Abstract
Auto-antibodies (auto-Abs) that neutralize type I interferons (IFNs) have been implicated in severe viral infections, including ~ 40% of cases of West Nile virus (WNV) neuroinvasive disease (WNND). Developing robust in vitro models to evaluate the protective effects of type I IFNs against viral infection, as well as the disruptive effects of auto-Abs, is essential for understanding disease pathogenesis and identifying patients at risk. In this study, we used Vero E6 and ARPE-19 cell lines to investigate the ability of type I (IFN-α, IFN-β, IFN-ω), type II (IFN-γ), and type III (IFN-λ1) IFNs to restrict WNV infection. Our results demonstrate that IFN-α, IFN-β, and IFN-ω effectively protect ARPE-19 cells from WNV infection, with IFN-β exhibiting the strongest antiviral effect. In contrast, Vero E6 cells required higher concentrations of IFN-ω to achieve comparable protection. Neither IFN-γ nor IFN-λ1 conferred protection in either cell line. We further screened serum samples from WNV-infected patients for auto-Abs neutralizing type I IFNs. Our findings confirm that the ARPE-19-based assay is consistent with other established methods for detecting neutralizing auto-Abs against type I IFNs. This simple and reliable assay offers a valuable tool for assessing the antiviral effects of type I IFNs and the neutralizing activity of auto-Abs in both research and clinical settings. Future studies should aim to validate the clinical utility of the ARPE-19-WNV infection model on a larger scale.
Similar content being viewed by others
Introduction
Type I interferons (IFNs) are a family of 16 antiviral cytokines that include 12 circulating interferon-alpha (IFN-α), encoded by 13 loci, along with IFN-ω, the high-affinity IFN-β, and the tissue-specific IFN-κ and IFN-ε, which have lower antiviral activity and are expressed in the skin and genitourinary tract, respectively. Type I IFNs contribute to protection against viral infections by restricting viral replication in cells, both in vitro and in vivo1,2,3. Single-gene disorders impairing toll-like receptor 3 (TLR3)- or TLR7-dependent production of, or the response to, type I IFNs underlie life-threatening viral infections in humans, including herpes simplex encephalitis (HSE), critical influenza A virus (IAV) pneumonia, and hypoxemic coronavirus disease 2019 (COVID-19)4,5. Additionally, type I IFN-neutralizing autoantibodies have recently been linked to approximately 10% of cases of tick-borne encephalitis, underscoring the significance of these autoantibodies in the pathogenesis of neuroinvasive viral diseases6. Auto-antibodies (auto-Abs) neutralizing type I IFNs were first described in 1983 and, starting in 2020, were shown to underlie ~ 15% of cases of hypoxemic COVID-19 pneumonia7, ~ 5% of critical IAV pneumonia, ~ 25% of hospitalizations for Middle East respiratory syndrome (MERS) pneumonia8,9,10, ~ 30% of adverse reactions to the live attenuated virus vaccine against yellow fever virus11, and ~ 40% of West Nile virus (WNV) encephalitis12. These auto-Abs pre-exist the infectious episode and phenocopy the genetic impairment of type I IFN production or signaling. They are found in 0.5-1% of individuals from the general population, while their prevalence sharply increases after 65 years of age, with ~ 4–7% of individuals 70 years old or older carrying these auto-Abs, in part explaining the increased incidence of severe viral infections in this age group7,13,14.
WNV belongs to arboviruses (arthropod-borne viruses), a group mostly composed of RNA viruses that are transmitted to vertebrate hosts by hematophagous arthropods, including mosquitoes, ticks, and sandflies15. The virus was first isolated in 1937 from a febrile patient in the West Nile sub-region of Uganda16,17,18. Initially perceived as causing only mild, subclinical infections in humans, it has been a significant source of morbidity and mortality19. Over the past few decades, WNV has been responsible for epidemic outbreaks and is currently endemic in ever increasing geographic regions across all continents20,21. The neuroinvasive forms (West Nile virus neuroinvasive disease, WNND), the most severe clinical manifestations of WNV infection, occur in less than 1% of human and equine WNV cases, and include meningitis, encephalitis, and acute flaccid paralysis/poliomyelitis22,23. Impairment of type I IFN signaling has been associated with increased severity of West Nile virus infection, including its detrimental effect on gut barrier integrity, which exacerbates disease susceptibility and progression24. Notably, ~ 30–50% of individuals with WNND, with at least ~ 40% with WNV encephalitis, carry auto-Abs neutralizing type I IFNs (IFN-I-NTAbs)12. A luciferase-based neutralization assay is currently used to test whether the auto-Abs against IFN-α, IFN-β, or IFN-ω in serum, plasma, or other body fluids display neutralizing activity as previously described12. These auto-Abs are typically detected by other techniques, such as enzyme-linked immunosorbent assay (ELISA) which allows detection but does not provide information on their neutralizing potential. The assay evaluates neutralizing activity at different concentrations: high (10 ng/mL) or low (100 pg/mL) for IFN-α or IFN-ω, and high (10 ng/mL) or intermediate (1 ng/mL) for IFN-β11. This assay is clinically relevant. IFN-I-NTAbs, in various combinations, significantly increase the risk of West Nile neuroinvasive disease (WNND). The risk ranges from ~ 20 to > 100 times higher compared to individuals without these auto-Abs in the general population. Notably, individuals with IFN-I-NTAbs neutralizing high concentrations of both IFN-α and IFN-ω display the highest risk of life-threatening WNV disease, ~ 127 times higher than in individuals without these auto-Abs12. The luciferase-based neutralization assay can demonstrate the blocking effect of the IFN-I-NTAbs on the ability of type I IFNs to activate the signaling downstream from the type I IFN receptor, thus indirectly suggesting the loss of type I IFN antiviral activity in the presence of these auto-Abs1,2,4. We previously described experiments on Vero E6 cells demonstrating the loss of antiviral activity of IFN-α2 against WNV in the presence of IFN-I-NTAbs neutralizing IFN-α212.
Here we provide a detailed series of experiments demonstrating the utility of a functional assay based on WNV infection in vitro to reproducibly assess both the blocking activity of IFN-I-NTAbs on IFN-α, IFN-β, and IFN-ω, and their detrimental effect on the antiviral activity of these type I IFNs against WNV. This assay can be used in clinical and research settings as an alternative to the luciferase-based method, or as a complementary test, to study samples from patients with severe WNV infection or infections other than WNV, whose severity could depend on the presence of these auto-Abs. It also allows screening of samples to detect individuals at risk for WNV neuroinvasive disease and other viral infections. Finally, it provides the methodological basis to systematically assess other cell lines for the sensitivity to the antiviral activity of type I IFNs, or other cytokines, against different viral infections, and to assess the detrimental activity of auto-Abs against these antiviral cytokines.
Results
In vitroprotection of Vero E6 and ARPE-19 cells against WNV infection by type I, but not type II or type III IFNs
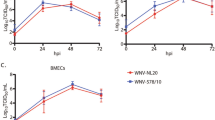
We first investigated the ability of type I IFNs to protect cells from WNV infection and determined the lowest protective concentration of IFN-α2, IFN-β, and IFN-ω against in vitro WNV infection in two mammalian cell lines, Vero E6 cells, a kidney epithelial cell line from African green monkey, and ARPE-19 cells, a human retinal epithelial cell line. Treatment with any of the three type I IFNs two hours following WNV inoculation did not provide protection at any of the tested concentrations. By contrast, pre-treatment (i.e., overnight treatment prior to infection with WNV) with IFN-α2, IFN-β, or IFN-ω provided protection to both Vero E6 and ARPE-19 cell lines against in vitro WNV infection, albeit to different extents for equal concentrations of each type I IFN. In ARPE-19 cells, IFN-β had the strongest antiviral effect, with protection at all the tested concentrations, followed by IFN-ω and IFN-α2 (Fig. 1A). Specifically, pre-treatment with IFN-β at 100 ng/mL, 10 ng/mL, 1 ng/mL, 500 pg/mL, 100 pg/mL, 50 pg/mL and 10 pg/mL conferred full protection (no cytopathic effect detected) to ARPE-19 cells against WNV replication. Pre-treatment with IFN-α2 or IFN-ω, tested at the same concentrations, conferred protection to ARPE-19 cells from 100 ng/mL to 50 pg/mL, while 10 pg/mL of IFN-α2 or IFN-ω only conferred partial protection (cytopathic effect observed at lower but not higher supernatant dilutions), and only slight differences depending on the viral inoculum (10,000, 1,000, or 100 TCID50). Conversely, Vero E6 cells, a more widely used cell line for WNV and other viral replication experiments, showed different sensitivity to each of the three type I IFNs (Fig. 1B). Pre-treatment with IFN-α2 or IFN-β, tested at the same serial concentrations, conferred protection to Vero E6 cells at 100 pg/mL or higher, while, surprisingly, pre-treatment with IFN-ω protected Vero E6 cells only at higher concentrations, 10 ng/mL or higher. Overall, we demonstrate that the widely used Vero E6 cells are protected by high (10 ng/mL) or low (100 pg/mL) concentrations of both human IFN-α2 and IFN-β at concentrations of 100 pg/mL or higher, while IFN-ω protects Vero E6 cells only at high concentrations, 10 ng/mL or higher. Conversely, ARPE-19 cells are protected by low concentrations of the three tested type I IFNs, with the strongest effect observed for IFN-β (protection from WNV infection at 10 pg/mL or higher), followed by IFN-ω and IFN-α2 (full protection at 50 pg/mL or higher, and partial protection at 10 pg/mL). Thus, ARPE-19 cells represent an optimal cell line to study the antiviral activity of IFN-α2, IFN-β, and IFN-ω against WNV and possibly other viruses.
Cytopathic effect of WNV infection in the presence of Type I IFNs. The rate of WNV infection was evaluated by measuring virus-induced cytopathic effects (CPE) in ARPE-19 cells (A) and Vero E6 cells (B) at 0, 24, and 48 h post-infection. Cells were pre-treated overnight with type I IFNs (IFN-β, IFN- ω, and IFN- α2) at concentrations of 10 ng/mL, 1 ng/mL, 100 pg/mL, 50 pg/mL, and 10 pg/mL, followed by infection with 100 TCID50 of WNV. In ARPE-19 cells, all type I IFNs effectively restricted WNV replication at concentrations as low as 50 pg/mL or higher. In contrast, in Vero E6 cells, IFN-α2 and IFN-β provided protection at concentrations of 100 pg/mL or higher, while IFN-ω was protective only at 10 ng/mL. All experiments were performed in triplicate. The figures have been simplified to display the most relevant time points and virus inoculum. Full data, including additional time points and concentrations, are available in the supplementary materials.
We also investigated the efficacy of type II (IFN-γ) and III (IFN-λ1) IFNs in protecting ARPE-19 and Vero E6 cells from WNV infection. In contrast to human type I IFNs, both post-treatment and pre-treatment with IFN-γ or IFN-λ1 did not confer protection against WNV infection in vitro in ARPE-19 and Vero E6 cell lines at any of the tested concentrations, from 10 ng/mL to 10 pg/mL, regardless of the viral inoculum (10,000, 1,000, or 100 TCID50) (Fig. 2). Despite evidence suggesting the antiviral role of IFN-λ1 in protection against WNV in vivo25, and a possible role of IFN-γ in viral infections26, our findings indicate that they have no antiviral effect against WNV in the two tested cell lines, Vero E6 and ARPE-19 cells, commonly used for in vitro experiments of viral infection.
Cytopathic effect of WNV infection in the presence of Type II (IFN-γ) and III (IFN-λ1) IFNs. The rate of WNV infection was assessed by measuring virus-induced cytopathic effects (CPE) in ARPE-19 cells (A) and Vero E6 cells (B) at 0, 24, and 48 h post-infection. Cells were pre-treated overnight with type II (IFN-γ) or type III (IFN-λ1) IFNs at concentrations of 10 ng/mL, 1 ng/mL, 100 pg/mL, 50 pg/mL, and 10 pg/mL, followed by infection with 100 TCID50 of WNV. Neither IFN-γ nor IFN-λ1 conferred protection against WNV infection in ARPE-19 or Vero E6 cells at any of the tested concentrations. All experiments were performed in triplicate. The figures have been simplified to display the most relevant time points and virus inoculum. Full data, including additional time points and concentrations, are available in the supplementary materials.
The main text figures have been simplified for conciseness, with full versions containing all experimental data available in Supplementary material 1 (Fig. S1, S2, S3).
WNV-NTAbs and IFN-I-NTAbs in patients with WNND and in asymptomatic blood donors
We collected serum samples from a cohort of 11 patients with WNND and 3 asymptomatic blood donors. The samples were collected between 2 and 3 weeks after onset of clinical signs for the WNND cases, and between 1 and 2 months after detection of WNV RNA during the screening prior to blood donation. WNV infection was documented in cases and controls by RT-PCR on serum, plasma or cerebrospinal fluid samples, or by serological demonstration of WNV-specific IgM or seroconversion to IgG, and/or WNV neutralization assays27. We detected WNV-NTAbs in 8/11 (73%) WNND serum samples, with a median WNV-NTAb titer of 1:20 (IQR: 1:10 − 1:40), and in all 3 serum samples from asymptomatic blood donors, with a median titer of 1:10 (IQR: 1:10 − 1:40), while 3/11 samples from WNND cases were negative (27%, titer < 1:10) (Fig. 3A). We used an enzyme-linked immunosorbent assay (ELISA) to detect IgG auto-Abs against IFN-α2, IFN-β, and/or IFN-ω, as defined by an optical density (OD) > 0.512, in serum samples from a cohort of 11 patients with WNND and 3 asymptomatic blood donors (Fig. 3B). We selected serum samples from 11 patients with WNND, 9/11 of whom had IFN-I-NTAbs, and 2/11 did not. The ability of these auto-Abs to neutralize the corresponding type I IFN was confirmed using a previously described luciferase-based assay (Fig. 3C and D). We detected auto-Abs neutralizing high concentrations (10 ng/mL) of both IFN-α2 and IFN-ω in 8/9 patients with WNND, and high concentrations of only IFN-ω in 1/9 patients, while none of the patients’ samples neutralized high concentrations of IFN-β, alone or in combination. By testing the neutralization of lower concentrations of type I IFNs, we demonstrated the presence of auto-Abs neutralizing low concentrations (100 pg/mL) of IFN-α2 and IFN-ω and intermediate concentrations (1 ng/mL) of IFN-β in 2/9 patients, and auto-Abs neutralizing low concentrations of IFN-α2 and IFN-ω but not intermediate concentrations of IFN-β in 7/9 patients (supplementary Table 1). Overall, we selected, for subsequent experiments, 2/11 serum samples with auto-Abs neutralizing at least low concentrations of IFN-α2, IFN-β and IFN-ω, 7/11 serum samples with auto-Abs neutralizing at least low concentrations of IFN-α2 and IFN-ω but not IFN-β, and 2/11 from WNND patients with no IFN-I-NTAbs. The concordance between the results on ELISA and luciferase-based assay, as well as the sex and age of patients and controls, are reported in supplementary Table 1. We also screened serum samples from 3 blood donors with recent, acute, asymptomatic WNV infection and demonstrated the absence of IFN-I-NTAbs. These samples were collected between 1 and 2 months after detection of WNV RNA during the screening prior to blood donation.
WNV-NTs Titer and IFN-I-NTAbs neutralizing type I IFNs in WNV-infected individuals. To characterize the antibodies present in serum samples: (A) WNV-neutralizing antibodies (WNV-NTAbs) were detected in serum samples from WNND patients and asymptomatic donors using a microneutralization assay. WNV-NTAbs were identified in 73% of WNND samples (8/11) and in all donor samples, with median titers of 1:20 and 1:10, respectively. Titers below 1:10 were considered negative, while those equal to or above 1:10 were considered positive. (B) Autoantibodies against IFN-α2, IFN-β, and IFN-ω were detected using an ELISA assay. A reading above 0.5 OD (dotted line) was considered positive, based on reference values from healthy donor serum/plasma. Each sample was tested once. (C) and (D) A luciferase-based assay was used to identify autoantibodies capable of neutralizing type I IFNs. Plasma samples were tested for their ability to neutralize IFN-α2, IFN-β, and IFN-ω at different concentrations. HEK293T cells were transfected with luciferase plasmids and treated with type I IFNs. Neutralizing activity was defined as a relative luciferase activity (RLA) below 15% of the control value (dotted line). Each sample was tested once.
Testing the neutralizing effect of the auto-Abs on IFN-α2, IFN-β and IFN-ω activity against WNV infection in ARPE-19 cells
We then assessed whether both ARPE-19 and Vero E6 cells subjected to WNV infection are suitable models to assess the neutralizing activity of anti-IFN-α2 auto-Abs in serum samples from individuals with WNND. From our cohort, we selected 5 serum samples from individuals with auto-Abs neutralizing high concentrations of IFN-α2. Prior to in vitro WNV infection, we pre-treated ARPE-19 cells with IFN-α2 at 10 ng/mL, 500 pg/mL, 100 pg/mL and 50 pg/ml) with or without 1:10 diluted sera from 5 cases and 2 controls. As expected, we observed viral replication and cytopathic effect, comparable to that observed in sham cells, in all the cells pretreated with a mixture of IFN-α2 and 1:10 serum from WNND patients carrying auto-Abs against IFN-α2 at all the tested concentrations (from 10 ng/mL to 50 pg/mL), while no cytopathic effect was observed in cells pretreated with a mixture of IFN-α2 at the same concentrations and 1:10 diluted serum from individuals not carrying the auto-Abs. The neutralizing capacity of the tested sera, with or without auto-Abs, was comparable in both ARPE-19 and Vero E6 cells (Fig. 4). In order to investigate the last concentration of sera with and without auto-Abs yielding a complete block of viral replication, we performed a titration dilution curve alongside a commercial monoclonal anti-IFN antibody. These serum samples were effective at dilutions up to approximately 1:300, similar to the monoclonal antibody, neutralizing the protective effect of IFN-α2. The experiment is described in the supplementary material 1 (Fig. S4).
Blocking of IFN-α2 activity utilizing sera from patients with WNV neuroinvasive disease. The rate of WNV infection was evaluated by measuring virus-induced cytopathic effects in ARPE-19 cells (A) at 24 h post-infection and Vero E6 cells (B) at 48 h post-infection, both treated with varying concentrations of IFN-α2 (10,000, 500, 100, and 50 pg/mL). Despite the presence of IFN-α2, increased WNV replication was observed when cells were treated with all concentrations of IFN-α2 in combination with sera from patients with neuroinvasive WNV disease and neutralizing autoantibodies (IFN-I-NTAbs) against IFN-α2 (n = 5). At 10,000 pg/mL, the neutralizing effect of these sera was reduced but not completely abolished, as the protective activity of IFN-α2 was only partially restored. This effect was compared to treatment with sera from WNV patients without IFN-I-NTAbs (n = 2). All sera were tested at a single dilution of 1:10. All experiments were performed in triplicate.
In a similar set of experiments, we tested the ability of anti-IFN-ω auto-Abs in serum samples from 4 individuals with WNND to block the protective effect of IFN-ω against WNV infection in ARPE-19 cells (Fig. 5). Likewise, we observed viral replication and cytopathic effect, comparable to that observed in control cells treated under identical conditions without exposure to the experimental variables, in all the cells pretreated with a mixture of IFN-ω and 1:10 serum from WNND patients carrying auto-Abs against IFN-ω at all the tested concentrations (from 10 ng/mL to 50 pg/mL), while no cytopathic effect was observed in the presence of 1:10 serum from 1 control not carrying the auto-Abs. Finally, we observed viral replication and cytopathic effect comparable to that observed in sham cells in ARPE-19 cells treated with 50 or 100 pg/mL of IFN-β, but not with 500 pg/mL or 10 ng/mL of IFN-β, in combination with 1:10 diluted serum from 1 WNND patient carrying auto-Abs neutralizing 100 pg/mL of IFN-β as assessed by the luciferase assay. By contrast, the two control sera did not block the protective effect of IFN-β at concentrations of 50 pg/mL through 10 ng/mL (Fig. 6). With an additional experiment, we confirmed that pre-treatment with 1:10 diluted serum alone did not provide protection against WNV infection in vitro (Figure S5). This result demonstrates that the washing step performed after cell pre-treatment effectively removed any residual serum, ensuring that the virus introduced later never came into contact with the serum components. Consequently, this allowed us to specifically assess the impact of auto-Abs without interference from WNV-NTAbs, even if they were present in the serum. These experiments demonstrate that the assay is suitable to test the neutralizing effect of auto-Abs targeting IFN-α2, IFN-β or IFN-ω in samples from patients with WNV infection and likely from patients with other viral infections caused by the presence of these auto-Abs, with results fully consistent with the previously validated luciferase-based neutralization assay (Fig. 7). It also allows to test the ability of serum samples to neutralize concentrations as low as 50 pg/mL of IFN-α2, IFN-β and IFN-ω, although the clinical relevance of the neutralization of such low concentrations of type I IFNs by 1:10 diluted serum or plasma samples remains to be defined. We also observed a strong correlation between the results obtained with the WNV-ARPE-19 cell line assay and those with the ELISA, with the former serving as a reliable platform for confirming the auto-antibody specificity and functional neutralization indicated by the latter. Thus, the blocking activity of type I IFNs antiviral activity by IFN-I-NTAbs neutralizing IFN-α and/or IFN-β and/or IFN-ω can be assessed in our in vitro model of WNV infection of ARPE-19 cells.
Blocking of IFN-ω activity utilizing sera from patients with WNV neuroinvasive disease. The rate of WNV infection, evaluated through virus-induced cytopathic effects 24 h post-infection in ARPE-19 cells treated with different concentrations of IFN-ω (10,000, 500, 100, and 50 pg/mL), was examined. Despite the presence of IFN-ω, increased WNV replication was observed when ARPE-19 cells were treated with all tested IFN-ω concentrations in conjunction with sera from patients with neuroinvasive WNV disease and neutralizing autoantibodies (IFN-I-NTAbs) against IFN-ω (n = 4). At 10,000 pg/mL, the neutralizing effect of these autoantibodies diminished but was not completely abolished, as the protective activity of IFN-ω was only partially restored. This effect was compared to treatment with sera from patients with WNV disease without IFN-I-NTAbs (n = 1). All sera were tested at a single dilution of 1:10. All experiments were performed in triplicate.
Blocking of IFN-β activity utilizing sera from patients with WNV neuroinvasive disease. The rate of WNV infection, evaluated through virus-induced cytopathic effects 24 h post-infection in ARPE-19 cells treated with different concentrations of IFN-β (10,000, 500, 100, and 50 pg/mL), was examined. Increased WNV replication was observed when ARPE-19 cells were treated with IFN-β in conjunction with serum from a neuroinvasive WNV disease patient carrying neutralizing auto-antibodies (IFN-I-NTAbs) against IFN-β (n = 1). The neutralizing activity of this serum was evident at IFN-β concentrations up to 100 pg/mL, as shown by the ARPE-NT assay. This effect was compared to cells treated with sera from WNV disease patients without IFN-I-NTAbs (n = 2), which served as controls. All sera were tested at a single dilution of 1:10. All experiments were performed in triplicate.
Correlation between IFN-I-NTAbs ARPE assay and ELISA/luciferase assays for neutralizing antibodies against type I IFNs. Correlation between the IFN-I-NTAbs ARPE cell-based assay and the ELISA and luciferase-based assays for detecting neutralizing antibodies against IFN-β (A), IFN-ω (B), and IFN-α2 (C). The Y-axis shows the percentage neutralization value of the IFN-I-NTAbs ARPE assay at different concentrations of type I IFNs (50 pg/mL, 100 pg/mL, 500 pg/mL, and 10 ng/mL). Red triangles represent positive results from the luciferase assay at 10 ng/mL. Green circles indicate positive results from the luciferase assay at 100 pg/mL. Black squares represent samples that were negative in the luciferase assay at 10 ng/mL but may neutralize at lower concentrations, as shown in the ARPE-NT assay. Purple diamonds indicate positive results from the ELISA assay. These data demonstrate the concordance between the newly developed IFN-I-NTAbs ARPE assay and established methods, highlighting the ability of the ARPE assay to detect and quantify the functional neutralizing activity of type I IFN auto-antibodies, even at varying IFN concentrations.
Discussion
We developed a simple in vitro WNV infection assay on ARPE-19 cells that is reproducible and suitable to assess the protective effect of type I IFNs against viral infection in these cells and to demonstrate that auto-Abs neutralizing type I IFNs in serum samples from patients with life-threatening viral disease impair the protective antiviral functions of type I IFNs. We first compared the sensitivity of both Vero E6 and ARPE-19 cells to type I, type II and type III IFNs, and demonstrated that ARPE-19 are protected even by very low concentrations (at least up to 50 pg/mL) of IFN-α, IFN-β or IFN-ω. Our system is not specifically designed to finely compare the antiviral activity of different type I IFNs. Nevertheless, we could observe that IFN-β retained its full antiviral effect at concentrations as low as 10 pg/mL, while IFN-α2 and IFN-ω fully protected ARPE-19 cells at 50 pg/mL and only partially protected these cells at 10 pg/mL, with a slightly higher protection conferred by IFN-ω over IFN-α2, in line with previous observations28. In our study, we identify the ARPE-19 cell line as the optimal cell line for these experiments to the current knowledge, and compared to the widely used Vero E6 cell line. We also demonstrate that, in ARPE-19 cells, type II and type III IFNs do not restrict viral replication, at least under the conditions tested. These observations highlight the limitations of using these cells for evaluating the efficacy of IFN-γ, and IFN-λ1 against WNV infection in vitro. Our findings are consistent with the notion that, in humans, IFN-γ has a major protective role against intracellular bacteria and mycobacteria and a more redundant role in protecting against many viral infections29. The lack of a protective effect of type III IFNs, despite their demonstrated antiviral activity, especially in epithelial tissues, is in line with the recent report on the lack of association between anti-type III IFNs auto-Abs and COVID-19 30. The role of type III IFNs in protecting against viral infection, including infection respiratory infections or other affecting mucosal tissues, and in arboviral infection, including WNV encephalitis, still remains to be elucidated.
In our ARPE-19 cell system, serum samples from patients with WNND containing auto-Abs anti-IFN-α2, IFN-β or IFN-ω neutralized the protective antiviral effect of the respective cytokines, whereas none of the samples without the auto-Abs blocked the protective function of the respective cytokine. During the pre-treatment phase, cells were exposed to a known concentration of IFN together with the sera to be tested. If the sera contained auto-Abs against IFNs, the amount of IFN available to protect the cells was reduced in proportion to the concentration of auto-Abs present. Following the pre-treatment, a critical washing step with PBS was performed to completely remove all traces of serum from the cells. This ensured that the sera, which might contain WNV-specific neutralizing antibodies (WNV-NTAbs), never came into direct contact with the virus. As a result, any observed effect on viral infection could be attributed exclusively to the activity of IFN-I-specific auto-Abs. Notably, pre-treatment with serum alone, without the addition of IFN, did not confer protection against WNV infection in vitro. This confirmed that the presence of WNV-NTAbs in the serum did not influence viral infection or the overall results of the assay. This experimental setup thus provides a robust and reliable framework to distinguish the specific neutralizing activity of IFN-I-specific auto-Abs from any potential interference by WNV-NTAbs in the context of WNND pathogenesis.
Our study is relevant for research purposes. First, it provides a model to study type I IFNs and test sera from patients with WNV infection for IFN-I-NTAbs. Importantly, our assay is consistent with and complementary to the luciferase-based neutralization assay, as it provides direct information on the antiviral function of type I IFNs and the detrimental effect of auto-Abs on this antiviral activity, allowing for the assessment of the IFN-I-NTAbs on actual viral replication and cell viability. This assay reflects the end-point effect of auto-Ab interference on the cell-intrinsic antiviral state, a crucial aspect that the ELISA and luciferase assays do not address directly, as they only measure only concentration and/or integrity in signaling, respectively. In addition, the WNV-ARPE-19 assay provides the experimental basis to a series of future studies aiming at identifying other human cell lines that could display different sensitivity to type I or type III IFNs in respect to different in vitro viral infections. Moreover, our study is clinically relevant. Our WNV infection-based in vitro assay with ARPE-19 cells is suitable for screening patients with life-threatening WNV infection for neutralization of type I IFN activity, either alone or in combination with IFN-I-NTAb screening by ELISA, or by using recently described whole blood type I IFN stimulation assays, which identify IP-10 as a key marker induced by both type I and II IFNs31. Our assay it is also equally suitable to test serum samples from patients with infection other than WNV, including hypoxemic COVID-19 pneumonia, MERS and critical influenza virus pneumonia, given the role of the same IFN-I-NTAbs in the pathogenesis of these life-threatening infections. Our findings align with previous studies highlighting the role of type I IFN-neutralizing autoantibodies in a range of severe viral infections, including arbovirus diseases such as Powassan and Usutu. These autoantibodies exacerbate disease severity by impairing IFN signaling32. To this purpose, validation in large patient cohorts is warranted. Given the prevalence of IFN-I-NTAbs in the general population and the associated risk of life-threatening COVID-19, Influenza pneumonia, WNND and other severe viral infections, it is important to set up screening programs with reliable methods to test the general population, or, at least, at risk individuals, including elderly individuals and those with autoimmune conditions.
Materials and methods
Virus propagation
Wild-type WNV (EG101 reference strain; Melnick et al., 1951) was propagated in a BSL-3 laboratory in African green monkey kidney Vero-E6 low-passage cells (VERO C1008 [Vero 76, clone E6, Vero E6]; ATCC CRL-1586). Virus stock titers were determined with the Reed-Muench method and presented as TCID50, as previously described27.
In vitro protective effect of type I IFNs against WNV
In order to assess pre-infection protective effect of type I IFNs, Vero-E6 cells or ARPE-19 Human retinal pigment epithelia (RPE) low-passage cells [ARPE-19 (ATCC CRL-2302)] were utilized to seed 24-well plates (COSTAR) at a density of 5 × 104 cells/well, with triplicate wells for each set of conditions. These included a virus control (no IFN), a cell test (+ IFN-α2, β or ω), and medium control (no virus, no IFN). The following day, cells were exposed to 100’000, 10’000, 1’000, 500, 100 or 50 pg/ml recombinant human IFN-α2 (ref. number 11101-2; R&D Systems, Minneapolis, United States) or recombinant human IFN-ω (ref. number 300–02 J; Peprotech, Waltham, United States) or recombinant human IFN- β (ref. number 300-02BC; Peprotech, Waltham, United States) for 24 h at 37 °C. The plates were then washed with PBS, and the cells were infected with 10,000, 1000 or 100 TCID50 WNV and incubated for 2 h at 37 °C under an atmosphere containing 5% CO2. After the removal of the WNV inoculum by washing with PBS, fresh medium was added to the wells. Supernatants were collected at 0, 24, 48, 72, and 96 h and titrated with twofold serial dilutions ranging from 1:1 to 1:128 in 96-well plates to score WNV CPE on confluent Vero-E6 cells after 5 days of incubation at 37 °C under an atmosphere containing 5% CO2. Viral WNV replicating percentage is calculated by normalizing the number of observed wells with cytopathic effect following the titration of the collected supernatant against the virus control, performing three replicates and serially diluting the supernatant by two-fold until 1:128. In order to asses post-infection protective effect of type I IFNs, Vero-E6 or ARPE-19 cells were infected as described above prior to IFN treatment, then were treated with IFN-α2 or ω or β under the same experimental conditions. The efficacy of type II (recombinant human IFN-γ, ref. number 300-02; Peprotech, Waltham, United States) and III (recombinant human IFN-λ1, ref. number 300–02 L; Peprotech, Waltham, United States) IFNs in protecting Vero E6 and ARPE-19 cells from WNV infection were tested at the same conditions described above.
Serum samples from patients with WNV infection
Serum samples from subjects with confirmed WNV infection, collected between 2014 and 2022 in northern Italy, were screened for inclusion in type I IFN neutralization experiments. Individuals with WNND were reported to have encephalitis (fever, acute signs of central or peripheral neurological dysfunction, including altered mental status and neurological deficits), meningitis (fever, pleocytosis, headache, nuchal rigidity), acute flaccid paralysis (poliomyelitis-like syndrome or Guillain-Barré-like syndrome), or other neurological syndromes. WNV-infected controls (WNVIC) were blood donors with documented WNV infection, diagnosed based on the detection of WNV RNA in blood during routine screening at the time of donation. These individuals remained asymptomatic or exhibited mild symptoms (e.g., headache) during follow-up. Informed consent was obtained from all subjects and/or their legal guardians. The experiments were conducted in Italy, in accordance with local regulations and guidelines from the Italian national data protection authority, and with the approval of the Institutional Review Board (IRB) of the San Matteo Research Hospital in Pavia.
WNV-NTAbs microneutralization assay
All sera used in the experiments underwent testing with the WNV-NTAb microneutralization assay for titration of WNV-NTAbs, as previously reported (Percivalle et al., 202027). Briefly, 50 µl of fourfold (1:10 to 1:640) serially diluted serum samples from patients with or without auto-Abs were added in duplicate to a flat-bottomed tissue culture microtiter plate (COSTAR, Corning Incorporated, New York, USA) with 50 µl (100 tissue culture infectious dose 50 [TCID50]) WNV. After incubation for 1 h at 37 °C under an atmosphere containing 5% CO2, Vero E6 cells (3 × 104 in 50 µl per well) were added. After 96 h, the plates were scored for cytopathic effect (CPE), and the NTAb titer was calculated as the concentration of Abs able to abolish CPE. A NTAb titer < 1:10 was considered negative, whereas a titer greater or equal to 1:10 was considered positive. To further evaluate the potential interference of WNV-NTAbs identified in the sera of infected patients, cells were pre-treated overnight with a 1:10 or 1:100 dilution of sera containing WNV NTAb as well as sera devoid of such antibodies. This pre-treatment was followed by the removal of the serum through a thorough washing procedure and subsequent infection of the cells with WNV. Using the same procedure subsequently employed to remove type I IFNs, the presence or absence of the patient’s serum containing WNV-NTAbs did not influence the results after overnight incubation, as described in the next paragraph. The viral replication from treated cells matched that of the virus control (i.e., not pre-treated with serum), demonstrating that pre-treatment with sera with WNV-NTAbs did not impact subsequent infection.
Inhibition of type I IFN activity using sera from patients with WNV neuroinvasive disease and containing auto-Abs against type I IFNs
The neutralization of type I IFNs by patients’ serum samples was assessed using Vero-E6 or ARPE-19 cells seeded in 96-well plates (COSTAR, 13 Corning Incorporated) at a density of 3 × 104 cells/well. Each sample was analyzed in triplicate (serum + IFN-α2, β or ω mixture), included a virus control (no IFN) and a medium control (no virus, no IFN) for each plate. The following day, an anti–IFN-α2 monoclonal Ab (21100-1; R&D Systems) or serial threefold dilutions of serum samples were incubated with 10,000, 400, 100 or 50 pg/ml recombinant IFN-α2 (11101-2; R&D Systems), IFN-β (300-02BC; Peprotech) or IFN-ω (300–02 J; PrepoTech) for 1 h at 37 °C under an atmosphere containing 5% CO2 (starting dilution of serum samples = 1:100 or 1:10). The culture medium was then removed from the cells by aspiration and replaced with the serum/Ab–IFN mixture. The plates were incubated for 24 h, after which the serum/Ab–IFN mixture was removed, and the plates were washed with PBS to eliminate any anti-WNV–neutralizing Abs present. This washing procedure eliminates the interference of WNV-NTAbs found in patient sera, preventing them from inhibiting infection in the assay. This enables the evaluation of solely the impact of anti-interferon antibodies. Importantly, the virus never comes into contact with the sera throughout the process, thereby excluding any neutralizing activity of WNV-NTAbs. Cells were then infected with 100 TCID50 WNV by directly dispensing the inoculum into the wells and incubating for 2 h at 37 °C. The plates were then washed with PBS to remove the WNV inoculum, and fresh medium was added to the wells. After 48 h, supernatants were collected and titrated with twofold serial dilutions ranging from 1:1 to 1:128 in 96-well plates for scoring WNV CPE on confluent Vero-E6 cells after 5 days of incubation at 37 °C.
ELISA
Enzyme-linked immunosorbent assays (ELISA) were performed as previously described (Puel et al., 2008). In brief, 96-well ELISA plates (MaxiSorp; Thermo Fisher Scientific) were coated by overnight incubation at 4 °C with 1 µg/mL rhIFN-α (Miltenyi Biotec, ref. number 130-108-984), rhIFN-ω (Peprotech, ref. number 300-02 J) or rhIFN-β (Peprotech, ref. number 300-02BC). Plates were then washed (PBS/0.005% Tween), blocked by incubation with the same buffer supplemented with 2% BSA, washed, and incubated with 1:50 dilutions of plasma samples from the patients or controls for 2 h at room temperature (or with specific mAbs as positive controls). Each sample was tested once. Plates were thoroughly washed (PBS/0.005% Tween). Horseradish peroxidase (HRP)–conjugated Fc-specific IgG fractions from polyclonal goat antiserum against human IgG (Nordic Immunological Laboratories) were added to a final concentration of 1 µg/mL. Plates were incubated for 1 h at room temperature and washed. Substrate was added and OD was measured. All the incubation steps were performed with gentle shaking (600 rpm).
Luciferase reporter assay
The blocking activity of anti-IFN-α2, anti-IFN-ω and anti-IFN-β auto-Abs was determined with a reporter luciferase activity, as previously described12. Briefly, HEK293T cells were transfected with a plasmid containing the firefly luciferase gene under the control of the human ISRE promoter in the pGL4.45 backbone, and a plasmid constitutively expressing Renilla luciferase for normalization (pRL-SV40). Cells were transfected in the presence of the X-tremeGene9 transfection reagent (Sigma-Aldrich, ref. number 6365779001) for 24 h. Cells in Dulbecco’s modified Eagle medium (DMEM, Thermo Fisher Scientific) supplemented with 2% fetal calf serum (FCS) and 10% healthy control or patient serum/plasma (after inactivation at 56 °C, for 20 min) were either left unstimulated or were stimulated with rhIFN-α2 (Miltenyi Biotech, ref. number 130-108-984 (not glycosylated) or Merck, ref. number H6041-10UG (glycosylated)), rhIFN-ω (Peprotech, ref. number 300–02 J (not glycosylated) or Origene, TP721113 (glycosylated)), at 10 ng/mL or 100 pg/mL (not glycosylated) or 1 ng/mL (glycosylated), or rhIFN-β (Peprotech, ref. number 300-02BC (glycosylated)) at 10 ng/mL or 1 ng/mL, for 16 h at 37 °C. Finally, cells were lysed for 20 min at room temperature and luciferase levels were measured with the Dual-Luciferase® Reporter 1000 assay system (Promega, ref. number E1980), according to the manufacturer’s protocol. Luminescence intensity was measured with a VICTOR-X Multilabel Plate Reader (PerkinElmer Life Sciences, USA). Firefly luciferase activity values were normalized against Renilla luciferase activity values. These values were then normalized against the median induction level for non-neutralizing samples and expressed as a percentage. Samples were considered neutralizing if luciferase induction, normalized against Renilla luciferase activity, was below 15% of the median values for controls tested the same day.
Data analysis
All analyses reported were performed using GraphPad Prism software (version 8; GraphPad Software Inc., La Jolla, CA), descriptive statistic included median, interquartile range (IQR) and numbers of samples analyzed (N).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request”.
References
Schoggins, J. W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485. https://doi.org/10.1038/nature09907 (2011).
Suthar, M. S., Diamond, M. S. & Gale, M. Jr West Nile virus infection and immunity. Nat. Rev. Microbiol. 11, 115–128. https://doi.org/10.1038/nrmicro2950 (2013).
Samuel, M. A. & Diamond, M. S. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 79, 13350–13361. https://doi.org/10.1128/JVI.79.21.13350-13361.2005 (2005).
Oleaga-Quintas, C. et al. Inherited GATA2 deficiency is dominant by haploinsufficiency and displays incomplete clinical penetrance. J. Clin. Immunol. 41, 639–657. https://doi.org/10.1007/s10875-020-00930-3 (2021).
Sologuren, I. et al. Lethal influenza in two related adults with inherited GATA2 deficiency. J. Clin. Immunol. 38, 513–526. https://doi.org/10.1007/s10875-018-0512-0 (2018).
Gervais, A. et al. Autoantibodies neutralizing type I IFNs underlie severe tick-borne encephalitis in ∼10% of patients. J. Exp. Med. 221, e20240637. https://doi.org/10.1084/jem.20240637 (2024).
Bastard, P. et al. Autoanti- bodies against type I IFNs in patients with life-threatening COVID-19. Science 370, eabd4585. https://doi.org/10.1126/science.abd4585 (2020).
Alotaibi, F. et al. Type I interferon autoantibodies in hospitalized patients with Middle East respiratory syndrome and association with outcomes and treatment effect of interferon beta-1b in MIRACLE clinical trial. Influenza Other Respir Viruses. 17, e13116. https://doi.org/10.1111/irv.13116 (2023).
Hale, B. G. Autoantibodies targeting type I interferons: Prevalence, mechanisms of induction, and association with viral disease susceptibility. Eur. J. Immunol. 53, e2250164. https://doi.org/10.1002/eji.202250164 (2023).
Puel, A., Bastard, P., Bustamante, J. & Casanova, J. L. Human auto- antibodies underlying infectious diseases. J. Exp. Med. 219, e20211387. https://doi.org/10.1084/jem.20211387 (2022).
Bastard, P. et al. Auto- antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 218, e20202486. https://doi.org/10.1084/jem.20202486 (2021).
Gervais, A. et al. Autoantibodies neutralizing type I IFNs underlie West Nile virus encephalitis in ∼40% of patients. J. Exp. Med. 220 (9), e20230661. https://doi.org/10.1084/jem.20230661 (2023).
O’Driscoll, M. et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 590, 140–145. https://doi.org/10.1038/s41586-020-2918-0 (2021).
Manry, J. et al. The risk of COVID-19 death is much greater and age dependent with type I IFN autoantibodies. Proc. Natl. Acad. Sci. USA. 119 (e2200413119). https://doi.org/10.1073/pnas.2200413119 (2022).
Petersen, L. R., Brault, A. C. & Nasci, R. S. West Nile virus: Review of the literature. JAMA 310 (3), 308–315. https://doi.org/10.1001/jama.2013.8042 (2013).
Petersen, L. R., Marfin, A. A. & Gubler, D. J. West Nile virus. JAMA 290 (4), 524–528. https://doi.org/10.1001/jama.290.4.524 (2003).
Phalen, D. N. & Dahlhausen, B. West Nile virus. Semin. Avian exot. Pet. Med. 13, 67–78. https://doi.org/10.1053/j.saep.2004.01.002 (2004).
van der Meulen, K. M., Pensaert, M. B. & Nauwynck, H. J. West Nile virus in the vertebrate world. Arch. Virol. 150 (4), 637–657. https://doi.org/10.1007/s00705-004-0463-z (2005).
Malkinson, M. & Banet, C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr. Top. Microbiol. Immunol. 267, 309–322. https://doi.org/10.1007/978-3-642-59403-8_15 (2002).
European Centre for Disease Prevention and Control. Factsheet about West Nile virus infection. (2023). Available at https://www.ecdc.europa.eu/en/ west-nile-fever/facts (Accessed Feb 20, 2024).
Centers for Disease Control and Prevention. West Nile virus. (2023). Available at https://www.cdc.gov/westnile/statsmaps/index.html (Accessed Feb 20, 2024).
Lim, S. M., Koraka, P., Osterhaus, A. D. & Martina, B. E. West Nile virus: Immunity and pathogenesis. Viruses 3 (6), 811–828. https://doi.org/10.3390/v3060811 (2011).
Debiasi, R. L. West Nile virus neuroinvasive disease. Curr. Infect. Dis. Rep. 13 (4), 350–359. https://doi.org/10.1007/s11908-011-0193-9 (2011).
Shrestha, B., Huynh, N. & Diamond, M. S. Blockade of interferon signaling decreases gut barrier integrity and promotes severe West Nile virus disease. Nat. Commun. 14, 5973. https://doi.org/10.1038/s41467-023-41600-3 (2023).
Ank, N. et al. Lambda Interferon (IFN-λ), a type III IFN, is Induced by viruses and IFNs and displays potent antiviral activity against select virus infections. Vivo J. Virol. 80, 4501–4509. https://doi.org/10.1128/JVI.80.9.4501-4509.2006 (2006).
Ning, Y. et al. Interferon-γ-directed inhibition of a novel high-pathogenic phlebovirus and viral antagonism of the antiviral signaling by targeting STAT1. Front. Immunol. 10, 1182. https://doi.org/10.3389/fimmu.2019.01182 (2019).
Percivalle, E. et al. West Nile or Usutu virus? A three-year follow-up of humoral and cellular response in a group of asymptomatic blood donors. Viruses 12, 157. https://doi.org/10.3390/v12020157 (2020).
Piehler, J., Thomas, C., Garcia, K. & Schreiber, G. Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation. Immunol. Rev. 250, 317–334. https://doi.org/10.1111/imr.12001 (2012).
Casanova, J., MacMicking, J. & Nathan, C. F. Interferon-γ and infectious diseases: Lessons and prospects. Science 384 https://doi.org/10.1126/science.adl2016 (2024).
Vanker, M. et al. Autoantibodies neutralizing type III interferons are uncommon in patients with severe coronavirus disease 2019 pneumonia. J. Interferon Cytokine Res. 43, 379–393. https://doi.org/10.1089/jir.2023.0003 (2023).
Menon, M. et al. A sensitive assay for measuring whole-blood responses to type I IFNs presents a diagnostic method for detecting inborn errors in type I interferon (IFN) immunity and autoantibodies neutralizing IFNs. J. Clin. Immunol. 44, 215–229. https://doi.org/10.1089/jci.2023.0015 (2023).
Gervais, A. et al. Auto-abs neutralizing type I IFNs in patients with severe Powassan, Usutu, or Ross River virus disease. J. Exp. Med. 221, e20240942. https://doi.org/10.1084/jem.20240942 (2024).
Funding
This research was supported by Fondazione IRCCS Policlinico San Matteo Pavia, grant number RC8048424 - “Cellular models to asses type I IFN’s protection against arboviruses infection” and partially supported by EU funding within the NextGeneration EU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT).
Author information
Authors and Affiliations
Contributions
Conceptualization, A.B., E.P., D.L. and A.F.; methodology, A.F., F.R,.; software, A.F. and L.D.; formal analysis, S.C., A.G., Z.S., and A.F.,; resources, F.B.; data curation, A.F. and I.C.; writing original draft preparation, A.F. and E.P.; writing review and editing, E.P., I.C., B.A., J.C. and F.B.; visualization, F.B.; investigation, F.R, B.A., and J.C.; funding acquisition, F.B.; *A.B. and F.B. contributed equally to this paper. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.-L. Casanova reported a patent to PCT/US2021/042741 pending. No other disclosures were reported from other authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ferrari, A., Cassaniti, I., Rovida, F. et al. Human type I interferons protect Vero E6 and ARPE-19 cells against West Nile virus and are neutralized by pathogenic autoantibodies. Sci Rep 15, 11271 (2025). https://doi.org/10.1038/s41598-025-89312-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89312-6