Abstract
Despite advances in neonatal and ophthalmological care, retinopathy of prematurity (ROP) continues to be a leading cause of childhood blindness worldwide. Investigating gene variants associated with vascular responses in ROP may provide valuable insights into its pathogenesis and identify risk or protective factors. Nitric oxide (NO) and endothelin-1 (ET-1) play roles in vascular regulation, influencing processes relevant to ROP development. Functional variants of genes encoding endothelial NO sythetase (NOS3 rs1799983, rs2070744), endothelin-1 (EDN1 rs5370), and endothelin receptor A (EDNRA rs5335) may influence ROP development or progression. The results of our study support the role of the rs2070744 variant in ROP. We identified the protective effect of the rs2070744C allele against the development of ROP requiring treatment, also after adjusting for covariates. Meta-analysis including 298 patients and 397 controls confirmed this protective role. The rs2070744CC homozygous genotype exhibited an odds ratio (OR) of 0.42 (adjusted P = 0.036). Additional meta-analysis results for NOS3 rs1799983 are presented, suggesting potential risk in a recessive model. No associations were found between EDN1, EDNRA variants, and ROP. Exploring genetic predispositions in ROP, including vascular regulation genes, can lead to personalized prevention and treatment approaches. Our results need to be replicated in a larger sample of premature infants.
Similar content being viewed by others
Introduction
Since its initial description by Terry in 1942, retinopathy of prematurity (ROP) continues to be a major cause of childhood visual impairment and blindness worldwide. ROP is characterized as a proliferative vascular disorder affecting the immature retina, which can progress to retinal detachment in severe cases. While it is widely recognized that low gestational age (GA) and birth weight (BW), along with prolonged oxygen supplementation, are significant risk factors for ROP, the exact mechanism of ROP development remains incompletely understood1.
The pathogenesis of ROP is multifactorial and includes genetic and environmental factors affecting hormones and growth factors that regulate the development of retinal blood vessels1,2. Among these factors, vascular endothelial growth factor (VEGF) is considered the most critical pro-angiogenic molecule, playing a key role in promoting the pathological neovascularization in ROP. Other factors include mediators of its interactions produced by endothelial cells, i.e. vasodilators (nitric oxide, NO) and vasoconstrictors (endothelin 1, ET-1)3,4. They interact during the two-step process of ROP development. After preterm birth, newborns are exposed to rapid changes in oxygen levels, leading to the production of reactive oxygen species that can impair the development of retinal blood vessels. This can initiate the vaso-obliterative phase of ROP, characterized by the suppression of new vessel formation. The inadequate vascularization of the retina results in hypoxia, which then triggers the pathologic neovascularization seen in the second phase of ROP.
Regular ophthalmological examinations are crucial for the early detection and management of ROP in preterm infants. However, it must be acknowledged that in some patients, despite screening and prompt treatment, the disease still progresses to severe stages, while in other patients with similar clinical characteristics, spontaneous regression occurs. This observation indicates the importance of genetic risk risk factors.
The role of NO signaling in the pathogenesis of ROP was demonstrated by RNA transcriptome studies in an animal model of this disease3. This study revealed that NO is crucial in mediating the angiogenic effects of VEGF by activating VEGF receptor 2 and initiating downstream signaling pathways. The activation of endothelial nitric oxide synthase (eNOS, also known as NOS3) was found to modulate NO signaling and thereby influence the progression of ROP4. Endothelial-derived NO acts as a vasodilator, promoting smooth muscle relaxation, enhancing blood flow, and regulating vascular tone. Conversely, endothelin 1 (ET-1), which is predominantly synthesized in endothelial cells, exhibits vasoconstrictive properties and is recognized as one of the most potent vasoconstrictors. The vascular effects of endothelin are mediated through various receptors, including endothelin receptor A (EDNRA), primarily located in vascular smooth muscle cells. Binding of ET-1 to EDNRA leads to increased vasoconstriction. An imbalance between NO and ET-1 is a characteristic feature of endothelial dysfunction5, which may contribute to the development of retinal vascular abnormalities in premature infants. Consequently, functional variants in the NOS3 gene, as well as in the genes encoding endothelin-1 (EDN1) and endothelin receptor A (EDNRA), have the potential to affect angiogenesis and play a role in the pathogenesis of ROP.
The human NOS3 gene is located on chromosome 7q35-36 and has several single nucleotide variants that can potentially impact the concentration or function of the encoded protein. Among them, the rs2070744 (− 786T > C) variant in the promoter region and the rs1799983 (896G > T; Glu298Asp) variant in the coding region have been extensively investigated6. The human EDN1 gene is located on chromosome 6p23-p247 and harbors the rs5370 (5665G > T, Lys198Asn) variant in the coding region, which has been shown to influence protein function8. The EDNRA gene, located on chromosome 4q31.22-q31.23, has the rs5335 (+ 70 C > G) variant within the 3′-untranslated region, which has been identified as affecting protein levels9,10.
Previous studies, including our preliminary analysis in a small cohort of 90 premature infants, with 14 advanced ROP cases, have reported a potential association between the NOS3 rs1799983 and rs2070744 variants and ROP11,12. The existing evidence remains inconclusive, however, because the results were obtained in analyses of relatively small groups of infants with ROP. Additionally, EDN1 and EDNRA variants have not been previously studied in the context of ROP.
In the present study, we hypothesized that selected variants of genes encoding endothelial NO synthetase (NOS3 rs1799983, rs2070744), endothelin-1 (EDN1 rs5370) and endothelin receptor A (EDNRA rs5335), either individually or in combination, are associated with ROP in preterm Caucasian infants. We conducted also a meta-analysis of all relevant studies to further investigate the role of the NOS3 gene variants in the occurrence and progression of ROP.
Results
Known risk factors and comorbidities
Table 1 provides detailed characteristics of the 285 premature infants included in the study. Among the group, 157 were males (55.1%). The mean GA of the study group was 27.6 weeks (range 22–33 weeks) and the mean BW was 1109.2 g (range 432–2 340 g). Based on the results of retinal screening examinations, the infants were divided into two groups: the control group (preterm infants without ROP; n = 105) and the ROP group (n = 180; 63.2%; 354 eyes). Within the ROP group, half of the cases consisted of infants who experienced spontaneous regression of ROP (31.6% of the total; 174 eyes), while the other half were infants with proliferative ROP requiring treatment (31.6% of the total; 180 eyes), including laser photocoagulation (n = 80), anti-VEGF injection (n = 5), or a combination of both methods (n = 5).
The incidence of ROP showed a negative correlation with GA (P < 0.001) and BW (P < 0.0001), but intrauterine hypotrophy seems to be a selective risk factor for ROP undergoing spontaneous remission. Additionally, there were significant associations between ROP development and Apgar scores at 1 min (P < 0.0001) and 5 min (P < 0.0001) after birth as well as with a number of red blood cell (RBC) transfusions (P < 0.0001). Significant associations were also observed between ROP development and parameters related to respiratory failure, such as surfactant treatment (P < 0.0001) and mechanical ventilation (P < 0.0001). The diagnosis of ROP was more frequent in patients who also developed other complications of prematurity, including necrotizing enterocolitis (NEC), patent ductus arteriosus (PBD), intraventricular hemorrhage (IVH) (all associations with P < 0.0001), respiratory distress syndrome (RDS) (P = 0.003), and diffuse white matter injury (DWMI, P = 0.016).
Genotypes and ROP in the studied population
We observed that the frequencies of all studied variants were consistent with Hardy–Weinberg equilibrium (HWE) (PHWE >0.05; Table 2), and significant linkage disequilibrium (LD) was detected between studied NOS3 variants (r2 = 0.153; P < 0.0001). Univariate analysis revealed an association between the NOS3 gene and the development of ROP requiring treatment.
NOS3 rs2070744 variant
A lower prevalence of the rs2070744C allele was observed in cases with ROP requiring treatment (0.311 vs. 0.410 in the rest of infants; P = 0.023), accompanied by a 67% reduction in the risk of treatment-requiring ROP in rs2070744CC homozygotes (odds ratio [OR] = 0.34; 95% confidence intervals [CI] 0.15–0.79, P = 0.009) and a 74% decrease in the risk in rs2070744CC/rs1799983GG + GT genotype carriers (OR = 0.34; 95% CI: 0.15–0.79, P = 0.009). To adjust for co-variates, risk factors and comorbidities relevant to ROP progression in the univariate analysis (Table 1) were selected for multivariate analysis (summarized in Table 2, footnote). In these analyses, the best-fitting models were selected to account for independent risk factors. In the first model, the following variables were analyzed: GA, GE < 28 weeks, BW, BW < 1000 g, Apgar at 1 min, Apgar at 1 min, number of RBC transfusions, surfactant treatment, resuscitation and mechanical ventilation. The final model includes: GA, BW < 1000 g, Apgar score at 1st minute, number of RBC transfusions, mechanical ventilation and surfactant treatment. It explained 78.7% of ROP progression. Both genotype effects remained statistically significant after accounting for co-variates (P = 0.040 and P = 0.023, respectively), and further explained up to 1.5% of the variation in risk factors in the study group. The second model examined the impact of GA, BW < 1000 g, NEC, BPD, IVH, RDS, and DWMI. The final analysis focused solely on comorbidities, as GA and BW < 1000 g caused statistical redundancy due to their strong correlation with the diseases studied. Comorbidities alone explained 72.3% of the risk for advanced ROP. Both genotype effects remained statistically significant after adjusting for comorbidities (P = 0.012 and P = 0.014, respectively), accounting for an additional 3% of the variation in risk factors. Genotype, GA, number of RBC transfusions, mechanical ventilation, surfactant treatment, BPD, and IVH were all statistically significant risk factors for advanced ROP in the models studied.
NOS3 rs1799983 variant
The strongest effects of the NOS3 rs1799983T allele on the risk of ROP requiring treatment were found for rs1799983TT homozygotes (OR = 1.4) and the rs2070744TT + CT/rs1799983TT genotype combination (OR = 1.95), although these effects did not reach statistical significance.
EDN1 rs5370 and EDNRA rs5335 variants
Additionally, no statistically significant associations were observed between the studied EDN1 and EDNRA variants, either individually or in combination, and the occurrence or progression of ROP.
NOS3 genotypes and risk factors of ROP and comorbidities
Individuals with homozygous genotypes for either of the studied NOS3 variants had fewer RBC transfusions (Table 3). The rs2070744CC homozygotes tended to have a higher GA (P = 0.166) and a lower stage of ROP (P = 0.089). In carriers of this genotype, significantly lower frequencies of RDS and sepsis were observed. No significant associations were found between the rs1799983 variant and comorbidities related to prematurity.
Meta-analysis of the effect of NOS3 genotypes on ROP
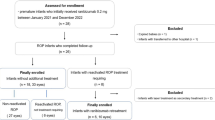
Our initial search of the databases yielded a total of 142 potentially relevant articles (Fig. 1). After evaluating the titles and abstracts, we excluded 68 duplicate and 63 obviously irrelevant studies. We then assessed the eligibility of the remaining studies and excluded 4 studies due to invalid data (review or letter to the editor) and overlapping data (also with the current study). Finally, a total of 7 cohort studies13,14,15,16,17,18,19, comprising 686 cases and 825 controls, were included in the meta-analysis, along with an additional 180 ROP cases and 105 controls analyzed in the present study.
Meta-analysis for the effect of NOS3 variants on the occurrence of any stage of ROP and ROP requiring treatment was performed. Table 4 presents the characteristics of the studies selected for the meta-analysis, and Table 5; Fig. 2 show the results.
The main characteristics of articles included in the meta-analysis are listed in Table 4. The studies were conducted in Asia (China, Turkey), Europe (Hungary, Italy, Poland, Serbia), and North America. Based on the quality assessment of NOS, seven studies13,15,16,17,18,19 including ours were of high quality while the other one study14 was of moderate quality. Furthermore, one study conducted by Shastry et al.20, which was excluded from the meta-analysis (as a letter to editors), was also assessed for its Newcastle-Ottawa Scale (NOS) score. It was confirmed to be of low quality for inclusion in the meta-analysis (NOS = 3). It should be noted that adjustments for potential confounding factors varied between studies, and the main factors adjusted were GA, BW, Apgar score, and parameters related to mechanical ventilation or other treatment.
As illustrated in Table 5; Fig. 2, our meta-analysis substantiates the role of the NOS3 rs2070744 variant in the development of proliferative-type ROP. The overall OR calculated from the three studies indicates an inverse correlation between the presence of the rs2070744CC genotype (recessive model) and the risk of ROP requiring treatment (fixed effects model [FEM]: I2 = 48%, OR 0.42, 95% CI 0.23–0.77, Pcrude = 0.005; Padj = 0.036). There was no statistically significant heterogeneity between the included studies (I2 = 48%, PH = 0.15); all three studies indicated a protective effect for the rs2070744CC genotype. The funnel plot representing this association displayed a symmetrical pattern (Supplemental Fig. S2), suggesting an absence of publication bias in the studies. This observation was further supported by Egger’s test (P = 0.427). In the allele assessment (Fig. 2a), the results were characterized by heterogeneity (I2 = 78%, PH = 0.01), that may have originated from heterozygous effects, which contributed to the lack of significance for differences in allele frequencies. Additional details concerning the conducted meta-analysis can be found in the Supplementary Materials (Supplemental Tables S1–S8 and Supplemental Figures S1–S28).
Discussion
Despite advancements in neonatal and ophthalmological care, ROP remains a major cause of blindness in children younger than 5 years even in developed countries with well-established screening and treatment protocols21. Currently, there is no reliable method to predict which patients will be more susceptible to severe complications of the disease. Evaluation of candidate gene variants that affect the vascular or angiogenic response in ROP may offer valuable insights into the disease’s pathogenesis and identify risk or protective factors associated with its development.
Both NO and ET-1 act as natural regulators of vascular function and exhibit physiological interactions that influence various cellular processes associated with the development of ROP. ET-1 can stimulate eNOS to produce NO, whereas NO can inhibit ET-1 production, thereby modulating its vascular effects5. The interaction between NO and ET-1 in the endothelium is mediated by the cGMP signaling pathway. Within the scope of these interactions, it is observed that ET-1 has an activity towards inducing expression of the NOS3 gene, leading to increased production of NO by human endothelial cells. It is believed that these effects are regulated by the activation of endothelin receptors, including EDNRA5,6.
Many previous studies have extensively investigated the association between NOS3 gene variants and various ophthalmological conditions. One area of interest has been the potential involvement of these variants in the susceptibility to retinal vascular disorders, such as diabetic retinopathy22,23,24. Similarities in the pathogenesis of diabetic retinopathy and ROP, provided the basis for the hypothesis that genetic alterations in the NOS3 gene could potentially contribute to the development of ROP. The involvement of this gene in pathways that lead to ROP development and progression is supported also by experimental studies on animal models3.
In our previous study, which was conducted on a relatively small study group, we observed a potential role of the NOS3 rs2070744T and rs1799983T alleles as risk factors for the progression of ROP to advanced stages11. The current analysis, conducted on a larger group of premature infants, provides further support for the role of the rs2070744 variant in ROP pathogenesis. We identified a protective effect of the rs2070744C allele against the development of ROP requiring treatment (OR 0.34, P = 0.009, in a recessive model). This association remained significant even after adjusting for the newborn’s condition at birth (including GA, BW, and Apgar score at 1 min) and the course of treatment (including the number of RBC transfusions, mechanical ventilation, and surfactant use; P = 0.040), as well as comorbidities (P = 0.012). NOS3 genotype, GA, number of RBC transfusions, mechanical ventilation, surfactant treatment, and the presence of BPD and IVH (both of which affect vital organs) were identified as independent risk factors for advanced ROP in this study.
Further confirmation of the role of this variant for the development of advanced stages of ROP was provided by the results of a meta-analysis. For this analysis, the results of our study, and two other studies from Turkish18 and Hungarian19 populations were available. All the data were obtained from newborns of Caucasian ethnicity and scored between seven and nine in the NOS quality assessment, indicating high quality. The meta-analysis did not reveal significant heterogeneity or any signs of publication bias. The FEM, which included 298 patients and 397 controls, yielded an OR of 0.42 for the rs2070744CC homozygotes (adjusted P-value = 0.036). The effect of rs2070744CC homozygotes, in our study, was strongest in carriers of the rs1799983G allele. In a phenotypic correlation study, it further showed an association with a lower incidence of sepsis, RDS and less intensive treatment with RBC transfusions.
Opposite results regarding the association between the rs207074C allele and advanced ROP were previously obtained by Shastry et al.20. This study was excluded from the meta-analysis due to its publication as a letter to the editor and its low NOS score (equal to 3). The primary objection to its quality was the use of a control group comprising a random population of newborns born at term rather than preterm infants without ROP.
For the meta-analysis of the impact of NOS3 rs2070744 variant on ROP occurrence, six studies comprising 478 cases and 723 controls were available. In the recessive model, both the FEM and the random effects model (REM) indicated no association (OR = 0.96, adjusted P-value was 1.0), with four out of the evaluated studies suggesting a protective role of the C allele, and two indicating the opposite effect of this allele. None of the individual studies showed statistical significance. The most significant protective function for C allele was demonstrated by Yu et al., who conducted a study in an Asian population (recessive model: OR = 0.50; 95% CI 0.20–0.27)16. Interestingly, these authors identified an interaction between the rs2070744CC and rs1799983GT genotypes, combined with a shorter duration of oxygen therapy (below 17 days), as protective factors against ROP.
Our meta-analysis did not find the association between the NOS3 rs1799983 variant and ROP (see to Supplementary Materials for details). For the study of ROP occurrence, five studies comprising 418 cases and 654 controls were available while for the study of treatment-requiring ROP occurrence, only two studies comprising 163 cases and 269 controls were available. The results suggested a potential role of the rs1799983T variant allele in recessive model as a risk factor for both the occurrence of any stage of ROP (FEM, OR = 1.45; 95% CI 0.80–2.61; P-value was nonsignificant), and ROP requiring treatment (FEM, OR = 1.55; 95% CI 0.71–3.39; P-value was nonsignificant). On the other hand, our study also found a correlation between the rs1799983TT genotype and lower RBC transfusion treatment intensity, suggesting its protective role. In the previous meta-analysis conducted by Gohari et al. in 2019, either rs2070744 nor rs1799983 were associated with ROP12. Including new data from the study of Ilguy 2021 et al.18, and ours, that have entered the results from additional 433 premature infants we had sufficient power to show the effect of the rs2070744CC genotype on the development of proliferative type of ROP.
In our study, we did not find any association between the EDN1 rs5370 and EDNRA rs5335 variants and the development of ROP. To our knowledge, these variants have not been previously investigated in ROP patients. However, in the literature, there are studies that have examined the role of these variants in other conditions. For example, Li et al. found a significant association between the EDN1 rs5370 variant and an increased risk of preeclampsia25. Szpecht et al. investigated the role of the EDN1 rs5370 variant in the development of intraventricular hemorrhage in preterm infants but found no association26. Buentello-Volante et al. reported a protective effect of the EDNRA rs5335CG genotype against primary open-angle glaucoma in the Mexican population27. These variants have mainly been studied in cardiovascular diseases, such as coronary heart disease28, ischemic stroke29 or intracerebral hemorrhage30.
It has been observed that the presence of NOS3 variants can decrease the biosynthesis of NO by the enzyme through different mechanisms. The rs2070744 СС genotype was found to be associated with the lowest levels of NO metabolites, as compared to CT and TT genotypes31, which corresponds to the observations that suppression of NO formation resulted in reduced retinal neoangiogenesis32. Our observations extend the previous finding on the role of hypoxia and antioxidants in the development of complications of prematurity33,34. By exploring the genetic predisposition to ROP, including variants of genes involved in the regulation of vascular function, we may gain new insights into the pathogenesis and progression of the disease. Such investigations can contribute to the development of personalized approaches for the prevention and treatment of ROP, ultimately improving the outcomes for affected infants. In details, the identification of low- and high-risk patient groups could allow for appropriate screening and early interventions. Additionally, personalized treatment strategies would be possible, e.g., infants with the high-risk genotype might benefit from special oxygen therapy protocols to minimize the risk of retinal damage. Phenotypic correlation studies suggested that infants with the NOS3 rs2070744 TT + TC genotype may require more intensive red blood cell transfusion treatment.
There are several limitations of our study that should be highlighted. Although the study presented in this paper was based on the analysis of one of the largest group of infants among those published to date on NOS3 variant and ROP, the sample size is still relatively small for genetic research. A post-hoc statistical power analysis for the demonstrated recessive effect of the NOS3 rs2070744C allele on the incidence of ROP requiring treatment showed a value of 72% for the Polish population analysis and 80% for the meta-analysis, indicating that both studies were reliable. Moreover, the strength of this work comes from the homogeneous population of Caucasian newborns. The findings of meta-analysis should be however interpreted cautiously due to the limited number of studies available for analysis.
In conclusion, we found that the NOS3 rs2070744C variant allele is a protective factor against the development of ROP requiring treatment, but not any stage of ROP. Since the rs2070744СС genotype has previously been found to be associated with lower levels of NO metabolites, local suppression of NO formation in infants with rs2070744 TT + TC risk genotypes may represent a protective strategy against the development of advanced ROP. We did not find any evidence to support the role of EDN1 or EDNRA in ROP. Nevertheless, this is the first study to address the potential involvement of variants in these genes in the pathogenesis of ROP.
Methods
Study population
The study included a population of 285 preterm infants who met the criteria for ROP screening as defined by the consensus of the Polish Neonatologists and Pediatric Ophthalmology Section. These criteria included infants with a GA of ≤ 33 weeks, a BW of ≤ 1800 g35. Of the 285 infants, 195 were collected between 2009 and 2014, and 90 infants were collected between 2009 and 2016 from the Neonatal Intensive Care Unit (NICU) at the Clinical Hospital of Gynecology and Obstetrics at Poznan University of Medical Sciences. This hospital is a tertiary referral center and the largest obstetrics center in Poland, with an annual birth rate exceeding 7000. All newborns included in the study were of Caucasian origin. Exclusion criteria for the study comprised infants born from multiple pregnancies and those with chromosomal abnormalities.
Clinical features and ROP management
Clinical factors that may impact the development of ROP were collected from the patients’ medical records. These factors included gender, GA (in weeks), BW (in grams), Apgar score at the 1st and 5th minute, mode of delivery (vaginal birth or cesarean section), presence of ruptured fetal bladder, intrauterine hypotrophy (defined as fetal birth weight below the 10th percentile for gestational age) and parameters related to respiratory failure such as surfactant treatment, resuscitation, or mechanical ventilation. Additionally, the presence of other complications of prematurity, including bronchopulmonary dysplasia (BPD), respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), and diffuse white matter injury (DWMI), was also recorded. The diagnostic criteria for RDS, NEC, IVH, BPD, and NEC have been described in previous studies36, while DWMI was diagnosed based on abnormal ultrasound or MRI findings.
The premature newborns included in the study underwent regular ROP screening, which involved a series of eye fundus examinations using binocular indirect ophthalmoscopy. The examinations were conducted under topical anesthesia (proxymetacaine) and after pupil dilation (tropicamide 1% and phenylephrine 2.5% drops). The initial examination was performed in the 4th week of chronological age, with subsequent follow-up examinations scheduled every 7–10 days, depending on the condition of the eyes. In cases where ROP was diagnosed, the fundus lesions were classified according to the International Classification of Retinopathy of Prematurity (ICROP). Treatment was administered based on the guidelines outlined in the Early Treatment for Retinopathy of Prematurity. Patients with any stage of ROP in zone I with plus disease, stage 3 ROP with no plus disease in zone I, or stage 2 or 3 ROP with plus disease in zone II were eligible for treatment. The treatment options included laser photocoagulation of the peripheral avascular retina or intravitreal administration of an anti-VEGF antibody (ranibizumab) within 72 h of diagnosis. The ROP screening continued until the vascularization reached zone III or until signs of ROP regression were observed in at least two consecutive examinations.
Genotyping
Genomic DNA was extracted from buccal swabs using the innuPREP DNA Kit (Analytik Jena AG, Jena, Germany) or from circulating blood lymphocytes using the QIAamp DNA Kit (Qiagen GmbH, Hilden, Germany) following the manufacturer’s instructions. Four variants in the NOS3, EDN1, and EDNRA genes were analyzed. The single nucleotide variants were determined using quantitative polymerase chain reaction (qPCR) with pre-designed TaqMan SNP Genotyping Assays (Thermo Fisher Scientific, Waltham, Mass., USA) on the ABI 7900HT Fast Real-Time PCR System (Life Technologies, Carlsbad, Calif., USA). The details of the variants and the genotyping tests used are presented in Table 6.
Meta- analysis for NOS3 variants and ROP
The meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement37. Associations with ROP development and progression were studied separately.
-
Search strategy
An electronic literature search was conducted in PubMed, Embase, Web of Science, and Scopus to identify relevant studies published in English on the association of the studied NOS3 variants with ROP. The search was performed up until June 01, 2023. Various combinations of keywords were used, including terms such as “endothelial nitric oxide synthase” or “NOS3”, “polymorphism” or “mutation” or “variant” or “786” or “rs2070744”, “polymorphism” or “mutation” or “variant” or “894” or “rs1799983” or “Glu298Asp”, and “retinopathy of prematurity” or “ROP”. Additionally, the reference lists of retrieved articles and previous reviews were manually searched to ensure the inclusion of all relevant studies.
-
Inclusion and exclusion criteria
The following criteria were applied to include studies in the meta-analysis: (a) studies with a case-control or cohort design; (b) studies focusing on the association between NOS3 variants and ROP; (c) availability of sufficient genotype data in the case and control groups to calculate crude ORs and 95% CIs. The exclusion criteria were as follows: (a) studies not designed as case-control or cohort studies; (b) lack of genotype data or inability to calculate it (no available genotyping data); (c) studies based on pedigree data, twins studies, linkage and family-based studies; (d) case reports, review articles, posters, abstracts, and animal studies; and (e) studies with incomplete or overlapping data. In cases of overlapping or duplicate publications on the same topic, only the largest or most recently updated sample data was included.
-
Data extraction
Two authors independently reviewed and extracted the following information from all included studies using a structured data collection: first author name, publication date, genotyping method, participant location, ethnicity, genotyping methods, sample sizes of cases and controls, genotype frequency distribution, minor allele frequency (MAF), and Hardy-Weinberg equilibrium (HWE) in controls. Any discrepancies were resolved through consensus among all authors. If the included studies did not provide detailed genotype or HWE information, we calculated them and provided the relevant information.
-
Quality assessment
The quality of studies was independently assessed by the Newcastle–Ottawa quality scale (NOS), which is a specific scale to assess the quality of non-randomized studies in meta-analyses38,39,40. It consisted of 3 parts which were the selection of study groups, the comparability of study groups, and the assessment of exposure or outcomes. We gave points if the studies met related condition. Studies with scores of 0–3, 4–6, 7–9 were, respectively, considered as low, moderate, and high quality.
Statistical analysis
Testing for HWE was conducted using a χ2 test available at the following URL: https://ihg.helmholtz-muenchen.de/cgi-bin/hw/hwa1.pl (accessed on 1 January 2023). To evaluate the individual effects of the studied factors on the onset and progression of ROP, a linear regression analysis was performed and the regression beta coefficients (βs) for the trend were calculated. In this analysis, the groups were categorized as follows: 0—no ROP, 1—ROP requiring treatment, and 2—ROP not requiring treatment. Factors showing deviations from a normal distribution in the Kolmogorov-Smirnov test were examined after being subjected to logarithmic transformation.
In the univariate analyses, qualitative variables were examined using the χ2 test or Fisher’s test, while quantitative variables were evaluated using the t-test or Mann–Whitney U test. ORs with 95% CIs were calculated for the genotypes, and the impact of MAF of the studied variants was assessed in these analyses. The recessive and dominant models of interactions were tested, along with the effect of combinations of genotypes. For multivariate analyses, multivariate logistic regression analysis was utilized to control for confounding factors.
For the meta-analysis, all statistical calculations were conducted using the METAGENYO software available at the following URL: http://bioinfo.genyo.es/metagenyo/ (accessed on June 10, 2023)41. For each variant, six comparisons were performed: allele contrast (A vs. B), recessive model (AA vs. AB + BB), dominant model (AA + AB vs. BB), overdominant model (AB vs. AA + BB), the effect of homozygotes AA vs. BB, and the effect of heterozygotes AB vs. BB. The Cochran’s Q test and I2 statistic were utilized to assess heterogeneity among the selected studies42. In cases where heterogeneity was evident (I2 > 50%), the random effects model (REM) was applied. In instances of low heterogeneity (I2 ≤ 50%), the fixed effects model (FEM) was employed. Additional tests were conducted using the fixed effect model for data with a heterogeneity P-value lower than 0.1. Since no new associations were identified in these tests, they are not presented here. For the tested associations, ORs, P-values (with 95% CIs), and adjusted P-values were computed. Additional tests for HWE were performed separately for each gene variant among control subjects. For each test, both P-values and adjusted P-values were calculated. The possibility of publication bias was assessed through visual inspection of the funnel plot and Egger’s test. The best-fitting model (fixed versus random effects) was selected automatically based on the heterogeneity test result (a function available in the software was used).
Results were calculated for both ROP and treatment-requiring ROP. All reported probabilities (P-values) were two-sided, and a significance level of P < 0.05 was considered statistically significant. Except for meta-analysis, STATISTICA version 10.0 and GraphPad Prism version 6.04 were used to perform statistical analyses. Post hoc power analysis for associations was conducted using the Quanto software.
Ethics statement
All procedures carried out on human participants in this study were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments (or comparable ethical standards). The study was approved by the Bioethics Committee of Poznan University of Medical Sciences (no. 1140/05, 1117/18, 45/22 and 126/22). Written prior-informed consent was obtained from the parents or guardians of the patients.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Chan-Ling, T., Gole, G. A., Quinn, G. E., Adamson, S. J. & Darlow, B. A. Pathophysiology, screening and treatment of ROP: A multi-disciplinary perspective. Prog. Retin Eye Res. 62, 77–119 (2018).
Lambert, S. R., Lyons, C. J., Hoyt, C. S. & Taylor, D. Taylor & Hoyt’s pediatric ophthalmology and strabismus. (2017).
Griffith, R. M. et al. Next-generation sequencing analysis of gene regulation in the rat model of retinopathy of prematurity. Doc. Ophthalmol. Adv. Ophthalmol. 127, 13–31 (2013).
Fujinaga, H. et al. Hyperoxia disrupts vascular endothelial growth factor-nitric oxide signaling and decreases growth of endothelial colony-forming cells from preterm infants. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L1160–1169 (2009).
Cardillo, C., Kilcoyne, C. M., Cannon, R. O., Panza, J. A. & 3rd & Interactions between nitric oxide and endothelin in the regulation of vascular tone of human resistance vessels in vivo. Hypertension 35, 1237–1241 (2000).
Demosthenous, M. et al. Endothelial nitric oxide synthase in the vascular wall: Mechanisms regulating its expression and enzymatic function. Artery Res. 5, 37–49 (2011).
Stow, L. R., Jacobs, M. E., Wingo, C. S. & Cain, B. D. Endothelin-1 gene regulation. Faseb j. 25, 16–28 (2011).
Palmo, T. et al. The EDN1 Missense Variant rs5370G > T Regulates Adaptation and Maladaptation under Hypobaric Hypoxia. Int. J. Environ. Res. Public. Health 19 (2022).
Ahmed, M. & Rghigh, A. Polymorphism in endothelin-1 gene: an overview. Curr. Clin. Pharmacol. 11, 191–210 (2016).
Okan, G. et al. G-231A and G + 70 C Polymorphisms of endothelin receptor type-a gene could affect the psoriasis area and severity index score and endothelin 1 levels. Indian J. Dermatol. 60, 211 (2015).
Choręziak-Michalak, A., Gotz-Więckowska, A., Chmielarz-Czarnocińska, A., Seremak-Mrozikiewicz, A. & Szpecht, D. Potential role of eNOS and EDN-1 gene polymorphisms in the development and progression of retinopathy of prematurity. BMC Ophthalmol. 23, 78 (2023).
Gohari, M. et al. Association of eNOS and ACE polymorphisms with retinopathy of prematurity: a systematic review and meta-analysis. Fetal Pediatr. Pathol. 39, 334–345 (2020).
Tekkeşin, F. et al. Endothelial nitric oxide synthase G894T, intron 4 VNTR, and T786C polymorphisms in retinopathy of prematurity. J. Neonatal Perinat. Med. 15, 249–255 (2022).
Pantelić, J. R. et al. Analysis of T-786 C and 4a/b endothelial nitric oxide synthase gene polymorphisms in retinopathy of prematurity. Genetika 48, 707–716 (2016).
Poggi, C. et al. Genetic Contributions to the Development of Complications in Preterm Newborns. PLoS One. 10, e0131741 (2015).
Yu, C. et al. Correlation of interactions between NOS3 polymorphisms and oxygen therapy with retinopathy of prematurity susceptibility. Int. J. Clin. Exp. Pathol. 8, 15250–15254 (2015).
Yanamandra, K., Napper, D., Pramanik, A., Bocchini, J. A. Jr. & Dhanireddy, R. Endothelial nitric oxide synthase genotypes in the etiology of retinopathy of prematurity in premature infants. Ophthalmic Genet. 31, 173–177 (2010).
Ilguy, S. et al. The relationship of retinopathy of prematurity with brain-derivated neurotrophic factor, vascular endotelial growth factor-A, endothelial PAD domain protein 1 and nitric oxide synthase 3 gene polymorphisms. Ophthalmic Genet. 42, 725–731 (2021).
Rusai, K. et al. Endothelial nitric oxide synthase gene T-786 C and 27-bp repeat gene polymorphisms in retinopathy of prematurity. Mol. Vis. 14, 286–290 (2008).
Shastry, B. S. Endothelial nitric oxide synthase gene promoter polymorphism (T-786 C) may be associated with advanced retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 251, 2251–2253 (2013).
Liu, L. et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379, 2151–2161 (2012).
Midani, F. et al. The role of genetic variants (rs869109213 and rs2070744) Of the eNOS gene and BglII in the α(2) subunit of the α(2)β(1) integrin gene in diabetic retinopathy in a Tunisian population. Semin. Ophthalmol. 34, 365–374 (2019).
Mihoubi, E. et al. T-786 C endothelial nitric oxide gene polymorphism and type 1 diabetic retinopathy in the Algerian population. J. Fr. Ophtalmol. 42, 579–585 (2019).
Bazzaz, J. T. et al. eNOS gene polymorphism association with retinopathy in type 1 diabetes. Ophthalmic Genet. 31, 103–107 (2010).
Li, J., Yin, W., Liu, M. S., Mao, L. J. & Wang, X. H. Potential correlation between EDN1 gene polymorphisms with preeclampsia. Eur. Rev. Med. Pharmacol. Sci. 24, 1602–1608 (2020).
Szpecht, D., Gadzinowski, J., Seremak-Mrozikiewicz, A., Kurzawińska, G. & Szymankiewicz, M. Role of endothelial nitric oxide synthase and endothelin-1 polymorphism genes with the pathogenesis of intraventricular hemorrhage in preterm infants. Sci. Rep. 7, 42541 (2017).
Buentello-Volante, B. et al. Association study of multiple gene polymorphisms with the risk of adult-onset primary open-angle glaucoma in a Mexican population. Exp. Eye Res. 107, 59–64 (2013).
Ellis, K. L. et al. Association between endothelin type A receptor haplotypes and mortality in coronary heart disease. Per Med. 9, 341–349 (2012).
Zhang, L. & Sui, R. Effect of SNP polymorphisms of EDN1, EDNRA, and EDNRB gene on ischemic stroke. Cell. Biochem. Biophys. 70, 233–239 (2014).
Zeng, Y. et al. Associations of EDNRA and EDNRB polymorphisms with intracerebral hemorrhage. World Neurosurg. 129, e472–e477 (2019).
Uryasev, O., Shakhanov, A., Belskikh, E. & Korshunova, L. NOS3 786 C/T polymorphism associated with plasma nitric oxide level and risk of development essential hypertension in patients with asthma. Eur. Respir. Soc. (2019).
Ninchoji, T. et al. eNOS-induced vascular barrier disruption in retinopathy by c-Src activation and tyrosine phosphorylation of VE-cadherin. Elife. 10 (2021).
Strauss, E. et al. Hypoxia-inducible pathway polymorphisms and their role in the complications of prematurity. Genes (Basel). 14 (2023).
Strauss, E., Januszkiewicz-Lewandowska, D. & Sobaniec, A. & Gotz-Więckowska, A. SELENOP rs3877899 variant affects the risk of developing advanced stages of retinopathy of prematurity (ROP). Int. J. Mol. Sci. 24 (2023).
Gotz-Więckowska, A., Bakunowicz-Łazarczyk, A., Hautz, W., Filipek, E. & Niwald, A. M. Polish ophthalmological society revised guidelines for the management of retinopathy of prematurity. Klinika Oczna/Acta Ophthalmol. Pol. 122, 14–16 (2020).
Kosik, K. et al. Single nucleotide vitamin D receptor polymorphisms (FokI, BsmI, ApaI, and TaqI) in the pathogenesis of prematurity complications. Sci. Rep. 10, 21098 (2020).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open. Med. 3, e123–130 (2009).
Wells, G. et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. vol. 2017 (2023).
Luchini, C., Stubbs, B., Solmi, M. & Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 5, 80–84 (2017).
Luchini, C. et al. Assessing the quality of studies in meta-research: Review/guidelines on the most important quality assessment tools. Pharm. Stat. 20, 185–195 (2021).
Martorell-Marugan, J., Toro-Dominguez, D. & Alarcon-Riquelme, M. E. Carmona-Saez, P. MetaGenyo: a web tool for meta-analysis of genetic association studies. BMC Bioinform. 18, 563 (2017).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Funding
The work has been supported by the Scientific Research Committee in Poland under grant no. 2 P05E 098 30 (A.G.-W., E.S.) and the Poznan University of Medical Sciences research grant for young employees (up to 35 years of age) and participants of four-year doctoral studies in 2021, no. 502-14-11121500-45047 (A.C.-M.).
Author information
Authors and Affiliations
Contributions
E.S., A.G.-W., D.S. A.C.-M., and A.C.-C. contributed to the design of the research. A.C.-M., E.S., and A.G.-W. participated in obtaining funding. A.G.-W., A.C.-M., A.C.-C., and D.S. selected and examined patients. A.G.-W., A.C.-M., D.S., P.G., and E.S. participated in data acquisition. A.C.-M. and E.S. performed genotyping. E.S. and T.W. conducted statistical analysis. A.C.-M., E.S., and P.G. analyzed and interpreted the data. A.C.-M., and E.S. drafted the manuscript. P.G., A.G.-W., T.W., and D.S. performed critical revision of the article. E.S. and A.G.-W. supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choręziak-Michalak, A., Szpecht, D., Woźniak, T. et al. Association of endothelial nitric oxide synthase (NOS3) rs2070744 variant with advanced retinopathy of prematurity: a case–control study and meta-analysis. Sci Rep 15, 329 (2025). https://doi.org/10.1038/s41598-024-83305-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83305-7