Abstract
Pathogens and vaccines that produce persisting antigens can generate expanded pools of effector memory CD8+ T cells, described as memory inflation. While properties of inflating memory CD8+ T cells have been characterized, the specific cell types and tissue factors responsible for their maintenance remain elusive. Here, we show that clinically applied adenovirus vectors preferentially target fibroblastic stromal cells in cultured human tissues. Moreover, we used cell-type-specific antigen targeting to define critical cells and molecules that sustain long-term antigen presentation and T cell activity after adenovirus vector immunization in mice. While antigen targeting to myeloid cells was insufficient to activate antigen-specific CD8+ T cells, genetic activation of antigen expression in Ccl19-cre-expressing fibroblastic stromal cells induced inflating CD8+ T cells. Local ablation of vector-targeted cells revealed that lung fibroblasts support the protective function and metabolic fitness of inflating memory CD8+ T cells in an interleukin (IL)-33-dependent manner. Collectively, these data define a critical fibroblastic niche that underpins robust protective immunity operating in a clinically important vaccine platform.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
scRNA-seq data are available in the ArrayExpress database (accession numbers E-MTAB-9558 and E-MTAB-9580). Ensembl GRCm38.94 was used as a reference to build index files for alignments in scRNA-seq analysis. Further information and requests for resources should be directed to and will be fulfilled by the lead contacts B.L. (burkhard.ludewig@kssg.ch) and P. Klenerman (paul.klenerman@medawar.ox.ac.uk). Source data are provided with this paper.
References
Chang, J. T., Wherry, E. J. & Goldrath, A. W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 15, 1104–1115 (2014).
Priddy, F. H. et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin. Infect. Dis. 46, 1769–1781 (2008).
Stephenson, K. E. et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 325, 1535–1544 (2021).
Snook, A. E. et al. Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced T-cell responses in colon cancer patients. J. Immunother. Cancer 7, 104 (2019).
Ewer, K. et al. Chimpanzee adenoviral vectors as vaccines for outbreak pathogens. Hum. Vaccin. Immunother. 13, 3020–3032 (2017).
Folegatti, P. M. et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396, 467–478 (2020).
Ewer, K. J. et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 27, 270–278 (2021).
Barnes, E. et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 4, 115ra111 (2012).
Logunov, D. Y. et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397, 671–681 (2021).
Klenerman, P. The (gradual) rise of memory inflation. Immunol. Rev. 283, 99–112 (2018).
Karrer, U. et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 170, 2022–2029 (2003).
Sierro, S., Rothkopf, R. & Klenerman, P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur. J. Immunol. 35, 1113–1123 (2005).
Holtappels, R., Pahl-Seibert, M. F., Thomas, D. & Reddehase, M. J. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62Llo memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J. Virol. 74, 11495–11503 (2000).
Grzimek, N. K., Dreis, D., Schmalz, S. & Reddehase, M. J. Random, asynchronous, and asymmetric transcriptional activity of enhancer-flanking major immediate-early genes ie1/3 and ie2 during murine cytomegalovirus latency in the lungs. J. Virol. 75, 2692–2705 (2001).
Snyder, C. M. et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29, 650–659 (2008).
Komatsu, H., Sierro, S., Cuero, A. V. & Klenerman, P. Population analysis of antiviral T cell responses using MHC class I–peptide tetramers. Clin. Exp. Immunol. 134, 9–12 (2003).
Klenerman, P. & Oxenius, A. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 16, 367–377 (2016).
Bolinger, B. et al. A new model for CD8+ T cell memory inflation based upon a recombinant adenoviral vector. J. Immunol. 190, 4162–4174 (2013).
Lee, L. N. et al. Adenoviral vaccine induction of CD8+ T cell memory inflation: impact of co-infection and infection order. PLoS Pathog. 13, e1006782 (2017).
Bassett, J. D. et al. CD8+ T-cell expansion and maintenance after recombinant adenovirus immunization rely upon cooperation between hematopoietic and nonhematopoietic antigen-presenting cells. Blood 117, 1146–1155 (2011).
Krishnamurty, A. T. & Turley, S. J. Lymph node stromal cells: cartographers of the immune system. Nat. Immunol. 21, 369–380 (2020).
Perez-Shibayama, C., Gil-Cruz, C. & Ludewig, B. Fibroblastic reticular cells at the nexus of innate and adaptive immune responses. Immunol. Rev. 289, 31–41 (2019).
Pikor, N. B., Cheng, H. W., Onder, L. & Ludewig, B. Development and immunological function of lymph node stromal cells. J. Immunol. 206, 257–263 (2021).
Torti, N., Walton, S. M., Murphy, K. M. & Oxenius, A. Batf3 transcription factor-dependent DC subsets in murine CMV infection: differential impact on T-cell priming and memory inflation. Eur. J. Immunol. 41, 2612–2618 (2011).
Torti, N., Walton, S. M., Brocker, T., Rülicke, T. & Oxenius, A. Non-hematopoietic cells in lymph nodes drive memory CD8 T cell inflation during murine cytomegalovirus infection. PLoS Pathog. 7, e1002313 (2011).
Busche, A. et al. Priming of CD8+ T cells against cytomegalovirus-encoded antigens is dominated by cross-presentation. J. Immunol. 190, 2767–2777 (2013).
Baumann, N. S. et al. Tissue maintenance of CMV-specific inflationary memory T cells by IL-15. PLoS Pathog. 14, e1006993 (2018).
Rowe, W. P. Studies on pathogenesis and immunity in lymphocytic choriomeningitis infection of the mouse. Navy Res. Rep. 12, 167–220 (1954).
Atasoy, D., Aponte, Y., Su, H. H. & Sternson, S. M. A FLEX switch targets channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008).
Chai, Q. et al. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity 38, 1013–1024 (2013).
Cheng, H. W. et al. Origin and differentiation trajectories of fibroblastic reticular cells in the splenic white pulp. Nat. Commun. 10, 1739 (2019).
Cupovic, J. et al. Central nervous system stromal cells control local CD8+ T cell responses during virus-induced neuroinflammation. Immunity 44, 622–633 (2016).
Cheng, H. W. et al. CCL19-producing fibroblastic stromal cells restrain lung carcinoma growth by promoting local antitumor T-cell responses. J. Allergy Clin. Immunol. 142, 1257–1271 (2018).
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Krebs, P., Scandella, E., Odermatt, B. & Ludewig, B. Rapid functional exhaustion and deletion of CTL following immunization with recombinant adenovirus. J. Immunol. 174, 4559–4566 (2005).
Novkovic, M. et al. Topological small-world organization of the fibroblastic reticular cell network determines lymph node functionality. PLoS Biol. 14, e1002515 (2016).
Zepp, J. A. et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 170, 1134–1148 (2017).
Xie, T. et al. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep. 22, 3625–3640 (2018).
Tsukui, T. et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 11, 1920 (2020).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
van der Windt, G. J. et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl Acad. Sci. USA 110, 14336–14341 (2013).
Rego, G. N. A. et al. Current clinical trials protocols and the global effort for immunization against SARS-CoV-2. Vaccines 8, 474 (2020).
Mercado, N. B. et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586, 583–588 (2020).
Bolinger, B. et al. Adenoviral vector vaccination induces a conserved program of CD8+ T cell memory differentiation in mouse and man. Cell Rep. 13, 1578–1588 (2015).
Gordon, C. L. et al. Induction and maintenance of CX3CR1-intermediate peripheral memory CD8+ T cells by persistent viruses and vaccines. Cell Rep. 23, 768–782 (2018).
Cohen, E. S. et al. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat. Commun. 6, 8327 (2015).
Mager, L. F. et al. IL-33 signaling contributes to the pathogenesis of myeloproliferative neoplasms. J. Clin. Invest. 125, 2579–2591 (2015).
Dominguez, D. et al. Exogenous IL-33 restores dendritic cell activation and maturation in established cancer. J. Immunol. 198, 1365–1375 (2017).
Bonilla, W. V. et al. The alarmin interleukin-33 drives protective antiviral CD8+ T cell responses. Science 335, 984–989 (2012).
Baumann, C. et al. Memory CD8+ T cell protection from viral reinfection depends on interleukin-33 alarmin signals. Front. Immunol. 10, 1833 (2019).
McLaren, J. E. et al. IL-33 augments virus-specific memory T cell inflation and potentiates the efficacy of an attenuated cytomegalovirus-based vaccine. J. Immunol. 202, 943–955 (2019).
Pikor, N. B. et al. Remodeling of light and dark zone follicular dendritic cells governs germinal center responses. Nat. Immunol. 21, 649–659 (2020).
Camara, A. et al. Lymph node mesenchymal and endothelial stromal cells cooperate via the RANK–RANKL cytokine axis to shape the sinusoidal macrophage niche. Immunity 50, 1467–1481 (2019).
Silva-Sanchez, A. & Randall, T. D. Role of iBALT in respiratory immunity. Curr. Top. Microbiol. Immunol. 426, 21–43 (2020).
Dahlgren, M. W. et al. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity 50, 707–722 (2019).
Alharbi, N. K. et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine 35, 3780–3788 (2017).
Grivel, J. C. & Margolis, L. Use of human tissue explants to study human infectious agents. Nat. Protoc. 4, 256–269 (2009).
McCarthy, D. J., Campbell, K. R., Lun, A. T. & Wills, Q. F. Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 33, 1179–1186 (2017).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Acknowledgements
We thank S. Caviezel-Firner and C. Engetschwiler for excellent technical support. This study received financial support from the Swiss National Science Foundation (grants 166500 and 159188 to B.L.), Swiss Cancer Research (KFS-4162-02-2017-R to P. Krebs), the Wellcome Trust (109965MA to P. Klenerman) and research fellowship grants from the British Infection Association (to J.M.C.) and the Wellcome Trust (099897/Z/12/A to J.M.C.).
Author information
Authors and Affiliations
Contributions
B.L., P. Klenerman and J.C. designed the study, discussed data and wrote the paper. J.C., J.M.C., S.S.R., L.O., A.D.M., H.-W.C. and D.E. conducted experiments and discussed data. M.L. performed bioinformatic analyses and discussed data. N.M.P. discussed data and provided reagents. P. Krebs and A.O. discussed data and provided reagents. L.F. and E.S. discussed data.
Corresponding authors
Ethics declarations
Competing interests
L.F. is a cofounder and shareholder of Hookipa Pharma. B.L., L.O. and H.-W.C. are cofounders and shareholders of Stromal Therapeutics. S.S.R. and H.-W.C. are part-time employees of Stromal Therapeutics. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Immunology thanks Stephen Jameson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. L. A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Adenoviral vectors ChAdOx1 and HuAd5 transduce human fibroblasts.
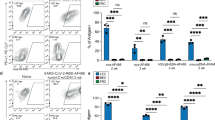
a, Preparation of sliced tissue cultures from human palatine tonsils following infection with adenoviral vectors. b, Flow cytometry-based gating strategy for PBMCs analysis. c and d, Frequency of CD3+ T cells, CD14+ HLA-DR+ monocytes or CD11c+ dendritic cells (DCs) within GFP+ cells following infection with (c) ChAdOx1-GFP or (d) HuAd5-GFP. e, Flow cytometry-based gating strategy for cultured tonsillar tissue stromal cell analysis. f and g, Frequencies of PDPN+ and CD31+ cells within GFP+ cultured tonsillar tissue stromal cells (TSC) following infection with (f) ChAdOx1-GFP or (g) HuAd5-GFP. h to m, Infection of cultured (h to j) skin- or (k to m) lung-derived stromal cells with ChAdOx1-GFP or HuAd5-GFP with representative FACS plots. (i and l) Frequency of GFP+ cells after infection with indicated adenoviral vectors at different multiplicity of infection (MOI). (j and m) Frequency of PDPN+ or CD31+ cells within GFP+ cells after infection with indicated adenoviral vectors. Pooled data from n = 3 PBMC samples [(c) and (d)] and n = 3 TSC samples [(f) and (g)]. Data from one experiment with single skin [(h-j)] or lung tissue donor [(k-m)]. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparison test [(c) and (d)] and unpaired two-tailored Student`s t test [(f) and (g)], with *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Exact P values are provided in the Source Data.
Extended Data Fig. 2 Induction of inflationary memory CD8+ T cells following Ad5-LacZ immunization.
a to c, B6 mice were immunized i.v. with Ad-LacZ. (a) Kinetics of the frequency of bgal96 or bgal497 tetramer+ CD8+ T cells in blood. Frequency of bgal96 or bgal497 tetramer+ CD8+ T cells in indicated organs on (b) day 21 or (c) day 50. d, Flow cytometry-based gating strategy for tetramer+ CD8+ T cell analysis. e, Ubi-Cre ERT2 mice were immunized i.v. with Ad-LacZ/FlexON. Frequency of bgal96 or bgal497 tetramer+ CD8+ T cells in blood after application of Tamoxifen. (f to g) LysM-Cre mice were immunized i.v. with Ad-LacZ/FlexON. f, Kinetics of bgal96 tetramer+ CD8+ T cell response with representative FACS plots. g, Kinetics of non-inflationary bgal497 tetramer+ CD8+ T cell response with representative FACS plots. Cre-negative mice were used as controls (Ctrl). Pooled data from 2 independent experiments with n = 4-8 mice [(a)]; n = 4 mice per group [(b) and (c)], representative of two experiments with n = 2 per group [(e)], n = 8 (LysM-Cre+) and n = 8 (Ctrl) [(f) and (g)]. Values indicate mean±s.e.m. for each time point. Statistical analysis was performed using two-way analysis of variance (ANOVA) with Bonferroni multiple comparison test [(f) to (g)] with *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Exact P values are provided in the Source Data.
Extended Data Fig. 3 Cross-talk between Ccl19-Cre+ cells and professional antigen presenting cells during the induction of LacZ-specific T cell responses.
The indicated bone marrow chimeric mice were generated and vaccinated i.v. with Ad-LacZ/FlexON. (a and b) The frequency of bgal497 tetramer+ CD8+ T cells was monitored in blood. Values indicate mean±s.e.m. for each time point. Pooled data from 2 independent experiments with n = 10 (Ctrl to Ccl19-Cre) to 8 (H2-Kb−/− to Ccl19-Cre) mice [(a)] and n = 5 (Ctrl to Ccl19-Cre) and 5 (Batf3−/− to Ccl19-Cre) mice [(b)]. Statistical analysis was performed using two-way analysis of variance (ANOVA) with Bonferroni multiple comparison test [(a) and (b)] *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Exact P values are provided in the Source Data.
Extended Data Fig. 4 4. Location of Ccl19-Cre+ cells that contribute to induction and maintenance of memory inflating CD8+ T cells.
a, Ccl19-EYFP mice were splenectomized and immunized i.v. with Ad-LacZ/FlexON. The frequency of tetramer+ CD8+ T cells was monitored in blood or the indicated organs. b to g, Ccl19-EYFP/iDTR mice were vaccinated i.v. with Ad-LacZ/FlexON and DT or PBS were given intranasal (in) or intraperitoneal (sys) on day 3 and 5. (c) Enumeration of EYFP+ cells in the lungs of day 7 Ad-LacZ/FlexON-vaccinated mice, following i.n. DT or PBS application. (d) Representative confocal microscopy image of EYFP+ FSCs in the liver and spleen after systemic or intranasal DT injection. Scale bar equals 30 μm. (e) Representative FACS plots of bgal96 tetramer+ CD8+ T cells in blood, lung, liver and spleen on day 21. (f) Frequency of bgal497 tetramer+ CD8+ T cells in the indicated organs. (g) Frequency of bgal497 tetramer+ CD8+ T cells in the blood at the indicated time points. h, Flow cytometry-based gating strategy for lung hematopoietic cells analysis used in Fig.4e. Dots represent individual mice. Bar graphs indicate mean ±s.e.m. Data from one [(a)] or pooled data from 2 independent experiments [(b) to (g)] with n = 2 mice per group [(a)]; n = 4 (DT intranasal) and n = 5 (PBS intranasal) [(c)]; representative data from n = 3 (DT systemic), n = 7 (DT intranasal) and n = 5 (PBS control) [(d)]; and n = 5 (8 blood) (DT systemic), n = 9 (DT intranasal) and n = 14 (PBS control) [(f)], n = 5 (days 7 and 50), 10 mice (day 14) and 8 (day 21) [(g)]. Statistical analysis was performed using two-way analysis of variance (ANOVA) with Bonferroni multiple comparison test [(a)], unpaired two-tailored Student`s t test [(c) and (g)] or one-way ANOVA with Tukey’s multiple comparison test [(f)] with *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Exact P values are provided in the Source Data.
Extended Data Fig. 5 Phenotypic changes in Ccl19-Cre+ cells after Ad-LacZ/FlexON immunization.
a to f, Ccl19-EYFP mice were immunized i.v. with Ad-LacZ/FlexON. On day 21 pulmonary FSCs were analyzed. (a) Gating for analysis of EYFP+ cells. (b to f) Representative FACS histograms with gMFI/MFI and frequencies of (b) PDPN+, (c) ICAM1+, (d) PDGFRa+, (e) PDGFRb+ or (f) VCAM1+ cells within EYFP+ cells. Dots represent individual mice and bar graphs indicate mean ±s.e.m. Pooled data from 2 independent experiments with n = 5 mice per group [(e)] and n = 9 mice per group [(f)]. Statistical analysis was performed using unpaired two-tailored Student`s t test [(e) and (f)] with *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Exact P values are provided in the Source Data.
Extended Data Fig. 6 Ad-LacZ/FlexON activates pulmonary Ccl19-expressing immune-stimulatory FSCs.
a, Averaged expression of cluster specific marker genes for pulmonary FSC clusters in merged naive and Ad-LacZ/FlexON-immunized Ccl19-EYFP mice. b, Heatmaps displaying indicated genes across all Lumhigh and Il33high FSCs in EYFP+ FSCs from Ad-LacZ/FlexON-immunized or naive mice. c, Prediction of differentiation dynamics based on RNA velocity analysis of EYFP+ FSCs constructed based on recent changes in the transcriptional rate of a gene to predict the future state of individual cells. d, Representative flow cytometric analysis of Sca-1+CD34+ cell populations within EYFP+ cells. e, Frequency Sca-1+ CD34+ cell within EYFP+ cells. f, Quantitative real-time PCR analysis of Il33 expression in pulmonary EYFP+ FSCs. g, Quantitative real-time PCR analysis of Il33 expression in EYFP+ pulmonary FSC populations. h, Quantitative real-time PCR analysis of LacZ mRNA copy numbers per 1,000 cells in the indicated EYFP+ pulmonary FSC populations. i, Reconstruction of IL-33 staining in lung EYFP+ cells of Ccl19-EYFP mice at indicated time points after Ad-LacZ/FlexON immunization, based on images shown in Fig. 5j. Scale bar equals 30 μm. Dots represent individual mice and bar graphs indicate mean ±s.e.m. Pooled data from two independent experiments with n = 8 naive and 9 AdLacZFlexON immunized [(e)]; n = 5 [(f)], n = 6 [(h)] mice; one experiment n = 6 mice [(g)]. Representative data from n = 4 mice per group (i). Statistical analysis was performed using unpaired two-tailored Student’s t test [(e)], Mann Whitney [(f), (g)], one-way ANOVA with Turkey`s multiple comparison test [(h)] with *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Exact P values are provided in the Source Data.
Extended Data Fig. 7 Bronchus-associated lymphoid tissues in the lungs of Ad-LacZ/FlexON immunized Ccl19-EYFP mice.
a, Overview image of three lung lobes (Sup, superior; Inf, inferior; Mid, middle) of the right lung. Dashed line in the schematic indicates approximate sagittal plane of sectioning. Arrowheads indicate lymphoid cell aggregates in the hilus region. Scale bar equals 2 mm. b, Images show hilus regions in lungs of Ccl19-EYFP mice. Scale bar equals 100 μm. Representative of 2 independent experiments, n = 4 mice per condition.
Extended Data Fig. 8 Pulmonary FSC-derived IL-33 preserves metabolic and functional fitness of inflationary memory CD8+ T cells.
(a to d, Ccl19-Cre-EYFP/iDTR mice were immunized i.v. with Ad-LacZ/FlexON and DT or PBS was given intranasally on day 3 and 5. Single cell RNA-seq analysis of bgal96 tetramer+ CD8+ T cells isolated from lungs on day 21. (a) Network plots displaying most significantly enriched gene ontologies based on transcriptional differences in bgal96 tetramer+ CD8+ T cells from DT vs PBS treated Ccl19-Cre/iDTR EYFP mice and with enriched genes annotated. (b) Violin plots show expression of Atp5b, Cox5a, Cox8a, Tomm20. (c) Representative FACS histograms of Ki67 staining on pulmonary bgal96 tetramer+ CD8+ T cells (left) and frequency of Ki67+ bgal96-specific CD8+ T cells (right). (d) Representative FACS dot-plot of live-dead stain negative pulmonary bgal96 tetramer+ CD8+ T cells (left) and frequency of Aqua-negative bgal96-specific CD8+ T cells (right). e, Quantitative real-time PCR analysis of Sdha, Uqcrc2 and Cox4i2 expression in bgal96-specifc CD8+ T cells sorted from the lung of Ccl19-Cre+ Il33fl/fl and Ccl19-Cre+ Il33+/+ mice, day 21 after Ad-LacZ/FlexON immunization. Dots represent individual mice. Bar graphs indicate mean ±s.e.m. (c-e) Pooled data from 2 independent experiments with n = 8 mice per group [(c)]; n = 7 mice [(d)]; and n = 4 mice [(e)]. Statistical analysis was conducted using unpaired two-tailored Student’s t test with *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Exact P values are provided in the Source Data.
Extended Data Fig. 9 Kinetics and tissue distribution of inflationary memory CD8+ T cells under conditions of Ccl19-Cre+ lung FSC depletion and Il33-ablation in Ccl19-Cre+ cells.
Ccl19-Cre-EYFP/iDTR mice and Ccl19-Cre Il33fl/fl mice (Il33fl/fl) were immunized i.v. with Ad-LacZ/FlexON and CD8+ T cells were analyzed on day 21. Ccl19-Cre-EYFP/iDTR mice were treated i.n. with DT or PBS on day 3 and 5 after immunization. a, Number of bgal96 tetramer+ CD8+ T cells in lung and spleen. b, Frequency of bgal497 tetramer+ CD8+ T cells in blood, lung and spleen. c, Frequency of KLRG1+/CX3CR1+ bgal497 tetramer+ CD8+ T cells in the lungs. d, IFN-g- and TNF-producing pulmonary bgal497-specific CD8+ T cells. Dots represent individual mice. Bar graphs indicate mean ±s.e.m. Pooled data from 2 independent experiments with n = 9 (DT intranasal), n = 9 (Ccl19-Cre Il33fl/fl) and n = 19 (Ccl19-EYFP/iDTR PBS i.n. and Ccl19-Cre Il33+/+) mice [(a)]; n = 9 (DT intranasal), n = 8 (Ccl19-Cre Il33fl/fl) and n = 19 (Ccl19-EYFP/iDTR PBS i.n. and Ccl19-Cre Il33+/+) mice [(b)]; n = 6 (DT intranasal), n = 9 (Ccl19-Cre Il33fl/fl) and n = 16 (Ccl19-EYFP/iDTR PBS i.n. and Ccl19-Cre Il33+/+) mice [(c)]; n = 7 (DT intranasal), n = 8 IFNg, n = 6 TNF (Ccl19-Cre Il33fl/fl) and n = 18 (Ccl19-EYFP/iDTR PBS i.n. and Ccl19-Cre Il33+/+) mice [(d)]. Statistical analysis was conducted using one-way ANOVA with Tukey’s multiple comparison test with *P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001. Exact P values are provided in the Source Data.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
About this article
Cite this article
Cupovic, J., Ring, S.S., Onder, L. et al. Adenovirus vector vaccination reprograms pulmonary fibroblastic niches to support protective inflating memory CD8+ T cells. Nat Immunol 22, 1042–1051 (2021). https://doi.org/10.1038/s41590-021-00969-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-021-00969-3
This article is cited by
-
Mucosal T-cell responses to chronic viral infections: Implications for vaccine design
Cellular & Molecular Immunology (2024)
-
Granzyme B + CD8 + T cells with terminal differentiated effector signature determine multiple sclerosis progression
Journal of Neuroinflammation (2023)
-
PI16+ reticular cells in human palatine tonsils govern T cell activity in distinct subepithelial niches
Nature Immunology (2023)
-
The advent of immune stimulating CAFs in cancer
Nature Reviews Cancer (2023)
-
Intestinal fibroblastic reticular cell niches control innate lymphoid cell homeostasis and function
Nature Communications (2022)