Abstract
As immune-checkpoint inhibitors (ICIs) therapy has made great strides in hepatocellular carcinoma (HCC) treatment, improving patient response to this strategy has become the main focus of research. Accumulating evidence has shown that m6A methylation plays a crucial role in the tumorigenesis and progression of HCC, while the precise impact of the m6A demethylase ALKBH5 on the tumor immune microenvironment (TIME) of HCC remains poorly defined. The clinical significance of ALKBH5 and TIM3 were evaluated in human HCC tissues. The biological function of ALKBH5 was analyzed in vitro and in vivo. The HCC molecular subtypes were identified based on key ALKBH5-regulated methylation-related genes (MRGs). The differences in survival, clinical features, TIME and immunotherapy response between these two subtypes were then evaluated. The regulation of ALKBH5 on TIM3 was detected by qPCR, western blotting and MeRIP. ALKBH5 was downregulated in HCC and associated with worse prognosis. ALKBH5 inhibited the proliferation and migration activities of HCC cells in vitro and in vivo. The HCC subtype with high expression of key MRGs was characterized by immunosuppression phenotypes and a worse response to ICIs. Moreover, TIM3 was identified as a target of ALKBH5. Upregulated TIM3 level was negatively correlated with survival in HCC. The results of this study suggest that ALKBH5 is an important regulator in HCC progression. ALKBH5 exerts its influence on the TIME and immunotherapy response by targeting TIM3 in HCC. This work provides new insight into the correlation between m6A modification and ICI response, which may help provide therapeutic benefits to HCC patients.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC), the most common subtype of primary liver cancer (PLC), is one of the most frequent malignant tumors and the third leading cause of cancer death worldwide1. Despite significant improvements in treatment approaches in recent years, the prognosis of HCC patients is remains unfavorable due to delayed diagnosis, frequent recurrence and distant metastasis2. Hence, further explication of the molecular pathogenesis and effective therapeutic strategies for HCC is urgently needed.
RNA N6methyladenosine (m6A), the methylation of adenosine at position 6, is the most abundant internal mRNA modification in mammalian eukaryotic mRNAs3,4. Mounting evidence indicates that m6A is involved in in various physiological and pathological biological processes, including the progression of malignant tumors5. ALKBH5 plays a crucial role in tumor initiation and progression as an important mammalian m6A demethylase6,7. Furthermore, its abnormal expression has a significant influence on the infiltration, activation, and effector functions of infiltrating immune cells in the TIME. For example, ALKBH5 inhibited lymphocyte infiltration by reducing IFNα secretion in head and neck squamous cell carcinoma8. ALKBH5-mediated m6A modification of AXIN2 increased dickkopf-related protein 1 (DKK1) to regulate myeloid-derived suppressor cells (MDSC) recruitment to drive immunosuppression in colorectal cancer9. In addition, ALKBH5 can modulate tumor metabolite contents to change the accumulation of Treg and myeloid-derived suppressor cells in tumor tissue to affect ICIs efficacy10. The findings suggest that ALKBH5 may play a significant role in tumor immunotherapy. However, the role of ALKBH5-induced m6A modification in the HCC TIME has not yet been explored.
Over the past decade, immune checkpoint inhibitors (ICIs) therapy has emerged as a promising strategy for treating advanced HCC11,12. The best-known ICIs are the monoclonal antibodies targeting programmed cell death-1 (PD-1) and its ligand (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) pathways, which exert influence on T-cell activation, exhaustion and tolerance to modulate the tumor immune microenvironment (TIME)13. As a new first-line treatment strategy, the combination of atezolizumab (anti-PD-L1) and bevacizumab (anti-VEGF) has shown superiority in improving overall survival and progression-free survival14. However, despite these unprecedented advancements, responses are observed in only a limited number of patients. Thus, there is an urgent need to improve ICIs therapy response by combining effective molecules and biomarkers. T cell immunoglobulin mucin-3 (TIM3) is a newly discovered immune checkpoint molecule and has been proven to exert a vital effect on antitumor immunity15. Its expression on TILs can increase effector T cell exhaustion, Treg-mediated immunity suppression and M2 polarization16,17. Thus, targeting TIM3 is considered as a promising strategy, and several TIM3 antibodies have shown efficacy to suppress tumor progression in some tumortypes18,19,20. However, the possible regulation of ALKBH5-mediated m6A modification on TIM3 expression is still not reported.
In this study, we unveiled the important role of ALKBH5 in HCC. And we identified two ALKBH5-regulated m6A methylation-related HCC molecular subtypes based on bioinformatics approaches. The subtypes are characterized by different immune microenvironments and immunotherapy responses. In addition, we found a regulating mechanism of ALKBH5 on the TIM3 level depending on m6A demethylation modification. This effort provides novel insight into the corsstalk between m6A modicfication and antitumor immunity.
Methods
HCC tissue specimens
HCC tissues and paired adjacent nontumor tissues were collected from a total of 50 patients who underwent curative surgery from 2016 to 2017 at Nanjing Drum Tower Hospital, China. Relevant clinicopathological features and follow-up data were obtained by referring to medical records. Written informed consent were acquired from all patients. Ethical approval was granted by the Institutional Ethics Committee of Nanjing Drum Tower Hospital.
Immunohistochemical (IHC) staining
The paraffin-embedded tumor tissue sections (3 μm) were incubated with ALKBH5 and TIM3 antibody overnight after dewaxed, antigen repaired, endogenous peroxidase blocked and nonspecific antigen blocked. The next day, sections were stained with DAB substrate and counterstained with hematoxylin after incubation with HRP-conjugated secondary antibody. IHC staining score was calculated from the score of staining intensity and the score of staining area. The cut-off score was the threshold with the objectively best sensitivity and specificity in the ROC curve.
Cell lines
The HCC cell lines HepG2 and Hep3B were obtained from the TCC Cell Bank (Shanghai, China), and cells were cultured with MEM supplemented with 10% fetal bovine serum, penicillin (100 U/ml)) and streptomycin (100 µg/ml) (Invitrogen, Carlsbad, CA, USA) in an atmosphere containing 5% CO2 at 37 °C.
Lentiviral construction and cell transfection
The overexpression and knockdown lentiviral plasmids of ALKBH5 were procured from GeneChem (Shanghai, China). Cells in the logarithmic growth phase were transfected with these lentiviral plasmids and subsequently subjected to puromycin selection (2 µg/ml) for a duration of 14 days. Cells were transfected with small interfering RNAs (siRNAs) oligonucleotides via Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA).
Cell proliferation and focus-formation assay
HepG2 and Hep3B cells were seeded at a density of 3000 cells/well into 96-well plates and added CCK-8 reagent (Beyotime, China) to each well at scheduled time. The optical density at 450 nm (OD450) was subsequently detected. For the focus-formation assay, the cells were cultured in six-well plates (500 cells/well) for 10 days and stained with 0.1% crystal violet (LEAGENE, China). Then, the stained colonies were counted.
Cell cycle assay
HCC cells were washed twice with cold PBS and subsequently fixed overnight in 70% ethanol. After centrifugation, the cells were incubated at 37 °C for 30 min in propidium (PI) staining buffer (Beyotime, China). The cell cycle was then analyzed using a FACSMelody flow cytometer (Becton Dickinson & Co., USA) and Modfit software.
Cell apoptosis assay
After being isolated with EDTA-free trypsin, HCC cells were washed three times with cold PBS and resuspended in a binding buffer. Then, the cells were incubated with PI and Annexin V-FITC (Beyotime, China) in the dark for 15 min. The detection of cell apoptosis was performed using flow cytometry (Becton Dickinson & Co., USA) and the data were analyzed with FlowJo software.
Cell migration assay
Cell migration was determined using a Transwell assay. The cells (1 × 105 cells/well) were resuspended in 200 µl serum-free medium and seeded into the upper chamber of 8.0 μm pore size Transwell inserts. A total of 700 µl of 10% FBS medium was added to the bottom chamber. After incubation for 48 h in 5% CO2 at 37 °C, the cells at the upper surface of Transwell inserts were removed with a cotton swab, and the cells at the bottom were fixed with 75% ethanol and then stained with 0.1% crystal violet (LEAGENE, China).
Subcutaneous xenograft model
Female nude mice aged 4 weeks were purchased from Yangzhou University Medical Center (Jiangsu, China). Hep3B cells (2 × 106 cells) were subcutaneously injected into the mice (n = 6 per group). Tumor size was recorded every 4 days beginning on day 10 after tumor cells injection. After 26 days, the mice were executed by CO2 gas, and their tumor tissues were collected for further analysis. All animal experiments were approved by the Use Committee for Animal Care of Jiangsu Province, and the studies were conducted in accordance with the approved guidelines and regulations. Furthermore, this study is reported in accordance with ARRIVE guidelines.
In vivo xenograft mouse model
4-week-old thymus-free BALB/c nude mice were raised under pathogen-free conditions (Yangzhou University Medical Center, China). 2 × 106 cells were injected into 0.2 mL phosphate buffer solution (PBS) via a tail-lateral vein (n = 6 per group). 4 weeks later, the mice were executed by CO2 gas, and the livers were immediately excised. Metastatic nodules were photographed and counted.
Western blotting
The total cellular protein of HCC cells was extracted using RIPA lysis buffer and the protein concentrations were determined with a bicinchoninic acid (BCA) protein assay (Beyotime, China). Proteins with the same quantities were incubated with antibodies. The antibody list was shown in Supplementary Table S2. Bands on the membranes were visualized, and the intensity was quantified using Image Lab software (Bio-Rad, Hercules, USA).
Quantitative real-time PCR (qRT-PCR)
The total RNA of HCC cells was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) followed by reverse transcription using a Reverse Transcription Kit (Takara, Dalian, China). qRT-PCR was performed with SYBR Green Master Mix in a StepOne Plus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). β-actin was used to normalize the mRNA expression levels. The primer sequences are shown in Supplementary Table S1.
MeRIP sequencing and identification of methylation-related differentially expressed genes
Total RNA of more than 250 µg was extracted from Hep3B cells transfected with ALKBH5-specific siRNAs and negative control (NC) using TRIzol (Invitrogen, Carlsbad, CA, USA). RNA was further purified and chemically fragmented into approximately 100 nt using a Magna MeRIP™ m6A kit (Millipore, Catalog No. 17-10499) according to the manufacturer’s instructions21. Then, 10% of the RNA was saved as input samples, and the remaining RNA was immunoprecipitated with anti-m6A antibodies and eluted. MeRIP sequencing and subsequent data analysis were mainly performed at the Lian-Chuan Genomic Facility (LC-bio, Hangzhou, Zhejiang, China)22,23. Differentially expressed genes were selected with the criteria q-value < 0.05 and |log2(fold-change)| ≥1, and differential m6A-peaks were selected with the criteria FDR < 0.05 and |log2(fold-change)| ≥1.
Functional enrichment analysis
Gene set enrichment analysis (GSEA) was conducted with Sangerbox 3.0 online tools (http://sangerbox.com/) using the ‘c8.all.v7.4. symbols’ gene sets of the MSigDB database. Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of differentially expressed genes and differentially methylated genes were performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID)(https://david.ncifcrf.gov/). The R package “ggplot2” was used to visualize the enrichment results.
Identification of the MRGs
A Venn diagram was used to combine the genes with both hyper-m6A peaks and increased expression and genes with both hyper-m6A peaks and decreased expression. The overlap was identified as methylation-related genes (MRGs).
Public data sources and preprocessing
The RNA-seq data (FPKM), clinicopathological characteristics and mutational information of HCC patients were downloaded from The Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/tcga). The GSE6764 dataset was downloaded from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?%20acc=GSE6764).
Survival analysis and the identification of key MRGs
The batch survival analysis of MRGs was evaluated by Sangerbox 3.0 online tools using TCGA data, and those genes with a P value < 0.01 were defined as the key MRGs for the following analysis.
Consensus clustering analysis
Unsupervised clustering was executed with the R package “Consensus Cluster Plus”, and the TCGA-LIHC samples were divided into two distinct molecular subtypes based on the expression of key MRGs. Principal component analysis (PCA) was applied to show the distribution difference between these two HCC subtypes.
Immune infiltration analysis
The composition of 22 immune cells in each HCC sample was quantified with the CIBERSORT deconvolution algorithm based on the TCGA expression data of key MRGs. The sum of the proportions of 22 immune cell types in each sample was 1. The single-sample gene set enrichment analysis (ssGSEA) method was applied to calculate the infiltration of 28 immune cell types with the R package “GSVA”. The R package “corrplot” was used to analyze Spearman’s correlation of the key MRGs and immune cell infiltration. The R package “estimate” was used to calculate the TIME of each HCC patient, including the stromal score, immune score and ESTIMATE score (synthetic mark of stroma and immune).
Prediction of immunotherapeutic responses of HCC subtypes
The R package “survival” was used to plot the K-M survival curves of overall survival (OS) and progression-free survival (PFS) for different HCC subtypes. The list of immunoinhibitory and immunostimulatory genes was downloaded from the TISIDB database (http://cis.Hku.hk/TISIDB/). The predictive indices for the immunotherapeutic responses, including TIDE, IFNG, MSI and T-cell dysfunction/exclusion, were calculated using the TIDE database (http://tide.dfci.harvard.edu/). The genetic mutation information of HCC patients was shown in oncoplots using the R package “maftools”. TMB was calculated based on the nucleotide variation of each patient. Markers of T-cell exhaustion were obtained from previous literature24.
Statistical analysis
The statistical analyses were carried out with R (4.1.1) and GraphPad Prism (9.0) software. All cytology experiments were independently repeated at least three times. The data were compared using the two-tailed Student’s t-test and are presented as the means ± SEMs. Box plots in bioinformatics analyses used the Wilcoxon rank-sum test. The clinical characteristics between the two clusters were compared using the chi-square test. The difference in survival curves between the two subtypes was estimated using the log-rank test. All hypothetical tests were two-sided, and a P value < 0.05 was considered significant (*P < 0.05, **P < 0.01, ***P < 0.001).
Results
ALKBH5 is downregulated in HCC and correlates with poor prognosis
To examine the expression of ALKBH5 in HCC, 50 pair of tumor and corresponding non-tumor tissues from HCC patients were analyzed. Immunohistochemistry (IHC) staining showed that ALKBH5 was mainly stained in the nucleus of HCC tumor cells. IHC staining further revealed that ALKBH5 was downregulated in HCC tissue compared to non-tumor tissues (Fig. 1A and B). To further evaluate the clinical prognostic values of ALKBH5, patients were divided into two groups based on IHC scores. The results showed that low expression of ALKBH5 was positively correlated with larger tumor size, higher ES grade and tumor encapsulation (Fig. 1C; Table 1). And survival analysis showed low ALKBH5 expression was associated with poor overall survival (OS) and progression-free survival (PFS) (Fig. 1D and E). Subsequently, exploration of the GSE6764 dataset confirmed that ALKBH5 expression in HCC tissue was lower than that in normal liver tissue (Fig. 1F). Furthermore, survival curve analysis based on HCC samples from the Kaplan-Meier Plotter dataset showed that patients with low ALKBH5 expression had poor OS and PFS (Fig. 1G and H). These results revealed that the downregulation of ALKBH5 has a significant correlation with a worse prognosis in HCC patients.
ALKBH5 is downregulated in HCC and correlates with a poor prognosis. (A) Representative images of ALKBH5 IHC staining in HCC tissues and adjacent non-tumor tissues. (B) IHC scores of ALKBH5 staining in HCC tissues and adjacent nontumor tissues (n = 50). (C) Correlation analysis of ALKBH5 level and clinical features. (D-E) The OS and PFS analysis of patients between low-ALKBH5 and high-ALKBH5 groups. (F) Analysis of differentially expressed ALKBH5 in the HCC-based GSE6764 dataset. (G-H) Kaplan‒Meier analysis showing the OS (left) and PFS (right) of HCC patients based on ALKBH5 expression in TCGA databases. (*P < 0.05, **P < 0.01, ***P < 0.001).
ALKBH5 inhibits proliferation of HCCin vitroandin vivo
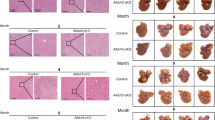
To investigate the function of ALKBH5 in HCC, we first analyzed ALKBH5 expression in HCC cells. Then, we established ALKBH5-overexpressing and ALKBH5-knockdown cell models in HepG2 and Hep3B cells, respectively. The transfection efficiency was validated by western blotting (Fig. 2A). The CCK-8 assay revealed that the overexpression of ALKBH5 resulted in a reduction in the proliferative capacity of HepG2 cells. Conversely, knockdown of ALKBH5 led to an increase in proliferation in Hep3B cells (Fig. 2B). Similarly, the focus-formation assay revealed that overexpression of ALKBH5 resulted in a reduction in colony formation ability in HepG2 cells, whereas knockdown of ALKBH5 led to an increase in colony formation in Hep3B cells (Fig. 2C and D). To evaluate the role of ALKBH5 in vivo, we constructed a subcutaneous implantation model in nude mice using control Hep3B and shALKBH5 Hep3B cells. As expected, ALKBH5 downregulation markedly promoted the tumor volume and weight (Fig. 2E-G).
ALKBH5 inhibits proliferation and migration and promotes apoptosis of HCC cells. (A) Western blot analysis showing ALKBH5 levels in HepG2-cells with stable ALKBH5 overexpression and Hep3B-cells with stable ALKBH5 knockdown. (B) CCK-8 assays showed that ALKBH5 overexpression inhibited cell proliferation and ALKBH5 knockdown promoted cell proliferation. (C, D) Colony formation assays of HCC cell lines with ALKBH5 overexpression or knockdown. (E) Images of subcutaneous tumor in Hep3B cells xenograft implantation model. (F-G) Tumor volume and weight of subcutaneous tumor (n = 6). (*P < 0.05, **P < 0.01, ***P < 0.001).
ALKBH5 inhibits migration and promotes apoptosis of HCC
Also, cell cycle experiments demonstrated that the overexpression of ALKBH5 led to an increase in the proportion of cells in the G1 phase, while reducing the proportions of cells in both the S and G2 phases (Fig. 3A), while the opposite result was observed in ALKBH5 knockdown cells (Fig. 3B). In addition, we performed Transwell assays to evaluate the effect of ALKBH5 on cell migration. The results showed that overexpression of ALKBH5 inhibited the migration capability of HepG2 cells, while downregulation of ALKBH5 promoted the migration activity of Hep3B cells (Fig. 3C and D). Moreover, the flow cytometry assay indicated that ALKBH5 overexpression enhanced apoptosis in HepG2 cells, while the number of apoptotic cells decreased after ALKBH5 knockdown (Fig. 3E and F). Subsequently, assessed the impact of ALKBH5 on HCC metastatic capacity in vivo. A liver metastasis model was established through tail vein injection. We established a liver metastatic model by tail vein injection. More metastatic nodules were observed in the Hep3B-shALKBH5 group than in the control group (Fig. 3G-I). Overall, these results proved that ALKBH5 acted as a tumor suppressor in HCC.
(A, B) Cell cycle assays showed that ALKBH5 overexpression induced cell cycle arrest at the G1/S phase and that ALKBH5 knockdown accelerated cell cycle progression at the G1/S phase. (C, D) Cell migration assays showed that ALKBH5 overexpression abolished the cell migration capabilities and ALKBH5 knockdown increased the cell migration capabilities. (E, F) Overexpression of ALKBH5 promoted cell apoptosis, and knockdown of ALKBH5 decreased cell apoptosis in cell apoptosis assays. (G-I)Downregulation of ALKBH5 promoted the metastasis of Hep3B cells in vivo, and the number of liver metastasis nodules and liver weights in the mice was determined (n = 6). (*P < 0.05, **P < 0.01).
Differentially expressed genes revealed ALKBH5 involvement in tumor immunity
Previous studies have reported that ALKBH5 is an important mammalian m6A demethylase that is involved in many types of tumors by affecting RNA export and metabolism of its target gene25,26,27. To explore the molecular mechanism of ALKBH5 in HCC, we performed m6A-seq combined with RNA-seq to comprehensively profile the target gene landscape in ALKBH5-downregulated and control Hep3B cells. m6A-seq identified 797 different transcripts (911 different m6A peaks) including 425 hypermethylated and 371 hypomethylated transcripts. RNA-seq uncovered 350 upregulated and 955 downregulated transcripts. These differentially expressed and methylated transcripts were visualized by volcano plots, respectively (Fig. 4A and F). Then, the top 10 upregulated genes and the top 10 downregulated genes were illustrated in a heatmap (Fig. 4B). Additionally, a heatmap was used to show the top 10 transcripts with hypermethylation and the top 10 with hypomethylation (Fig. 4G). GSEA based on all expressed and methylated transcripts enriched the liver NK and NKT cell signatures (Fig. 4C and H). GO analysis indicated that the differentially regulated transcripts were primarily enriched in the T-cell activation and T cell differentiation pathways (Fig. 4D and I). KEGG analysis showed that the P53, NF-κB and TNFsignaling pathways were enriched (Fig. 4E and J). Overall, these results suggested that ALKBH5 may participate in tumor immunity in HCC by affecting m6A modification of its downstream target genes.
Differentially expressed genes revealed that ALKBH5 is involved in tumor immunity. (A) Volcano plot of differentially expressed transcripts regulated by ALKBH5. (B) Heatmap of the top 10 positively regulated genes and the top 10 negatively regulated genes. (C) GSEA of transcripts from RNA-seq. (D) GO analysis of differentially expressed transcripts. (E) KEGG analysis of differentially expressed transcripts. (F) Volcano plot of differentially methylated transcripts regulated by ALKBH5. (G) Heatmap of the top 10 genes with hypermethylation and top 10 with hypomethylation. (H) GSEA of transcripts from m6A-seq. (I) GO analysis of differentially methylated transcripts. (J) KEGG analysis of differentially methylated transcripts.
Identification of the MRGs and HCC molecular subtypes
The flowchart presents the main design of our research (Fig. 5A). Considering the demethylation activity of ALKBH5, we intersected the hypermethylated genes with the high/low expressed genes and obtained 39 MRGs for further exploration (Fig. 5B). We examined the expression levels of these 39 genes in the TCGA-LIHC dataset containing 369 HCC samples and 50 normal samples. The results showed that most of these genes were highly expressed in HCC tissues (Fig. 5C). Six prognosis-related MRGs (key MRGs) were chosen for the following investigations (Fig. 5D). Based on the expression levels of these 6 key MRGs, HCC patients in the TCGA-LIHC dataset were divided into two regulation clusters by the consensus clustering algorithm, including 270 patients in Cluster 1 and 99 patients in Cluster 2 (Fig. 5E). Furthermore, PCA confirmed the remarkable difference between the two subtypes (Fig. 5F). The 6 key MRGs were highly expressed in Cluster 2 (Fig. 5G). The survival analysis of patients with complete survival information suggested that patients in Cluster 1 had longer OS and PFS compared with those in Cluster 2 (Fig. 5H and I). In addition, we explored the difference in the clinicopathological parameters of these two HCC subtypes (Table 2). More patients with histologic grade G1/2, TNM stage I/II and lower AFP level were in Cluster 1, which means that patients in Cluster 1 had a better prognosis. In summary, ALKBH5-mediated m6A methylation of core genes may be associated with the progression of HCC.
Identification of the MRGs and HCC molecular subtypes. (A) The overall design of the whole study. (B) Upregulated/downregulated (blue circle) and hypermethylated (red circle) transcripts were combined using a Venn diagram. 39 transcripts containing in the overlap were identified as MRGs. (C) Expression of MRGs in HCC tissues and normal tissues based on the TCGA database. (D) Batch survival analysis of MRGs and 6 prognosis-related MRGs (P value < 0.01) were defined as the key MRGs. (E) Consensus clustering of HCC patients in the TCGA cohort according to the expression of key MRGs. (F) PCA of HCC subtypes. (G) The expression of 6 key MRGs in different HCC subtypes. (H, I) Kaplan–Meier curves for OS and PFS of different HCC subtypes. (*P < 0.05, **P < 0.01, ***P < 0.001).
Patients in the two subtypes exhibit different TIME
We found that these key MRGs used for consensus clustering may be involved in tumor immunity in HCC based on functional enrichment analysis. We estimated the association between the HCC molecular subtypes and TIME using CIBERSORT and ESTIMATE analyses. In Cluster 1, the infiltration degree of CD8+ T cells, CD4+ T cells, monocytes, resting mast cells and M1 macrophages was higher than that in Cluster 2. Nevertheless, opposite results were found for memory B cells, follicular helper T cells, M0 macrophages and neutrophils (Fig. 6A). The TIME scores indicated that Cluster 1 had higher stromal scores and ESTIMATE scores than Cluster 2. However, the immune scores between the two clusters exhibited no significant difference (Fig. 6B). Then, we checked the correlations between these 6 MRGs and immune cell infiltration. Most MRGs were associated with immune cells (Fig. 6C). In addition, we explored the correlation between our molecular subtypes and the 6 immune subtypes of HCC identified in previous research: wound healing (C1), IFN-γ dominant (C2), inflammatory (C3), lymphocyte depleted (C4), immunologically quiet (C5), and TGF-β dominant (C6)28. Cluster 1 was enriched in C3 and C4, while Cluster 2 was significantly enriched in C1, C4 and C6 (Fig. 6D).
Patients in the two subtypes exhibit different immune infiltration and TIME. (A) The immune cell infiltration in the two HCC subtypes. (B) The TME score in the two HCC subtypes. (C) The relationship between HCC subtypes and immune subtypes. (D) Correlation analysis of the key MRGs and immune cell infiltration. (*P < 0.05, **P < 0.01, ***P < 0.001).
Immunotherapy response evaluation of patients in the two subtypes
Targeting therapy with immune checkpoint inhibitors (ICIs) has been approved for advanced malignant tumors, and some extensively studied biomarkers, such as PD-L1, TMB and some immune-related genes, can be used to predict the response of these patients to ICIs therapy29. Therefore, we evaluated the difference in these immune-associated biomarkers between the two clusters. Most immunostimulatory genes were more highly expressed in Cluster 2 (Fig. 7A). Moreover, immunoinhibitory genes, including PD-1, PD-L1 and CTLA4, were highly expressed in Cluster 2 (Fig. 7B). In addition, some other immune response indicators of Cluster 2 were higher than those of Cluster 1, such as the TIDE score level, which means that patients in Cluster 2 would have worse immunotherapy efficacy (Fig. 7C). These results were also evidenced by the lower MSI score in Cluster 2 (Fig. 7C). Also, the values of T-cell exclusion and MDSC in Cluster 2 were higher than those in Cluster 1(Fig. 7D and E).Moreover, we compared the genetic mutational landscapes of the two HCC subtypes. Patients in Cluster 2 had more mutation events than those in Cluster 1 (88.54% versus 81.51%), and TP53 mutation was the most common alteration in Cluster 2, which occurred in 57% of patients (Fig. 7F and G). CTNNB1 was the major mutated gene in Cluster 1 patients (Fig. 7F). Moreover, the mutation rate of CTNNB1 in Cluster 1 was higher than that in Cluster 2. TMB was calculated based on the mutation profiles, and Cluster 2 obtained a higher TMB score than Cluster 1 (Fig. 7F and G). T-cell exhaustion is a new concept in understanding ICIs responses30. The expression of markers for exhausted CD8+ T cells in Cluster 2 was higher than that in Cluster 1 (Fig. 7H). WNT/β-catenin signaling pathway activation has been reported to be related to immune exclusion, which leads to the failure of ICIs therapy31. We examined the gene expression of key regulators in the WNT/β-catenin signaling pathway to evaluate the difference between the two subtypes. The results revealed that all these regulators were highly expressed in Cluster 2 (Fig. 7I). These results also indicated that Cluster 1 patients benefitted more from ICIs therapy. Taken together, our results suggested that ALKBH5-mediated m6A demethylation could affect immunotherapy response in HCC patients.
Immunotherapy response evaluation of patients in the two subtypes. (A, B) Expression of immunostimulatory and immunoinhibitory genes in the two HCC subtypes. (C) Differences in TIDE score, IFNG and MSI in the two HCC subtypes. (D) Differences in T-cell dysfunction and exclusion in the two HCC subtypes based on the TIDE database. (E) Differences in MDSC of the two HCC subtypes. (F, G) Landscape of genomic mutations in the two HCC subtypes. (H) The expression of T-cell exhaustion markers in the two HCC subtypes. (I) The expression of key regulators in WNT/β-catenin signaling between the two HCC subtypes. (*P < 0.05, **P < 0.01, ***P < 0.001).
TIM3 is a target of ALKBH5-mediated m6A demethylation
To elucidate the regulatory role of ALKBH5 in antitumor immunity, we focused on the immunity-related molecules regulated by ALKBH5 through m6A-seq and RNA-seq data. TIM3 (encoded by HAVCR2) was an immune checkpoint and upregulated when ALKBH5 was silenced among the ALKBH5 potential target genes (Fig. 8A). Therefore, we hypothesized that ALKBH5 regulates the m6A modification of TIM3 involved in HCC antitumor immunity. And m6A-seq data showed m6A level of TIM3 was upregulated in siALKBH5 HCC cells (Fig. 8B). The m6A residue was located in TIM3 5′-DRACH-3′ motif (D = A/G/U, R = G/A, and H = C/A/U) (Fig. 8C). The expression of TIM3 was downregulated by ALKBH5 as demonstrated by qPCR and western blot analysis. In contrast, the expression level of TIM3 was restored when ALKBH5 was silenced. The results revealed that TIM3 expression was inversely regulated by ALKBH5 (Fig. 8D and E). Taken together, these results suggested TIM3 was the target of ALKBH5.
TIM3 is a target of ALKBH5. (A)TIM3 was upregulated in siALKBH5 cells. (B) The m6A peak analysis of TIM3 in siControl and siALKBH5 cells. (C) The m6A residues prediction of TIM3 in SRAMP website (http://www.cuilab.cn/sramp/). (D-E) RNA and protein level of TIM3 in overexpression or knockdown ALKBH5 cells. (F) Representative IHC images of TIM3 in paired HCC tissues. (G) Comparison of IHC score of TIM3 in paired HCC tissues (n = 50). (H) Survival analysis between low-TIM3 and high-TIM3 groups. (I) Correlation between protein level of ALKBH5 and TIM3 in HCC tissues. (*P < 0.05, **P < 0.01).
To further investigate the expression and clinical implications of TIM3 in HCC, we conducted IHC staining on a cohort of 50 HCC tumor tissues and corresponding non-tumor tissues. Our findings revealed significantly elevated levels of TIM3 expression in HCC tissues compared to non-tumor tissues (Fig. 8F and G). Survival analysis showed that OS and PFS of patients exhibiting high TIM3 expression were inferior compared to those with corresponding low expression (Fig. 8H). What’s more, the patients with low expression of ALKBH5 showed high TIM3 expression in HCC (Fig. 8I). These data indicated high expression of TIM3 in HCC tissues was associated with poor prognosis and its expression was inversely correlated with ALKBH5 expression.
Discussion
HCC is the third leading cause of cancer-related death worldwide with low early diagnosis and high mortality1,32. The dysregulation of epigenetic modification has contributed greatly to HCC progression. In recent years, emerging evidence has demonstrated that the N6-methyl-adenosine (m6A) modification of RNA is substantially involved in the pathogenesis of HCC as a new layer of epigenetic regulation. m6A is a dynamic and reversible process regulated by three different functional enzymes: m6A methyltransferases (“writers”), m6A demethylases (“erasers”) and m6A binding proteins (“readers”)33,34. RNA m6A modification enzymes play a prominent role in regulating mRNA stability, degradation, splicing and translation in liver cancer35,36. For example, the m6A demethylases FTO increased the m6A abundance of GPNMB and stabilized its mRNA to inhibit CD8 T cell activation, thus leading to the proliferation and migration of HCC cells37. The m6A reading enzyme YTHDF1 directly binds to the m6A modified NOTCH1 mRNA, and plays a role in promoting CSCs renewal and resistance to targeted therapies by enhancing its stability and translation in HCC38. However, as an important m6A demethylase, the mechanism of ALKBH5 involvement in the regulation of HCC is still not detailed, especially its role in HCC anti-tumor immunity.
A few reports have suggested that ALKBH5 is involved in HCC pathogenesis, but the results remain contradictory26,39,40. This may be attributed to different factors, such as cell lineage selection, viral infection and the immune microenvironment. This laterally confirms that the study of ALKBH5 requires a comprehensive consideration of the pertinent underlying mechanisms. In our study, we dissected the involvement of ALKBH5 in the malignant progression of HCC from clinical features, in vitro and in vivo experiments, and bioinformatics analysis of m6A sequencing combined with RNA sequencing, which enhances the persuasiveness of the evidence. Based on clinicopathological analysis of our collected clinical samples, we found that ALKBH5 was downregulated in HCC tissues and that its downregulation was associated with poor survival of HCC patients, which generally coincided with previous work26. The cytologic assay revealed that overexpression of ALKBH5 strikingly inhibited the proliferation and migration of HCC cells and enhanced the apoptosis of HCC cells, whereas knockdown of ALKBH5 had the opposite effects. Also, knockdown ALKBH5 increased the tumor growth and migration of tumors in vivo. Thus, through clinicopathological validation and in vitro and in vivo experiments, our study further clarifies the pivotal role of ALKBH5 in HCC.
Epigenetic therapy of the immune response has been brought to the forefront in recent studies. m6A modification has been proven to regulate the complexity and diversity of the TIME41,42. After functional enrichment analysis of our m6A-seq and RNA-seq data in Hep3B cells with ALKBH5 knockdown, we found that some immune-related pathways were enriched, such as T-cell activation signaling pathways. This implies that ALKBH5 influences HCC tumor immunity. Then, we identified two HCC molecular subtypes (Cluster 1 and Cluster 2) based on the expression of 6 key MRGs in the TCGA database. Furthermore, the analysis of survival and clinical characteristics suggested that Cluster 1 had a better prognosis.
Immune cells are essential components of the TIME that can be utilized to predict the prognosis of cancer patients43. Activated CD4+ T cells and CD8+ T cells in the tumor are associated with favorable prognosis in HCC patients11. Macrophages (M0) can be differentiated into two different phenotypes (M1 and M2) in response to cytokine or pathogenic signals, in which M1 macrophages have antitumor immunity effects, while M2 macrophages inhibit tumor immunity44. Tumor-associated monocytes and neutrophils are viewed as “double-edged swords” according to recent studies. Increased monocytes can induce HCC cell death in the early stage, and neutrophils drive HCC progression by immunosuppression11,45. Similarly, infiltration of follicular helper T cells is associated with unfavorable survival in tumors46. Inhibition of mast cells are considered as a promising strategy to improve ICIs efficiay47. We analyzed the difference in tumor-infiltrating immune cells between these two subtypes according to distinct m6A methylation modifications regulated by ALKBH5. The results showed that antitumor immune cells, such as CD8+ T cells and CD4+ T cells, were less abundant in Cluster 2, while the abundance of infiltrating immunosuppressive cells, such as monocytes and neutrophils, was high. Additionally, the stromal score in Cluster 2 was lower than that in Cluster (1) These results indicated that patients in Cluster 2 were in an immunosuppressive state, which mainly accounts for the poor survival of Cluster (2) In summary, we comprehensively analyzed the characteristics of the TIME based on different m6A methylation modification patterns and transcriptional features under the impact of ALKBH5.
Tumor cells can upregulate inhibitory immunoreceptors to induce immune suppression and immune evasion48. PD-L1 and TMB expression are considered to be predictive biomarkers for ICIs49,50,51. However, there is still an urgent need to find effective biomarkers for diagnostic treatment because of the low incidence of high TMB levels or high PD-L1 expression in HCC24. In this study, our results showed that immunoinhibitory factors were highly expressed in Cluster 2. Although TMB and PD-L1 expression in Cluster 2 were higher than those in Cluster 1, their levels were fairly low. Therefore, TMB and PD-L1 are not sufficient as valid biomarkers for HCC patients. Recently, TIDE has been shown to serve as a more accurate biomarker for ICIs response52. We compared some indices of the two subtypes in the TIDE database. Cluster 2 had a high TIDE score, MDSC and T-cell exclusion score as well as a low MSI score. This demonstrates that Cluster 2 had immune escape phenotypes and poor response to ICIs.
Activation of the Wnt/β-catenin signaling pathway is correlated with exhaustion of T cells53. β-catenin downregulates the expression of CCL4 to inhibit CD8+ T-cell activation by repressing the recruitment of CD103+ dendritic cells54. Markers of T-cell exhaustion were highly expressed in Cluster 2. Genes in the Wnt/β-catenin signaling pathway were also upregulated in Cluster 2. These results also revealed that Cluster 2 patients would not benefit from immunotherapies.
TIM3 is a novel immune checkpoint protein and has been considered a marker of exhausted CD4 and CD8 T cells2. Emerging evidence of preclinical and clinical trials suggests antibodies targeting TIM3, especially combined with other ICIs, is a promising treatment option for HCC patients12,55. Given that the antitumor effect of immune checkpoints is closely related to their surface expression, modulating the surface expression by epigenetic modification gains great attention. We found that ALKBH5 could act as an upstream regulator of TIM3. ALKBH5-mediated m6A demethylation decreased the m6A level of TIM3, which in turn decreased its mRNA and protein levels. Moreover, HCC patients with elevated TIM3 expression had worse survival.
In conclusion, downregulated ALKBH5 was associated with a poor prognosis in HCC. And ALKBH5 acts as a tumor suppressor in vitro and in vivo. The subtype with low expression of 6 key MRGs was associated with a good prognosis, an antitumor immune microenvironment and a better response to immunotherapy. Furthermore, low expression of ALKBH5 leads to elevated levels of m6A methylation on TIM3, which was responsible for the TIM3 upregulation in HCC (Fig. 9). We discovered the directly regulation of ALKBH5 on immune checkpoint TIM3 for the first time. Our work This effort provided new insigmht into the correlation between m6A methylation and tumor immunity.
Though we have disclosed the relation between ALKBH5-mediated m6A and TIM3, several limitations should be noted in this study. Firstly, whether ALKBH5 affects TIME and immunotherapy response by targeting TIM3 need to be confirmed in the additional animal experiments. Secondly, several studies have found soluble TIM3 facilitates tumor progression and confers anti-PD-1 resistance in HCC56. We cannot confirm whether the regulation of ALKBH5 in TIM3 expression affect soluble TIM3 level in current study.
Data availability
The original datasets used in this study are available in The Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/tcga) and the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE6764). Further inquiries can be directed to the corresponding author.
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Yang, C. et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 20, 203–222. https://doi.org/10.1038/s41575-022-00704-9 (2023).
Sivasudhan, E., Blake, N., Lu, Z. L., Meng, J. & Rong, R. Dynamics of m6A RNA methylome on the hallmarks of hepatocellular carcinoma. Front. Cell. Dev. Biol. 9, 642443. https://doi.org/10.3389/fcell.2021.642443 (2021).
Yang, Y. et al. O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N(6)-methyladenosine-dependent manner. Signal. Transduct. Target. Ther. 8, 63. https://doi.org/10.1038/s41392-023-01316-8 (2023).
Ma, H. et al. N(6)-methyladenosine (m(6)A) modification in hepatocellular carcinoma. Biomed. Pharmacother. 173, 116365. https://doi.org/10.1016/j.biopha.2024.116365 (2024).
Lv, D. et al. EGFR promotes ALKBH5 nuclear retention to attenuate N6-methyladenosine and protect against ferroptosis in glioblastoma. Mol. Cell 83, 4334–4351 e4337, (2023). https://doi.org/10.1016/j.molcel.2023.10.025
Cheng, C. et al. Smoking-induced M2-TAMs, via circEML4 in EVs, promote the progression of NSCLC through ALKBH5-regulated m6A modification of SOCS2 in NSCLC cells. Adv. Sci. (Weinh) 10, e2300953. https://doi.org/10.1002/advs.202300953 (2023).
Jin, S. et al. The m6A demethylase ALKBH5 promotes tumor progression by inhibiting RIG-I expression and interferon alpha production through the IKKepsilon/TBK1/IRF3 pathway in head and neck squamous cell carcinoma. Mol. Cancer 21, 97. https://doi.org/10.1186/s12943-022-01572-2 (2022).
Zhai, J. et al. ALKBH5 drives immune suppression via targeting AXIN2 to promote colorectal cancer and is a target for boosting immunotherapy. Gastroenterology 165, 445–462. https://doi.org/10.1053/j.gastro.2023.04.032 (2023).
Li, N. et al. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc. Natl. Acad. Sci. USA 117, 20159–20170. https://doi.org/10.1073/pnas.1918986117 (2020).
Donne, R. & Lujambio, A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology 77, 1773–1796. https://doi.org/10.1002/hep.32740 (2023).
Vanmeerbeek, I. et al. Targeting conserved TIM3(+)VISTA(+) tumor-associated macrophages overcomes resistance to cancer immunotherapy. Sci. Adv. 10, eadm8660. https://doi.org/10.1126/sciadv.adm8660 (2024).
Xiao, Y. et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 13, 758. https://doi.org/10.1038/s41467-022-28279-8 (2022).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905. https://doi.org/10.1056/NEJMoa1915745 (2020).
Ausejo-Mauleon, I. et al. TIM-3 blockade in diffuse intrinsic pontine glioma models promotes tumor regression and antitumor immune memory. Cancer Cell 41, 1911–1926e https://doi.org/10.1016/j.ccell.2023.09.001 (2023).
Acharya, N., Sabatos-Peyton, C. & Anderson, A. C. Tim-3 finds its place in the cancer immunotherapy landscape. J. Immunother Cancer 8 https://doi.org/10.1136/jitc-2020-000911 (2020).
Joller, N., Anderson, A. C. & Kuchroo, V. K. LAG-3, TIM-3, and TIGIT: Distinct functions in immune regulation. Immunity 57, 206–222. https://doi.org/10.1016/j.immuni.2024.01.010 (2024).
Edwards, S. C. et al. PD-1 and TIM-3 differentially regulate subsets of mouse IL-17A-producing gammadelta T cells. J. Exp. Med. 220 https://doi.org/10.1084/jem.20211431 (2023).
Das, M., Zhu, C. & Kuchroo, V. K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 276, 97–111. https://doi.org/10.1111/imr.12520 (2017).
Guo, Q. et al. Cancer cell intrinsic TIM-3 induces glioblastoma progression. iScience 25, 105329. https://doi.org/10.1016/j.isci.2022.105329 (2022).
Shi, Y. et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat. Commun. 10, 4892. https://doi.org/10.1038/s41467-019-12801-6 (2019).
Ni, W. et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)a reader YTHDF3. Mol. Cancer 18, 143. https://doi.org/10.1186/s12943-019-1079-y (2019).
Yang, L. et al. Melatonin restores the pluripotency of long-term-cultured embryonic stem cells through melatonin receptor-dependent m6A RNA regulation. J. Pineal Res. 69, e12669. https://doi.org/10.1111/jpi.12669 (2020).
Hsu, C. L. et al. Exploring markers of exhausted CD8 T cells to predict response to Immune checkpoint inhibitor therapy for hepatocellular carcinoma. Liver Cancer. 10, 346–359. https://doi.org/10.1159/000515305 (2021).
Zheng, G. et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 49, 18–29. https://doi.org/10.1016/j.molcel.2012.10.015 (2013).
Chen, Y. et al. ALKBH5 suppresses malignancy of hepatocellular carcinoma via m(6)A-guided epigenetic inhibition of LYPD1. Mol. Cancer 19, 123. https://doi.org/10.1186/s12943-020-01239-w (2020).
Hu, Y. et al. Demethylase ALKBH5 suppresses invasion of gastric cancer via PKMYT1 m6A modification. Mol. Cancer 21, 34. https://doi.org/10.1186/s12943-022-01522-y (2022).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830 e814. https://doi.org/10.1016/j.immuni.2018.03.023 (2018).
He, Q., Yang, J. & Jin, Y. Immune infiltration and clinical significance analyses of the coagulation-related genes in hepatocellular carcinoma. Brief. Bioinform. 23 https://doi.org/10.1093/bib/bbac291 (2022).
Baessler, A. & Vignali, D. A. A. T cell exhaustion. Annu. Rev. Immunol. 42, 179–206. https://doi.org/10.1146/annurev-immunol-090222-110914 (2024).
Wang, K. et al. Hyperactivation of beta-catenin signal in hepatocellular carcinoma recruits myeloid-derived suppressor cells through PF4-CXCR3 axis. Cancer Lett. 586, 216690. https://doi.org/10.1016/j.canlet.2024.216690 (2024).
Foerster, F., Gairing, S. J., Ilyas, S. I. & Galle, P. R. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology 75, 1604–1626. https://doi.org/10.1002/hep.32447 (2022).
Yang, Y. et al. Dysregulated m6A modification promotes lipogenesis and development of non-alcoholic fatty liver disease and hepatocellular carcinoma. Mol. Ther. 30, 2342–2353. https://doi.org/10.1016/j.ymthe.2022.02.021 (2022).
Boulias, K. & Greer, E. L. Biological roles of adenine methylation in RNA. Nat. Rev. Genet. 24, 143–160. https://doi.org/10.1038/s41576-022-00534-0 (2022).
Pan, X. Y., Huang, C. & Li, J. The emerging roles of m(6)a modification in liver carcinogenesis. Int. J. Biol. Sci. 17, 271–284. https://doi.org/10.7150/ijbs.50003 (2021).
Wang, X. et al. Crosstalk between N6-methyladenosine modification and circular RNAs: Current understanding and future directions. Mol. Cancer 20, 121. https://doi.org/10.1186/s12943-021-01415-6 (2021).
Chen, A. et al. Targeting the oncogenic m6A demethylase FTO suppresses tumourigenesis and potentiates immune response in hepatocellular carcinoma. Gut https://doi.org/10.1136/gutjnl-2024-331903 (2024).
Zhang, X. et al. N6-Methyladenosine reader YTHDF1 promotes stemness and therapeutic resistance in Hepatocellular Carcinoma by enhancing NOTCH1 expression. Cancer Res. 84, 827–840. https://doi.org/10.1158/0008-5472.CAN-23-1916 (2024).
Qu, S. et al. A positive-feedback loop between HBx and ALKBH5 promotes hepatocellular carcinogenesis. BMC Cancer. 21, 686. https://doi.org/10.1186/s12885-021-08449-5 (2021).
You, Y. et al. ALKBH5/MAP3K8 axis regulates PD-L1 + macrophage infiltration and promotes hepatocellular carcinoma progression. Int. J. Biol. Sci. 18, 5001–5018. https://doi.org/10.7150/ijbs.70149 (2022).
Zhang, B. et al. M(6)a regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol. Cancer 19, 53. https://doi.org/10.1186/s12943-020-01170-0 (2020).
Liu, Y. et al. Allosteric regulation of IGF2BP1 as a Novel Strategy for the activation of tumor immune microenvironment. ACS Cent. Sci. 8, 1102–1115. https://doi.org/10.1021/acscentsci.2c00107 (2022).
Pandey, P. R., Young, K. H., Kumar, D. & Jain, N. RNA-mediated immunotherapy regulating tumor immune microenvironment: Next wave of cancer therapeutics. Mol. Cancer 21, 58. https://doi.org/10.1186/s12943-022-01528-6 (2022).
Wanderley, C. W. et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 Profile in a TLR4-dependent manner. Cancer Res. 78, 5891–5900. https://doi.org/10.1158/0008-5472.CAN-17-3480 (2018).
Geh, D. et al. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 19, 257–273. https://doi.org/10.1038/s41575-021-00568-5 (2022).
Bai, X. et al. Reciprocal regulation of T follicular helper cells and dendritic cells drives colitis development. Nat. Immunol. 25, 1383–1394. https://doi.org/10.1038/s41590-024-01882-1 (2024).
Somasundaram, R. et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat. Commun. 12, 346. https://doi.org/10.1038/s41467-020-20600-7 (2021).
He, X. & Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell. Res. 30, 660–669. https://doi.org/10.1038/s41422-020-0343-4 (2020).
Sangro, B. et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 73, 1460–1469. https://doi.org/10.1016/j.jhep.2020.07.026 (2020).
Tang, B. et al. Diagnosis and prognosis models for hepatocellular carcinoma patient’s management based on tumor mutation burden. J. Adv. Res. 33, 153–165. https://doi.org/10.1016/j.jare.2021.01.018 (2021).
Zhao, Q. et al. Crosstalk between colorectal CSCs and immune cells in tumorigenesis, and strategies for targeting colorectal CSCs. Exp. Hematol. Oncol. 13, 6. https://doi.org/10.1186/s40164-024-00474-x (2024).
Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 24, 1550–1558. https://doi.org/10.1038/s41591-018-0136-1 (2018).
Zhang, N. et al. Biomarkers and prognostic factors of PD-1/PD-L1 inhibitor-based therapy in patients with advanced hepatocellular carcinoma. Biomark. Res. 12, 26. https://doi.org/10.1186/s40364-023-00535-z (2024).
Xue, J. et al. Intrinsic beta-catenin signaling suppresses CD8(+) T-cell infiltration in colorectal cancer. Biomed. Pharmacother. 115, 108921. https://doi.org/10.1016/j.biopha.2019.108921 (2019).
Dixon, K. O., Lahore, G. F. & Kuchroo, V. K. Beyond T cell exhaustion: TIM-3 regulation of myeloid cells. Sci. Immunol. 9, eadf2223. https://doi.org/10.1126/sciimmunol.adf2223 (2024).
Chen, C. et al. Soluble Tim-3 serves as a tumor prognostic marker and therapeutic target for CD8(+) T cell exhaustion and anti-PD-1 resistance. Cell. Rep. Med. 5, 101686. https://doi.org/10.1016/j.xcrm.2024.101686 (2024).
Acknowledgements
The authors acknowledge the contributions from The Cancer Genome Atlas database and Gene Expression Omnibus database.
Funding
This study was supported by the National Natural Science Foundation of China (82002984, 31371399 and 81803132), Natural Science Foundation of Jiangsu Province (BK20200119), Anhui Province Key Laboratory of Translational Cancer Research (Bengbu Medical College) (KFDX202201).
Author information
Authors and Affiliations
Contributions
J. L. and W. S. designed the study. H. M. completed the cytology experiments and performed the data analysis. Clinical specimen collection was performed by Y. H., Z. X., Z. W. and Y. Y. Figures and table were edited by D. J., Z. C. and X. Z. The first manuscript was revised by Z. X. and F. F. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Ethics Committee of Nanjing Drum Tower Hospital. Written informed consents was obtained from all patients.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, H., Hong, Y., Xu, Z. et al. ALKBH5 acts a tumor-suppressive biomarker and is associated with immunotherapy response in hepatocellular carcinoma. Sci Rep 15, 55 (2025). https://doi.org/10.1038/s41598-024-84050-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-84050-7
Keywords
This article is cited by
-
The m6A modification in cancer: roles, implications, and its potential in therapy
Molecular Biomedicine (2025)
-
RNA methylation in hepatocellular carcinoma: from metabolic reprogramming and immune escape mechanisms to small molecule inhibitor development
Journal of Translational Medicine (2025)