACA retrogradely labels neurons in DPs, but not DPd. i, PHAL/CTB coinjections into BMAa and COAa produce anterograde labelling in DP layer 1 and retrograde labelling in DPs. j, In the same brain, coinjections of PHAL/CTB into the parataenial thalamic nucleus (PT) and AAV-RFP/FG into the RE revealed distinct labelling patterns in DP (see Fig. 1c). k, FG injection into MPO results in retrogradely labelled neurons in DPd. l, FG injection into LHA labels neurons in DPd and deep DPs (see Fig. 1c).
Extended Data Fig. 2 Gene expression defining regional and laminar specificity of DP.
a, RNAscope images of marker genes, Cux2, Etv1, and Tle4, reveal distinct laminar expression in DPs and DPd. b-g, Allen Brain Atlas in situ hybridization data (mouse.brain-map.org/)73 across rostral (top) and caudal (bottom) DP levels. Cacna1h and Plxnd1 (L5 markers; Plxnd1 also in L2) extend into DPs; Tle4 (L6 CT marker) is restricted to DPd. Etv1 and Fezf2 (L5 CPT/IT markers) are expressed in both DPs and DPd. Cux2 (L2/3 IT marker) does not extend into DP, marking its dorsal boundary; Lrmp2 (TTd marker) defines the ventral border. Htr2c and Pamr1 are expressed in DPd and deep DPs, while Fxyd6 is enriched in DPd.
Extended Data Fig. 3 RNAscope combined with retrograde tracing confirms PT identity of DP projection neurons.
a-d, show retrograde tracer injection labels colocalized with RNAscope hybridization of Fezf2 (left), a gene marker for cortical PT cells. 60x confocal images (1 µm optic z; middle panels) were taken through the DP and were used to quantify the degree of tracer and gene colocalization (right panels). Boxed regions in left panels are magnified in the middle panels, and boxed cells in middle panels are magnified in the right panels. a, A retrograde tracer FG MPN injection back-labelled neurons in the DPd. 92% of the FG labelled cells (DPd→MPN) colocalized with Fezf2. b, A FG LHA injection backlabeled neurons in the DPd and in the deeper layers of DPs. 86% of the FG labelled cells (DPd/DPs→LHA) colocalized with Fezf2. c, A FG DMH injection back-labelled neurons in the DPd. 86% of the FG labelled cells (DPd→DMH) colocalized with Fezf2. d, A rabies PVH injection back-labelled neurons in the DPd. 89% of the rabies labelled cells (DPd→PVH) colocalized with Fezf2. Together, this data shows that the large majority of the subcortical projecting neurons in the DP are PT neurons, lending more support for labelling the DP as a primary cortex. Left panel scale bars = 200 µm.
Extended Data Fig. 4 Morphological analysis and clustering of DP neurons.
a, General design of Cre-dependent MORF3 reporter mouse line25. b, Lightsheet images and digital reconstructions of MORF-labelled DP projection neurons. These neurons exhibit detailed dendritic morphology. c, Reconstructed DP neurons coloured by distance of soma from the midline. Left: continuous colour gradient (blue = closest, red = farthest). Middle and right: neurons clustered into superficial (blue) and deep (red) groups based on distance-based classification. d, Left: classification of DP neurons into two morphological clusters: Cluster 1 neurons (blue) have smaller, less complex dendrites (average total dendritic length: 3024 ± 833 µm, average number of branches: 38 ± 12). Cluster 2 neurons (red) have larger, more complex dendrites (average total dendritic length: 5821 ± 958 µm, average number of branches: 76 ± 13). Right: differences between DP neurons with cell bodies in the superficial vs. deep layer. We initially measured the distance of each DP neuron’s cell body from the brain midline. Subsequently, we grouped all 47 neurons into two categories based on their distance from the midline (using k-means clustering method): (i) superficial (26 neurons, average distance of 288 ± 81 µm from the midline) and (ii) deep (21 neurons, average distance of 605 ± 106 µm from the midline). When comparing the superficial and deep clusters with the morphological clusters, the majority of deep layer DP neurons (18/21 neurons) fell within the smaller, less complex (deep layer average length: 3472 ± 1285 µm, average number of branches: 42 ± 16) morphological cluster, whereas the morphology of superficial DP neurons were significantly larger and more complex (average length: 4275 ± 1717 µm, average number of branches: 56 ± 24).
Extended Data Fig. 5 Representative stereotaxic injections in the DP and other MPF areas.
a, Drawings illustrate the locations of representative injection sites, their surgical case IDs, and tracer types over the Allen Reference Atlas (ARA11). The atlas wire frame for ARA levels 38-41 incorporated updated DP boundaries, ILA and TTd. A total of 18 anterograde and 15 retrograde injections in DPs, DPd, ILA, PL, ACAv, and ACAd were quantitatively analysed. Additional 35 cases (10 in DPs/DPd; 25 in other MPF areas) supported validation and whole-brain wiring diagram construction. See SI Fig. 1 for refined DP borders. b, Representative microphotographs of retrograde (CTB) and anterograde (PHAL, AAV) tracer injections made into DPd, DPs, ILA, PL, ACAd and ACAv. Scale bars = 500 µm.
Extended Data Fig. 6 Our informatics data processing workflow.
a, Overview of our informatics data processing workflow. b, Microphotographs of representative PHAL and CTB coinjections in the ACAv. Left panel shows a composed view of two channels where the injection site is overexposed. Middle and right panels show the same PHAL and CTB injection sites following their rescan with lower exposure parameters. Note the reduction of the injection site size following the rescan. All our injection sites are rescanned to attain an improved assessment of the injection site spread. c, Mapping of raw images onto their corresponding level of the ARA11 atlas using our registration pipeline. d, Accuracy of the registration pipeline demonstrated by precise mapping of CTB labelling in small nuclei, such as CLA, and laminar structures, such as cortex and SUB. e, Quantitative comparison showing that the semi-automatic registration resulted in fewer errors compared to automatic registration. f, Global connectivity matrix from representative cases with injections in the DPs, DPd, ILA, PL, ACAv, and ACAd. Despite individual variability, injections in the same MPF areas exhibit consistent connectivity patterns, while different MPF areas display distinct patterns. This consistency highlights the reliability and accuracy of our injections, supporting the validity of the experimental approach. Refer to the Supplementary Methods sections Statistical Model of Brain Connectivity and Quantifying Global MPF Brain Connectivity for further details on the quantification and construction of the connectivity matrix.
Extended Data Fig. 7 Quantitative analysis of MPF whole brain connectivity.
a, 2D hierarchical clustering of the Projection Fraction Matrix for different MPF areas, namely DPs, DPd, ILA, PL, ACAd, and ACAv. b, 2D hierarchical clustering of the MPF Input Fraction Matrix. These matrices illustrate that each MPF area exhibits distinct brain-wide input and output connectivity patterns. The “_i” denotes brain structures located on the ipsilateral side of the injection site, while “_c” indicates structures in the contralateral hemisphere. Cortical areas labelled with “_s” correspond to superficial layers (layers 2-4), whereas those labelled with “_d” represent deep layers (layers 5/6). Refer to the Supplementary Methods sections Statistical Model of Brain Connectivity and Quantifying Global MPF Brain Connectivity for further details on the quantification and construction of the connectivity matrix.
Extended Data Fig. 8 Flatmaps showing global input-output pathways of MPF areas.
Top: DPs and DPd; middle: PL and ILA; bottom: ACAd and ACAv. Solid dots mark input sources; solid lines indicate major projection pathways; thinner lines denote lighter projections. Flatmap adapted with permission from Hahn et al.73, Wiley. See Extended Data Fig. 7 for quantitative connectivity data.
Extended Data Fig. 9 Connectional input specificities of MPF areas with olfactory cortical areas and the claustrum (CLA).
a, A 2D cluster map highlights the distinct olfactory inputs to MPF areas and TTd, based on the connectivity data shown in b (for injection sites, see Extended Data Fig. 5). The brightness of the grids corresponds to strength of input. The brighter the grid, the stronger the connection. Numbers 1-4 refer to the corresponding panels of retrograde labelling in different olfactory regions in b, Note the consistency between the raw image data and the results of the clustermap. For example, strongest input to MPF from the COApm (#3) is to DPd, which is evident from both the clustermap in a and the raw image in b. c-e, Comparisons of quantified olfactory inputs to different MPF areas from the AON, PIR, and COApm, respectively. f, A summary of average projections to MPF areas from olfactory regions. Collectively, these data illustrate distinct connectivity patterns of the TTd, DPs, DPd, and other MPF areas. While TTd (as part of the olfactory cortical area) receives dense input from PIR, AON, and TTv, DPs receives dense inputs from PIR and AON. DPd uniquely receives dense inputs from COApm, in addition to other olfactory inputs. ILA receives weak olfactory inputs, while other MPF areas remain void of olfactory inputs. g, Retrograde labelling in CLA after CTB or FG injections into DPd, DPs, ILA, or PL reveals quantitatively distinct connectivity patterns shown in h, DPs receives no inputs from CLA, whereas DPd, ILA, and PL receive robust CLA input. Note the consistency between the raw and quantified datasets. See SI Fig. 7 for connectional output specificities of MPF areas with cortical areas and the CLA.
Extended Data Fig. 10 Cortical inputs to DPs and DPd.
a, CTB injection into the DPs (injection site is shown in top left image) resulted in dense retrograde labelling in PIR, AON, dorsal endopiriform nucleus (EPd), posterior agranular insular area (AIp), AUDv, temporal association area (TEa), ectorhinal area (ECT), perirhinal area (PERI), and ENTl, as well as BMAa and its adjacent COAa. Right panel shows close-up images of labelled neurons in these cortical areas. Other MPF areas (e.g., ACAv) showed only sparse labelling. Scale bars = 500 µm. b, Coinjection of CTB (retrograde) and PHAL (anterograde) into the DPd labelled neurons in the medial orbitofrontal area (ORBm), dorsal, ventral, and posterior agranular insular areas (AId, AIv, AIp), BLAa, COApm, ventral subiculum (SUBv), and caudal CA1. Some of these regions also contained anterogradely labelled axons, indicating bidirectional connectivity (e.g., ORBm, BMAp, BLAp). c, The same injection resulted in retrograde and anterograde labelling in subcortical structures, such as the lateral septal nucleus (LS), nucleus accumbens (ACB), substantia innominata (SI), superior mammillary nucleus (SUM), and superior central nucleus of raphe (CS). For other abbreviations, see Supplementary Table 1. Scale bars = 500 µm.
Extended Data Fig. 11 Validation of DPd and DPs retrograde injection data.
a, Validation of neural inputs to DPd and DPs using anterograde tracing. Anterograde tracers (AAV or PHAL) were injected into various structures that showed labelled neurons following retrograde tracer MPF injections (see Extended Data Fig. 10), such as PIR, EPd, COApm, AUDv, CLA, PERI/ECT, SUBv, CA1v, and AIp. These injections resulted in specific labelling in either DPs or DPd and validated all the retrograde tracing data. b, Left panels show BLAa subdivisions as defined in Hintiryan et al.21. Images adapted from ref. 21, Springer Nature Limited. Right panels show projection specificities of the different BLAa subdivisions to DP. BLA.al and BLAp densely innervate DPs, BLAac and BLAam, (Figs. 1a, 2e,f) project densely to DPd and ILA, but avoid DPs. c-d, Quantified data showing the ILA and PL share similar input and output connectivity strength with one another, demonstrating their reciprocity. e, Raw and quantified data is shown for axonal projections from DPs to RSPd, PTLp, and VISam. f, Cre-dependent TVA receptor mediated rabies tracing (schematic in top left panel) reveals monosynaptic inputs to DP neurons that project to the ACA. AAVretro-Cre was injected into the ACA, and a Cre-dependent TVA- and RG-expressing helper virus (AAV8-hSyn-FLEX-TVA-P2A-GFP-2A-oG) along with mCherry-expressing G-deleted rabies virus was injected into the DP. This allowed for the labelling of ACA-projecting neurons in the DP (1st-order DP→ACA neurons) and brain-wide (2nd-order) neurons that provide monosynaptic inputs to these DP → ACA projecting neurons. f1 shows AAVretro-Cre ACA injection. f2 shows starter cells in DP through co-labelling of Cre, AAV-GFP, and mCherry (rabies). f3–f6, Representative rabies labelled neurons in AON, PIR, SUB, CA1, and ENT, demonstrating that DP→ACA receive monosynaptic inputs from these structures.
Extended Data Fig. 12 Thalamic connectivity of the DP compared to other MPF areas.
a, Composite map showing distribution of thalamic neurons projecting to MPF (left) and MPF projections to thalamus (right) based on tracer injections into DPs, DPd, ILA, PL, ACAv, and ACAd. b, DPs CTB injection labels RE neurons, indicating dense RE→DPs input. c, Coinjection of PHAL/CTB into DPd shows dense reciprocal connectivity with midline/medial thalamic nuclei (PVT, PT, RE, MDm). d, DPd shows distinct thalamic connectivity compared to PL (top) and ILA (bottom), based on tracer coinjections into DPd, PL, or ILA. DPd and ILA share strong connections with PT, PVT, and RE, while PL and ILA exhibit dense reciprocal connections with AMd/AMv, which are sparsely connected to DPd. e, 2D hierarchical clustering of MPF to thalamic projections represented in a fraction matrix reveals three major clusters of thalamic nuclei: DPd and ILA connect densely with RE, PVT, PT, MDm, and IMD; ACAv with LP, LD, IAD, AMd, AMv, and CL; PL and ACAd with MD, PF, VAL, MDl, and VM. Clustering performed on Maximum Likelihood Estimates (MLE; Supplementary Methods) yields similar results to clustering performed on projection fractions (SI Fig. 9a). f, Dissociative inputs to RE (CTB) and PVT (FG) (see Extended Data Fig. 13b1, b2 for injection sites). Distinct bidirectional connections were observed with DP, ILA, PL, AId, and AIv (left). PVT projecting neurons were in the median preoptic nucleus (MEPO), BST rhomboid nucleus (BSTrh), substantia innominata (SI), central amygdalar nucleus (CEA), lateral hypothalamic area (LHA), periaqueductal gray (PAG), and PB. CTB RE projecting neurons were in medial amygdalar nucleus (MEA) and dorsal premammillary nucleus (PMd). g, Schematic illustrating PVT, PT, and RE as network hubs linking DPs, DPd, and ILA to distinct functional circuits. PVT connects DP/ILA with visceral sensorimotor regions, while RE/PT link DP/ILA to networks regulating social behaviours. PT connects with reproductive-related regions (PA, LSv, MEAad/pd, BSTpr/if, MPN, VMH, PMv)43, whereas RE receives input from defensive behaviour-associated regions (LSr, MPO, AHN, VMHdm, PMd), navigation-related nuclei (LM, MM), and visual/oculomotor areas (MPT, OP, SC, LGv)43. See Supplementary Table 1 for abbreviations. Scale bars = 500 µm.
Extended Data Fig. 13 Additional cortico-thalamic connectivity of MPF.
a, PT (PHAL/CTB) and RE (FG/AAV-RFP) coinjections (a1-a2). CTB and FG labelled neurons were in DPd and ILA L5/6 (a3). PT axons (PHAL) densely innervated ILA L1-3 but sparsely labelled DPd; RE axons (AAV-RFP) targeted DP layer 1, DPd, and ILA L6 (a4). RE projecting neurons were found in ORBvl, ORBl, ACAv, and AIp (a3-a5) and in SUBd and SUBv, while PT projecting neurons were mainly in SUBv (a10-a11). PT projecting neurons were also in MEApd and hypothalamic nuclei related to reproductive behaviours (MPN, PMd), while RE projecting neurons targeted PMd (a6-a8) linked to defensive behaviour. In brainstem, CTB/FG labelled neurons were in PAG and Kolliker-Fuse nucleus (KF) (a9), involved in respiratory and cardiovascular regulation. b, PHAL/CTB was injected into the RE (b1) while FG into the PVT (b2), revealing distinct bidirectional connections of both nuclei with the DP, ILA, and other MPF regions. Retrograde labelling showed divergent input sources: PVT receives input mainly from visceral sensorimotor-related structures (b3–4), while RE receives input from PMd (b5), mammillary nuclei (MM, LM; b6), SC (b7), ventral lateral geniculate nucleus (LGv) (b8), and SUB (b9). c, Monosynaptic inputs to RE → DP neurons was investigated (schematic). AAVretro-Cre was injected into DP, and a Cre-dependent TVA- and RG-expressing helper virus (AAV8-hSyn-FLEX-TVA-P2A-GFP-2A-oG) and mCherry-expressing G-deleted rabies virus into RE. c1, AAVretro-Cre DP injection. c2, shows RE starter cells via colabeling of Cre, AAV-GFP, and mCherry. c3-c11, Representative images with rabies labelling in (1) cortical areas (ACAv, ACAd, MOs); (2) thalamic nuclei [PVT, rhomboid (RH), medial mediodorsal (MDm), dorsal anteromedial (AMd), ventromedial (VM), and reticular thalamic nuclei (RT)]; (3) BLAa, (4) hippocampal CA1, SUB, ENT; and (5) superior colliculus (SC) and periaqueductal grey (PAG). d, Schematic of RE as a hub integrating and relaying an array of neural inputs to DP. See Supplementary Table 1 for abbreviations.
Extended Data Fig. 14 Cortico-cerebral nuclei projections from MPF areas.
a, Axonal projections from DPs (top), DPd (middle), and ILA (bottom) show distinct but overlapping patterns targeting BST, CEA, SI, MEAad, and MEApd. Scale bars = 200 µm. b, Projections from DPd, DPs, ILA, and PL to nucleus accumbens (ACB) and CP striosomes. Scale bars = 500 µm. c, Comparison of quantified MPF projections to CP, ACB, OT, and striatal-like amygdalar nuclei (CEA, MEA). d, Schematic summarizing MPF projections to cerebral nuclei. e, Quantified projections to BST subregions (BSTal, BSTam, BSTif) based on data in a-b.
Extended Data Fig. 15 Topographic projections of MPF to the hypothalamus.
a, Microscopic images show topographic projections from DPs, DPd, ILA, PL, ACAv, and ACAd to hypothalamus across different rostral-caudal levels (anterior, tuberal, and posterior). Note that DPd generates dense projections to the periventricular zone. The DPd and ILA generate projections to different hypothalamic nuclei in the medial zone. DPs, DPd, ILA, and PL generate dense, partially overlapping, projections to LHA, while the ACAv and ACAd project densely to the zona incerta and much less to LHA. Also see Supplementary Results section Topographic projections from MPF to LHA. b, Quantified comparison of projections from MPF areas to the preautonomic PVHd, which includes the PVHlp and PVHf. Data shows that PVHd receives input from DPs. c, Comparison of quantified projections from MPF to LHA tuberal level. Data is visualized in a pareto chart, which orders the number of projections in descending order, shows that densest projections from MPF to LHA are from DPs. The cumulative total of projections is represented by the curved line. Right y axis denotes cumulative percentage.
Extended Data Fig. 16 DP regulation of preautonomic outputs via LHA.
a, AAV-RFP/FG coinjection in LHA reveals dense retrograde labelling in MPF, AId, and TTd. Notably, DPs still contains the densest labelling (left panels). Anterogradely labelled axons project to widespread targets, including forebrain, thalamus, PAG, DMX, NTS, and parvicellular reticular nucleus (PARN). b, AAV1-Cre was injected into the DP and Cre-dependent AAV-FLEX-GFP into LHA (schematic, upper left) enabling examination of the downstream projections of DP → LHA neurons. b1, the AAV1-Cre DP injection site; b2-b3, AAV-FLEX-GFP LHA injection site. Cre-labelled starter cells express GFP and send broad axons to brain structures that regulate autonomic and behavioural activities (b4-b19), including BST, SI, PAG, PB, DMH/NTS, ventromedial medulla (RM, RPA), and reticular nuclei (PARN, IRN, PGRN near AMB). Compared to non-selective LHA projections (panel a), these DP-recipient LHA neurons show sparse thalamic projections, with the notable exception of dense input to the lateral habenula (LH; b9). See Supplementary Table 1 for abbreviations. c, Microphotographs showing axonal labelling in DMX following PHAL injections into ILA. Scale bars = 500 µm. d, Schematic illustrating the neural network through which the DPs and ILA regulate vagal parasympathetic outputs.
Extended Data Fig. 17 Additional evidence for DPd projections to hypothalamic neuroendocrine zone.
a, Anterograde tracing confirms specificity of DPd’s projections to hypothalamic neuroendocrine zone and periventricular structures including MPO (GnRH neurons), PVa (somatostatin neurons), and SO (oxytocin, vasopressin neurons). DPd innervates periventricular and medial hypothalamic structures involved in homeostatic regulation, like the anteroventral periventricular nucleus (AVPV) and MPN, two sexually dimorphic areas essential for sex hormone control and reproductive behaviours. Scale bars = 500 µm. b, 2D hierarchical clustering illustrates the comparative projections from MPF areas and TTd to the hypothalamic periventricular zone (PVZ). The DPd generates unique projections throughout the PVZ. The DPs and other MPF areas display minimal to no projections to the PVZ. c, Bar graph shows DPd, unlike other MPF areas, generates dense projections to neuroendocrine PVHmpd and PVHpv regions, compared to the graph showing DPs projections to autonomic PVHlp and PVHf (Extended Data Fig. 15b). d, Flatmap showing the spatial distribution of hypothalamic neuroendocrine cell populations across rostral-caudal levels. Gonadotropin-releasing hormone (GnRH) neurons localize in preoptic area; somatostatin (SS) neurons in intermediate periventricular nucleus (PVi) and PVHpv (periventricular part of the PVH); thyrotropin-releasing hormone (TRH) and corticotropin-releasing hormone (CRH) neurons in PVH medial parvicellular division (PVHmpd); dopamine and growth hormone–releasing hormone (GRH) neurons are in arcuate nucleus (ARH), where dopamine regulates prolactin secretion. Two magnocellular neuron types, oxytocin (OXY) and vasopressin, distribute across the magnocellular division of PVH (PVHm), supraoptic nucleus (SO), and accessory supraoptic nucleus (ASO). The longitudinal organization of the hypothalamus contains three major zones: periventricular (PVZ), medial (MEZ), and lateral (LZ). e-g, Pareto charts (left) compare quantified labelling in arcuate (ARH, e), medial preoptic nucleus (MPN, f), and dorsomedial hypothalamic nuclei (DMH, g) following anterograde MPF tracer injections. Right y axis denotes cumulative percentage. Strongest projectionss to these hypothalamic regions are from DPd. Right panels validate these strong DP→ARH/MPN/DMH connections with retrograde tracer injections (CTB, FG), supporting unique DPd projections to the periventricular hypothalamic region.
Extended Data Fig. 18 Additional data supporting DPd’s projections to the hypothalamic neuroendocrine zone.
a, Additional evidence showing that DPd VGLUT2+ neurons directly innervate hypothalamic neuroendocrine cells. In VGLUT2 Cre mice, a Cre-dependent AAV-FLEX-RFP was injected into DPd (upper left), resulting in robust anterograde labelling in the hypothalamic neuroendocrine zone. FG was administered intravenously to reveal neuroendocrine cells48. Neuroendocrine cells in the periventricular nucleus (PVpo) and PVH intermingle with dense AAV-RFP labelled axonal terminals from DP (Fig. 4d). Intermingling was also evident in the dorsomedial arcuate nucleus (ARH; b) and dorsal supraoptic nucleus (SO; c), indicating direct synaptic input from DPd VGLUT2+ neurons to diverse neuroendocrine cell populations. d, VGLUT2+ axons from the DPd closely appose OXY neurons in PVH (revealed with fluorescent immunostaining method). Close-up image shows putative synaptic appositions at 1 µm optical z-resolution. e, Monosynaptic rabies tracing reveals direct inputs from DPd to CRH-expressing neuroendocrine neurons in PVH. In CRH Cre transgenic mice, Cre-dependent helper virus (AAV8-hSyn-FLEX-TVA-P2A-GFP-2A-oG) was injected into the PVH to express TVA and rabies glycoprotein (RG), followed by injection of mCherry-expressing G-deleted rabies virus (left panel). This approach selectively labels neurons providing direct monosynaptic input to CRH neurons. Rabies-labelled neurons were observed in DPd (right panels), but not in other MPF areas, despite the known limitation of rabies virus in labelling deep-layer cortical neurons. These data provide anatomical evidence that DPd directly innervates CRH neuroendocrine neurons. f, BSTam PHAL/CTB coinjection labels neurons in DPd and ILA, as well as other structures (CEA, MEA, BMAa). Anterogradely labelled axons target multiple hypothalamic nuclei, including PVH, ARH, AHN, DMH, VMH, and LHA. These findings suggest that DPd and ILA modulate neuroendocrine, autonomic, and behavioural functions via their projections to BSTam. Scale bars = 500 µm.
Extended Data Fig. 19 Feedforward projections from DPd to hypothalamic neuroendocrine zone and autonomic related structures.
a, Anterograde tracing reveals that DPd axons innervate BST, anteroventral preoptic nucleus (AVPV), medial preoptic nucleus (MPNm), ventrolateral preoptic area (MPO), and dorsomedial hypothalamic nucleus (DMH). Tracer injections into each of these DPd targets reveal downstream projections to: (1) the hypothalamic neuroendocrine zone (PVH, ARH); (2) medial hypothalamic nuclei involved in social behaviour, including the VMH, TU, PMv, and PMd; and (3) brainstem autonomic structures such as the PAGvl and PB. The DMH also projects to Barrington’s nucleus (B), raphe nuclei (RM, RPA), and DMX. b, Schematic summarizing the feedforward network architecture through which DPd modulates neuroendocrine and autonomic outputs via the BST, AVPV, and DMH. Projections from MPNm, MPO, and other downstream hypothalamic nuclei are omitted for clarity. Scale bars = 500 µm. Refer to Supplementary Results section DPd connections with neuroendocrine structures.
Extended Data Fig. 20 DPd regulation of HPA axis via hippocampus and amygdala, and additional electrophysiological and behavioural data regarding DP neurons.
a, DP regulation of the hypothalamic–pituitary–adrenal (HPA) axis via inputs from ventral subiculum (SUBv) and anterior basolateral amygdala (BLAa). b, Dual-tracing strategy to map disynaptic pathways from SUBv and BLAa onto DPd→PVH neurons. c-e, In MORF3 mice, AAV1-Cre was injected into SUBv (c) and Cre-dependent AAV-FLEX-RFP into DP (e). Cre trans-synaptically transported to postsynaptic DP neurons. d-f, BLAa PHAL injection labelled projections to DPd and ILA. g-h, RFP-labelled SUBv→DP neurons project to PVHmpd (CRH neuroendocrine neurons). i–n, Confocal imaging of close appositions between BLAa-derived PHAL labelled terminals and AAV-RFP (or MORF) labelled DPd neurons. n, A single DPd neuron co-labelled with AAV-RFP and MORF3, receives convergent input from SUBv and BLAa. PHAL terminal boutons form close appositions onto the soma of this neuron (1 µm optical z), supporting convergence of hippocampal and amygdalar inputs onto DPd→PVH. o, Related to Fig. 5a–c. Top trace shows a response evoked by optogenetic stimulation of ENTl inputs in the presence of Na+ channel blocker tetrodotoxin (TTX, 1 µM) to abolish activity-dependent neurotransmitter release and 4-aminopyridine (4-AP, 100 µM) to block A-type K+ channels and enhance neurotransmitter release. This allowed isolation of monosynaptic responses. Middle trace demonstrates application of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 µM) and DL-2-amino-5-phosphonovaleric acid (APV, 50 µM), blockers of AMPA and NMDA receptors respectively, almost eliminates the excitatory response. Remaining small current was abolished with bicuculline (10 µM), a GABAA receptor antagonist (data not shown). Bottom traces show the merged responses. p-q, Related to Fig. 5d–k. Ratio of excited or inhibited cells out of the total behaviourally-responsive cells (Excited+Inhibited). Neuronal responsiveness was quantified using the area under the ROC curve (auROC). Direction of modulation (inhibited vs. excited) significantly affects ratio of responsive cells for all social and non-social behaviours in DP [p; Two-way ANOVA: DP, F(1, 24) = 11.13, **p = 0.0028] and ILA [q; F(1, 30) = 13.58, ***p = 0.0009]. Dots = n/group. Boxes represent average, error bars = s.e.m.
Supplementary information
Supplementary Information
In the Supplementary Methods, additional details are provided regarding the data processing pipeline (registration, segmentation, annotation) that was used to analyse the neuroanatomical tracing data. The Supplementary Results and Supplementary Figs. 1–13 expand on those included in the manuscript. The Supplementary Discussion extends the discussion on the purported unified MPF model. A table of contents is included.
Supplementary Table 1
A list of all structure abbreviations.
Supplementary Video 1
This video is related to Fig. 3b and shows a single AAV-RFP-labelled axon originating from the DP forming putative synapses with the soma and dendritic branches of a single LHA neuron that projects to the spinal cord validating the DP to LHA to spinal cord connection.
Supplementary Data
Source data for all the plots presented in the Supplementary Figures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hintiryan, H., Zhu, M., Zhao, P. et al. Neural networks of the mouse visceromotor cortex. Nature (2025). https://doi.org/10.1038/s41586-025-09360-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-025-09360-w