Abstract
Hepatoblastoma is a rare pediatric liver malignancy usually treated with surgery and chemotherapy. To explore new treatment options for hepatoblastoma, drug screening was performed using six cell models established from aggressive hepatoblastoma tumors and healthy pediatric primary hepatocytes. Of the 527 screened compounds, 98 demonstrated cancer-selective activity in at least one hepatoblastoma model. The kinesin spindle protein (KSP) inhibitor filanesib was effective in all models and was further evaluated. Filanesib induced G2/M arrest and apoptosis in hepatoblastoma cells at concentrations tolerable to primary hepatocytes. Prominent nuclear fragmentation was observed in filanesib-treated hepatoblastoma cells. Genes participating in cell cycle regulation were noted to be differentially expressed after filanesib treatment. Filanesib reduced the rate of tumor growth in 4/5 hepatoblastoma mice models. One of these models showed complete growth arrest. Our results suggest that filanesib is a potential candidate for hepatoblastoma treatment and should be investigated in future clinical trials.
Similar content being viewed by others
Introduction
Hepatoblastoma (HB) is the most common type of pediatric liver malignancy. The annual incidence is 1.5–2.2 cases per million and is slowly rising1,2. Low birth weight and preterm birth are known risk factors of HB3. Genetic risk factors include familial adenomatous polyposis (APC), Beckwith–Wiedemann syndrome (hemihypertrophy), and Li–Fraumeni syndrome (TP53)4,5,6,7. HB is usually diagnosed in children under 5 years8. Current European standard-of-care treatment protocols are based on the International Society of Paediatric Oncology Epithelial Liver Tumor Group (SIOPEL) trials and depend on the risk stratification of disease9. Treatment usually entails a combination of surgery and chemotherapeutics, and cure can only be achieved with surgical intervention. First-line chemotherapeutic treatment consists of preoperative and postoperative administration of cisplatin, carboplatin, and doxorubicin. Liver transplantation is an option for unresectable HB. Currently, there is no standard-of-care treatment for relapsed or refractory HB, and 5-year overall survival is less than 50% in this patient group10.
Conventional chemotherapeutics pose risks to young patients. Cisplatin treatment is associated with a significant risk of hearing impairment11. Anthracyclines, such as doxorubicin, are known to be cardiotoxic and can cause heart failure years after the initial exposure12. In principle, targeted drugs and personalized therapies could result in fewer adverse effects; however, such treatments have not been widely used in HB. Exploitation of mutation-targeting therapies has proven difficult because the mutation frequency in HB is low13. An exception is CTNNB1, a gene encoding β-catenin, which is known to have a high mutation frequency in HB14. However, drugs targeting Wnt/β-catenin signaling have shown little potential, and no HB trials have been conducted using these agents15. Drug screening is a useful tool to discover new potential treatment modalities, but only one extensive drug screening with three-dimensional (3D) HB models has been published. Kluiver et al. screened HB organoids with over 200 compounds and identified sensitivity to histone deacetylase (HDAC) inhibitors across HB subtypes16.
This study aimed to identify new potential compounds with an acceptable toxicity profile for the management of HB and especially for high-risk HB tumors. We used six previously established HB models derived from aggressive tumors and healthy pediatric primary hepatocytes, all cultured as spheroids, to perform an extensive drug screen with over 500 compounds. To validate and further assess the effects of the potential drugs, both in vitro and in vivo experiments were conducted.
Results
Characteristics of HB PDX models
Cancer tissue samples for the PDX models were obtained from high-risk HB patients. The cell models used in this study (n = 6) were established from the PDX by XenTech (Evry, France)17. Clinical information and basic demographics of the models are shown in Supplementary Table 1. Two models were derived from the same patient (HB-279 from primary tumor resection, HB-284 from intraperitoneal metastasis). Of the six HB models, five carry CTNNB1 mutations.
Overview of the drug screen and identification of effective drugs
The drug screen consisted of 528 approved or emerging oncological compounds and combinations (527 individual drugs, one combination) (Supplementary Table 2). The screened compounds have varying mechanisms of action, including both agents with a specific target as well as conventional chemotherapeutics (Supplementary Fig 1A). Z-factors were calculated to assess the robustness of the screen18. Z-factors of >0.5 were found for all screens, demonstrating sufficient quality (Supplementary Fig 1B). Drug sensitivity score (DSS) was calculated for each tested compound, as previously described19. A DSS ≥ 10 was considered effective. General drug sensitivity varied between the HB cell models (Fig. 1A). HB-303 and HB-295 cells, representing fetal HB, had the highest number of hits considered effective, 149 (28%) and 157 (30%), respectively (Fig. 1B). The other four HB cell models, with predominantly embryonal histology, demonstrated responses to 62–119 compounds with DSS ≥ 10 (Fig. 1B). Seventy out of 527 drugs (13%) also reduced the viability of control primary hepatocytes at this cut-off (Fig. 1A, B).
Efficacy of tested drugs in six HB models grown as spheroids was evaluated using DSS. DSS ≥ 10 was considered effective (dotted red line) (A). The number of compounds with DSS ≥ 10 (B). Taking into account the effect of the drug on healthy primary hepatocytes, sDSS was calculated. Drugs with sDSS ≥ 10 were considered effective and non-toxic (C). The top 10 compounds with the highest sDSS of each cell line are shown (D) intensity of color depicts DSS. Venn diagram shows overlapping compounds with sDSS ≥ 10 (E). The dose–response curves of these eight overlapping compounds are shown in panel F. DSS drug sensitivity score, sDSS selective drug sensitivity score, PH primary hepatocytes.
Ninety-eight compounds reduced HB cell viability while being non-toxic to normal hepatocytes
To evaluate the tumor-selective effect of the drugs, compounds toxic to the age-matched healthy primary hepatocytes were excluded by calculating a selective drug sensitivity score (sDSS). The list of potential candidate compounds was narrowed down using a sDSS ≥ 10 as a cutoff (Fig. 1C). The highest-scoring compounds included epigenetic and metabolic modifiers, mitotic inhibitors, modulators of apoptosis, protein kinases, and heat shock protein (Hsp) inhibitors (Fig. 1D). The conventional compounds in HB treatment (carboplatin, doxorubicin, cisplatin, irinotecan, vincristine, and etoposide) demonstrated only a minor effect (Supplementary Fig 2), though it should be noted that all PDX models were established from pre-treated patient tissues (Supplementary Table 1).
After the above-described exclusion criteria were applied, 98 compounds with sDSS ≥ 10 in at least one of the HB cell models remained (Fig. 1C). Eight compounds were found to be effective in all six HB models (Fig. 1E). These were filanesib (kinesin spindle protein [KSP] inhibitor), onalespib (Hsp inhibitor), fimepinostat (PI3K/HDAC inhibitor), idasanutlin (MDM2-antagonist), cabazitaxel (microtubule inhibitor), BIIB021 (Hsp inhibitor), eribulin (microtubule inhibitor), and luminespib (Hsp inhibitor) (Fig. 1E, F). While all eight compounds met the criteria of sDSS ≥ 10, fimepinostat and eribulin showed some toxicity in healthy control cells even at lower concentrations and were thus excluded. Response to both cabazitaxel and idasanutlin was observed across the models. The remaining four compounds (filanesib, onalespib, BIIB021, and luminespib) consisted of three Hsp inhibitors and one KSP inhibitor. Of these, the KSP inhibitor filanesib and one Hsp inhibitor, luminespib, were chosen for further investigation.
Filanesib and luminespib reduced cell survival and triggered apoptosis in HB spheroids
In the initial screen, cells were seeded on pre-drugged U-bottom plates, where they formed spheroids for 72 h under drug treatment. To simulate the treatment of existing tumors and validate the results, the cells were first allowed to form spheroids and then treated with either filanesib or luminespib. The concentration of ATP was decreased at least 30% in all cell models after 10 or 100 nM filanesib treatment. HB-279 showed an especially strong response to filanesib, with the ATP-concentration decreasing 80% already with 1 nM of filanesib (Fig. 2A). Luminespib-treated HB models showed remarkable, 60–99% decrease in viability with the highest 100 nM concentration (Fig. 2B). Lower luminespib concentrations of 1 nM and 10 nM were less effective.
In contrast to DSRT, spheroids were first established for 72 h and after that chosen compounds were administered. Cell viability was measured by relative ATP concentration and decreased in all cell models after 72 h of filanesib treatment (A). Luminespib exposure decreased spheroid survival notably with the highest tested dose (B). While filanesib decreased cell viability in all HB models, the mechanism of cell death seemed to be partly caspase 3/7 mediated but varied among the models (C). Luminespib increased caspase 3/7 activity in a dose-dependent manner (D). * p < 0.05, ** p < 0.01 (Student’s t-test), (N = 4).
The magnitude of apoptotic cell death after treatment was assessed using a caspase 3/7 assay. HB-284 showed the highest activation of caspase 3/7 activity after filanesib treatment (Fig. 2C). Interestingly, while filanesib-treated HB-279 cells showed the greatest decrease in cell viability, caspase 3/7 activity did not increase remarkably, suggesting an alternative method of cell death (Fig. 2C). Luminespib-treated models showed increased caspase 3/7 activity in a dose-dependent manner (Fig. 2D).
Filanesib and luminespib caused alterations in cell morphology
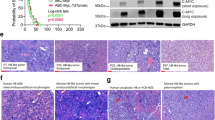
Cell Painting, a phenotypic, morphological profiling assay for cellular components/organelles was used to visualize the morphological changes caused by filanesib and luminespib treatments. First, UMAPs were used to show a nonlinear 2D projection of 300-dimensional single-cell data with each dot representing one cell, where cells grouped based on the similarity of their morphological features. Filanesib-treated cells clustered by dose and separately from the DMSO controls (Fig. 3A–D). Similarly, luminespib-treated cells clustered together especially at the highest concentration of 100 nM (Fig. 3A–D). In HB-279 cells treated with 10 nM filanesib, the cells became round (Fig. 4A-E), and the nuclei appeared fragmented (Fig. 4A) resembling mitotic catastrophe. This was also observed in HB-284 cells (Supplementary Fig 3C). In HB-243 and HB-282 cells, the fragmentation of nuclei was not as distinct (Supplementary Fig 3A, B). Luminespib induced changes in the mitochondrial morphology (Fig. 4D, Supplementary Fig 3A–C). The lowest (1 nM) concentration of filanesib or luminespib did not cause marked changes in cell morphology (Supplementary Fig 3A–C). The most notable changes caused by filanesib were those in the nuclei shape. Heatmap and clustering of the features (averages, normalized to the controls of each cell line) showed that, in general, samples clustered according to their treatment (Supplementary Fig 4).
Representative images of HB-279 cells treated with either 10 nM filanesib, 10 nM luminespib, or control (DMSO) (A–E). Four different wave lengths were used to capture the morphological features. Different stains were used to visualize the nuclei (A) endoplasmic reticulum (B) cellular membranes and microfilaments (C) and mitochondria (D). Merged images are presented in (E). The nuclei of filanesib-treated cells displayed a fragmented morphology (A). Scale bar = 50 µm, scale bar in close-up images = 10 µm.
Filanesib caused G2/M cell cycle arrest in HB cells
To investigate the effect of the candidate drugs on cell cycle, HB-279, HB-284, and HB-243 cells were treated with 10 nM filanesib for 24 h or 50 nM luminespib for 48 h or with DMSO. Next, the cell cycle was analyzed using flow cytometry. We found that treatment with filanesib induced a significant increase in the percentage of cells in the G2/M phase in all cell lines (Fig. 5A–C). In DMSO treated HB-279 cells, 16% (SD = 0.2) of the cells were in G2/M phase while in filanesib treated cells, the percentage of G2/M cells was 64% (SD = 4.4) (Fig. 5A). In HB-284 cells, the corresponding percentages were 27% (SD = 0.9) (DMSO) and 56% (SD = 1.9) (filanesib) (Fig. 5B), and in HB-243 cells 16% (SD = 1.1) (DMSO) and 61% (SD = 2.9) (filanesib) (Fig. 5C). Luminespib also caused an increase in the percentage of cells in the G2/M phase, even though the effect was milder than with filanesib (Fig. 5D–F). In DMSO treated HB-279 cells the percentage of cells in the G2/M phase was 13% (SD = 0.6) while 16% (SD = 1.1) of luminespib treated cells were in G2/M phase (Fig. 5D). The corresponding percentages in HB-284 cells were 10% (SD = 1.0) (DMSO) and 17% (SD = 1.2) (luminespib) (Fig. 5E), and in HB-243 cells 16% (SD = 1.0) (DMSO) and 19% (SD = 0.7) (luminespib) (Fig. 5F).
Representative histograms of flow cytometry analysis after 10 nM filanesib treatment of HB-279 cells (A) HB-284 cells (B) and HB-243 (C) for 24 h. Representative histograms of flow cytometry analysis after 50 nM luminespib treatment of HB-279 cells (D) HB-284 cells (E), and HB-243 cells (F) for 48 h. Bar plots show the percentage of cells in each phase of the cell cycle of four independent experiments presented as mean ± SD. ** p < 0.01 (Student’s t-test).
Silencing KIF11 caused apoptosis and G2/M cell cycle arrest in HUH6 HB cells
To validate the significance of KIF11, the target protein of filanesib, in HB pathobiology the impact of KIF11 on HB cells was assessed by silencing it in HUH6 HB cells. Silencing efficiency was verified at both RNA and protein level. Following transient siRNA transfections, KIF11 expression was reduced 80% at the mRNA and protein level in HUH6 cells (Fig. 6A, B). Next, we assessed the effect of KIF11 silencing on HB cell viability and apoptosis. KIF11 silencing caused a 50% reduction in cell viability (Fig. 6C). Apoptosis was assessed with two different methods. KIF11 silencing resulted in 1.6-fold increase in Caspase 3/7 activity (Fig. 6D) and a 4.3-fold increase in the number of late apoptotic cells (Fig. 6E). Furthermore, the cell cycle analyses revealed that KIF11 silencing promotes G2/M cell cycle arrest in HUH6 HB cells, mirroring the effect of filanesib treatment (Fig. 6F). Finally, the impact of filanesib on HUH6 cell viability was assessed. Both 10 and 100 nM filanesib caused ∼40% reduction in HUH6 cell viability (Fig. 6G).
In HUH6 cells, KIF11 mRNA expression was reduced 80% after 72 h of KIF11 siRNA transfection compared to NT control (A). Protein band intensity of KIF11 was 80% lower in KIF11 siRNA treated cells in contrast to NT control cells (B). ATP concentration decreased 50 % (C), caspase 3/7 activity increased 1.6-fold (D), and the number of late apoptotic cells increased 4.3-fold (E) after KIF11 silencing. KIF11 silencing induces G2/M cell cycle arrest in HUH6 cells. Representative histograms of flow cytometry analysis after NT siRNA or KIF11 siRNA transfection (F). Bar plots show the percentage of cells in each phase of the cell cycle (F). Filanesib reduced HUH6 cell viability measured by relative ATP concentration after 72 h treatment (G). Bar plots are presented as relative values of mean ± RSD. Band intensity is normalized to total protein expression of each lane. Normalization factor (NF) describing the amount of total protein in lane in relation to other lanes is given beneath the bands (B). **p < 0.01 (relative to DMSO control). NT non-targeting.
Filanesib decreased HB growth in vivo
To assess the efficacy of filanesib and luminespib in vivo, we used five HB PDXs. Mice with HB tumors were randomly assigned to receive either vehicle, filanesib, or luminespib. Tumors treated with vehicle grew rapidly (Fig. 7A–E). Filanesib reduced the growth of tumor volume in 4/5 PDX tumors (Fig. 7A–D). Of these, HB-279 showed a distinctively strong response to filanesib (Fig. 7B). The tumor volume of HB-279 did not increase substantially in any of the filanesib-treated mice during the 18-day treatment period. HB-295 did not show any response to filanesib (Fig. 7E). Only one PDX model, HB-243, showed reduction in tumor growth when treated with luminespib (Fig. 7A). Mice that responded to the treatments did not show a significant change in body weight (Fig. 7A–E, right panel).
Changes in tumor volume and bodyweight of mice treated with oral 20 mg/kg filanesib every fourth day (red squares), 30 mg/kg luminespib three times per week (blue triangles) or with vehicle (black circles) are shown (left panel, A–E). Each point is the mean ± SEM. Each of the five study arms included three mice. Bodyweights are shown in right panel (A–E).
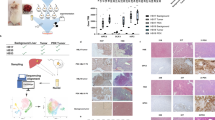
Tumor tissues resected from filanesib-treated mice were immunostained with KIF11, a target protein of the compound. Hematoxylin and eosin (H&E) staining was performed to show the histology of the tumors (Fig. 8A–E). All untreated tumor tissues showed relatively high expression of KIF11, and the staining was mainly localized in the cytoplasm (Fig. 8A–E). In dividing cells showing mitotic spindle formation, expression was also localized in the nuclei (Supplementary Fig 5A–E). In HB-279 and HB-284 tumors, the number of KIF11-positive cells was lower in filanesib-treated tumor tissues than in controls (Figs. 8B–D).
KIF11 expression in HB PDX FFPE tissue samples was evaluated by immunohistochemistry. Baseline KIF11 expression was high in all samples (A–E). A decrease in KIF11 expression after filanesib treatment was evident in 2/5 HB PDX tumors, HB-279 (B) and HB-284 (D). H&E-staining shows the histology of the tumors (A–E). Scale bar = 50 μm. Close-ups in the corners: scale bar = 50 μm.
Expression of KIF11 in RNA sequencing data
To assess the mRNA expression of KIF11, RNA sequencing of the six HB models was performed. KIF11 was overexpressed in all six HB PDX models at mRNA level compared to healthy hepatocytes (Fig. 9A). Five models showed notable KIF11 overexpression varying between 3.61 and 4.70 log2FC, while the overexpression in HB-279 was the lowest at 1.97 log2FC (Fig. 9A).
KIF11 RNA expression in six PDX cell models relative to primary hepatocytes is shown in panel A Unsupervised principal component analysis of the expression of the most variably expressed genes (B) and of a subset of mitotic spindle-related genes (C) shows the relation of untreated HB-279 cells to the other HB models. Each dot represents a single replica sample. Gene set enrichment analysis comparing the RNA-sequencing results of HB-279 to the other models using Hallmark gene sets was done. D shows the top 10 highest and lowest scoring Hallmark gene sets with FDR < 0.25. PH primary hepatocytes, PC principal component, NES normalized enrichment score.
HB-279 has lower expression of mitotic spindle-related genes compared to the other models
To investigate why HB-279 was more sensitive to filanesib compared to the other models, differentially expressed genes between HB-279 and the other five HB models were analyzed. In total, 729 genes were significantly upregulated and 1819 were downregulated (adjusted p ≤ 0.1). Unsupervised principal component analysis (PCA) showed that HB-279 differed from the other models and that HB-284, the metastasis of HB-279, clustered between HB-279 and the other models (Fig. 9B). When only looking at Hallmark Mitotic Spindle genes, HB-279 clustered even further from the other models (Fig. 9C).
Gene set enrichment analysis (GSEA) was performed to identify enriched gene sets among the differentially expressed genes between HB-279 and other HB models. From the Hallmark gene set collection, 18 gene sets with a false discovery rate (FDR) ≤ 0.25 were found, of which 17/18 had lower expression in HB-279 than in the other models (Fig. 9D). The most negatively enriched gene set was Hallmark E2F Targets, in which the genes had a consistently lower RNA expression in HB-279 than in the other HB models. (Supplementary Fig 6; FDR < 0.005). This gene set includes 200 genes encoding cell cycle -related targets of the E2F transcription factors20. It includes four KIF genes (KIF18B, KIF22, KIF2C, and KIF4A) but not the filanesib target KIF11. The only significantly positively enriched Hallmark gene set in HB-279 vs. other HBs was Wnt/β-catenin signaling (Fig. 9D).
Next, expression of the Hallmark Mitotic Spindle gene set was investigated. Among the 200 genes included in this gene set, 41 were significantly differentially expressed in HB-279 compared to the other HB models (Supplementary Table 3). Of these 41 genes, six were upregulated and 35 were downregulated in HB-279. The expression of Hallmark Mitotic Spindle genes clearly separated HB-279 from the other HB models (Fig. 9C). Eleven KIF genes are among the Hallmark Mitotic Spindle genes. 7/11 of them had significantly lower expression in HB-279 than in the other HB samples, including KIF11.
A correlation analysis with DESeq2 was performed to identify genes whose mRNA expression correlated with the selective drug response rate of filanesib in the in vitro testing in each of the six HB models. No genes significantly correlating with filanesib drug response were identified.
Filanesib-induced changes on mRNA level
To assess the transcriptomic changes caused by filanesib, RNA sequencing of filanesib and DMSO-treated HB-279 and HB-284 cells was performed. KIF11 mRNA expression was not significantly affected by filanesib treatment (Fig. 10A, B). However, filanesib treatment increased the expression of other members of the KIF protein family, including KIF1A, KIF2C, KIF4A, KIF5C, KIF14, KIF18A, and KIF20A (Fig. 10A, B). In contrast, KIF12 expression was downregulated in both HB-279 and HB-284 cells following filanesib treatment (Fig. 10A, B).
Expression of KIF-protein family members after treatment with 10 nM filanesib in HB-279 (A) and HB-284 (B) cells. Unsupervised principal component analysis of the expression of all genes (C) and of a subset of mitotic spindle related genes (D) shows the relation between filanesib-treated and DMSO-treated HB-279 and HB-284 models. Gene set enrichment analysis comparing the mRNA expression profiles of filanesib-treated cells (HB-279 and HB-284 combined) compared to untreated cells was done. Top 10 highest and lowest enriched Hallmark gene sets with FDR < 0.25 are shown (E). PC principal component, NES normalized enrichment score.
An unsupervised PCA of the most variably expressed genes across these two treated and untreated HB models showed that the variance between the treatments (filanesib and DMSO) was greater than the variance between the models (HB-279 and HB-284), with treatment explaining 56% of the variance (PC1) and model 37% (PC2) (Fig. 10C). When analyzing Hallmark Mitotic Spindle genes only, PC1 separated the models and PC2 separated the treatments (Fig. 10D). A paired GSEA analysis was performed to determine the pathways affected by filanesib treatment (Fig. 10E). Filanesib induced overexpression of genes included in Hallmark gene sets for TNFα signaling, G2M Checkpoint, Epithelial-mesenchymal Transition (EMT), and Mitotic Spindle (Fig. 10E).
Discussion
Few adjustments have been proposed for the pharmaceutical management of HB during the past two decades, and targeted agents have not been incorporated into routine care. Chemotherapeutics utilized in HB treatment predispose the patient to adverse effects, which may be irreversible and affect the health of the individual far into the future. Moreover, patients with high-risk HBs demonstrate a 3-year event-free survival rate below 60%21, underscoring the need for better and safer treatment modalities. Herein, we performed an extensive drug screening with 527 compounds using 3D HB cell models established from PDXs with aggressive tumors. Eight of the 527 compounds were found to be effective in all six HB cell models without notable toxicity toward primary pediatric hepatocytes. Two compounds, filanesib and luminespib, were further investigated in this study. Filanesib decreased cell survival in vitro and reduced growth of HB tumors in vivo, suggesting that it could be a potential candidate for future clinical trials.
High-throughput DSRT is utilized to efficiently discover and investigate the effects of multiple compounds simultaneously. Here, we chose to utilize 3D spheroids, which have been shown to better resemble tumor characteristics and behavior in vivo compared to traditional monolayer cultures22,23,24,25. To the best of our knowledge, this extensive screening has not been performed with 3D HB cell cultures. Over 200 compounds were investigated in a recent study employing patient-derived HB organoids16. Similar to our screen, HDAC inhibitors were shown to be effective for multiple HB subtypes16. Five of the six HB models used in our study have been investigated earlier in a screen encompassing 60 compounds17. There has also been a small screen consisting of 12 drugs using HB organoids26. In this screen BET bromodomain inhibitor JQ1 showed some preclinical potential26. Our drug library included JQ1; however, it was not among the most effective compounds in our study. Pre-drugged plates were used in our initial drug screen and the results were validated on chosen compounds using readily formed spheroids. The results from these two approaches were consistent as previously demonstrated also by Feodoroff et al.22.
In our drug screen, eight of the initial 527 compounds were effective in all six aggressive HB models and showed little to no toxicity toward healthy, age-matched hepatocytes. These eight compounds represented several different mechanisms of action and included an HDAC inhibitor, Hsp inhibitors, an MDM2-antagonist, microtubule inhibitors, and the KSP inhibitor filanesib. Epigenetic modifiers such as HDAC and DNA methyltransferase inhibitors have already previously been under extensive investigation for management of high-risk HBs16,27,28. In our study, the HDAC inhibitor fimepinostat (CUDC-907) reduced viability of all HB cell models representing different subtypes. However, we also observed a decrease in the survival of healthy hepatocytes already at concentrations below 100 nM and thus decided to exclude it from further testing.
Three out of eight compounds that were effective in all HB cell models target heat shock protein Hsp90, a molecular chaperone protein that participates in multiple processes in oncogenesis and cancer progression29. An elevated Hsp90 expression has been demonstrated in HBs, underlining the reasonableness of this target30. One of the three Hsp90 inhibitors, luminespib (also known as AUY922), which has been previously investigated in phase 2 trials for other indications, was chosen for additional analyses31. Increased apoptosis was detected in HB spheroids treated with luminespib, but only one model, HB-243, responded slightly to luminespib treatment in vivo. In a previous study conducted using a hepatocellular carcinoma murine model with Huh7 cells, luminespib caused a notable decrease in in vivo tumor growth32. Our dosing schedule was less frequent (30 mg/kg of luminespib five times a week for 15 days versus 30 mg/kg three times per week for 18 days), and the route of administration was different from that in the study by Augello et al.32 (intraperitoneal injection versus oral gavage administration), which may have affected responses. While results from hepatocellular carcinoma cannot be interpolated directly to HB, more frequent administration should be considered in future HB study designs using Hsp90 inhibitors.
We observed that several mitotic inhibitors decreased the viability of all investigated HB cell models while reducing the survival of primary hepatocytes only at the highest concentrations. Effective drugs included the conventional microtubule inhibitors cabazitaxel and eribulin, as well as the targeted agent filanesib. As of today, the only mitotic inhibitor that has been widely used for HB treatment is vincristine. However, its side effects, such as peripheral neuropathy, limit the use33,34. The search for more targeted mitotic inhibitors with fewer side effects has led to the discovery of kinesin spindle protein inhibitors, such as filanesib, litronesib, and ispinesib35. Kinesin-like protein 11 (KIF11, also known as kinesin-5 and Eg5) is part of the kinesin superfamily and plays many roles in mitosis, including chromosome positioning, centrosome separation, and the establishment of the bipolar spindle36,37,38. In pediatric cancers, KIF11 has been shown to be overexpressed and to have a prognostic role in Wilms tumors and in acute lymphocytic leukemia39,40. Our RNA sequencing data analysis showed overexpression of KIF11 in all HB PDX cell models. However, there was no correlation between the magnitude of KIF11 upregulation and response to KIF11 inhibition with filanesib. Thus, predicting who may benefit from KSP inhibitor treatment may be more complex than merely observing the abundance of the target.
It has been shown that inhibition of KIF11 causes mitotic arrest and blocks the cell cycle in metaphase41. Consistently, HB cells treated with filanesib in our study were predominantly arrested in the G2/M phase. KSP inhibitors and direct depletion of KIF11 have been demonstrated to cause formation of abnormal monopolar spindles (monoastral phenotype) and mitotic catastrophe, a process leading either to cell death or senescence42,43,44. We observed that the nuclei of HB cells became shattered after filanesib exposure, resembling a typical morphology for mitotic catastrophe.
Filanesib treatment reduced tumor growth in four of five HB PDX models in vivo. These four models predominantly represented the embryonal subtype. Filanesib showed distinctively strong activity in the PDX model HB-279. A remarkable reduction in KIF11 expression was observed in treated HB-279 PDX tissues, confirming that filanesib acts at least partly through KIF11. To explore the mechanisms underlying the strong sensitivity of HB-279 to filanesib, we compared the mRNA expression profiles of all of the HB models and examined the differences in gene expression before and after filanesib treatment. HB-279 clearly differed from the other five HB models by presenting lower expression of many cell cycle related genes and the filanesib-target KIF11. Compared to the rest of the models, genes belonging to the Hallmark Mitotic Spindle, Hallmark E2F Targets and Hallmark G2M Checkout sets were mainly downregulated in HB-279 cells compared to the other models. We wonder if the lower baseline expression level of KIF11 and other related genes makes HB-279 more vulnerable to filanesib compared to the rest. The low KIF11 RNA expression level in HB-279 was reversed when the cells were treated with filanesib, as we observed the upregulation of genes belonging to the aforementioned sets. Interestingly, increased mRNA expression of KIFs other than KIF11 was observed after filanesib treatment, possibly indicating a compensatory mechanism. KIF25 and KIF16A have been hypothesized to function similarly to KIF11, but little is known about their actual functions45,46. Further research is required to identify which genes or gene signatures could be employed to identify patients who may benefit from KSP inhibitor treatment.
The only model that showed no response to filanesib in vivo was HB-295 representing fetal HB subtype. However, our in vivo study included only five HB models, and it could be a coincidence that the only PDX with fetal HB histology did not respond to filanesib. The fetal HB models (HB-295, HB-303) had the highest number of hits in our drug screen, suggesting that there are many other compounds to consider for this HB subtype. A more heterogeneous set of HB tumor models is needed for further investigation.
Clinical studies on filanesib have focused on adult multiple myeloma. Currently, there are eight completed phase 1 and phase 2 filanesib trials, and four have published results47,48,49,50. Adverse events, such as neutropenia, anemia, and fatigue, have mostly been non-cumulative and reversible51. There have been no clinical trials with filanesib in HB or other pediatric solid tumor patients. A preclinical neuroblastoma study revealed that filanesib prolongs the survival of orthotopic mice52. The only KSP inhibitor that has undergone a phase 1 trial with pediatric solid tumors is ispinesib, which was unfortunately not included in the predefined screening panel used in this study53. Our drug screen included three KSP inhibitors: filanesib, SB-743921, and litronesib. SB-743921 has previously been shown to be effective towards pediatric Ewing sarcoma in a preclinical setting43. In our screen, SB-743921 showed no toxicity towards healthy controls, but was considered effective in only one of the models, HB-279. To the best of our knowledge, litronesib has not been previously tested in pediatric tumors. In the present study, litronesib demonstrated good efficacy and specificity, with sDSS > 10 in 5/6 of the models. Overall, better understanding of treatment biomarkers and more clinical trials are needed to determine the safety and efficacy of KSP inhibitors in the pediatric population. Based on our findings, we propose that HB should be among the investigated tumor types. Prior to proceeding to phase 1 clinical trials, the potential biomarkers for patient stratification should be further searched, given the variability in treatment responses observed in our study. Clinical trials could then be focused on the HB patient group most likely to benefit from filanesib treatment.
Herein, we investigated 527 compounds using six 3D HB cell models established from PDXs with aggressive tumors, alongside healthy primary cells to assess general toxicity. Our screen revealed eight agents that were effective in all HB models with relatively low toxicity. None of these eight compounds are currently used in the routine care of HB, suggesting that there are novel approaches that may improve patient outcomes. HDACs, proteins mediating mitosis, and Hsp90 were found to be common targets in all HB subtypes. Luminespib was effective in vitro but showed very limited effect in vivo. Filanesib, a KSP inhibitor, efficiently suppressed HB cell viability in vitro and in vivo, particularly in HBs of embryonal origin. Taken together, filanesib is a promising candidate for use in future clinical trials.
Methods
Cell models and cell culture
The six HB cell lines established from patient-derived xenografts (PDX; HB-243, HB-279, HB-282, HB-284, HB-295, HB-303) were provided by XenTech (Evry, France)17. Human HB cell line HUH6 was obtained from Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). PDX cells were cultured in Advanced DMEM/F12 (Gibco, Waltham, MA, USA) that was supplemented with 8% fetal bovine serum, 2 nM glutaMAX (Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin sulfate and 20 μM rock kinase inhibitor Y-27632 (Cat#1049; Selleck Chemicals, Houston, TX, USA). HUH6 cells were maintained with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 nM glutaMAX, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate (all from Gibco). Primary hepatocytes from 10-year-old white male (HUM191501; Lonza, Basel, Switzerland) were used as controls and cultured as instructed by the manufacturer’s protocol “Guide to spheroid formation using Verified for Spheroids human pediatric hepatocytes”. All cell lines were authenticated through short tandem repeat profiling.
3D high-throughput drug sensitivity and resistance testing (DSRT)
Cells were seeded with Biotek MultiFlo FX using a single-channel cassette into 25 µl final volume per well to 384-well U-bottom plates (Corning 3830), pre-drugged using the Echo 550 acoustic dispenser (Labcyte) with a FO5A library of 527 approved and investigational oncology drugs and one combination, each in five concentrations (Supplementary Table 2) [by the High Throughput Biomedicine core unit (FIMM, HiLIFE, University of Helsinki, Finland)]. Cell seeding densities were as follows: HB-303, HB-282, HB-284, and HB-279: 750 cells/well, HB-295 and HB-243: 1000 cells/well. After 72 h incubation, 15 µl CellTiter-Glo 2.0 reagent (CTG, Promega, Madison, WI) was added with Biotek MultiFlo FX. Cell viability (intracellular ATP) was measured using BMG Labtech PHERAstar FS plate reader. Pediatric healthy primary hepatocytes were similarly screened (HUM191501, 500 cells/well, 384-well plate) with FO5A library to evaluate the general toxicity/specificity of the compounds. The quality control for the screens as well as IC50, EC50, and drug sensitivity scores (DSS) were determined for every individual drug from the raw data using FIMM-UH analytics tool Breeze (version 1.0, available https://breeze.fimm.fi/)19,54. To assess the robustness of the screen, Z-factors were calculated for the difference in viability at each cell density using dimethyl sulfoxide (DMSO; 0.1%) as a negative control and the antiseptic benzethonium chloride (100 µM) as a positive control. Selective DSS (sDSS) was counted by subtracting the DSS value of similarly screened healthy control hepatocytes from DSS value of cancer cells, method and controls previously described by Chen et al.55. Drugs with sDSS ≥ 10 were considered effective.
Confirmatory viability and apoptosis assays
For validation experiments, 10 mM filanesib (HY-15187) and 10 mM luminespib (HY-10215) in DMSO stocks were obtained from MedChemExpress (New Jersey, USA). Cells were treated with 1, 10, and 100 nM of filanesib or luminespib in all cell experiments if not otherwise described.
HB-243, HB-282, HB-284, HB-279, HB-295, HB-303, and HUH6 cells were seeded at density of 2000 cells/well to low attachment CellCarrier spheroid ULA 96-well-plates (PerkinElmer, Waltham, MA, USA). After 48 h incubation, spheroids were established and treatment with filanesib, luminespib, or control medium with corresponding DMSO concentration was initiated. Viability of spheroids was assessed with ATPlite™ 3D monitoring system (PerkinElmer) as described in the manufacturer’s instructions at treatment timepoint of 72 h. Caspase 3/7 Glo assay (Promega, Madison, WI, USA) was used to measure apoptosis in 3D cultures as instructed. Luminescence was measured with a GloMax microplate reader (Promega, Madison, WI, USA). In HUH6 cells the number of apoptotic cells after KIF11 silencing was determined by Nicoletti assay56.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from HUH6 cells utilizing NucleoSpin™ RNA, Mini kit (Macherey-Nagel, Düren, Germany) as instructed. Reverse transcription was carried out using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, United States). Quantitative polymerase chain reaction was performed using PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, Fremont, CA, United States). Relative gene expression was assessed using the 2−ΔΔCT method. The geometric mean of Cyclophilin, GAPDH, and PPIB served as a reference. Primer sequenced were designed as follows: Cyclophilin 5′ -CAATGGCCAACAGAGGGAAG—3′ (forward), 5′ -CCAAAAACAACATGATGCCC—3′ (reverse); GAPDH 5′ -GGTCATCCATGACAACTTTGG—3′ (forward), 5′ -CCATCCACAGTCTTCTGGGT—3′ (reverse); PPIB 5′ -CAATGGCCAACAGAGGGAAG—3′(forward), 5′—CCAAAAACATGATGCCCA—3′ (reverse); KIF11 5′ -GATGGACGTAAGGCAGCTCA —3′ (forward), 5′ -TGTGGTGTCGTACCTGTTGG—3′ (reverse).
Protein extraction and western blotting
Proteins were extracted utilizing ProteoSpin Detergent-free total protein isolation kit (Norgen Biotek Corp., Thorold, Canada). Fifteen micrograms of protein was separated by electrophoresis using Mini-Protean TGX Stain-Free Gels (Bio-Rad). Proteins were transferred onto polyvinyl fluoride membrane and non-specific binding was blocked with 5% non-fat milk in 0.1% Tris-buffered Tween saline buffer. Membranes were incubated with the following primary antibodies at +4 °C for overnight: anti-human EG5 rabbit mAb in dilution 1:750 (#14404S, Cell Signaling Technology, Danvers, MA, USA). Next, goat anti-rabbit IgG secondary antibody (#111-035-144 in dilution 1:10,000; Jackson ImmunoResearch, West Grove, PA, USA) incubation was performed at room temperature for 1 h. Protein bands were illuminated utilizing the Enhanced Chemiluminescence detection kit (Amersham ECL reagent; GE Healthcare, Barrington, IL) and analyzed with Image Lab Software 6.0 (Bio-rad). KIF11 band intensities were normalized to amount of total protein in corresponding lane (Supplementary Fig 7) utilizing stain-free technology57.
Cell phenotype analysis and imaging
HB cell lines HB-243, HB-279, HB-282, and HB-284 were cultured and treated with filanesib or luminespib as described above. Cells were seeded on 96-well PhenoVue plates (PerkinElmer, Waltham, MA, USA); 6000–13,000 cells per well, and after 24 h treated with filanesib or luminespib for another 24 h. DMSO 0,1% was used as control. PhenoVue Cell Painting JUMP kit (PerkinElmer) was used to stain the cells according to the Cell Painting protocol with some modifications (JUMP protocol v2)58. In brief, two separate staining solutions with mitochondrial stain, wheat germ agglutin (WGA), concavalin A, Hoechst and nuclear acid stain were prepared. Cells were first stained with mitochondrial stain and incubated at 37 °C, after which they were fixed with methanol-free PFA, washed with Hank’s balanced salt solution (HBSS) and then stained with the rest of the stains.
Cells were then imaged with a high content confocal microscope (PerkinElmer Opera Phenix; FIMM High Content Imaging and Analysis Unit FIMM-HCA, FIMM, HiLIFE, UH, Finland) using a 40x water immersion objective (NA 1.1) and 7 optical Z-planes with 1 µm interval to optimize the spatial resolution of features in filanesib-treated cells. In this system, we used the confocal mode for imaging which utilizes four separate channels. The used labels and wavelengths were Hoechst 33342 for DNA (405 nm ex/435–480 nm em); Concavalin A/Alexa Fluor 488 for endoplasmic reticulum (488 ex/500–550 em); PhenoVue Fluor 568 phalloidin, PhenoVue Fluor 555—WGA and PhenoVue 512 Nuclear Acid stain conjugate (561 ex/570–630 em) and PhenoVue 641 for mitochondria (640 ex/650–760 em).
High-content image analysis
The images obtained from Cell Painting assay by high-content microscopic imaging were analyzed in single-cell level using CellProfiler 3.1.859. The Hoechst channel was first converted to binary image using minimum cross entropy (MCE) based automated thresholding. Individual nuclei were labeled using marker-based watershed transform from the seed points of local maxima of the distance transformed binary image. The outcome label image was masked with the thresholded Hoechst channel to get the final segmented nuclei objects. All nuclei with a diameter of less than 40px or more than 240px were discarded. The cells were segmented by propagating from the segmented nuclei objects to MCE thresholded Alexa488 (endoplasmic reticulum) channel. In total, 300 intensity, morphological and texture features were measured from each segmented cell for downstream analysis.
Jupyter Notebook using Python 3.6.13 was developed for downstream analysis. As a preprocessing step, all features were normalized plate-wise using standard scaling of the negative control samples (DMSO). The scaled single-cell features were visualized separately for each PDX model using Uniform Manifold Approximation and Projection (UMAP)60. The similarity of treatment responses was studied using data of all PDX models by hierarchical clustering with Euclidean distance metric.
Cell cycle analysis
HB-279, HB-284, HB-243, and HUH6 cells (5.0 × 105) were seeded in six-well plates and allowed to adhere for 24 h. Next, HB-279, HB-284, and HB-243 cells were treated with 10 nM filanesib or 50 nM luminespib or DMSO for 24 h (filanesib) or 48 h (luminespib), trypsinized, and fixed with 70% ethanol at −4 °C. HUH6 cells were transfected with NT siRNA or KIF11 siRNA for 72 h, trypsinized, and fixed with 70% ethanol at −4 °C. Fixed cells were washed with 1% BSA in PBS, followed by incubation with FxCycle PI/RNase Staining Solution for 30 min at RT. Cell cycle analysis was conducted using Novocyte Quanteon (Agilent, CA, USA), flow cytometer, and the results were analyzed with NovoExpress software (Agilent). The flow cytometry analysis was performed at the HiLife Flow Cytometry Unit, University of Helsinki.
KIF11 silencing
KIF11 expression was inhibited in HUH6 cells via small interfering RNA (siRNA) transfection. Briefly, adherent HUH6 cells were exposed to 25 nM of KIF11 ON-TARGETplus SMARTpool siRNA or ON-TARGETplus non-targeting (NT) control siRNA (both from Horizon Discovery, Cambridge, UK). Dharmafect 1 (Horizon Discovery) was used to deliver siRNAs into the HUH6 cells. Knockdown efficacy was evaluated at mRNA and protein level 72 h after initiation of transfection. Cells were transfected following manufacturer’s instructions.
In vivo experiments
Each study arm consisted of three mice per PDX. Five different PDXs in total were used. Thus, filanesib arm consisted of 15 mice, luminespib arm had 15 mice and the control group 15 mice. 20 mm3 tumor pieces of HB-243, HB-279, HB-282, HB-284, and HB-295 were subcutaneously implanted into the interscapular area of each female athymic nude mouse (Athymic Nude-Foxn1 nu, ENVIGO, Gannat, France). Mice that met the inclusion criteria of 60–200 mm3 tumors were then after the latency period randomly assigned to one of the study arms. Drugs were administered via oral gavage. Dose of filanesib was 20 mg/kg every 4th day and for luminespib 30 mg/kg three times per week. The treatment period was 18 days or shorter if sacrification criteria was met earlier. A mixture of 10% DMSO, 40% PEG300, 5% tween 80, 45% NaCl 0.9% was used as the vehicle. Animals were weighted at tumor measurement times and monitored daily for physical changes, behavior, and clinical observations. Tumor volume (TV) was evaluated by measuring diameters with a caliper 2–3 times a week using the formula TV (mm3) = [length (mm) × width (mm)2]/2 (length and width being the longest and the shortest diameters of the tumor). The antitumoral effect was assessed by the ratio of mean tumor volume in treated versus control groups. Animals were euthanized by cervical dislocation or by CO2 inhalation followed by observation of rigor mortis. No analgesic drug was used for pain. All experiments were performed in accordance with French legislation concerning the protection of laboratory animals and in accordance with a currently valid license for experiments on vertebrate animals, issued by the French Ministry of Higher Education, Research and Innovation (APAFIS#27068-2017090717367058 v2).
Fresh tumor samples were collected from all mice for FFPE at the end of the study or at the group endpoint, without specific delay after the last dosing. The tumor was fixed in 10% formalin for 24 h, transferred in 70% ethanol for maximum 2 weeks and transferred to further sampling for immunohistochemistry
Immunohistochemistry
Murine HB tumor sections were stained as follows: samples were deparaffinized and 10 mM Tris-1mM EDTA pH9, 20 at min +99 °C was used for antigen target retrieval. 0.9% H2O2 in TBS, 15 min RT was used to block endogenous peroxidase activity. Nonspecific binding was blocked using TBST/ 10% Normal Goat Serum, 15 min RT. Following primary antibodies were used: Rabbit anti KIF11 (PA5-82394, Invitrogen, dilution 1:300) and rabbit anti KIF-15 (PA5-57305, Invitrogen, dilution 1:150) for 1 h RT. Secondary antibody was BrightVision (DPVR55HRP, ImmunoLogic). BrightDAB (BS04-110, ImmunoLogic) was used to visualize the bound antibody.
Images were generated using 3DHISTECH Pannoramic 250 FLASH II digital slide scanner at the FIMM Digital Microscopy and Molecular Pathology Unit (Helsinki Institute of Life Science-HiLIFE, University of Helsinki, and Biocenter Finland).
RNA sequencing and analysis
RNA sequencing protocols for all six untreated HB PDX models are previously described by Kats et al.17. Raw RNA sequencing datasets from previously published studies were obtained from European Genome-phenome Archive (EGA) (https://ega-archive.org/). Accession number for untreated HB-282, HB-295, HB-279, HB-284 and HB-243 cells is EGAS00001004827/EGAD00001006621 and for HB-303 EGAS50000000928/EGAD5000000135. Comparison of untreated HB cell lines to normal hepatocytes has been previously described by Nousiainen et al.61.
Cell models HB-279 and HB-284 were cultured and treated with 10 nM filanesib or DMSO as described above. Total protein and RNA were extracted after 24 h incubation using NucleoSpin RNA/Protein extraction kit (Macherey-Nagel, Düren, Germany). Instructions provided by the manufacturer were followed. Sequencing of filanesib-treated HB-279 and HB-282 cells was completed by Genewiz, Leipzig, Germany. Samples went through quality control by fragment analysis before sequencing. RNA libraries were prepared applying polyA selection, after which Illumina compatible cDNA libraries were constructed. Raw RNA sequencing datasets have been deposited to EGA, accession number EGAS50000000899/EGAD50000001314.
The online tool Chipster was used for the analysis of the RNA sequencing data (version 4.0, available https://chipster.rahtiapp.fi/)62. The tool FastQC was used for quality control. Adapters were trimmed using Trimmomatic. Reads were then mapped to human reference genome Homo_sapiens GRCh38.95 using HISAT2 for paired end reads63. Aligned reads per genes were counted using HTseq64. Differential gene expression analyses were performed using DESeq2 (R 4.2.1, DESeq2 1.38.3). Genes were considered significantly differentially expressed with an adjusted p ≤ 0.1 for a minimum absolute log2 fold change of 0.6. Gene set enrichment analysis was performed with GSEA (4.3.2; preranked on the DESeq2 log2FC estimate and using the weighted scoring scheme). FDR < 0.25 was considered significant.
Statistical analyses
For viability assays, apoptosis measurements, and cell cycle analysis, statistical significance was assessed using Student’s t-test. *p < 0.05 was considered as a statistically significant and **p < 0.01 as a statistically highly significant. All experiments were performed at least in triplicates.
Data availability
The RNA sequencing datasets generated and/or analyzed during the current study are publicly available in the European Genome-phenome Archive (EGA) (https://ega-archive.org/, accession number EGAS00001004827/EGAD00001006621 for untreated HB-282, HB-295, HB-279, HB-284, and HB-243, accession number EGAS50000000928/EGAD5000000135 for untreated HB-303 and accession number EGAS50000000899/EGAD50000001314 for filanesib-treated HB-279 and HB-284). Other data, such as detailed drug screen results, can be provided by the corresponding author upon justified request.
Code availability
Publicly available codes have been cited in the related Methods sections. Scripts used in the high-content image analysis are available via GitHub repository (https://github.com/lopaavol/filanesib-hepatoblastoma).
References
Feng, J. et al. Incidence trends and survival prediction of hepatoblastoma in children: A population-based study. Cancer Commun. 39, https://doi.org/10.1186/S40880-019-0411-7 (2019).
Linabery, A. M. & Ross, J. A. Trends in childhood cancer incidence in the US. (1992-2004). Cancer 112, 416–432 (2008).
Spector, L. G., Feusner, J. H. & Ross, J. A. Hepatoblastoma and low birth weight. Pediatr. Blood Cancer 43, 706 (2004).
Hirschman, B. A., Pollock, B. H. & Tomlinson, G. E. The spectrum of APC mutations in children with hepatoblastoma from familial adenomatous polyposis kindreds. J. Pediatr. 147, 263–266 (2005).
Curia, M. C. et al. Sporadic childhood hepatoblastomas show activation of b-catenin, mismatch repair defects and p53 mutations. Mod. Pathol. 21, 7–14 (2008).
Nichols, K. E., Malkin, D., Garber, J. E., Fraumeni, J. F. Jr & Li, F. P. Germ-line p53 mutations predispose to a wide spectrum of early-onset cancers. Cancer Epidemiol. Biomark. Prev. 10, 83–87 (2001). Accessed October 3, 2023.
Kim, S. Y. et al. Genomic profiles of a hepatoblastoma from a patient with Beckwith-Wiedemann syndrome with uniparental disomy on chromosome 11p15 and germline mutation of APC and PALB2. Oncotarget 8, 91950–91957 (2017).
Finegold, M. J. et al. Liver tumors: pediatric population. Liver Transpl. 14, 1545–1556 (2008).
Meyers, R. L. et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 18, 122–131 (2017).
Espinoza, A. F. et al. Navigating relapsed hepatoblastoma: predictive factors and surgical treatment strategy. Cancer Med. 12, 21270–21278 (2023).
Brock, P. R. et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N. Engl. J. Med. 378, 2376–2385 (2018).
Kremer, L. C. M., Van Dalen, E. C., Offringa, M. & Voûte, P. A. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Ann. Oncol. 13, 503–512 (2002).
Gröbner, S. N. et al. The landscape of genomic alterations across childhood cancers. Nature 555, 321–327 (2018).
Eichenmüller, M. et al. The genomic landscape of hepatoblastoma and their progenies with HCC-like features. J. Hepatol. 61, 1312–1320 (2014).
Yu, F., et al. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 6, 1–24 (2021).
Kluiver, T. A. et al. Divergent WNT signaling and drug sensitivity profiles within hepatoblastoma tumors and organoids. Nat. Commun. 15, 8576 (2024).
Kats, D. et al. Volasertib preclinical activity in high-risk hepatoblastoma. Oncotarget 10, 6403–6417 (2019).
Zhang, J. H., Chung, T. D. & Oldenburg, K. R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. SLAS Discov. 4, 67–73 (1999).
Yadav, B. et al. Quantitative scoring of differential drug sensitivity for individually optimized anticancer therapies. Sci. Rep. 4, https://doi.org/10.1038/SREP05193 (2014).
Liberzon, A. et al. The molecular signatures database hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Cairo, S. et al. A combined clinical and biological risk classification improves prediction of outcome in hepatoblastoma patients. Eur. J. Cancer 141, 30–39 (2020).
Feodoroff, M. et al. Comparison of two supporting matrices for patient-derived cancer cells in 3D drug sensitivity and resistance testing assay (3D-DSRT). SLAS Discov. 28, 138–148 (2023).
Mehta, G., Hsiao, A. Y., Ingram, M., Luker, G. D. & Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control Release 164, 192–204 (2012).
Fontoura, J. C. et al. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C. 107, 110264 (2020).
Kapałczyńska, M. et al. 2D and 3D cell cultures—a comparison of different types of cancer cell cultures. Arch. Med. Sci. 14, 910–919 (2018).
Saltsman, J. A. et al. A Human organoid model of aggressive hepatoblastoma for disease modeling and drug testing. Cancers12, 1–18 (2020).
Clavería-Cabello, A. et al. Identification and experimental validation of druggable epigenetic targets in hepatoblastoma. J. Hepatol. 79, 989–1005 (2023).
Espinoza, A. F. et al. A novel treatment strategy utilizing panobinostat for high-risk and treatment-refractory hepatoblastoma. J. Hepatol. 80, 610–621 (2024).
Trepel, J., Mollapour, M., Giaccone, G. & Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 10, 537–549 (2010).
Hurley, E. H. et al. Inhibition of heat shock factor 1 signaling decreases hepatoblastoma growth via induction of apoptosis. Am. J. Pathol. 193, 148–160 (2023).
Felip, E. et al. Phase 2 study of the HSP-90 inhibitor AUY922 in previously treated and molecularly defined patients with advanced non–small cell lung cancer. J. Thorac. Oncol. 13, 576–584 (2018).
Augello, G. et al. Targeting HSP90 with the small molecule inhibitor AUY922 (luminespib) as a treatment strategy against hepatocellular carcinoma. Int J. Cancer 144, 2613–2624 (2019).
Thompson, P. A. et al. Vincristine/irinotecan/temsirolimus upfront window treatment of high-risk hepatoblastoma: a report from the Children’s Oncology Group AHEP0731 Study Committee. Pediatr. Blood Cancer 70, e30365 (2023).
Mora, E., Lavoie Smith, E. M., Donohoe, C. & Hertz, D. L. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am. J. Cancer Res. 6, 2416–2430 (2016). Accessed March 14, 2024.
Shahin R., Aljamal S. Kinesin spindle protein inhibitors in cancer: from high throughput screening to novel therapeutic strategies. Future Sci. OA. 8, https://doi.org/10.2144/FSOA-2021-0116/ASSET/IMAGES/LARGE/FIGURE4.JPEG (2022).
Sawin, K. E., Leguellec, K., Philippe, M. & Mitchison, T. J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature 359, 540–543 (1992).
Heck, M. M. S. et al. The kinesin-like protein KLP61F is essential for mitosis in Drosophila. J. Cell Biol. 123, 665–679 (1993).
Konjikusic, M. J., Gray, R. S. & Wallingford, J. B. The developmental biology of kinesins. Dev. Biol. 469, 26–36 (2021).
Luo, Y. et al. KIF11 as a potential cancer prognostic marker promotes tumorigenesis in children with Wilms tumor. Pediatr. Hematol. Oncol. 39, 145–157 (2022).
Zhu, L. et al. KIF11 serves as a cell cycle mediator in childhood acute lymphoblastic leukemia. J. Cancer Res. Clin. Oncol. 149, 15609–15622 (2023).
Cox, C. D. et al. Kinesin spindle protein (KSP) inhibitors. Part 1: the discovery of 3,5-diaryl-4,5-dihydropyrazoles as potent and selective inhibitors of the mitotic kinesin KSP. Bioorg. Med. Chem. Lett. 15, 2041–2045 (2005).
Chen, Y., Chow, J. P. H. & Poon, R. Y. C. Inhibition of Eg5 acts synergistically with checkpoint abrogation in promoting mitotic catastrophe. Mol. Cancer Res. 10, 626–635 (2012).
Turaga, S. M. et al. Inducing mitotic catastrophe as a therapeutic approach to improve outcomes in Ewing sarcoma. Cancers15, 4911 (2023).
Jungwirth, G. et al. KIF11 inhibitors filanesib and ispinesib inhibit meningioma growth in vitro and in vivo. Cancer Lett. 506, 1–10 (2021).
Decarreau, J. et al. A tetrameric kinesin, Kif25, suppresses centrosome separation to establish proper spindle orientation. Nat. Cell Biol. 19, 384 (2017).
Torres, J. Z. et al. The STARD9/Kif16a kinesin associates with mitotic microtubules and regulates spindle pole assembly. Cell 147, 1309–1323 (2011).
Pan, D. et al. Filanesib plus bortezomib and dexamethasone in relapsed/refractory t(11;14) and 1q21 gain multiple myeloma. Cancer Med. 11, 358–370 (2022).
Shah, J. J. et al. A Phase 1 and 2 study of Filanesib alone and in combination with low-dose dexamethasone in relapsed/refractory multiple myeloma. Cancer 123, 4617–4630 (2017).
Khoury, H. J. et al. A phase 1 dose-escalation study of ARRY-520, a kinesin spindle protein inhibitor, in patients with advanced myeloid leukemias. Cancer 118, 3556–3564 (2012).
Ocio, E. M. et al. Filanesib in combination with pomalidomide and dexamethasone in refractory MM patients: safety and efficacy, and association with alpha 1-acid glycoprotein (AAG) levels. Phase Ib/II Pomdefil clinical trial conducted by the Spanish MM group. Br. J. Haematol. 192, 522–530 (2021).
Lorusso, P. M. et al. First-in-human phase 1 study of filanesib (ARRY-520), a kinesin spindle protein inhibitor, in patients with advanced solid tumors. Investig. New Drugs 33, 440–449 (2015).
Hansson, K. et al. Therapeutic targeting of KSP in preclinical models of high-risk neuroblastoma. Sci. Transl. Med. 12, https://doi.org/10.1126/scitranslmed.aba4434 (2020).
Souid, A. K. et al. A pediatric phase I trial and pharmacokinetic study of ispinesib: a Children’s Oncology Group phase I consortium study. Pediatr. Blood Cancer 55, 1323–1328 (2010).
Potdar, S. et al. Breeze: an integrated quality control and data analysis application for high-throughput drug screening. Bioinformatics 36, 3602–3604 (2020).
Chen, Y. et al. Robust scoring of selective drug responses for patient-tailored therapy selection. Nat. Protoc. 19, 60–82 (2024).
Nicoletti, I., Migliorati, G., Pagliacci, M. C., Grignani, F. & Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139, 271–279 (1991).
Gürtler, A. et al. Stain-Free technology as a normalization tool in Western blot analysis. Anal. Biochem. 433, 105–111 (2013).
Bray, M. A. et al. Cell Painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat. Protoc. 11, 1757–1774 (2016).
McQuin, C. et al. CellProfiler 3.0: next-generation image processing for biology. PLoS Biol. 16, e2005970 (2018).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2018).
Nousiainen, R. et al. UBE2C expression is elevated in hepatoblastoma and correlates with inferior patient survival. Front. Genet. 14, https://doi.org/10.3389/FGENE.2023.1170940/FULL (2023).
Kallio, M. A. et al. Chipster: user-friendly analysis software for microarray and other high-throughput data. BMC Genom. 12, 507 (2011).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Acknowledgements
We thank the DDCB core facility FIMM High Throughput Biomedicine (HTB) unit, and FIMM High Content Imaging and Analysis (HCA) unit (supported by Helsinki Institute of Life Science-HiLIFE, University of Helsinki, Biocenter Finland and EuroBioImaging). KIF11 immunostaining was performed at the FIMM Digital Microscopy and Molecular Pathology Unit (supported by Helsinki Institute of Life Science-HiLIFE/Faculty of Medicine, University of Helsinki, and Biocenter Finland). Flow cytometry analysis was performed at the HiLIFE Flow Cytometry Unit, University of Helsinki. The study was financially supported by Sigrid Jusélius Foundation (M.H.), Helsinki University Hospital Funds (M.H.), Päivikki and Sakari Sohlberg Foundation (M.P., V.P.), AAMU Suomen Lasten Syöpäsäätiö (M.P., V.P.), Emil Aaltonen Foundation (R.N.), Mary and Georg Ehrnrooth Foundation (R.N.), Cancer Foundation Finland (V.P.), Kymenlaakso Regional Fund/Finnish Cultural Foundation (V.P.), Lasten Syöpäsäätiö Väre (R.N., V.P.) and Research Council of Finland (LP).
Author information
Authors and Affiliations
Contributions
Study concept and design: K.E., M.H., M.P., V.P.; acquisition of data: R.N., K.E., J.S., A.H., T.J.L., S.P., M.J.F., L.P., M.P.; analysis and interpretation of data: R.N., K.E., J.S., A.H., T.J.L., S.P., J.L., L.P., V.P., M.P.; drafting of the manuscript: R.N., K.E.; critical revision of the manuscript for important intellectual content: K.E., V.P., D.B.W., M.H., M.P.; statistical analysis: R.N., K.E., S.P., L.P.; obtained funding: R.N., M.H., M.P., V.P.; administrative, technical, or material support: E.I., S.C.; study supervision: K.E., V.P., M.H., M.P.
Corresponding author
Ethics declarations
Competing interests
E.I. is currently employed by the company XenTech. S.C. has formerly been employed by the company XenTech and is currently employed by the company Champions Oncology. No other competing interests to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nousiainen, R., Eloranta, K., Saarela, J. et al. Functional screening identifies kinesin spindle protein inhibitor filanesib as a potential treatment option for hepatoblastoma. npj Precis. Onc. 9, 122 (2025). https://doi.org/10.1038/s41698-025-00915-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-00915-8