Abstract
Targeting drugs to cancer cells via overexpressed cell-surface receptors has emerged as an effective therapeutic strategy for several cancers. However, identifying cell-surface receptors that allow selective uptake of targeting ligands by cancer cells—while sparing normal cells—remains a challenge, especially for triple-negative breast cancer (TNBC), which lacks a well-defined receptor for targeted delivery. In this study, immunohistochemical (IHC) analysis revealed that human TNBC patient tissues have significantly higher levels of keratin 1 (K1) compared to normal breast tissues. Among TNBC tissues, grade 3 tumors showed significantly higher (threefold) K1 expression compared to grade 2 tumors. We analyzed human TNBC and normal mammary epithelial cells to detect K1 from cell lysates using three methods: mass spectrometry, peptide mass fingerprinting, and Western blot. TNBC cell lysates confirmed the presence and high expression levels of 67 kDa K1. Importantly, intact cells showed that K1 is uniformly present on the surface of TNBC cells, while no or minimal cell-surface K1 was found in normal mammary epithelial cells using immunofluorescence confocal microscopy. Further, we show that cell-surface K1 was utilized by TNBC-selective peptide 18–4 for its uptake via cell-surface receptor (K1)-mediated endocytosis in TNBC cells, and the presence of peptide 18–4 did not affect the assembly of endogenous cytoplasmic K1. Taken together, our results demonstrate that K1 is overexpressed in human TNBC, and cell-surface K1 represents a promising new target for directed delivery in TNBC using targeting ligands such as peptide 18–4.
Similar content being viewed by others
Introduction
Targeting cancer cells via their overexpressed cell-surface receptors (CSRs) for drug delivery has emerged as a promising therapeutic strategy for treatment of several cancers1,2,3,4,5,6. In this approach, drug conjugates are designed by linking a drug to a targeting ligand through a linker to bind selectively to an overexpressed CSR in cancer cells (Fig. 1). The approach involves careful consideration of four key factors: (i) targeting ligand e.g., a peptide or an antibody, (ii) cytotoxic drug or payload such as chemotherapy or radionuclide agent, (iii) linker chemistry like cleavable carbonate or non-cleavable succinimidyl thioether bond, and (iv) target protein or CSR on cancer cells such as somatostatin receptor or human epidermal growth factor receptor 2 (HER2). This approach has seen significant clinical success, evident from the FDA approval of both antibody–drug conjugates (ADCs)2,3,5 and peptide-radionuclide conjugates (PRCs)7,8,9,10. Similarly, peptide-drug conjugates (PDCs) that target CSRs in cancer cells for targeted delivery of chemotherapy (payload) are being developed1,4,6. The success of this therapeutic approach relies on the identification of CSRs as targets that are expressed at higher levels in cancer cells – with minimal or no expression in normal cells. There are currently a handful of overexpressed CSRs in cancer cells, including TROP-2, HER-2, Nectin-4, and somatostatin receptor, that are targeted using FDA-approved conjugates for specific delivery of payloads to solid tumors7,11.
TNBC is an aggressive subtype of breast cancer with the highest 5-year mortality rate of 18.5%12. TNBC is more common in younger women, premenopausal African American and Hispanic women, and women of lower socioeconomic status when compared to other breast cancer subtypes13. TNBC is characterized by the absence of estrogen and progesterone receptors (ER/PR), and without the overexpression of HER2. Due to the absence of major drug interacting receptors, and its heterogeneity and aggressive nature, TNBC is considered a difficult-to-treat disease. Chemotherapy is the main treatment for TNBC but is not selective, causing toxicities in non-malignant tissues14,15. Thus, targeted delivery of chemotherapy to TNBC cells using CSRs is an emerging area of importance in oncology.
We previously identified keratin 1 (K1, Fig. 2a), a type II intermediate filament protein, as the receptor for cancer cell targeting peptides, such as p160 and analogues (Fig. 2b), in estrogen-receptor positive (ER +) breast cancer MCF-7 cells16. Additionally, we found higher K1 expression in breast cancer MCF-7 and melanoma MDA-MB-435 cells compared to the non-cancerous breast tissue derived MCF-10A cells16. Keratins (K) serve as epithelial cell markers, and expression of keratins is linked with cancer prognosis and is being used as a diagnostic marker17,18. K1 in particular belongs to non-hair keratins (K1-K24), which have been extensively studied for their role in human disease18,19,20,21. K1 expression is modulated during cell transformation events, such as tumorigenesis, and such events lead to its increased expression22. Attallah et al. detected significantly higher levels of K1 in hepatocellular carcinoma (HCC) patients compared to healthy individuals23. Upregulation of K1 in resistant cells from nasopharyngeal carcinoma (NPC) is also reported24.
Tumor-selective peptides for targeting keratin 1 (K1). (a) Schematic of K1 showing conserved rod region flanked by N-terminal head and C-terminal tail domains. The rod domain is divided into four α-helical units, 1a, 1b, 2a, and 2b linked via L1, L12, and L2 loops. a.a. stands for amino acid. (b) Sequence of peptide p160 and the engineered peptide analogues that display high binding to breast cancer cells and low/minimal binding to non-cancerous cells. X is Norleucine, lower case stands for D-amino acids, and cy stands for cyclic peptide with N- to C-terminal cyclization. (c) Chemical structure of peptide 18–4. L- and D-amino acids are shown in black and blue, respectively.
In addition to increased expression of keratins in cancer cells, it is also reported that keratins are present on the surface of cancer cells22. Godfroid et al. reported the presence of three cell-surface keratins (CSKs), namely CSK8, CSK18 and CSK19, in human mammary MCF-7 carcinoma cells with absence from the cell-surface of normal human mammary epithelial cells more than 30 years ago25; however, since then little is known about these cell-surface proteins. While the role of cytoplasmic keratins is well known, the function of CSKs is less clear. It is proposed that increased keratin (such as modified keratins 8 and 18) expression by transformed cells may expose keratins to the cell-surface due to their lack of integration into intermediate filaments26. Cell-surface K1 (CSK1) was previously discovered by our group as an overexpressed CSR in ER + breast cancer MCF-7 cells during our efforts to determine the uptake mechanism of cancer cell-targeting peptides we developed16. In addition, Doljak and colleagues reported that a monoclonal antibody raised against MCF-7 breast cancer cell lysates recognizes a common epitope present on keratins (K1, K2, K8, K10, and K18) in MCF-7 cells27. The study suggested that these keratins are exposed on the surface of MCF-7 cells and facilitate antibody binding. The presence of CSK1 has also been reported on neuroblastoma NMB7 cells by Chuang et al.28. Aside from this, not much is known about K1 expression, while other keratins such as K8, K18, and K19 are better studied in cancer18,22.
Cancer cell-targeting peptide p16029 and its analogues we designed, like 18 and 18–430,31, have shown high specific uptake by cancer cells (Fig. 2b). Peptide 18–4 was engineered for selective-uptake by TNBC cells. Starting with dodecapeptide p160 as a lead, we identified linear peptide 18 (1st generation) that displayed high affinity for breast cancer cells while showing low binding for non-cancerous HUVECs30. Peptide 18 was not proteolytically stable. Therefore its sequence was optimized by substitution with two D-amino acids to obtain 2nd generation proteolytically stable linear peptide 18–4 (Fig. 2c)31. Peptide 18–4 showed up to 3.5-fold enhanced binding to breast cancer cells compared to 18 and minimal/no uptake by non-cancerous cells. In addition, peptide 18–4 was found to be safe with minimal cellular toxicity31. Next, we obtained a 3rd generation cyclic peptide named cy18-4. Cy18-4 was proteolytically stable and showed 2 to threefold higher uptake by the breast cancer cells compared to the linear 18–432. Peptide cy18-4 showed minimal uptake by the non-cancerous cells, and it accumulated primarily at the tumor site in mice bearing orthotopic MDA-MB-231 (TNBC) tumors32. Further, we developed peptide 18–4 – doxorubicin (Dox) conjugates for targeted delivery of chemotherapy to TNBC cells33,34. These conjugates were specifically toxic to TNBC cells and xenograft tumors in mice35,36, and up to 30-fold less toxic to normal breast epithelial cells34. In mice, we found K1 was upregulated in tumors, and with low levels in normal mammary fat pad and liver tissues36.
In this study, we sought out to validate CSK1 as a target protein in human TNBC. While keratins are cytoplasmic proteins expressed in epithelial cells, we demonstrate here that both total and cell-surface expression of K1 are elevated in human TNBC compared to normal breast epithelial cells. Here, we report that (i) K1 is significantly upregulated in human TNBC tissues, with its expression being dependent on the TNBC grade. Grade 3 tumors exhibit a threefold higher K1 expression compared to grade 2 tumors. Further, K1 is absent in HER2 + breast cancer tissues, while ER + patient tissues show K1 expression. (ii) Human TNBC cells display higher levels of K1 expression compared to normal breast epithelial cells. (iii) K1 is uniformly present on the surface of human TNBC cells, while absent or minimally present on surface of normal mammary epithelial cells. (iv) The uptake of a TNBC-selective peptide, 18–4, takes place via CSK1-mediated endocytosis in TNBC cells, and (v) the presence of peptide 18–4 does not affect the assembly of endogenous cytoplasmic intermediate filaments.
The significance of this work lies in validating CSK1 targeting as a viable mechanistic approach for the selective delivery of therapeutics to TNBC. This builds on a large body of our previous research over the past decade, demonstrating the feasibility of K1 targeting using peptides and PDCs in both in vitro and in vivo models16,31,32,33,34,35,36. Collectively, our findings demonstrate CSK1 as a valuable target in human TNBC.
Results
Human TNBC tissues show significantly elevated K1 levels compared to normal breast tissues
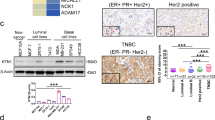
We examined K1 expression levels in human tissues from different breast cancer subtypes (TNBC, ER + /PR + , and HER2 +) and normal breast using IHC staining for K1. As shown in Figs. 3a–c, S1 (supplementary information), the TNBC tissue images displayed the strongest staining for K1, followed by ER + /PR + , while no staining was observed for HER2 + or normal breast tissues. Comparison of the immunoreactive score (IRS) for K1 expression from different tissues showed that average K1 expression was significantly elevated in TNBC and ER + /PR + tissues compared to normal breast and HER2 + tissues (Fig. 3d). The average expression of K1 in TNBC tissues was higher than in ER + /PR + tissues, though the difference was not statistically significant. These results suggest that K1 could serve as a target receptor for both TNBC and ER + /PR + breast cancer subtypes. However, the significance of K1 overexpression is particularly notable in TNBC, as this subtype has been challenging to target and treat, whereas several specific treatments are available for the ER + /PR + subtype, such as selective ER modulators (e.g., tamoxifen) and aromatase inhibitors.
K1 levels are significantly increased in human TNBC compared to normal breast and other breast cancer subtypes. (a) Representative breast tissue images showing IHC staining for K1. IHC analysis of human breast tissue array (TissueArray.com) was performed using rabbit monoclonal [EPR17744] to cytokeratin 1 (Abcam) and goat anti-rabbit IgG H&L (HRP polymer) secondary antibody (Abcam). Images of tissues from two patients each with TNBC, ER + /PR +, HER2 + or normal breast are shown (scale bar 50 μm). (b) IHC images of a full TNBC tissue core (scale bar 200 μm) and a 10X magnified region of the tissue. (c) Data for human tissues (from TissueArray.com) used for the IHC images shown above. IC invasive carcinoma (d) Quantification of K1 staining (dark brown) determined as immunoreactive score (IRS) from IHC images of tissues from normal breast (n = 8), HER2 + (n = 14), ER + /PR + (n = 20), and TNBC (n = 24) breast cancer subtypes (refer to Fig. S1 for patient details). Mean ± SEM; ns not significant; ***p < 0.001, Welch’s one-way ANOVA followed by Dunnett’s T3 post-hoc test. (e) Quantification of K1 staining (IRS) of grade 2 (n = 12) and grade 3 (n = 10) TNBC tissues compared to normal breast (n = 8) tissues. Mean ± SEM; *p < 0.05, **p < 0.01, Welch’s one-way ANOVA, followed by Dunnett’s T3 post-hoc test.
Remarkably, the data showed that TNBC tissues with grade 3 tumors have significantly more K1 expression (3.13-fold) compared to grade 2 tumors (Fig. 3e). More TNBC patients have grade 3 tumors (68%), followed by grade 2 (17%) and grade 1 (4%)12. The survival rates for highly abnormal and poorly differentiated grade 3 tumors are generally low for most aggressive cancer types as grade 3 tumors have a high risk of recurrence12,37. Higher levels of K1 in grade 3 TNBC could be targeted with PDCs or ADCs for targeted delivery of chemotherapy to these tumors. Overall, the IHC results presented here provide evidence for the overexpression of K1 in human TNBC tissues.
Overexpression of K1 in Human TNBC cells
Three human TNBC cell lines (MDA-MB-231, MDA-MB-436, and MDA-MB-468) and two normal mammary epithelial cell lines (MCF-10A and MCF-12A) were used to evaluate K1 expression. Cells were lysed with non-denaturing cell lysis buffer to keep the cell-surface proteins intact, and cell lysates were analyzed by three methods. First, the mass analysis using matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry of all three TNBC cell lysates showed a peak at ~ 67 kDa, which is in the range of predicted mass for K1. Lysate of MDA-MB-468 cells showed a large peak at ~ 66,819 (Fig. 4a). Similarly, lysates of MDA-MB-231 and MDA-MB-436 cells showed a peak at ~ 66,889 and ~ 66,878 (Fig. S2), respectively. The corresponding mass peak was not detected in normal breast cell lysates. The appearance of mass peak directly from the cell lysates suggests high concentration of K1 in TNBC cells. Second, the cell lysate proteins were resolved on SDS–polyacrylamide gel (Figs. 4b, S3) and the band corresponding to 67 kDa were excised and analyzed by in-gel trypsin digestion followed by liquid chromatography – tandem mass spectrometry and proteomics (peptide mass fingerprinting). K1, along with human albumin, was detected as the top two proteins in bands from all three TNBC cell lines. For instance, thirteen peptide sequences (highlighted in red) matched K1 sequence with a coverage of 22% for MDA-MB-468 cell lysate (Figs. 4c, S4). Further, the Western blot (WB) analysis of the whole cell lysates showed large bands at 67 kDa corresponding to K1 protein for the three TNBC cell lysates, while these bands were not present for the normal breast cell lysates (Figs. 4d, S5). These analyses confirmed that K1 is highly expressed in TNBC cells compared to normal breast cells.
Human TNBC cells overexpress K1. (a) Mass analysis of cell lysate (MDA-MB-468) using MALDI-TOF mass spectrometry. The mass spectrum was obtained in positive linear mode and shows a mass peak for K1 (found 66819, expected ~ 67 kDa). Peak for housekeeping β-actin (~ 42 kDa) was also observed. (b) Coomassie stained gel showing resolved protein bands from lysates of normal (MCF-10A and MCF-12A) and TNBC (MDA-MB-231, MDA-MB-436, and MDA-MB-468) cells. (c) Identification of keratin 1 (K1) after in-gel trypsin digestion of the 67 kDa band for MDA-MB-468 cell lysate and LC MS/MS analysis of peptide fragments (in red). Thirteen peptide sequences matched K1 which corresponds to 22% protein sequence coverage. (d) Western blot analysis using anti-K1 antibody (LHK1 clone) of cell lysates (15 μg) shows overexpression of K1 (at 67 kDa) in TNBC cells versus no/minimal K1 expression in normal breast cells. β-actin was analyzed for all cell lysates (TNBC and normal breast) as a loading control.
Previously, we and others reported higher expression of K1 in ER + breast cancer MCF-716, melanoma MDA-MB-43516, and other cancer cells compared to the non-cancerous breast tissue derived MCF-10A cells23,24,28. Further, Trask et al. showed large amounts of K8, K18, and K19 produced by several tumor-derived mammary epithelial cells, such as ER positive MCF-7, T-47D, ZR-75–1, and ER negative SK-BR-3, BT-20, 21 T series38. Notably, their data showed that TNBC cell line MDA-MD-231 produced low levels of K8, K18, and K19. Also, it was observed that K8, K18, and K19 were not present in the normal cells.
Increased expression of CSK1 in human TNBC cells compared to normal mammary epithelial cells
To determine CSK1 expression, cells were incubated with mouse monoclonal antibody to human K1 under non-permeabilizing conditions, and the cell membrane was stained with a red cytoplasmic membrane staining dye. The representative Z-section confocal microscopy images showed that K1 is uniformly present on the surface of all three TNBC cell lines as evidenced by the colocalization of K1 (green) and the membrane (red) dye (Fig. 5). Some K1 (green) was also observed inside the TNBC cells suggesting the antibody diffused into the cytoplasm during the 90-min incubation time; however, intense staining at the cell periphery supports K1 is associated with the cell-surface. No or minimal staining on the surface of non-cancerous mammary epithelial cells indicates low levels of CSK1 in normal cells. This is consistent with the CSK1 expression reported in other cancer cells like ER positive MCF-7 breast cancer cells16 and neuroblastoma NMB7 cells28, as well as cell-surface expression of other intermediate filament proteins like keratin 8/1825,26 and vimentin39,40,41.
Immunofluorescence analysis of CSK1 expression (green and orange) in human TNBC cells (MDA-MB-231, MDA-MB-436, and MDA-MB-468). (a) Z-section confocal microscopy images of a few representative cells show higher CSK1 expression in TNBC cells compared to the normal mammary epithelial cells (MCF-10A and MCF-12A). Cells were stained with anti-K1 mAb (green), cell-membrane stain (red), and the overlay is shown in orange. DAPI (blue) was used to stain the nucleus. Scale bar 15 μm. (b) Fluorescence intensity of CSK1 (% of cell membrane stain intensity) for TNBC and normal cells. Mean ± SEM; ***p < 0.001 (c) Representative Z-section confocal microscopy images show overlay (orange) of K1 and cell-membrane staining in a large number of TNBC cells. Scale bar 25 μm.
Uptake of cancer cell targeting peptide 18–4 by TNBC cells takes place via CSK1-mediated endocytosis
We hypothesized that the high and specific uptake of peptide 18–4 and corresponding PDCs (peptide 18–4-Dox conjugates) is due to the presence of CSK1 in TNBC cells. Here, we validated our hypothesis by following labeled 18–4 (structure shown in Fig. S6) in TNBC cells in the presence and absence of K1 mAb. First, TNBC cells incubated with 18–4 peptide showed colocalization of the peptide (labeled green) and lysosomes (labeled orange) using confocal microscopy (Fig. 6), suggesting that the peptide entered the cells by the endocytic pathway. Next, the cells were incubated with K1 mAb prior to addition of the peptide. The presence of K1 mAb eliminated uptake of peptide 18–4 by TNBC cells indicating the peptide binds to CSK1 on cancer cells and is transported to intracellular lysosomes by CSK1-mediated endocytosis. The incubation of a control scrambled peptide with the TNBC cells showed almost no uptake of the control peptide (Fig. S7) highlighting the specificity of peptide 18–4 for CSK1 in TNBC cells.
CSK1 receptor-mediated endocytosis of peptide 18–4 by TNBC cells. Cells (MDA-MB-231 and MDA-MB-468) were incubated with FITC labeled peptide 18–4 (green) in the absence (-) and presence (+) of K1 mAb, followed by incubation with LysoView 640 (orange) for labeling lysosomes. Nuclei were stained with DAPI (blue). (a) Confocal Z-section images of representative cells along with overlay (yellow) are shown. Scale bar 20 μm. (b) Fluorescence intensity of FITC-peptide (% of lysosomes intensity) in TNBC cells in the absence and presence of K1 mAb. Mean ± SEM; ****p < 0.0001.
Peptide 18–4 does not bind to cytoplasmic K1
To demonstrate the specificity of peptides 2 and 18–4 for CSK1, and not cytoplasmic K1/K10 complex, we used an in vitro K1/K10 filament assembly assay and electron microscopy (EM) to show that K1/K10 filament assembly is not disrupted by 2 or 18–4 (Fig. 7). This supports our finding that peptide 18–4-Dox conjugates are less toxic to normal breast tissue33,34. Normal filament assembly was also observed when peptides 2 and 18–4 were incubated with K8/K18 during assembly; K8/K18 is also expressed cytoplasmically in breast tissue42.
We have determined four K1/K10 x-ray crystal structures to date, representing all of the K1-based structures in the Protein Data Bank (PDB)43,44,45. Importantly, two structures exist of K1 helix 2b (Fig. 2a), where peptides 2 and 18–4 are expected to bind based on our prior studies16. The coiled-coil region of keratins is historically divided into four helical domains (1a, 1b, 2a, and 2b) that are flanked by N-terminal head and C-terminal tail regions46. The fact that K1 targeting peptides did not perturb cytoplasmic IF assembly (Fig. 7) is a further indication that these peptides bind to CSK1 and not cytoplasmic K1, which enhances the therapeutic potential for targeting and treating TNBC cells while preserving host tissues.
Positive correlation between KRT1 and EGFR gene expression
TNBC is classified into four subtypes, basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal (M) and luminal androgen receptor (LAR)47. Each TNBC subtype displays unique gene ontologies involving different signaling pathways48. For instance, the BL2 and M subtypes are enriched in growth factor receptor gene expression such as EGFR, MET, and EPHA248. We found a positive correlation (R = 0.29, p-value 0.004) between KRT1 and EGFR expression in basal breast cancer subtypes (Fig. S8). In contrast, the KRT1 expression was negatively associated with EGFR expression in the normal adjacent breast tissue samples. Further, ERBB2 (gene for HER2) showed a negative correlation with KRT1 across basal, luminal A and luminal B breast cancer subtypes which is in line with no K1 expression in human HER2 + breast cancer tissues (Fig. 3a). These results suggest that K1 interacts with EGFR signaling, specifically in a basal subtype of TNBC. Future studies are warranted to explore the role of K1 in cancer progression using differential gene expression with extensive pathway analysis.
Discussion
As intermediate filament proteins, keratins contribute to the structural integrity of cells; moreover, their rich expression patterns make them valuable diagnostic markers for epithelial malignancies17,18. However, the modern paradigm is that keratins are intricately involved in human disease processes, not merely performing important cellular functions or serving as diagnostic markers18,19,20,21. The keratin family includes 28 Type I (acidic) and 26 Type II (basic or neutral) proteins, which pair to form heterodimers (e.g., K1/K10, K5/K14, and K8/K18) that make up intermediate filaments22. Basal and luminal cells in mammary epithelium differ in keratin expression: basal cells (majority of TNBCs are basal)48 express K5 and K14, while luminal cells (e.g., ER + MCF7) primarily express K8, K18, and K1922. Keratins have been linked to over 60 different disorders and display altered expression levels, mutations, or posttranslational modifications in disease. Further, altered cellular localization of keratins K8, K18, and K19 on the surface of cancer cells has been observed in disease for decades25 and yet remains poorly characterized. A study showed that CSK8 may help with immune escape by masking MHC class I molecules on cancer cell-surface, thereby preventing recognition by the cytotoxic CD8 + T cells and facilitating metastasis49. Other studies point to phosphorylation of CSK8 by kinases leading to enhanced solubility of CSKs which may correlate with cancer progression50. Previously, we and others found increased expression of CSK1 in ER + MCF-7 breast cancer cells16,27 and NMB7 neuroblastoma28. In this study, we advance the understanding of K1 in breast cancer by demonstrating that K1 is overexpressed in human TNBC compared to normal breast cells and tissues, and K1 is uniformly present on the surface of TNBC cells (Figs. 3 and 5).
With the validation of CSK1 as a receptor in TNBC, CSK1 can now be targeted for selective delivery of cytotoxic drugs or imaging agents to TNBC cells. To this end, we previously developed peptides (Fig. 2b), such as linear 18–4 (18–4), designed for specific binding and uptake by TNBC cells31,32. TNBC-selective peptide 18–4 was engineered in two steps31. First, phage display screening by Zhang et al. identified a cancer cell-selective peptide (p160, Fig. 2b) with no uptake by normal cells29. In the second step, starting with p160 as a lead we synthesized a library of 70 peptides in an array format on functionalized cellulose membrane. The peptide array was screened for binding to breast cancer cells, which ultimately led to 18–430,31. All peptides in the array were covalently linked from the C-terminus to the cellulose membrane, and therefore the C-terminal of the peptides was blocked30. Soluble peptides with free C-terminal were later synthesized separately that maintained specific binding to the breast cancer cells31. Similarly, peptides with FITC labeling in the N-terminal or N- to C-terminal cyclized peptide (cy18-4, Fig. 2b) also maintained breast cancer cell binding31,32. This suggested that the binding domain of peptide 18–4 is located in the middle of the sequence. Blocking the N-terminus, the C-terminus, or both termini of 18–4 did not affect its binding to the target receptor in breast cancer cells. Also, both charged residues, Glu3 and Arg8, were retained in 18–4 as they were found important for selective binding to cancer cells51.
Subsequently, identification of the target receptor using pull-down assay for peptide 18–4 in ER + MCF-7 breast cancer cells led to the discovery of CSK116. K1 knockdown in MCF-7 cells (via siRNA) resulted in almost no uptake of peptide 18–416. The binding specificity between peptide 18–4 and K1 was confirmed using surface plasmon resonance (SPR) experiments, and the binding constant (Kd) for 18–4 and K1 fragment (387–496 aa, K1 2b domain) interaction was found to be 0.98 μM16. Here in this study, the binding of peptide 18–4 with CSK1 in TNBC cells specifically is supported by the observation that cellular uptake of 18–4 was eliminated in the presence of K1 mAb, indicating that 18–4 needs CSK1 to bind to and therefore enter TNBC cells. Here we specifically show that this tumor-selective peptide (18–4) also targets CSK1 in human TNBC (Fig. 6), which is a novel avenue of molecular targeting for TNBC. Such a stepwise platform—screening cancer cell-selective peptides followed by receptor identification—can be adopted to identify novel CSRs for different cancer types, where targeted drug delivery could improve treatment outcomes.
Peptide 18–4 was previously conjugated to the chemotherapeutic drug Dox using different linkers, to synthesize PDCs33,34. Dox is an anthracycline that is used as both adjuvant and neoadjuvant therapy for breast cancer treatment15. The PDCs were specifically toxic to TNBC cells (IC50 1.2 − 4.7 μM for TNBC cells versus 15 − 39 μM for normal breast cells)34, and demonstrated improved efficacy and reduced uptake by non-tumor tissues compared to free Dox in mice bearing MDA-MB-231 TNBC xenografts35,36. Significantly more Dox accumulated in tumors of PDC treated mice (sevenfold more) compared to the Dox treated mice, while lower levels of Dox was observed in the liver, heart, and lungs of PDC treated mice (up to threefold less) than Dox treated mice36. Based on our previous and current findings, Fig. 8 illustrates the mechanism of selective toxicity of PDCs toward TNBC, following their uptake by TNBC cells via CSK1-mediated endocytosis (Fig. 6). The specific endocytic pathway, such as clathrin-mediated, caveolae-mediated, or macropinocytosis52, responsible for the uptake of peptide 18–4 or its PDCs remains to be determined for a more detailed mechanistic understanding of internalization. Additionally, evaluation of the therapeutic efficacy of PDCs in patient-derived xenograft (PDX) mouse models is warranted to substantiate CSK1 as a viable target receptor in TNBC and support its potential for clinical translation.
Schematic of a TNBC cell showing CSK1-mediated endocytosis of a PDC targeting K1. First, the peptide domain of PDC binds CSK1 (1), leading to endocytosis of PDC (2). Once internalized, the K1-PDC complex undergoes proteolytic cleavage and degradation in lysosomes (3) followed by release of the cytotoxic drug in the cytoplasm (4). Finally, the drug enters the nucleus to intercalate with the DNA (5) eventually causing TNBC cell death.
In addition to PDCs, future studies could explore the use of antibodies against CSK1 to develop ADCs for TNBC treatment. Several ADCs are FDA-approved for the treatment of various cancers, and PDCs are under developmental stages1,2,3,4,5,6. Notably two peptide-radionuclide conjugates (PRCs), 177Lu DOTA-TATE (FDA-approved in 2018) and 177Lu vipivotide tetraxetan (FDA-approved in 2022), that target somatostatin receptor and prostate-specific membrane antigen (PSMA), respectively, were recently approved for treatment of somatostatin or PSMA overexpressing cancers, respectively7,8,9,10. The approval of PRCs brings tremendous hope for the success and approval of PDCs (or peptide-chemotherapy conjugates) as treatments for different cancer types in the near future.
TNBCs have a poor prognosis and remain a therapeutic challenge as they do not respond well to anti-hormonal or anti-HER2-targeted therapies, unlike other breast cancer subtypes11. Additionally, the humanized anti-PD-1 antibody pembrolizumab, used in combination with chemotherapy for treating PD-L1 positive metastatic TNBC, shows effectiveness in only some patients. The ADC sacituzumab govitecan targeting TROP-2 was recently (2021) approved as a third line treatment for metastatic TNBC patients53,54. Finding new CSRs in cancer cells that can be targeted using the ADC or PDC approach will increase the efficacy and reduce off-target side effects of otherwise extremely potent chemotherapy drugs or other anti-cancer agents such as oncolytic peptides55,56. The findings delineated herein, alongside our prior results with the PDCs, corroborate CSK1 as a target receptor for TNBC, thereby underscoring its potential utility in targeted delivery strategies.
Conclusions
This study highlights the distinctive expression pattern of K1 in human TNBC. First, we show that TNBC patient tissues exhibit significantly higher levels of K1 compared to normal breast tissues, with grade 3 tumors showing markedly elevated K1 expression relative to grade 2 tumors. Second, human TNBC cells exhibit increased K1 expression relative to normal breast epithelial cells. Third, K1 is also uniformly localized on the surface of TNBC cells while absent or minimally present on surface of normal mammary epithelial cells. Finally, we show that the uptake of a tumor-selective peptide 18–4 by TNBC cells is mediated through CSK1. These findings provide first unequivocal evidence of elevated levels of CSK1 in human TNBC.
We also show that assembly of K1/K10 intermediate filament is not disturbed in the presence of peptide 18–4. This indicates that the peptide likely does not interact with the cytoplasmic K1, supporting its selective binding to TNBC cells via CSK1. Given the challenges in targeting and treating TNBC, CSK1-targeted delivery represents a promising therapeutic strategy. The differential expression of CSK1 in TNBC cells compared to normal cells highlights the potential for developing TNBC-specific delivery systems for drugs or imaging agents. Such interventions, where CSK1 is targeted using our peptides, are being pursued by us (such as PDCs targeting K1)34,35,36 and other researchers for imaging aggressive cancers57 or creating prodrugs58. In conclusion, our findings uncover CSK1 as an attractive target in TNBC, offering opportunities for the directed delivery of cytotoxic agents to tumors.
Materials and methods
Peptides and antibodies
Peptides and their fluorescently labeled analogues were synthesized following Fmoc solid-phase peptide synthesis as described previously31. Peptides were purified on a reversed-phase (RP) HPLC system (Prominence-I, Shimadzu Corp., Kyoto, Japan) using semipreparative C18 HPLC column (10 × 250 mm, flow rate 1 mL/min, monitored at 220 nm) and a gradient of 10–50% acetonitrile in 0.05% aqueous TFA over a period of 1 h. Pure peptides were characterized on an analytical C18 column (4.6 mm × 250 mm, flow rate 0.8 mL/min) using a Shimadzu LCMS-2020 system (Fig. S6). CellBrite Fix 555 Membrane Stain (Cat# 30,088) and LysoView 640 (Cat# 70,085) dyes were purchased from Biotium. All antibodies used in this study and their sources of purchase are listed in Table S1.
Cell culture
Three TNBC (MDA-MB-231, MDA-MB-436 and MDA-MB-468) and two normal breast tissue derived epithelial (MCF-10A and MCF-12A) cell lines, obtained from ATCC, were used in this study. Their culture conditions are described in Table S2. Hank’s balanced salt solution (HBSS) (without calcium chloride, magnesium chloride and magnesium sulfate) (Gibco, USA) was used to wash the cells before trypsinization. Mycoplasma contamination of cells was monitored using MycoAlert Detection Kit (Lonza).
Immunohistochemical (IHC) analyses of human tissues
Human tissue microarrays (Cat# BR087e, BR251f, and BR1191), two of each, were purchased from TissueArray.com (USA). Characterization, such as IHC for ER, PR, HER2 and Ki-67, as well as age, stage and grade of cancer, for each tissue on the array, was provided by the vendor (Fig. S1). Before use, the tissue on microarray slides were deparaffinized and rehydrated. Heat-induced epitope retrieval was performed, followed by blocking and then incubation with rabbit cytokeratin 1 mAb (1:200) overnight at 4 °C. Sections were washed, and incubated with 3% hydrogen peroxide to block endogenous peroxidase activity, followed by incubation with goat anti-rabbit IgG H&L. The sections were developed using a DAB Substrate Kit (Abcam), counterstained with hematoxylin to stain the nuclei and were mounted with coverslips. Tissues were imaged with a fluorescence microscope (Keyence BZ-X710). K1 expression levels were quantified using IRS59. IRS for K1 was obtained by multiplying the K1 staining intensity (dark brown), determined using NIS-Elements AR Analysis software (Nikon), with the percentage of positive cells in five gradations (0–4). IHC analyses were repeated for each tissue (core) on a separate day.
Cell lysis using non-denaturing lysis buffer
TNBC and normal breast cells were lysed, and the cell lysates were analyzed using three methods (MALDI-TOF, in-gel trypsin digestion followed by peptide mass fingerprinting, and Western blot). Cells were grown in T25 flasks until 80–90% confluent. Cells were washed with ice cold HBSS (without calcium and magnesium) and lysed with 1 mL of non-denaturing lysis buffer containing 50 mM Tris–HCl (pH 7.4), 5 mM MgCl2, 250 mM sucrose, 1X HALT protease inhibitor cocktail, and 1 mM PMSF, at 4 ºC for 45 min16. The cell lysates were then centrifuged at 6000 g for 15 min at 4 ºC, whereby the supernatant was collected as the soluble nuclear and membrane fraction60. Protein concentration of supernatant from each cell lysate was determined with BCA Assay Kit.
MALDI-TOF mass analysis
The supernatants from the cell lysates were analyzed using MALDI-TOF mass spectrometry using autoflex speed MALDI-TOF mass spectrometer (Bruker, USA). The mass spectra were acquired in positive linear mode over a mass range of 30–210 kDa for analyses of proteins. The matrix was prepared using sinapinic acid in water/acetonitrile (70:30, v/v) containing 0.1% trifluoroacetic acid and mixed with the cell lysate in 1:1 ratio. The resulting solution was spotted on MALDI plate and allowed to air dry. The data from 2000 laser shots was summed to generate the mass spectrum. MALDI-TOF for each cell lysate was repeated at least 3 times on different days.
In-gel trypsin digestion and peptide mass fingerprinting
Supernatant (15 μg) from each cell lysate was loaded onto an SDS–polyacrylamide gel (4–20%). The gel was run for 70 min at 110 V to separate the proteins, followed by visualization with Coomassie Brilliant Blue G250. Protein band of interest (between 50 and 75 kDa) was excised, digested with trypsin as described before16 and peptides were collected. The digestion mixture was then subjected to LC–MS/MS (Bruker Impact II™ Ultra-High Resolution Qq-Time-Of-Flight, USA) and the MS/MS data were analyzed by the MASCOT search engine to identify proteins in the band. The experiment was repeated 2 times.
Western blot analysis
The gradient gel to resolve proteins was repeated for analysis by Western blot. The protein from the gel were transferred onto a 0.45 μm PVDF membrane. After blocking with 3% milk, the membrane was incubated with either 1:500 primary K1 antibody (LHK1 clone) or 0.03 µg/mL mAb against β-actin, in 1X TBST with 3% milk at 4 ºC overnight. The next day, membranes were washed with 1X TBST (3 times) and incubated in 1:5,000 of IgG (H + L) Poly-HRP goat anti-mouse in 1X TBST with 3% milk for 1 h. Membranes were washed again (3 times) followed by ECL Ultra solution (TMA-100, Lumigen), and imaging with BioRad imager (ChemiDoc XRS +). The experiment was repeated at least 5 times.
Immunofluorescent confocal microscopy for monitoring CSK1 expression
The cell-surface or cell membrane K1 expression in TNBC versus normal breast cells was determined using immunofluorescent confocal microscopy. Cells (~ 105 cells/mL) were plated on a 4-chamber cell culture microscope slide (Corning™ Falcon™, USA) for 24 h. After rinsing the cells, the cell membrane was stained with a staining dye (CellBrite Fix 555 Membrane Stain). The slide was rinsed with HBSS and cells were fixed with 4% formaldehyde. The cells were washed, blocked (2% BSA) and 34BetaB4 clone K1 mAb (1:20) was added for 90 min in dark. Next, secondary antibody Alexa 488 goat anti-mouse IgG (H + L) in PBS (1:250) was added, followed by addition of DAPI. Cells treated with secondary antibody alone served as controls. Each sample was analyzed with a confocal microscope (Nikon Eclipse). For each chamber, 10 randomized fields were photographed and analyzed with NIS-Elements AR software. Three independent experiments were performed each in quadruplicate (with > 50 cells analyzed). Fluorescence intensities for the cell membrane stain and CSK1 were measured from the images using ImageJ software version 1.53t (Bethesda, MD, USA). The cell membrane stain intensity was set to 100%, and the CSK1 intensity, relative to membrane stain, was plotted for TNBC and normal cells.
Confocal microscopy of colocalization of lysosomes and peptide in TNBC cells
Stock solutions for fluorescein labeled peptides, 18–4 (WxEAAYQrFL) and control scrambled (EPAAYQRFT), were prepared in sterile DMSO/water (5:95). The concentration of the stock solution was measured using Quickdrop34. Cells (1.5 × 105/well) were grown on a four-chambered culture slide for 72 h. The primary K1 antibody (1:100 dilution in 0.5% BSA in serum free media, LHK1 clone) was added to two wells while serum free media was added to the other two wells, for 20 min at 37 \(^\circ\)C in dark. Next, peptide 18–4 solution (1 μM) was added to each of the four wells on the slide and incubated for 1 h at 37 \(^\circ\)C in dark. Cells were washed, and LysoView 640 (1:750) was added followed by DAPI with mounting medium and analysis with a confocal microscope. In a separate culture slide, control scrambled peptide was added to the cells, followed by washing and incubation with LysoView as above. Images were captured at 10 randomized fields for each sample and examined with NIS-Elements AR software. The experiment was repeated at least three times, and > 50 cells were analyzed for each experiment. Fluorescence intensity was measured from images using ImageJ 1.53t. Peptide intensity was plotted relative to lysosomes set to 100%.
Electron microscopy of K1/K10 filament
Stock solutions of peptides 2 and 18–4 (Fig. 2), were prepared in acetonitrile:water (1:9). K1 and K10 full-length proteins were produced and purified as described44. K1 and K10 were mixed with 10 mM Tris–HCl buffer (pH 8.5) containing 9 M urea and 2 mM DTT to 0.5 mg/mL final concentration. This solution was added to a Pur-A-Lyzer Midi dialysis tube (molecular weight cut-off 1000) and placed in solution of 10 mM Tris–HCl buffer (pH 8.5) containing 4 M urea and 2 mM DTT at r.t. for 2 h. The dialysis tube was next moved to a similar solution but with 2 M urea for 2 h. Lastly, the dialysis tube containing the filament solution was left stirring in the final dialysis buffer of 0.7 mM NaH2PO4 (pH 7.5) containing 1 mM DTT at 4 °C overnight. To assemble mature filaments prior to imaging, dialyzed filament solution was mixed with activation solution: 0.7 mM NaH2PO4 buffer (pH 7.5) containing 40 mM KCl. The tube was incubated in a 37 °C water bath for 30 min, and then 12 µL of termination solution (0.7 mM NaH2PO4 buffer (pH 7.5) containing 40 mM KCl, 0.2% glutaraldehyde) was added. To assess for peptide inhibition of IF assembly, peptides (2 or 18–4) targeting CSK1 were added either: (i) prior to dialysis, (ii) after dialysis steps, or (iii) after 15 min of assembly (but before termination). Control experiments included IF without the addition of inhibitory peptide and inhibitory peptide without IF. A drop of the assembled filament solution was then placed on a carbon type-B support film copper grid for electron microscopy and the sample negatively stained using a 1% uranyl acetate solution. Grids were visualized on a Talos L120C transmission electron microscope. Several wells in every quadrant of each grid were visualized and representative images collected.
Statistical analysis
All statistical analyses for the quantification of the imaging data were performed using the GraphPad Prism 9.0.0 software. Significant differences among three or more groups were assessed using Welch’s one-way ANOVA, followed by Dunnett’s T3 post-hoc test. The Welch’s t-test was utilized to compare two groups with unequal variances and/or unequal sample sizes. The error bars are representative of the mean ± standard error of the mean (SEM), from at least two independent experiments. p < 0.05 was considered statistically significant.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
Abbreviations
- ADC:
-
Antibody–drug conjugate
- BL1:
-
Basal-like 1
- BL2:
-
Basal-like 2
- CSK:
-
Cell-surface keratin
- CSK1:
-
Cell-surface keratin 1
- CSR:
-
Cell-surface receptor
- Dox:
-
Doxorubicin
- ER + /PR +:
-
Estrogen and progesterone receptor positive
- HER2:
-
Human epidermal growth factor receptor 2
- IHC:
-
Immunohistochemical
- IRS:
-
Immunoreactive score
- IF:
-
Intermediate filament
- K:
-
Keratins
- K1:
-
Keratin 1
- LAR:
-
Luminal androgen receptor
- LC–MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- M:
-
Mesenchymal
- MALDI-TOF:
-
Matrix assisted laser desorption/ionization-time of flight
- mAb:
-
Monoclonal antibody
- PDC:
-
Peptide-drug conjugate
- PRC:
-
Peptide-radionuclide conjugate
- r.t.:
-
Room temperature
- TNBC:
-
Triple-negative breast cancer
References
Alas, M., Saghaeidehkordi, A. & Kaur, K. Peptide-drug conjugates with different linkers for cancer therapy. J. Med. Chem. 64(1), 216–232 (2021).
Chau, C. H., Steeg, P. S. & Figg, W. D. Antibody-drug conjugates for cancer. Lancet 394(10200), 793–804 (2019).
Drago, J. Z., Modi, S. & Chandarlapaty, S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 18(6), 327–344 (2021).
Rizvi, S. F. A., Zhang, L., Zhang, H. & Fang, Q. Peptide-drug conjugates: Design, chemistry, and drug delivery system as a novel cancer theranostic. ACS Pharmacol. Transl. Sci. 7(2), 309–334 (2024).
Tsuchikama, K., Anami, Y., Ha, S. Y. Y. & Yamazaki, C. M. Exploring the next generation of antibody-drug conjugates. Nat. Rev. Clin. Oncol. 21(3), 203–223 (2024).
Zhang, B. et al. Recent advances in targeted cancer therapy: Are PDCs the next generation of ADCs?. J. Med. Chem. 67(14), 11469–11487 (2024).
Banerjee, S., Pillai, M. R. & Knapp, F. F. Lutetium-177 therapeutic radiopharmaceuticals: linking chemistry, radiochemistry, and practical applications. Chem. Rev. 115(8), 2934–2974 (2015).
Fallah, J. et al. FDA approval summary: Lutetium Lu 177 vipivotide tetraxetan for patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 29(9), 1651–1657 (2023).
Hope, T. A., Pavel, M. & Bergsland, E. K. Neuroendocrine tumors and peptide receptor radionuclide therapy: When is the right time?. J. Clin. Oncol. 40(24), 2818–2829 (2022).
Shah, H., Ravi, P., Sonpavde, G. & Jacene, H. Lutetium Lu 177 vipivotide tetraxetan for metastatic castration-resistant prostate cancer. Expert Rev. Anticancer Ther. 22(11), 1163–1175 (2022).
Dumontet, C., Reichert, J. M., Senter, P. D., Lambert, J. M. & Beck, A. Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 22(8), 641–661 (2023).
Nelson, D. R., Brown, J., Morikawa, A. & Method, M. Breast cancer-specific mortality in early breast cancer as defined by high-risk clinical and pathologic characteristics. PLoS ONE 17(2), e0264637 (2022).
Narod, S. A. & Dent, R. Triple-negative breast cancers. Expert Rev. Anticancer Ther. 23(10), 1041–1043 (2023).
Garrido-Castro, A. C., Lin, N. U. & Polyak, K. Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov. 9(2), 176–198 (2019).
Lee, J. S., Yost, S. E. & Yuan, Y. Neoadjuvant treatment for triple negative breast cancer: Recent progresses and challenges. Cancers (Basel) 12(6), 1404 (2020).
Soudy, R., Etayash, H., Bahadorani, K., Lavasanifar, A. & Kaur, K. Breast cancer targeting peptide binds keratin 1: A new molecular marker for targeted drug delivery to breast cancer. Mol. Pharm. 14(3), 593–604 (2017).
Ho, M. et al. Update of the keratin gene family: evolution, tissue-specific expression patterns, and relevance to clinical disorders. Hum. Genomics 16(1), 1 (2022).
Toivola, D. M., Boor, P., Alam, C. & Strnad, P. Keratins in health and disease. Curr. Opin. Cell Biol. 32, 73–81 (2015).
Karantza, V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene 30(2), 127–138 (2011).
Loschke, F., Seltmann, K., Bouameur, J. E. & Magin, T. M. Regulation of keratin network organization. Curr. Opin. Cell Biol. 32, 56–64 (2015).
Sharma, P., Alsharif, S., Fallatah, A. & Chung, B. M. Intermediate filaments as effectors of cancer development and metastasis: A focus on keratins, vimentin, and nestin. Cells 8(5), 497 (2019).
Ogunnigbagbe, O., Bunick, C. G. & Kaur, K. Keratin 1 as a cell-surface receptor in cancer. Biochim. Biophys. Acta Rev. Cancer 1877(1), 188664 (2022).
Attallah, A. M. et al. Evaluation of cytokeratin-1 in the diagnosis of hepatocellular carcinoma. Clin. Chim. Acta 412(23–24), 2310–2315 (2011).
Tang, S. et al. Identification Keratin 1 as a cDDP-resistant protein in nasopharyngeal carcinoma cell lines. J. Proteomics 75(8), 2352–2360 (2012).
Godfroid, E., Geuskens, M., Dupressoir, T., Parent, I. & Szpirer, C. Cytokeratins are exposed on the outer surface of established human mammary carcinoma cells. J. Cell Sci. 99(Pt 3), 595–607 (1991).
Ditzel, H. J. et al. Modified cytokeratins expressed on the surface of carcinoma cells undergo endocytosis upon binding of human monoclonal antibody and its recombinant Fab fragment. Proc. Natl. Acad. Sci. U. S. A. 94(15), 8110–8115 (1997).
Doljak, B., Obermajer, N., Jamnik, P. & Kos, J. Monoclonal antibody to cytokeratin VKIALEVEIATY sequence motif reduces plasminogen activation in breast tumour cells. Cancer Lett. 267(1), 75–84 (2008).
Chuang, N. N. & Huang, C. C. Interaction of integrin beta1 with cytokeratin 1 in neuroblastoma NMB7 cells. Biochem. Soc. Trans. 35(5), 1292 (2007).
Zhang, J., Spring, H. & Schwab, M. Neuroblastoma tumor cell-binding peptides identified through random peptide phage display. Cancer Lett. 171(2), 153–164 (2001).
Ahmed, S., Mathews, A. S., Byeon, N., Lavasanifar, A. & Kaur, K. Peptide arrays for screening cancer specific peptides. Anal. Chem. 82(18), 7533–7541 (2010).
Soudy, R., Gill, A., Sprules, T., Lavasanifar, A. & Kaur, K. Proteolytically stable cancer targeting peptides with high affinity for breast cancer cells. J. Med. Chem. 54(21), 7523–7534 (2011).
Raghuwanshi, Y. et al. Proteolytically stable cyclic decapeptide for breast cancer cell targeting. J. Med. Chem. 60(12), 4893–4903 (2017).
Soudy, R., Chen, C. & Kaur, K. Novel peptide-doxorubucin conjugates for targeting breast cancer cells including the multidrug resistant cells. J. Med. Chem. 56(19), 7564–7573 (2013).
Ziaei, E. et al. Targeting triple negative breast cancer cells with novel cytotoxic peptide-doxorubicin conjugates. Bioconjug. Chem. 30(12), 3098–3106 (2019).
Saghaeidehkordi, A., Chen, S., Yang, S. & Kaur, K. Evaluation of a keratin 1 targeting peptide-doxorubicin conjugate in a mouse model of triple-negative breast cancer. Pharmaceutics 13(5), 661 (2021).
Ziaei, E. et al. Peptide-drug conjugate targeting keratin 1 inhibits triple-negative breast cancer in mice. Mol. Pharm. 20(7), 3570–3577 (2023).
Chen, X. & Wang, Z. Q. The differences between pancreatic neuroendocrine tumors grade 2 and grade 3-letter. Clin. Cancer Res. 25(14), 4580–4580 (2019).
Trask, D. K. et al. Keratins as markers that distinguish normal and tumor-derived mammary epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 87(6), 2319–2323 (1990).
Batth, I. S. & Li, S. Discovery of cell-surface vimentin (CSV) as a sarcoma target and development of CSV-targeted IL12 immune therapy. Adv. Exp. Med. Biol. 1257, 169–178 (2020).
Satelli, A. & Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 68(18), 3033–3046 (2011).
Suprewicz, L. et al. Extracellular vimentin as a target against SARS-CoV-2 host cell invasion. Small 18(6), e2105640 (2022).
Bozza, W. P., Zhang, Y. & Zhang, B. Cytokeratin 8/18 protects breast cancer cell lines from TRAIL-induced apoptosis. Oncotarget 9(33), 23264–23273 (2018).
Bunick, C. G. & Milstone, L. M. The X-ray crystal structure of the keratin 1-keratin 10 helix 2B heterodimer reveals molecular surface properties and biochemical insights into human skin disease. J. Invest. Dermatol. 137(1), 142–150 (2017).
Eldirany, S. A., Ho, M., Hinbest, A. J., Lomakin, I. B. & Bunick, C. G. Human keratin 1/10–1B tetramer structures reveal a knob-pocket mechanism in intermediate filament assembly. EMBO J. https://doi.org/10.15252/embj.2018100741 (2019).
Lomakin, I. B., Hinbest, A. & Bunick, C. G. The crystal structure of keratin 1/10(Cys401Ala) helix 2B heterodimer determined at 20 Å resolution. J. Invest. Dermatol. 138(5), S121 (2018).
Herrmann, H. & Aebi, U. Intermediate filaments: Structure and assembly. Cold Spring Harb. Perspect. Biol. 8(11), a018242 (2016).
Lehmann, B. D. et al. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS ONE 11(6), e0157368 (2016).
Lehmann, B. D. et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 121(7), 2750–2767 (2011).
Wu, M. S., Li, C. H., Ruppert, J. G. & Chang, C. C. Cytokeratin 8-MHC class I interactions: a potential novel immune escape phenotype by a lymph node metastatic carcinoma cell line. Biochem. Biophys. Res. Commun. 441(3), 618–623 (2013).
Gires, O., Andratschke, M., Schmitt, B., Mack, B. & Schaffrik, M. Cytokeratin 8 associates with the external leaflet of plasma membranes in tumour cells. Biochem. Biophys. Res. Commun. 328(4), 1154–1162 (2005).
Askoxylakis, V. et al. Characterization and development of a peptide (p160) with affinity for neuroblastoma cells. J. Nucl. Med. 47(6), 981–988 (2006).
Komin, A., Russell, L. M., Hristova, K. A. & Searson, P. C. Peptide-based strategies for enhanced cell uptake, transcellular transport, and circulation: Mechanisms and challenges. Adv. Drug Deliv. Rev. 110–111, 52–64 (2017).
Jeong, J. H. & Kim, S. B. Antibody-drug conjugates targeting Trop-2: Clinical developments in early breast cancer therapy. Breast 66, 199–203 (2022).
Shaffer, C. Trop2 deal heats up antibody-drug conjugate space in cancer. Nat. Biotechnol. 39(2), 128–130 (2021).
Fu, X. Y. et al. Three rounds of stability-guided optimization and systematical evaluation of oncolytic peptide LTX-315. J. Med. Chem. 67(5), 3885–3908 (2024).
Yin, H. et al. Design, synthesis and anticancer evaluation of novel oncolytic peptide-chlorambucil conjugates. Bioorg. Chem. 138, 106674 (2023).
Subiros-Funosas, R. et al. Fluorogenic Trp(redBODIPY) cyclopeptide targeting keratin 1 for imaging of aggressive carcinomas. Chem. Sci. 11(5), 1368–1374 (2019).
He, X. et al. An all-in-one tetrazine reagent for cysteine-selective labeling and bioorthogonal activable prodrug construction. Nat. Commun. 15(1), 2831 (2024).
Specht, E. et al. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology 67(3), 368–377 (2015).
Cox, B. & Emili, A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat. Protoc. 1(4), 1872–1878 (2006).
Acknowledgements
The National Cancer Institute of the National Institutes of Health (Award Number R15CA208656 to K.K.) supported this research. This work was also supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R03AR076484 and R01AR079428 (to C.G.B.). We thank Dr. Sherif A. Eldirany for work on filament assembly assays. We thank the core labs at the Chapman University School of Pharmacy for access to all instrumentation.
Author information
Authors and Affiliations
Contributions
K.K. designed research; S.Y., F.A., E.Z., A.S., M.R.R., K.S., A.S., R.K.S., S.M.N., and C.G.B. performed research; S.Y., C.G.B. and K.K. analyzed data and wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The patient tissue samples used in this study were purchased from TissueArray.com. All ethical considerations were followed when using human tissue samples.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yao, SJ., Amirrad, F., Ziaei, E. et al. Surface keratin 1, a tumor-selective peptide target in human triple-negative breast cancer. Sci Rep 15, 21644 (2025). https://doi.org/10.1038/s41598-025-05351-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05351-z