Abstract
The ProGlide system represents the most common approach to close the main access site during transcatheter aortic valve implantation (TAVI). A crossover balloon occlusion technique (CBOT) has been reported in small case-series to possibly provide a controlled, safe, and successful hemostasis during TAVI. We sought to investigate the comparative performance of a combined (CBOT + ProGlide) compared with a ProGlide only approach. Primary endpoint was VARC-3 type ≥ 2 bleeding. Secondary outcomes included red blood cells transfusion, vascular complications, acute kidney injury (AKI), length of hospital stay (LOS), and death. Our study retrospectively included 229 consecutive patients admitted to “Villa Verde” Clinic (Taranto, Italy) and “Mater Dei” Hospital (Bari, Italy) and treated with TAVI. The study population was divided based on the access closure strategy in two groups: the combined and the ProGlide suture only group. The CBOT + ProGlide treated group presented lower incidence of primary endpoint, as well as less red blood cells transfusions, AKI stages ≥ 2, and shorter LOS. After propensity-score matching, the CBOT + ProGlide group confirmed lower incidence of primary endpoint (2 (2.4%) vs. 10 (11.9%), p = 0.016), of AKI stage ≥ 2 (0 vs. 5 (6%), p = 0.029), and shorter LOS (6.9 ± 4.0 vs. 8.9 ± 6.1, p = 0.015); moreover, the multivariate logistic regression analysis confirmed the protective role of the combined approach against VARC-3 type ≥ 2 bleeding (CI 0.04–0.99; OR 0.2; p = 0.05). Data from this multicenter comparative study highlight that a routinary percutaneous closure of the main TAVI access with ProGlide coupled with CBOT is possible and safe. Moreover, this combined approach seems promising in terms of reduced bleeding, RBC transfusions, AKI incidence, and shortening of hospitalizations.
Similar content being viewed by others
Introduction
Aortic stenosis (AS) represents the most prevalent primary valve disease in Europe1. Over the past 15 years, the significant rise in the total number of aortic valve replacements (AVR) is partly due to the development of the transcatheter aortic valve implantation (TAVI) procedure. TAVI was firstly performed in patients with prohibitive surgical risk2but demonstrated afterward to be non-inferior to surgical AVR (SAVR) at 5 years follow-up also in high- and intermediate- surgical risk patients3,4,5. Moreover, at the same mid-term follow-up, the recent NOTION trial demonstrated comparable major clinical outcomes between TAVI and SAVR also in a low surgical risk population6.
The transfemoral access is the current guidelines suggested approach for TAVI7,8. Transfemoral TAVI is nowadays feasible in more than 95% of patients thanks to improved expertise and technical progress9: (1) new generation of transcatheter heart valves (THVs) which have become smaller and more deliverable than previously; (2) new hydrophilic, small bore, expandable, and atraumatic sheaths10; (3) the widespread possibility to perform advanced peripheral transluminal angioplasty (PTA) in patients with peripheral artery disease (PAD). However, recent data suggest that major bleeding, major vascular complications, and need for transfusion still account for 4.6%, 5.6%, and 10% respectively11,12,13,14 in transfemoral TAVI. Moreover, all these adverse events have been associated with worse short- and long-term clinical outcomes including all-cause mortality15. The pre-loading in the main femoral access site of a Perclose ProGlide (Abbott Vascular, CA) system, a suture-mediated closure device, remains the most common choice for vascular closure during TAVI. This approach may require the use of additional closure devices in up to 40% of patients and endovascular intervention in up to 5%16.
The crossover balloon occlusion technique (CBOT), added to the pre-loading of a Perclose ProGlide (Abbott Vascular, CA) system, has been described in small case-series and proposed as a safe, successful, and more controlled access closure technique after TAVI17 (Fig. 1). However, the performance of a routinary application of this technique has not been investigated. This study sought to compare the combined CBOT + ProGlide vs. the standard ProGlide only approach for the main access closure during TAVI in terms of technical success and clinical outcomes among which bleeding, need for red blood cells (RBCs) transfusions, major and minor vascular complications, acute kidney injury (AKI), death, and length of stay (LOS).
Steb-by-step CBOT. A Before complete withdrawal of the TAVI delivery system, an appropriate sized balloon for PTA is placed in the external iliac artery, upstream of the main access site, over the 0.018” protection wire. B When TAVI sheath is retracted, the balloon is inflated to assure complete hemostasis during sheath removal. C The main arterial access is closed using the pre-implanted ProGlide, the balloon is deflated, and, if sufficient hemostasis is achieved, a final angiogram is performed to confirm the absence of vascular complications.
Methods
This multicenter case-control study, designed and written in accordance to the STROBE checklist18retrospectively included all consecutive patients who accessed the Cardiology Divisions of the “Villa Verde” Clinic (Taranto, Italy) and “Mater Dei” Hospital (Bari, Italy) with the diagnosis of severe AS and who underwent elective TAVI from September 2021 to May 2023. Both divisions are high-volume and high expertise centers according to the 2018 AATS/ACC/SCAI/STS Expert Consensus Systems of Care Document19 since they have been involved in TAVI procedures for more than 10 years performing more than 100 TAVIs per year. Informed consent was obtained according to the study protocol. The study protocol was approved by the ethics committee of the participating centers (Territorial Ethics Committee of the “Azienda Ospedaliera Universitaria Consorziale Policlinico di Bari” – resolution dated March 6, 2024 protocol n. 0022011). All methods were performed in accordance with the relevant guidelines and regulations. In all patients the puncture of the main arterial access was executed under fluoroscopic guidance, based on the anatomical assessment of the arterial axis obtained from the pre-procedural TAVI-dedicated CT scan. Per internal protocol one of the two enrolling center adopts a default approach of combined (2 ProGlides + CBOT) hemostasis, while the other a 2 ProGlides technique. The internal protocol reflects a standardized local practice developed from institutional experience and consensus among TAVI operators within the heart team of each center. As stated before, in one of the two enrolling division this includes also the routine use of balloon-assisted hemostasis (2 ProGlides + CBOT); despite this technique is not mandated by current guidelines, it is widely adopted and was consistently applied to all eligible cases during the study period. Exclusion criteria were: need for surgical access, acute decompensated heart failure requiring urgent valvuloplasty before TAVI, PAD requiring propaedeutic PTA of the main access, severe tortuosity of ileo-femoral artery, enrollment in other clinical studies, planned occlusion method of the main access different from the default approach of the enrolling center. Demographic data, baseline clinical features, and hospital outcomes were gathered.
Primary endpoint was the incidence of Valve Academic Research Consortium-3 (VARC-3) type ≥ 2 bleedings. Secondary endpoints included RBCs transfusion, vascular complications, AKI, LOS, and death. Major vascular complications, minor vascular complications, and AKI were defined according the VARC-3 criteria20. Both Bleeding Academic Research Consortium (BARC) and VARC-3 criteria were used to define bleeding events20,21.
The sample size calculation was based on the expected incidence of the primary endpoint, which can reach up to 20% in high-risk populations22. To adopt a conservative approach, we assumed an event rate of 15% in the control group and 4% in the intervention group. Under these assumptions, with a two-sided alpha of 0.05 and a statistical power of 80%, a total sample size of 220 patients was calculated.
The study population was divided according to the vascular closure strategy in two groups: patients treated with both ProGlide and CBOT (CBOT group) and those only receiving the suture-based device (ProGlide group). Baseline characteristics, procedural features, and follow-up data were reported for the whole population and per group. All endpoints were assessed at the time of discharge or afterward. The Excel software (Microsoft Corporation, Redmond, Washington, USA) was used to build the database; statistical analysis was performed using both SPSS version 26 software (IBM, Inc., Armonk, NY) and R4.2.2 (R Foundation; Wirtschafts universitat Wien Welthandelsplatz, Vienna, Austria). Continuous variables are presented as means ± standard deviations (SD) and compared using paired Student’s t-tests. Categorical variables are shown as numbers with percentages and compared using the chi-square analysis and Fisher’s exact test for counts < 5.
For VARC-3 type ≥ 2 bleeding the association with baseline characteristics and procedural features has been tested with a univariate logistic regression analysis; odds ratios (ORs) were calculated with 95% confidence intervals (CIs). Each of the statistically significant (p ≤ 0.05) predictor of outcome was entered into a multivariable logistic regression model.
A propensity score (PS) analysis, performed with R4.2.2 (R Foundation; Wirtschafts universitat Wien Welthandelsplatz, Vienna, Austria) was also used to adjust for differences in patients’ baseline and procedural characteristics. The following parameters were selected: age, sex, hemoglobin (Hb), creatinine (Cr), diabetes mellitus (DM), oral anticoagulation (OAC), double antiplatelet therapy (DAPT), and sheath size. These factors were chosen among those significantly different between the CBOT and ProGlide groups in our sample and/or significantly linked with VARC-3 type ≥ 2 bleeding in the multivariate logistic regression model and/or known to be predictors of bleeding from the literature. The 1:1 nearest neighbor matching without replacement method was used (standard deviation and caliper value were 0.1 and 0.2 respectively). Standardized differences were used to confirm negligible differences in the mean or prevalence of the selected covariates between treatment groups. For all tests significance was set for a 2-tailed value of p ≤ 0.05. The data that support the findings of this study are available from the corresponding author, MP, upon reasonable request.
Results
CBOT and ProGlide groups included 135 and 94 patients respectively. Table 1 depicts the baseline clinical characteristics of patients as a whole and by group. Mean age of the population was 81.8 ± 5.7 years, while aortic mean gradient was 47.1 ± 14.4 mmHg. Patients in the ProGlide group showed higher prevalence, though not statistically significant, of cardiac revascularization and DAPT. All other variables were comparable between groups. Tables 2 and 3 describe the procedural characteristics and main access related complications respectively. In the ProGlide group, the radial artery was prevalently used as secondary TAVI access. The differences in valve types between the two groups were reasonably mainly due to the corporate agreements of each institution. However, most procedures (71.2%) were performed with 14Fr sheath’s size devices which were equally distributed between groups (supplementary Table 2). Minor access-related complications were significantly lower in CBOT group (p = 0.029), but major vascular complications did not differ between groups.
In hospital follow-up data are presented in Table 4: CBOT was associated with lower need for RBC transfusion (p = 0.012), fewer VARC-3 type ≥ 2 bleeding (p = 0.010), and shorter hospitalization (p = 0.014). Moreover, AKI resulted significantly lower in the CBOT treated group (p = 0.033), being the difference mainly driven by the AKI stages 2 and 3 (p = 0.017).
In the univariate logistic regression analysis (Table 5) only Hb, axillary arterial access, and CBOT were associated with VARC-3 type ≥ 2 bleeding. In the multivariate logistic regression analysis (Table 5) only Hb and CBOT showed a protective role against VARC-3 type ≥ 2 bleeding [p = 0.003 (95% C.I. 0.349–0.805) and p = 0.045 (95% C.I. 0.079–0.974) respectively].
After a PS-matching, a population of 168 patients was identified (baseline features are shown in supplementary Table 1). Standardized differences confirmed negligible differences in the mean or prevalence of the selected covariates (age, sex, DM, Hb, Cr, OAC, DAPT, and sheath size) between groups (supplementary Fig. 1).
Table 6 displays the main TAVI access-related complications and in-hospital follow-up data of the PS-matched population: a statistically significant difference between the two groups was confirmed in terms of VARC-3 type ≥ 2 bleeding and mean hospitalization. In addition, the adjusted analysis confirmed the higher prevalence of AKI stage ≥ 2 in the ProGlide treated group. The univariate logistic regression analysis (Table 7) showed that only Hb, Cr, and CBOT were significantly associated with VARC-3 type ≥ 2 bleeding. The multivariate logistic regression analysis proved that both Hb and CBOT had a protective role against VARC-3 type ≥ 2 bleeding in a PS-matched population, [p = 0.003 (95% C.I. 0.284–0.769) and p = 0.050 (95% C.I. 0.040–0.998) respectively], while Cr had a predictive role [p = 0.011 (95% C.I. 1.144–2.896)].
Discussion
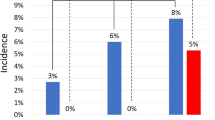
The main findings of our study are the following: (1) in trans-femoral or trans-axillary TAVI procedures the main access percutaneous closure with ProGlide coupled with CBOT is possible and safe; (2) the unadjusted analysis suggests a possible association between the combined approach and a lower incidence of VARC-3 type ≥ 2 bleeding, RBC transfusions in the first post-procedural 72 h, and AKI; (3) the potential protection against VARC-3 type ≥ 2 bleeding of the combined approach was confirmed in the adjusted analysis; (4) the CBOT group displayed shorter time-to-discharge after TAVI.
The study explores the use of CBOT as endoclamping technique in addition to ProGlide for the main arterial access closure after TAVI (Fig. 2). To the best of authors knowledge, this is the first real-world comparison of the CBOT performance vs. the standard closure using the ProGlide device.
The crossover balloon occlusion technique (CBOT), in which a balloon is placed in the external iliac artery, upstream of the main TAVI access site, over the 0.018” protection wire, assures complete hemostasis during sheath removal and access closure with the pre-implanted ProGlide. In this multicenter comparative study, 229 consecutive patients treated with TAVI were divided based on the main access closure strategy in two groups: the combined (CBOT + ProGlide) and the ProGlide suture only group. The CBOT + ProGlide treated group presented lower incidence of minor vascular complications, red blood cells transfusions, Valve Academic Research Consortium-3 (VARC-3) type ≥ 2 bleeding and acute kidney injury stages ≥ 2, and reduced LOS. Even after a propensity score matching, the combined approach was associated with fewer major bleedings (CI 0.04–0.99; OR 0.2; p = 0.05). Prospective, pragmatic, randomized trials would be valuable to possibly confirm the clinical benefit of this endoclamping technique.
We purposively restricted the analysis to patients admitted after September 2021 because TAVI represents a rapidly evolving field due to the quick improvement of operators’ expertise and of technological progresses. We thus focused on recent bioprosthetic devices with reduced sheath size and high deliverability; as a proof, most of the procedures were performed with 14 Fr devices which represent the best technology currently available10,23,24. However, even if a dramatic reduction in vascular complications and bleeding rates has been observed with newer generation devices25, to date the incidence of major bleeding during TAVI is still reported to be 4.3–11.3%, and its management remains difficult11,12,14,26. Major bleeding, major vascular complications, and transfusions unfavorably impact on patients’ short- and long-term clinical outcomes, including all-cause death11,12,13,15. In this study, the incidence of major vascular complications in the overall population was 0.9%, which confirms the high-level expertise of the two high volume enrolling centers. Minor vascular complications and RBC transfusions occurred in 4.8% and 9.2% of patients, respectively. Notably, although the two groups differed in the prosthetic valves used, the size of the delivery systems, which is a known predictor of vascular complications and bleeding27, was comparable between groups with slight advantage in terms of lower profile observed in the ProGlide treated population (supplementary Table 2).
Interestingly, in the present study most of the transfusions were unrelated to vascular complications (major vascular complications and VARC-3 type ≥ 2 bleeding were 0.9% and 7.4% respectively). As a consequence, the main advantage of CBOT seems to come from the complete occlusion of the artery during the access closure which provides a clean tightening of the pre-implanted ProGlides and an effective temporary hemostasis in cases of ProGlide failure, thus allowing the operator to safely deliver an additional closure device system. Moreover, routinary use of the endoclamping technique can favor the quick management of any complications occurring after the removal of the prosthetic delivery system. These advantages seem to result in reduced bleedings and fewer post-operative transfusions. Notably, the balloon inflation may also eventually reduce the arterial stenosis related to the ProGlide sutures.
Intraoperative bleeding is one of the most consistent contributing factors for AKI development28,29. In turn, a recent meta-analysis suggested that even AKI stage 1 negatively impacts on both the one year and three year survival rates after TAVI30. Yan-biao Liao et al. demonstrated that significant bleedings and number of RBC transfusions predict in a directly proportional manner the incidence of AKI and long term mortality, mainly due to structural and functional alterations in the preserved RBCs and the buildup of pro-inflammatory mediators31. Moreover, a 1.8-fold increased risk of AKI was also associated to anemia after TAVI32. In agreement with the above cited literature, the lower incidence of AKI in the CBOT treated population may reasonably occur as the result of less transfusions and major bleedings observed in this group.
However, because of the non-randomized nature of enrolment, our findings must be interpreted with caution. Purposively, PS matching was used to partly overcome disparities in the baseline clinical profile of the two patients’ groups. The adjusted analysis confirmed the protective role of CBOT against VARC-3 type > 2 bleeding and the correlation between this technique and lower mean hospitalization time. Moreover, a slight tendency to higher incidence of AKI was observed in the ProGlide group after PS-matching, mainly driven by stage 2 and 3 AKI.
Conclusively, these findings represent the first report on a relatively large population of a possible protective effect against bleeding of a routine endoclamping technique adjunctive to conventional sutures. On the other hand, this technique carries an increase in procedural time and costs due to the need for additional devices; future investigations will clarify if the hypothesized reduction in vascular complications and in consequent additional unplanned interventions may ultimately offset in terms of clinical and economic net benefit the above-mentioned drawbacks.
Some limitations of the present study should be acknowledged. Firstly, sample size, despite larger than previous reports, is in absolute terms small. Second, this was a non-randomized study and, even if PS-matching was used to adjusting for potential confounding variables, it was not able to correct for the unmeasured variables. Third, the choice of prostheses was left to the discretion of operators in each institution, and not all of the devices currently in use were included in this study. Finally, patients with severe PAD or significant tortuosity of the iliofemoral axes were excluded from the analysis; thus, the routine application of CBOT was not assessed in these cases.
Furthermore, despite the favorable interplay between novel devices and trans-axillary access, TAVI represents a challenging procedure requiring careful patient selection and management28. –29 As a result, our findings should be regarded only as hypotheses generating and would require and, to authors opinion, deserve further confirmation from a large randomized trial30.
Conclusions
This multicenter comparative study highlights that in patients undergoing trans-femoral or trans-axillary TAVI a routinary percutaneous access closure with ProGlide paired with supplementary CBOT is possible and safe. Moreover, our data suggest that CBOT effectively contributed in reducing in-hospital major bleedings, RBC transfusions, AKI, and hospitalization time in patients undergoing TAVI. Prospective, pragmatic, randomized trials would be valuable to possibly confirm the clinical benefit of this endoclamping technique.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Zipes, D. P. et al. Braunwald’s heart disease: a textbook of cardiovascular medicine. Eleventh edition. edn, (Elsevier/Saunders, 2019).
Leon, M. B. et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl. J. Med. 363, 1597–1607. https://doi.org/10.1056/NEJMoa1008232 (2010).
Makkar, R. R. et al. Five-Year outcomes of transcatheter or surgical Aortic-Valve replacement. N Engl. J. Med. 382, 799–809. https://doi.org/10.1056/NEJMoa1910555 (2020).
Mack, M. J. et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 385, 2477–2484. https://doi.org/10.1016/s0140-6736(15)60308-7 (2015).
Pepe, M. et al. Comparison of outcomes of transcatheter aortic valve implantation in patients ≥ 85 years versus those < 85 years. Am. J. Cardiol. 129, 60–70. https://doi.org/10.1016/j.amjcard.2020.05.033 (2020).
Thyregod, H. G. H. et al. Five-Year clinical and echocardiographic outcomes from the NOTION randomized clinical trial in patients at lower surgical risk. Circulation 139, 2714–2723. https://doi.org/10.1161/circulationaha.118.036606 (2019).
Vahanian, A. et al. ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 43, 561–632. https://doi.org/10.1093/eurheartj/ehab395 (2021).
Morello, A. et al. The best way to transcatheter aortic valve implantation: from standard to new approaches. Int. J. Cardiol. 322, 86–94. https://doi.org/10.1016/j.ijcard.2020.08.036 (2021).
Corcione, N. et al. TAVI-CT score to evaluate the anatomic risk in patients undergoing transcatheter aortic valve implantation. Sci. Rep. 12, 7612. https://doi.org/10.1038/s41598-022-11788-3 (2022).
Corcione, N. et al. The novel flexnav delivery system for transcatheter aortic valve implantation with the portico device: A case series. J. Invasive Cardiol. 33, E474–e478 (2021).
Thourani, V. H. et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 387, 2218–2225. https://doi.org/10.1016/S0140-6736(16)30073-3 (2016).
Thyregod, H. G. H. et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-Year results from the All-Comers NOTION randomized clinical trial. J. Am. Coll. Cardiol. 65, 2184–2194. https://doi.org/10.1016/j.jacc.2015.03.014 (2015).
Costa, F. & Cohen, M. G. Transfusion and mortality after transcatheter aortic valve replacement. Circulation: Cardiovasc. Interventions. 13, e010225. https://doi.org/10.1161/CIRCINTERVENTIONS.120.010225 (2020).
Corcione, N. et al. Baseline, procedural and outcome features of patients undergoing transcatheter aortic valve implantation according to different body mass index categories. Minerva Med. 112, 474–482. https://doi.org/10.23736/s0026-4806.21.07379-1 (2021).
Sherwood, M. W. et al. Temporal trends, and associated outcomes of vascular and bleeding complications in patients undergoing transfemoral transcatheter aortic valve replacement: insights from the society of thoracic surgeons/american college of cardiology transcatheter valve therapies registry. Circ. Cardiovasc. Interv. 13, e008227. https://doi.org/10.1161/circinterventions.119.008227 (2020). Incidence.
Bazarbashi, N. et al. The utilization of single versus double perclose devices for transfemoral aortic valve replacement access site closure: insights from Cleveland clinic aortic valve center. Catheter Cardiovasc. Interv. 96, 442–447. https://doi.org/10.1002/ccd.28585 (2020).
Genereux, P. et al. Clinical outcomes using a new crossover balloon occlusion technique for percutaneous closure after transfemoral aortic valve implantation. JACC Cardiovasc. Interv. 4, 861–867. https://doi.org/10.1016/j.jcin.2011.05.019 (2011).
Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 13, S31–s34. https://doi.org/10.4103/sja.SJA_543_18 (2019).
Bavaria, J. E. & AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter aortic valve replacement: a Joint Report of the American Association for Thoracic Surgery, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 73, 340–374 (2018). https://doi.org/10.1016/j.jacc.2018.07.002.
Généreux, P. et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. J. Am. Coll. Cardiol. 77, 2717–2746. https://doi.org/10.1016/j.jacc.2021.02.038 (2021).
Ben-Yehuda, O. & Redfors, B. Validation of the bleeding academic research consortium bleeding definition. J. Am. Coll. Cardiol. 67, 2145–2147. https://doi.org/10.1016/j.jacc.2016.03.505 (2016).
Avvedimento, M. et al. Validation of the valve academic research consortium high bleeding risk definition in patients undergoing TAVR. Circ. Cardiovasc. Interv. 18, e014800. https://doi.org/10.1161/circinterventions.124.014800 (2025).
Pepe, M. et al. Assessing the best prognostic score for transcatheter aortic valve implantation (from the RISPEVA Registry). Am. J. Cardiol. 144, 91–99. https://doi.org/10.1016/j.amjcard.2020.12.068 (2021).
Corcione, N. et al. Comparing the safety and effectiveness of five leading New-Generation devices for transcatheter aortic valve implantation: Twelve-Month results from the RISPEVA study. J. Invasive Cardiol. 33, E320–e329 (2021).
Winter, M. P. et al. Evolution of outcome and complications in TAVR: a meta-analysis of observational and randomized studies. Sci. Rep. 10, 15568. https://doi.org/10.1038/s41598-020-72453-1 (2020).
Thieme, M. et al. Interventional treatment of access site complications during transfemoral TAVI: A single center experience. Front. Cardiovasc. Med. 8, 725079. https://doi.org/10.3389/fcvm.2021.725079 (2021).
Barbanti, M. et al. Impact of low-profile sheaths on vascular complications during transfemoral transcatheter aortic valve replacement. EuroIntervention 9, 929–935 (2013).
Canet, E. & Bellomo, R. Perioperative renal protection. Curr. Opin. Crit. Care. 24, 568–574. https://doi.org/10.1097/mcc.0000000000000560 (2018).
Liu, W. et al. Impact of major bleeding on the risk of acute kidney injury in patients undergoing off-pump coronary artery bypass grafting. J. Thorac. Dis. 10, 3381–3389. https://doi.org/10.21037/jtd.2018.05.98 (2018).
Liao, Y. B. et al. Predictors and outcome of acute kidney injury after transcatheter aortic valve implantation: a systematic review and meta-analysis. EuroIntervention 12, 2067–2074. https://doi.org/10.4244/eij-d-15-00254 (2017).
Sinning, J. M. et al. Systemic inflammatory response syndrome predicts increased mortality in patients after transcatheter aortic valve implantation. Eur. Heart J. 33, 1459–1468. https://doi.org/10.1093/eurheartj/ehs002 (2012).
Arai, T. et al. Impact of pre- and post-procedural anemia on the incidence of acute kidney injury and 1-year mortality in patients undergoing transcatheter aortic valve implantation (from the French aortic National corevalve and Edwards 2 [FRANCE 2] Registry). Catheter Cardiovasc. Interv. 85, 1231–1239. https://doi.org/10.1002/ccd.25832 (2015).
Acknowledgements
N/A.
Funding
none.
Author information
Authors and Affiliations
Contributions
GN, MP and RT wrote the main manuscript text; GN and GN prepared tables; GN and PLN did statistical analysis; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Giuseppe Biondi-Zoccai has consulted for Abiomed, Advanced Nanotherapies, Aleph, Amarin, AstraZeneca, Balmed, Cardionovum, Cepton, Crannmedical, Endocore Lab, Eukon, Guidotti, Innovheart, Meditrial, Menarini, Microport, Opsens Medical, Terumo, and Translumina, outside the present work.All other authors report no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Napoli, G., Ausiello, A., Pestrichella, V. et al. Crossover balloon occlusion technique for access closure during transcatheter aortic valve implantation: a multicenter observational study. Sci Rep 15, 28998 (2025). https://doi.org/10.1038/s41598-025-13765-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13765-y