Abstract
Metabolomics enables the characterisation of pathogenic and diagnostic biomarkers in cardiovascular disease (CVD). Given the high prevalence of rheumatic heart disease (RHD) and degenerative aortic stenosis (AS) in Africa, we investigated potential discriminant metabolic biomarkers in individuals with severe RHD and AS undergoing valve replacement and compared them to those in matched controls. Untargeted metabolomics of serum samples showed that seven metabolites that were differentially expressed in RHD patients were independent of the patients’ baseline characteristics (covariates) and could differentiate RHD patients from healthy controls (AUC > 0.7). Four metabolites could differentiate AS patients from controls (AUC ≥ 0.7). Of the perturbed metabolites in RHD and AS, 7-HOCA and deoxycholate showed a moderate association with left ventricular ejection fraction. Furthermore, acylcarnitine and ketone body levels were correlated with the left ventricular mass index in RHD and left atrial area in AS patients. Elevated levels of cortisol were associated with the presence of valve calcification in RHD and AS patients. This is a pilot study on rarely studied CVD in sub-Saharan Africa. This study suggested that the metabolites altered in RHD and degenerative AS are not only associated with cardiac remodelling but also involved in major energetic pathways, amino acid metabolism, and inflammation regulation processes.
Similar content being viewed by others
Introduction
Rheumatic heart disease (RHD) is the most common contributor to cardiovascular disease (CVD) and cardiovascular-related morbidity in people aged 20–25 years in sub-Saharan Africa (SSA)1,2. Specifically, RHD is reported to affect approximately 30 people per 100,000 people in SSA and other low- and middle-income countries (LMICs)1. RHD develops after repeated acute rheumatic fever (ARF) episodes due to oropharyngeal infection with group A Streptococcus (GAS)3. Poor housing, low education levels, poor diet, alterations in the gut microbiome, and genetic mutations are risk factors for the development of ARF and worsening of RHD3. The pathological mechanisms of RHD are not fully understood, especially the molecular and metabolic processes involved4. Chronic RHD is initiated by autoimmune reactions that lead to increased collagen accumulation, remodelling of the extracellular matrix, and mineralisation of the heart valves4. The prevalence of calcific aortic stenosis (AS) is increasing in SSA1 with age, bicuspid aortic valve (BAV) disease, smoking, hypertension, genetic mutations, and dyslipidemia, which are considered risk factors5. AS progression is associated with osteoblast differentiation, myofibroblast activation, or high shear forces across valves6. Abnormalities in specific lipid classes, especially glycerophospholipids and lipoproteins, are associated with the progression of AS7. Both AS and RHD may progress to heart failure, left ventricular hypertrophy, diastolic dysfunction, myocardial fibrosis, and impaired energetics4,6.
There are no existing therapeutic strategies to halt the progression of RHD and AS4. The only reliable intervention for RHD and AS is valve replacement or implantation, which are not readily available or accessible to many individuals, especially in LMICs. Further, delayed valve replacement diminishes the chances of recovery and increases mortality8,9. Given the similarities in the clinical presentation of RHD and AS, the ability to distinguish them in the clinic using low-cost, easily available testing without the need for specialised expertise or equipment is a clinically unmet need.
Metabolomics is a robust technology that has the potential to identify metabolic pathways and provides an opportunity to describe metabolic and genetic alterations associated with disease progression and for the discovery of diagnostic markers10. Metabolomics studies can be designed as targeted or untargeted approaches. Targeted metabolomics tests existing hypotheses and measures a specific panel of predetermined metabolites, ideally with methods optimised for the detection and quantification of these metabolites. In contrast, the untargeted metabolomics approach is a hypothesis-generating study capable of identifying potential novel diagnostic biomarkers and therapeutic targets or identifying mechanisms that may explain the pathogenesis of the disease11. Several metabolomics studies have explored the pathophysiological mechanisms and diagnostic biomarkers of AS. However, to the best of our knowledge, very few studies have explored metabolic biomarkers in RHD6,12. Changes in the gut microbiome are associated with metabolic changes as well as the progression of RHD and AS13,14. Prior studies using metabolomics have evaluated metabolic changes associated with ventricular remodelling in CVD15,16. RHD and degenerative AS have different mechanisms of development: RHD is an autoimmune response, while degenerative AS may be associated with low grade inflammation due to oxidative stress, age-related calcification, shear stress, and lipid deposition3,5. An inflammatory state shifts metabolic processes to support continued elevated inflammatory biomarkers17. To date, few studies have directly investigated metabolic profiles between individuals with RHD and those with degenerative AS and compared them to those of healthy controls. We therefore hypothesised that RHD and degenerative AS will elicit different metabolic findings due to different pathomechanisms. Therefore, we applied an untargeted metabolomics approach involving ultra-performance liquid chromatography with quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS) to detect serum metabolomic biomarkers in RHD and AS patients undergoing valve replacement. In addition, we characterised the associations of RHD and AS cardiac remodelling with changed biomarkers.
Results
Baseline characteristics of the study participants
The baseline characteristics of the enrolled participants are summarised in Table 1. The mean age in years was 44.7 ± 13.7 years for RHD patients, 64.2 ± 12.8 years for patients with degenerative AS, and 52.1 ± 8.11 years for controls (p < 0.001). As expected, AS patients had an elevated mean systolic blood pressure of 143.0 ± 23.1 mmHg compared to RHD patients (121.0 ± 24.2 mmHg) and controls (138.0 ± 23.7 mmHg) (p = 0.01). Furthermore, patients with AS had an elevated BMI of 33.6 ± 7.58 kg/m2 compared to those with RHD (27.4 ± 6.07 kg/m2) and controls (30.0 ± 4.07 kg/m2) (p = 0.01). Compared with AS patients and controls, RHD patients had impaired systolic function; the mean left ventricular ejection fraction (LVEF) of RHD patients was 44.8 ± 14.7%, that of AS patients was 57.0 ± 4.07%, and that of controls was 56.1 ± 12.1% (p = 0.02). Both the RHD and AS patients had dilated left atria compared to the controls (p < 0.001). Degenerative AS patients had greater left ventricular mass than did RHD patients and controls (p = 0.01). Histologically, AS was predominantly associated with calcification (p < 0.01) and fibrosis, while RHD was mostly associated with neovascularisation (p = 0.01), fibrosis and acute rheumatic valvulitis. Aortic stenosis patients presented with severe aortic stenosis (p < 0.001), while RHD patients were mostly characterised by severe mitral regurgitation and mitral stenosis (p < 0.001).
Discovery and exploratory analysis of potential biomarkers
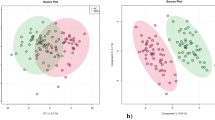
Initially, 41,738 and 31,772 features were detected in the positive (ESI+) and negative (ESI-) ionisation modes, respectively. After peak picking, alignment, and initial filtering, 3,692 (ESI+) and 2,864 (ESI-) features were extracted. The median RSD of the QC (mRSD) was used as a measure of the technical reproducibility and was 29.99% (ESI+) and 35.85% (ESI-), respectively, following data preprocessing. After batch normalisation and filtration based on the QC RSD cut-off (≤ 30%), 1,847 (ESI+, mRSD = 21.87%) and 1,093 (ESI-, mRSD = 18.27%) features (m/z, retention time, and intensity) were extracted and used for statistical analysis. When PCA was used for dimensionality reduction, the RHD samples clustered together in PC1 and PC2, although there was no clear separation between the three groups at this level. PLS-DA clearly distinguished RHD from degenerative AS and controls, suggesting that there are clear metabolic differences between these two groups and the other two groups (Fig. 1a,b). The results suggested that there were m/z features that could be used to differentiate valvular heart disease patients from controls. The multidimensional reduction models reported a PLS-DA cross-validation predictive relevance score (Q2) of 0.49, which is not unusual for a human-based study with high interindividual variability (Fig. 1c). Permutation testing of the model (1000 times) suggested that the modelled data were significantly different (empirical p value = 0.042) from those of a random model (Fig. 1d). ANOVA and Tukey’s HSD post hoc analyses revealed that 26 metabolites were significantly different among the RHD, AS and control groups (p < 0.05) (Table 2).
Chemometric analysis of metabolites dysregulated in the RHD, AS, and control groups. (a) PCA score plot of unsupervised separation of RHD patients (red asterisks), AS patients (green circles), and controls (blue diamonds). (b) PLS-DA score plot of the supervised separation of RHD patients, AS patients, and controls. (c) PLS-DA score model cross-validation based on component 1; R2 = 0.72* and Q2 = 0.49. (d) Permutation test analysis of the model separating RHD patients, AS patients, and controls based on 1000 permutations (empirical p = 0.042). (AS Aortic stenosis, RHD rheumatic heart disease, R2 coefficient of determination, Q2 predictive coefficient of determination).
Characterisation of metabolites altered in RHD patients compared to controls
The chemometric analysis revealed which metabolites were altered between the RHD patients and controls, as summarised in Table 3, indicated by their fold changes (FC and log2FC), crude p values (p), FDR values (q), and covariate-adjusted p values (Covariate adj. p), and the area under the ROC curve (AUC-ROC). Univariate chemometric analysis of the annotated metabolites between RHD patients and controls indicated that 18 metabolites were significantly altered (log2FC > 2, p < 0.05) (Table 3). Of the 18 significantly altered signatures, 4 metabolites:- 3-formylindole, tryptophan, ursodeoxycholic acid, and 2,4-dihydroxy-butanoic acid, were shown to be biomarkers moderately capable of differentiating RHD patients from controls based on AUC-ROC analysis and significance level testing after adjusting for covariates (sex, age, race, BMI, hypertension, diabetes, and batch effects) (AUC > 0.7, covariates adjusted p < 0.05) (Table 2). Moreover, linoleic acid was significantly altered and elevated in RHD patients (Table 3; Fig. 2). Linoleic acid is considered to play a role in regulating the inflammatory response (Fig. 2c). In addition, LysoPE (0:0/22:5) (lysophosphatidylethanolamine) was increased in RHD patients, while LysoPC (18:1/0:0) (lysophosphatidylcholine) was reduced in RHD patients compared to controls (Table 3; Fig. 2a). The altered phospholipids are associated with the glycerophospholipid metabolic pathway (Supp Table S2). Other metabolites that were significantly different between RHD patients and controls were amino acids and their derivatives (valine, tryptophan, and taurine) (Fig. 2b); these metabolites are involved in aminoacyl tRNA biosynthesis and the taurine and hypotaurine metabolism pathways (Supp Table S2).
Exploration of the important metabolites that were detected and perturbed in RHD patients compared to controls. (a) Profile of perturbed amino acids and derivatives. (b) Profile of glycerophospholipid metabolites in RHD patients compared to controls. (c) Profile of the detected metabolites that regulate certain inflammatory responses. (For all the plots, RHD samples, n = 33; control samples, n = 16; *p value < 0.05; **p value < 0.01). (RHD rheumatic heart disease, LysoPE lysophosphatidylethanolamine, LysoPC lysophosphatidylcholine).
Profile of altered metabolites between patients with degenerative aortic stenosis and controls
Univariate chemometric analysis of metabolites that were significantly different between AS patients and controls revealed 12 metabolites (log2FC > 2, p < 0.05) (Table 4). Four of these metabolites (palmitoylcarnitine, isocaproic acid, taurine, and ornithine) have moderate potential to be robust discriminant biomarkers that are largely independent of the effects of sex, age, race, BMI, hypertension, diabetes, and batch differences (AUC ≥ 0.7, covariates adj. p < 0.05) (Table 4). Furthermore, PA (8:0/13:0) (phosphatidic acid) was altered between AS patients and controls (log2FC > 2, p < 0.05) (Fig. 3a). The altered phosphatidic acid and lysophosphatidylcholine are associated with the phosphatidylinositol signalling system and the glycerophospholipid metabolic pathway respectively (Supp Table S3). Taurine levels were lower in AS patients than in controls (Fig. 3b). The pathways associated with the metabolites perturbed in RHD and AS had low coverage by the metabolites detected in this study (Fig. 3c).
Profiling of the important metabolites detected and altered in AS patients compared to controls. (a) The detected perturbations of phosphatidic acid. (b) Changes in taurine between AS patients and controls. (c) Exploration of the pathways associated with the metabolites detected in RHD, AS, and control samples showing the percentage coverage of the detected significant metabolites against the total metabolites within a KEGG pathway (for all the plots, control samples n = 16, AS samples n = 9; *raw p value < 0.05; **raw p value < 0.01). (AS aortic stenosis, PA phosphatidic acid, RHD-HC rheumatic heart disease vs. healthy controls, AS-HC aortic stenosis vs. healthy controls, RHD-AS rheumatic heart disease vs. aortic stenosis).
Metabolites showing associations with ventricular remodelling parameters in RHD and degenerative AS
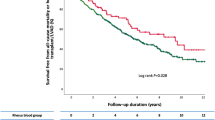
Robust correlation analyses were performed between the changed metabolites (or their ratios) and cardiac physiological measurements (including the left ventricle mass index (LVMI), left ventricle end-systolic volume (LVESV), LVEF, and left atrial (LA) volume) in RHD patients and degenerative AS patients while controlling for covariates (sex, age, race, BMI, hypertension status, diabetes status, and batch effects). In RHD, the value and ratio of 7-HOCA (7-alpha-hydroxy-3-oxo-4-cholestenoate) to deoxycholate were associated with the LVEF (7-HOCA - deoxycholate rs = -0.64, p < 0.001; 7-HOCA/deoxycholate rs = 0.57, p = 0.002) (Fig. 4a,b). In addition, butyrylcarnitine was moderately associated with LVEF in RHD (rs = -0.54, p = 0.004) (Fig. 4c). The propionylcarnitine to acetoacetate ratio was moderately associated with the LVMI in RHD (rs = -0.57, p = 0.003) (Fig. 4d). Other metabolites showed a weak association with the LVMI, LVESV, LVEF, and LA area in patients with RHD (Supp Table S5). In patients diagnosed with degenerative AS, the ratio of acetoacetate to propionylcarnitine was strongly negatively correlated with the LA area (rs = -1, p = 0.028) (Fig. 4e). Cases of calcification in RHD or AS patients were not associated with specific metabolites. However, elevated levels of cortisol were associated with the presence of valve calcification in all samples (Fig. 4f), and a point-biserial analysis showed a weak but statistically significant association (r = 0.46, p = 0.005). Other metabolites showed associations in AS patients; however, they were not robust after adjusting for covariates (Supp Table S6).
Correlation analysis of the cardiac remodelling parameters and valve calcification with metabolites detected in RHD patients and degenerative AS patients after adjustment for sex, age, race, BMI, hypertension, diabetes, and batch effects. Correlation of LVEF in RHD patients with (a) differences in the levels of 7-HOCA and deoxycholate, (b) the ratio of 7-HOCA to deoxycholate, and (c) butyrylcarnitine. (d) The correlation of the LVMI in RHD patients with the propionylcarnitine and acetoacetate ratio. (e) The correlation of the left atrial area with acetoacetate and the propionylcarnitine ratio in patients with degenerative aortic stenosis. (f) Levels of cortisol in valvular heart disease patients (RHD and AS) with and without valve calcifications on histology. (For all the plots, RHD n = 33, AS samples n = 9; *p value < 0.05; adj. rs = adjusted Spearman’s rank correlation coefficient). (RHD rheumatic heart disease, AS aortic stenosis, LVEF left ventricular ejection fraction, LVMI left ventricular mass index, LA left atrium, 7-HOCA 7α-Hydroxy-3-oxo-4-cholestenoic acid).
Discussion
To the best of our knowledge, this is the first study to compare metabolic markers between RHD patients and patients with degenerative AS. Here, we have described metabolic biomarkers that were differentially expressed in patients with RHD and degenerative AS and healthy controls. In this study, metabolites supporting proliferation of inflammation processes were shown to be differentially changed in RHD and degenerative AS. Furthermore, metabolites involved in cholesterol and lipid metabolism showed moderate associations with cardiac hypertrophy and ventricular function in patients with degenerative AS and RHD. When RHD and AS were considered together, higher cortisol was associated with incidence of valvular calcification.
Chronic RHD mainly presents with pure mitral regurgitation, mitral stenosis, mixed mitral valve disease, and/or aortic regurgitation, while degenerative AS primarily presents with aortic stenosis8,18,19. RHD patients have a decreased LVEF; in contrast, AS patients present with a normal LVEF, a hypertrophied LV and a dilated LA. In this study, untargeted metabolomics was successfully applied to identify metabolites with biological relevance that can be used to potentially differentiate patients with severe RHD, patients with degenerative AS, and healthy controls even after adjusting for patients’ baseline characteristics. In addition, the study included a set of matched controls for a disease with similar clinical parameters, which could support the idea that the potential biomarkers we found are genuinely discriminatory and not just a marker of a sick heart. The observed metabolite profiles in patients with RHD and degenerative AS who underwent valve replacement may suggest alterations in major energetic pathways, amino acid metabolism, or inflammatory response processes. The findings also suggest that in addition to the metabolites that differentiate RHD and degenerative AS patients from healthy individuals, some specific metabolites are correlated with cardiac remodelling in severe RHD and degenerative AS patients. Upon further assessment of the correlation patterns between metabolites and certain physiological parameters, there was a distinction between the strength of correlation for RHD and AS. Here, we have shown that metabolomics can help in understanding the pathomechanisms of RHD and degenerative AS and provide biomarkers for the diagnosis, identification of these two conditions and assessment of disease progression.
Furthermore, this study revealed changes in metabolites often involved in the regulation of inflammatory responses. RHD and degenerative AS have different mechanistic processes where autoimmunity drives subsequent developments in RHD. On the other hand, low-grade inflammation in degenerative AS is mostly caused by lipid accumulation, calcification, and oxidative stress. Linoleic acid was altered in RHD patients and was relatively unaffected by the patients’ baseline characteristics. To date, there have been very few metabolomics studies on RHD. Das and colleagues recently reported the dysregulation of the linoleic acid metabolism pathway in patients with RHD12. Similar to our study, they also found linoleic acid to be elevated in RHD patients compared to controls. Although not validated, elevated levels of linoleic acid are associated with inflammation20. However, Froyen and colleagues proposed that increased linoleic acid consumption reduces cardiovascular risk, suggesting that linoleic acid metabolism could instead be a protective mechanism in the body20. Our results are observational; therefore, we cannot confirm the role of linoleic acid; however, since it was elevated in RHD patients compared to controls, we hypothesised that it could be involved in the inflammatory response.
Here, we found metabolites with immunomodulation capabilities being changed in RHD patients. The previously reported findings on continued inflammation in patients with chronic RHD6,21,22 corroborate our findings that proinflammatory metabolites are elevated in RHD patients compared to controls. Furthermore, isocaproic acid, which is primarily a gut microbiome-derived short-chain fatty acid, was elevated in AS patients compared to controls. Isocaproic acid has been shown to have immunomodulatory and endothelial function restoration capabilities23,24. In RHD patients, we observed elevated 3-formylindole and tryptophan; 3-formylindole is a product of gastrointestinal bacteria (Lactobacillus or Clostridium genus) breaking down tryptophan25,26. In humans, 3-formylindole has been reported to stimulate intestinal immune cells to produce interleukin-2227,28. Previous studies have also reported alterations in Lactobacillus and Clostridium levels in the subgingival plaque microbiota that differentiated RHD patients from controls29. The observed perturbations in 3-formylindole and tryptophan could be caused by alterations in the microbiota and could sustain the valve inflammation observed in RHD patients. It would have been expected that in the autoimmune response driven pathology there would be changes in gluconeogenesis and dysregulated cholesterol pathways as compared to changed fatty acid metabolism in chronic degenerative AS7. However, our data could not clearly differentiate the two processes perhaps because the RHD and degenerative AS patients were recruited while undergoing valve replacement after chronic stages of the valve pathology. In addition, based on the study design and ethical considerations, this study could not determine whether the identified potential biomarkers were causal or only associated with the diseases studied. Therefore, further studies using in vitro or in vivo models are warranted to determine the causal effect of the reported metabolites and pathways. Furthermore, this was an exploratory study from one centre; multicenter studies with larger cohorts and multiple analytic platforms are required to validate the described potential diagnostic metabolic biomarkers and pathways.
In this study, some of the altered metabolites were linked to glycerophospholipid metabolism, which is one of the major cardiac energy pathways. Elevated phospholipids and oxidised phospholipids have previously been associated with the extent of calcification in cardiovascular diseases; however, the exact mechanism is not well understood30,31. RHD and degenerative AS have distinct aetiopathogenesis, yet both present with valve stenosis, predominantly due to fibrosis in RHD and calcification in degenerative aortic stenosis, although there are isolated cases of calcification in RHD4,6. Whether calcification in RHD results from processes like those in atherosclerosis and degenerative AS remains to be determined4. Alterations in glycerophospholipid metabolism in RHD patients have not been reported elsewhere, but changes in phospholipid metabolism have been associated with shifts in energy metabolism32. Patients in this study were symptomatic because of chronic RHD, which is associated with ventricular remodelling, which translates into energy metabolism derangements33,34. Phospholipid and ketone body dysregulation also indicates shifts in energy metabolism via the acetyl-CoA–TCA cycle to produce ATP35. The metabolism of long-chain fatty acids provides 70–80% of heart energy requirements36. It is also worth noting that obesity and diabetes, both risk factors for heart disease, shift cardiac energy metabolism toward overreliance on fatty acids because of reduced glucose uptake37. Changes in phospholipid, lysophosphatidic acid, and antioxidant levels have been linked to lipid metabolism in calcific aortic stenosis7,15,38. Therefore, the observed changes in phospholipid metabolism could be associated with the incidence of calcification observed in both RHD and AS patients. Palmitoylcarnitine and ornithine differentiated degenerative AS patients from controls. Furthermore, the differences in and ratios of 7-HOCA and deoxycholate were associated with left ventricle function in RHD. 7-HOCA and deoxycholate are metabolites of cholesterol metabolism and are associated with bile acid production, and deoxycholate is traditionally viewed as a method of cholesterol excretion. At high levels, it can also be toxic. Our findings revealed that an increase in 7-HOCA compared with deoxycholate was associated with an increase in the ejection fraction. Increased levels of circulatory 7-HOCA may indicate cholesterol uptake, which could aid in cholesterol homeostasis39. Propionylcarnitine and butyrylcarnitine are acylcarnitines that play essential roles in fatty acid transport into the mitochondria during beta-oxidation. The ratios of propionylcarnitine to acetoacetate, a ketone body, may indicate the dysregulation of β-oxidation processes. In this study, increased levels of acetoacetate compared to those of propionylcarnitine were associated with increased ventricular remodelling. Ketone body use as an energy source has previously been shown to increase in the failing heart40. Reliance on ketone bodies depletes the citric acid cycle of available coenzyme A, and recent studies have shown that exogenous propionylcarnitine provides an additional source of succinate for the citric acid cycle, with a corresponding increase in contractile function41. In concordance with the decreased use of fatty acids as a cardiac energy source, van Driel and colleagues previously reported that acylcarnitine levels are elevated in AS patients and showed that this method was capable of differentiating patients with calcific aortic stenosis from controls15. Unsurprisingly, we also found that elevated cortisol was associated with the incidence of valve calcification in RHD and AS patients. Patients with valve calcification had severe valve stenosis and were at advanced stages of heart failure. Previous studies have shown that patients with chronic CVD have high levels of psychological stress, anxiety, and depression42. Whether the observed cortisol levels were a precursor to the observed valve pathology due to chronic stress was beyond the scope of this study.
Alterations in the metabolism of amino acids and associated metabolites were observed in RHD and AS patients compared to controls. This study reported alterations in taurine and hypotaurine metabolism in which taurine was decreased in RHD and AS patients compared to controls. Most of the included AS patients were hypertensive and had elevated BMIs and elevated left ventricular myocardium mass indices compared to controls. Taurine is a sulfonic amino acid that is essential for energy metabolism, protects mitochondria, and is produced in small quantities by the healthy heart; taurine deficiencies in animal models have been associated with cardiac remodelling and altered cardiomyocyte morphology43. Changes in energy metabolism are a hallmark phenomenon in a failing heart43,44. Patients with severe RHD often present with mitral valve disease, a reduced LVEF, and a dilated LA, which may lead to cardiac hypertrophy, ischaemic stroke, or eventually heart failure17. Previous studies have reported that shifts in taurine levels and energetic metabolism processes are associated with strained/hypertrophied hearts43,45. Supplementation with taurine has been proposed to lead to cardiac ion homeostasis and antioxidant-like activities45. The significance of the taurine and hypotaurine metabolic pathways identified in the present study may suggest that perturbations in energy metabolism occur through impaired mitochondrial or endoplasmic reticulum function in RHD and AS patients. Ornithine was elevated in AS and RHD patients compared to controls and in differentiated AS patients compared to controls. Ornithine is an alpha amino acid that is a byproduct of arginine metabolism by arginase. Increased arginase expression has been associated with endothelial dysfunction and collagen deposition, which are common in RHD and AS patients46,47. Creatine is elevated in AS patients and has been reported elsewhere to be associated with increased LV systolic pressure48. In this study, the observed changes may suggest that as valve pathologies worsen, the heart needs to pump harder and therefore requires a quick supply of ATP supplied by increasing levels of creatine. In addition, increased inosine and valine may further suggest shifts in ATP catabolism48,49.
In conclusion, the current pilot study is the first to describe metabolic biomarkers expressed in poorly studied valvular heart diseases (RHD and degenerative AS) in SSA patients. This study observed that RHD and AS patients had differences in metabolic biomarkers that could cause shifts in immune response. Furthermore, these results suggest that the perturbation of several metabolites in RHD and AS is associated with energy metabolism, amino acid regulation, and immune response regulation. The linoleic acid metabolism and glycerophospholipid metabolic pathways were some of the key pathways affected in RHD and AS patients. The study also reports metabolites that are associated with valve calcification, cardiac function, and altered pathological parameters in RHD and AS patients. We acknowledge limitations associated with the study in that the results are based on a small sample size since collection of samples from the studied patient cohort was difficult. In addition, we did not perform validation of the reported biomarkers since we did not have enough sample size to independently validate the reported signatures. We, however, believe our findings should allow for the planning of larger longitudinal cohort studies to allow targeted assessment of the protective and causal mechanisms of the reported metabolic biomarkers.
Methods
Study population
Patients with RHD and AS who were scheduled to undergo valve replacement surgery at the Division of Cardiothoracic Surgery were recruited from the Cardiac Clinic Groote Schuur Hospital, Cape Town, from 26th September 2018 to 20th February 2023. Patients were classified with either RHD or degenerative AS based on transthoracic echocardiography (TTE) and cardiovascular magnetic resonance (CMR) as per the World Heart Federation and American College of Cardiology (ACC)/American Heart Association (AHA) and the European Society of Cardiology (ESC)/European Association of Cardiothoracic Surgery (EACTS) guidelines on the diagnosis and management of RHD and valvular heart disease8,17,18. In all patients, the diagnosis was confirmed via histopathological assessment. Blood samples were collected from RHD (n = 39) and AS (n = 10) patients and from age-, sex-, ethnicity-, and comorbidity-matched healthy controls (n = 19). The estimated sample size using G*Power118 (version 3.1.9.7) was 13 and 27 in the two groups assuming normal distribution in the population, with an accepted significance level of less than 0.05 (p value < 0.05). Multivariate studies mostly focus on the number of variables that will be significantly different given a certain sample size and with less emphasis on the effect size of the differences. The allowed minimum fold change of the significant metabolites was > 2 which translated to an effect size (d = 1.5), which was suitable to give an expected 95% power (1-β). Although the estimations were thought sufficient to predict the desired sample size for economic and planning purposes, there are inherent limitations associated with using unimodal effect size and standard deviation estimations in multidimensional metabolomics dataset. The study was conducted in accordance with the principles of the Declaration of Helsinki and was reviewed and approved by the University of Cape Town Faculty of Health Sciences Human Research Ethics Committee (HREC REF: 574/2018 & HREC REF: 061/2018). Individuals younger than 18 years and older than 80 years, those with atherosclerosis, HIV infection, cardiomyopathy, congenital valve lesions, other inflammatory conditions, previous cardiac surgery or valve repair, and those unable to consent were excluded.
Blood sampling and processing
Venous blood samples were collected from healthy controls at recruitment and after providing written informed consent. Venous blood samples were collected from RHD and AS patients before they were subjected to general anaesthesia in VACUETTE® CAT serum clot activator tubes (Greiner Bio-One, Austria), allowed to clot at room temperature for 30 min and spun at 2,000 × g for 15 min in a cooled centrifuge at 4 °C. The serum samples were stored in a -80 °C freezer until analysis (1–2 years after collection).
Liquid chromatography (LC), positive ionisation (ESI+), and negative ionisation (ESI-) mass spectrometry (MS) methods were adapted from Dunn et al.50 Briefly, frozen serum samples were thawed on ice. Quality control (QC) samples were prepared by pooling all samples. The NIST SRM 1950 (NIST, USA) reference standard was used as a long-term external reference QC. To extract the metabolites, 100 µL of sample, QC, and external standard were mixed with 400 µL of an ice-cold mixture of HPLC-grade acetonitrile and methanol (ACN: MeOH 9:1 v/v) supplemented with 1% formic acid (ESI+) or 1 mM acetic acid (ESI-). The mixture was vortexed at high speed for 2 min and subsequently centrifuged at 14,000 × g for 15 min at 4 °C for protein precipitation. The supernatant was transferred to 3 mL glass tubes and dried with nitrogen gas. Dried extracts were reconstituted with 100 µL of HPLC-grade deionised water spiked with 0.1% formic acid (ESI+) or 1 mM acetic acid (ESI-) and centrifuged at 14,000 × g for 15 min. The supernatant was transferred to low-recovery-volume flat bottom inserts placed in 2 mL vials, and 5 µL was injected into the UPLC column. Chromatographic separation was achieved using an ExionLC™ AD series UPLC system with an Omega Polar C18 column (particle size of 1.6 μm, pore size of 100 Å, length of 10 cm, internal diameter of 3 mm; Phenomenex Inc., USA) kept at 40 °C. Mobile phase A was composed of HPLC-grade water, and mobile phase B was composed of a mixture of 100% acetonitrile and methanol (1:1, v/v) and was modified with 2 mM ammonium formate and 0.1% formic acid for ESI+. The mobile phases for the ESI- mode were similar to those for the ESI + mode but were modified with 1 mM acetic acid. The mobile phases for both modes were run with a 60-minute gradient at a rate of 0.4 mL/min. More details are provided as an online supplement (Supp Table S1). The autosampler injection volume was 5 µL, and the temperature was set at 15 °C with multiple 2 s rinsing cycles between injections at 35 µL/s using a mixture of water, methanol, and isopropanol (6:3:1, v/v).
The data were acquired in positive and negative ionisation mode on a Sciex X500R Q-TOF mass spectrometer operated with Sciex OS software ver.1.4 (Sciex, USA) with a dual ESI ion source system. After calibration, the data were collected using the data-dependent acquisition (DDA) mode. The total analysis duration was set at 60 min with a total scan time of 0.6672 s and approximately 5355 duty cycles. Ion source gas 1 was set at 40 psi, ion source gas 2 was set at 65 psi, and the source temperature was set at 500 °C. The curtain gas pressure was set at 25 psi. To obtain the MS1 data, the ion spray voltage was set at 5000 V, the CAD gas pressure was set at 7 psi, and the data were obtained in profile format with a mass range of 50 − 1200 Da. The TOFMS accumulation time was 200 milliseconds, the declustering potential was 70 V, the spread voltage was 0 V, the collision energy was 10 V, the collision energy spread voltage was 0 V, and the data acquisition rate was 4 scans/second. The TOF‒MS/MS data of the fragment ions were obtained in a data-dependent format as part of the same scan cycle, and the following settings were used: maximum candidate ions, 7; intensity threshold, 50 counts/second; mass tolerance, 50 mDa; ion spray voltage, 70 V; target mass range, 50–1200 Da; accumulation time, 60 milliseconds; declustering potential, 70 V; declustering potential spread, 0 V; collision energy, 35 V; collision energy spread, 15 V; Q1 unit resolution; and data acquisition rate, 8 scans/second.
Data processing and statistical analysis
Raw positive and negative mode liquid chromatography-tandem mass spectrometry (LC‒MS/MS) data were processed with MS-Convert in ProteoWizard 3.0.190851 and MSDIAL 4.3852. The peaks were detected and aligned using MS-DIAL, where a peak was discarded if it included more than 30% missing values. In addition, peaks were excluded if they were missing in more than 30% of the samples per group in the ESI + and ESI- modes. Batch drift adjustment was performed using the pooled QC samples that were injected every 5 samples in all batches, including one pooled QC sample injected at the beginning and end of each batch. The raw data were preprocessed and aligned to the pooled QC samples, and the batches were normalised with the LOESS algorithm based on the QC samples. The data were filtered by removing background noise based on the blank samples (maximum sample/blank < 5-fold change). The missing (zero) values were then replaced with 1/10 of the minimum peak height for that m/z feature across all samples. The extracted features were further filtered based on the technical reproducibility of individual features: features were removed where the quality control (QC) relative standard deviation (RSD) was greater than 30%. To obtain the significant features, data were first normalised per sample based on the sum intensities in each sample before transformation per feature by mean centring and dividing by the standard deviation (autoscaling) of each feature using MetaboAnalyst 5.053. To identify potentially important features between groups, univariate ANOVA and volcano plot analysis were used, while multidimensional reduction (PCA and PLS-DA) analyses were used to visualise group differences. Chemical annotation of the selected features was performed by matching their mass spectra and product ion mass spectra to publicly available spectral libraries (HMDB, PubChem, CheBI, MassBank, etc.) with GNPS 28.254 and SIRIUS 4.5.355. A similarity score above 70% between the queried features and the library molecular formula, structure, and adducts was considered during annotation. Important features lacking product ion mass spectra data were matched to public databases based on their accurate mass, retention time, and adduct formation patterns using the CEU Mass Mediator available at http://ceumass.eps.uspceu.es/56. Important features with and without product ion mass spectra data were putatively annotated at Metabolomics Society Initiative (MSI) levels 2 and 3 of identification, respectively. Data was additionally manually curated where an annotation could not be correct e.g. because of retention time. Furthermore, potential biomarkers were analysed using receiver operating characteristic (ROC) curve analysis. To determine the robustness of the potential biomarkers, the effect of clinical metadata (covariates) on their significance level was assessed by analysing the correlation of the p values with and without covariate adjustments using multivariate linear regression models. In addition, correlation analyses between perturbed metabolites and cardiac hypertrophy and functional parameters were performed while adjusting for covariates. The m/z features and metabolites with a p value < 0.05 and log2-fold change (log2FC) > ± 1.5 were considered significantly altered.
Functional analysis of significant metabolites
To explore the functional relevance of the annotated significant metabolites, pathway enrichment and topology analyses were performed in MetaboAnalyst 5.054. The HMDB and KEGG identifiers of the annotated metabolites were uploaded and mapped to the KEGG human pathway library for pathway analysis. The mapped pathways were visualised with scatter plots while testing the significance level. The enrichment was analysed using the hypergeometric test, while the topology analysis was performed using relative betweenness centrality on the Homo sapiens KEGG pathway library57,58. Pathways with a false discovery rate (FDR)-adjusted p value (q) < 0.05 were significantly overrepresented.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon request.
References
Chen, J., Li, W. & Xiang, M. Burden of valvular heart disease, 1990–2017: results from the global burden of disease study 2017. J. Glob Health. 10 (2), 020404 (2020).
Essop, M. R. & Nkomo, V. T. Rheumatic and nonrheumatic valvular heart disease. Circulation 112 (23), 3584–3591 (2005).
Muhamed, B., Parks, T. & Sliwa, K. Genetics of rheumatic fever and rheumatic heart disease. Nat. Rev. Cardiol. 17 (3), 145–154 (2020).
Passos, L. S. A., Nunes, M. C. P. & Aikawa, E. Rheumatic heart valve disease pathophysiology and underlying mechanisms. Front. Cardiovasc. Med. 7, 612716 (2021).
Chen, H. Y., Engert, J. C. & Thanassoulis, G. Risk factors for valvular calcification. Curr. Opin. Endocrinol. Diabetes Obes. 26 (2), 96–102 (2019).
Blaser, M. C., Kraler, S., Luscher, T. F. & Aikawa, E. Multi-omics approaches to define calcific aortic valve disease pathogenesis. Circ. Res. 128 (9), 1371–1397 (2021).
Capoulade, R. et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 66 (11), 1236–1246 (2015).
Otto, C. M. et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation 143 (5), e72–e227 (2021).
Everett, R. J., Clavel, M-A., Pibarot, P. & Dweck, M. R. Timing of intervention in aortic stenosis: a review of current and future strategies. Heart 104 (24), 2067 LP – 2076. (2018).
Beger, R. D. et al. Metabolomics enables precision medicine: A white paper, community perspective. Metabolomics 12 (10), 149 (2016).
Jacob, M., Lopata, A. L. & Dasouki, M. Abdel Rahman A.M. Metabolomics toward personalized medicine. Mass Spectrom. Rev. 38 (3), 221–238 (2019).
Das, S. et al. An untargeted LC–MS based approach for identification of altered metabolites in blood plasma of rheumatic heart disease patients. Sci. Rep. 12 (1), 5238 (2022).
Amrein, M. et al. Gut microbiota-dependent metabolite trimethylamine N-oxide (TMAO) and cardiovascular risk in patients with suspected functionally relevant coronary artery disease (fCAD). Clin. Res. Cardiol. 111 (3), 692–704 (2022).
Neculae, E. et al. The oral microbiota in valvular heart disease: current knowledge and future directions. Life 13 (1), 182 (2023).
van Driel, B. O. et al. Metabolomics in severe aortic stenosis reveals intermediates of nitric oxide synthesis as most distinctive markers. Int. J. Mol. Sci. 22 (7), 3569 (2021).
Xiong, T-Y. et al. Differences in metabolic profiles between bicuspid and tricuspid aortic stenosis in the setting of transcatheter aortic valve replacement. BMC Cardiovasc. Disord. 20, 229 (2020).
Wang, T. et al. Inosine is an alternative carbon source for CD8+-T-cell function under glucose restriction. Nat. Metab. 2 (7), 635–647 (2020).
Reményi, B. et al. World heart federation criteria for echocardiographic diagnosis of rheumatic heart disease-an evidence-based guideline. Nat. Rev. Cardiol. 9 (5), 297–309 (2012).
Vahanian, A. et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European society of cardiology (ESC) and the European association for Cardio-Thoracic surgery (EACTS). Eur. Heart J. 43 (7), 561–632 (2022).
Farvid, M. S. et al. Dietary Linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation 130 (18), 1568–1578 (2014).
Froyen, E. & Burns-Whitmore, B. The effects of Linoleic acid consumption on lipid risk markers for cardiovascular disease in healthy individuals: a review of human intervention trials. Nutrients 12 (8), 2329 (2020).
Zietzer, A., Düsing, P., Reese, L., Nickenig, G. & Jansen, F. Ceramide metabolism in cardiovascular disease: a network with high therapeutic potential. Arterioscler. Thromb. Vasc Biol. 42 (10), 1220–1228 (2022).
Habeeb, N. M. & Al Hadidi, I. S. Ongoing inflammation in children with rheumatic heart disease. Cardiol. Young. 21 (3), 334–339 (2011).
Mutithu, D. W., Roberts, R. & Manganyi, R. Ntusi N.A.B. Chronic rheumatic heart disease with recrudescence of acute rheumatic fever on histology: a case report. Eur. Hear. J. - Case Rep. 6 (7), ytac278 (2022).
Richards, L. B., Li, M., van Esch, B. C. A. M., Garssen, J. & Folkerts, G. The effects of short-chain fatty acids on the cardiovascular system. PharmaNutrition 4 (2), 68–111 (2016).
Zhao, P., Zhao, S., Tian, J. & Liu, X. Significance of gut microbiota and short-chain fatty acids in heart failure. Nutrients 14 (18), 3758 (2022).
Roager, H. M. & Licht, T. R. Microbial Tryptophan catabolites in health and disease. Nat. Commun. 9 (1), 3294 (2018).
Zhang, L. S. & Davies, S. S. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 8, 46 (2016).
Shi, X-R. et al. Microbiota in gut, oral cavity, and mitral valves are associated with rheumatic heart disease. Front. Cell. Infect. Microbiol. 11, 643092 (2021).
Vorkas, P. A. et al. Perturbations in fatty acid metabolism and apoptosis are manifested in calcific coronary artery disease: an exploratory lipidomic study. Int. J. Cardiol. 197, 192–199 (2015).
Tintut, Y., Hsu, J. J. & Demer, L. L. Lipoproteins in cardiovascular calcification: potential targets and challenges. Front. Cardiovasc. Med. 5, 172 (2018).
van der Veen, J. N. et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta - Biomembr. 1859 (9), 1558–1572 (2017).
Jameel, M. N. & Zhang, J. Myocardial energetics in left ventricular hypertrophy. Curr. Cardiol. Rev. 5 (3), 243–250 (2009).
Wu, J. et al. Variations in energy metabolism precede alterations in cardiac structure and function in hypertrophic preconditioning. Front. Cardiovasc. Med. 7, 602100 (2020).
Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell. Biochem. Biophys. 68 (3), 475–478 (2014).
Chabowski, A. et al. Protein-mediated fatty acid uptake in the heart. Curr. Cardiol. Rev. 4 (1), 12–21 (2008).
An, D. & Rodrigues, B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiol. Circ. Physiol. 291 (4), H1489–H1506 (2006).
Kamstrup, P. R., Hung, M-Y., Witztum, J. L., Tsimikas, S. & Nordestgaard, B. G. Oxidized phospholipids and risk of calcific aortic valve disease: the Copenhagen general population study. Arterioscler. Thromb. Vasc Biol. 37 (8), 1570–1578 (2017).
Duan, Y. et al. Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics. Signal. Transduct. Target. Ther. 7 (1), 265 (2022).
Manolis, A. S., Manolis, T. A. & Manolis, A. A. Ketone bodies and cardiovascular disease: an alternate fuel source to the rescue. Int. J. Mol. Sci. 24 (4), 3534 (2023).
Haijes, H. A., Jans, J. J. M., van der Ham, M., van Hasselt, P. M. & Verhoeven-Duif, N. M. Understanding acute metabolic decompensation in propionic and methylmalonic acidemias: a deep metabolic phenotyping approach. Orphanet J. Rare Dis. 15 (68), 1–13 (2020).
Chauvet-Gelinier, J-C. & Bonin, B. Stress, anxiety and depression in heart disease patients: A major challenge for cardiac rehabilitation. Ann. Phys. Rehabil Med. 60 (1), 6–12 (2017).
Jong, C. J., Sandal, P. & Schaffer, S. W. The role of taurine in mitochondria health: more than just an antioxidant. Molecules 26 (16), 4913 (2021).
Schaffer, S. W., Ju Jong, C., Azuma, J. & KC R. & Physiological roles of taurine in heart and muscle. J. Biomed. Sci. 17 (Suppl 1), S2 (2010).
Bkaily, G. et al. Taurine and cardiac disease: state of the Art and perspectives. Can. J. Physiol. Pharmacol. 98 (2), 67–73 (2019).
Li, Z. et al. Arginase: shedding light on the mechanisms and opportunities in cardiovascular diseases. Cell. Death Discov. 8 (1), 413 (2022).
Ryoo, S. et al. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ. Res. 102 (8), 923–932 (2008).
Balestrino, M. Role of creatine in the heart: health and disease. Nutrients 13 (4), 1215 (2021).
Farthing, D. E., Farthing, C. A. & Xi, L. Inosine and hypoxanthine as novel biomarkers for cardiac ischemia: from bench to point-of-care. Exp. Biol. Med. 240 (6), 821–831 (2015).
Dunn, W. B. et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 6 (7), 1060–1083 (2011).
Chambers, M. C. et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30 (10), 918–920 (2012).
Tsugawa, H. et al. MS-DIAL: data-independent MS/MS Deconvolution for comprehensive metabolome analysis. Nat. Methods. 12 (6), 523–526 (2015).
Pang, Z. et al. MetaboAnalyst 5.0: narrowing the gap between Raw spectra and functional insights. Nucleic Acids Res. 49 (W1), W388–W396 (2021).
Wang, M. et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 34 (8), 828–837 (2016).
Dührkop, K. et al. SIRIUS 4: a rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods. 16 (4), 299–302 (2019).
de la Gil, A. et al. Knowledge-based metabolite annotation tool: CEU mass mediator. J. Pharm. Biomed. Anal. 54, 138–149 (2018).
Kanehisa, M. & Goto, S. K. E. G. G. Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Acknowledgements
The authors would like to thank Prof. Dhiren Govender, Dr. Riyaadh Roberts, and Subash Govender for H&E sectioning, staining, and slides review; Alicia Evans for UPLC-QTOF-MS analysis support; Dr. Rodgers Manganyi for providing surgery samples. The authors would like to acknowledge and thank Promolab (Pty) Ltd., trading as Separations, South Africa, for their contribution to the Sciex X500R QTOF instrument used.
Funding
This research was funded through a Blue Skies research grant from the National Research Foundation to Sebastian Skatulla (Grant Numbers 104839 and 105858). In addition, Ntobeko Ntusi gratefully acknowledges funding from the National Research Foundation, South African Medical Research Council, and the Lily and Ernst Hausmann Trust. HAA thanks the South African Medical Research Council (SAMRC) for a mid-career scientist and self-initiated research grants and the South African National Research Foundation (NRF) for a research development grant.
Author information
Authors and Affiliations
Contributions
DWM conducted the experiments, analysed the data, and developed the draft manuscript; NABN, JAK, and HAA reviewed the data and the draft manuscript; OOA, ENL, MF, LW, SS and RN reviewed the draft manuscript; and NABN conceived, funded, and supervised the project. All coauthors made substantial contributions to the design of the draft, reviewed it, and contributed to the intellectual content of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors agreed on the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Faculty of Health Science, Human Research Ethics Committee, University of Cape Town (HREC REF: 574/2018 & HREC REF: 061/2018).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mutithu, D.W., Kirwan, J.A., Adeola, H.A. et al. Serum metabolic profiling in rheumatic heart disease and degenerative aortic stenosis. Sci Rep 15, 31470 (2025). https://doi.org/10.1038/s41598-025-03149-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03149-7