Abstract
A multicenter cross-sectional study was conducted to investigate the magnification-corrected association between fovea-disc distance (FDD) and optical coherence tomography (OCT)-measured macular retinal layer thickness in eyes with and without primary open-angle glaucoma (POAG). A 12.0 × 9.0-mm-wide swept-source OCT scan, which includes both the macula and optic disc, was performed in 190 eyes from 124 healthy subjects (normal group) and 149 eyes from 117 POAG patients (POAG group). The FDD and thickness of the macular retinal nerve fiber layer (mRNFL), ganglion cell inner plexiform layer (GCIPL), and outer retina (OR, total retina minus (mRNFL plus GCIPL)) were measured and corrected for magnification effects. The mixed-effects models, accounting for potential confounding factors, revealed two significant associations between a longer FDD and retinal layer thickness: thinner mRNFL in the normal group (coefficients, -3.14, 95% confidence intervals (CI), -4.75 to -1.53; p = 0.0001) and thinner GCIPL in the POAG group (coefficients, -4.26; 95% CI, -6.85 to -1.67; p = 0.0013). The association between FDD and macular retinal layer thickness varies by retinal layer and the presence of POAG. FDD can significantly affect OCT-determined macular retinal layer thickness, especially GCIPL in POAG eyes and mRNFL in normal eyes.
Similar content being viewed by others
Introduction
Myopia is a risk factor for glaucoma1. Structural deformity in the optic disc, including changes in size, ovality, or torsion due to axial elongation, may mimic glaucomatous optic neuropathy (GON) or increase susceptibility to GON2,3. Stretching of the posterior fundus by axial elongation may change the fovea–disc relationship. Two parameters that describe the fovea-disc relationship are fovea-disc distance (FDD) and fovea-disc angle (FDA) (the angle between the line connecting the fovea and the optic disc center and the horizontal reference line). A significant relationship between FDD and spherical equivalent (SE)4 or axial length (AXL)5,6,7 has been reported: A longer FDD is associated with myopic SE4, a longer AXL5,6 or a shorter estimated AXL7. Regarding the clinical significance of FDD in glaucoma management, longer FDD was associated with changes in surrogate parameters of glaucomatous damage such as a smaller rim area8 or thinner circumpapillary retinal nerve fiber layer (cpRNFL) in normal eyes9. Longer FDD was associated with the false-positive classification of glaucoma diagnosis in the thickness assessment of the macular inner retinal layers by optical coherence tomography (OCT)10. Central visual field (VF) involvement was related to shorter FDD in patients with normal tension glaucoma and mild VF defects11. Conversely, larger FDD was associated with visual acuity loss in glaucoma patients with myopia12.
Although measuring the thickness of the inner retinal layer using OCT is crucial for diagnosing and monitoring glaucoma progression13, few studies have addressed the association between FDD and inner retinal layer thickness in the macula; these studies have been limited to healthy subjects and have not provided any findings in patients with glaucoma. Larger FDD was significantly associated with thinner inner retinal layers in the macula of normal eyes in a multivariate analysis adjusting for confounding factors related to macular retinal thickness (age14,15,16,17, sex14,15,16,17, VF mean deviation (MD), AXL14,15,16,17, cpRNFL thickness16,17, and image quality16) and those with FDD (FDA4 and optic disc area5)10,18. However, other potential confounders were not considered in the previous studies. These include optic disc torsion19 and the retinal artery angle (the angle between the supratemporal and infratemporal major retinal arteries)20 for macular retinal layer thickness and ovality index for FDD21. Furthermore, previous studies did not consider the magnification effects on OCT scan areas, which can lead to artificial thinning of the measured thickness of macular inner retinal layers22.
This study investigated the magnification-corrected association between FDD and OCT-measured macular retinal layer thickness in normal and primary open-angle glaucoma (POAG) eyes accounting for all possible confounding factors4,5,14,15,16,17,18,19,20,21. Furthermore, we investigated whether the relationship between FDD and the macular retinal layer thickness varied based on the presence of POAG.
Results
Characteristics of the normal and POAG groups
Data from 190 eyes of 124 healthy subjects and 149 eyes of 117 patients with POAG were included in this study. The demographics and fundus parameters of the normal and POAG groups are shown in Table 1. FDD was significantly longer in POAG eyes than in normal eyes (4.95 ± 0.39 mm vs. 4.78 ± 0.38 mm, p = 0.0005). The difference was significant after the Bonferroni correction. There were no significant differences in age or AXL between the two groups (p = 0.07, 0.10, respectively). Besides variables related to GON (i.e., MD and thickness of cpRNFL, macular RNFL and GCIPL), there were no factors that had significant Bonferroni-corrected p-values for the difference between the two groups. The intra-observer reproducibility (ICC) of FDD, disc area, ovality index, torsion angle, FDA, and retinal artery angle were 0.986, 0.987, 0.883, 0.896, 0.984, and 0.995, respectively. The inter-observer reproducibility (ICC) of FDD, disc area, ovality index, torsion angle, FDA, and retinal artery angle were 0.989, 0.994, 0.890, 0.819, 0.981, and 0.998, respectively.
The association between FDD and retinal layer thickness
Adjusted for all potential confounders in the multivariate analysis, a longer FDD was significantly associated with thinner mRNFL in the normal group (coefficients, -3.14; 95% confidence intervals (CI), -4.75 to -1.53, p = 0.0001) and thinner GCIPL in POAG group (coefficients, -4.26; 95% CI, -6.85 to -1.67; p = 0.0013) (Tables 2 and 3). According to the multivariate model, the GCIPL in eyes with POAG was thinner by 4.26 μm, corresponding to a 1 mm longer FDD (Table 3; Fig. 1). The p-values were significant after the Bonferroni correction. The effect of FDD on retinal layer thickness was compared between two groups as an interaction term between FDD and diagnosis (normal or POAG) in the multivariate analysis with all participants adjusted for all other variables. The difference in the coefficient of FDD between the two groups was significant for GCIPL thickness (p = 0.005), but not for mRNFL or OR (p = 0.70 and 0.53, respectively) (Table 4).
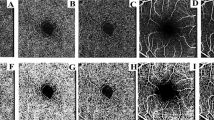
Comparison of magnification-corrected fundus images between POAG eyes with long and short FDD. Representative POAG eyes with long (a, b) and short (c, d) FDD. (a, c) En-face OCT images, corrected for magnification, displaying defects in the inferior arcuate retinal nerve fiber layer. Both eyes exhibited a comparable level of glaucomatous optic neuropathy based on MD and cpRNFL thickness measurements. (b, d) The shadowgram OCT images of a and c, respectively. A 6.0 mm x 6.0 mm square map of GCIPL thickness is overlaid on the image. GCIPL thickness was thinner by 4 μm in the eye with a longer FDD (b) compared to the eye with a shorter FDD (d), corresponding to the 1 mm difference in FDD. POAG primary open-angle glaucoma, FDD fovea-disc distance, MD mean deviation, cpRNFL circumpapillary retinal nerve fiber layer, GCIPL ganglion cell inner plexiform layer.
Other factors significantly associated with retinal layer thickness
In addition to FDD, various other factors showed a significant association with the thickness of the macular retinal layers in multivariate analysis of normal or POAG groups (Tables 2 and 3). In both groups, the thicker cpRNFL was significantly associated with thicker mRNFL (p < 0.001) and GCIPL (p < 0.001). In the normal group, smaller retinal artery angles and higher image quality scores were significantly associated with thicker mRNFL (p < 0.0001 for both). Males had significantly thicker OR than females (p = 0.0007). In the POAG group, a smaller disc area was significantly associated with thicker mRNFL (p = 0.0031). These p-values were significant after the Bonferroni correction.
Discussion
In the current study, the effects of FDD on macular retinal layer thickness differed between the eyes with and without POAG. In a multivariate analysis adjusted with all potential confounders, a significant association was found between longer FDD and thinner mRNFL in normal eyes. In contrast, longer FDD was significantly associated with thinner GCIPL in POAG eyes. Furthermore, the relationship between FDD and GCIPL was significantly different between normal and POAG groups. Accordingly, a connection between longer FDD and thinner GCIPL may be a specific finding related to glaucoma.
Previous studies demonstrated that longer FDD was significantly associated with thinner macular retinal layers including mRNFL and GCIPL in the eyes of normal subjects10,18. The discrepancy in GCIPL findings in normal eyes between our study and previous studies may be due to magnification correction, participant background, or confounding parameters for adjustments. It is well known that the actual size of OCT images is larger in eyes with longer AXL owing to magnification effects. Higashide et al. found that, without accounting for magnification, the mRNFL was thicker and the GCIPL was thinner in normal eyes with longer AXL22. However, the relationship became insignificant when magnification effects were corrected. The findings from magnification-uncorrected data may be explained by the relationship between the normal thickness profile of each retinal layer and the analytical area of OCT. For example, GCIPL has an annular-shaped thickest portion in the pericentral region15. The proportion of the GCIPL’s thick area to the total OCT analytical area reduces in eyes with longer AXL, leading to thinner GCIPL on average.
Regarding the significant association between FDD and mRNFL thickness found in the normal group in our study, we confirmed the results of Qiu et al.18 using the magnification-corrected data. AXL elongation may cause retinal stretching in the macula, along with FDD enlargement. The mechanical changes may explain the thinning of the mRNFL in normal eyes with longer FDD, considering the parallel trajectory of retinal nerve fibers in the macula to the measurement line of FDD. Similarly, the relationship between FDD and mRNFL thickness had a p-value of 0.019 in the POAG group, although it became insignificant after the Bonferroni correction. Furthermore, the difference in the coefficient of FDD between the two groups was negligible for mRNFL (Table 4). Thus, thinning of the mRNFL due to retinal stretching in eyes with longer FDD can occur regardless of the presence of POAG.
On the other hand, GCIPL was affected by enlargement of FDD in POAG eyes, but not in normal eyes. However, the reason why FDD-related macular GCIPL thinning occurs in glaucomatous eyes is currently unknown. According to the results of multivariate analysis, the significant relationship between GCIPL and FDD in POAG eyes was independent of the parameters related to the severity of GON, i.e., cpRNFL thickness or MD. Therefore, the susceptibility of the GCIPL to thinning caused by the elongation of FDD may be an inherent feature of POAG eyes. Given that FDD positively correlates with AXL5,6, scleral changes due to AXL elongation may cause retinal stretching in the macula, along with FDD enlargement. Axial elongation causes significant remodeling in the sclera that underlies the retina and choroid23. Differences in scleral microstructure between glaucomatous and non-glaucomatous donors have also been reported in the macular region24. These differences in scleral properties between myopic eyes with and without glaucoma may play a role in the different associations between FDD and macular inner retinal layer thickness.
Other variables were also associated with macular layer thickness. Male sex was significantly associated with thicker OR in normal eyes. The results were consistent with a previous study involving healthy Japanese participants15. The study reported that the inner nuclear layer, outer plexiform layer, and outer nuclear layer were significantly thicker in men than in women. Smaller retinal artery angles were significantly associated with thicker mRNFL in normal eyes. The result confirms previous findings that RNFL thickness increases when retinal arteries shift toward the fovea25. A higher image quality score was associated with thicker GCIPL in an earlier study using the Cirrus spectral-domain OCT26. In the current study using a swept-source OCT, image quality scores were significantly associated with mRNFL thickness in normal groups, indicating an advantage of GCIPL over mRNFL as a clinically more convenient marker.
This study had several limitations. As the majority of the participants were Asian, these findings may not be generalizable to other races. Given the upper limit of AXL to 28 mm, the results may not be applicable to eyes with AXL > 28 mm. However, it was expected to be difficult to enroll eyes with AXL > 28 mm without any possible structural abnormalities related to pathologic myopia27. The FDD in this study is a two-dimensional parameter that cannot account for the depth in the Z-axis between the fovea and the optic disc center. The true FDD would be longer in eyes with a posteriorly protruded fundus than in those with a flat fundus, even if the two-dimensional FDD was the same. Kim et al. reported that the mean depth of the deepest point in the fundus relative to the temporal disc margin was 0.17 ± 0.16 mm using a three-dimensional volumetric scan of swept source OCT in eyes with myopic glaucoma (AXL, 26.2 ± 1.3 mm)28. Assuming a two-dimensional FDD of 5.0 mm and a depth of 0.2 mm at the fovea relative to the disc center, the difference between the two- and three-dimensional FDD would be less than 0.1%, suggesting that two-dimensional FDD is a reasonable approximation of true FDD in the current subjects. Most previous studies have employed the optic disc center in the fundus photograph as one end of the FDD, and the present study utilized the previous described method to measure FDD4,5,6,7,8,9,10,11,12,18 to compare the obtained results to the previous one under the same conditions.
In conclusion, the association between FDD and macular retinal layer thickness varies according to the retinal layer and the presence of glaucoma. Notably, the relationship between thinner GCIPL and longer FDD was only observed in POAG eyes, and the effect of FDD on GCIPL thickness was significantly different between normal and POAG eyes. FDD was the only significant factor associated with GCIPL thickness other than parameters for GON, i.e., cpRNFL thickness and MD, in the multivariate analysis. Given that GCIPL thickness is a representative OCT parameter for glaucoma diagnosis, further investigation is needed to determine if adjusting with FDD improves the diagnostic accuracy of magnification-corrected GCIPL thickness. FDD should be considered a confounding factor to be adjusted for OCT measurements of macular retinal layer thickness, especially for GCIPL in eyes with glaucoma.
Methods
Study participants
The Topcon Myopia Study, a multicenter cross-sectional study, was conducted at eight study sites: five in Japan, one each in the USA, Korea, and China (Kanazawa University, Osaka University, Tajimi Iwase Eye Clinic, Toho University Ohashi Medical Center, Tohoku University, Seoul National University Bundang Hospital, University of California, San Diego, and University of Hong Kong)29,30,31. The study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Kanto Central Hospital (R1-06-005). Written informed consent was obtained from all the participants.
Emmetropic and myopic participants in the normal and POAG groups were recruited and refraction (ARK-900; NIDEK, Aichi, Japan), best-corrected visual acuity (BCVA), and AXL (IOL Master; Carl Zeiss Meditec Inc., Dublin, CA, USA) were measured. Slit-lamp examination, intraocular pressure (IOP) measurements using a Goldmann applanation tonometer, and visual field testing with a Humphrey Field Analyzer (HVF) 24 − 2 Swedish Interactive Threshold Algorithm Standard program (Carl Zeiss Meditec, Inc.) were conducted. Following pupil dilation, fundoscopy, fundus photography (including stereo photographs), and swept-source OCT scans (DRI OCT Triton; Topcon Corp., Tokyo, Japan) were performed.
The subjects in the normal group had non-glaucomatous optic disc appearance and normal HVF results [normal or borderline glaucoma hemifield test (GHT) and pattern standard deviation (PSD) ≥ 1%], astigmatism < 2D, IOP ≤ 21 mmHg, and best corrected visual acuity ≥ 0.7 in both eyes and no family history of glaucoma. Optic disc appearance was assessed using stereo fundus photographs by experts in glaucoma or myopia (MA, AI, GT, and KOM). The inclusion criteria for POAG group were as follows: glaucomatous optic disc appearance, abnormal HVF results (abnormal GHT, PSD < 5% and/or a cluster of 3 points with a probability < 5% on a pattern deviation map in at least one hemifield and including at least 1 point with a probability < 1%), MD > -12.0 dB and IOP < 21 mmHg at the visit for OCT examination. The exclusion criteria were subjects with a narrow angle (Schaffer grade ≤ 2), unreliable HVF (fixation loss ≥ 20% or false positive rate ≥ 15%) results, low-quality OCT images (image quality score ≤ 40 or significant artifacts), history of intraocular or refractive surgery, AXL > 28 mm, or ocular disease (i.e., pathologic myopia, clinically significant cataract, diabetic retinopathy, age-related macular degeneration, epiretinal membrane, optic nerve, or retinal abnormality) in either eye or systemic disease (i.e., systemic use of steroids or anticancer drugs, clinically significant hyper-or hypotension). Types of glaucoma other than POAG were excluded. Both eyes were included when they were eligible.
Swept-source OCT measurements
Two types of raster scans, one for both the macula and optic disc (12.0 × 9.0-mm) and the other for the optic disc (6.0 × 6.0-mm), were performed. Three images of each OCT scan type were acquired, and the data with the best quality were selected by meticulous inspection by four experienced investigators (GT, AM, TH, and TK). The errors in automated retinal layer segmentation32 by the built-in software have been corrected as necessary, including the location of Bruch’s membrane opening. Thickness data of three retinal layers, i.e., macular RNFL (mRNFL), ganglion cell inner plexiform layer (GCIPL), and total retina, in a 6.0 × 6.0 mm square area centered on the fovea were obtained from the best macular OCT scan. The average thickness of the mRNFL, GCIPL, and outer retina (OR, i.e., total retina minus (mRNFL plus GCIPL)) was determined. The cpRNFL thickness was measured along a 3.4 mm diameter circle from the center of the Bruch’s membrane opening-based optic disc using the best OCT scan of the optic disc. The FDD (distance between the fovea and the centroid of the ellipse-fitted disc contour), FDA (deviation of the fovea-disc center axis from the horizontal reference line), and parameters of the optic disc [disc area, ovality index (short axis of the ellipse-fitted disc contour/long axis), and torsion angle (deviation of the long axis of the ellipse-fitted disc contour from the reference line vertical to the fovea-disc center axis)] were measured on a shadowgram OCT image of the best macular OCT scan using ImageJ (ver. 1.53t August 24, 2022) by an experienced examiner (SU). The shadowgram is an en-face method for obtaining high-contrast images of light-blocking objects such as blood vessels and the optic disc in raster OCT scans33. This technique helps identify the position of the fovea and the clinical optic disc margin using OCT images (Fig. 2). The optic disc contour was determined using the ellipse-fitting function of the ImageJ software. The retinal artery angle, that is, the angle between the supratemporal and infratemporal major retinal arteries, was measured at a 3.4 mm circumpapillary circle centered on the optic disc on fundus photographs using custom software, which was reported to be highly correlated with the peak angles of cpRNFL thickness25. Magnification effects were corrected for OCT images, FDD, and disc area based on the method using refractive error, corneal radius, AXL, and lay-tracing method developed for Topcon fundus camera34,35. The details of magnification correction are described in a previous study35.
Parameters measured in shadowgram images of optical coherence tomography. The FDD (distance between the fovea and the centroid of ellipse-fitted disc contour), FDA (deviation of the FDD line from the horizontal reference line), and parameters of the optic disc [ellipse-fitted disc area, ovality index (short axis of the ellipse-fitted disc contour/long axis), and torsion angle (deviation of the long axis of the ellipse-fitted disc contour from the reference line vertical to the fovea-disc center axis)] were measured on shadowgram images of optical coherence tomography using ImageJ (ver. 1.53t 24 August 2022). FDD fovea-disc distance, FDA fovea-disc angle.
Statistical analysis
Linear mixed-effects models were used in all association analyses to account for possible correlations between fellow eyes, both of which were included, if eligible. The relationship between FDD and macular retinal layers (mRNFL, GCIPL, and OR) was examined using multivariate models for the normal or POAG groups adjusting for all possible confounders: age, sex, MD, AXL, corneal radius, IOP, disc area, ovality index, torsion angle, FDA, retinal artery angle, average cpRNFL thickness, and OCT image quality scores. According to the correlation matrix of coefficients between variables, there was a high correlation between AXL and SE in the normal (r = -0.77) and POAG groups (r = 0.78). Therefore, SE was not included in the multivariate models that included AXL and corneal radius, which have the most significant influence on SE. An additional analysis was performed for all participants, where an interaction term between FDD and diagnosis (normal or POAG) was added to the multivariate model adjusted for all other variables to test whether the association between FDD and each macular retinal layer thickness differed by diagnosis.
The intra- and inter-observer reproducibility of manual measurements of FDD, disc area, ovality index, torsion angle, FDA, and retinal artery angle were determined using intraclass correlation coefficients. Two examiners (TH and SU) measured the parameters using OCT and fundus photographic images from 30 randomly selected participants.
All statistical analyses were performed using STATA software (version 17.0, StataCorp, College City, TX, USA). P-values < 0.05 were considered significant. However, Bonferroni-corrected p-values < 0.05 were deemed significant, accounting for multiple p-values (Tables 1, 2 and 3).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Marcus, M. W., de Vries, M. M., Junoy Montolio, F. G. & Jansonius, N. M. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology 118 (10), 1989–1994e2 (2011).
Chang, R. T. & Singh, K. Myopia and glaucoma: diagnostic and therapeutic challenges. Curr. Opin. Ophthalmol. 24 (2), 96–101 (2013).
Wang, Y. X., Panda-Jonas, S. & Jonas, J. B. Optic nerve head anatomy in myopia and glaucoma, including parapapillary zones alpha, beta, gamma and delta: histology and clinical features. Prog Retin Eye Res. 83, 100933 (2021).
Mataki, N., Tomidokoro, A., Araie, M. & Iwase, A. Morphology of the optic disc in the Tajimi study population. Jpn J. Ophthalmol. 61 (6), 441–447 (2017).
Jonas, R. A. et al. Optic Disc-Fovea distance, axial length and parapapillary zones. The Beijing eye study 2011. PloS One 10(9), e0138701 (2015).
Qiu, K. et al. Influence of optic disc-fovea distance on macular thickness measurements with OCT in healthy myopic eyes. Sci. Rep. 8 (1), 5233 (2018).
Hood, D. C. et al. Anatomical features can affect OCT measures used for clinical decisions and clinical trial endpoints. Transl Vis. Sci. Technol. 13 (4), 27 (2024).
Iwase, A., Sawaguchi, S., Tanaka, K., Tsutsumi, T. & Araie, M. Relationship between ocular risk factors for glaucoma and optic disc rim in normal eyes. Br. J. Ophthalmol. 104 (8), 1120–1124 (2020).
Jonas, J. B. et al. Retinal nerve fibre layer thickness in association with gamma zone width and disc-fovea distance. Acta Ophthalmol. 100 (6), 632–639 (2022).
Qiu, K. et al. Effect of optic disc-fovea distance on the normative classifications of macular inner retinal layers as assessed with OCT in healthy subjects. Br. J. Ophthalmol. 103 (6), 821–825 (2019).
Lee, M., Jin, H. & Ahn, J. Relationship between disc margin to fovea distance and central visual field defect in normal tension glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 252 (2), 307–314 (2014).
Kim, S. A., Park, C. K. & Park, H. L. Factors affecting visual acuity and central visual function in glaucoma patients with myopia. Am. J. Ophthalmol. 253, 106–118 (2023).
Mohammadzadeh, V. et al. Macular imaging with optical coherence tomography in glaucoma. Surv. Ophthalmol. 65 (6), 597–638 (2020).
Song, W. K., Lee, S. C., Lee, E. S., Kim, C. Y. & Kim, S. S. Macular thickness variations with sex, age, and axial length in healthy subjects: a spectral domain-optical coherence tomography study. Invest. Ophthalmol. Vis. Sci. 51 (8), 3913–3918 (2010).
Ooto, S. et al. Effects of age, sex, and axial length on the three-dimensional profile of normal macular layer structures. Invest. Ophthalmol. Vis. Sci. 52 (12), 8769–8779 (2011).
Mwanza, J. C. et al. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 52 (11), 7872–7879 (2011).
Koh, V. T. et al. Determinants of ganglion cell-inner plexiform layer thickness measured by high-definition optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 53 (9), 5853–5839 (2012).
Qiu, K. et al. Effect of optic disc-fovea distance on measurements of individual macular intraretinal layers in normal subjects. Retina 39 (5), 999–1008 (2019).
Sung, M. S., Kang, Y. S., Heo, H. & Park, S. W. Characteristics of optic disc rotation in myopic eyes. Ophthalmology 123 (2), 400–407 (2016).
Omoto, T. et al. Relationship between macular ganglion cell thickness and ocular elongation as measured by axial length and retinal artery position. Invest. Ophthalmol. Vis. Sci. 61 (11), 16 (2020).
Nonaka, A. et al. Biometric features of peripapillary atrophy beta in eyes with high myopia. Invest. Ophthalmol. Vis. Sci. 52 (9), 6706–6713 (2011).
Higashide, T. et al. Influence of clinical factors and magnification correction on normal thickness profiles of macular retinal layers using optical coherence tomography. PloS One 11(1), e0147782 (2016).
Jonas, J. B. et al. IMI-Nonpathological human ocular tissue changes with axial myopia. Invest. Ophthalmol. Vis. Sci. 64 (6), 5 (2023).
Danford, F. L. et al. Differences in the region- and depth-dependent microstructural organization in normal versus glaucomatous human posterior sclerae. Invest. Ophthalmol. Vis. Sci. 54 (13), 7922–7932 (2013).
Yamashita, T. et al. Relationship between position of peak retinal nerve fiber layer thickness and retinal arteries on sectoral retinal nerve fiber layer thickness. Invest. Ophthalmol. Vis. Sci. 54 (8), 5481–5488 (2013).
Xu, X. et al. Determinants of macular ganglion cell-inner plexiform layer thickness in normal Chinese adults. BMC Ophthalmol. 21 (1), 267 (2021).
Flitcroft, D. I. et al. IMI – Defining and classifying myopia: A proposed set of standards for clinical and epidemiologic studies. Invest. Ophthalmol. Vis. Sci. 60 (3), M20–M30 (2019).
Kim, Y. C., Jung, K. I., Park, H. L. & Park, C. K. Three-Dimensional evaluation of posterior pole and optic nerve head in myopes with glaucoma. Sci. Rep. 7 (1), 18001 (2017).
Saito, H. et al. Deep optic nerve head structures associated with increasing axial length in healthy myopic eyes of moderate axial length. Am. J. Ophthalmol. 249, 156–166 (2023).
Saito, H. et al. Association of bergmeister papilla and deep optic nerve head structures with prelaminar schisis of normal and glaucomatous eyes. Am. J. Ophthalmol. 257, 91–102 (2024).
Kambayashi, M. et al. Effects of deep optic nerve head structures on Bruch’s membrane Opening- minimum rim width and peripapillary retinal nerve fiber layer. Am. J. Ophthalmol. 263, 99–108 (2024).
Yang, Q. et al. Automated layer segmentation of macular OCT images using dual-scale gradient information. Opt. Express. 18 (20), 21293–21307 (2010).
Jiao, S., Knighton, R., Huang, X., Gregori, G. & Puliafito, C. Simultaneous acquisition of sectional and fundus ophthalmic images with spectral-domain optical coherence tomography. Opt. Express. 13 (2), 444–452 (2005).
Iwase, A. et al. A new method of magnification correction for accurately measuring retinal vessel calibers from fundus photographs. Invest. Ophthalmol. Vis. Sci. 58 (3), 1858–1864 (2017).
Iwase, A. et al. Aging-associated changes of optical coherence tomography-measured ganglion cell-related retinal layer thickness and visual sensitivity in normal Japanese. Jpn J. Ophthalmol. 68 (2), 117–125 (2024).
Funding
Hitomi Saito: Topcon and Japan Society for the Promotion of Science (Project number: 20K18368). Atsuya Miki: Council for Science, Technology and Innovation (CSTI), Cross-ministerial Strategic Innovation Promotion Program (SIP) “Innovative AI Hospital System”. Christopher Kai Shun Leung: Topcon. Tae-Woo Kim: Topcon. Linda Zangwill: National Eye Institute EY027510, Topcon. Robert Weinreb: Topcon Corporation.
Author information
Authors and Affiliations
Contributions
TH and MAraie designed the study. TH, SU, HS, DT, KS, GT, AM, AI, TN, MAihara, KOM, TK, CL, LZ and RW collected the data. MAraie, AI, KOM, GT, AM, TH and TK verified the data. TH, SU, HS, and MAraie did manual measurements of parameters and the statistical analysis. TH and MAraie interpreted the data and wrote the first draft. All authors critically revised the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Tomomi Higashide: Heidelberg Engineering (Grants); Allergan Japan, Bayer, HOYA, Kowa, Nitto Medic, Novartis, Otsuka, Pfizer, Santen, Senju, Viatris (Honoraria); Heidelberg Engineering (Research Instruments)Sachiko Udagawa: CHUGAI PHARMACEUTICAL CO., LTD., RE Medical, Inc., CREWT Medical Systems, Inc., Carl Zeiss Meditec Co., Ltd. (Honoraria)Makoto Araie: Pfizer, Santen, Topcon, Senju, Aerie, Kowa (Consultants); Pfizer, Senju, Kowa (Honoraria); GSTK-DiscAnalysis (License)Hitomi Saito: Carl Zeiss Meditec, Topcon, Senju, Otsuka, Santen, Kowa, Novartis, Abbvie, Viatris (Honoraria)Daisuke Takemoto: noneKazuhisa Sugiyama: Santen, Otsuka, Senju, Kowa, Novartis, Viatris, Bayers, Inami Company (Honoraria)Goji Tomita: Senju, Viatris, Nitto Medic (Honoraria)Atsuya Miki: Alcon, Ellex, Kowa, Menicon, Nitten, Nitto Medic, Otsuka, Rohto, SEED, Senju, Viatris, Topcon (Honoraria); Santen (Consultant)Tsutomu Kikawa: Topcon (employee)Aiko Iwase: Topcon Corp (Patent); Carl Zeiss Meditec, CREWT Medical Systems, Heidelberg Engineering, Santen, Senju, Otsuka, Novartis (Honoraria); Santen (Consultant)Toru Nakazawa: Topcon Corp, Nidek, Senju (Grants); Topcon Corp, Nidek, Senju (Honoraria)Makoto Aihara: Santen, Alcon, Pfizer, Otsuka, HOYA, TOMEY, CREWT Medical systems, Carl Zeiss Meditec, Senju, Novartis, Kowa, Johnson & Johnson, Glaukos, Iridex, Canon (Honoraria); Santen, Alcon, Pfizer, Otsuka, Johnson & Johnson, TOMEY, CREWT medical systems, Senju, Novartis, Kowa, Wakamoto, Glaukos, Ono, Sato (Grants); Santen, Alcon, Kowa, Wakamoto, Glaukos, Astellas, Senju, Pfizer, Otsuka, HOYA, IRIDEX (Consultant); Santen, HOYA, Senju, Kowa (Advisory Board)Kyoko Ohno-Matsui: Santen, CooperVision (Consultant)Tae-Woo Kim: noneChristopher Kai Shun Leung: Carl Zeiss Meditec (Licenses); Topcon, Santen (Honoraria); Santen, Janssen (Advisory Board); Carl Zeiss Meditec, Heidelberg Engineering (Equipment loan)Linda M Zangwill: Heidelberg Engineering, Carl Zeiss Meditec (Grants); Carl Zeiss Meditec (License); Abbvie, Digital Diagnostics (Consultants); Topcon, Optovue, Heidelberg Engineering, Carl Zeiss Meditec (Research instruments)Robert N Weinreb: Abbvie, Aerie Pharmaceuticals, Alcon, Allergan, Amydis, Editas, Equinox, Eyenovia, Iantrek, Implandata, IOPtic, iStar Medical, Nicox, Santen, Topcon (Consultant); National Eye Institute, National Institute of Minority Health and Health Disparities (Research Support); Heidelberg Engineering, Topcon, Zeiss Meditec, Optovue, Centervue, Zilia; Patents (licensed by UCSD), Zeiss Meditec, Toromedes (Research Instruments).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Higashide, T., Udagawa, S., Araie, M. et al. Relationships between distance from the fovea to the disc and macular retinal layer thickness differ between normal and glaucomatous eyes. Sci Rep 15, 11554 (2025). https://doi.org/10.1038/s41598-025-96741-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96741-w