Abstract
Xanthomonas axonopodis pv. vasculorum (Xav), the causative agent of sugarcane gumming disease, represents a significant threat to global sugarcane production due to its systemic and destructive nature. A field-deployable tool specific to Xav is required for rapid infection detection and timely disease management. This resulted in a loop-mediated isothermal amplification (LAMP) assay targeting the gene region, unique to Xav strains, as a rapid and precise diagnostic assay. The selection of a target gene region was informed by comprehensive in silico genomes analyses of Xav and other closely related Xanthomonas species. The target gene region’s specificity was validation against the NCBI GenBank database and internally sequenced genomes. Primers for both endpoint PCR and LAMP assays were designed using this unique gene. The LAMP assay underwent extensive testing against inclusivity and exclusivity panels. Use of exclusivity panel, comprising 81 strains from related species, other bacterial genera, and host genomes, demonstrated the assay’s specificity with no false positives. The assay exhibited a detection limit of 1 pg, and its effectiveness was unimpeded by crude host lysate (sugarcane). Further validation through multi-device and multi-operator testing underscored the assay’s 100% reproducibility and robustness. Application to infected plant samples resulted in the detection of all infected specimens without any false positives or negatives. This novel LAMP assay is accurate and reliable tool for Xav detection, with promising applications in routine diagnostics, biosecurity measures, microbial forensics, and epidemiological research.
Similar content being viewed by others
Introduction
Members of the Gamma proteobacterial genus Xanthomonas, composed of 36 validly published species, predominantly infect plants and have caused severe damage to a wide variety of economically important vegetable and field crops and tree plant species1,2,3. Sugarcane (Saccharum officinarum L.) is among these plant species affected by Xanthomonas species2,4. Xanthomonas axonopodis pv. vasculorum (Xav), a Gram-negative phytopathogenic bacterium, poses a particularly significant threat to the agricultural industry due to its capability to cause gumming diseases in sugarcane, a tropical grass native to Asia. Sugarcane holds a vital position in global sugar production, contributing to around 75% of the industry5,6. Gumming disease is a vascular ailment distinguished by its unique symptoms, including distinctive lesions, red discoloration at nodes, gum pockets at growing points, and damage to nodal and internodal tissues6,7. These symptoms are followed by chlorosis of new leaves in mature plants, reducing photosynthetic efficiency and consequently decreasing crop yield. Xav demonstrates clear phylogenetic differentiation from other sugarcane-infecting xanthomonads, such as X. vasicola pv. vasculorum, X. sacchari, and X. albilineans2. Therefore, an accessible, highly specific, and rapid identification method is crucial for reliable diagnosis, efficient disease management, timely intervention, and developing strategies to protect agricultural productivity.
Traditional serological assays, such as the enzyme-linked immunosorbent assay (ELISA), have been used since the 1970s to detect microbial proteins associated with specific pathogens. Although genomic data is not required, ELISA is labour-intensive and prone to high rates of false negatives and false positives, increasing the risk of inaccurate diagnosis, which can lead to disease transmission and crop loss8. The advent of PCR has enabled efficient processing, allowing for the precise quantification of amplification products and facilitating the rapid detection of pathogens9. Various reliable identification methods, such as endpoint PCR and qPCR assays, have been established for Xanthomonas species, offering improved accuracy10,11,12. However, certain limitations have been identified regarding point-of-need applications. Most notably, PCR requires intricate, costly machinery and precise operating conditions13.
In contrast, isothermal methods are user-friendly and field-deployable, making them highly advantageous for point-of-need applications15. Among these isothermal methods, loop-mediated isothermal amplification (LAMP) is particularly favorable for point-of-need assays16,17. LAMP assays are widely recognized for their exceptional cost-efficiency and have gained prominence for their utility in various applications13,18. Due to its high sensitivity, inhibitor resistance, ease of use, and quick reaction time (under 20 min), LAMP ensures effectiveness and reliability, even in challenging conditions19,20,21.
LAMP operates by utilizing a DNA polymerase that can displace strands and synthesize DNA, enabling sequence amplification under isothermal conditions13,22. This process relies on four primers comprised two inner primers, the forward inner primer (FIP) and backward inner primer (BIP), and two outer primers, F3 and B313. In the initial stages of LAMP, the inner primer, either FIP or BIP, hybridizes to its target, allowing the polymerase to begin synthesizing the complementary strand. Next, the outer primer, F3 or B3, hybridizes to its target site and displaces the synthesized complementary strand, releasing the strand that serves as a template for the other inner primer. Loop primers, developed by Nagamine and Notomi22accelerate the reaction by hybridization of regions between the binding sites of F3 and FIP, or B3 and BIP, enhancing the formation of the characteristic “dumb-bell” stem-loop structure of the LAMP product with improved selectivity22,23. Results of LAMP can be verified by electrophoresis, precipitation of magnesium pyrophosphate byproduct, or SYBR green assay17,24. SYBR green assays use a fluorescent intercalating dye present in the solution that binds to double-stranded DNA and can be confirmed visually with the naked eye or under UV light18. The assays designed using unique and conserved genomic regions provide robustness and reliability17,19.
The objective of this study was to develop a LAMP assay for the specific detection and identification of Xav in infected plant tissues. The developed assay exhibits potential applications in plant disease diagnostics, seed certification, agricultural surveys, farm management, and plant biosecurity.
Results
Target selection and primer design
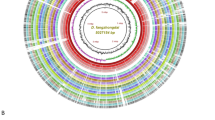
In silico analyses of genomes (Fig. 1) identified potential target regions specific to Xav. A gene region emerged as distinctive to Xav and exhibited conservation across all six strains, thereby establishing its utility for diagnostic assay development. In silico validation of the primers designed for both PCR and LAMP showed high specificity (Table 1). BLASTn analysis against an in-house custom database, which included genomes of Xav and closely related species, confirmed the primers’ specificity. The location of unique target gene region/primers is shown in Fig. 1.
A ring plot highlighting the unique sequence region employed in developing a loop-mediated isothermal amplification (LAMP) assay for the specific detection of Xanthomonas axonopodis pv. vasculorum. This circular graphic illustrates multiple genome alignments that include six X. axonopodis pv. vasculorum genomes (three of which were obtained from our lab) and ten other Xanthomonas species that share the same ecological niche or infect the same host, sugarcane. The first three innermost layers of the graphic represent the genome coordinates (in megabase pairs, mbp), the GC content (depicted as a zigzag black line), and the GC skew (shown as a zigzag purple + / green - line) of the reference genome X. axonopodis pv. vasculorum strain NCPPB 796. Subsequent color-coded rings display the BLASTn pairwise comparisons of various X. axonopodis pv. vasculorum strains, including NCPPB 796 (NZ_CP053649), NCPPB 900 (NZ_JPHD00000000), CFBP5823 (MDCD00000000), LMG894, LMG901, and LMG903. These are followed by the position of the unique target region conserved across all X. axonopodis pv. vasculorum strains, which is pointed out and labeled in red. Additionally, the plot includes genomes from other Xanthomonas species, such as X. campestris pv. campestris ATCC 33,913 (NC_003902), X. campestris pv. musacearum NCPPB 4379 (NZ_AKBF00000000), X. citri pv. citri MN12 (NZ_CP008998), X. euvesicatoria pv. alfalfae CFBP3836 (NZ_CP072268), X. oryzae pv. oryzae ICMP3135 (NZ_CP031697), X. oryzae pv. oryzicola GX01 (NZ_CP043403), X. phaseoli pv. dieffenbachiae LMG695 (NZ_CP014347), X. perforans GEV872 (NZ_CP116305), X. vasicola pv. vasculorum Xv1601 (NZ_CP025272), and X. sacchari DJ16 (NZ_CP121698). This image was generated using the BLAST Ring Image Generator (BRIG) v 0.9525.
End-point PCR primers specificity
The endpoint PCR primer set’s specificity was validated in vitro by testing against several strains included in both inclusivity and exclusivity panels. The inclusivity assay utilized genomic DNA from six strains of Xav (Table 2), while the exclusivity panel comprised three strains of Stenotrophomonas and 47 strains of various Xanthomonas species (Table 2) confirmed the high specificity. The targeted gene region was found in all strains of Xav, and there was no off-target amplification with the strains in the exclusivity panel. The PCR amplification of the target region yielded a product size of 208 nucleotides, as predicted with Primer3.
LAMP assay specificity
We employed an extensive panel of strains to demonstrate the specificity of the LAMP primers designed in this study (Tables 1 and 2). After amplification, the assay successfully detected all six Xav strains from the inclusivity panel, with no false positives observed in the exclusivity panel. Real-time LAMP assay cycling data produced sigmoidal curves indicating a positive result surpassing a threshold of 0.2 normalized fluorescence (Fig. 2A). Positive samples displayed Ct values ranging from 8.76 (LMG8715) to 14.45 (LMG894) (Fig. 2A). Amplified DNA was confirmed colorimetrically by the appearance of fluorescent green (visual observation; Fig. 2B), and also detectable through exposure to UV light fluorescence (Fig. 2C). SYBR Green detection was concordant with the results of sigmoidal amplification curves.
The specificity of the loop-mediated isothermal amplification (LAMP) assay, designed for the selective amplification of Xanthomonas axonopodis pv. vasculorum, was validated using 87 bacterial strains, as outlined in the inclusivity and exclusivity panels in Table 2. Identify A as a real-time LAMP assay, detected using a qPCR machine, while B and C are visual and under UV detection methods. (A) Curves surpassing the threshold of 0.2 normal fluorescence indicate a positive result. All strains from the inclusivity panel were positive, and all strains from the exclusivity panel tested negative. (B) SYBR Green I dye was added to each tube, with Xav strains in tubes 1–6 (LMG901, LMG894, LMG897, LMG903, LMG8709, LMG8715) and exclusivity strains in tubes 7–12 (A6252 - X. citri pv. aracearum, A5592 - X. sacchari, A6237 - X. sonti, A6256 - X. phaseoli pv. dieffenbachiae, A1809 - X. euvesicatoria, A4724 - X. campestris pv. campestris). Positive results changed to green, while negative results remained orange. (C) Positive SYBR Green binding was verified by fluorescence emission detection under UV light.
Limit of detection
The LAMP assay demonstrated high sensitivity, detecting purified genomic DNA down to 1 pg per reaction, with or without the addition of host crude lysate (Fig. 3). The introduction of crude host lysate extracted from the leaves and sets (stems) of sugarcane showed no adverse effect on detection, confirming the applicability of this assay for in-field detection without purifying high-quality genomic DNA. Validation across multiple detection chemistries, including SYBR Green fluorescence, UV, and gel electrophoresis, revealed consistent outcomes, with no discrepancies among the respective results. The limit of detection with positive control was 1 fg.
The limit of detection (LoD) for the loop-mediated isothermal amplification (LAMP) assay with serially diluted purified genomic DNA and genomic DNA spiked with crude host lysate derived from sugarcane stems. The genomic DNA of strain LMG901 was subjected to tenfold serial dilution, ranging from 10 ng to 1 fg, and added to the LAMP reaction tubes (numbered 1–8). For the spiked assay, 5 µl of crude host lysate was added to each tube containing the serially diluted genomic DNA. Identify A/D as a real-time LAMP assay, detected using a qPCR machine, while B/E and C/F are visual and under UV detection methods.
Tube N: Non-template control (NTC) water. Figure 3A–C and D–F represent results from sensitivity and spiked sensitivity assays, respectively. A standardized sigmoidal curve (A and D); SYBR Green verification of results performed by adding SYBR Green I dye to the amplified reaction tubes—a color change from orange to bright green indicates positive amplification (B and E); UV fluorescence confirmation of SYBR Green added tubes - positive amplification emit fluorescence (C and F). The LAMP assay detected amounts as low as 1 pg with both sensitivity and spiked sensitivity assays.
Multi-operator blind validation
The LAMP assay demonstrated high robustness in a blind multi-operator test. Two independent operators analyzed ten unknown samples, correctly identifying all three Xav strains and seven non-Xav samples, including a non-template control, with 100% accuracy (Fig. 4).
Multi-operator blind tests were conducted to assess the reproducibility and robustness of the loop-mediated isothermal amplification (LAMP) assay, designed for the specific detection of Xanthomonas axonopodis pv. vasculorum (Xav). Ten representative samples from inclusivity and exclusivity panels were used in each blind test. Identify A as a real-time LAMP assay, detected using a qPCR machine, while (B) and (C) are visual detection method. (A) Standardized sigmoidal curve, incorporating results from both operators. (B,C) results from operator 1 and operator 2, respectively, after the addition of SYBR Green dye I. The color change from orange to bright green indicates positive amplification. Tubes 1, 5, and 10 were correctly identified as Xav (strains LMG903, LMG 901, LMG894). Non-template control (NTC; tube 4).
Multi-device validation
The developed assay demonstrated high reproducibility across multiple devices, including the T100 thermal cycler (Bio-Rad, Hercules, CA), Rotor-Gene Q real-time PCR system (Qiagen, Germantown, MD), and a digital heat block (VWR). When tested with ten samples, the assay consistently detected all five Xav strains (LMG901, LMG894, LMG897, LMG903, and LMG8709) while correctly identifying X. campestris pv. campestris (A4724), X. sacchari (A5592), X. citri pv. aracearum (A6252), X. sontii (A6237), and the non-template control as negative. The results were fully concordant across all devices, with no false positives or negatives, confirming the assay’s robustness and reliability (Fig. 5).
Multi-device validation of Xanthomonas axonopodis pv. vasculorum was conducted using three different instruments: (A) Rotor-Gene Q real-time PCR (Qiagen), (B) T100 thermal cycler (Bio-Rad), and (C) dry bath (VWR). Three independent operators performed the assays on the aforementioned platforms, maintaining a consistent temperature of 65 °C for 20 min. The positive samples comprised cell lysate directly from Xav colonies (LMG901, LMG894, LMG897, LMG903, LMG8709). The change to green color after addition of SYBR Green dye and subsequent fluorescence under UV light indicated positive amplification. Five non-target samples were included: X. campestris pv. campestris (A4724), X. sacchari (A5592), X. citri pv. aracearum (A6252), X. sontii (A6237), and a non-template control. After the addition of SYBR Green dye, these samples remained orange and showed no fluorescence under UV light, indicating a negative reaction.
Detection from artificially inoculated samples
The LAMP assay successfully detected Xav in sugarcane plant slices inoculated with six strains of Xav (LMG894, LMG897, LMG901, LMG903, LMG8709, and LMG8715), as well as other Xanthomonas species (X. campestris pv. campestris (A4724), X. hawaiiensis sp. nov. (A2111), X. citri pv. aracearum (A6252), X. sacchari (A5592), and X. sontii (A6237))26. Control groups consisting of healthy sugarcane and a non-template control showed no amplification. These results confirm the assay’s ability to detect Xav in infected plant tissue (Fig. 6).
Detection of Xanthomonas axonopodis pv. vasculorum from infected sugarcane plant slices. LAMP products were visualized after addition of SYBR Green I dye—green color represents positive amplification. Tube 1 is a positive control (LMG901); tubes 2–7 are sugarcane plant slice samples infected with six different Xav strains: LMG894, LMG897, LMG901, LMG903, LMG8709, and LMG8715; tubes 8–12 are sugarcane slices inoculated with other Xanthomonas species: Xanthomonas campestris pv. campestris (A4724), Xanthomonas hawaiiensis sp. nov. (A2111), Xanthomonas citri pv. aracearum (A6252), Xanthomonas sacchari (A5592), Xanthomonas sontii (A6237); tube 13 contains healthy sugarcane, and tube 14 is non-template control (NTC—sterile water).
Discussion
The genus Xanthomonas poses a significant threat to many important crops, with the potential for substantial economic losses3,27,28. Given the risks associated with this genus, various assays suitable for point-of-need detection of many Xanthomonas spp. have been developed29,30,31,32. However, no assay is currently available for the detection of Xav, despite the considerable impact of gumming disease in sugarcane. The field-deployable LAMP is a widely used and well-established method for pathogen detection and diagnosis due to its cost-effectiveness, high sensitivity, and ease of use16,29,33. In response to this gap, we have developed a robust LAMP assay for specific detection of the sugarcane systemic pathogen Xav.
Selecting a unique gene region as the target in the development of a plant pathogen detection assay is critically important for ensuring the reliability, robustness, and specificity of the diagnostic test14,34,35,36. The uniqueness of the gene region helps to avoid cross-reactivity with non-target organisms, including closely related pathogens or the host plant itself, thereby increasing the accuracy of detection. To identify a Xav-specific genomic region, the genomes of Xav were compared against those of closely related species and other bacterial genera. This comparative genomics analysis revealed a unique gene region conserved exclusively across all Xav strains. LAMP and endpoint PCR primers designed from this unique gene region demonstrated specificity to Xav strains only. Precise target selection, therefore, underpins the overall effectiveness of the diagnostic tool.
In cases where a common host is affected by multiple pathogens—particularly those from closely related taxa that cause complex diseases—diagnostic techniques must be carefully optimized for high specificity. This is crucial to avoid cross-reactivity with non-target species, which can lead to false positives and misdiagnosis. Accurate identification of the causal pathogen is essential for implementing appropriate and effective disease management strategies, especially in field settings where timely intervention is critical37,38. Conventional laboratory-based diagnostic methods, although accurate, are typically time-consuming and may not provide the rapid turnaround needed for immediate intervention. In contrast, molecular techniques such as LAMP have emerged as powerful tools for field diagnostics due to their speed, sensitivity, and ease of use. Previous studies have demonstrated the high selectivity and sensitivity of LAMP assays developed for various plant pathogens29,33,39. In our study, the specificity of the Xav-specific LAMP assay was rigorously validated through inclusivity and exclusivity testing. This involved multiple strains of Xav as well as 81 non-target bacterial strains (Table 2). The assay consistently detected only Xav strains, confirming its high specificity and robustness for in-field and in lab detection.
High sensitivity was confirmed through four Limit of Detection (LoD) assays. The use of APC allowed for a more accurate comparison and validation of the LoD, eliminating uncertainties related to the presence of inhibitors or variations in nucleic acid extraction procedure34. As plant biosecurity measures often restrict the import of many pathogens, the development of an APC enables regions that cannot obtain a positive control for Xav to instead utilize the non-infectious plasmid for assay development and validation, thereby streamlining the assay adaptation process in different labs nationally and internationally34,40. The LoD was determined to be 1 fg with plasmid DNA. In a prior study by Arif et al.14. it was observed that the LAMP assay is relatively less susceptible to plant inhibitors than PCR, however not as effective as the recombinase polymerase amplification (RPA) assay. The LoD of the LAMP assay was verified using purified Xav genomic DNA and two spiked assays with different sugarcane tissues (leaf and culm). These tissue-based assays were crucial for assessing whether inhibitors commonly found in plant material could interfere with detection. The consistent detection of Xav down to 1 pg across all LoD experiments—with no evidence of inhibition—highlights the assay’s robustness and suitability for field use. The low LoD achieved by the LAMP assay, combined with its speed and simplicity, underscores its potential as a reliable, field-deployable tool for early detection and rapid response.
Reproducibility is a critical requirement for any diagnostic assay to be considered reliable and suitable for routine use34. In this study, the LAMP assay demonstrated high reproducibility and robustness through comprehensive performance evaluations. Multi-operator testing yielded consistent and accurate detection of Xav, indicating minimal user-related variability (Fig. 4). Furthermore, the assay maintained consistent performance across different amplification platforms, underscoring its adaptability to a range of devices (Fig. 5). Importantly, the assay was also resilient to potential inhibitors present in plant tissues, accurately detecting the pathogen in crude lysates. This ability to function effectively without the need for highly purified DNA suggests the assay is well-suited for resource-limited settings where rapid and simplified sample preparation is essential19,30. Overall, these findings highlight the assay’s strong diagnostic potential, not only for laboratory-based testing but also for broader deployment in field conditions. Its straightforward protocol enhances accessibility and ease of adoption across diverse settings, making it a practical tool for early and reliable disease detection.
In conclusion, we have developed a Xav-specific LAMP assay targeting a highly conserved and unique genomic region exclusive to Xav strains. The assay demonstrated high specificity and robustness, reliably detecting the target DNA down to 1 pg. Its sensitivity, simplicity, and resistance to inhibitors make it suitable for a wide range of applications, including disease diagnosis, farm-level management, quarantine screening, border biosecurity, epidemiological surveillance, and certification of planting materials. Importantly, the assay is adaptable to both laboratory and field settings, supporting its potential for broad implementation in plant health diagnostics.
Materials and methods
Any plant and plant materials used in this research comply with international, national, and institutional guidelines.
Bacterial isolates used for DNA extraction
This study analyzed 87 bacterial strains from diverse hosts and geographic regions to represent the six strains of Xav and other closely related species. The inclusivity panel consisted of 6 Xav strains (procured from BCCM/LMG; Bacteria Collection, Belgium), while the exclusivity panel consisted of 81 bacterial strains (various species of Xanthomonas, Stenotrophomonas, Pantoea, Pectobacterium, Erwinia, Clavibacter, Dickeya, and Klebsiella) from the Pacific Bacterial Collection and Phytobacteriology culture collection (The University of Hawaii at Manoa) and other sources (Table 2). The bacterial strains used in the exclusivity panel were taken from storage (-80 ºC) and plated onto various media, including nutrient agar (NA) (BD, Becton Dickinson), glucose NAG media (NA medium supplemented with 0.4% glucose), dextrose peptone agar (DPA) media (10 g 1− 1 peptone, 5 g 1− 1 dextrose, 17 g 1− 1 agar), and 2,3,5-tetrazolium chloride (TZC) media (10 g 1− 1 peptone, 5 g 1− 1 dextrose, 17 g 1− 1 agar, and 0.001% TZC).
The six Xav strains included in the inclusivity panel (strains LMG894, LMG897, LMG901, LMG903, LMG8709, and LMG8715) were revived from lyophilized tubes. Each tube was aseptically broken inside a biosafety cabinet II and then resuspended in 200 µl of dextrose peptone broth and plated on M009 plates (10 g glucose, 5 g yeast extract, 30 g CaCO3, 15 g agar, pH adjusted to 7.0 and final volume to 1 L) and incubated at 28 ºC for 48–72 h. Single colonies were picked and re-streaked onto another M009 plate. Approximately a quarter of a loop (10 µl) of pure bacterial cells from M009 plates was suspended in phosphate-buffered saline (PBS) in 1.5 ml centrifuge tubes, 200 µl of alkaline lysis buffer was added, and genomic DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen). The same kit was used to isolate DNA from other strains. The DNA of certain strains included in the study were from other studies (S. Dobhal, S. Chuang and M. Arif; unpublished information). DNA of Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicola and Ralstonia solanacaerum R3B2 were used from the previous study (Dobhal et al., unpublished information).
Target gene selection
The genomes of three Xav strains, NCPPB796 (NZ_CP053649), CFBP5823 (MCDC00000000), and NCPPB900 (NZ_JPHD00000000), and genomes of other closely related species, were retrieved from the NCBI GenBank genome database. Fragments of strain NCPPB900 were concatenated for alignment. All genomes were aligned using Mauve41 and a unique gene region shared between the Xav strains was identified using Geneious Prime 2023.2.1 software. Gene regions unique to Xav were verified utilizing the nucleotide BLASTn tool. The gene region was consistently present across all available genomes, including three available in our lab and three from NCBI GenBank (NCPPB796, CFBP5823, and NCPPB900).
Primer specificity with endpoint PCR
The unique target gene region was utilized to design endpoint PCR and LAMP primers. Endpoint PCR primers were designed using Primer3 v4.1.0 (https://bioinfo.ut.ee/primer3-0.4.0), following the procedures outlined by Arif and Ochoa-Corona42. The specificity of the designed primers was validated in silico using the NCBI database, and a local custom database was created using Geneious Prime. Both primers demonstrated high specificity for Xav.
Each 20 µl endpoint PCR reaction contained 10 µl of Gotaq Green Master Mix (2X) (Promega, Madison, WI), 1 µl (5 µM) of each forward and reverse primer with final concentration of 0.25 µM, 7 µl of nucleus-free water, and 1 µl of DNA template. PCR amplification was carried out in a T100 thermal cycler (Bio-Rad) with an initial denaturation step at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 57 °C for 30 s, extension at 72 °C for 30 s, and a final extension at 72 °C for 3 min.
For electrophoresis, 10 µl of the PCR product was electrophoresed on a 1.5% (w/v) agarose gel pre-stained with ethidium bromide (Invitrogen, Carlsbad, CA). The electrophoresis was conducted at 100 V for 40 min. PCR results were visualized under UV light in a gel documentation system (Bio-Rad Gel Doc-XR+; Bio-Rad).
LAMP assay
LAMP primers were designed using PrimerExplorer V5 (https://primerexplorer.jp/e/). The primers comprised the forward inner primer (XCV1-FIP), backward inner primer (XCV1-BIP), forward outer primer (XCV1-F3), backward outer primer (XCV1-B3), loop forward primer (XCV1-LF), and loop backward primer (XCV1-LB). Two additional primers were designed manually to expedite the reaction. All primers are detailed in Table 1. In silico validation was performed following the aforementioned procedure, demonstrating specificity with Xav.
Each 25 µl LAMP reaction contained 15 µl of Isothermal Master Mix (Optigene; ISO-001), 2.0 µl of the LAMP Primer Mix, 7.0 µl of nucleus-free (NF) water, and 1 µl of template DNA. The LAMP primer mix comprised LAMP primers (final concentration): XCV1-FIP (1.6 µM), XCV1-BIP (1.6 µM), XCV1-F3 (0.2 µM), XCV1-B3 (0.2 µM), XCV1-LF (0.4 µM), and XCV1-LB (0.4 µM). The positive control included the Xav strain LMG901, while the non-template control (NTC) consisted of nuclease-free water. For detection, the reactions were carried out at 65 ºC for 20 min using the Rotor-Gene Q (Qiagen). Melt curve analysis was conducted from 99 ºC to 80 ºC, increasing at 0.2 ºC per second, using Rotor-Gene Q software v.2.3.1 (Built 49). During amplification, samples at the Ct value crossing the fluorescence intensity threshold of 0.2 were considered as positive.
Amplification of the target region was confirmed by adding 3 µl of SYBR Green I solution (Life Technologies Corporation, Eugene, OR) to each reaction. Positive results were manifested by a green color, indicating successful binding of the dye to the amplified DNA, whereas negative samples retained an orange color. SYBR Green binding was further validated through fluorescence detection in a gel doc system under UV light.
Artificial positive control (APC)
An artificial positive control was generated by amplifying the target Xav DNA with F3 and B3 primers. The amplified product was ligated into pJET 1.2/blunt vector using a CloneJET PCR cloning kit (Thermo Fischer Scientific Inc., Worcester, MA) and the ligated product was transformed into E. coli DH5 alpha by heat shock. Transformed cells were plated on LB media containing carbenicillin (100 µg/ml) to confirm the successful integration. The plates were incubated at 37 ºC for 24 h. Subsequently, 17 bacterial colonies were randomly selected and confirmed carrying the target gene region using the aforementioned primers. The colonies were further inoculated in LB broth supplemented with carbenicillin and incubated overnight in a shaker at 120 rpm at 37ºC. The plasmid DNA was extracted using QIAprep Spin Miniprep Kit (Qiagen) and used as positive control.
Limit of detection
Four assays were conducted to determine the LoD of the LAMP assay. The first LoD was confirmed using tenfold serially diluted APC (10 ng to 1 fg). The LoD with genomic DNA, ranging from 10 ng to 1 fg, was assessed by tenfold serially diluted genomic DNA of Xav strain LMG901 (pathotype strain). Also, two independent assays spiked with sugarcane leaf tissues and cane were performed. In each spiked assay, 5 µl of crude lysate from healthy sugarcane leaf tissue or cane was added to each tube containing tenfold serially diluted genomic DNA from 10 ng to 1 fg. The crude lysate (unpurified DNA) was extracted using a plant material lysis kit (Optigene, West Sussex). An NTC was included in each reaction.
Multi-operator blind test
For additional validation of the LAMP assay, two independent operators conducted blinded LAMP assays. Ten blind samples were prepared, encompassing strains from both inclusivity and exclusivity panels, along with a no-template control (NTC). The operators followed the protocol outlined above. The outcomes were corroborated through Rotor-Gene Q melting curve analysis, and SYBR Green I was added for further validation.
Multi-device test
Three independent operators conducted a multi-device assessment utilizing three distinct platforms, each maintained at a temperature of 65 °C for 20 min. The three incubation platforms employed in this study were the Bio-Rad T100 thermal cycler, Qiagen Rotor-Gene Q, and a dry bath system. Positive samples consisted of crude lysate extracted from Xav colonies (LMG901, LMG894, LMG897, LMG903, LMG9708), while four strains of Xanthomonas spp. were employed as negative samples (A4724, A5592, A6252, A6237), with one sample acting as the NTC. For this assay, the strains were cultured on M009/NAG media at 28 °C for 2 days. The colonies were picked from the media plate, suspended in 50 µL of nuclease-free water, and subjected to heating at 95 °C for 10 min in a thermocycler to obtain crude cell lysate. All tubes were placed on ice, followed by a brief spin for 1 min, and the supernatant was transferred to a new 200 µl PCR tube. One microliter of crude cell lysate was used directly in the LAMP reaction.
Detection in artificially inoculated plant samples
To evaluate LAMP assay’s accuracy for on-site detection, six Xav strains (LMG894, LMG897, LMG901, LMG903, LMG8709, and LMG8715) were used for sugarcane plant slice inoculation. Prior to inoculation, healthy sugarcane stalks were washed with 10% sodium hypochlorite solution for 5 min, rinsed three times with sterile water, and sliced. Each slice was inoculated with 10 µl of 108 CFU/ml of the Xav strains (Table 2, inclusivity panel) or other Xanthomonas spp.: X. campestris pv. campestris (A4724), X. hawaiiensis sp. nov. (A2111), X. citri pv. aracearum (A6252), X. sacchari (A5592), and X. sontii (A6237)26. The plates were incubated for 2 days at 28 ºC. To evaluate the field applicability of LAMP assay, uninfected and infected sugarcane tissues were processed using the Plant Material Lysis Kit following the manufacturer’s protocol, and 5 µL of crude lysate was directly used in the LAMP reaction. Each sample was amplified using the Rotor-Gene Q at 65 °C for 20 min.
Data availability
The genomes were retrieved from the NCBI GenBank database and are available under the following accession numbers: X. axonopodis pv. vasculorum NCPPB796 (NZ_CP053649) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_013177355.1/; X. axonopodis pv. vasculorum CFBP5823 (MCDC00000000) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_002939725.1/; X. axonopodis pv. vasculorum NCPPB900 (NZ_JPHD00000000) https://www.ncbi.nlm.nih.gov/datasets/taxonomy/325777/; X. campestris pv. campestris ATCC 33,913 (NC_003902) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000007145.1/; X. campestris pv. musacearum NCPPB 4379 (NZ_CP034655) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000277895.2/; X. citri pv. citri MN12 (NZ_CP008998) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000961215.1/; X. euvesicatoria pv. alfalfae CFBP 3836 (NZ_CP072268) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_017724035.1/; X. oryzae pv. oryzae ICMP3135 (NZ_CP031697) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_004136375.1/; X. oryzae pv. oryzicola GX01 (NZ_CP043403) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_008370835.2/; X. phaseoli pv. dieffenbachiae LMG 695 (NZ_CP014347) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_001564415.1/; X. perforans GEV872 (NZ_CP116305) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_028010245.1/; X. vasicola pv. vasculorum Xv1601 (NZ_CP025272) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_003949975.1/; X. sacchari DJ16 (NZ_CP121698) https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_029761895.1/.
References
Ryan, R. P. et al. Pathogenomics of Xanthomonas: Understanding bacterium–plant interactions. Nat. Rev. Microbiol. 9, 344–355 (2011).
Harrison, J. & Studholme, D. J. Draft genome sequence of Xanthomonas axonopodis pathovar vasculorum NCPPB 900. FEMS Microbiol. Lett. 360, 113–116 (2014).
Dhakal, U., Dobhal, S., Alvarez, A. M. & Arif, M. Phylogenetic analyses of Xanthomonads causing bacterial leaf spot of tomato and pepper: Xanthomonas euvesicatoria revealed homologous populations despite distant geographical distribution. Microorganisms 7, 462 (2019).
Davis, M. J., Rott, P., Baudin, P. & Dean, J. L. Evaluation of selective media and immunoassays for detection of Xanthomonas albilineans, causal agent of sugarcane leaf scald disease. Plant. Dis. 78, 78 (1994).
Baucum, L. E., Rice, R. W. & Schueneman, T. J. An Overview of Florida Sugarcane: SS-AGR-232/SC032, rev. 8/2009. EDIS (2009). (2009).
Dookun, A., Stead, D. E. & Autrey, L. J. C. Variation among strains of Xanthomonas campestris pv. vasculorum from Mauritius and other countries based on fatty acid analysis. Syst. Appl. Microbiol. 23, 148–155 (2000).
Mehnaz, S. Microbes – friends and foes of sugarcane. J. Basic. Microbiol. 53, 954–971 (2013).
Mitra, D. Emerging plant diseases: research status and challenges. in Emerging Trends in Plant Pathology (eds Singh, K. P., Jahagirdar, S. & Sarma, B. K.) 1–17 (Springer, doi:https://doi.org/10.1007/978-981-15-6275-4_1. (2021).
Hartung, J. S., Daniel, J. F. & Pruvost, O. P. Detection of Xanthomonas campestris pv. citri by the polymerase chain reaction method. Appl. Environ. Microbiol. 59 (4), 1143–1148 (1993).
Cho, M. S. et al. Sensitive and specific detection of Xanthomonas oryzae pv. oryzae by Real-Time Bio-PCR using Pathovar-Specific primers based on an Rhs family gene. Plant. Dis. 95, 589–594 (2011).
Mondal, K. et al. The reliable and rapid PCR diagnosis for Xanthomonas axonopodis pv. punicae in pomegranate. Afr. J. Microbiol. Res. 6, 5950–5956 (2012).
Adriko, J. et al. Multiplex PCR for specific and robust detection of Xanthomonas campestris pv. musacearum in pure culture and infected plant material. Plant. Pathol. 61, 489–497 (2012).
Notomi, T. et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28, e63 (2000).
Arif, M., Busot, G. Y., Mann, R., Rodoni, B. & Stack, J. P. Field-Deployable recombinase polymerase amplification assay for specific, sensitive and rapid detection of the US select agent and toxigenic bacterium, Rathayibacter toxicus. Biology 10, 620 (2021).
Lobato, I. M. & O’Sullivan, C. K. Recombinase polymerase amplification: basics, applications and recent advances. Trends Anal. Chem. 98, 19–35 (2018).
Yasuhara-Bell, J. et al. Comparative genomic analysis confirms five genetic populations of the select agent, Rathayibacter toxicus. Microorganisms 8, 366 (2020).
Ocenar, J. et al. Development of a robust, field-deployable loop-mediated isothermal amplification (LAMP) assay for specific detection of potato pathogen Dickeya dianthicola targeting a unique genomic region. PLOS ONE. 14, e0218868 (2019).
Rahman, A. M. A., Ransangan, J. & Subbiah, V. K. Improvements to the rapid detection of the marine pathogenic bacterium, Vibrio harveyi, using Loop-Mediated isothermal amplification (LAMP) in combination with SYBR green. Microorganisms 10, 2346 (2022).
Domingo, R. et al. Genome-informed loop-mediated isothermal amplification assay for specific detection of Pectobacterium Parmentieri in infected potato tissues and soil. Sci. Rep. 11, 21948 (2021).
Amambo, G. N. et al. Application of loop mediated isothermal amplification (LAMP) assays for the detection of Onchocerca volvulus, Loa loa and Mansonella perstans in humans and vectors. Front. Trop. Dis. 3, 1016176 (2023).
Misawa, Y. et al. Application of loop-mediated isothermal amplification technique to rapid and direct detection of methicillin-resistant Staphylococcus aureus (MRSA) in blood cultures. J. Infect. Chemother. 13, 134–140 (2007).
Nagamine, K., Hase, T. & Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes. 16, 223–229 (2002).
Kubota, R., Vine, B. G., Alvarez, A. M. & Jenkins, D. M. Detection of Ralstonia solanacearum by loop-mediated isothermal amplification. Phytopathology 98, 1045–1051 (2008).
Lai, M. Y., Ooi, C. H. & Lau, Y. L. Validation of SYBR green I based closed-tube loop‐mediated isothermal amplification (LAMP) assay for diagnosis of Knowlesi malaria. Malar. J. 20, 166 (2021).
Alikhan, N. F., Petty, N. K., Zakour, B., Beatson, S. A. & N. L. & BLAST ring image generator (BRIG): simple prokaryote genome comparisons. BMC Genom. 12, 402 (2011).
Chuang, S. C., Dobhal, S., Alvarez, A. M. & Arif, M. Three new species, Xanthomonas Hawaiiensis sp. nov., Stenotrophomonas aracearum sp. nov., and Stenotrophomonas oahuensis sp. nov., isolated from Araceae family. 2023.09.17.558166 Preprint at (2023). https://doi.org/10.1101/2023.09.17.558166.
Jones, J. B., Lacy, G. H., Bouzar, H., Stall, R. E. & Schaad, N. W. Reclassification of the Xanthomonads associated with bacterial spot disease of tomato and pepper. Syst. Appl. Microbiol. 27, 755–762 (2004).
Coutinho, T. A., Jacques, M. A., Jones, J. & Editorial Emergence and re-emergence of plant diseases caused by Xanthomonas species. Front Microbiol 13, (2022).
Lang, J. M. et al. Sensitive Detection of Xanthomonas oryzae pathovars oryzae and oryzicola by Loop-Mediated Isothermal Amplification. Appl. Environ. Microbiol. 80, 4519–4530 (2014).
Li, W., Lee, S. Y., Back, C. G., Ten, L. N. & Jung, H. Y. Loop-Mediated isothermal amplification for the detection of Xanthomonas arboricola pv. pruni in peaches. Plant. Pathol. J. 35, 635–643 (2019).
Stehlíková, D., Beran, P., Cohen, S. P. & Čurn, V. Development of Real-Time and colorimetric loop mediated isothermal amplification assay for detection of Xanthomonas gardneri. Microorganisms 8, 1301 (2020).
Luo, M., Meng, F. Z., Tan, Q., Yin, W. X. & Luo, C. X. Recombinase Polymerase Amplification/Cas12a-Based Identification of Xanthomonas arboricola pv. pruni on Peach. Front. Plant Sci. 12 (2021).
Larrea-Sarmiento, A. et al. Development of a genome-informed loop-mediated isothermal amplification assay for rapid and specific detection of Xanthomonas euvesicatoria. Sci. Rep. 8, 14298 (2018).
Arif, M., Busot, G. Y., Mann, R., Rodoni, B. & Stack, J. P. Multiple internal controls enhance reliability for PCR and real time PCR detection of Rathayibacter toxicus. Sci. Rep. 11, 8365 (2021).
Ramachandran, S., Dobhal, S., Alvarez, A. M. & Arif, M. Improved multiplex TaqMan qPCR assay with universal internal control offers reliable and accurate detection of Clavibacter michiganensis. J. Appl. Microbiol. 131, 1405–1416 (2021).
Arizala, D. et al. Development of a multiplex TaqMan qPCR targeting unique genomic regions for the specific and sensitive detection of Pectobacterium species and P. parmentieri. J. Appl. Microbiol. 132, 3089–3110 (2022).
Landa, B. B., Montes-Borrego, M., Muñoz-Ledesma, F. J. & Jiménez-Díaz, R. M. Phylogenetic analysis of downy mildew pathogens of opium poppy and PCR-Based in planta and seed detection of Peronospora arborescens. Phytopathology 97, 1380–1390 (2007).
EPPO. PM 7/76. Use of EPPO diagnostic protocols. EPPO Bull. 44 (3), 335–337. https://doi.org/10.1111/epp.12158 (2014).
Ash, G. J. et al. Development of a Genomics-Based LAMP (Loop-Mediated isothermal Amplification) assay for detection of Pseudomonas fuscovaginae from rice. Plant. Dis. 98, 909–915 (2014).
Caasi, D. R. J. et al. A multi-target, non-infectious and clonable artificial positive control for routine PCR-based assays. J. Microbiol. Methods. 95, 229–234 (2013).
Arif, M. & Ochoa-Corona, F. M. Comparative assessment of 5′A/T-rich overhang sequences with optimal and sub-optimal primers to increase PCR yields and sensitivity. Mol. Biotechnol. 55 (1), 17–26 (2013).
Darling, A. E., Mau, B., Perna, N. T. & Progressivemauve Multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5, (2010).
Acknowledgements
This work was supported by the USDA-ARS Agreement no. 58-2040-9-011, Systems Approaches to Improve Production and Quality of Specialty Crops Grown in the U.S. Pacific Basin; sub-project: Genome Informed Next Generation Detection Protocols for Pests and Pathogens of Specialty Crops in Hawaii. This work is also supported by USDA-NIFA Award No. 2023-70440-40179, the Barry and Barbara Brennan Endowment of the University of Hawaii, and the Oklahoma’s Sarkeys Foundation Professorship III. This research is a product of the course “PEPS/MBBE 627 Molecular Diagnostics: Principles and Practices”. The mention of specific trade names or commercial products in this publication does not constitute an official endorsement or recommendation by the University of Hawaii or the USDA.
Author information
Authors and Affiliations
Contributions
M.A. conceived and designed the study and reviewed the first draft; M.M., J.H., S.B., S.D., M.M.P and Y.D. performed the experiment, compiled the data and wrote the first draft of the manuscript; S.D. provided training, supervised the students for the lab experiment and helped in compiling data; D.A. helped in lab experiments, in silico analyses and created BRIG image; S.M. helped in lab exercises; S.A.A., F.O.C., J.P.B, J.O., D.J., L.M.M., J.F., J.P.S. and M.A. revised the manuscript and provided ideas and support for the final submission; all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Marabella, M., Howard, J., Bhandari, S. et al. Genome guided LAMP assay for rapid and reliable detection of Xanthomonas axonopodis pv. vasculorum. Sci Rep 15, 23093 (2025). https://doi.org/10.1038/s41598-025-08291-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08291-w