Abstract
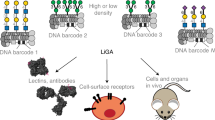
Glycans constitute a significant fraction of biomolecular diversity on cellular surfaces across all kingdoms of life. As the structure of glycans is not directly encoded by the organism’s DNA, it is impossible to use high-throughput DNA technologies to study the role of cellular glycosylation or to understand how glycocalyx is recognized by glycan-binding proteins (GBPs). To address this gap, we recently described a liquid glycan array (LiGA) platform that allows profiling of glycan–GBP interactions on the surface of live cells in vitro and in vivo using next-generation sequencing. LiGA is a library of DNA-barcoded bacteriophages, where each clonal bacteriophage displays 5–1,500 copies of a glycan and the distinct DNA barcode inside each bacteriophage clone encodes the structure and density of the displayed glycans. Deep sequencing of the glycophages associated with live cells yields a glycan-binding profile of GBPs expressed on the surface of cells. This protocol provides detailed instructions for how to use LiGA to probe cell surface receptors and includes information on the preparation of glycophages, analysis by MALDI–TOF mass spectrometry, the assembly of a LiGA library and its deep sequencing. Using this protocol, we measure glycan-binding profiles of the immunomodulatory sialic acid-binding immunoglobulin-like lectins‑1, -2, -6, -7 and -9 expressed on the surface of different cell types. Compared with existing methods that require complex specialist equipment, this method allows users with basic molecular biology expertise to measure the precise glycan-binding profile of GBPs on the surface of any cell type expressing exogenous GBP within 2–3 d.
Key points
-
This protocol describes the preparation of a liquid glycan array (LiGA) platform, a library of DNA-barcoded bacteriophages displaying 5–1,500 copies of a glycan. Deep sequencing of the glycophages associated with live cells yields a glycan-binding profile of GBPs displayed on the surface of cells.
-
The development of this technology enables testing of the biological role of multivalent glycan–lectin interactions in a multiplexed fashion, which was not previously possible.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data relating to differential enrichment analysis are submitted as source data. All raw deep-sequencing data are publicly available in a searchable format on https://48hd.cloud/; for example, search ‘EF Sig9 jurkat’ to obtain raw sequencing data of LiGA EF post panning against Siglec-9+ Jurkat cells. Additionally, raw deep-sequencing data can also be requested from the corresponding author. DNA sequences of the three LiGA phage constructs with the reporter genes LacZ, mNeonGreen and mCherry have been deposited to GenBank (MN865131, MN865132, MN872303). Source data are provided with this paper.
Code availability
MATLAB and R scripts used to generate MALDI–TOF spectra and differential enrichment analysis, respectively, have been deposited to https://github.com/derdalab/liga.
References
Oyelaran, O. & Gildersleeve, J. C. Glycan arrays: recent advances and future challenges. Curr. Opin. Chem. Biol. 13, 406–413 (2009).
Cobb, B. A. & Kasper, D. L. Coming of age: carbohydrates and immunity. Eur. J. Immunol. 35, 352–356 (2005).
Smith, B. A. H. & Bertozzi, C. R. The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 20, 217–243 (2021).
Blixt, O. et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl Acad. Sci. USA 101, 17033–17038 (2004).
Razi, N. & Varki, A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc. Natl Acad. Sci. USA 95, 7469–7474 (1998).
Collins, B. E. et al. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc. Natl Acad. Sci. USA 101, 6104–6109 (2004).
Kiessling, L. L. & Grim, J. C. Glycopolymer probes of signal transduction. Chem. Soc. Rev. 42, 4476–4491 (2013).
Frenz, T. et al. Antigen presenting cell-selective drug delivery by glycan-decorated nanocarriers. Eur. J. Pharm. Biopharm. 95, 13–17 (2015).
Alam, M. M. et al. Glycan-modified virus-like particles evoke T helper type 1-like immune responses. Acs Nano 15, 309–321 (2021).
Sojitra, M. et al. Genetically encoded multivalent liquid glycan array displayed on M13 bacteriophage. Nat. Chem. Biol. 17, 806–816 (2021).
Tjhung, K. F. et al. Silent encoding of chemical post-translational modifications in phage-displayed libraries. J. Am. Chem. Soc. 138, 32–35 (2016).
Lin, C.-L. et al. Chemoenzymatic synthesis of genetically-encoded multivalent liquid N-glycan arrays. Nat. Commun. 14, 5237 (2023).
Thomas, B. et al. Application of biocatalysis to on-DNA carbohydrate library synthesis. ChemBioChem 18, 858–863 (2017).
Yan, M. et al. Next-generation glycan microarray enabled by DNA-coded glycan library and next-generation sequencing technology. Anal. Chem. 91, 9221–9228 (2019).
Kondengaden, S. M. et al. DNA encoded glycan libraries as a next-generation tool for the study of glycan–protein interactions. Preprint at bioRxiv https://doi.org/10.1101/2020.03.30.017012 (2020).
Schmidt, E. N. et al. Siglec-6 mediates the uptake of extracellular vesicles through a noncanonical glycolipid binding pocket. Nat. Commun. 14, 2327 (2023).
Crocker, P. R., Paulson, J. C. & Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 (2007).
Nicoll, G. et al. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via Siglec-7-dependent and -independent mechanisms. Eur. J. Immunol. 33, 1642–1648 (2003).
Jandus, C. et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J. Clin. Invest. 124, 1810–1820 (2014).
Shenoy, G. N. et al. Sialic acid-dependent inhibition of T cells by exosomal ganglioside GD3 in ovarian tumor microenvironments. J. Immunol. 201, 3750–3758 (2018).
Laubli, H., Nalle, S. C. & Maslyar, D. Targeting the Siglec–sialic acid immune axis in cancer: current and future approaches. Cancer Immunol. Res. 10, 1423–1432 (2022).
Gray, M. A. et al. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat. Chem. Biol. 16, 1376–1384 (2020).
Gonzalez-Gil, A., Li, T. A., Kim, J. & Schnaar, R. L. Human sialoglycan ligands for immune inhibitory Siglecs. Mol. Asp. Med. 90, 101110 (2023).
Jung, J. et al. Carbohydrate sulfation as a mechanism for fine-tuning Siglec ligands. ACS Chem. Biol. 16, 2673–2689 (2021).
Feinberg, H., Castelli, R., Drickamer, K., Seeberger, P. H. & Weis, W. I. Multiple modes of binding enhance the affinity of DC-SIGN for high mannose N-linked glycans found on viral glycoproteins. J. Biol. Chem. 282, 4202–4209 (2007).
Matochko, W. L., Cory Li, S., Tang, S. K. Y. & Derda, R. Prospective identification of parasitic sequences in phage display screens. Nucleic Acids Res. 42, 1784–1798 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Chen, Y., Lun, A. & Smyth, G. From reads to genes to pathways: differential expression analysis of RNA-seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Research 5, 1438 (2016).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Rodrigues, E. et al. A versatile soluble Siglec scaffold for sensitive and quantitative detection of glycan ligands. Nat. Commun. 11, 5091 (2020).
McCord, K. A. et al. Dissecting the ability of Siglecs to antagonize Fcγ receptors. ACS Cent. Sci. 10, 315–330 (2024).
Zeng, F. Y. & Gabius, H. J. Sialic acid-binding proteins: characterization, biological function and application. Z. Naturforsch. C. J. Biosci. 47, 641–653 (1992).
Collins, B. E. et al. Binding specificities of the sialoadhesin family of I-type lectins—sialic acid linkage and substructure requirements for binding of myelin-associated glycoprotein, Schwann cell myelin protein, and sialoadhesin. J. Biol. Chem. 272, 16889–16895 (1997).
Ishida, H. & Kiso, M. Synthetic study on neural Siglecs ligands: systematic synthesis of alpha-series polysialogangliosides and their analogues. J. Synth. Org. Chem. Jpn 58, 1108–1113 (2000).
Chen, W. C. et al. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood 115, 4778–4786 (2010).
Purohit, S. et al. Multiplex glycan bead array for high throughput and high content analyses of glycan binding proteins. Nat. Commun. 9, 258 (2018).
Dixit, A. et al. Perturb-seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 167, 1853–1866 (2016).
Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017).
Blair, J. D. et al. Phospho-seq: integrated, multi-modal profiling of intracellular protein dynamics in single cells. Preprint at bioRxiv https://doi.org/10.1101/2023.03.27.534442 (2023).
Celik, E. et al. Glycoarrays with engineered phages displaying structurally diverse oligosaccharides enable high-throughput detection of glycan–protein interactions. Biotechnol. J. 10, 199–209 (2015).
Celik, E., Fisher, A. C., Guarino, C., Mansell, T. J. & DeLisa, M. P. A filamentous phage display system for N-linked glycoproteins. Protein Sci. 19, 2006–2013 (2010).
Kaltgrad, E. et al. On-virus construction of polyvalent glycan ligands for cell-surface receptors. J. Am. Chem. Soc. 130, 4578–4579 (2008).
Polonskaya, Z. et al. T cells control the generation of nanomolar-affinity anti-glycan antibodies. J. Clin. Invest. 127, 1491–1504 (2017).
Raja, K. S., Wang, Q. & Finn, M. G. Icosahedral virus particles as polyvalent carbohydrate display platforms. ChemBioChem 4, 1348–1351 (2003).
Cairo, C. W., Gestwicki, J. E., Kanai, M. & Kiessling, L. L. Control of multivalent interactions by binding epitope density. J. Am. Chem. Soc. 124, 1615–1619 (2002).
Kearney, C. J. et al. SUGAR-seq enables simultaneous detection of glycans, epitopes, and the transcriptome in single cells. Sci. Adv. 7, eabe3610 (2021).
Minoshima, F., Ozaki, H., Odaka, H. & Tateno, H. Integrated analysis of glycan and RNA in single cells. iScience 24, 102882 (2021).
Odaka, H., Ozaki, H. & Tateno, H. scGR-seq: integrated analysis of glycan and RNA in single cells. STAR Protoc. 3, 101179 (2022).
Ng, S., Jafari, M. R., Matochko, W. L. & Derda, R. Quantitative synthesis of genetically encoded glycopeptide libraries displayed on M13 phage. ACS Chem. Biol. 7, 1482–1487 (2012).
Chou, Y. et al. Genetically-encoded fragment-based discovery (GE-FBD) of glycopeptide ligands with differential selectivity for antibodies related to mycobacterial infections. Org. Biomol. Chem. 16, 223–227 (2018).
Ng, S. et al. Genetically-encoded fragment-based discovery of glycopeptide ligands for DC-SIGN. Bioorg. Med. Chem. 26, 5368–5377 (2018).
Krag, D. N. et al. Selection of tumor-binding ligands in cancer patients with phage display libraries. Cancer Res. 66, 7724–7733 (2006).
Aida, Y. & Pabst, M. J. Removal of endotoxin from protein solutions by phage-seperation using triton X-114. J. Immunol. Methods 132, 191–195 (1990).
Adam, O., Vercellone, A., Paul, F., Monsan, P. F. & Puzo, G. A nondegradative route for the removal of endotoxin from exopolysaccharides. Anal. Biochem. 225, 321–327 (1995).
Crimmins, D. L., Mische, S. M. & Denslow, N. D. Chemical cleavage of proteins in solution. Curr. Protoc. Protein Sci. 11, 11.4.1–11.4.11 (2005).
He, B. F. et al. Compositional bias in naive and chemically-modified phage-displayed libraries uncovered by paired-end deep sequencing. Sci. Rep. 8, 1214 (2018).
Acknowledgements
We thank the staff at the University of Alberta mass spectrometry facility (chemistry department) for help with MALDI analysis and S. Dang at the molecular biology service unit for assistance with Illumina sequencing. We acknowledge funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) (RGPIN-2018-03815 to M.S.M. and RGPIN-2016-402511 to R.D.), the NSERC Accelerator Supplement (to R.D.), NSERC (RGPIN-2022-04484 to R.D.), Canadian Institutes of Health Research (CIHR) (no. 180445 to R.D.), GlycoNet (CR-29 and TP−22 to R.D.), and the Alberta Innovates Strategic Research Project to R.D. Infrastructure support was provided by the Canada Foundation for Innovation New Leader Opportunity (to R.D. and M.S.M.). Many compounds were prepared by the Consortium for Functional Glycomics, supported by National Institutes of Health (NIH) GM061126. G.M.L. acknowledges funding from Alberta Innovates Graduate Student Scholarship.
Author information
Authors and Affiliations
Contributions
M.S. performed modifications of phages by glycans, MALDI analysis and cell binding assays. G.M.L. and A.A. optimized the cell binding assay. E.N.S., K.A.M. and M.S.M. generated the Siglec expressing cell lines. M.S., E.J.C. and R.D. performed statistical analysis and wrote software in R and MATLAB. R.D. and M.S. wrote the manuscript. R.D. and M.S.M. edited the final manuscript and contributed intellectual and strategic input. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
R.D. is a shareholder of the start-up company 48Hour Discovery Inc. that licensed the patent application (WO2018141058A1) describing LiGA technology. The other authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Zheng Li, Ruijun Tian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Sojitra, M. et al. Nat. Chem. Biol. 17, 806–816 (2021): https://doi.org/10.1038/s41589-021-00788-5
Lin, C.-L. et al. Nat. Commun. 14, 5237 (2023): https://doi.org/10.1038/s41467-023-40900-y
Schmidt, E. N. et al. Nat. Commun. 14, 2327 (2023): https://doi.org/10.1038/s41467-023-38030-6
Extended data
Extended Data Fig. 1 Describes the workflow for generating the encoding glycan using SDB M13 phage.
a) The M13KE SDB SVEK library is a combination of two degenerate codon regions in the phage DNA: SB1 and SB2. The combination of SB1 and SB2 yields a total of 1.3 × 1010 DNA possible barcoded phages that are phenotypically identical. b) The M13KE SDB SVEK library was plated at ~100 plaques per plate. Each plaque (clone) was isolated, individually amplified, and purified with Triton X-100 and PEG to remove lipopolysaccharides. c) Schematic showing unmodified phage, DBCO-modified phage, and phage with azido-glycan. d) Workflow for modification of clonal phage with a distinct barcode. First, the phage is reacted with DBCO-NHS and verified by MALDI-TOF. Azide glycan is ligated with the DBCO on the phage. e) Typical MALDI-TOF spectra of unmodified phage, phage modified with DBCO, and after cycloaddition of azide glycan.
Extended Data Fig. 2 Optimizing LiGA binding, washing, elution, and PCR.
a) Incubation at 37 °C consistently showed higher amounts of eluted phage particles, n=3. b) Titer results describe the binding of a specific reporter phage decorated with DC-SIGN binding glycan (αMan-green) over multiple washes, n=3. Also shown are lactose (red), blank phage (white) and a pool of glyco-phages (LiGA YY-blue). c) RNase I treatment to degrade cellular RNA. d) The amount of phage eluted was consistently higher with Proteinase K treatment compared to boiling the cell pellet. e) Gel describes a one-step and two-step PCR product. Lanes 1 and 16 are DNA 50 bp ladder. Lanes 2-9 are PCR of phage sample in decreasing concentration of phage from 108 to 101 PFU using NF10 and -96 primers. Lanes 10-18 are PCR of phage sample in decreasing concentration of phage from 108 to 101 PFU using F1 and R1 primers. f) PCR using no-overhang primers annealing to regions outside of the SB1 and SB2 regions. g) Second PCR appends the Illumina adapters and multiplexing barcodes to the PCR product from the first step (f). h) Sequence of the final DNA fragment. All respective n values are independent biological replicates.
Extended Data Fig. 3 LiGA profiling of hSiglec-9 on the surface of U937 and CHO cells.
a) Visual representation of the glycans in the LiGA. b) Siglec-9 expressing U937 cells with LiGA-100 enriched broad range of sialylated glycans, n = 5 test and n = 5 control samples. c) Siglec-9 expressing CHO cells showed a higher level of enrichment of the sialylated glycans and minor preference for a2-6 linked and poly-sialylated glycans, n = 5 test and n = 10 control samples. All respective n values are independent biological replicates. In b,c, the FC was calculated by Bioconductor edgeR DE analysis using the negative binomial model, TMM normalization, and BH correction for FDR. Error bars represent s.d. propagated from the variance of the TMM-normalized sequencing data. Red bars indicate glycans with non-background responses in the CHO cells but not in the U937 cells.
Extended Data Fig. 4 Arginine mutant of Siglec -7 -9 and hCD22.
Data mirrored from Fig. 3for better visualization. a) Visual representation of glycans in the library. b) Siglec-7 R124A showed a lower enrichment compared to the wild-type Siglec on CHO cells binding, n = 10 test and n = 15 control samples. c) Siglec-9 R120A binding to LiGA, n = 10 test and n = 15 control samples. d) hCD22 R120A mutant showed a lack of binding, n = 5 test and n = 15 control samples. All respective n values are independent biological replicates. In b–d, the FC was calculated by Bioconductor edgeR DE analysis using the negative binomial model, TMM normalization, and BH correction for FDR (n= 5 for each cell type). Error bars represent s.d. propagated from the variance of the TMM-normalized sequencing data.
Extended Data Fig. 5 LiGA binding to purified Siglec-6 and Siglec-6+ CHO cells.
a) Visual representation of the glycans in the LiGA. b) The binding pattern of LiGA on Siglec-6 expressing CHO cells showed no observable binding pattern, n = 5 test and n = 4 control samples. c) Binding of LiGA-100 purified Siglec 6 coated in a well, n = 5 test and n = 11 control samples. d) Purified Siglec-6 R122A mutant binding with LiGA-100. e) Differential enrichment of Siglec-6 compared to R122A mutant, n = 6 test and n = 11 control samples. The AzOH enrichment shows non-specific interaction among LiGA-100. All respective n values are independent biological replicates. In b–e, the FC was calculated by Bioconductor edgeR DE analysis using the negative binomial model, TMM normalization, and BH correction for FDR. Error bars represent s.d. propagated from the variance of the TMM-normalized sequencing data.
Supplementary information
Supplementary Information
Supplementary Note 1, Method 1, Tables 1–9 and Figs. 1 and 2.
Supplementary Data
1. Folder ‘Comparison Campaigns’. Comparison campaigns in csv format. 2. Folder ‘Dictionaries’. Contains excel files of LIGA mixtures, which provide the 1:1 correspondence between DNA sequence and glycan for glycan–phages. Each file represents a library of glycan–phage conjugates. 3. Folder ‘Differential Enrichment files’. Contains files of enrichment analysis between GBP+ and GBP−. 4. Folder ‘Differential-testing_Scripts’. Contains files and folders related to the differential enrichment pipeline. The analysis software is written in R programming language. 5. Folder ‘Maldi’. Contains information about each glycan–phage conjugate used in this manuscript. MATLAB script to analyze MALDI–TOF spectra. MALDI.pdf, which contains spectra for all the glycophages used in the manuscript. 6. Supplementary Data for Supplementary Table 1.
Source data
Source Data Fig. 3
Differential enrichment analysis of sequencing data. Each panel’s source data is tab-separated in the excel file.
Source Data Fig. 4
Differential enrichment analysis of sequencing data. Each panel’s source data is tab-separated in the excel file.
Source Data Fig. 5
Differential enrichment analysis of sequencing data. Each panel’s source data is tab-separated in the excel file.
Source Data Extended Data Fig. 1
Unprocessed gel for panel e.
Source Data Extended Data Fig. 1
Plaque count used to generate data for panels a,b.
Source Data Extended Data Fig. 3
Differential enrichment analysis of sequencing data. Each panel’s source data is tab-separated in the excel file.
Source Data Extended Data Fig. 4
Differential enrichment analysis of sequencing data. Each panel’s source data is tab-separated in the excel file.
Source Data Extended Data Fig. 5
Differential enrichment analysis of sequencing data. Each panel’s source data is tab-separated in the excel file.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sojitra, M., Schmidt, E.N., Lima, G.M. et al. Measuring carbohydrate recognition profile of lectins on live cells using liquid glycan array (LiGA). Nat Protoc 20, 989–1019 (2025). https://doi.org/10.1038/s41596-024-01070-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-024-01070-3