Abstract
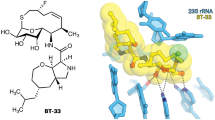
Growing resistance toward ribosome-targeting macrolide antibiotics has limited their clinical utility and urged the search for superior compounds. Macrolones are synthetic macrolide derivatives with a quinolone side chain, structurally similar to DNA topoisomerase-targeting fluoroquinolones. While macrolones show enhanced activity, their modes of action have remained unknown. Here, we present the first structures of ribosome-bound macrolones, showing that the macrolide part occupies the macrolide-binding site in the ribosomal exit tunnel, whereas the quinolone moiety establishes new interactions with the tunnel. Macrolones efficiently inhibit both the ribosome and DNA topoisomerase in vitro. However, in the cell, they target either the ribosome or DNA gyrase or concurrently both of them. In contrast to macrolide or fluoroquinolone antibiotics alone, dual-targeting macrolones are less prone to select resistant bacteria carrying target-site mutations or to activate inducible macrolide resistance genes. Furthermore, because some macrolones engage Erm-modified ribosomes, they retain activity even against strains with constitutive erm resistance genes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Coordinates and structure factors were deposited to the Research Collaboratory for Structural Bioinformatics (RCSB) PDB under the following accession codes: 8VTU for the wild-type T. thermophilus 70S ribosome in complex with macrolone MCX-66, mRNA, aminoacylated A-site Phe-tRNAPhe, aminoacylated P-site fMet-tRNAiMet and deacylated E-site tRNAPhe; 8VTV for the wild-type T. thermophilus 70S ribosome in complex with macrolone MCX-91, mRNA, aminoacylated A-site Phe-tRNAPhe, aminoacylated P-site fMet-tRNAiMet and deacylated E-site tRNAPhe; 8VTW for the wild-type T. thermophilus 70S ribosome in complex with macrolone MCX-128 and protein Y; 8VTX for the m26A2058 T. thermophilus 70S ribosome in complex with macrolone MCX-128, mRNA, aminoacylated A-site Phe-tRNAPhe, aminoacylated P-site fMet-tRNAiMet and deacylated E-site tRNAPhe; 8VTY for the wild-type T. thermophilus 70S ribosome in complex with CIP and protein Y. All previously published structures that were used in this work for structural comparisons were retrieved from the RCSB PDB under accession codes 6XHW, 6XHX and 7ZTA. No sequence data were generated in this study. Source data are provided with this paper.
References
Fernandes, P. Use of antibiotic core structures to generate new and useful macrolide antibiotics. In Antibiotics Current Innovations and Future Trends (eds Sánchez, S. & Demain, A. L.) (Caister Academic Press, 2015).
Bush, N. G., Diez-Santos, I., Abbott, L. R. & Maxwell, A. Quinolones: mechanism, lethality and their contributions to antibiotic resistance. Molecules 25, 5662 (2020).
Agouridas, C. et al. Synthesis and antibacterial activity of ketolides (6-O-methyl-3-oxoerythromycin derivatives): a new class of antibacterials highly potent against macrolide-resistant and -susceptible respiratory pathogens. J. Med. Chem. 41, 4080–4100 (1998).
Seiple, I. B. et al. A platform for the discovery of new macrolide antibiotics. Nature 533, 338–345 (2016).
Pavlovic, D., Fajdetic, A. & Mutak, S. Novel hybrids of 15-membered 8a- and 9a-azahomoerythromycin A ketolides and quinolones as potent antibacterials. Bioorg. Med. Chem. 18, 8566–8582 (2010).
Fan, B. Z. et al. Design, synthesis and structure–activity relationships of novel 15-membered macrolides: quinolone/quinoline-containing sidechains tethered to the C-6 position of azithromycin acylides. Eur. J. Med. Chem. 193, 112222 (2020).
Barry, A. L. & Jones, R. N. Comparative in vitro activity of amifloxacin and five other fluoroquinolone antimicrobial agents and preliminary criteria for the disk susceptibility test. Eur. J. Clin. Microbiol. 6, 179–182 (1987).
Yourassowsky, E., Van der Linden, M. P., Crokaert, F. & Glupczynski, Y. In vitro activity of pefloxacin compared to other antibiotics. J. Antimicrob. Chemother. 17, 19–28 (1986).
Dinos, G. P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 174, 2967–2983 (2017).
Vazquez-Laslop, N. & Mankin, A. S. How macrolide antibiotics work. Trends Biochem. Sci. 43, 668–684 (2018).
Lin, J., Zhou, D., Steitz, T. A., Polikanov, Y. S. & Gagnon, M. G. Ribosome-targeting antibiotics: modes of action, mechanisms of resistance, and implications for drug design. Annu. Rev. Biochem. 87, 451–478 (2018).
Schlunzen, F. et al. Structural basis for the antibiotic activity of ketolides and azalides. Structure 11, 329–338 (2003).
Dunkle, J. A., Xiong, L., Mankin, A. S. & Cate, J. H. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc. Natl Acad. Sci. USA 107, 17152–17157 (2010).
Bulkley, D., Innis, C. A., Blaha, G. & Steitz, T. A. Revisiting the structures of several antibiotics bound to the bacterial ribosome. Proc. Natl Acad. Sci. USA 107, 17158–17163 (2010).
Svetlov, M. S. et al. Structure of Erm-modified 70S ribosome reveals the mechanism of macrolide resistance. Nat. Chem. Biol. 17, 412–420 (2021).
Beckert, B. et al. Structural and mechanistic basis for translation inhibition by macrolide and ketolide antibiotics. Nat. Commun. 12, 4466 (2021).
Tu, D., Blaha, G., Moore, P. B. & Steitz, T. A. Structures of MLSBK antibiotics bound to mutated large ribosomal subunits provide a structural explanation for resistance. Cell 121, 257–270 (2005).
Hansen, J. L. et al. The structures of four macrolide antibiotics bound to the large ribosomal subunit. Mol. Cell 10, 117–128 (2002).
Svetlov, M. S. et al. High-resolution crystal structures of ribosome-bound chloramphenicol and erythromycin provide the ultimate basis for their competition. RNA 25, 600–606 (2019).
Chen, C. W. et al. Structural insights into the mechanism of overcoming Erm-mediated resistance by macrolides acting together with hygromycin-A. Nat. Commun. 14, 4196 (2023).
Jenni, S. & Ban, N. The chemistry of protein synthesis and voyage through the ribosomal tunnel. Curr. Opin. Struct. Biol. 13, 212–219 (2003).
Kannan, K., Vázquez-Laslop, N. & Mankin, A. S. Selective protein synthesis by ribosomes with a drug-obstructed exit tunnel. Cell 151, 508–520 (2012).
Davis, A. R., Gohara, D. W. & Yap, M. N. Sequence selectivity of macrolide-induced translational attenuation. Proc. Natl Acad. Sci. USA 111, 15379–15384 (2014).
Kannan, K. et al. The general mode of translation inhibition by macrolide antibiotics. Proc. Natl Acad. Sci. USA 111, 15958–15963 (2014).
Sothiselvam, S. et al. Binding of macrolide antibiotics leads to ribosomal selection against specific substrates based on their charge and size. Cell Rep. 16, 1789–1799 (2016).
Almutairi, M. M. et al. Co-produced natural ketolides methymycin and pikromycin inhibit bacterial growth by preventing synthesis of a limited number of proteins. Nucleic Acids Res. 45, 9573–9582 (2017).
Franceschi, F., Kanyo, Z., Sherer, E. C. & Sutcliffe, J. Macrolide resistance from the ribosome perspective. Curr. Drug Targets Infect. Disord. 4, 177–191 (2004).
Versalovic, J. et al. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 40, 477–480 (1996).
Wand, G. & Taylor, D. E. Site-specific mutations in the 23S rRNA gene of Helicobacter pylori confer two types of resistance to macrolide-lincosamide-streptogramin B antibiotics. Antimicrob. Agents Chemother. 42, 1952–1958 (1998).
Shallom, S. J. & Zelazny, A. M. Detection of mixed populations of clarithromycin-susceptible and -resistant Mycobacterium abscessus strains. J. Clin. Microbiol. 60, e0169421 (2022).
McGuire, J. M. et al. Ilotycin, a new antibiotic. Antibiot. Chemother. (Northfield) 2, 281–283 (1952).
Kirst, H. A. Introduction to the macrolide antibiotics. In Macrolide Antibiotics (eds Schönfeld, W. & Kirst, H. A.) (Birkhäuser Verlag, 2002).
Iacoviello, V. R. & Zinner, S. H. Macrolides: a clinical overview. In Macrolide Antibiotics (eds Parnham, M. J. & Bruinvels, J.) (Birkhäuser Verlag, 2002).
Tanikawa, T. et al. Synthesis and antibacterial activity of acylides (3-O-acyl-erythromycin derivatives): a novel class of macrolide antibiotics. J. Med. Chem. 44, 4027–4030 (2001).
Liang, J. H. et al. Structure–activity relationships of novel alkylides: 3-O-arylalkyl clarithromycin derivatives with improved antibacterial activities. Eur. J. Med. Chem. 49, 289–303 (2012).
Magee, T. V. et al. Novel 3-O-carbamoyl erythromycin A derivatives (carbamolides) with activity against resistant staphylococcal and streptococcal isolates. Bioorg. Med. Chem. Lett. 23, 1727–1731 (2013).
Tang, D. et al. Design, synthesis, and antibacterial activities of novel 3,6-bicyclolide oximes: length optimization and zero carbon linker oximes. Bioorg. Med. Chem. Lett. 18, 5078–5082 (2008).
Bryskier, A. & Denis, A. Ketolides: novel antibacterial agents designed to overcome resistance to erythromycin A within Gram-positive cocci. In Macrolide Antibiotics (eds Schönfeld, W. & Kirst, H. A.) (Birkhäuser Verlag, 2002).
Fernandes, P., Martens, E., Bertrand, D. & Pereira, D. The solithromycin journey—it is all in the chemistry. Bioorg. Med. Chem. 24, 6420–6428 (2016).
Capobianco, J. O. et al. Studies of the novel ketolide ABT-773: transport, binding to ribosomes, and inhibition of protein synthesis in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44, 1562–1567 (2000).
Llano-Sotelo, B. et al. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob. Agents Chemother. 54, 4961–4970 (2010).
Douthwaite, S. Structure–activity relationships of ketolides vs. macrolides. Clin. Microbiol. Infect. 7, 11–17 (2001).
Svetlov, M. S., Cohen, S., Alsuhebany, N., Vazquez-Laslop, N. & Mankin, A. S. A long-distance rRNA base pair impacts the ability of macrolide antibiotics to kill bacteria. Proc. Natl Acad. Sci. USA 117, 1971–1975 (2020).
Ma, C. X. et al. Design, synthesis and structure–activity relationships of novel macrolones: hybrids of 2-fluoro 9-oxime ketolides and carbamoyl quinolones with highly improved activity against resistant pathogens. Eur. J. Med. Chem. 169, 1–20 (2019).
Liu, X. P. et al. Design and synthesis of novel macrolones bridged with linkers from 11,12-positions of macrolides. Bioorg. Med. Chem. Lett. 68, 128761 (2022).
Hutinec, A. et al. Novel 8a-aza-8a-homoerythromycin–4′-(3-substituted-amino)propionates with broad spectrum antibacterial activity. Bioorg. Med. Chem. Lett. 20, 3244–3249 (2010).
Drlica, K., Malik, M., Kerns, R. J. & Zhao, X. Quinolone-mediated bacterial death. Antimicrob. Agents Chemother. 52, 385–392 (2008).
Aldred, K. J., Kerns, R. J. & Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 53, 1565–1574 (2014).
Miotto, P., Cirillo, D. M. & Migliori, G. B. Drug resistance in Mycobacterium tuberculosis: molecular mechanisms challenging fluoroquinolones and pyrazinamide effectiveness. Chest 147, 1135–1143 (2015).
Machalek, D. A. et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect. Dis. 20, 1302–1314 (2020).
Lungu, I. A., Moldovan, O. L., Biris, V. & Rusu, A. Fluoroquinolones hybrid molecules as promising antibacterial agents in the fight against antibacterial resistance. Pharmaceutics 14, 1749 (2022).
Pavlovic, D. & Mutak, S. Discovery of 4′-ether linked azithromycin–quinolone hybrid series: influence of the central linker on the antibacterial activity. ACS Med. Chem. Lett. 2, 331–336 (2011).
Cipcic Paljetak, H. et al. Macrolones are a novel class of macrolide antibiotics active against key resistant respiratory pathogens in vitro and in vivo. Antimicrob. Agents Chemother. 60, 5337–5348 (2016).
Weisblum, B. Insights into erythromycin action from studies of its activity as inducer of resistance. Antimicrob. Agents Chemother. 39, 797–805 (1995).
Ramu, H., Mankin, A. & Vazquez-Laslop, N. Programmed drug-dependent ribosome stalling. Mol. Microbiol. 71, 811–824 (2009).
Subramanian, S. L., Ramu, H. & Mankin, A. S. Inducible resistance to macrolide antibiotics. In Antibiotic Drug Discovery and Development (eds Dougherty, T. J. & Pucci, M. J.) (Springer Publishing Company, 2011).
Vazquez-Laslop, N. et al. Role of antibiotic ligand in nascent peptide-dependent ribosome stalling. Proc. Natl Acad. Sci. USA 108, 10496–10501 (2011).
Liang, J. H. et al. Synthesis and antibacterial activities of 6-O-methylerythromycin A 9-O-(3-aryl-2-propenyl) oxime ketolide, 2,3-enol ether, and alkylide analogues. Eur. J. Med. Chem. 45, 3627–3635 (2010).
Osterman, I. A. et al. Sorting out antibiotics’ mechanisms of action: a double fluorescent protein reporter for high-throughput screening of ribosome and DNA biosynthesis inhibitors. Antimicrob. Agents Chemother. 60, 7481–7489 (2016).
Orelle, C. et al. Tools for characterizing bacterial protein synthesis inhibitors. Antimicrob. Agents Chemother. 57, 5994–6004 (2013).
Morgan-Linnell, S. K., Becnel Boyd, L., Steffen, D. & Zechiedrich, L. Mechanisms accounting for fluoroquinolone resistance in Escherichia coli clinical isolates. Antimicrob. Agents Chemother. 53, 235–241 (2009).
Hooper, D. C. & Jacoby, G. A. Topoisomerase inhibitors: fluoroquinolone mechanisms of action and resistance. Cold Spring Harb. Perspect. Med. 6, a025320 (2016).
Gellert, M., Mizuuchi, K., O’Dea, M. H. & Nash, H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc. Natl Acad. Sci. USA 73, 3872–3876 (1976).
Paton, J. H. & Reeves, D. S. Fluoroquinolone antibiotics. Microbiology, pharmacokinetics and clinical use. Drugs 36, 193–228 (1988).
Quan, S., Skovgaard, O., McLaughlin, R. E., Buurman, E. T. & Squires, C. L. Markerless Escherichia coli rrn deletion strains for genetic determination of ribosomal binding sites. G3 (Bethesda) 5, 2555–2557 (2015).
Triman, K. L., Peister, A. & Goel, R. A. Expanded versions of the 16S and 23S ribosomal RNA mutation databases (16SMDBexp and 23SMDBexp). Nucleic Acids Res. 26, 280–284 (1998).
Malik, M. et al. Suppression of gyrase-mediated resistance by C7 aryl fluoroquinolones. Nucleic Acids Res. 44, 3304–3316 (2016).
Gupta, P., Sothiselvam, S., Vazquez-Laslop, N. & Mankin, A. S. Deregulation of translation due to post-transcriptional modification of rRNA explains why erm genes are inducible. Nat. Commun. 4, 1984 (2013).
Vazquez-Laslop, N. & Mankin, A. S. Context-specific action of ribosomal antibiotics. Annu. Rev. Microbiol. 72, 185–207 (2018).
Horinouchi, S. & Weisblum, B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc. Natl Acad. Sci. USA 77, 7079–7083 (1980).
Gryczan, T. J., Grandi, G., Hahn, J., Grandi, R. & Dubnau, D. Conformational alteration of mRNA structure and the posttranscriptional regulation of erythromycin-induced drug resistance. Nucleic Acids Res. 8, 6081–6097 (1980).
Sutcliffe, J. & Leclercq, R. Mechanisms of resistance to macrolides, lincosamides, and ketolides. In Macrolide Antibiotics (eds Schönfeld, W. & Kirst, H. A.) (Birkhäuser Verlag, 2002).
Mitcheltree, M. J. et al. A synthetic antibiotic class overcoming bacterial multidrug resistance. Nature 599, 507–512 (2021).
Osterman, I. A. et al. Tetracenomycin X inhibits translation by binding within the ribosomal exit tunnel. Nat. Chem. Biol. 16, 1071–1077 (2020).
Leroy, E. C., Perry, T. N., Renault, T. T. & Innis, C. A. Tetracenomycin X sequesters peptidyl-tRNA during translation of QK motifs. Nat. Chem. Biol. 19, 1091–1096 (2023).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Bundy, B. C. & Swartz, J. R. Site-specific incorporation of p-propargyloxyphenylalanine in a cell-free environment for direct protein–protein click conjugation. Bioconjug. Chem. 21, 255–263 (2010).
Svetlov, M. S., Vazquez-Laslop, N. & Mankin, A. S. Kinetics of drug–ribosome interactions defines the cidality of macrolide antibiotics. Proc. Natl Acad. Sci. USA 114, 13673–13678 (2017).
Cheng, Y. & Prusoff, W. H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108 (1973).
Orelle, C. et al. Identifying the targets of aminoacyl-tRNA synthetase inhibitors by primer extension inhibition. Nucleic Acids Res. 41, e144 (2013).
Bailey, M., Chettiath, T. & Mankin, A. S. Induction of ermC expression by ‘non-inducing’ antibiotics. Antimicrob. Agents Chemother. 52, 866–874 (2008).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Selmer, M. et al. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313, 1935–1942 (2006).
Polikanov, Y. S., Blaha, G. M. & Steitz, T. A. How hibernation factors RMF, HPF, and YfiA turn off protein synthesis. Science 336, 915–918 (2012).
Polikanov, Y. S., Steitz, T. A. & Innis, C. A. A proton wire to couple aminoacyl-tRNA accommodation and peptide-bond formation on the ribosome. Nat. Struct. Mol. Biol. 21, 787–793 (2014).
Polikanov, Y. S., Melnikov, S. V., Soll, D. & Steitz, T. A. Structural insights into the role of rRNA modifications in protein synthesis and ribosome assembly. Nat. Struct. Mol. Biol. 22, 342–344 (2015).
Syroegin, E. A., Aleksandrova, E. V. & Polikanov, Y. S. Insights into the ribosome function from the structures of non-arrested ribosome-nascent chain complexes. Nat. Chem. 15, 143–153 (2023).
Aleksandrova, E. V. et al. Structural basis of Cfr-mediated antimicrobial resistance and mechanisms to evade it. Nat. Chem. Biol. https://doi.org/10.1038/s41589-023-01525-w (2024).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Schuttelkopf, A. W. & van Aalten, D. M. PRODRG: a tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 60, 1355–1363 (2004).
Acknowledgements
We thank M. Svetlov for valuable discussions and assistance with the data processing. We thank the Analysis and Testing Center at the Beijing Institute of Technology for collecting and analyzing the spectral data. We thank the staff at Northeastern Collaborative Access Team (NE-CAT) beamlines 24ID-C and 24ID-E for help with X-ray diffraction data collection, especially M. Capel, F. Murphy, S. Banerjee, I. Kourinov, D. Neau, J. Schuermann, N. Sukumar, A. Lynch, J. Withrow, K. Perry, A. Kaya and C. Salbego. This work is based upon research conducted at the NE-CAT beamlines, which are funded by the National Institute of General Medical Sciences from the National Institutes of Health (NIH; grant P30-GM124165 to NE-CAT). The Eiger 16M detector on the 24ID-E beamline is funded by an NIH-ORIP HEI grant (S10-OD021527 to NE-CAT). This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. This work was supported by the National Institute of General Medical Sciences of the NIH (grant R35-GM127134 to A.S.M.), the National Institute of Allergy and Infectious Diseases of the NIH (grant R21-AI137584 to A.S.M. and Y.S.P.), the Illinois State startup funds (to Y.S.P.), the National Key Research and Development Program of China (grant 2018YFA0901800 to J.-H.L.) and the National Natural Science Foundation of China (grant 81673335 to J.-H.L.). The funders had no role in study design, data collection and analysis, decision to publish or manuscript preparation.
Author information
Authors and Affiliations
Contributions
C.-X.M. performed the chemical synthesis, purification and microbiological characterization of MCX compounds. D.K. and F.A. performed the in vivo dual-reporter assay, mutant selection and microbiological characterization of the selected MCX-resistant mutant strains. D.K. also performed the in vitro translation inhibition, gyrase inhibition and toeprinting assays. E.V.A. and Y.S.P. designed and performed X-ray crystallography experiments. A.S.M., N.V.-L., Y.S.P. and J.-H.L. designed and supervised the experiments. All authors interpreted the results. A.S.M., N.V.-L., Y.S.P. and J.-H.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Graeme Conn, Suparna Sanyal and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Inhibition of translation and ribosome binding of macrolones.
(a) Inhibition of production of the green flourescent protein (GFP) in cell-free translation system by varying concentrations of macrolones relative to uninhibited reaction. Shown are the results of two independent experiments. (b) Competitive binding of [14C]-ERY and macrolones to the E. coli ribosome. Unlabeled ERY was used as a control (black circles). Experimental details are presented in the Online Methods section. Shown are the results of two independent experiments.
Extended Data Fig. 2 Effects of macrolones on in vitro translation.
Mapping the sites of macrolone-mediated ribosome arrests (blue arrows) at the early codons of the model ORF derived from the E. coli yrbA gene. The classic macrolide ERY is included for comparison. Due to the presence of the Thr-RS inhibitor borrelidin, the ribosomes that did not stall at the early codons are eventually trapped at the Gln12 codon when Thr13 needs to be incorporated into the growing protein (grey arrow). The AUG start codon is marked with a black arrow. The sample labeled as ‘NONE’ contained only borrelidin but no ERY or macrolones. Amino acid and nucleotide sequences of yrbA gene are shown on the left. Sequencing lanes are marked as C, U, A, G. This experiment was repeated independently twice and produced similar results.
Extended Data Fig. 3 Electron density maps of ribosome-bound MCX-66, MCX-91, and MCX-128.
(a-c) 2Fo-Fc Fourier electron density maps of MCX-66 (a, magenta), MCX-91 (b, green), and MCX-128 (c, yellow) in complex with the T. thermophilus 70S ribosome (blue mesh) shown in two mutually perpendicular views. The refined models of ribosome-bound MCX compounds are displayed in their respective electron density maps after the refinement contoured at 1.0σ. Carbon atoms are colored magenta (MCX-66), green (MCX-91), or yellow (MCX-128); nitrogen atoms are blue; oxygen atoms are red; fluorine atoms are dark green. Note that the locations of fluoroquinolone side chains can be unambiguously determined from the obtained electron density maps.
Extended Data Fig. 5 Structure of ciprofloxacin (CIP) in complex with the 70S ribosome.
(a) 2Fo-Fc Fourier electron density map of ciprofloxacin (CIP, greencyan) in complex with the T. thermophilus 70S ribosome (blue mesh). The refined model of ribosome-bound CIP is displayed in its respective electron density map after the refinement contoured at 1.0σ. Carbon atoms are colored greencyan; nitrogen atoms are blue; oxygen atoms are red; fluorine atom is dark green. (b, c) Close-up views of CIP bound in the NPET of the 70S ribosome, highlighting its stacking (red arrows) interactions with the nucleotides of the 23S rRNA. (d) Superposition of the structures of ribosome-bound CIP and MCX-128 (yellow). The structures were aligned based on domain V of the 23S rRNA.
Extended Data Fig. 6 Macrolones inhibit DNA gyrase activity in vitro.
(a) Chemical structures of the macrolones used in the assay. (b) Effect of macrolones on the activity of DNA gyrase. Supercoiled or relaxed plasmid DNA bands are marked on the left. CIP and ERY were used as positive and negative controls, respectively. The sample labeled as ‘NONE’ contained no antibiotics. A control sample where gyrase was not added is marked as ‘-’.The experiment was repeated independently and produced similar results.
Extended Data Fig. 7 Electron density maps of A2058 nucleotide in Erm-modified and wild-type T. thermophilus 70S ribosome.
(a, c) Unbiased Fo-Fc (grey and green mesh) and (b, d) 2Fo-Fc (blue mesh) electron difference Fourier maps of nucleotide A2058 in the T. thermophilus 70S ribosome contoured at 3.0σ and 1.0σ, respectively. Grey mesh shows the Fo-Fc map after refinement with the entire modified nucleotide omitted. Green mesh, reflecting the presence of the two methyl groups at N6 position of the nucleobase, shows the Fo-Fc electron density map after refinement with the nucleotide A2058 built as a regular unmethylated adenine. The refined models of Erm-modified N6-dimethylated (a, b) or wild-type unmodified (c, d) nucleotide A2058 are displayed in the corresponding electron density maps. Both structures carry MCX-128 compound (not shown). Carbon atoms are colored dark blue for the Erm-modified A2058 and light blue for the unmodified A2058; nitrogens are dark blue; oxygens are red.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2, Note, Figs. 1–6 and References.
Source data
Source Data Fig. 2
Raw experimental data for Fig. 2a.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aleksandrova, E.V., Ma, CX., Klepacki, D. et al. Macrolones target bacterial ribosomes and DNA gyrase and can evade resistance mechanisms. Nat Chem Biol 20, 1680–1690 (2024). https://doi.org/10.1038/s41589-024-01685-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41589-024-01685-3
This article is cited by
-
Exploring GNRA tetraloop-like motifs in nucleic acid 3D structures
Scientific Reports (2025)
-
Selective silencing of antibiotic-tethered ribosomes as a resistance mechanism against aminoglycosides
Nature Communications (2025)
-
Structural insights into context-specific inhibition of bacterial translation by macrolides
Nature Communications (2025)
-
Evading resistance at the double
Nature Chemical Biology (2024)