Abstract
Intracranial electrical kilohertz stimulation has recently been shown to achieve similar therapeutic benefit as conventional frequencies around 140 Hz. However, it is unknown how kilohertz stimulation influences neural activity in the mammalian brain. Using cellular calcium imaging in awake mice, we demonstrate that intracranial stimulation at 1 kHz evokes robust responses in many individual neurons, comparable to those induced by conventional 40 and 140 Hz stimulation in both the hippocampus and sensorimotor cortex. The evoked responses at the single-cell level are shaped by prominent network inhibition and critically depend on brain region. At the network level, all frequencies lead to pronounced population suppression except 1 kHz in the cortex, which evokes balanced excitatory and inhibitory population effects. Thus, kilohertz stimulation robustly modulates neural activity at both the single-neuron and population network levels through mechanisms distinct from conventional frequency stimulation, highlighting the clinical potential of intracranial kilohertz neuromodulation.

Similar content being viewed by others
Introduction
The efficacy of electrical deep brain stimulation (DBS) in treating movement disorders has prompted its widespread application across neurological and psychiatric diseases, including depression, epilepsy, and Alzheimer’s disease1,2,3,4,5. However, the therapeutic mechanisms of DBS remain poorly understood, resulting in the adoption of standardized pulse parameters based on precedent, irrespective of the stimulated brain region or targeted neuropathology1,2,3,4,5,6. The parameter space for electrical stimulation pulse patterns is essentially infinite, but one of the most widely analyzed characteristics is frequency. An earlier study showed that higher frequencies (~130–5000 Hz) effectively reduced tremor in essential tremor patients7. Based on this clinical observation, Benabid et al. chose 130 Hz because it was the “maximum frequency available” on the patients’ implanted pulse generators at the time7. This early adoption of 130 Hz ultimately led to its selection as a clinical standard8,9,10.
Lower stimulation frequencies have been widely explored to address intolerance or unresponsiveness to standard high-frequency stimulation. For example, some epilepsy patients are intolerant to high-frequency stimulation (≥100 Hz) in the anterior nucleus of the thalamus or the epileptogenic zone11,12,13,14. A recent retrospective case study revealed that 5 s bursts of 7 Hz responsive neural stimulation (RNS) reduced seizure frequency in focal drug-resistant epilepsy patients unresponsive to high-frequency stimulation15. Lower stimulation frequencies have also been tested for potential cognitive improvement. One study delivered 20 Hz DBS in the nucleus basalis of Meynert and reported cognitive improvement in patients with Parkinson’s disease dementia16. Similarly, theta-burst DBS in hippocampal and associated structures improved memory performance in epilepsy patients17,18. Likewise, theta-frequency stimulation of the subthalamic nucleus (STN) ameliorated symptoms of Parkinson’s disease dementia and enhanced working memory19.
In contrast to the active exploration of lower-frequency intracranial stimulation, studies on ultrahigh-frequency (>333 Hz) stimulation have been limited to the peripheral systems20 despite the initial suggestion of efficacy in 19917. Thus far, implantable clinical neurostimulation systems like DBS and RNS have an FDA-approved upper-frequency limit of 333 Hz21,22,23. Even though battery consumption may be higher compared to lower frequencies, ultrahigh-frequencies are increasingly employed in spinal cord stimulation for pain management, as they reduce paresthesia and associated sleep disturbance and jolting effects which commonly occur during lower frequency stimulation24,25,26. Ultrahigh-frequencies have also been used in peripheral nerve and dorsal root ganglia stimulation to manage pain and in vagal nerve stimulation to treat morbid obesity27,28. Recently, Harmsen et al. investigated the clinical efficacy of 10 kHz DBS in the STN and ventral intermediate nucleus for movement disorders29. Their findings revealed no difference in therapeutic efficacy between conventional 130 Hz and 10 kHz DBS, but, notably, 10 kHz DBS produced fewer speech impairments and less stimulation-induced transient paresthesia. Thus, ultrahigh-frequencies in the kilohertz range have the potential to mitigate side effects while preserving therapeutic efficacy20,24,25,26,29.
The biophysical properties of neurons limit membrane voltage responses to high-frequency stimulation30,31. We recently demonstrated that 140 Hz DBS poorly entrained hippocampal neurons’ membrane potentials, in sharp contrast to the robust entrainment observed during 40 Hz DBS32. In the kilohertz range, electrical stimulation blocks nerve conduction, potentially produces local thermal effects, and alters synaptic transmission such as synaptic depletion, fatigue, and facilitation/depression20,33. Despite the clinical potential of intracranial kilohertz stimulation7,29, experimental analysis of kilohertz stimulation has been limited to nerves27,34,35,36, in vitro studies37,38, and computational modeling30,33. Recent studies in isolated brain tissue confirmed that kilohertz stimulation alters mammalian neuron activity, likely through direct excitation at the soma rather than the axon37,38. However, it is unknown whether and how kilohertz stimulation engages neurons in the mammalian brain.
We compared the effects of kilohertz and conventional frequency stimulation on individual neurons in awake, head-fixed mice. Using cellular calcium imaging, free from electrical stimulation artifacts, we optically measured the real-time responses of individual neurons in the hippocampal CA1 and sensorimotor cortex to local electrical stimulation delivered at 40 Hz, 140 Hz, and 1 kHz. We found that 1 kHz evoked precisely timed somatic calcium changes in many individual neurons, comparable to those induced by conventional 40 Hz and 140 Hz stimulation. As somatic calcium dynamics are critical for cellular signaling and are related to spiking39,40,41, these results provide direct experimental evidence that kilohertz stimulation alters neuronal activity at the single-cell level. Furthermore, the temporal dynamics of the evoked responses depended on brain region and stimulation frequency. Across the population of recorded neurons, conventional frequencies suppressed overall activity in both brain regions. In contrast, 1 kHz stimulation only suppressed population network activity in CA1, but not in cortex, highlighting distinct mechanisms engaged by ultrahigh-frequency stimulation. These experimental results, with single neuron resolution, underscore the potential of kilohertz frequency stimulation in clinical neuromodulation.
Results
Electrical stimulation at 40 Hz, 140 Hz and 1 kHz evoked prominent and heterogenous intracellular calcium changes across individual neurons in the CA1 and the sensorimotor cortex
To investigate how kilohertz and conventional frequency electrical stimulation modulate individual neurons across brain regions in awake mammals, we performed calcium imaging in the hippocampal CA1 and the superficial layers of the sensorimotor cortex in freely locomoting, head-fixed mice. A custom imaging window was surgically placed over the CA1 or the cortex, providing optical access to neurons transduced with the genetically encoded calcium sensor GCaMP7f via AAV9-syn-jGCaMP7f (Fig. 1a, b). Using a custom microscope (Fig. 1c), we imaged a large field-of-view containing tens to hundreds of neurons while delivering electrical stimulation via a nearby intracranial electrode (Fig. 1d). Each recording session included 10–20 trials, with each trial consisting of 5 s baseline, 5 s stimulation, and 10 s post-stimulation (Fig. 1e–j). An 18 s intertrial interval was included to minimize photobleaching of GCaMP7f.
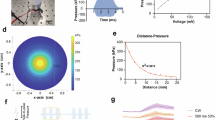
a, b An illustration of the animal preparation for the (a) CA1 and (b) cortex, showing optical imaging chambers with nearby stimulation and ground electrodes. c Schematic of the widefield calcium imaging experimental setup in freely locomoting head-fixed mice. d (left) An example max-min projected imaging field of view of the GCaMP7f fluorescence during a CA1 recording session testing 140 Hz stimulation, (middle) a heatmap depicting the theoretical electric field strength dissipation, and (right) the overlay of the left and middle panels. White dotted circles mark every 50 µm from the center of the electrode tip. The fluorescence traces of four example modulated neurons (outlined in yellow in the left and right panels) are shown in f. e The normalized fluorescence traces of example CA1 neurons during 40 Hz stimulation, aligned to stimulation onset. Every trial is shown in gray (10 trials total); the average fluorescence trace across all trials is shown in red for activated neurons and blue for suppressed neurons. f–j Additional example modulated neurons for the other experimental conditions, with electrical stimulation period highlighted by the bar on the top (green, for CA1; yellow for cortex). (e) 40 Hz in the CA1, (f) 140 Hz in the CA1, (g) 1 kHz in the CA1, (h) 40 Hz in the cortex, (i) 140 Hz in the cortex, and (j) 1 kHz in the cortex.
Clinical electrical stimulation parameters vary widely and are tailored to each patient to balance therapeutic benefits, side effects, and battery lifetime21,22,23. Comparing stimulation parameters between mice and humans is difficult, as the mouse brain is over 2000 times smaller than that of humans. Thus, to compare the stimulation-evoked effects across frequencies and brain regions, we kept the stimulation pulse-width constant as in general clinical practice. For each mouse, we first empirically determined the effective stimulation current by gradually increasing amplitude until a detectable change in GCaMP7f fluorescence was elicited without inducing noticeable behavioral responses. Stimulation pulses were current-controlled cathode-leading biphasic square waves delivered at 40 Hz (200 µs/phase), 140 Hz (200 µs/phase), or 1 kHz (45 µs/phase) (Fig. 1e–j). For 40 Hz, the average current amplitude was 66.6 ± 42.6 µA (mean ± standard deviation, n = 11 mice, ranged 7–175 µA, corresponding to a charge density of 11.1–276.3 µC/cm2/phase at the electrode tip). Similarly, for 140 Hz, it was 62.7 ± 40.8 µA (n = 11 mice, ranged 5–175 µA, 7.9–276.3 µC/cm2/phase), and for 1 kHz, it was 85.6 ± 73.1 µA (n = 10 mice, ranged 5–340 µA, 1.8–120.8 µC/cm2/phase). Stimulation amplitude was largely consistent for a given frequency within each mouse across recording days that lasted up to ~1 month for cortex and ~8 months for CA1 (Suppl. Figure 1g,h). However, the effective current amplitude varied by mouse, and the cortex generally required higher amplitudes than CA1 (Suppl. Figure 1a,d). All charge densities used were below the tissue-damaging threshold observed previously in similar rodent studies42,43,44.
Our imaging field-of-view covered a large area of ~1 mm2 with many simultaneously recorded neurons being separated by tens to hundreds of microns. As an electric field dissipates rapidly from the electrode tip in a 3-dimensional tissue environment, neurons within each recording were exposed to highly variable stimulation strength. To estimate the electric field decay under our experimental condition, we modeled the spatial gradient of the electric field from the electrode tip using the inverse square law. The electrode tip was modeled as a point source and the electric field decay was approximated as change in uniform charge density in homogenous isotropic brain matter with a frequency-dependent permittivity (ε40Hz = 1.64 × 107, ε140Hz = 2.16 × 107, ε1kHz = 1.64 × 105)45. The modeled electric field decayed rapidly from the center of the electrode tip (Fig. 1d), dropping by 3 orders of magnitude at 50 µm, and by 5 orders at 300 µm. Consequently, the variability in current amplitude across recording sessions was comparable to the variation in electric field strength across our imaging field-of-view.
To quantify stimulation-evoked cytosolic Ca2+ changes across individual neurons, we processed the recorded videos, segmented neuronal soma, and extracted GCaMP7f fluorescence traces for each neuron (details in Methods, Fig. 1d,f). A total of 11,289 neurons were imaged, revealing robust GCaMP7f fluorescence changes in many neurons during electrical stimulation at all three frequencies in both brain regions (Fig. 1e-j, Suppl. Figure 2). The evoked cellular responses were highly diverse, with some neurons displaying immediate GCaMP7f fluorescence increases and others prominent decreases, consistent with the general observation of both excitatory and inhibitory effects upon electrical stimulation in animal models and human patients46,47,48,49. Surprisingly, 1 kHz stimulation evoked robust and reliable cellular responses similar to those evoked by 40 Hz and 140 Hz (Fig. 1g, j, Suppl. Figure 2).
Neurons were reliably modulated by electrical stimulation across trials, and the proportion of modulated neurons depended on brain region and stimulation frequency
To determine whether a neuron was modulated by stimulation, we compared the mean fluorescence during stimulation (0–5 s) to baseline (–5-0s) across trials using Wilcoxon signed-rank test (details in Methods). Neurons were classified as activated if the mean fluorescence increased during stimulation or suppressed if it decreased (Fig. 2a). Across test conditions, 18–28% of cells were modulated (Fig. 2b–g). In the CA1, 1 kHz stimulation modulated a greater fraction of neurons than 40 Hz or 140 Hz (Fig. 2b–d,h,i), primarily through increased suppression rather than activation (Fig. 2h(i), h(iii)). This was observed consistently across recording sessions (Fig. 2l). While a greater fraction of neurons was suppressed than activated by 1 kHz stimulation (Fig. 2l), 40 and 140 Hz modulated a balanced fraction of activated and suppressed neurons (Fig. 2j, k). The prominent suppression evoked by 1 kHz stimulation suggests distinct cellular processes engaged by 1 kHz versus conventional frequencies, likely through better recruitment of inhibitory mechanisms.
a Heatmap of 140Hz-evoked responses across 3058 CA1 neuron. Every row is the mean normalized fluorescence of a neuron across trials. The color corresponds to the change relative to baseline, with red indicating increases, blue indicating decreases, and white indicating minimal change. Of the neurons recorded, 318 were activated (red, +, top), 2441 were unchanged (gray, Ø, middle), and 299 were suppressed (blue, -, bottom). b–g The fraction of activated, suppressed, and unchanged neurons for (b) 40 Hz in the CA1, (c) 140 Hz in the CA1, (d) 1 kHz in the CA1, (e) 40 Hz in the cortex, (f) 140 Hz in the cortex, and (g) 1 kHz in the cortex. h Comparison across stimulation frequencies for the fraction of (i, ii) activated and (iii, iv) suppressed neurons in CA1 and cortex. Bar plots are ± 95% confidence intervals. (Chi-squared test, statistical results in Suppl. Table 1). i Comparison of the fraction of modulated neurons for CA1 and cortex. (Chi-squared test, statistical results in Suppl. Table 1). j–o The number of activated vs. suppressed neurons across experimental sessions. Each circle corresponds to a recording session. Solid line is the linear regression with R2 value indicated, and the dotted line corresponds to the diagonal for reference. p Variance of dynamic time warping (DTW) distance across neurons that were activated, suppressed, or unchanged at the three stimulation frequencies in the two brain regions. (Kruskal-Wallis with Dunn-Sidak correction, statistical results in Suppl. Table 1). Violin plots depict the kernel density overlaid with box plots showing the interquartile range (1x, 1.5x). The white lines in the boxes are the median. All tests are two-tailed. n.s., not significant, *p < 0.05, **p < 0.01, ***p < 0.005.
Intriguingly, in the cortex, suppression consistently dominated across all stimulation frequencies (Fig. 2e–i), in contrast to the more balanced activation and suppression observed in CA1. 40 Hz modulated more cortical neurons than the higher frequencies (Fig. 2i), again, primarily due to a larger fraction of suppressed neurons (Fig. 2h(iv), h(ii)), which was also evident across recording sessions (Fig. 2m–o). Furthermore, the proportion of activated cortical neurons increased with frequency, with 1 kHz activating more neurons than 40 Hz (Fig. 2h(ii)). However, the correlation between the fraction of activated and suppressed neurons across sessions was weak for 40 Hz and 140 Hz (Fig. 2m,n), in contrast to 1 kHz (Fig. 2o), or any stimulation frequency in CA1 (Fig. 2j–l). To account for the variability in stimulation current amplitude across recording sessions, we examined the fraction of activated (Suppl. Figure 1c,f) and suppressed (Suppl. Figure 1b,e) neurons relative to charge density and total charge delivered, detecting no correlation. Together, these results demonstrate that the stimulation-evoked responses exhibited greater suppression in the cortex than CA1, which became less prominent as stimulation frequency increased.
Despite substantial variations in response profiles across different neurons, the evoked responses in the same neuron were largely consistent across trials (Fig. 1e–j). To quantify the consistency of the evoked responses for each neuron, we computed the variance of dynamic time warping (DTW) distance50 between the GCaMP7f fluorescence during each trial and the trial-average fluorescence trace. If stimulation elicited similar responses across trials, the variance of the evoked responses would be smaller compared to spontaneous fluctuations. As expected, the variance of DTW distances for both activated and suppressed neuron populations was significantly smaller than the unmodulated neurons across all conditions, except 1 kHz in the activated cortical population (Fig. 2p). The more random responses evoked by 1 kHz stimulation were consistent with a previous modeling study30, further underscoring the unique cellular effects of kilohertz stimulation. It is intriguing to observe consistent GCaMP7f fluorescence drop across trials in suppressed neurons (Figs. 1e-j, 2p), which most likely reflects changes in cellular signaling related to calcium dynamics, as it is difficult to detect spike-inhibition mediated reduction of the generally low cytosolic calcium concentrations51. Together, these results demonstrate that 1 kHz robustly and consistently modulated cytosolic calcium in individual CA1 and cortical neurons, evoking responses in a comparable or higher fraction of neurons than 40 Hz and 140 Hz. The fraction of suppressed neurons is sensitive to stimulation frequency in both brain regions, with 1 kHz suppressing more CA1 neurons and 40 Hz suppressing more cortical neurons.
1 kHz evoked excitation and inhibition in CA1 do not correlate with electric field strength, unlike 40 Hz and 140 Hz
The direct response of a neuron to electrical stimulation critically depends on its biophysical properties and synaptic inputs. For example, low-intensity stimulation that mildly depolarizes membrane potentials could increase spiking in response to inputs, whereas high-intensity stimulation that leads to strong and prolonged depolarization could inactivate sodium channels and thereby block spiking. Furthermore, electrical pulse trains could recruit non-physiological cellular ionic currents and complex network effects. As simultaneously recorded neurons were exposed to highly variable electric field strength depending on their distance to the electrode tip, we further analyzed the recordings obtained in a CA1 mouse whose electrode tip was within the imaging field-of-view (Fig. 3, Suppl. Figure 3).
a Example fields-of-view showing max-min projected GCaMP7f fluorescence in the CA1 in a mouse with visible electrode tip during (i) 40 Hz, (ii) 140 Hz, and (iii) 1 kHz stimulation. The electrode is outlined with a white dashed rectangle, and its tip marked with a white cross. White dashed circles mark every 200 µm from the electrode tip. Every neuron is marked with a small colored circle (red = activated, blue = suppressed, gray = unchanged) outlined in white. (b) The fraction of neurons being activated (red) or suppressed (blue) within each distance bin across recordings (0–200 µm, 200–400 µm, and >400 µm) during (i) 40 Hz, (ii) 140 Hz, and (iii) 1 kHz stimulation (±95% confidence interval; Chi-squared test, statistical results in Suppl. Table 1). c The mean GCaMP7f fluorescent traces (±95% confidence interval) of activated (red) and suppressed (blue) neurons in each bin (0–200 µm = lightest shade, 200–400 µm = middle shade, and >400 µm = darkest shade) during (i) 40 Hz, (ii) 140 Hz, and (iii) 1 kHz stimulation. d Quantification of the area under the curve (AUC) during stimulation for the traces shown in c during (i) 40 Hz (ii) 140 Hz, and (iii) 1 kHz stimulation (Kruskal-Wallis with Dunn-Sidak correction, statistical results in Suppl. Table 1). Violin plots depict the kernel density overlaid with box plots showing the interquartile range (1x, 1.5x). The white lines in the boxes are the median. All tests are two-tailed. n.s., not significant, *p < 0.05, **p < 0.01, ***p < 0.005.
Since most imaged neurons were located within 600 µm of the electrode tip (Fig. 3a, Suppl. Figure 3a–c), we binned neurons within 200 µm, 200–400 µm and >400 µm from the electrode tip. The theoretically estimated electric field strength at 200, 400 and 600 µm is around 1.1 × 10−10, 2.8 × 10−11, and 1.3 × 10−11 V/m, respectively. We found that more neurons within 200 µm of the electrode tip were activated than in the farther bins during 40 Hz (30 µA) and 140 Hz (20–25 µA) stimulation, consistent with previous studies showing stronger activation closer to the electrode tip in anesthetized mice44,52. In contrast, during 1 kHz (20 µA) stimulation, activation was more prominent >400 µm from the electrode tip (Fig. 3b), again supporting that 1 kHz stimulation activates neurons through distinct mechanisms than conventional 40 and 140 Hz stimulation.
Previous studies in anesthetized mice did not report the effect of field strength on neuronal suppression, likely because of the difficulty in detecting a reduction of the already low spontaneous activity under anesthesia44,52. Interestingly, we found that the fraction of suppressed neurons showed little change over the spatial bins across all frequencies (Fig. 3b), aside from a slight elevation during 140 Hz stimulation within 200 µm of the tip. To further probe the spatial-dependence of the strength of the evoked responses, we measured the area under the GCaMP7f fluorescent curve (AUC) for each neuron during stimulation. We found that AUC varied substantially across neurons (Suppl. Figure 3c). However, grouping neurons into spatial bins revealed similar reduction of AUC amplitude over distance (Fig. 3c,d). Finally, a similar reduction of AUC over distance to electrode tip was also observed in one cortical recording session where the electrode was visible (Suppl. Figure 4). This result again supports the involvement of network mechanisms in mediating the evoked suppression.
All stimulation conditions suppressed population dynamics despite heterogeneous single-cell effects, except during 1 kHz stimulation in the cortex
After detecting robust modulation in individual neurons, we next evaluated how population network dynamics respond to different stimulation frequencies. Stimulation-evoked network responses ultimately impact the therapeutic outcomes of clinical neuromodulation. As we recorded several hundred neurons for each stimulation condition, we estimated the population response for each condition as the mean across all neurons recorded, regardless of whether the neuron was modulated or not (details in Methods). We then compared the distribution of AUC from these neurons to its corresponding baseline for each stimulation condition (Fig. 4b, d).
a, c The population fluorescence across all neurons (±95% confidence interval) during 40 Hz (darkest shade), 140 Hz (middle shade), and 1 kHz (lightest shade) stimulation in the (a) CA1 (green) and (c) cortex (yellow). b, d Area under the curve (AUC) during stimulation in the (b) CA1 (Wilcoxon signed-rank test, statistical results in Suppl. Table 1), and (d) cortex (Wilcoxon signed-rank test, statistical results in Suppl. Table 1). Violin plots depict the kernel density overlaid with box plots showing the interquartile range (1x, 1.5x). The white lines in the boxes are the median. All tests are two-tailed. n.s., not significant, *p < 0.05, **p < 0.01, ***p < 0.005.
In CA1, we noted that population fluorescence dropped immediately at stimulation onset across all frequencies, reached peak suppression at stimulation offset, and remained suppressed for seconds afterward (Fig. 4a). The evoked suppression was significant for all frequencies (Fig. 4b). Interestingly, in the cortex, population fluorescence was only suppressed by 40 Hz and 140 Hz, but not by 1 kHz (Fig. 4c, d). Furthermore, the peak suppression evoked by 40 and 140 Hz stimulation was stronger in the cortex (~2%) than in CA1 (~0.8%), consistent with the greater fraction of suppressed cortical neurons (Fig. 2h(ii), h(iv)). Finally, the temporal dynamics of the cortical suppression varied from that of the CA1, peaking before stimulation offset and followed by a strong rebound (Fig. 4c). Thus, despite single-cell level heterogeneity, population dynamics were consistently suppressed regardless of stimulation frequency or brain region, except during 1 kHz in the cortex (Fig. 4c, d). The unique population effect evoked by 1 kHz cortical stimulation further supports the distinct cellular and network mechanisms of kilohertz stimulation compared to conventional frequencies.
40 Hz and 140 Hz evoked largely similar excitatory and inhibitory responses in both brain regions
It is interesting that 40 and 140 Hz stimulation led to a greater fraction of suppressed neurons in the cortex than in the CA1, which may have contributed to the more pronounced population suppression observed in the cortex (Fig. 4). To further understand how the stimulation-evoked individual cell responses relate to population dynamics, we examined the evoked excitation and suppression separately. Across the population of activated neurons in both CA1 and cortex (Fig. 5a–d), 40 and 140 Hz stimulation elicited an immediate rise at stimulation onset and reached a peak towards offset (Fig. 5e, k). At the individual neuron level, the time-to-peak activation varied substantially and many neurons did not reach a peak until seconds after stimulation offset (Fig. 5g, m). The peak time was slightly higher in CA1 than the cortex (Fig. 5g, m), but the evoked AUC showed no difference between the two brain regions (Fig. 5f, l).
a–d Heatmaps showing the normalized fluorescence across all neurons during (a–b) 40 Hz or (c-d) 140 Hz stimulation in the (a, c) CA1 and (b, d) cortex. The neurons were grouped based on modulation classification: activated (top, red, +), unchanged (middle, gray, Ø), or suppressed (bottom, blue, -). e Average fluorescence trace (± 95% confidence interval) in the CA1 (dark green) and cortex (dark yellow) for activated neurons during 40 Hz stimulation. f, g 40 Hz stimulation-evoked (f) AUC and (g) time to peak (Wilcoxon rank-sum, statistical results in Suppl. Table 1). h Average fluorescence trace (± 95% confidence interval) in the CA1 (dark green) and cortex (dark yellow) for suppressed neurons during 40 Hz stimulation. i, j Same as f, g, but for suppressed neurons. (Wilcoxon rank-sum, statistical results in Suppl. Table 1). (k) Average fluorescence trace (± 95% confidence interval) in the CA1 (green) and cortex (yellow) for activated neurons during 140 Hz stimulation. l–m 140 Hz stimulation-evoked response (l) AUC and (m) time to peak (Wilcoxon rank-sum, statistical results in Suppl. Table 1). n Average fluorescence trace (± 95% confidence interval) in the CA1 (green) and cortex (yellow) for suppressed neurons during 140 Hz stimulation. o, p Same as l and m but for suppressed neurons (Wilcoxon rank-sum, statistical results in Suppl. Table 1). Violin plots depict the kernel density overlaid with box plots showing the interquartile range (1x, 1.5x). The white lines in the boxes are the median. All tests are two-tailed. n.s., not significant, *p < 0.05, **p < 0.01, ***p < 0.005.
Across the population of suppressed neurons in both CA1 and cortex, 40 Hz and 140 Hz stimulation elicited an immediate drop at stimulation onset (Fig. 5h, n) and reached peak suppression at stimulation offset. Individual neurons again showed wide variation in peak time with many reaching peak after stimulation offset (Fig. 5j, p). Cortical neurons reached peak sooner than CA1 neurons (Fig. 5j, p), and exhibited a negligible but nonetheless stronger suppression during 40 Hz stimulation than CA1, but not during 140 Hz stimulation (Fig. 5i, o). Thus, the more pronounced population suppression observed in the cortex during 40 and 140 Hz stimulation likely resulted from the higher ratio of suppressed to activated neurons in the cortex compared to the CA1 (Fig. 2j–o). Additionally, the stronger single-cell inhibition evoked by 40 Hz stimulation may have further contributed to the particularly strong population suppression during 40 Hz cortical stimulation (Fig. 4c, d).
1 kHz stimulation-evoked excitation and suppression were both stronger in cortical neurons than in CA1 neurons
It is intriguing that 1 kHz stimulation produced no population changes in the cortex, but robust population suppression in CA1 (Fig. 4), even though a similar fraction (~20%) of neurons were modulated in both regions (Fig. 2d-g). Across activated neurons (Fig. 6a, b), 1 kHz evoked more pronounced population excitation in the cortex than the CA1 (Fig. 6c), which was accompanied by a greater AUC across individual cortical neurons (Fig. 6d). However, peak activation time was the same between activated neurons in the two brain regions (Fig. 6e). Similarly, 1 kHz stimulation also evoked stronger population suppression in the cortex than the CA1 (Fig. 6f), accompanied by a greater AUC across individual cortical neurons (Fig. 6g). Additionally, individual cortical neurons reached a peak earlier than CA1 neurons (Fig. 6h). While a greater fraction of cortical neurons were suppressed by 1 kHz stimulation (Fig. 2g, o), the more prominent and larger magnitude activation (Fig. 6c, d) likely resulted in a lack of overall population effects during 1 kHz stimulation (Fig. 4c, d).
a, b Heatmaps showing the normalized fluorescence for all neurons upon 1 kHz stimulation in the (a) CA1 and (b) cortex. The neurons were grouped based on modulation classification (activated (top, red, +), unchanged (middle, gray, Ø), or suppressed (bottom, blue, -)). (c) Average fluorescence trace (± 95% confidence interval) in the CA1 (light green) and cortex (light yellow) for activated neurons during 1 kHz stimulation. d, e 1 kHz stimulation-evoked (d) AUC and (e) time to peak (Wilcoxon rank-sum, statistical results in Suppl. Table 1). f Average fluorescence trace (± 95% confidence interval) in the CA1 (light green) and cortex (light yellow) for suppressed neurons during 1 kHz stimulation. g, h Same as d and e but for suppressed neurons (Wilcoxon rank-sum, statistical results in Suppl. Table 1). Violin plots depict the kernel density overlaid with box plots showing the interquartile range (1x, 1.5x). The white lines in the boxes are the median. All tests are two-tailed. n.s., not significant, *p < 0.05, **p < 0.01, ***p < 0.005.
The stimulation-evoked excitatory and inhibitory responses were similar in magnitude but differ in temporal dynamics across frequencies within each brain region
After comparing the excitatory and inhibitory effects between brain regions, we further investigated these effects within the same brain regions (Fig. 7a–d). We detected no consistent trends across frequencies within either region (Fig. 7e–l). However, various temporal differences in stimulation-evoked responses were observed (Fig. 7f, j, l), suggesting that distinct network elements may be differentially engaged by different stimulation frequencies in each brain region. In particular, 1 kHz evoked stronger activation than 40 Hz and 140 Hz in cortical neurons (Fig. 7i). Together, these results demonstrate that although there are small differences, 1 kHz evoked excitatory and inhibitory responses within a brain region were largely similar to conventional frequencies, underscoring the importance of considering brain-region specific stimulation effects.
a–d Population fluorescence traces as shown in Figs. 5e,h,k,n and 6c,f are replotted here but grouped by brain region for (a, b) activated neurons and (b, d) suppressed neurons. Solid lines were the mean, and the shaded areas were 95% confidence interval. e-l Violin plots quantifying the difference between 40 Hz, 140 Hz and 1 kHz for the (e-f, i-j) activated neurons and (g-h, k-l) suppressed neurons. e, f AUC and Time to peak for activated neurons in the CA1 (Kruskal-Wallis, statistical results in Suppl. Table 1). (g, h) (g) AUC and (h) time to peak for suppressed neurons in the CA1 (Kruskal-Wallis, statistical results in Suppl. Table 1). (i, j) (i) AUC and (j) time to peak for activated neurons in the cortex (Kruskal-Wallis, statistical results in Suppl. Table 1). (k, l) (k) AUC and (l) time to peak for suppressed neurons in the cortex (Kruskal-Wallis, statistical results in Suppl. Table 1). Violin plots depict the kernel density overlaid with box plots showing the interquartile range (1x, 1.5x). The white lines in the boxes are the median. All Kruskal-Wallis tests were performed with a Dunn-Sidak multiple comparisons correction. n.s., not significant, *p < 0.05, **p < 0.01, ***p < 0.005.
Discussion
Kilohertz electrical stimulation is increasingly used in spinal cord and peripheral nerve stimulation and has recently been explored in the brain for reducing stimulation-evoked side effects20,24,29,34. However, it is unclear how kilohertz electrical stimulation modulates neurons in the mammalian brain. We examined responses evoked by pulsed electrical stimulation at 1 kHz, 140 Hz and 40 Hz using cellular calcium imaging in the CA1 and sensorimotor cortex in awake head-fixed mice. At the individual neuron level, we observed that 1 kHz stimulation, like conventional 40 Hz and 140 Hz, consistently modulated ~20% of neurons, inducing heterogeneous excitatory and inhibitory cellular calcium responses. At the population-level, 1 kHz stimulation selectively suppressed responses in CA1 but not cortex, contrasting with the consistent suppression observed during 40 Hz and 140 Hz stimulation in both brain regions. Stimulation-evoked responses exhibited significant temporal variations across brain regions and frequencies, with the inhibitory responses showing little dependence on electric field strength, suggesting robust network effects. These results provide direct experimental evidence that kilohertz stimulation, like conventional frequency stimulation, reliably modulates individual neuron activities in the awake mammalian brain, though via distinct mechanisms, highlighting the therapeutic potential of kilohertz intracranial electrical stimulation.
Experimental testing of kilohertz stimulation on peripheral nerves has consistently revealed an action potential blockade effect, often called nerve blockade20,34. Biophysical modeling has attributed such effects to the dynamic interplays between the activation and inhibition of voltage-gated sodium and potassium channels34. As neuronal action potentials are limited to a few hundred hertz and membrane capacitance serves as a low pass filter, kilohertz stimulation is unlikely to pace or entrain neuronal spiking20,31,53,54. However transient changes in spiking, particularly at stimulation onset, may drive the observed increase in cytosolic calcium via direct activation of voltage-gated calcium channels or indirect recruitment of calcium signaling downstream of voltage-gated channels. One recent study in hippocampal brain slices found that kilohertz stimulation consistently excited CA1 pyramidal cells, even after blocking excitatory glutamatergic inputs37, supporting a direct cellular effect in addition to synaptic effects. We found that kilohertz stimulation evoked robust and reliable cellular changes across trials, with some neurons consistently showing increased, and others decreased, somatic cytosolic calcium (Fig. 2, Fig. 6).
It is intriguing to detect pronounced reduction in GCaMP7f fluorescence in many neurons in both brain regions. Transient calcium rise has been anecdotally linked to increased spiking, but suppression of cytosolic calcium has not been broadly reported. A lack of calcium transient has been associated with pauses of neuronal firing51. However, we observed consistent drop in GCaMP7f fluorescence that tracked the stimulation duration, instead of a lack of increase in GCaMP7f fluorescence, which cannot be easily explained by reduced spiking (Fig. 1, Suppl Fig. 2). Furthermore, as we used non-specific neuronal labelling with AAV9-syn-jGCaMP7f (Fig. 1d), most of the recorded neurons were excitatory with low spontaneous firing and low basal calcium concentrations55,56,57,58, making it difficult for calcium imaging to detect decreases in somatic calcium associated with suppressed spiking51,59. Thus, the observed calcium suppression most likely reflect changes in cellular signaling related to calcium dynamics, which has been reported in CA1 neurons after mild blast injury60. For example, the membrane potential of mitochondria, a key calcium regulator61, may be directly altered during kilohertz stimulation. Further, kilohertz stimulation has been hypothesized to induce transient and modest heating of local tissue, but this effect remains unvalidated in the brain20,33,62. Finally, kilohertz stimulation may also induce vesicle deformation63,64,65 via electrical tension across the membrane, though its impact on neuronal activity remains unclear. Additionally, we cannot rule out the possibility of changes in cytosolic pH or other factors that may impact GCaMP7f fluorescence. Nonetheless, if such drops in fluorescence were due to changes in GCaMP7f conformation or binding to calcium, it would still support a direct effect of electrical stimulation on protein functions, again underscoring direct engagement of cellular signaling. Thus, in addition to directly suppressing action potentials via nerve blockade, kilohertz stimulation in the brain can evoke diverse yet robust neuronal responses, similar to conventional 40 Hz and 140 Hz stimulation, but through different cellular mechanisms.
While kilohertz stimulation evoked diverse effects on individual neurons, it did not alter the overall population dynamics in the cortex, in contrast to the prominent suppression observed with the two lower frequencies in the cortex and all frequencies in CA1 (Fig. 4). This lack of population suppression, along with stronger stimulation-evoked excitation and suppression at the single-neuron level (Fig. 6), further supports a distinct cellular effect engaged by 1 kHz stimulation compared to the conventional frequencies (Fig. 5). Similarly, analysis of electric field strength revealed that kilohertz-evoked responses were independent of stimulation strength, distinct from 40 Hz and 140 Hz (Fig. 3). Although we were only able to perform this analysis in one CA1 mouse, the electric field dependent effect at conventional frequencies is consistent with previous two-photon calcium imaging studies showing stronger activation closer to the electrode in anesthetized rodents44,52. In awake mice, which exhibit higher levels of spontaneous activity than anesthetized mice, we also observed prominent inhibitory effects that showed minimal dependence on stimulation strength, further supporting the presence of network effects (Fig. 3, Suppl. Figures 3, 4).
Different brain regions have distinct cell-type compositions and tissue architectures. Unsurprisingly, we found that electrical stimulation-evoked responses exhibited prominent differences between brain regions. Notably, far more cortical neurons were suppressed than activated by all stimulation frequencies, whereas a more balanced fraction of suppression and activation was observed in CA1 (Fig. 2). However, further characterization of the excitatory and inhibitory population effects revealed subtle differences across brain regions, both in magnitude and in temporal dynamics, consistent with the brain region-specific individual neuron-level effects (Figs. 5–6). As fast-spiking interneurons are known to better support high-frequency firing than pyramidal cells66, it is possible that fast-spiking interneurons in the cortex were more sensitive to electrical stimulation than CA1 at the frequencies tested here, leading to prominent cortical network inhibition. It is also possible that as stimulation frequency increases, entrainment of cortical fast-spiking interneurons may decrease, leading to less network inhibition, which would be consistent with the observed smaller magnitude population suppression by 140 Hz and a complete lack of population suppression by 1 kHz. Future cell-type-specific analysis is needed to explore these possibilities. The frequency-dependent cortical network suppression underscores the intricate balance between excitatory and inhibitory components recruited by electrical stimulation.
We observed large variation in the temporal kinetics of the stimulation-evoked responses across individual neurons (Fig. 1, Suppl Fig. 2). Interestingly, stimulation-evoked GCaMP7f fluorescence in many neurons, increasing or decreasing, tracked the entire 5 s stimulation period, in contrast to the physiologic calcium transients possessing a rapid rise followed by a slow decay on the order of a second or two. Thus, electrical stimulation could engage cellular signaling distinct from those naturally occurring under physiological conditions. While our previous voltage imaging experiments demonstrated that 40 and 140 Hz had distinct effects on CA1 neuronal firing32, the stimulation-evoked calcium changes observed here showed little difference between these frequencies. The relationship between somatic calcium dynamics and neuronal spiking is complex and likely differs across cell types. Future studies using voltage imaging as in Lowet et al.32 are needed to provide a more detailed understanding of how stimulation-evoked single-neuron voltage dynamics relate to the calcium dynamics observed here, and to population local field potential dynamics observed in electrophysiological studies67,68.
To characterize the stimulation-evoked responses, we varied current amplitude between recording sessions to consistently elicit neural responses without producing noticeable behavioral effects. The variation in stimulation amplitude across CA1 and cortical sites was likely related to differing tissue properties and the placement of the electrode relative to local neurons and fiber tracts. Even though the current amplitude and charge density varied over a wide range, the total charge delivered across conditions was comparable (Suppl. Figure 1d), and the variation between recording sessions was similar to the estimated electric field variation across the ~1 mm2 imaging field-of-view. The effective stimulation current showed little change in the same mice over the study time course (Suppl. Figure 1g, h). Furthermore, the stimulation-evoked suppression showed little correlation with charge density or total charge delivered (Suppl. Figure 1b,e), and there was a lack of total suppression near the electrode where the electric field was strongest (Fig. 3, Suppl. Figures 3, 4). Thus, the inhibition observed cannot be explained by depolarization induced action potential blockade alone, supporting a network mechanism.
It is also worth noting that in the cortex, only a handful of neurons were activated, resulting in a potential under-sampling of the stimulation-evoked cortical activation. Furthermore, electrical stimulation was delivered for 5 s in each trial, and many neurons’ responses peaked and then decayed during stimulation, suggesting that the transient response to electrical stimulation may differ from that during chronic stimulation43,69. Finally, neurons consistently responded across trials, indicating no detrimental effects on cell health at the stimulation intensities used in our study, including during 1 kHz stimulation, which delivered slightly higher total charge. Nonetheless, future exploration is needed to assess safety concerns especially at higher frequencies and current amplitudes. Sinusoidal kilohertz carrier frequencies have been used to target deep brain structures using electrical interference phenomenon53,54, assuming that kilohertz sinusoidal electrical stimulation does not alter neural activity. Future studies are needed to examine how neural activity responds to sinusoidal waves versus biphasic pulse waves.
Methods
Animal Preparation
We have complied with all relevant ethical regulations for animal use. All animal experiments were performed in accordance with the National Institute of Health Guide for Laboratory Animals and approved by the Boston University Institutional Animal Care and Use and Biosafety Committees. Same-sex littermates were housed together prior to surgery and singly housed post-surgery. Cages were enriched with igloos or running wheels. Animal facilities were maintained around 70° F and 50% humidity and mice were kept on a 12 hr light/dark cycle. A total of 14 adult mice, 10 female and 4 male, 10–31 weeks old at the start of experiments were used in this study.
One group of 8 C57BL/6 mice (n = 5 female, n = 3 male) were used for CA1 study. Under isoflurane general anesthesia, these mice first underwent the surgical preparation to affix a preassembled, sterilized recording apparatus over CA1(stereotaxic coordinates: AP −2.0 mm, ML 1.8 mm, DV −1.5 mm from pia) by gently removing the overlying cortical tissue and thinning the corpus callosum. The recording apparatus consisted of a custom imaging window attached to a guide infusion cannula and a stimulation electrode. The imaging window consisted of a stainless-steel cannula (outer diameter 0.317 cm, inner diameter 0.236 cm, height 1.75 mm; Item 3ADD3; Grainger), adhered to a circular coverslip (size 0, outer diameter 3 mm; No. CS-3R-0; Warner Instruments) using an ultraviolet-curable adhesive (Norland Products). The infusion guide cannula (6 mm, 26 gauge; No. 8IC315DCS4SP; P1) was soldered at a 30° angle relative to the coverslip with the end of the guide cannula flush to the base of the imaging cannula. The stimulation electrode was assembled by soldering polyamide-coated steel core wire (diameter 127 µm No. 7N003736501F; P1), cut to ~1 inch in length, to a dip pin (No. ED85100-ND; DigiKey). This electrode was then bent so that the male end of the dip pin was perpendicular, and the wire parallel, to the infusion cannula; the electrode was secured to the infusion cannula with adhesive gel (Loctite 454; Loctite SF 713) and cut to terminate below the middle of the imaging window. During the same surgery, a ground electrode, made by soldering a dip pin (No. ED85100-ND; DigiKey) to a skull screw (No. F000CE094, J.I. Morris Co.), was placed in the motor cortex of the same hemisphere as the imaging apparatus, and a custom aluminum head-plate was attached to the skull posterior to the window. Mice were provided with buprenorphine (0.1 mg/kg) or sustained release (SR) buprenorphine (3.25 mg/kg) pre-operatively and for 48 h following surgery. Mice were singly housed to avoid damage to the recording apparatus after surgery.
After full recovery (14–21 days after surgery), mice were infused with 600–800 nL of AAV9-Syn-jGCaMP7f-WPRE (titer 2 × 1013 GC/mL; 104488-AAV9, Addgene), at a speed of 100 nL per minute. Virus was delivered using a 10 µL syringe (World Precision Instruments, NANOFIL 10 µL syringe) controlled by a micro-infusion pump (Micro-2T, World Precision Instruments) attached to 28 gauge PTFE tubing (No. STT-28; Component Supply Co.) with a 33 gauge infusion cannula (6 mm with 1 mm projection; No. 8IC315IS4SPC; P1). The infusion cannula was removed 10–15 min after the infusion had been completed to improve AAV spread.
A second group of 6 mice was used for sensorimotor cortex study (4 C57BL/6 female; 1 male and 1 female PV-TdTomato mice, crossed between B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (Jax, stock number: 007914) and B6.129P2-Pvalbtm1(cre)Arbr/J (Jax, stock number: 017320) (Jackson Laboratory, Bar Harbor, ME). Under isoflurane general anesthesia, a small craniotomy was made over the sensorimotor cortex (stereotactic coordinate: AP 1.5 mm, ML 1.5 mm), and a total of 350 nL of AAV9-syn-jGCaMP7f-WPRE was injected via a 10 µL syringe (World Precision Instruments, NANOFIL 10 µL syringe) controlled by a micro-infusion pump (MicroPump4, World Precision Instruments) in 3-4 locations within the craniotomy, avoiding major blood vessels, ~250 µm below pia. After viral injection the syringe was left in place for 10–15 min at each location, and then the syringe was removed and a circular glass coverslip (size 0, outer diameter 3 mm; No. CS-3R-0; Warner Instruments) was placed flush with the skull. The coverslip was pressed onto the cortex to ensure stable optical recordings and was secured with ultraviolet-curable cement (Tetric EvoFlow; Ivoclar). Then, a small craniotomy was made posterior to the window, through which a stimulation electrode was inserted at an angle of ~30° towards the center of the imaging window and terminated at ~ 400 µm below the pia. A ground screw was implanted posterior to the window and a custom aluminum head-plate was attached to the skull posterior to the window as that described above for CA1 surgeries. Mice were provided with buprenorphine (0.1 mg/kg) or sustained release (SR) buprenorphine (3.25 mg/kg) pre-operatively and for 48 h following surgery. Mice were singly housed after the surgery.
Electrical Stimulation
Electrical stimulation was delivered by a constant current stimulator (Model 2100/4100; A-M Systems). The stimulation waveform was a cathode-leading biphasic square wave pulse delivered at either 40 Hz or 140 Hz with a 400 µs pulse width (200 µs/phase) or at 1 kHz with a 90 µs pulse width (45 µs/phase). Because of the variation in local tissue matter composition, electrode impedance, and the spatial distance of the implanted stimulation electrode to the optical imaging field-of-view across mice, the current amplitude used was empirically determined for each mouse at the beginning of each experiment as detailed in Lowet et al.32 Briefly, we first tested a handful of neurons in each mouse to determine the effective electrical stimulation current amplitude range for a given mouse by delivering a short burst of stimulation with a current amplitude starting at 10 µA and increasing in 5–10 µA steps. Once we observed any change in GCaMP7f fluorescence in the selected neurons, we deemed that current amplitude as the putative current threshold for that mouse. This process was preformed during the first recording session of each stimulation frequency in each mouse. For subsequent recording sessions, we tested a smaller range of current amplitudes starting at 20 µA below the putative current threshold and increasing in steps of 5–10 µA until we saw an obvious calcium response. See Table 1 for electrical stimulation parameters used.
The stimulation waveforms were verified using an oscilloscope (TDS 2002B; Tektronix). Stimulation pulse train patterns were generated in MATLAB (Mathworks Inc.), and MATLAB triggered the stimulator at the onset of each electrical stimulation train using a digital transistor-transistor logic (TTL) pulse delivered by a NI DAQ board (USB-6259, National Instruments). These TTL pulses were recorded at 20 kHz sampling rate using the Open Ephys system (http://open-ephys.org).
GCaMP7f calcium imaging
Animals were imaged starting 1-2 weeks post viral infusion in the CA1, or 3–4 weeks post viral injection in the sensorimotor cortex, to ensure sufficient GCaMP7f expression. GCaMP7f calcium imaging was performed with a custom wide-field fluorescence microscope equipped with a Hamamatsu ORCA Fusion Digital sCMOS camera (C14440-20UP; Hamamatsu Photonics) and a 10x, 0.3NA water immersion objective (CFI Plan Fluorite; Nikon). In cases where the orientation of the guide cannula blocked part of the field-of-view with the water immersion objective, a 10x, 0.2NA air objective (Epiplan; Zeiss) was used instead. To capture the GCaMP7f fluorescence signal, a blue 470 nm LED (M470L3; Thorlabs Inc.) controlled by a T-Cube LED driver (LEDD1B, low gain; Thorlabs Inc.) was coupled to a 470/25 nm excitation filter, a 495 nm dichroic mirror and a 520/50 nm emission filter.
A custom MATLAB script first triggered frame capture, then triggered electrical stimulation 5 s later, and then repeated the process for each trial after an inter-trial interval of 18 s. TTL trigger pulses were generated in MATLAB and delivered to both the camera and the stimulator via an NI DAQ (USB-6259, National Instruments). We set the image file for each trial to be a length of 400 frames captured at 20 Hz (50 ms exposure, 16 bits, 2 × 2 binning) using HCImage Live (Hamamatsu Photonics) and stored as DCAM image files (DCIMG). Each trial was saved in a separate DCAM file. The first image frames were excluded from all analysis, due to the uncontrolled exposure time. The files were analyzed offline with MATLAB.
Calcium imaging data preprocessing: motion correction
Calcium imaging videos were converted from DCAM image files to TIF image files in MATLAB, and then motion corrected as described in Tseng et al.70. Briefly, motion correction was performed using cross-correlation of each contrast-enhanced and normalized image frame to a reference frame. The reference frame was created by taking the average pixel values across all frames in the first trial. After motion correcting each frame in the first trial, an updated reference frame was created by averaging the pixel values across all motion-corrected frames from the first trial. Each trial then underwent the same motion correction algorithm while continually updating the reference frame. The algorithm outputted the motion-corrected files as HDF5 files and a projection image of the difference between the maximum and minimum intensity of each pixel (max-min projection image) as a TIF to be used for region of interest selection.
Region of interest (ROI) identification
ROIs were first automatically identified using a custom deep learning network developed in Python with Tensorflow/Keras as described in Tseng et al.70. The max-min projection image TIF from our motion correction algorithm was fed into the network and it outputted an ROI mask. The automatically segmented ROIs were then manually inspected and either kept or rejected in a custom MATLAB script. If neurons were not identified automatically by the network, they were manually identified as ROIs in MATLAB using a custom script. Automatically identified ROIs had morphological shapes owing to the segmentation algorithm used, but manually identified ROIs were defined as circles with radius of 15 pixels, corresponding to the neuron.
GCaMP7f fluorescence trace extraction and normalization
After ROI identification, raw GCaMP7f fluorescence traces were extracted from the motion-corrected videos using the mean pixel fluorescence value of all the pixels in a given ROI for each frame. The mean fluorescence value of all the pixels surrounding a given ROI in a donut shape with a 50-pixel outer radius and a 15-pixel inner radius was used to calculate the local background associated with that ROI for each frame. The pixels in the middle of the “donut” correspond to the identified ROI. If there were any other ROIs that overlapped with the surrounding “donut” region, those overlapped pixels were not included in the background fluorescence calculation for the “donut”. This background “donut-with-bites” background trace was then subtracted from the corresponding raw GCaMP7f fluorescence trace to obtain the ROI fluorescence traces.
Because there is an inter-trial interval of 18 s during which GCaMP7f fluorescence was not collected, we computed the normalized FOI fluorescence (Ftrial normalized) using the following equation.
Where Ftrial is the ROI fluorescence during each trial, \({\bar{F}}_{{trial\; baseline}}\) is the average baseline fluorescence of the trial, defined as the mean fluorescence during the 5-s-long baseline period before the onset of the electrical stimulation pulse train, and \({\bar{F}}_{{all\; trials\; baseline}}\) is the mean baseline fluorescence across all trials.
Finally, to compare across ROIs, trial normalized ROI traces were scaled between 0 and 1, where 0 represents the lowest fluorescence value of the entire recorded trace (all trials) and 1 represents the highest fluorescence value of the entire recorded trace. After scaling, the traces were normalized by subtracting the mean fluorescence during the baseline periods before stimulation onset. Thus, the average baseline fluorescence maintained at 0 and all fluorescence changes are equivalently a percent change relative to baseline.
Classification of calcium responses in individual neurons
Individual neuron’s GCaMP7f responses during electrical stimulation were classified into one of three categories: (1) activated, (2) unmodulated, and (3) suppressed. Specifically, for each neuron, we first computed the average fluorescence during baseline and during stimulation across all trials. Wilcoxon signed-rank test was used to determine whether there was a significant difference between baseline and stimulation over all trials for a neuron. If a neuron had a significant change in fluorescence, it was further classified as activated if the average fluorescence over all trials during stimulation was greater than during baseline or suppressed if smaller than during baseline.
Quantifying stimulation-evoked waveform consistency across trials using dynamic time warping (DTW)
To quantify consistency of stimulation-evoked responses across trials in each neurons, we first calculated the dynamic time warping (DTW) distance using the fastdtw library in Python (version 3.12.0)50, using individual GCaMP7f traces around each stimulation trial starting 3 frames before stimulation onset and ending 7 frames after stimulation offset50 and the mean GCaMP7f trace across all trials during the same period. The DTW distance represents the cost of warping stimulation-evoked responses during each individual trial to the overall mean stimulation-evoked response of the neuron. We then computed the variance of the DTW distance across all trials to assess consistency of the stimulation-evoked responses across trials.
Statistics and Reproducibility
All statistical tests were performed in MATLAB 2023a using the standard statistical functions unless otherwise specified. All data distributions were non-normal, therefore only non-parametric tests were used throughout. All sample sizes and test details are included in their corresponding figure legends. A summary of statistical tests performed including sample size, multiple comparison correction test, p-value, and Cliff’s δ value is provided in Suppl. Table 1. Because hundreds to thousands of neurons were recorded under each condition, all statistical tests may be overpowered to detect minimal differences, so we further evaluated Cliff’s δ value to estimate the observed size for any significant tests71.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Example recordings are available along with the software/code at the Github repository: https://github.com/HanLabBU/Ravasio-Nature-Communications-Biology-2024. Additional experimental data are available from the lead contact upon request. The source data for all relevant statistics are provided in Supp. Data 1.
Code availability
Code used for data analysis is available at the Github repository: https://github.com/HanLabBU/Ravasio-Communications-Biology-2024.
References
Alector Inc. A Phase 2 Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Efficacy and Safety of AL002 in Participants With Early Alzheimer’s Disease. https://clinicaltrials.gov/ct2/show/NCT04592874 (2021).
McKinnon, C. et al. Deep brain stimulation: potential for neuroprotection. Ann. Clin. Transl. Neurol. 6, 174–185 (2018).
Lozano, A. M. et al. A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer’s Disease. J. Alzheimers Dis. 54, 777–787 (2016).
Nair, D. R. et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology 95, e1244–e1256 (2020).
Li, M. C. H. & Cook, M. J. Deep brain stimulation for drug-resistant epilepsy. Epilepsia 59, 273–290 (2018).
Mohan, U. R. et al. The effects of direct brain stimulation in humans depend on frequency, amplitude, and white-matter proximity. Brain Stimulation: Basic, Transl., Clin. Res. Neuromodulat. 13, 1183–1195 (2020).
Benabid, A. L. et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337, 403–406 (1991).
Cavallieri, F., Mulroy, E. & Moro, E. The history of deep brain stimulation. Parkinsonism & Related Disorders 105980 https://doi.org/10.1016/j.parkreldis.2023.105980 (2023).
Krauss, J. K. et al. Technology of deep brain stimulation: current status and future directions. Nat. Rev. Neurol. 17, 75–87 (2021).
Lee, D. J., Lozano, C. S., Dallapiazza, R. F. & Lozano, A. M. Current and future directions of deep brain stimulation for neurological and psychiatric disorders: JNSPG 75th Anniversary Invited Review Article. J. Neurosurg. 131, 333–342 (2019).
Heck, C. N. et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 55, 432–441 (2014).
Fisher, R. et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51, 899–908 (2010).
Peltola, J. et al. Deep Brain Stimulation of the Anterior Nucleus of the Thalamus in Drug-Resistant Epilepsy in the MORE Multicenter Patient Registry. Neurology 100, e1852–e1865 (2023).
Yu, T. et al. High-frequency stimulation of anterior nucleus of thalamus desynchronizes epileptic network in humans. Brain 141, 2631–2643 (2018).
Alcala-Zermeno, J. L., Starnes, K., Gregg, N. M., Worrell, G. & Lundstrom, B. N. Responsive Neurostimulation with Low Frequency Stimulation. Epilepsia 64, e16–e22 (2023).
Gratwicke, J. et al. Bilateral Deep Brain Stimulation of the Nucleus Basalis of Meynert for Parkinson Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 75, 169–178 (2018).
Titiz, A. S. et al. Theta-burst microstimulation in the human entorhinal area improves memory specificity. eLife 6, e29515 (2017).
Miller, J. P. et al. Visual-spatial memory may be enhanced with theta burst deep brain stimulation of the fornix: a preliminary investigation with four cases. Brain 138, 1833–1842 (2015).
Salehi, N. et al. Theta frequency deep brain stimulation in the subthalamic nucleus improves working memory in Parkinson’s disease. Brain 147, 1190–1196 (2024).
Neudorfer, C. et al. Kilohertz-frequency stimulation of the nervous system: A review of underlying mechanisms. Brain Stimul. 14, 513–530 (2021).
Premarket Approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p100026.
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P960009 Premarket Approval (PMA).
Health, C. for D. and R. VerciseTM PC, Vercise GeviaTM and Vercise GenusTM Deep Brain Stimulation (DBS) System – P150031/S040. FDA (2021).
Kapural, L. et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology 123, 851–860 (2015).
Van Buyten, J.-P., Al-Kaisy, A., Smet, I., Palmisani, S. & Smith, T. High-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back Pain Patients: Results of a Prospective Multicenter European Clinical Study. Neuromodulation: Technol. Neural Interface 16, 59–66 (2013).
Abraham, M. E. et al. High Frequency 10 kHz Spinal Cord Stimulation as a First Line Programming Option for Patients With Chronic Pain: A Retrospective Study and Review of the Current Evidence. Cureus 13, e17220 (2021).
Billet, B. et al. Wireless high-frequency dorsal root ganglion stimulation for chronic low back pain: A pilot study. Acta Anaesthesiol. Scandinavica 62, 1133–1138 (2018).
Sarr, M. G. et al. The EMPOWER Study: Randomized, Prospective, Double-Blind, Multicenter Trial of Vagal Blockade to Induce Weight Loss in Morbid Obesity. OBES SURG 22, 1771–1782 (2012).
Harmsen, I. E. et al. Ultra-high-frequency deep brain stimulation at 10,000 Hz improves motor function. Mov. Disord. 34, 146–148 (2019).
Couto, J. & Grill, W. M. Kilohertz Frequency Deep Brain Stimulation Is Ineffective at Regularizing the Firing of Model Thalamic Neurons. Front. Comput. Neurosci. 10, 22 (2016).
Hutcheon, B. et al. Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 23, 216–222 (2000).
Lowet, E. et al. Deep brain stimulation creates informational lesion through membrane depolarization in mouse hippocampus. Nat. Commun. 13, 7709 (2022).
Khadka, N., Harmsen, I. E., Lozano, A. M. & Bikson, M. Bio-Heat Model of Kilohertz-Frequency Deep Brain Stimulation Increases Brain Tissue Temperature. Neuromodulation: Technol. Neural Interface 23, 489–495 (2020).
Kilgore, K. L. & Bhadra, N. Reversible nerve conduction block using kilohertz frequency alternating current. Neuromodulation 17, 242–254; discussion 254–255 (2014).
Lee, K. Y., Lee, D., Kagan, Z. B., Wang, D. & Bradley, K. Differential Modulation of Dorsal Horn Neurons by Various Spinal Cord Stimulation Strategies. Biomedicines 9, 568 (2021).
Lee, K. Y. et al. Low-intensity, Kilohertz Frequency Spinal Cord Stimulation Differently Affects Excitatory and Inhibitory Neurons in the Rodent Superficial Dorsal Horn. Neuroscience 428, 132–139 (2020).
Karnup, S. V., Groat, W. D., Beckel, J. & Tai, C. Effect of Biphasic kHz Field Stimulation on CA1 Pyramidal Neurons in Slices. Med. Res. Arch. 10, (2022).
Lesperance, L. S. et al. Artifactual hyperpolarization during extracellular electrical stimulation: Proposed mechanism of high-rate neuromodulation disproved. Brain Stimulation: Basic, Transl., Clin. Res. Neuromodulat. 11, 582–591 (2018).
Huang, L. et al. Relationship between simultaneously recorded spiking activity and fluorescence signal in GCaMP6 transgenic mice. eLife 10, e51675 (2021).
Dana, H. et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657 (2019).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Piallat, B. et al. Monophasic but not biphasic pulses induce brain tissue damage during monopolar high-frequency deep brain stimulation. Neurosurgery 64, 156–162; discussion 162–163 (2009).
Spix, T. A. et al. Population-specific neuromodulation prolongs therapeutic benefits of deep brain stimulation. Science 374, 201–206 (2021).
Michelson, N. J., Eles, J. R., Vazquez, A. L., Ludwig, K. A. & Kozai, T. D. Y. Calcium activation of cortical neurons by continuous electrical stimulation: Frequency dependence, temporal fidelity, and activation density. J. Neurosci. Res. 97, 620–638 (2019).
Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies. https://apps.dtic.mil/sti/citations/ADA305826.
Steiner, L. A. et al. Persistent synaptic inhibition of the subthalamic nucleus by high frequency stimulation. Brain Stimulat. 15, 1223–1232 (2022).
Liu, L. D. et al. Frequency-dependent effects of electrical stimulation in the globus pallidus of dystonia patients. J. Neurophysiol. 108, 5–17 (2012).
Bowles, S. et al. Vagus nerve stimulation drives selective circuit modulation through cholinergic reinforcement. Neuron 110, 2867–2885.e7 (2022).
van den Boom, B. J. G. et al. Unraveling the mechanisms of deep-brain stimulation of the internal capsule in a mouse model. Nat. Commun. 14, 5385 (2023).
Salvador, S. & Chan, P. FastDTW: Toward Accurate Dynamic Time Warping in Linear Time and Space. Intell. Data Anal. 11, 561–580 (2007).
Ali, F. & Kwan, A. C. Interpreting in vivo calcium signals from neuronal cell bodies, axons, and dendrites: a review. Neurophotonics 7, 011402 (2020).
Wu, G. K. et al. Amplitude- and frequency-dependent activation of layer II/III neurons by intracortical microstimulation. iScience 26, 108140 (2023).
Grossman, N. et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 169, 1029–1041.e16 (2017).
Violante, I. R. et al. Non-invasive temporal interference electrical stimulation of the human hippocampus. Nat Neurosci 1–11 https://doi.org/10.1038/s41593-023-01456-8 (2023).
Mohammed, A. I. et al. An integrative approach for analyzing hundreds of neurons in task performing mice using wide-field calcium imaging. Sci. Rep. 6, 20986 (2016).
Shemesh, O. A. et al. Precision Calcium Imaging of Dense Neural Populations via a Cell-Body-Targeted Calcium Indicator. Neuron 107, 470–486.e11 (2020).
Keaveney, M. K. et al. CaMKIIα-Positive Interneurons Identified via a microRNA-Based Viral Gene Targeting Strategy. J. Neurosci. 40, 9576–9588 (2020).
Mount, R. A. et al. Distinct neuronal populations contribute to trace conditioning and extinction learning in the hippocampal CA1. eLife 10, e56491 (2021).
Forli, A. et al. Two-Photon Bidirectional Control and Imaging of Neuronal Excitability with High Spatial Resolution In Vivo. Cell Rep. 22, 3087–3098 (2018).
Hansen, K. R. et al. Mild Blast Injury Produces Acute Changes in Basal Intracellular Calcium Levels and Activity Patterns in Mouse Hippocampal Neurons. J. Neurotrauma 35, 1523–1536 (2018).
Kowaltowski, A. J. et al. Mitochondrial morphology regulates organellar Ca2+ uptake and changes cellular Ca2+ homeostasis. FASEB J. 33, 13176–13188 (2019).
Zannou, A. L. et al. Tissue Temperature Increases by HF10 Spinal Cord Stimulation System: A Phantom Study and Bioheat Model. Neuromodulation 24, 1327–1335 (2021).
Riske, K. A. & Dimova, R. Electric Pulses Induce Cylindrical Deformations on Giant Vesicles in Salt Solutions. Biophys. J. 91, 1778–1786 (2006).
Ye, H. & Curcuru, A. Vesicle biomechanics in a time-varying magnetic field. BMC Biophys. 8, 2 (2015).
Cepkenovic, B., Friedland, F., Noetzel, E., Maybeck, V. & Offenhäusser, A. Single-neuron mechanical perturbation evokes calcium plateaus that excite and modulate the network. Sci. Rep. 13, 20669 (2023).
Cardin, J. A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009).
Buzsáki, G. Theta Oscillations in the Hippocampus. Neuron 33, 325–340 (2002).
Buzsáki, G. & Draguhn, A. Neuronal Oscillations in Cortical Networks. Science 304, 1926–1929 (2004).
Eles, J. R. & Kozai, T. D. Y. In vivo imaging of calcium and glutamate responses to intracortical microstimulation reveals distinct temporal responses of the neuropil and somatic compartments in layer II/III neurons. Biomaterials 234, 119767 (2020).
Tseng, H. et al. Region-specific effects of ultrasound on individual neurons in the awake mammalian brain. iScience 24, 102955 (2021).
Romano, J. & Kromrey, J. Appropriate Statistics for Ordinal Level Data: Should We Really Be Using t-test and Cohen’s d for Evaluating Group Differences on the NSSE and other Surveys?, (2006).
Acknowledgements
We thank members of Han Lab for providing invaluable support throughout the study. X.H. acknowledges funding from NIH (1R01NS115797, 1RF1NS129520) and NSF (DIOS-2002971, CIF-1955981). C.R. acknowledges funding from NSF Graduate Research Fellowship 2021324226. E.L. acknowledges funding from Boston University Center for Systems Neuroscience. R.A.M. acknowledges NIH NRSA fellowship F31MH123008. S.L.Z and E.S.A acknowledge support from the NIH 5T32GM145455. S.K.B acknowledges funding from the Boston University Undergraduate Research Opportunity Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
C.R., K.K., S.Z., and E.L. performed all imaging experiments. C.R. analyzed the data. C.R., K.K., S.Z., R.A.M. prepared the animals for experiments. S.K.B and E.S. provided technical assistance. X.H. supervised the study. C.R. and X.H. wrote the manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Kristin K Sellers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ravasio, C.R., Kondabolu, K., Zhou, S. et al. Kilohertz electrical stimulation evokes robust cellular responses like conventional frequencies but distinct population dynamics. Commun Biol 8, 19 (2025). https://doi.org/10.1038/s42003-024-07447-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-07447-0