Abstract
Doxorubicin (DOX) is an anthracycline class of chemotherapy drug, the application of which is limited due to its cardiotoxic effects. Recombinant Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9), is a serine protease pivotal in lipid metabolism and has a profound correlation with the onset of cardiovascular diseases. This study uncovers a connection between PCSK9 and DOX-induced cardiotoxicity (DIC). This research found that injection of DOX in mice caused cardiac toxicity. DOX treatment up-regulated the expression of PCSK9 protein in myocardial tissue. Evolocumab (PCSK9 inhibitors) improved cardiac function, myocardial injury, and fibrosis in DOX-treated mice, indicating a protective effect against DIC. The mechanism involved modulation of cardiomyocyte apoptosis and regulation of apoptosis-related proteins, including Bax/Bcl-2 ratio and Cleaved Caspase-3/Pro Caspase-3 ratio. DOX exhibited concentration- and time-dependent cytotoxic effects on H9C2 cardiomyocytes, promoting apoptosis. PCSK9 nuclear aggregation occurred in H9C2 cardiomyocytes after DOX treatment, and PCSK9 interacted with the Importin subunit beta-1 (KPNB1) protein. Interference with PCSK9 up-regulated KPNB1 expression, affecting apoptosis-related proteins and improving DOX-induced H9C2 cardiomyocyte apoptosis. In short, the elucidation of this mechanism is helpful involve that PCSK9 inhibitor may be a potential drug for improving DIC.
Similar content being viewed by others
Introduction
In the past decade, new cancer treatment methods have been effectively used, which has greatly improved the prognosis of patients. The number of tumor survivors has increased significantly, and the new subspecialty of Oncology Cardiology has emerged1. In the course of cancer treatment, although the new treatment can significantly reduce the mortality rate of cancer patients, it may cause severe cardiotoxicity2. Many chemotherapeutic drugs can cause cardiotoxicity, the most common of which include anthracyclines, anthraquinones, monoclonal antibodies, tyrosine kinase inhibitors, alkylating agents, and interferon-α.
DOX is one of the anthracycline drugs. As one of the effective anti-tumor drugs, it is often used to treat different types of tumors, including Kaposi’s sarcoma, acute lymphoblastic leukemia, breast cancer, lymphoma and bladder cancer. DOX is an inhibitor of DNA topoisomerase II, which inhibits tumor growth by causing damage to DNA. When the dose range of DOX is 400–700 mg/m2, it can cause dose-dependent cardiotoxicity3. Apoptosis and oxidative stress are considered to be important causes of DIC. Some drugs can be used to treat DIC. For example, dexrazoxane can reduce DIC and heart failure by binding to poisomerase 2β. Angiotensin-converting enzyme inhibitors, β-blockers and some antioxidants have shown positive effects in protecting against DIC4,5.
The cardiac complications of DOX are directly related to the increase of oxidative stress in heart tissue. The increase of oxidative stress will lead to the production of Reactive oxide species (ROS), which will cause damage to the heart6. DIC also involves changes in calcium levels, which in turn leads to apoptosis. With the activation of Caspase, apoptosis occurs in cardiomyocytes and vascular endothelial cells3,7. The molecular mechanisms of DIC also involve DNA damage, intracellular signal transduction, transcription factors, epigenetic regulators, autophagy, pyroptosis, and ferroptosis8.
DOX can induce apoptosis in endothelial cells and cardiomyocytes, which is characterized by Caspase activation and DNA degradation between nucleosomes9,10. DOX-induced apoptosis is beneficial in tumor therapy. However, the pro-apoptotic effect of DOX on cardiomyocytes and endothelial cells is the main cause of cardiotoxicity. Due to the different cell types and stimulation intensity, there may be a variety of signal transduction pathways involved in DOX-induced apoptosis.
The use of drugs in the treatment of tumors is a double-edged sword. Chemotherapy drugs can not only treat tumors, but also cause serious cardiac toxicity. The cardiotoxicity of DOX limits its clinical application. Its main clinical manifestations are left ventricular systolic dysfunction and induced heart failure. In preliminary work, we found that while DOX induces cardiomyocyte toxicity in mice, the expression of PCSK9 in myocardial tissue is significantly up-regulated. PCSK9 is the ninth member of the subtilisin proteolytic enzyme family, located on human chromosome 1p32, mainly expressed in liver, kidney, intestine and other tissues. In 2003, it was first discovered in the brain and was named PCSK9 according to standard nomenclature11. As a secretory protein, PCSK9 protein is secreted into the circulation in the form of heterodimer after its precursor protein in the liver is autocatalytically cleaved in the molecule. Then, it binds and internalizes the precursor domain of the low-density lipoprotein receptor and regulates its circulation on the surface of the liver cell, thereby regulating the concentration of low-density lipoprotein cholesterol12. In addition to lipid-lowering effects, PCSK9 is closely associated with a variety of cardiovascular diseases, including ischemic myocardial injury13, heart failure14, arrhythmia15 and hypertension16. More and more studies have found that PCSK9 can be used as one of the risk factors of cardiovascular disease, which plays an important role in the research field of cardiovascular disease. Therefore, inhibiting the activity of PCSK9 is of great value for the treatment of lowering blood lipids and cardiovascular-related diseases. At present, the main PCSK9 inhibitors used in clinical practice are Evolocumab, Alirocumab and Inclisiran. Although the safety of these drugs has been proven after clinical use, adverse reactions such as nasopharyngitis and mild self-limited injection site reactions still exist17. However, there is no report on the relationship between PCSK9 and DIC. Whether it is possible to improve DIC by regulating PCSK9 is a field worthy of further study. This study can provide a theoretical basis for the new application of PCSK9 inhibitors and has potential application value.
Materials and methods
Animal and procedures
Wild-type female C57BL/6J mice were purchased from Yisi Laboratory Animal Technology company (Changchun, China), 8–10 weeks old, with an average weight of 20–22 g. Mice were fed under specific pathogen free conditions. Before the start of the experiment, mice had 1 week to adapt to the environment. The mice were placed separately in a plastic cage with padding to ensure that the mice could eat water and food at will. Keep the ambient temperature at 22 ± 2 °C, keep the humidity at 50–60%, and take a 12 h light/dark cycle to simulate the mice biological clock. the experiment was approved by the Animal Experimental Ethics Committee of the Fourth Affiliated Hospital of Harbin Medical University, ethical number (2022-DWSYLLCZ-45). All methods were performed in accordance with the relevant guidelines and regulations, as well as in accordance with the ARRIVE guidelines.
The mice DOX cardiotoxicity model is mostly by the intraperitoneal injection of DOX. DOX (HY-15142, Med Chem Express) was fully dissolved in 0.9% normal saline and the concentration of DOX was 20 mg/kg in a single intraperitoneal injection18,19,20. The control group was injected with the same amount of 0.9% normal saline. PCSK9 inhibitors were selected using Repatha (Amgen), and the drug was named Evolocumab (Evo). According to the literature, the dose of Evo used in some studies is 10 mg/kg21,22. In this experiment, the same dose of Evo was used for the experiment. Evo was dissolved in sterile water for injection to make the drug concentration reach 10 mg/kg, and the injection method was subcutaneous injection. Evo was subcutaneously injected 1 week before DOX injection and half an hour before DOX injection, respectively. IgG antibodies (ab90284, Abcam) were subcutaneously injected at the same time in the control group and the DOX treatment group.
Twenty C57BL/6J mice were divided into two groups: Control group (Con, n = 10) and DOX treatment group (DOX, n = 10). The mice in the DOX treatment group were intraperitoneally injected with DOX at a single dose of 20 mg/kg, and control group was injected with the same amount of normal saline. The survival rate of mice was observed and body weight was recorded every day within 5 days after injection. Then, echocardiography was performed on the 5th day, and cardiac tissue was collected for histopathological examination (H&E) (Fig. S1A).
Forty C57BL/6J mice were divided into three groups: Control group (Con, n = 10), DOX treatment group (DOX, n = 20), DOX + PCSK9 inhibitor group (DOX + Evo, n = 10). On the 1st and 7th day, DOX + Evo group was subcutaneously injected with 10 mg/kg Evo, Control group and DOX group were subcutaneously injected with the same dose of IgG antibody. On the 7th day, DOX group and DOX + Evo group were given a single intraperitoneal injection of 20 mg/kg DOX, and Control group was injected with the same dose of normal saline. The three groups of mice continued to feed for 5 days. The survival rate of mice within 12 days was observed and the body weight was recorded. On the 12 th day, the three groups of mice underwent echocardiography. The mice were sacrificed by spinal dislocation and the heart weight was recorded. Heart tissue samples were collected for subsequent experiments (Fig. S1B).
Echocardiography
The mice were anesthetized with tribromoethanol, and the chest of the anesthetized mice was depilated, placed on the operating table and fixed. Cardiac function was assessed by M-mode echocardiography, and echocardiography was performed using a Vevo1100 high-resolution device. Interventricular septal thickness diastolic (IVSd), interventricular septal thickness systolic (IVSs), left ventricular internal diameter at end-diastole (LVIDd), left ventricular internal diameter at end-systole (LVIDs), left ventricular posterior wall thickness diastolic (LVPWd), left ventricular posterior wall thickness systolic (LVPWs), left ventricular ejection fraction (LVEF) and left ventricular fraction shortening (LVFS) were accurately recorded.
Hematoxylin and Eosin (H&E) staining
The heart tissue was fixed in 4% paraformaldehyde (30525-89-4, Sigma) for 12–16 h, and the tissue was embedded in paraffin. The section thickness was 4 μm, and sections were dried at 37 °C for 24 h. Xylene dewaxing 10 min × 2 times. The sections were immersed in anhydrous ethanol, 90% ethanol, 80% ethanol, 70% ethanol, 50% ethanol for 5 min, and washed with distilled water for 5 min. HE staining was performed using a modified HE staining kit (G1121, Solarbio). Hematoxylin solution was used for 8 min, differentiation solution was differentiated for 3 s, bluing solution was returned to blue for 1 min, eosin solution was performed for 2 min, neutral gum was sealed and observed under microscope (Olympus Corporation, Japan).
Masson trichrome staining
The thickness of paraffin section was 4 μm. The operation was performed according to the modified Masson trichrome staining kit (G1346, Solarbio). The sections were immersed in mordant solution, treated at 60 °C for 1 h, and rinsed with distilled water for 10 min. Celestite blue solution droplets were stained for 3 min and rinsed with distilled water 15 s×2 times. Mayer Hematoxylin solution was dripped for 3 min and rinsed with distilled water 15 s × 2 times.The acid differentiation solution was differentiated for 5 s, and the distilled water was washed for 10 min. Ponceau-Acid Funchsin solution was carried out in drops for 10 min and washed with distilled water 15 s×2 times. Phosphomolybdic acid solution was dripped for 10 min, and aniline blue solution was directly dripped for 5 min without washing with water. Neutral gum seals, microscopic observation (Olympus Corporation, Japan).
Immunohistochemistry (IHC)
The thickness of paraffin section was 5 μm. The sections were immersed in xylene and gradient alcohol, and the experimental steps were referred to the HE staining process. The sections were immersed in antigen repair solution (P0081, Beyotime), 95 °C for 15 min, and washed with 1×PBS 5 min×3 times. The sections were immersed in 3% hydrogen peroxide (7722-84-1, Nanjing Reagent), kept away from light for 15 min, and washed with 1×PBS 5 min×3 times. The sections were added with an appropriate amount of 10% Donor Equine Serum (SH30074.03, HyClone) and blocked at 37℃ for 1 h. PCSK9 (1:200, ab31762, Abcam) and Caspase-3 (1:200, WL02117, Wanleibio) primary antibody solution were incubated overnight at 4 °C, and washed with 1×PBS 5 min×3 times. The primary antibody solution was removed, the secondary antibody solution (1:100, AS014, ABclonal) was incubated at 37℃ for 1 h, and washed with 1×PBS 5 min×3 times. An appropriate amount of DAB color developing solution (ZLI-9018, ZSGB-BIO) was added to the tissue surface in the dark, and hematoxylin solution was performed with reference to the HE staining process. Neutral gum seals, microscopic observation (BX43, Olympus Corporation, Japan).
Cell culture
The cell line used in the experiment was rat H9C2 myocardial cell line, which was purchased from Procell Life Science and Technology Co.,Ltd (CL-0089, Wuhan). The culture conditions of H9C2 were Dulbecco’s Modified Eagle’s Medium (DMEM, 12100046, Gibco), 10% fetal bovine serum (FBS, BS-1101, Inner Mongolia Opcel Biotechnology Co.,Ltd) and 1% Penicillin-Streptomycin solution (SV30010, Hyclone). The cell culture environment was a sterile incubator at 37℃ and 5% CO2 concentration. The suitable cell passage ratio was 1:2–1:4, the cell fusion degree was maintained between 30 and 90% during the culture process, and the frequency of medium change was 2–3 times/week.
Cell viability assay
The cells were seeded in a 96-well plate with about 5000 cells per well and cultured in a CO2 incubator at 37 °C for 24 h. DOX was treated at 0, 0.5, 1, 2.5, 5, 10 and 20µM for 12 h, 24 h and 48 h to detect the cytotoxicity of DOX. Each group of experiments was repeated for 6 times, and the control group was added with an equal volume of DMSO (D2650, Sigma). Replace the medium in the 96-well plate and add 10 µL CCK8 solution (K1018, APE×BIO) to each well, and culture in a CO2 incubator at 37 °C for 2 h. The absorbance value of each hole under the condition of 450 nm was detected by microplate reader (Bio-Rad). Cell viability = [ ( experimental hole absorbance-blank hole absorbance ) / ( control hole absorbance-blank hole absorbance ) ] ×100%.
RNA extraction and reverse transcription-quantitative (RT-q) PCR analyses
RNAiso Plus (9108, Takara) was used to extract total RNA from H9C2 cells. The total RNA was reverse transcribed into cDNA for qPCR using the HiScript III 1st Strand cDNA Synthesis Kit (R312-02, Vazyme). The reaction conditions for reverse transcription of cDNA: 37 °C, 15 min, 85 °C, 5 s. After reverse transcription, 2 µL of cDNA was used for subsequent experiments, and real-time fluorescence quantitative detection was performed using the ChamQ Universal SYBR qPCR Master Mix kit (Q711, Vazyme). The rat GAPDH was used as the internal reference gene for qPCR. The specific primers of Rat PCSK9 gene (Gene ID: 298296) were PCSK9 forward (F) 5′-ACAGACAGGCGAGCAAGTGT-3′ and PCSK9 reverse (R) 5′-TATGAGGGTGCCGCTGAC TG-3′. The GAPDH gene (Gene ID: 24383)-specific primers were GAPDH forward (F) 5′-GACATGCCGCCTGGAGA AAC-3′ and GAPDH reverse (R) 5′-AGCCAGGATGCCCTTTAGT-3′. According to the manufacturer ‘s plan, the 7500 Real Time PCR System (ABI, USA) was used for detection, and each sample was subjected to three repeated experiments. The following reaction conditions were used for qPCR: 95 °C, 30 s, followed by 95 °C, 10 s, 60 °C, 30 s, 40 cycles, 95 °C, 15 s, 60 °C, 60 s. Relative quantitative analysis of mRNA expression levels was performed using the 2−ΔΔCt.
Western blot analysis
RIPA (P0013B, Beyotime) and PMSF (P0100, Solarbio) were prepared in a ratio of 100: 1, and the prepared RIPA lysate was used to fully homogenize the heart tissue and the treated H9C2 cells. The protein concentration was determined by BCA kit (P0012, Beyotime), and 1/4 volume of SDS-PAGE protein loading buffer (P0286, Beyotime) was added and boiled. The gel was prepared using 7.5%, 10% and 12.5% PAGE Gel Fast Preparation Kits (PG111, PG112, PG113, Shanghai Epizyme Biomedical Technology Co.,Ltd), and the loading amount of protein per well was 30 µg. After gel electrophoresis, the protein was transferred to the PVDF membrane (IPVH00010, Millipore), which was blocked at 37 °C for 1 h in 5% skim milk (232100, BD Difco) prepared with PBST (0.05%Tween-20 in PBS). After blocking, the milk was removed, and the primary antibody PCSK9 (1:1000, ab31762, Abcam), Caspase-3/Cleaved Caspase-3 (1:500, WL02117, Wanleibio), Bax (1:1000, 2772, Cell Signaling Technology), Bcl-2 (1:1000, ab196495, Abcam), KPNB1 (1:1500, 10077-1-AP, Proteintech) and GAPDH (1:10,000, AC001, ABclonal) prepared with the primary antibody diluent (P0023A, Beyotime) were added and incubated overnight at 4 °C.The primary antibody was removed and PBST was used to wash the membrane 5 min × 4 times. The membrane was placed in HRP goat anti-rabbit IgG secondary antibody (AS029, ABclonal) prepared with secondary antibody dilution (P0023D, Beyotime), incubated at 37 °C for 1 h, and washed with PBST for 5 min × 4 times. The chemiluminescence imager (Beijing Sage Creation Science Co.,Ltd, China) was used for imaging, and ImageJ software was used for grayscale scanning.
Immunofluorescence
H9C2 cells were seeded in glass bottom cell culture dish (801001, NEST). The cells were treated with 1 µM DOX for 24 h and 48 h, respectively, and the control group was added with the same volume of DMSO. Add pre-cooled 4% paraformaldehyde fixed for 20 min, and PBS was washed for 3 min × 3 times. The cells were treated with 0.5% Ttiton-100 for 10 min, shaken for 10 min, and PBS was washed for 3 min × 3 times. The cells were blocked with 5% BSA (A6020, Biotopped) (5% BSA in PBSTX) in an incubator at 37 °C for 1 h. The primary antibody PCSK9 (1:100, ab31762, Abcam) prepared by adding BSA was incubated overnight at 4 °C. Remove the primary antibody, PBSTX (0.5% Ttiton-100 in PBS) was washed for 5 min × 3 times. The Goat Anti-Rabbit IgG H&L/FITC antibody (bs-0295G-FITC, Bioss) prepared with BSA was added and incubated in an incubator at 37 °C for 1 h. Remove the secondary antibody and PBSTX was washed for 5 min × 3 times. DAPI (C1006, Beyotime) staining for 5 min, PBSTX was washed for 5 min×3 times. Add an appropriate amount of anti-fluorescence quenching agent (P0126, Beyotime) and observe under ultra-high resolution microscope (GE DeltaVision OMX SR, USA).
Co-immunoprecipitation
H9C2 cells were seeded in a large dish, treated with 1 µM DOX for 24 h, and washed with PBS × 2 times. Cells were collected by adding 1 ml RIPA lysis solution, standing on ice for 1 h, then 4 °C, 14,000 rpm, centrifugation for 10 min, and the supernatant was collected for subsequent experiments. The supernatant was divided into three groups, Input group: 200 µL, IP group: 400 µL, IgG group: 400 µL. Take 60 µL of Protein A/G magnetic beads (HY-K0202, Med Chem Express), add PBSTX to rinse the magnetic beads, magnetically separate and remove the supernatant. PCSK9 antibody (1:40, ab31762, Abcam) prepared with PBSTX was added, incubated at 4 °C for 2 h, and PBSTX washed magnetic beads 4 times. 400 µL IP group and IgG group samples were added to the rinsed magnetic beads, fully mixed, and incubated at 4 °C for 2 h. After magnetic separation, the supernatant was discarded and the magnetic beads were collected, and the magnetic beads were rinsed with PBSTX × 4 times. 30 µL SDS-PAGE protein loading buffer was added to the magnetic beads and boiled for 10 min. The supernatant was collected for subsequent Western blot experiments. Western blotting gel Coomassie brilliant blue staining was used to observe the protein bands, and the gel was cut and sent to Shanghai Bai pu Biotechnology Co., Ltd (Shanghai, China) for protein spectrum identification. According to the identification results, the same steps were used to verify the protein interaction.
TUNEL staining
After the myocardial tissue and H9C2 cardiomyocytes were treated, One-step TUNEL FITC Apoptosis Detection Kit (K1133, APE×BIO) was used to operate according to the standard procedure. Each sample was added with 100µL of Proteinase K working solution and rinsed with PBS for 5 min×3 times. Sample was added with 1 × Equilibration Buffer working solution and incubated at room temperature for 30 min. Each sample was added with 50 µL of the labeled reaction solution (ddH2O + 5×Equilibration Buffer + FITC-12-dUTP Labeling Mix + TdT Enzyme), incubated at 37℃ for 1 h, and rinsed with PBS for 5 min × 3 times. An appropriate amount of anti-fluorescence quencher containing DAPI (P0131, Beyotime) was added to each sample and sealed. The green fluorescence was observed under an inverted fluorescence microscope (Olympus Corporation, Japan) at 517 nm, and the blue fluorescence of DAPI was observed at 460 nm. The positive rate of TUNEL staining was counted by Image J cell counting.
RNA interference
H9C2 cells were seeded in 6-well plates and transfected when the cell fusion reached 30-50%. Three sets of small interfering (si)RNA oligos for rat PCSK9 gene were synthesized by General Biosystems (Anhui, China) Co.,Ltd. Target sequence for PCSK9-2179 is: (sense strand: 5′GGACAGGACCAGUGAAGAATT; antisense strand: 5′UUCUUCACUGGUCCUGUCCTT); for PCSK9-979 is: (sense strand: 5′CCUCAUAGGCCUGGAGUUUTT; antisense strand: 5′ AAACU CCAGGCCUAUGAGGTT); and for PCSK9-606 is: (sense strand: 5′UGGAGUACAUCGAGGA AGATT; antisense strand: UCUUCCUCGAUGUACUCCATT ). Opti-MEM™ (31985070, Gibco) was mixed with PCSK9 siRNA or Control siRNA for 3–5 times, and Opti-MEM™ was mixed with Lipofectamine 2000 (11668019, Invitrogen) for 3–5 times. Mix the above liquid, blow 3–5 times, and stand at room temperature for 20 min. Serum-free and antibiotic-free medium was added to each well of the 6-well plate, and the above mixed liquid was added dropwise to each well. After 6 h of culture at 37 °C, the fresh medium was replaced. 1 µM DOX was added to each well for 24 h, and the cells were collected for Western blot to detect the transfection efficiency.
Statistical analysis
The experimental data were statistically analyzed and plotted using Graphpad Prism 8.0.2. The data were expressed as mean ± standard deviation (SD). Statistical methods such as unpaired Student’s t test was used for comparison between the two groups, and One-way ANOVA followed by the Tukey’s post hoc test was used for comparison between multiple groups. ImageJ and AI were used for mapping. P < 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Results
DOX induce cardiotoxicity in mice
After a single intraperitoneal injection of DOX, the physiological status and survival rate of mice within 5 days were observed and compared with the control group. From the third day of DOX injection in mice, the food intake and water intake of mice decreased, the exercise ability decreased, the hair lost luster, irritability, and some mice showed shivering. From the 4th day, a large number of deaths occurred in the DOX group compared with the control group. The survival rate of the DOX group was significantly lower than that of the control group (Fig. 1A, B). The changes in body weight of the two groups of mice were observed within 5 days after injection of DOX. Compared with the control group, the body weight of mice in the DOX group decreased significantly (Fig. 1C). Compared with the control group, the heart volume of the DOX group was significantly reduced, and the appearance of the heart was decreased (Fig. 1D). Compared with the control group, the M-mode ultrasound images of the DOX group changed significantly (Fig. 1E). There was no significant difference in IVSd (Fig. 1F) and IVSs (Fig. 1G) between the DOX group and the control group. Compared with the control group, LVIDd (Fig. 1H) and LVIDs (Fig. 1I) were significantly increased in the DOX group. Compared with the control group, LVPWd (Fig. 1J) and LVPWs (Fig. 1K) were significantly decreased in the DOX treatment group. Compared with the control group, LVEF (Fig. 1L) and LVFS (Fig. 1M) were significantly decreased in the DOX group. Cardiac ultrasound showed that the cardiac function of mice was significantly decreased after DOX treatment. Compared with the control group, the HE staining of myocardial tissue in the DOX treatment group was uneven, the myocardial fibers were arranged in disorder, the stripes disappeared, the nucleus was arranged in disorder, and the local vacuolization of myocardial tissue and the surrounding inflammatory infiltration were observed (Fig. 1N). DOX treatment caused pathological changes in myocardial tissue. In summary, DOX induce cardiotoxicity in mice.
DIC in mice. A Show the survival rate over 5 days (n = 10). B Bar graph of survival rate (n = 10). C Body weight of mice in both groups over 5 days (n = 10). D Images of the heart size of both groups of mice (n = 10). E Representative echocardiography images in the both groups of mice (n = 7). F–M The Statistical results are IVSd, IVSs, LVIDd, LVIDs, LVPWd, LVPWs, LVEF and LVFS respectively (n = 7). N HE staining of myocardial tissue from both groups of mice (n = 6), scale bar = 50 μm. Data are presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns. No significant vs. Control group.
PCSK9 inhibitor improved DIC
PCSK9 is closely related to the occurrence of various cardiovascular diseases such as heart failure and atrial fibrillation, and is a risk factor for cardiovascular diseases. Therefore, this study detected the expression of PCSK9 protein in myocardial tissue after DOX treatment. Western Blot results showed that the expression of PCSK9 protein in myocardial tissue of DOX-treated mice was significantly up-regulated compared with the control group (Fig. 2A, B). In order to clarify the relationship between DOX-induced up-regulation of PCSK9 expression and cardiotoxicity, this study used PCSK9 inhibitors for mouse animal experiments to prove that PCSK9 inhibitors can improve DIC. Experimental studies have found that PCSK9 inhibitors can improve DOX-induced decreased survival rate (Fig. 2C, D) and weight loss (Fig. 2E) in mice. The heart volume of the DOX treatment group was significantly reduced, and the appearance of the heart was decreased. PCSK9 inhibitors can improve heart volume and appearance (Fig. 2F). Moreover, heart weight (HW) was measured and the ratio of heart weight to body weight (HW/BW) was calculated. Compared with the control group, the HW and HW/BW of the DOX group were significantly reduced. HW and HW/BW were significantly restored after the application of PCSK9 inhibitors (Fig. 2G, H).
The expression of PCSK9 protein in myocardial tissue of mice was up-regulated after DOX treatment, and PCSK9 inhibitor improved DIC. A WB images of PCSK9 protein expression in cardiac tissue of both groups of mice (n = 8). B Gray scale scanning analysis of PCSK9/GAPDH protein expression (n = 8). C Show the survival rate over 12 days (n = 10). D Bar graph of survival rate (n = 10). E Body weight of the three groups of mice over 12 days (n = 10). F Images of the heart size of three groups of mice (n = 10). G Heart weight (n = 10). H Heart weight to body weight ratio (n = 10). Data are presented as mean ± SD, *P < 0.05, ***P < 0.001, vs. Control group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. DOX group.
PCSK9 inhibitor improved DOX-induced cardiac dysfunction, myocardial injury and myocardial fibrosis in mice
In order to further clarify the effect of PCSK9 inhibitors on cardiac function in DOX-treated mice, cardiac ultrasound was used to evaluate cardiac function in mice. The previous results showed that the cardiac function of DOX-treated mice was significantly reduced. However, compared with the DOX group, the M-mode ultrasound images of the DOX + Evo group were significantly reversed (Fig. 3A). There was no significant difference in IVSd (Fig. 3B) and IVSs (Fig. 3C) between the control group, DOX group and DOX + Evo group. Compared with the DOX group, LVIDd (Fig. 3D) and LVIDs (Fig. 3E) in the DOX + Evo group were decreased. Compared with the DOX group, there was no significant difference in LVPWd (Fig. 3F) in the DOX + Evo group, and the LVPWs (Fig. 3G) in the DOX + Evo group increased. Compared with the DOX group, LVEF (Fig. 3H) and LVFS (Fig. 3I) were significantly improved in the DOX + Evo group. The results showed that PCSK9 inhibitors could improve DOX-induced cardiac function decline. In order to explore the effect of PCSK9 inhibitors on the pathological indexes of heart tissue in DOX-treated mice, HE and masson staining were used to stain the myocardial tissue of three groups of mice (Fig. 3J). Compared with the control group, obvious myocardial injury and fibrosis occurred in the myocardial tissue of the DOX group. Compared with the DOX group, myocardial injury and myocardial fibrosis were significantly alleviated in the DOX + Evo group. The results showed that PCSK9 inhibitors could improve DOX-induced myocardial injury and myocardial fibrosis in mice.
PCSK9 inhibitor improved DOX-induced cardiac dysfunction, myocardial injury and myocardial fibrosis in mice. A Representative echocardiography images in the three groups of mice (n = 7). B–I The Statistical results are IVSd, IVSs, LVIDd, LVIDs, LVPWd, LVPWs, LVEF and LVFS respectively (n = 7). J HE staining and Masson staining of myocardial tissue from the three groups of mice (n = 6), scale bar = 50 μm, myocardial fibers are shown in red, collagen fibers are shown in blue. Data are presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001 vs. Control group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. DOX group.
PCSK9 inhibitor improved DOX-induced myocardial apoptosis
TUNEL staining was used to detect the effect of PCSK9 inhibitor on DOX-induced myocardial apoptosis. Compared with the control group, the degree of myocardial tissue apoptosis in the DOX group was significantly increased, and the positive percentage of TUNEL was significantly increased. Compared with the DOX group, the positive signal of the DOX + Evo group was significantly reduced, and the percentage of TUNEL positive was significantly reduced. TUNEL staining experiments showed that PCSK9 inhibitors significantly improved DOX-induced myocardial apoptosis (Fig. 4A, B).
PCSK9 inhibitor improved DOX-induced myocardial apoptosis. A TUNEL staining images of myocardial tissues of the three groups of mice (n = 6), scale bar = 100 μm. B The percentage of TUNEL positive in the three groups of mice (n = 6). C Immumohis tochemical staining images of PCSK9 and Caspase-3 (n = 8), scale bar = 50 μm. D WB images of PCSK9 and apoptosis-related protein expression in myocardial tissue of the three groups of mice (n = 5). E–G Results of gray-scale scanning analysis of relative protein expression of PCSK9/GAPDH, Bax/Bcl-2 and Cleaved Caspase-3/Pro Caspase-3 (n = 5). Data are presented as mean ± SD, **P < 0.01, ***P < 0.001 vs. Control group, ##P < 0.01, ###P < 0.001 vs. DOX group.
In order to explore the molecular mechanism of PCSK9 inhibitor to improve cardiotoxicity, this study used immunohistochemical method to stain PCSK9 protein and apoptosis-related protein Caspase-3 in mouse myocardial tissue and observe the changes of protein expression and localization. The results showed that PCSK9 protein and Caspase-3 protein in the control group showed a small amount of scattered brown positive signals and were mainly concentrated in the cytoplasm. Compared with the control group, the expression of PCSK9 protein and Caspase-3 protein in the DOX group was significantly increased, showing a large number of dark brown positive signals. At the same time, the positive signals of PCSK9 (Fig. 4C) in the DOX group were mainly distributed in the cytoplasm and nucleus, and the positive signals of Caspase-3 (Fig. 4C) were mainly distributed in the cytoplasm. Compared with the DOX group, the expression of PCSK9 and Caspase-3 in the DOX + Evo group was significantly decreased, and the localization of PCSK9 and Caspase-3 showed a regional uneven distribution trend. Western Blot was used to detect the expression of apoptosis-related proteins in myocardial tissue, and to explore the molecular mechanism of PCSK9 inhibitor in improving DIC. Moreover, compared with the control group, the relative protein expression of PCSK9, Bax/Bcl-2 and Cleaved Caspase-3/Pro Caspase-3 in the myocardial tissue of the DOX group was significantly up-regulated. However, compared with the DOX group, the relative protein expression of PCSK9, Bax/Bcl-2 and Cleaved Caspase-3/Pro Caspase-3 in the DOX + Evo group was significantly down-regulated (Fig. 4D–G). In summary, PCSK9 inhibitors may improve DIC in mice by reducing the expression of PCSK9 and apoptosis-related protein. However, whether the accumulation of PCSK9 in the nucleus after DOX induction is related to DOX-induced cardiomyocyte apoptosis and its molecular mechanism need to be further explored.
The changes of PCSK9 gene and protein and the localization of PCSK9 in H9C2 cardiomyocytes after DOX treatment
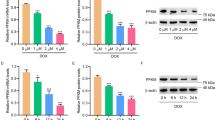
In this study, the role and molecular mechanism of PCSK9 in DIC were investigated by using H9C2 cardiomyocytes cultured in vitro. H9C2 cells were treated with DOX at concentrations of 0, 0.5, 1, 2.5, 5, 10, and 20 µM, and cell viability was detected by CCK8 for 12 h, 24 h, and 48 h, respectively. The results showed that different concentrations of DOX could significantly down-regulate cell viability. At the same time, with the gradual increase of DOX concentration and time, the cell viability gradually decreased (Fig. 5A). The half maximal inhibitory concentration (IC50) at 24 h was calculated to be about 1 µM. H9C2 cardiomyocytes were treated with DOX at a drug concentration of 1 µM for 12 h, 24 h and 48 h, respectively. The expression of PCSK9 was detected by RT-PCR and Western Blot. The results showed that there was no significant difference in the relative expression of PCSK9 mRNA and the expression of PCSK9 protein in the 12 h DOX group compared with the 12 h control group. Compared with the 24 h control group, the relative expression of PCSK9 mRNA (Fig. 5B) and the expression of PCSK9 protein (Fig. 5C, D) in the 24 h DOX group were significantly increased, and the difference was statistically significant. The results of 48 h of treatment were the same as those of 24 h, that is, DOX treatment could significantly up-regulate the expression levels of PCSK9 mRNA and PCSK9 protein. DOX could up-regulate the expression of PCSK9 in H9C2 cells. According to the results of immunohistochemical staining in vivo, PCSK9 aggregated in the nuclear region after DOX treatment. In order to further explore the effect of DOX treatment on the localization of PCSK9 cells, this study used immunofluorescence staining and ultra-high resolution microscopy to observe the expression and localization of PCSK9. The results showed that in the normal control group, the nucleus morphology was normal and the nucleus staining was uniform. The expression of PCSK9 was mainly concentrated in the cytoplasm and a small amount was distributed in the nucleus. The nuclei of the 24 h DOX treatment group and the 48 h DOX treatment group became larger and the nuclear staining was uneven, indicating that DOX treatment destroyed the structure of H9C2 cells, and the expression of PCSK9 was mainly concentrated in the nucleus (Fig. 5E). PCSK9, as a secretory protein, is not secreted and localized around the cytoplasm, but accumulates in the nucleus, indicating that PCSK9 may have a new biological mechanism in the process of DOX-induced cardiomyocyte apoptosis, which is worthy of further study.
The changes of PCSK9 gene and protein and the localization of PCSK9 in H9C2 cardiomyocytes after DOX treatment. A The cells were treated with DOX for 12 h, 24 h and 48 h (n = 6). B Changes in relative expression of PCSK9 mRNA after DOX treatment (n = 6). C WB images of PCSK9 protein expression after DOX treatment (n = 6). D Results of gray-scale scanning analysis of relative protein expression of PCSK9/GAPDH after DOX treatment (n = 6). E Ultra-high resolution images of changes in PCSK9 localization after 24 h and 48 h of DOX treatment (n = 5), scale = 10 μm. Data are presented as mean ± SD, **P < 0.01, ***P < 0.001 vs. 0µM group, #P < 0.05, ##P < 0.01 vs. 24 h control group, &&P < 0.01, &&&P < 0.001 vs. 48 h control group.
Interfering with PCSK9 improved DOX-induced apoptosis of H9C2 cardiomyocytes
Western Blot was used to detect the expression of apoptosis-related proteins when PCSK9 was up-regulated. The results showed that compared with the corresponding control group, the ratios of Cleaved Caspase-3/Pro Caspase-3 (Fig. 6A, B) and Bax/Bcl-2 (Fig. 6A, C) were up-regulated at 12 h, 24 h and 48 h after DOX treatment. The results showed that DOX-induced up-regulation of PCSK9 expression may be positively correlated with H9C2 cardiomyocyte apoptosis. H9C2 cells were seeded in 6-well plates and transfected with negative control si-NC, PCSK9 si-606, PCSK9 si-979 and PCSK9 si-2179, respectively. After 6 h of transfection, the serum-containing medium was replaced and cultured for 24 h. After 24 h of treatment with 1µM DOX, the protein samples were collected for Western Blot experiments to verify the interference effect of PCSK9. The results showed that the expression of PCSK9 protein could be significantly down-regulated (Fig. 6D), and the interference effect of PCSK9 si-979 (Fig. 6E) was the best. Therefore, PCSK9 si-979 was selected for subsequent experiments to inhibit the expression of PCSK9. In order to verify the relationship between the expression of PCSK9 and the apoptosis of H9C2 cardiomyocytes after DOX treatment, 1 µM DOX was used to treat H9C2 cardiomyocytes for 24 h and 48 h respectively and TUNEL staining was performed. The results showed that the positive signal of TUNEL staining in the 24 h DOX treatment group and the 48 h DOX treatment group was significantly higher than that in the corresponding control group, and the positive signal intensity of the 48 h DOX treatment group was the highest (Fig. 6F). By calculating the percentage of TUNEL positive cells, it was found that compared with the corresponding control group, the percentage of TUNEL positive cells in the DOX treatment group was significantly increased at 24 h and 48 h. TUNEL assay showed that the apoptosis of H9C2 cells was significantly increased after DOX treatment for 24 h and 48 h, and the positive percentage of apoptosis was similar (Fig. 6G), but the TUNEL positive signal intensity of DOX treatment group was the highest at 48 h. In order to further verify that PCSK9 interference can improve DOX-induced cardiomyocyte apoptosis in H9C2 cardiomyocytes, TUNEL staining was used for observation. In the control group, the TUNEL positive signal intensity was high, the nuclear staining was uneven and the morphology changed significantly, and the chromatin was fragmented. Compared with the control group, the number of cells in the PCSK9 si-979 group increased, the TUNEL positive signal intensity in the PCSK9 si-979 group decreased significantly, and the nucleus staining was more uniform and the morphology was restored (Fig. 6H). The percentage of apoptosis in the PCSK9 si-979 group was significantly lower than that in the control group (Fig. 6I). Studies have shown that interference with PCSK9 can improve DOX-induced apoptosis of H9C2 cardiomyocytes.
Interfering with PCSK9 improved DOX-induced apoptosis of H9C2 cardiomyocytes. A WB images of apoptosis-related protein expression after DOX treatment (n = 6). B Results of gray-scale scanning analysis of relative protein expression of Cleaved Caspase-3/Pro Caspase-3 after DOX treatment (n = 6). C Results of gray-scale scanning analysis of relative protein expression of Bax/Bcl-2 after DOX treatment (n = 6). D WB images of PCSK9 gene interference effect after DOX treatment (n = 6). E Results of gray-scale scanning analysis of relative protein expression interference PCSK9 gene after DOX treatment (n = 6). F TUNEL staining images of cells treated with DOX at different times (n = 6), scale bar = 100 μm. G Percentage of TUNEL positive cells treated with DOX at different times (n = 6). H TUNEL staining showed that PCSK9 interference improved DOX-induced apoptosis in H9C2 cardiomyocytes (n = 6), scale bar = 50 μm. I Percentage statistics of apoptosis after PCSK9 interference (n = 6). Data are presented as mean ± SD, *P < 0.05 vs. 12 h control group, **P < 0.01, ***P < 0.001 vs. si-NC group, ##P < 0.01, ###P < 0.001 vs. 24 h control group, &&&P < 0.001 vs. 48 h control group.
The mechanism of PCSK9 in DOX-induced apoptosis of H9C2 cardiomyocytes
To explore the molecular mechanism of PCSK9 in DOX-induced cardiomyocyte apoptosis. In this study, Co-IP combined with mass spectrometry was used to detect the proteins that bind to PCSK9 protein in H9C2 cardiomyocytes after DOX treatment. Since PCSK9 mRNA, PCSK9 protein and apoptosis-related proteins increased significantly after 24 h of DOX treatment, 1 µM DOX treatment for 24 h was selected for Co-IP experiment and mass spectrometry sequencing. Mass spectrometry sequencing was identified by Shanghai Bai pu Biotechnology Co., Ltd. A total of 510 binding proteins were identified by mass spectrometry sequencing, of which 247 proteins were bound to PCSK9 but not to IgG (Table-S1). Among them, the binding score was greater than 20 and the reliability was high, with a total of 83. The partial results of sequencing are as follows (Table 1). Since the ultra-high resolution results showed that PCSK9 was localized in the nucleus, suggesting that PCSK9 may promote cardiomyocyte apoptosis by binding to nuclear proteins, this study selected KPNB1 for subsequent experiments. At the same time, mass spectrometry results showed that KPNB1 and PCSK9 had a high binding score.
In this study, the interaction between PCSK9 and KPNB1 protein was detected by co-immunoprecipitation. Western Blot results showed that KPNB1 protein was precipitated in the experimental group with PCSK9 antibody, and KPNB1 protein was not detected in the IgG negative control group (Fig. 7A). PCSK9 protein was precipitated in the experimental group with KPNB1 antibody and PCSK9 protein was not detected in the IgG negative control group (Fig. 7B). The experiment verified that the protein spectrum sequencing results were accurate, and PCSK9 and KPNB1 could interact with each other. KPNB1 is a member of the nuclear transport protein family, which binds and assists nucleoproteins to enter and exit the nucleus through nuclear pores and plays an important role in cell growth and development. In this study, Western Blot was used to detect the expression of KPNB1 in H9C2 cardiomyocytes after DOX treatment. After 12 h, 24 h and 48 h of DOX treatment, the protein expression of KPNB1 in the DOX group was significantly down-regulated compared with the corresponding control group (Fig. 7C, D). It is speculated that DOX treatment up-regulates the expression of PCSK9 protein, and PCSK9 may bind to and hydrolyze KPNB1 protein and affect the transport of nuclear proteins, thereby inducing apoptosis. Western Blot results showed that compared with the si-NC group, the expression level of PCSK9 protein in the PCSK9 si-979 group was significantly decreased, while the expression level of KPNB1 protein was significantly increased. At the same time, the expression levels of Bax/Bcl-2 and Cleaved Caspase-3/Pro Caspase-3 proteins were significantly down-regulated (Fig. 7E, F). It is speculated that the down-regulation of PCSK9 may reduce the hydrolysis of KPNB1, suggesting that interference with PCSK9 may up-regulate the expression of KPNB1 and may regulate the expression of apoptosis-related proteins to improve DOX-induced cardiomyocyte apoptosis.
The mechanism of PCSK9 in DOX-induced apoptosis of H9C2 cardiomyocytes. A CO-IP forward verification (n = 5). B CO-IP reverse verification (n = 5), whole protein sample of H9C2 cardiomyocytes in Input group (positive control), Nonspecific IgG-binding proteins in IgG group (negative control). C WB images of KPNB1 protein expression after DOX treatment (n = 6). D Results of gray-scale scanning analysis of relative protein expression of KPNB1 after DOX treatment (n = 6). E WB images of KPNB1 and apoptosis-related proteins after interfering with PCSK9 (n = 5). F Results of gray-scale scanning analysis of relative protein expression of PCSK9, KPNB1, Bax/Bcl-2 and Cleaved Caspase-3/Pro Caspase-3 after interfering PCSK9 gene (n = 5). Data are presented as mean ± SD, *P < 0.05 vs. 12 h control group, **P < 0.01, ***P < 0.001 vs. si-NC group, ##P < 0.01 vs. 24 h control group, &&P < 0.01 vs. 48 h control group.
Discussion
Chemotherapeutic drugs have been widely used in tumor therapy, but their use is limited by drug concentration-related cardiotoxicity. DOX is a chemotherapeutic drug used to treat solid tumors and hematological tumors, which inhibits tumor progression by inhibiting topoisomerase. However, the side effects of DOX chemotherapy can lead to a decline in the quality of life of cancer patients. Among them, cardiotoxicity is one of the common side effects, which is mainly manifested as cardiac dysfunction, and may be combined with other cardiovascular system diseases23. DOX has been reported to induce cardiotoxicity through multiple programmed cell death pathways. In the DIC rat model, cell death in association with autophagy/mitophagy, apoptosis, necroptosis, pyroptosis, and ferroptosis occurred simultaneously in the heart and could potentially be mainly responsible for impaired LV function due to DOX treatment24,25. Inhibit fission or promoting fusion of mitochondria protects the heart from DOX-induced myocardial damage26. Moreover, the molecular mechanism of cardiotoxicity is mainly manifested as DOX treatment induces oxidative stress and increases ROS, which further triggers changes in intracellular signal transduction at the level, and ultimately leads to a series of changes in Bax, Bcl-2 and Caspase-3, which leads to apoptosis8.
PCSK9 is closely related to the occurrence of various cardiovascular diseases27,28,29. In the past few decades, PCSK9, as a secreted protein, has closely regulated the occurrence of cardiovascular diseases through transcription, secretion, clearance or extracellular inactivation30. In addition to participating in cholesterol metabolism, PCSK9 also plays an important role in heart, brain and kidney tissues. In recent years, PCSK9 inhibitors are increasingly used in clinical practice and have a very important position31. At present, there is no relevant report on the relationship between PSCK9 and DOX. The purpose of this study is to explore the role and mechanism of PCSK9 in DIC. In vivo studies have found that a single intraperitoneal injection of 20 mg/kg DOX for 5 days can induce cardiac toxicity in mice. DOX treatment significantly up-regulated the expression of PCSK9 protein in myocardial tissue. PCSK9 inhibitors improve DIC by regulating cardiomyocyte apoptosis, improving cardiac function, reducing myocardial injury and myocardial fibrosis.
There are two ways of administration in the clinical application of DOX. The first way of administration is 50–60 mg/time, once every 3–4 weeks, and the second way of administration is 20–30 mg/time for 3 weeks. Single-dose induction of cardiac toxicity is more obvious and single-dose treatment is often used in clinical practice. Therefore, this study selected a single-dose method to establish an animal model of acute cardiac toxicity. In this study, the survival rate of mice treated with DOX was significantly reduced, the food intake and water intake of mice decreased, the hair lost luster, and the body weight was significantly reduced, indicating that a series of pathophysiological changes occurred in mice after DOX treatment. HE staining of myocardium after DOX treatment showed disordered arrangement of myocardial fibers, disappearance of transverse striations, disordered arrangement of nuclei, visible myocardial vacuolization and surrounding inflammatory infiltration. At the same time, Masson staining showed that interstitial fibrous precipitation increased significantly. Considering that DOX can lead to the occurrence of myocardial injury, it is consistent with previous studies. At the same time, a series of changes occurred in cardiac ultrasound of mice after DOX treatment, manifested as the increase of LVIDd and LVIDs, and the decrease of LVPWd, LVPWs, LVEF and LVFS, among which the changes of LVEF and LVFS were the most significant. The results showed that DOX induction could lead to a significant decrease in cardiac function and an increase in left ventricular systolic and diastolic diameter, which was consistent with previous studies32. The main molecular mechanisms of DIC are oxidative stress and apoptosis. Similar to the molecular mechanism of ischemic myocardial injury, it is considered that the expression of PCSK9 can be verified in the DIC model. The results of this study showed that the expression of PCSK9 protein in mouse myocardial tissue was significantly up-regulated after DOX treatment. The possible mechanism is that DOX treatment induces oxidative stress and apoptosis, which affects the expression of PCSK9.
The intervention experiment of PCSK9 was carried out according to the injection method in the study of ischemic myocardial injury. The mice were subcutaneously injected with 10 mg/kg PCSK9 inhibitor 1 week before injection of DOX and half an hour before injection of DOX21,33. This study showed that PCSK9 inhibitors changed doxorubicin-induced cardiomyocyte apoptosis by affecting the Bax/Bcl-2/Caspase-3 pathway. At the same time, Western Blot results showed that the expression of PCSK9 protein in the PCSK9 inhibitor group was significantly down-regulated, which may involve the following possibilities. First of all, PCSK9 inhibitors can regionally bind to PCSK9 protein in the blood, thereby blocking the role of PCSK9 protein, thereby reducing the release of pro-inflammatory cytokines from macrophages, hepatocytes and various tissue cells34. Secondly, recent studies have shown that cardiomyocytes can express and release PCSK9 and act on cardiomyocytes in an autocrine manner and impair their function. PCSK9 inhibitors may reduce the functional damage caused by cardiomyocyte autocrine35. Finally, PCSK9 inhibitors may affect their ability to resist DIC by affecting changes in a series of related pathways. In summary, PCSK9 inhibitors may improve DIC by reducing the release of inflammatory factors, reducing autocrine damage and regulating related pathway changes. Therefore, the expression of PCSK9 protein in the PCSK9 inhibitor group showed a downward trend.
PCSK9 is a secreted protein strictly controlled by a variety of regulations. Previous studies have shown that PCSK9 is located on chromosome 1p32, contains 11 introns and 12 exon regions, and synthesizes a 692-amino-acid glycoprotein in the endoplasmic reticulum. Its structure contains a signal peptide, an N-terminal domain, and a catalytic domain36. In this study, it was found that the immunohistochemical results of mice treated with DOX showed that the expression of PCSK9 was significantly up-regulated and the localization was mainly concentrated in the nucleus, which was different from previous studies. PCSK9 may have a special molecular mechanism in DIC. In this study, cell experiments were used to explore the molecular mechanism. In this study, H9C2 cardiomyocytes were used as the research object. After DOX treatment of H9C2 cells, it was found that DOX had toxic effects on H9C2 cells in a concentration-dependent and time-dependent manner. The IC50 of 24 h was about 1 µM. The results of this study are the same as those of previous studies37. After H9C2 cells were treated with DOX, the expression of PCSK9 mRNA and protein was significantly up-regulated, which was consistent with the results of in vivo experiments. At the same time, it was found that apoptosis-related proteins were significantly up-regulated after DOX treatment of H9C2 cells and TUNEL staining also confirmed this result. After interfering with PCSK9, it was found that TUNEL staining showed that apoptosis was significantly alleviated and the nuclear damage after DOX treatment was restored. Studies have shown that regulation of PCSK9 at the gene level can also improve cardiomyocyte apoptosis induced by DOX treatment, which is the same phenomenon as the use of PCSK9 inhibitors in vivo experiments.
In vitro studies found that PCSK9 was mainly concentrated in the nucleus after DOX treatment, which was consistent with the results of immunohistochemical experiments in vivo, which may be a new mechanism of PCSK9. In order to explore its molecular mechanism, CO-IP combined with mass spectrometry sequencing experiments were used to explore 510 possible binding proteins with PCSK9 in the cell model, of which 83 had higher scores. The proteins involved were mainly related enzymes in the tricarboxylic acid cycle, mitochondrial-related enzymes, cytoskeletal proteins, membrane proteins and nuclear transporters. Because this study found that PCSK9 localization was mainly concentrated in the nucleus, the nuclear transporter KPNB1 with high score was selected for in-depth study of the mechanism. This study found that CO-IP interaction verified that PCSK9 and KPNB1 could interact with each other, which had not been reported before. KPNB1 is a member of the nuclear transport protein β family, which mediates the transport of proteins from the cytoplasm to the nucleus. In a variety of tumor studies, it has been found that up-regulation of KPNB1 expression can promote tumor growth and progression. Down-regulation of KPNB1 level and inhibition of KPNB1 activity can prevent tumor-related transcription factors from entering the nucleus, thereby inhibiting tumor cell proliferation and metastasis and promoting tumor cell apoptosis38. Previous studies have shown that KPNB1 is necessary for the maintenance of homeostasis in glioblastoma cells. Inhibition of KPNB1 can induce apoptosis in glioblastoma cells by affecting the imbalance of Bcl-2 family members39. The research on KPNB1 mainly focuses on the field of tumors. In vitro experiments in this study found that the expression of KPNB1 was significantly down-regulated after DOX treatment. At the same time, the expression of KPNB1 was significantly up-regulated after interference with PCSK9 and the apoptosis-related pathways were significantly changed.
This study showed that interference with PCSK9 could improve DOX-induced cardiomyocyte apoptosis, and the results of in vitro experiments were consistent with those of in vivo experiments. The possible molecular mechanism is that by interfering with the PCSK9 gene, the expression of PCSK9 protein is reduced, which leads to a decrease in the binding of PCSK9 to KPNB1 or a decrease in the hydrolysis ability, both of which up-regulate the protein level of KPNB1, thereby improving DOX-induced cardiomyocyte apoptosis. Although this study found that PCSK9 and KPNB1 can interact and bind to each other, and PCSK9 may trigger the hydrolysis of KPNB1 to promote cardiomyocyte apoptosis, the binding and interaction between PCSK9 and KPNB1 still need to be further investigated.
In this study, a single intraperitoneal injection of DOX was used to establish an acute cardiotoxicity model. Whether PCSK9 has the same phenomenon and molecular mechanism in DOX-induced chronic cardiotoxicity model is still under study. In this study, we found that PCSK9 affects myocardial apoptosis by affecting nuclear transport protein KPNB1. The apoptosis of cardiomyocytes is also closely related to energy metabolism and mitochondria. Whether PCSK9 plays a regulatory role in energy metabolism and mitochondrial damage will be verified in future. PCSK9 may interact with KPNB1 to induce apoptosis in cardiomyocyte and PCSK9 inhibitor may be a potential drug for improving DIC.
Conclusion
DOX has the potential induce cardiotoxicity in mice, and PCSK9 inhibitors can improve DIC by inhibiting myocardial apoptosis. Molecular investigation into the underlying mechanism have revealed that DOX induction leads to an up-regulate the expression of PCSK9 and make it accumulate in the nucleus. Subsequently, PCSK9 binds to KPNB1 and affects the expression of apoptotic genes such as Bax, Bcl-2 and Caspase-3 to promote cardiomyocyte apoptosis (Fig. 8).
Data availability
Data is provided within the manuscript or supplementary information files.
References
Grumbach, I. M. Cardio-oncology at the beginning of a new decade. J. Am. Heart Assoc. 9, e015890. https://doi.org/10.1161/JAHA.120.015890 (2020).
Deo, S. V., Al-Kindi, S. G. & Oliveira, G. H. Management of advanced heart failure due to cancer therapy: the present role of mechanical circulatory support and cardiac transplantation. Curr. Treat. Opt. Cardiovasc. Med. 17, 388. https://doi.org/10.1007/s11936-015-0388-8 (2015).
Renu, K., Abilash, V. G. & Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy—an update. Eur. J. Pharmacol. 818, 241–253. https://doi.org/10.1016/j.ejphar.2017.10.043 (2018).
Henriksen, P. A. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 104, 971–977. https://doi.org/10.1136/heartjnl-2017-312103 (2018).
Yarmohammadi, F., Rezaee, R. & Karimi, G. Natural compounds against doxorubicin-induced cardiotoxicity: a review on the involvement of Nrf2/AREsignaling pathway. Phytother. Res. 35, 1163–1175. https://doi.org/10.1002/ptr.6882 (2021).
Navarro-Hortal, M. D. et al. Role of flavonoids against adriamycin toxicity. Food Chem. Toxicol. https://doi.org/10.1016/j.fct.2020.111820 (2020).
Ye, X. Y. et al. Endogenous hydrogen sulfide persulfidates caspase-3 at cysteine 163 to inhibit doxorubicin-induced cardiomyocyte apoptosis. Oxid. Med. Cell. Longev. https://doi.org/10.1155/2022/6153772 (2022).
Kitakata, H. et al. Therapeutic targets for DOX-induced cardiomyopathy: role of apoptosis vs. ferroptosis. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23031414 (2022).
Jiang, C. et al. Pyrroloquinoline quinine ameliorates doxorubicin-induced autophagy-dependent apoptosis via lysosomal-mitochondrial axis in vascular endothelial cells. Toxicology 425 https://doi.org/10.1016/j.tox.2019.152238 (2019).
Faridvand, Y. et al. Human Amnion membrane proteins prevent doxorubicin-induced oxidative stress injury and apoptosis in rat H9c2 cardiomyocytes. Cardiovasc. Toxicol. 20, 370–379. https://doi.org/10.1007/s12012-020-09564-8 (2020).
Seidah, N. G. et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA 100, 928–933. (2003). https://doi.org/10.1073/pnas.0335507100
Scrimali, C. et al. Pcsk9-D374y mediated Ldl-R degradation can be functionally inhibited by Egf-a And truncated Egf-a peptides. An in vitro study. Atherosclerosis 315, E258–E258 (2020).
Ding, Z. et al. PCSK9 expression in the ischaemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc. Res. 114, 1738–1751. https://doi.org/10.1093/cvr/cvy128 (2018).
Bayes-Genis, A. et al. The PCSK9-LDL receptor axis and outcomes in heart failure: BIOSTAT-CHF subanalysis. J. Am. Coll. Cardiol. 70, 2128–2136. https://doi.org/10.1016/j.jacc.2017.08.057 (2017).
Cammisotto, V. et al. PCSK9 regulates Nox2-mediated platelet activation via CD36 receptor in patients with atrial fibrillation. Antioxidants (Basel). https://doi.org/10.3390/antiox9040296 (2020).
Sharotri, V., Collier, D. M., Olson, D. R., Zhou, R. F. & Snyder, P. M. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9). J. Biol. Chem. 287, 19266–19274. https://doi.org/10.1074/jbc.M112.363382 (2012).
Khoshnejad, M. et al. Development of novel DNA-encoded PCSK9 monoclonal antibodies as lipid-lowering therapeutics. Mol. Ther. 27, 188–199. https://doi.org/10.1016/j.ymthe.2018.10.016 (2019).
Wang, Y. et al. NSUN2 alleviates doxorubicin-induced myocardial injury through Nrf2-mediated antioxidant stress. Cell. Death Discov. 9, 43. https://doi.org/10.1038/s41420-022-01294-w (2023).
Zhang, J. et al. Resolvin E1 protects against doxorubicin-induced cardiotoxicity by inhibiting oxidative stress, autophagy and apoptosis by targeting AKT/mTOR signaling. Biochem. Pharmacol. 180, 114188. https://doi.org/10.1016/j.bcp.2020.114188 (2020).
Wang, Z. et al. Inhibition of TRPA1 attenuates doxorubicin-induced acute cardiotoxicity by suppressing oxidative stress, the inflammatory response, and endoplasmic reticulum stress. Oxid. Med. Cell. Longev. : 5179468. (2018). https://doi.org/10.1155/2018/5179468 (2018).
Huang, G. W. et al. PCSK9 Inhibition protects against myocardial ischemia-reperfusion injury via suppressing autophagy. Microvasc. Res. 142, 104371. https://doi.org/10.1016/j.mvr.2022.104371 (2022).
Qi, Z. Y. et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 143, 45–61. https://doi.org/10.1161/Circulationaha.120.046290 (2021).
Li, X. F. Doxorubicin-mediated cardiac dysfunction: revisiting molecular interactions, pharmacological compounds and (nano)theranostic platforms. Environ. Res. 234, 116504. https://doi.org/10.1016/j.envres.2023.116504 (2023).
Prathumsap, N. et al. Vagus nerve stimulation exerts cardioprotection against doxorubicin-induced cardiotoxicity through Inhibition of programmed cell death pathways. Cell. Mol. Life Sci. 80, 21. https://doi.org/10.1007/s00018-022-04678-4 (2022).
Khuanjing, T. et al. Acetylcholinesterase inhibitor ameliorates doxorubicin-induced cardiotoxicity through reducing RIP1-mediated necroptosis. Pharmacol. Res. 173, 105882. https://doi.org/10.1016/j.phrs.2021.105882 (2021).
Maneechote, C. et al. Promoting mitochondrial fusion in doxorubicin-induced cardiotoxicity: a novel therapeutic target for cardioprotection. Clin. Sci. (Lond). 136, 841–860. https://doi.org/10.1042/CS20220074 (2022).
Ding, Z. F. et al. PCSK9 expression in the ischaemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc. Res. 114, 1738–1751. https://doi.org/10.1093/cvr/cvy128 (2018).
Cammisotto, V. et al. PCSK9 regulates Nox2-mediated platelet activation via CD36 receptor in patients with atrial fibrillation. Antioxidants (Basel) 9, 296. https://doi.org/10.3390/antiox9040296 (2020).
Langsted, A., Nordestgaard, B. G., Benn, M., Tybjærg-Hansen, A. & Kamstrup, P. R. PCSK9 R46L loss-of-function mutation reduces lipoprotein(a), LDL cholesterol, and risk of aortic valve stenosis. J. Clin. Endocr. Metab. 101, 3281–3287. https://doi.org/10.1210/jc.2016-1206 (2016).
Gupta, J. & Gupta, R. PCSK9 biomarker and key modulator for cardiovascular disorders: heralding a new therapeutic era and their future perspectives. Curr. Mol. Pharmacol. 16, 832–854. https://doi.org/10.2174/1874467216666221202144813 (2023).
Sener, Y. Z. & Tokgözoglu, L. Pleiotropy of PCSK9: functions in extrahepatic tissues. Curr. Cardiol. Rep. 25, 979–985. https://doi.org/10.1007/s11886-023-01918-2 (2023).
Sabatino, J. et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc. Diabetol. 19 https://doi.org/10.1186/s12933-020-01040-5 (2020).
Zou, Y. Q. et al. Targeting PCSK9 ameliorates graft vascular disease in mice by inhibiting NLRP3 inflammasome activation in vascular smooth muscle cells. Front. Immunol. 13, 894789. https://doi.org/10.3389/fimmu.2022.894789 (2022).
Ding, Z. F., Pothineni, N. V. K., Goel, A., Lüscher, T. F. & Mehta, J. L. PCSK9 and inflammation: role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc. Res. 116, 908–915. https://doi.org/10.1093/cvr/cvz313 (2020).
Wolf, A. et al. Autocrine effects of PCSK9 on cardiomyocytes. Basic. Res. Cardiol. 115, 65. https://doi.org/10.1007/s00395-020-00824-w (2020).
Barale, C., Melchionda, E., Morotti, A. & Russo, I. PCSK9 biology and its role in atherothrombosis. Int. J. Mol. Sci. 22, 5880. https://doi.org/10.3390/ijms22115880 (2021).
Wu, Y. Z., Zhang, L., Wu, Z. X., Shan, T. T. & Xiong, C. Berberine ameliorates doxorubicin-induced cardiotoxicity via a SIRT1/p66Shc-mediated pathway. Oxid. Med. Cell. Longev. 2019, 2150394. https://doi.org/10.1155/2019/2150394 (2019).
Shi, Q. W. et al. KPNB1-mediated nuclear import in cancer. Eur. J. Pharmacol. 955, 175925. https://doi.org/10.1016/j.ejphar.2023.175925 (2023).
Zhu, Z. C., Liu, J. W., Li, K., Zheng, J. & Xiong, Z. Q. KPNB1 Inhibition disrupts proteostasis and triggers unfolded protein response-mediated apoptosis in glioblastoma cells. Oncogene 37, 2936–2952. https://doi.org/10.1038/s41388-018-0180-9 (2018).
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Shuai Shi: Writing-original draft, Methodology, Investigation, Formal analysis, Data curation, Supervision. Zhihui Qin: Investigation, Formal analysis. Chang liu: Investigation, Data curation. Yanru Zhao: Investigation. Xiaopeng Bai: Formal analysis. Chaoyu Sun: Investigation. Xu Li: Investigation, Methodology. Wanting Cong: Methodology. Xinyue yuan: Investigation. Lixiu Sun: Formal analysis, Data curation. Bingchen Liu: Writing-review & editing, Methodology, Investigation. Xueqi Li: Writing-review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, S., Qin, Z., Liu, C. et al. The function of PCSK9 in doxorubicin-induced cardiotoxicity and its underlying mechanism. Sci Rep 15, 22067 (2025). https://doi.org/10.1038/s41598-025-03419-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03419-4