Abstract
Wilms Tumor (WT), a prevalent pediatric renal malignancy, exhibits marked heterogeneity and variable clinical outcomes. Epithelial-mesenchymal transition (EMT), a biological process enabling epithelial cells to acquire mesenchymal traits associated with enhanced migratory and invasive capacities, plays a crucial role in cancer progression. Protein Regulator of Cytokinesis 1 (PRC1) is a critical protein in cell division, whose overexpression is linked to poor prognosis in various cancers. This study investigates the role of PRC1 as a key prognostic factor in WT and explore the mechanism through comprehensive bioinformatic and experimental approaches. Through bulk RNA-seq data from the TARGET database, we identified PRC1 as significantly up-regulated in WT and associated with poor overall survival. Functional enrichment analyses (GO, KEGG, GSEA) demonstrated PRC1’s involvement in cell division, chromatin dynamics, and activation of oncogenic pathways including Wnt/β-catenin, PI3K/AKT/mTOR, and Hedgehog signaling. Immunological analysis showed that elevated PRC1 expression correlates with diminished immune cell activity, particularly in NK cells, suggesting potential immune evasion mechanisms. Single-cell RNA-seq analysis (GSE200256) confirmed PRC1’s elevated expression in anaplastic Wilms tumor (AWT) compared to favorable Wilms tumor (FWT), and highlighted its involvement in intercellular communication and metastasis via the EMT process. Genomic analyses identified copy number variations (CNVs) and downregulated PRC1-targeting microRNAs as drivers of its overexpression. In vitro, PRC1 knockdown in WIT-49 cells significantly impaired migratory capacity, invasive potential, EMT progression, and glycolytic metabolism. These findings collectively position PRC1 as a promising therapeutic target and prognostic biomarker in WT.
Similar content being viewed by others
Introduction
Nephroblastoma, also known as Wilms tumor (WT), is the most common renal tumor among infants and young children1. While modern multi-modal therapies have improved prognoses for most WT patients, a subset remains vulnerable to mortality or long-term treatment-related complications, including renal failure, pulmonary dysfunction, cardiotoxicity, infertility, and secondary malignancies1,2,3. These persistent risks underscore the critical need for novel therapeutic targets to enable individualized treatment in WT management.
Epithelial-mesenchymal transition (EMT), a dynamic cellular reprogramming process enabling epithelial cells to acquire mesenchymal phenotypes, plays crucial roles in both physiological and pathological processes such as embryogenesis, wound healing, fibrosis, and cancer metastasis4. EMT and mesenchymal-epithelial transition (MET) are crucial processes in kidney embryogenesis. In renal development, balanced EMT and mesenchymal-epithelial transition (MET) drive the differentiation of metanephric mesenchyme into functional nephron components5. WT arises from arrested differentiation of metanephric blastema, yielding triphasic tumors with epithelial, stromal, and blastemal elements1. EMT may have a dual role in WT: While EMT activation facilitates tumor invasion and metastasis through characteristic marker changes including decreased E-cadherin and increased N-cadherin/Vimentin6, it may also participate in the differentiation process of undifferentiated blastema into differentiated epithelium or mesenchyme in WT, thereby reducing its malignancy. This paradoxical role amplifies tumor heterogeneity and complicates therapeutic strategies.
Currently, the exact mechanism of EMT in WT is unclear, but several studies provide clues. For instance, WT often presents with activating mutations in β-catenin, a key factor in the Wnt signaling pathway and a major inducer of EMT7,8. Dysregulation of other EMT-related signaling pathways and transcription factors, such as Snail, Slug, Twist and Notch, have been observed in WT9,10,11. Interactions between epithelial and mesenchymal components in WT may also regulate EMT or MET by secreting factors or extracellular matrix11. In summary, these findings position EMT dysregulation as a critical yet underexplored role in WT progression.
This study aims to identify core EMT-related genes influencing WT prognosis and elucidate their potential functions and mechanisms. Through integrative bioinformatics analysis of TARGET database-based transcriptomic and clinical data, we identified Protein Regulator of Cytokinesis1 (PRC1) as a key EMT-associated prognostic biomarker, with elevated expression correlating with adverse outcomes. Single-cell RNA sequencing data from the GEO database (GSE200256) were integrated to analyze cell-type heterogeneity in WT tissue. The in vitro experiment of WIT-49 cells confirmed the pathogenic role of PRC1 in WT, nominating it as a promising target for WT diagnosis, therapy, and prognostication.
Materials and method
Data extraction
RNA-seq transcriptome data and clinical information for 126 WT and 6 normal renal samples were retrieved from the TARGET-WT project via UCSC Xena12. Expression values were log2(FPKM + 1)-transformed and annotated using official gene symbols. Single-cell RNA-seq data from GSE200256 was retrieved from the Gene Expression Omnibus (GEO). As this study utilized de-identified public data, institutional review board (IRB) approval, institutional consent, and informed consent were not required. Additionally, we sequenced the transcriptome of WIT-49 cells treated with shRNA against PRC1 and control plasmids through Sangon Biotech (Shanghai, China). No institutional review board (IRB) approval was required for this secondary analysis of de-identified public data.
Screening for differentially expressed EMT-Related genes (DEEGs)
Differentially expressed genes (DEGs) between WT and normal renal tissues were identified using the ‘limma’ package (|log2FC| > 2, FDR < 0.05) and visualized via ‘pheatmap’ and ‘ggplot2’ packages in R13. EMT-related genes were obtained from the HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION gene set from the Molecular Signatures Database (MSigDB)14. Pearson correlation analysis (|r| > 0.8, p < 0.05; ‘corrplot’ package visualization) identified prognosis-associated EMT-related DEGs, with PRC1 expression validation using GSE66405.
Prognostic value analysis of PRC1
TARGET-derived PRC1 expression data and clinical data were stratified by median and quartile expression. Kaplan-Meier analysis (‘survival/survminer’ packages) assessed survival differences (p < 0.05)15. ROC (Receiver Operating Characteristic) curve was drawn (‘survivalROC’ package), with AUCs (Area Under the Curve) calculated for 1-, 3-, and 5-year survival16. Univariate and multivariate Cox regression analyses identified independent prognostic factors, visualized in a forest plot (‘ggforest’ package). A nomogram for prognosis prediction was generated (‘rms’ package)17.
Functional analysis of PRC1 in WT
DEGs between high and low PRC1 expression groups were identified by ‘limma’ and Wilcoxon tests (|log2FC| > 0.5 and FDR < 0.05). Functional enrichment analyses including GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) were conducted for the DEGs (‘ClusterProfiler’ package)18,19. Gene set enrichment analysis (GSEA) was performed using ‘h.all.v2024.1.Hs.symbols.gmt’ gene sets from MSigDB with 1000 permutations (FDR < 0.05)20.
Immunological characteristic analysis
ESTIMATE (Estimation of STromal and Immune cells in MAlignant Tumours using Expression data) is an algorithm that uses gene expression signatures to infer the fraction of stromal and immune cells in tumor samples21. CIBERSORT (Cell Type Identification by Estimating Relative Subsets of RNA Transcripts) is a method for characterizing cell composition of complex tissues from their gene signature matrix22. We applied the ESTIMATE method to calculate the tumor microenvironment scores, and used CIBERSORT with the LM22 signature matrix (22 immune cell types) to quantify the proportions of tumor-infiltrating immune cells in each WT sample. In addition, we performed GSEA enrichment analysis in the c7 collection (immunologic signature gene sets) of MSigDB.
Single-cell RNA sequencing analysis
The expression files and clinical information of GSE200256 (10x Genomics) in the GEO database were obtained for single-cell RNA sequencing analysis. We first filtered the single-cell RNA-seq raw data (‘Seurat’ package), setting each feature to be expressed in at least 3 cells and each cell to express at least 300 features. The percentages of mitochondria and rRNA were calculated by the ‘PercentageFeatureSet’ function and ensured that each cell expressed more than 300 features and less than 10,000 features, while keeping the mitochondrial and ribosomal features expressed at less than 20%.
The qualified data were then normalized and scaled to perform principal component analysis (PCA) for dimensionality reduction23. We removed batch effects by ‘harmony’ method and visualize it using the t-distributed Stochastic Neighbor Embedding (t-SNE) method24,25. The cells were finally clustered using the ‘FindNeighbors’ and ‘FindClusters’ functions to obtain cell subgroups. The Uniform Manifold Approximation and Projection (UMAP) and t-SNE algorithm were used to visualize the cell clusters26.
The ‘SingleR’ package, CellMarker database, and ToppGene database were used for cell type annotation27,28. Besides, functional enrichment of ‘HALLMARK’ gene set was performed on cancer cells with high/low PRC1 expression (‘irGSEA’ and ‘GSVA’ packages). Intercellular communication and pathways were analyzed by ‘CellChat’ package.
Mechanism of upregulation of PRC1 expression in WT
We used cBioPortal database to analyze PRC1 copy number variation (CNV) and DNA methylation levels in WT29. The TargetScan database was used to predict microRNAs that can bind to the 3’UTR region of the PRC1 gene30. Concurrently, the microRNA sequencing data was downloaded from the TARGET-WT database, and we performed differential analysis to detect differentially expressed microRNAs between tumor and normal tissues (‘limma’ package). Intersection analysis via Venn diagrams revealed miRNAs that both target PRC1 and exhibit differential expression in WT. On this basis, we analyzed the correlation between these microRNAs and PRC1 expression using the Pearson method. We selected the miRNA most likely to target PRC1 and verified it by RT-qPCR and WB experiments in cells. The miRNA mimic, inhibitor, and corresponding negative controls (mimic-NC and inhibitor-NC) were purchased from RiboBio (Guangdong, China). The sequences of these oligonucleotides are shown in Supplementary Table 3.
In vitro cell validation experiments
Cell line and cell culture
HEK293T (Chinese Academy of Sciences Cell Bank), SK-NEP-1 (Chinese Academy of Sciences Cell Bank), and WIT-49 cells (The Affiliated Hospital of Qingdao University) were cultured in DMEM supplemented with 10% fetal bovine serum (FBS; Gibco) and 100 U/ml penicillin-streptomycin at 37 °C under 5% CO₂. All cell lines were confirmed mycoplasma-free.
Plasmids construction and cell transfection
Two PRC1-targeting shRNAs (sequences in Supplementary Table 1; Chen et al., 2016a; Zhan et al., 2017) were inserted into the PLKO.1 vector, with original vector as negative control. WIT-49 cells at 70–80% confluence in 6-well plates were transfected using Lipo8000 Transfection Reagent (C0533, Beyotime).
Reverse transcription quantitative PCR (RT-qPCR)
Total RNA from WIT-49 and HEK293 cells was extracted (Fastagen, 220011) and reverse-transcribed using the Evo M-MLV RT Kit (AG11705, AG Accurate Biology). SYBR Green Pro Taq HS Premix II (AG11702, AG Accurate Biology) was used for RT-qPCR with primers listed in Supplementary Table 1. Relative gene expression was calculated via the 2^(-ΔΔCT) method.
Cell viability assay
Transfected WIT-49 cells (3 × 103 cells/well in 96-well plates) were incubated with CCK-8 reagent (GK10001, GlpBio) at 0, 24, 48, and 72 h post-seeding. Absorbance at 450 nm was measured 2 h after reagent addition.
Cell scratch assay and transwell assay
Scratch assay: Confluent monolayers (cells at 95–100% density) in 6-well plates were scratched with sterile pipette tips. Wound closure was monitored at 0, 24, and 48 h using microscopy.
Transwell assay: Cells (2 × 105/ml in serum-free DMEM) were seeded into 8-µm Transwell chambers (Millipore). After 16 h migration toward 20% FBS-containing medium, cells were fixed with methanol, stained with 0.1% crystal violet, and quantified using ImageJ.
Western blotting
Cell lysates prepared with RIPA buffer (P0013J, Beyotime) containing 1% PMSF (ST507, Beyotime) were separated on 4–12% SDS-PAGE gels and transferred to PVDF membranes (HVLP2932A, Millipore). Membranes were blocked with 5% skim milk (P0216, Beyotime), incubated with primary antibodies (Proteintech) overnight at 4 °C, followed by HRP-conjugated secondary antibodies (Proteintech). Signals were detected using ECL reagent (P0018S, Beyotime).
Glycolysis analysis
Glucose kit (glucose oxidase method) and Lactic Acid assay kit were purchased from Nanjing Jiancheng Bioenginieering Institute. Each group was repeated three times. Protein concentrations were determined via enhanced BCA protein assay kit (P0010S, Beyotime).
Statistical analysis
The statistical analysis and plotting of the bioinformatics part of this paper were completed with the assistance of R (version 4.2.1) and SangerBox 3.0 online platform31. The statistical analysis and plotting of the experimental part of this paper were completed in GraphPad Prism 10.2.3 software. A p value or adjusted p value less than 0.05 was considered statistically significant.
Results
Identification of DEEGs related to the prognosis of patients with WT
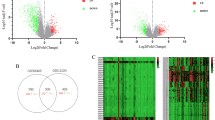
Through the bioinformatic method of differential expression analysis, we identified 1125 differentially expressed genes (DEGs) between WT and normal tissues. These DEGs were visualized via a heatmap (Supplementary Fig.S1) and a volcano plot (Fig. 1A). Subsequently, Pearson’s correlation analysis was performed between the 1,125 DEGs and 200 EMT-related genes, with partial results visualized in Supplementary Fig.S2. 84 differentially expressed EMT-related genes (DEEGs) were screened out by |r|>0.8 and p < 0.05, among which 27 up-regulated genes in WT were identified as candidate genes (Fig. 1B). Survival analysis and univariate Cox regression analysis revealed that only PRC1 and FBN2 among the 27 genes were significantly associated with WT prognosis. While upregulation of FBN2 was correlated with increased overall survival in WT patients, indicating its role as a protective factor (Supplementary Fig.S3). Therefore, we selected PRC1 as the focus for further studies due to its potential pathogenicity.
PRC1 is an independent adverse prognostic factor in WT
The differential expression of PRC1 mRNA in WT tissues is shown in the box plot (Fig. 1C), demonstrating significant upregulation of PRC1 in WT compared to normal tissues (p < 0.05). External validation using the GSE66405 dataset from the GEO database further confirmed elevated PRC1 expression in WT (Supplementary Fig.S4). Kaplan-Meier survival curves were generated to evaluate the association between PRC1 expression levels and overall survival (OS), with quartiles and median values used as cut-offs to define high/low expression groups (Fig. 1D; Supplementary Fig.S5). Receiver operating characteristic (ROC) curve analysis revealed moderate predictive accuracy of PRC1 for 1-, 3-, and 5-year survival, with corresponding AUC values of 0.694, 0.701, and 0.660, respectively (Fig. 1E; Supplementary Fig.S5).
The Wilcoxon signed-rank test and logistic regression showed that there was no significance between the expression level of PRC1 and clinical stage (Fig. 1F). Univariate Cox hazard regression analysis presented that gender, stage and PRC1 expression were significantly related to OS (Supplementary Table 2). Subsequent multivariate Cox regression analysis confirmed these three factors as independent prognostic indicators for WT (Fig. 1G). A nomogram integrating gender, stage, and PRC1 expression was developed to visualize predicted 1-, 3-, and 5-year OS probabilities (Fig. 1H).
To investigate the pathogenicity and mechanism of PRC1 in WT, we performed Pearson correlation analysis on the mRNA levels of PRC1 and WT predisposition and driver genes1 (Supplementary Fig.S11). Results showed that PRC1 exhibited moderate correlations with kidney development-related genes WT1 (r = 0.36) and SIX2 (r = 0.50). Notably, PRC1 was strongly associated with WT driver genes related to chromatin biology or epigenetic modifiers (KDM3B, CHD4, BCOR, ARID1A), related to microRNA processing and RNA metabolism (NONO, DROSHA, XPO5, MAP3K4), and related to genome maintenance (BUB1B, BRCA2, BLM, PALB2) (r > 0.6).
PRC1 is an independent adverse prognostic factor in WT. (A) Volcano map of DEGs between normal and tumor samples in the TARGET-WT database (|log2FC| ≥ 2, FDR < 0.05). (B) The Venn diagram was obtained by taking the intersection of 200 EMT-related genes, 84 DEEGs, and 487 DEGs up-regulated in the WT group. (C) The PRC1 mRNA expression by boxplot in WT from TARGET (N = 6; T = 126). (D) K–M survival analysis of PRC1 in WT from TARGET. (E) ROC curves and 1-, 3-, and 5-year AUCs of PRC1 in WT from TARGET. (F) Associations between PRC1 mRNA expression and clinical stage in WT from TARGET. (G) Multivariate Cox hazard regression analysis of three clinical variables (gender, stage, PRC1) in in WT from TARGET. (H) Nomogram based on three clinical variables (gender, stage, PRC1) in WT from TARGET.
Role and downstream mechanism of PRC1 in WT
Differential expression analysis between high- and low- PRC1 expression groups initially identified 40 DEGs under the Wilcoxon test with |log2FC|>1 (Fig. 2A–B). Given the limited gene count, we relaxed the threshold to |log2FC| > 0.5, yielding 1,513 DEGs for downstream analyses (Supplementary Fig.S6). GO functional enrichment analysis results showed (Fig. 2C): In the BP (biological process) category, DEGs were mainly involved in biological processes associated with cell division such as nuclear division, mitotic cell cycle phase transition, chromosome segregation. In the CC (cellular component) category, DEGs were mainly located in cellular components such as chromosome, spindle and ribosome. In the MF (molecular function) category, DEGs mainly had molecular functions such as DNA − binding transcription factor binding, tubulin binding and structural constituent of ribosome. KEGG pathway enrichment analysis results showed that DEGs were mainly involved in metabolic pathways such as cell cycle, DNA replication and ribosome function (Fig. 2D). Taken together, these results indicated that PRC1 may promote the rapid growth and proliferation of Wilms tumor by affecting cell division, chromatin remodeling, and ribosome function.
In order to further explore the mechanism and molecular pathway of PRC1 in WT, we performed GSEA based on ‘h.all.v2024.1.Hs.symbols.gmt’ gene set from the MSigDB. We found that 11 gene sets were significantly enriched in the high-PRC1 expression group with NES > 1.5 and FDR q values < 0.05, and 8 of them were visualized (Fig. 2E). Among these signaling pathway, the Hedgehog (Hh) signaling is necessary for normal embryonic development, and aberrant Hh signaling leads to the development and progression of many types of cancer, including basal cell carcinomas (BCCs) and medulloblastoma32. The Wnt/β-catenin pathway also plays critical roles in embryonic development and adult tissue homeostasis, and its deregulation often leads to various serious diseases, including cancer and non-cancer diseases33. The Wnt/β-catenin and the PI3K/AKT/mTOR signaling pathways are both related to EMT process and carcinogenesis34.
To further verify the downstream pathway of PRC1, we treated WIT-49 cells with shRNA targeting PRC1, extracted RNA and sent it to the company for transcriptome sequencing. We then performed GO, KEGG, and GSVA enrichment analysis on the sequencing results (Supplementary Fig.S12). Comparing the enrichment results of the TAEGET-WT database data with the sequencing data, we found that the functions and pathways related to cell division and cell cycle were highly consistent. At the same time, the PIK3/AKT signaling pathway, Hedgehog signaling pathway, and Wnt/β-catenin signaling pathway were downregulated in the shPRC1 group, which is also consistent with the above database analysis results.
Role and downstream mechanism of PRC1 in WT. (A, B) Volcano plot and heat map illustrating 1513 DEGs under |log2FC|>0.05 threshold and 40 DEGs under |log2FC|>1 between high and low PRC1 expression groups. (C) GO functional enrichment analysis based on the PRC1 mRNA expression in WT from TARGET. (D) KEGG pathway enrichment analysis based on the PRC1 mRNA expression in WT from TARGET. (E) Gene Set Enrichment Analysis (GSEA) using ‘h.all.v2024.1.Hs.symbols.gmt’ gene set in MSigDB based on the PRC1 mRNA expression in WT from TARGET.
Immunological characteristic analysis
To investigate PRC1’s association with tumor microenvironment (TME) and tumor-infiltrating immune cells (TIICs), we analyzed 126 WT samples using ESTIMATE and CIBERSORT algorithm. ESTIMATE analysis revealed high tumor purity across all WT samples, while the proportion of non-tumor cells was low. The high-PRC1 expression group had lower both stromal and immune scores than the low-PRC1 expression group with a significant statistical difference (p < 0.05) (Fig. 3B). CIBERSORT immune deconvolution identified activated NK cells as the only TIIC subset showing significant differences between high- and low- PRC1 expression groups, with marked depletion in high-PRC1 expression group (Fig. 3A). In addition, spearman correlation analysis further confirmed a strong negative association between PRC1 expression and activated NK cell infiltration (r = -0.63, p < 0.05; Fig. 3C–D).
Then, we performed GSEA based on ‘c7.immunesigdb.v2024.1.Hs.symbols.gmt’ gene set from the MSigDB (Fig. 3E). It showed that Elevated PRC1 expression patterns resembled gene upregulation induced by absence of IL-2/IL-15 stimulation in unactivited NK cells. Moreover, IL-2 stimulation exhibited stronger activation effects compared to IL-15. IL-2 and IL-15 are lymphocyte growth factors, which promote the development and proliferation of NK cells and enhance the cytotoxicity and secretory function of NK cells35,36. Therefore, PRC1 may preferentially accumulate in quiescent NK cells, where its overexpression could impair NK cell activation by antagonizing cytokine signaling. Mechanistically, IL-2/IL-15 stimulation might restore NK cell function through PRC1 suppression.
Immunological characteristic analysis based on PRC1 expression in WT. (A) CIBERSORT analysis comparing 22 types of immune cell types between high and low PRC1 expression groups in WT. (B) Tumor Microenvironment (TME) Scores by ESTIMATE analysis between high and low PRC1 expression groups in WT. (C) Correlation analysis using Spearman method between PRC1 mRNA levels and 22 types of immune cells. (D) Scatter plot depicting the negative correlation between PRC1 expression and the number of activated NK cells (r = -0.63, p < 0.05). (E) Gene Set Enrichment Analysis (GSEA) using the ‘c7.immunesigdb.v2024.1.Hs.symbols.gmt’ gene set to analyze PRC1 expression in NK cells.
Single-cell RNA sequencing analysis
Single-cell RNA sequencing data from the GEO dataset GSE200256 (10x Genomics) were analyzed using R software. We selected two age- and gender-matched samples: GSM6025615 (anaplastic Wilms tumor, AWT) and GSM6025622 (favorable Wilms tumor, FWT). Quality control was performed with the ‘Seurat’ package to filter out low-quality droplets (doublets, dead cells, and empty droplets) (Fig. 4A–B), followed by normalization and identification of the top 2,000 highly variable genes (Fig. 4C). After z-score standardization and principal component analysis (PCA), batch effects were removed by harmony method, with t-SNE visualization (Fig. 4D). Clustering analysis (top 20 principal components, resolution = 1.0) identified 20 distinct cell clusters, which were visualized via UMAP and t-SNE (Fig. 4E–G).
Cell type annotation using singleR, CellMarker, and ToppGene databases revealed predominant PRC1 expression in cancer cells (Fig. 5A–D). Specific genes expression in every cell type was shown in a heatmap (Fig. 5E). The expression of PRC1 of cancer cells in AWT was higher than that in FWT, while NK cells showed no intergroup differences (Fig. 5F–G). Stratifying cancer cells by median PRC1 expression level, the functional enrichment of hallmark gene sets (‘irGSEA’ and ‘GSVA’ packages) revealed that the high-PRC1 expression group was enriched in the Wnt/β-catenin, TGF-β, PI3K/AKT/mTOR, Notch and Hedgehog signaling (Fig. 5H). This is consistent with the previous GSEA analysis of bulk RNA-seq, suggesting that PRC1 may promote WT malignant progression by activating these pathways. Besides, potential PRC1 overexpression mechanisms included E2F transcription factor activation, which may also increase tumor blood supply by stimulating angiogenesis. GO and KEGG enrichment analysis of cancer cells showed that the differential expression of PRC1 may be closely related to ribosome, GTPase regulator activity, cadherin binding, ubiquitin mediated proteolysis, among others (Fig. 5I–J).
Furthermore, we explored intercellular communication networks according to specific pathways and ligand receptors by ‘CellChatDB.human’ Database (Fig. 6A). The heatmap and cell communication map showed (Fig. 6B–C): In the FWT group, cell communication occurred more frequently in NK cells, fibroblastic cells and B cells. Compared with the FWT group, the overall level of intercellular communication in the AWT group significantly increased. We speculate that anaplastic nephroblastoma may activate the immune system, resulting in increased communication between immune cells. At the same time, the mobilization of endothelial cells in AWT may promote angiogenesis and vascular repair processes in tumor. The activation of mesenchymal cells may play a key role in promoting the proliferation and migration of tumor cells. In addition, we found that the IGF (insulin-like growth factor) signaling pathway was increased in both groups (Fig. 6D). It was more pronounced in NK cells of FWT, while it was more complex in AWT. Growth factors commonly involved in the PI3K/AKT/mTOR pathway include PDGF, IGF, VEGF, etc. These growth factors activate this signaling pathway by binding to their corresponding receptors37,38. Therefore, IGF may on the one hand enhance the function of NK cells in FWT to inhibit tumor progression, and on the other hand promote the PI3K/AKT/mTOR pathway of cancer cells in AWT to promote tumor development.
Single-cell sequencing analysis workflow of WT samples in GSE200256. (A, B) Quality control results showing filtering of unqualified droplets including doublets, dead cells, and empty droplets. (C) The variance plot shows the variation of genes expression in all cells of WT to identification of highly variable genes (HVGs). The red dots represent the top 2000 HVGs. (D) Visualization of batch effect removal between the AWT and FWT groups using the Harmony method, shown in a t-SNE map. (E) Cell clustering trees at different resolutions after principal component analysis (PCA). (F, G) 20 independent cell clusters (resolution = 1.0) were visualized using the Uniform Manifold Approximation and Projection (UMAP) and t-distributed Stochastic Neighbor Embedding (t-SNE) algorithm.
Annotation of the different cell clusters and functional enrichment analysis of Cancer cells. (A) Bubble plots showing mRNA expression levels of marker genes for each cell type. (B, C) 11 cell types were annotated based on marker gene expression and information from other databases. The annotation results were visualized using t-SNE and UMAP plots. (D) Distribution of PRC1 expression in different cell types by UMAP plots. (E) Heatmap displaying specific gene expression in each cell type. (F, G) Comparison of PRC1 expression levels in cancer cells and NK cells between AWT and FWT groups. (H) Functional enrichment analysis of hallmark gene sets was performed on cancer cells with high and low PRC1 expression groups. (I, J) GO and KEGG enrichment analysis was performed on cancer cells with high and low PRC1 expression groups.
Intercellular communication analysis of AWT and FWT. (A) The proportion of each category in the CellChatDB.human database. (B, C) Heatmap and communication map illustrating the frequency of intercellular interactions, including numbers of interactions and interaction weights. (D) Analysis of the IGF signaling pathway through ligand-receptor interactions in AWT and FWT groups.
Mechanism of upregulation of PRC1 expression in WT
Genomic structural variations that result in a change in the number of copies of a DNA sequence (DNA dosage) are often called copy number variations (CNVs)39. Specifically, it is a duplication or deletion event that affects a large number of base pairs40. CNV is one of the important reasons for abnormal gene expression in tumor41, so we analyzed the changes of PRC1 at genome level. Through the analysis of cBioPortal database, it was found that PRC1 genomic mutations were present in 7% of WT samples, and amplification was present in half of samples with high mRNA levels (Fig. 7A). PRC1 mRNA expression levels in gene amplification and gain samples were significantly higher than diploid and shallow deletion samples (Fig. 7B). These indicate that CNV of PRC1 may play an important role in the occurrence and development of WT by regulating its mRNA expression level.
Epigenetics is the study of heritable traits or a stable change of cell function that occur without altering the DNA sequence42. Epigenetic factors can also lead to cancer43. DNA methylation is one of the most common epigenetic modifications and it frequently occurs in repeated sequences to suppress the expression and mobility of ‘transposable elements’44. We analyzed the correlation between methylation level and PRC1 expression through cBioPortal database, and the results showed that there was no significant correlation (Spearman: r = -0.31; Pearson: r = -0.23) (Fig. 7C).
Another important epigenetic mechanism is microRNA. MicroRNAs (miRNAs) are endogenous non-coding RNA molecules that can bind to complementary target mRNAs, resulting in mRNA translational inhibition or degradation45. MicroRNAs play an important role in cell differentiation, proliferation and survival, and microRNA therapeutics is a promising area for tumors and other diseases46. Using TargetScan database, we identified 356 miRNAs potentially targeting PRC1. The sequencing data of 1,881 Wilms tumor miRNAs were downloaded from the TARGET-WT database. Next, differential analysis of normal tissues and tumor tissues was performed to obtain differential miRNAs, and 49 miRNAs whose expression was significantly downregulated in the WT group were screened out by FDR < 0,05, log2FC < −0.5 (Fig. 7D). These 49 differential miRNAs were intersected with the above 356 predicted PRC1-targeting miRNAs. Intersection analysis yielded 12 candidate miRNAs whose reduced expression may drive PRC1 overexpression in WT (Fig. 7E). Among these, 9 miRNAs exhibited comparatively strong negative correlations (r < -0.3) with PRC1 levels (Supplementary Fig.S10, Fig. 7F(a)). It was verified that the expression of these 9 miRNAs in the WT group was significantly lower than that in the normal tissue group (Fig. 7F(b)). By consulting the literature, we found that miR-143-3p has been verified in liposarcoma to target PRC1 to inhibit its expression47. We verified the interaction between the two again in WIT-49 cells. miR-143-3p significantly reduced both PRC1 mRNA and protein levels (Fig. 7G), indicating that its binding to the PRC1 3’UTR promotes mRNA degradation while simultaneously inhibiting translation through canonical miRNA-mediated mechanisms, thereby suppressing PRC1 expression.
Mechanism of upregulation of PRC1 expression in WT. (A) Analysis of PRC1 copy number variations (CNVs) in WT using the cBioPortal database. (B) Comparison of PRC1 mRNA expression levels in WT samples with shallow deletion, diploid, gain, and amplification using the cBioPortal database. (C) Correlation analysis between PRC1 expression and DNA methylation levels using the cBioPortal database (Spearman: r = -0.31; Pearson: r = -0.23; both p < 0.05). (D) Volcano plot showing differentially expressed miRNAs between normal tissues and Wilms tumor tissues in TARGET database (FDR < 0.05, |log2FC| < 0.5). (E) Venn plot showing intersection of the 49 down-regulated miRNAs in WT with 356 PRC1-binding miRNAs predicted by TargetScan database, yielding 12 candidate miRNAs that may contribute to PRC1 upregulation in WT. (F) (a) Violin plots showing the comparison of mRNA expression levels of the selected 9 miRNAs in WT and normal tissues in the TARGET database; (b) Pearson correlation analysis between hsa-miR-143-3p and PRC1 expression in WT. (G) (a, b) The protein expression levels of PRC1 detected by western blotting (p < 0.05); (c) The mRNA expression levels of PRC1 detected by RT-qPCR (p > 0.05).
In vitro cell validation experiments
RT-qPCR analysis revealed significantly elevated PRC1 mRNA levels in WIT-49 and SK-NEP-1 cells compared to HEK293T cells (Fig. 8A). We selected the WIT-49 cell line to represent WT for subsequent experiments. Transfection with shPRC1-1 or shPRC1-2 plasmids (vs. pLKO.1-puro control) achieved high efficiency (> 80% fluorescent cells at 48 h), with RT-qPCR confirming significant PRC1 knockdown in both shRNA groups (Fig. 8B–C). Next, a series of cell function experiments were performed to explore the changes after knockdown of PRC1. Cell scratch assay found that the cell migration ability of shPRC1-1 and shPRC1-2 groups was significantly lower than that of the control group after 24 h and 48 h (Fig. 8D–F, supplementary Fig.S7). Transwell assay found that the number of cells that passed through the transwell chambers in the shPRC1-1 and shPRC1-2 groups was significantly less than that in the control group, indicating that knockdown of PRC1 reduced the cell invasion ability (Fig. 8G–H, supplementary Fig.S8). Cell viability assay found that the OD at 450 nm on the 3rd and 4th days after adding CCK-8 reagent in the shPRC1-1 and shPRC1-2 groups was significantly lower than that in the control group, indicating that cell proliferation ability decreased after downregulating PRC1 (Fig. 8I).
In addition, we examined the effect of PRC1 knockdown on the expression of EMT-related core proteins via western blotting. Compared with the control group, the expression of N-cadherin protein was decreased and the expression of E-cadherin protein was increased in the shPRC1-1 and shPRC1-2 groups (Fig. 8J–L, supplementary Fig.S9). N-cadherin and E-cadherin are members of the classical family of cadherins. E-cadherin is the component of the adherens junctions and the principal organizer of the epithelial phenotype48. Loss of E-cadherin expression has been shown to be important in EMT and increased migratory and invasive behavior of tumor cells49. N-cadherin mainly exists in nerve tissue, muscle and fibroblasts, while it is expressed at low levels in other normal cells. However, N-cadherin was found to be aberrantly expressed in various human malignancies recently50. The hallmark of EMT is the upregulation of N-cadherin followed by the downregulation of E-cadherin, and EMT process is crucial in tumorigenesis where the EMT program enhances metastasis, chemoresistance, and tumor stemness51. Therefore, we inferred that PRC1 may promote the occurrence and development of WT by facilitating the EMT process.
Besides, we also measured the metabolic levels of glucose and lactic acid. Compared with the control group, the glucose and lactic acid levels in the shPRC1-1 and shPRC1-2 groups decreased (Fig. 8M–N). To ensure the reliability of the results, we also measured the protein level using metabolic samples, and the results showed that there was no significant difference in the protein sample concentrations among the three groups (Fig. 8O–P). Therefore, the differences in the metabolic levels of glucose and lactic acid were significant. PRC1 may promote the growth of WT by promoting the glycolytic pathway of tumor cells.
In vitro experiments to explore the role of PRC1 in WIT-49 cells. (A) The relative mRNA expression levels of PRC1 in HEK293T, WIT-49, and SK-NEP-1 cell lines detected by RT-qPCR (p < 0.05). (B) Verification of PRC1 knockdown efficiency in WIT-49 cells transfected with shPRC1-1 and shPRC1-2 plasmids compared to the control group by RT-qPCR (p < 0.05). (C) Fluorescence photos under a microscope to obtain the transfection efficiency of WIT-49 cells. (D, E, F) Assessment of cell migration ability: measurement of the migration area of each group at 24 and 48 h after scratching (p < 0.05). (G, H) Transwell assay indicating cell invasion ability (p < 0.05). (I) Cell viability measured by CCK-8 assay, revealing lower OD values at 450 nm in shPRC1-1 and shPRC1-2 groups on days 3 and 4 compared to the control group (p < 0.05). (J, K, L) The expression levels of EMT-related core proteins detected by western blotting (p < 0.05). (M) Measurement of glucose levels in WIT-49 cells. (N) Measurement of lactic acid levels in WIT-49 cells. (O) BSA protein standard curve: Y = 1.107*X + 0.1883, R2 = 0.9914. (P) Comparison of protein sample concentrations in Control, shPRC1-1, and shPRC1-2 groups.
Discussion
Protein Regulator of Cytokinesis 1 (PRC1) is a microtubule-associated protein critical in cell division by aiding the final stages of cytokinesis. Disruption in PRC1 regulation leads to cytokinesis errors, resulting in chromosomal instability (CIN) that fosters tumor diversity and drives cancer progression52. PRC1 overexpression has been linked with various cancers, where it frequently contributes to poor prognosis53. Studies show that PRC1 promotes oncogenic processes by enhancing cell proliferation, migration, and survival, often involving key signaling pathways such as Wnt/β-catenin, PI3K/AKT, and the EMT pathways53,54. Elevated PRC1 levels also correlate with immune checkpoint activation and contribute to the suppressive immune microenvironment in liver hepatocellular carcinoma (LIHC)55. These studies suggest that PRC1 is a possible target for novel cancer therapies in multiple types of cancer. However, there is a lack of research on the role and mechanism of PRC1 in Wilms tumor. In our study, we identified and characterized the role of PRC1 as a crucial prognostic factor in Wilms Tumor (WT). Through mining of bulk RNA-seq data in the TARGET database, we found 14 EMT-related differential genes that were significantly up-regulated in WT. Among these, PRC1 was selected for further investigation due to its significant upregulation in WT tissues and its correlation with poor overall survival.
Our findings revealed that PRC1 is an independent adverse prognostic factor in WT, confirmed through Kaplan-Meier survival curves and multivariate Cox regression analysis. The high expression of PRC1 was associated with lower survival probabilities, indicating its potential as a therapeutic target. It is worth noting that PRC1 is co-expressed with many confirmed WT driver genes such as WT1 and SIX2, which further illustrates that PRC1 promotes the pathogenesis and development of WT and also suggests the possible mechanism behind it.
Functionally, Enrichment analysis of public database and sequencing data added a layer of insight by identifying key signaling pathways, including the Hedgehog and Wnt/β-catenin pathways, which are well-known for their roles in embryonic development and cancer progression32,33. The deregulation of these pathways in high PRC1-expressing tumors may indicate that PRC1 influences the tumorigenic process by altering developmental signaling networks that become aberrant in cancer. Moreover, the connection of PRC1 with the EMT process via the Wnt/β-catenin and PI3K/AKT/mTOR pathways suggests that PRC1 might facilitates metastasis by promoting EMT, a critical step in cancer progression. In vivo and in vitro experiments have demonstrated that the PI3K/AKT pathway plays a key role in the growth and metastasis of Wilms tumor56. WNT/β-catenin signaling is essential for the initiation of nephrogenesis and MET process, and mutations in WT1 and CTNNB1 (encoding β-catenin) detected in 15-20% of WTs lead to sustained activation of the Wnt/β-catenin pathway57.
The immunological analysis revealed that high PRC1 expression correlates with lower immune and stromal scores, alongside a significant reduction in activated NK cells. This observation raises important questions regarding the immune evasion capabilities of WT, particularly in relation to PRC1’s role in modulating the tumor microenvironment (TME). The inverse relationship between PRC1 expression and NK cell activation suggests that PRC1 may inhibit the immune response, potentially allowing tumor cells to escape immune surveillance. These findings also make us pay more attention to the analysis of NK cells in the following single-cell sequencing data mining.
Single-cell sequencing analysis of GSE200256 provides valuable insights into the differential roles of PRC1 in anaplastic Wilms tumor (AWT) and favorable Wilms tumor (FWT). The higher expression of PRC1 in AWT cancer cells suggests a potential link between PRC1 and the aggressive behavior of this tumor subtype. Functional enrichment results indicate that PRC1 high-expression groups in cancer cells are significantly associated with pathways such as Wnt/β-catenin, PI3K/AKT/mTOR, and Hedgehog signaling, all of which are implicated in promoting tumor growth and metastasis. These findings echo our previous bulk RNA-seq analysis, reinforcing the notion that PRC1 drives tumor progression by activating key oncogenic pathways.
An intriguing aspect of the study is the role of PRC1 in modulating intercellular communication. In AWT, we observed enhanced communication between immune, endothelial, and mesenchymal cells, which may reflect the tumor’s ability to mobilize its microenvironment for angiogenesis and immune system activation. The increased involvement of mesenchymal cells in AWT is particularly noteworthy, as it suggests that PRC1 might facilitate tumor proliferation and migration through stromal interactions and promoting EMT process.
Genomic studies have found that PRC1 upregulation in Wilms tumors may be driven by copy number variation (CNV), especially gene amplification, which significantly increases PRC1 expression. DNA methylation did not significantly correlate with PRC1 levels, suggesting that it plays a minor role in regulation. However, we found 9 miRNAs that were down-regulated in WT. Among them, we conducted cell experiments to verify whether miR-143-3p targets PRC1. The downregulation of these miRNAs may be one of the reasons for the overexpression of PRC1 through loss of repression in WT. These findings suggest that both CNV amplification and miRNAs downregulation are key mechanisms driving PRC1 upregulation in WT, providing potential targets for therapeutic intervention.
In vitro experiments confirmed the role of PRC1 in the progression of WT, particularly through its influence on the EMT process. PRC1 knockdown significantly impaired cell migration, invasion, and proliferation, suggesting a critical role in promoting tumor invasiveness. Meanwhile, the changes in EMT markers observed after knockdown of PRC1—upregulation of E-cadherin and downregulation of N-cadherin—highlight the involvement of PRC1 in promoting the EMT process, which may enhance metastasis, chemoresistance and tumor stemness51. Moreover, the reduction in glucose and lactic acid levels upon PRC1 knockdown points to its role in enhancing the glycolytic pathway, a hallmark of tumor metabolism. This metabolic reprogramming could further support tumor growth and survival. These results suggest that PRC1 not only drives tumor progression through EMT but also by promoting glycolysis, offering potential therapeutic targets for WT treatment.
Collectively, we found that high PRC1 expression is an independent risk factor for poor prognosis in Wilms tumor (WT), exerting its effects by affecting cell division, chromatin dynamics, and multiple oncogenic signaling pathways. Although our findings provide new insights into the role of PRC1 in tumor proliferation, immune evasion, and metastasis, there are still some limitations that need to be addressed. First, the specific mechanism by which PRC1 affects the behavior of immune cells (especially NK cells) remains unclear and warrants further study. In addition, although we demonstrated the role of PRC1 in promoting EMT and glycolysis using simple in vitro experiments, more in vitro experiments are needed to explore the specific signaling pathways affected by PRC1, as well as in vivo studies needed to confirm these effects in a physiological context. Future studies should focus on elucidating the therapeutic potential of targeting PRC1, especially in combination with immune-based therapies, thereby providing new therapeutic targets for Wilms tumor and improving the prognosis of children with the disease.
Conclusion
In conclusion, our study identifies PRC1 as a significant prognostic factor in Wilms tumor (WT), where its overexpression is associated with poor survival outcomes and aggressive tumor features. Elevated PRC1 levels promote tumor progression by driving key oncogenic pathways, including Wnt/β-catenin, PI3K/AKT/mTOR, and Hedgehog signaling, and by facilitating the epithelial-mesenchymal transition (EMT) and glycolysis. Furthermore, PRC1 appears to contribute to immune evasion in WT, particularly by reducing NK cell activation within the tumor microenvironment. These findings underscore the potential of PRC1 as a therapeutic target, suggesting that inhibition of PRC1, particularly in combination with immune-based therapies, could improve treatment outcomes for children with WT. Further studies are needed to clarify the exact mechanisms of PRC1’s effects on immune cells and to validate these findings through in vivo models.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author or the first author upon reasonable request. If someone wishes to request the data from this study, please contact the corresponding author (Fengyin Sun: tjcollege2014@163.com) or the first author (Yanping Wang: 202435956@mail.sdu.edu.cn).
References
Spreafico, F. et al. Wilms tumour. Nat. Rev. Dis. Primers 7, 75 (2021).
Theilen, T. M. et al. Multidisciplinary treatment strategies for Wilms tumor: Recent advances, technical innovations and future directions. Front. Pediatr. 10, 852185 (2022).
Wong, K. F. et al. Risk of adverse health and social outcomes up to 50 years after Wilms tumor: The British childhood cancer survivor study. J. Clin. Oncol. 34, 1772–1779 (2016).
Yang, J. et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 21, 341–352 (2020).
McMahon, A. P. Development of the mammalian kidney. Curr. Top. Dev. Biol. 117, 31–64 (2016).
Huang, Y., Hong, W. & Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 15, 129 (2022).
Drake, K. A. et al. Stromal β-catenin activation impacts nephron progenitor differentiation in the developing kidney and May contribute to Wilms tumor. Development 147, (2020).
Debnath, P., Huirem, R. S., Dutta, P. & Palchaudhuri, S. Epithelial-mesenchymal transition and its transcription factors. Biosci. Rep. 42, (2022).
Wang, Y., Shi, J., Chai, K., Ying, X. & Zhou, B. P. The role of snail in EMT and tumorigenesis. Curr. Cancer Drug Targets 13, 963–972 (2013).
Li, Y. et al. Regulation of EMT by Notch signaling pathway in tumor progression. Curr. Cancer Drug Targets. 13, 957–962 (2013).
Wilms Tumor. Codon Publications, (2016). https://doi.org/10.15586/codon.wt.2016
Goldman, M. J. et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 38, 675–678 (2020).
Ritchie, M. E. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47–e47 (2015).
Liberzon, A. et al. The molecular signatures database hallmark gene set collection. Cell. Syst. 1, 417–425 (2015).
Rich, J. T. et al. A practical guide to understanding Kaplan–Meier curves. Otolaryngology–Head Neck Surg. 143, 331–336 (2010).
Mandrekar, J. N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 5, 1315–1316 (2010).
Balachandran, V. P., Gonen, M., Smith, J. J. & DeMatteo, R. P. Nomograms in oncology: More than Meets the eye. Lancet Oncol. 16, e173–e180 (2015).
Ashburner, M. et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Kanehisa, M. K. E. G. G. Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Subramanian, A. et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. 102, 15545–15550 (2005).
Yoshihara, K. et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 4, 2612 (2013).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457 (2015).
Tsuyuzaki, K., Sato, H., Sato, K. & Nikaido, I. Benchmarking principal component analysis for large-scale single-cell RNA-sequencing. Genome Biol. 21, 9 (2020).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with harmony. Nat. Methods 16, 1289–1296 (2019).
Linderman, G. C., Rachh, M., Hoskins, J. G., Steinerberger, S. & Kluger, Y. Fast interpolation-based t-SNE for improved visualization of single-cell RNA-seq data. Nat. Methods. 16, 243–245 (2019).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2019).
Zhang, X. et al. CellMarker: A manually curated resource of cell markers in human and mouse. Nucleic Acids Res. 47, D721–D728 (2019).
Chen, J., Bardes, E. E., Aronow, B. J. & Jegga, A. G. ToppGene suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 37, W305–W311 (2009).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, (2013).
McGeary, S. E. et al. The biochemical basis of MicroRNA targeting efficacy. Sci. (1979) 366, (2019).
Shen, W. et al. Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. iMeta 1, (2022).
Jiang, J. Hedgehog signaling mechanism and role in cancer. Semin Cancer Biol. 85, 107–122 (2022).
Liu, J. et al. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal. Transduct. Target. Ther. 7, 3 (2022).
Ang, H. L. et al. Mechanism of epithelial-mesenchymal transition in cancer and its regulation by natural compounds. Med. Res. Rev. 43, 1141–1200 (2023).
Dunne, J. et al. Selective expansion and partial activation of human NK cells and NK receptor-positive T cells by IL-2 and IL-15. J. Immunol. 167, 3129–3138 (2001).
Ranson, T. et al. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood 101, 4887–4893 (2003).
Tewari, D., Patni, P., Bishayee, A., Sah, A. N. & Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin Cancer Biol. 80, 1–17 (2022).
Omolekan, T. O., Chamcheu, J. C., Buerger, C. & Huang, S. PI3K/AKT/mTOR signaling network in human health and diseases. Cells 13, 1500 (2024).
Hollox, E. J., Zuccherato, L. W. & Tucci, S. Genome structural variation in human evolution. Trends Genet. 38, 45–58 (2022).
Sharp, A. J. et al. Segmental duplications and copy-number variation in the human genome. Am. J. Hum. Genet. 77, 78–88 (2005).
Zhang, F., Gu, W., Hurles, M. E. & Lupski, J. R. Copy number variation in human health, disease, and evolution. Annu. Rev. Genomics Hum. Genet. 10, 451–481 (2009).
Dupont, C., Armant, D., Brenner, C. & Epigenetics Definition, mechanisms and clinical perspective. Semin Reprod. Med. 27, 351–357 (2009).
Sharma, S., Kelly, T. K. & Jones, P. A. Epigenetics in cancer. Carcinogenesis 31, 27–36 (2010).
Slotkin, R. K. & Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 8, 272–285 (2007).
Bartel, D. P. MicroRNAs Cell. 116, 281–297 (2004).
Rupaimoole, R. & Slack, F. J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 16, 203–222 (2017).
Ugras, S. et al. Small RNA sequencing and functional characterization reveals MicroRNA-143 tumor suppressor activity in liposarcoma. Cancer Res. 71, 5659–5669 (2011).
Gumbiner, B. M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell. Biol. 6, 622–634 (2005).
Mendonsa, A. M., Na, T. Y. & Gumbiner, B. M. E-cadherin in contact inhibition and cancer. Oncogene 37, 4769–4780 (2018).
Cao, Z. Q., Wang, Z. & Leng, P. Aberrant N-cadherin expression in cancer. Biomed. Pharmacother. 118, 109320 (2019).
Loh, C. Y. et al. The E-Cadherin and N-Cadherin switch in Epithelial-to-Mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 8, 1118 (2019).
Li, J., Dallmayer, M., Kirchner, T., Musa, J. & Grünewald, T. G. P. PRC1: Linking cytokinesis, chromosomal instability, and cancer evolution. Trends Cancer 4, 59–73 (2018).
Li, S. et al. Protein regulator of cytokinesis 1: A potential oncogenic driver. Mol. Cancer 22, 128 (2023).
Chen, J. et al. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/β-catenin signalling pathway. Gut 65, 1522–1534 (2016).
Zhang, C. et al. Protein regulator of cytokinesis 1 (PRC1) upregulation promotes immune suppression in liver hepatocellular carcinoma. J. Immunol. Res. 1–27 (2022). (2022).
Polosukhina, D. et al. Functional KRAS mutations and a potential role for PI3K/AKT activation in Wilms tumors. Mol. Oncol. 11, 405–421 (2017).
Perotti, D. et al. Is Wilms tumor a candidate neoplasia for treatment with WNT/β-Catenin pathway Modulators?—A report from the renal tumors Biology-Driven drug development workshop. Mol. Cancer Ther. 12, 2619–2627 (2013).
Acknowledgements
We would like to acknowledge support and resources provided by the Stomatology Laboratory, Shandong University. Additionally, we also would like to acknowledge TARGET (Therapeutically Applicable Research to Generate Effective Treatments) database and GEO (Gene Expression Omnibus) database for providing a public data platform.
Funding
This work was supported by Grant ZR2020MH076 from Natural Science Foundation of Shandong Province, and by Grant 6010121111 from Horizontal project of Shandong Qidu Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Contributions
Yanping Wang: Writing—original draft, Software, Formal analysis, Conceptualization. Hongjie Gao: Writing—original draft, Validation, Formal analysis, Conceptualization. Xuetian Li: Visualization, Investigation, Data curation. Ding Li & Fan Huang: Writing—review & editing, Resources, Methodology. Yuqiang Sun & Xingjian Liu: Writing—review & editing, Validation. Junli Yang: Writing—review & editing, Supervision. Fengyin Sun: Writing—review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Gao, H., Li, X. et al. PRC1 as an independent adverse prognostic factor in Wilms tumor via integrated bioinformatics and experimental validation. Sci Rep 15, 13282 (2025). https://doi.org/10.1038/s41598-025-98030-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98030-y