Abstract
Dysregulated endometrial receptivity is a well-established critical factor that contributes to recurrent implantation failure (RIF). Decidualization of stromal cells and differentiation of epithelial cells in the endometrium are crucial processes for achieving endometrial receptivity. Menin, the unique subunit of the H3K4 methyltransferase complex, exhibits cell-specific effects on gene expression through chromatin modification by histone 3 lysine 4 trimethylation (H3K4me3). We have previously reported the significant role of Menin-regulated modifications in H3K4me3 in the maintenance of early pregnancy in mice. However, the physiological function of Menin and its interaction with H3K4me3 in regulating human endometrial receptivity remain poorly understood. Here, we report that Menin expression is reduced in the endometrial stroma of RIF patients. Stromal Menin deficiency not only impairs the decidualization of stromal cells but also negatively impacts the differentiation of epithelial cells through HAND2-FGFs-FGFR axis. Transcriptome analysis reveals that MEN1 knockdown in stromal cells induces the aberrant activation of the WNT signaling pathway, and in vivo experiments show it is associated with a significant reduction in the weight of implantation sites. Mechanistically, Menin deficiency suppresses the expression of SFRP2 and DKK1, which are negative regulators of the WNT signaling pathway, through H3K4me3. In summary, our study identifies Menin as a critical regulator of endometrium receptivity, advances our understanding of its molecular mechanisms, and highlights its potential role in the pathogenesis of RIF.
Similar content being viewed by others
Introduction
Successful embryo implantation depends on a competent blastocyst, a receptive endometrium, and precise communication between the embryonic and maternal interfaces1. Recurrent implantation failure (RIF) is defined as the inability to achieve clinical pregnancy after transfer of at least four good-quality embryos across a minimum of three fresh or frozen cycles in women under 40 years of age2. Despite significant advancements in in vitro embryo transfer technology, approximately 10% of women undergoing in vitro fertilization (IVF) treatment still experience RIF2. In addition to chromosomal abnormalities, two-thirds of implantation failures are considered to result from suboptimal endometrial receptivity3. Clinical diagnosis of endometrial receptivity has posted a challenge. Recently, for RIF patients, endometrial receptivity array (ERA) has been developed to evaluate the expression of 238 endometrial receptivity-associated genes on luteinizing hormone (LH) + 7 day4,5. However, The ERA focuses on determining an individualized optimal timing for blastocyst transfer but does not address the issue of impaired endometrial receptivity in some patients. Many studies have shown limited benefit of this test6. Further studies are required to identify key regulatory molecules and potential therapeutic targets, as well as to elucidate the underlying molecular mechanisms of abnormal endometrial receptivity in RIF.
Endometrial receptivity is a complex process that makes the endometrium suitable for embryo implantation. This phenomenon occurs during the window of implantation (WOI), which spans days 21–24 of the menstrual cycle7,8. During the WOI, the endometrium undergoes morphological, cytoskeletal, biochemical, and genetic changes to become functionally competent9. Endometrial stromal cells differentiate into specialized secretory decidual cells, which are characterized with rounding of the nucleus, increased number of nucleoli, and the accumulation of lipid droplets and glycogen in the expanding cytoplasm10. This process is termed as decidualization, which is driven by the postovulatory increase in progesterone levels and an increase in local cyclic AMP production10. The epithelium simultaneously undergoes major remodeling. This remodeling process results in the cessation of epithelial cell proliferation, enhanced secretion, and the loss of cell polarity and surface microvilli11,12. Notably, the successful remodeling of epithelial cells depends on functional signals from decidual stromal cells8. For instance, HAND2, a downstream effector of stromal progesterone signaling and an established marker of decidualization, suppresses epithelial cell proliferation via fibroblast growth factors (FGFs)13. Given the critical role of proper endometrial remodeling during WOI, the molecular mechanisms of decidualization and intercellular communications between stromal cells and epithelial cells should be further investigated.
Menin, encoded by Men1, interacts with the histone methyltransferase MLL1/2 to catalyze histone 3 lysine 4 trimethylation (H3K4me3) and plays a pivotal role in embryogenesis, cell proliferation and differentiation, and cell senescence14,15. Menin is also involved in modulating multiple signaling pathways, including the Nuclear Factor Kappa B (NF-κB), Transforming growth factor beta (TGF-β), Hedgehog, mTOR and DNA damage repair signaling pathways16,17,18. Our previous study demonstrated that uterine deletion of Men1 in mice did not influence embryo implantation, while it caused incomplete development of the secondary decidual zone (SDZ) during decidualization and increased the incidence of early miscarriage19. In mice, the decidualization process begins with embryo implantation. However, in contrast to mice, human endometrial decidualization is an independent process that occurs in the absence of an embryo. The important functions of Menin in human endometrium decidualization and the establishment of endometrial receptivity have not yet been fully elucidated.
In the present study, we found that Menin expression was downregulated in the stroma of human patients with RIF. MEN1 knockdown in primary human endometrial stromal cells (hESCs) impaired decidualization and hindered HAND2-FGFs-FGFR mediated epithelial cell differentiation. According to the results of RNA-seq analysis, MEN1 knockdown in hESCs caused substantial aberrant downregulation of negative regulators of the WNT signaling pathway. We further confirmed β-catenin nuclear accumulation in human and mouse decidual cells following MEN1 downregulation, indicating WNT pathway activation. In addition, we used lithium chloride (LiCl) stimulation to mimic classical activation of the WNT signaling pathway and found that excessive activation of this pathway impaired decidua formation in vivo. Notably, secreted frizzled-related protein 2 (SFRP2) and dickkopf WNT signaling pathway inhibitor 1 (DKK1), which are negative regulators of the WNT signaling pathway, are target genes of Menin-H3K4me3 interaction. Thus, this study provides fundamental insights into understanding the complex interplay between the WNT signaling pathway and uterine epigenome-transcriptome during RIF.
Results
Aberrantly decreased level of Menin in stromal cells was associated with RIF
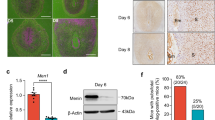
MEN1 was expressed in both endometrial epithelial and stromal cells and exhibited a dynamic expression pattern throughout the menstrual cycle. MEN1 showed high expression in stromal cells during the early to mid-secretory phase but displayed reduced expression in the late secretory phase (Fig. 1a). WB assay further demonstrated that Menin expression was downregulated following artificial decidualization of primary hESCs (Fig. 1b, Supplementary Fig. 5a). Notably, the WOI, which opens at 6–7 days after ovulation and aligns with the early-mid secretory phase. The results suggested a potential role of Menin in endometrial remodeling during this critical period. Analysis of public datasets GSE103465 and GSE205398 revealed a significant decrease in Menin expression in the WOI-phase endometrium of RIF patients as compared to that in controls (Fig. 1c). To validate these findings, we collected the WOI-phase endometrial samples from six RIF patients and six patients undergoing in vitro embryo transfer for male chromosomal factors. The detailed information is provided in Supplementary Table 3. Protein and mRNA expression analysis confirmed significantly reduced expression of Menin in the endometrium of RIF patients during the WOI as compared to that in controls (Fig. 1d, e). Immunohistochemical analysis further revealed retained Menin expression in epithelial cells but significant depletion in stromal cells in RIF patients during WOI (Fig. 1f). These findings led us to hypothesize that Menin plays a critical role in the pathogenesis of RIF by modulating the biological functions of endometrial stromal cells during WOI.
a Immunohistochemical (IHC) staining images of Menin expression in human endometrium in the early, mid, and late secretory phases and quantification of Menin-positive cells in stroma of different phases (n = 3); scale bar = 50 µm. GE: glandular epithelium, S: stroma. b Western blotting analysis of Menin and IGFBP1 protein expression in hESCs treated with estrogen (E2), medroxyprogesterone acetate (MPA), and 8Br-cAMP for 0, 2, 4, and 6 days (n = 3). c mRNA expression levels of MEN1 in human endometrial samples from the control and RIF groups in the Gene Expression Omnibus (GEO) datasets (GSE103465 and GSE205398). d Western blotting assay of Menin in human endometrial samples from the control and RIF groups (n = 5); β-actin served as the loading control. e Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of MEN1 mRNA expression in human endometrial samples from the control and RIF groups (n = 6). f Representative IHC staining images of Menin expression in the mid secretory phase human endometrium from the control and RIF groups, and quantification of Menin-positive cells in epithelium and stroma of these groups (n = 3); scale bar = 200 μm. GE glandular epithelium, S stroma. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t test.
MEN1 depletion in hESCs impaired decidualization
To determine the function of MEN1 in decidualization, we performed lentivirus-mediated MEN1 knockdown in primary hESCs before decidualization. The results of qPCR and WB assay confirmed the successful knockdown of MEN1 in primary hESCs (Fig. 2a, b). MEN1 depletion significantly downregulated the mRNA expression levels of PRL and IGFBP1 (Fig. 2a), decreased the secretion of PRL (Fig. 2b), and reduced the protein expression level of IGFBP1 (Fig. 3c, Supplementary Fig. 5b) in primary hESCs after artificial decidualization. Morphological examination revealed round-shaped and larger hESCs following their transformation into decidual stromal cells. IF staining of F-actin showed that stromal cells with MEN1 knockdown remained elongated even after artificial decidualization (Fig. 2d). To investigate Menin’s regulatory role in stromal cells, we conducted RNA sequencing on MEN1-knockdown hESCs versus the control group following artificial decidualization. Compared to the control group, shMEN1 hESCs showed upregulation and downregulation of 637 and 807 genes, respectively (Fig. 2e). MEN1 knockdown significantly downregulated decidualization marker genes, including PRL and IGFBP1, as well as other genes critical for achieving decidualization, such as HAND2, EGR1, CEBPB, WNT4, and FST (Fig. 2f). This finding was also confirmed by qPCR results (Fig. 2g). Thus, these observations revealed that MEN1 depletion impaired the decidualization process. During decidualization, endometrial stromal cells shift from a proliferative to a differentiated state. EdU staining and CCK-8 assays showed increased proliferation in shMEN1 hESCs (Fig. 2h, i), indicating that Menin depletion disrupts this transition, resulting in excessive proliferation.
a qRT-PCR analysis of MEN1, PRL, and IGFBP1 mRNA expression in human endometrial stromal cells (hESCs) treated with E2, MPA, and 8Br-cAMP for 0, 2, and 4 days (n = 3). b ELISA analysis of the secreted levels of PRL in spent medium at D0 and D4 of decidualization in the shCtrl and shMEN1 groups (n = 3). c Western blotting assay of Menin and IGFBP1 protein expression in the shCtrl and shMEN1 groups treated with E2, MPA, and 8Br-cAMP for 4 days (n = 3). d Immunofluorescence (IF) staining of F-actin in primary hESCs transfected with shCtrl or shMEN1 following 96 h of EPC treatment (n = 3). e Volcano plot of differentially expressed genes detected by RNA sequencing (RNA-seq) in hESCs decidualized for 4 days with shCtrl and shMEN1. f Heatmap of decidualization-related genes in the shCtrl and shMEN1 groups treated with E2, MPA, and 8Br-cAMP for 4 days (n = 3) as determined by RNA-seq. g qRT-PCR analysis of HAND2, FST, CEBPB, and EGR1 expression in the shCtrl and shMEN1 groups treated with E2, MPA, and 8Br-cAMP for 4 days (n = 3). h IF staining for EdU in the shCtrl and shMEN1 groups and EdU-positive proportion (%) of the shCtrl and shMEN1 groups (n = 3); scale bar = 200 µm. i CCK-8 assay of the shCtrl and shMEN1 groups treated with E2, MPA, and 8Br-cAMP for 0 and 2 days (n = 4). Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t test, one-way ANOVA and two-way ANOVA.
a Gene Ontology analysis of the differential upregulated genes as determined by RNA-seq. b Heatmap of differentially expressed fibroblast growth factors (FGFs) following MEN1 knockdown in decidualized hESCs (n = 3). c qRT-PCR analysis of FGFs in the shCtrl and shMEN1 groups treated with E2, MPA, and 8Br-cAMP for 0 and 4 days (n = 3). d Schematic representation of the culture of assembloids. e Immunostaining of Ki67 and GFP in assembloids from the EEO+shCtrl and EEO+shMEN1 groups (n = 3); scale bar = 200 μm. f qRT-PCR analysis of PAEP and HSD17β2 in assembloids from the EEO+shCtrl and EEO+shMEN1 groups before and after treatment with E2, MPA, and 8Br-cAMP (n = 3). g Representative IHC staining images of Ki67 and p-ERK1/2 expression in the mid secretory phase human endometrium from the control and RIF groups (n = 3); scale bar = 200 μm. GE glandular epithelium, S stroma. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, one-way ANOVA.
MEN1 depletion in stromal cells upregulated FGFs and promoted the proliferation of endometrial epithelial cells
Next, the genes with upregulated expression following MEN1 depletion were subjected to GO analysis. Functional annotation showed that these upregulated genes were mainly enriched in cell-cell adhesion, cytoskeleton organization, cell migration, and activation of the MAPK cascade (Fig. 3a). Interestingly, the upregulated genes included a group of FGFs (Fig. 3b). According to the qPCR results, the mRNA expression levels of FGF2, FGF9, and FGF17 were markedly increased in shMEN1 hESCs as compared to those in shCtrl hESCs (Fig. 3c). Previous studies have shown that HAND2 in stromal cells inhibits the proliferation of epithelial cells by downregulating the expression of paracrine FGFs13. Stromal-derived FGFs bind to epithelial FGFRs, triggering ERK pathway activation13,20. In the present study, following MEN1 knockdown, HAND2 expression was remarkably decreased (Fig. 2f). To determine how MEN1 knockdown in stromal cells affects epithelial cells, we co-cultured normal endometrial epithelial-like organoids with MEN1 knockdown stromal cells, which were termed assembloids (Supplementary Figs. 1a, b). These assembloids expressed the epithelial marker E-cadherin and showed GFP positivity, which confirmed the high efficiency of lentiviral transfection (Supplementary Fig. 1c). The assembloids treated with E2, MPA, and cAMP exhibited increased IGFBP1 transcription, decreased transcription levels of the epithelial stemness marker SOX9, and elevated transcription levels of the secretory markers PAEP and HSD17β2 (Supplementary Fig. 1d). These findings were in line with the changes observed in the endometrium during the secretory phase in vivo. Based on these observations, we concluded that the assembloids can simulate the secretory-phase endometrium to a certain extent. IF staining for Ki67 was conducted to assess epithelial cell proliferation and differentiation. Following hormone treatment, endometrial epithelial organoids in the shMEN1 group exhibited a larger diameter, higher Ki67 positivity (Fig. 3e, Supplementary Fig. 5c), and remarkably decreased mRNA expression levels of PAEP and HSD17β2 (Fig. 3f) as compared to the control group. This finding demonstrates that stromal Menin orchestrates epithelial receptivity acquisition during WOI, wherein Menin deficiency disrupts stromal paracrine mediators (e.g., FGFs) critical for stromal-epithelial crosstalk. To further confirm these findings, we conducted the immunohistochemical analysis of the window-phase endometrium of RIF patients. The RIF group displayed higher epithelial Ki67 positivity and enhanced nuclear p-ERK1/2 translocation compared to controls, suggesting aberrant epithelial proliferation and ERK pathway activation (Fig. 3g, Supplementary Fig. 5d). These observations suggested that Menin not only guaranteed the decidualization of stromal cells but also facilitated the transition of endometrial epithelial cells into a receptive state through the HAND2-FGFs-FGFR axis.
MEN1 depletion overactivated the WNT/β-catenin signaling pathway
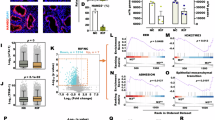
Next, we performed GO analysis to elucidate the underlying mechanisms through which MEN1 knockdown impaired the decidualization of stromal cells. Functional annotation analysis revealed that genes downregulated following MEN1 depletion were primarily enriched in angiogenesis, hypoxic response, negative regulation of the WNT signaling pathway, and N-acetylglucosamine metabolism (Fig. 4a). Consistent with these results, GSEA showed significant differences between the control and shMEN1 groups in gene sets related to the negative regulation of the WNT signaling pathway (Fig. 4b). It suggests that MEN1 knockdown compromises negative regulation of the WNT signaling pathway. This observation aligned with our findings in mice with uterine deletion of Men1(Men1d/d), where KEGG pathway analysis and GSEA revealed significant enrichment of the WNT signaling pathway (Supplementary Fig. 2a, b) and RNA-seq analysis indicated a downregulation of genes that antagonize WNT ligands, such as Sfrp1, Sfrp2, Dkk1, Dkk3(Supplementary Fig. 2c). These findings demonstrated that MEN1 knockdown may promote aberrant activation of the WNT signaling pathway. WNT pathway activation is mediated by nuclear translocation of β-catenin. To determine the effect of MEN1 knockdown on WNT signaling, Men1d/d uteri were subjected to IF assay for β-catenin. The results revealed abnormal intranuclear aggregation of β-catenin in Men1d/d uteri (Fig. 4c, Supplementary Fig. 5e). Consistent with this observation, shMEN1 hESCs also showed remarkable nuclear accumulation of β-catenin as compared to shCtrl cells in the IF assay (Fig. 4d, Supplementary Fig. 5f). Furthermore, Western blot analysis of nuclear and cytoplasmic proteins revealed significantly elevated levels of non-phosphorylated (active) β-catenin and total β-catenin in the nucleus of the shMEN1 group (Fig. 4e, Supplementary Fig. 5g). Moreover, Luciferase reporter assay of TCF/LEF binding sites showed remarkably increased luciferase activity in shMEN1 hESCs compared to shCtrl group (Fig. 4f). Collectively, these results indicated that the WNT signaling pathway was overactivated following MEN1 knockdown.
a Gene Ontology analysis of the differential downregulated genes as determined by RNA-seq. b Gene set enrichment analysis (GSEA) plot of negative regulation of the WNT signaling pathway in the shMEN1 group as compared to that in the shCtrl group. c IF assay of β-catenin expression in stroma of MEN1f/f and MEN1d/d mice on D8; scale bar = 200 μm. d IF analysis of β-catenin expression in the shCtrl and shMEN1 groups treated with E2, MPA, and 8Br-cAMP for 4 days (n = 3); scale bar = 200 μm. e Western blotting assay of cytoplasmic and nuclear expression of β-catenin in the shCtrl and shMEN1 groups treated with E2, MPA, and 8Br-cAMP for 4 days (n = 3). f Measurement of luciferase activity in TOPflash-transfected and FOPflash-transfected hESCs treated with shMEN1 or shCtrl during decidualization (n = 3). Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t test.
Abnormal activation of the WNT signaling pathway impaired decidualization
The spatiotemporal specific expression of WNT ligands and Frizzled (FZD) receptors in stromal cells demonstrates that the WNT signaling pathway is indispensable for decidualization21. However, the potential effect of the hyperactivated WNT signaling pathway on stromal cell decidualization is unclear. LiCl, a classical agonist that activates the WNT signaling pathway, can inhibit GSK3β activity (by increasing GSK3β phosphorylation at Ser9) and thereby effectively stabilize the level of free cytosolic β-catenin. Luciferase reporter assays of TCF/LEF binding sites showed a dose-dependent increase in luciferase activity following LiCl treatment (Fig. 5a). And the LiCl-treated group showed β-catenin accumulation in nucleus (Fig. 5b), confirming activation of the WNT signaling pathway. LiCl treatment led to decreased mRNA expression of PRL, IGFBP1, and HAND2, indicating impaired decidualization (Fig. 5c, Supplementary Fig. 3a). CCK-8 assays and Ki67 immunostaining showed significantly increased proliferation in stromal cells treated with 10 mmol/L LiCl following hormone exposure (Supplementary Fig. 3b, c). SKL2001 disrupts the AXIN/β-catenin interaction and acts as a small molecule activator of the WNT signaling pathway. In SKL2001-treated group, β-catenin expression increased in a dose-dependent manner, while the expression levels of PRL, IGFBP1 and HAND2 all decreased when the concentration of SKL2001 reached 20 nM (Supplementary Fig. 3d, e). These results suggested that the abnormal activation of the WNT signaling pathway disrupted decidualization and promoted excessive proliferation of hESCs. In the in vivo study, female mice with vaginal plugs were subjected to a 3-day intraperitoneal administration of LiCl (60 mg/kg), commencing on D5 of pregnancy (D1 = vaginal plug). The uterine samples were then collected on D8 (Fig. 5d). Immunofluorescence staining of β-catenin revealed increased nuclear β-catenin levels in stromal cells in the LiCl-treated group compared to controls (Supplementary Fig. 3f). Although the number of implantation sites was similar between groups (Fig. 5g), the LiCl-treated group exhibited smaller implantation sites (Fig. 5e) and decreased wet weight of implantation sites (Fig. 5h). Histological analysis also showed smaller decidual nuclei and lower polyploidy rates within the SDZ in the LiCl-treated group (Fig. 5f). Additionally, a higher Ki67 positivity rate indicated excessive cellular proliferation (Fig. 5i). Moreover, Prl8a2 expression, a classical marker of mouse endometrial decidualization, was decreased in the LiCl-treated group (Fig. 5j). To further confirm that overactivation of the WNT signaling pathway affects decidualization, we utilized an oil-induced artificial decidualization model (Fig. 5k). The LiCl-treated group exhibited a remarkably diminished or absent decidual response at 96 h following intraluminal oil administration (Fig. 5l, m, n) and significantly decreased Prl8a2 mRNA expression level (Fig. 5o). These results confirmed that endometrial decidualization is largely influenced by the homeostasis of the WNT signaling pathway and that the overactivation of this pathway impairs decidualization.
a Measurement of luciferase activity in TOPflash-transfected and FOPflash-transfected hESCs treated with LiCl or control during decidualization (n = 3). b IF assay of β-catenin expression in hESCs treated with LiCl or control (n = 3); scale bar = 50 μm. c qRT-PCR analysis of PRL and IGFBP1 expression in the control and LiCl-treated groups treated with E2, MPA, and 8Br-cAMP for 4 days (n = 3). d Schematic representation of the natural decidualization procedures with LiCl treatment. e Gross morphology of D8 uterine from the PBS and LiCl treatment (60 mg/kg) groups; scale bar = 1 cm. f Hematoxylin-eosin staining of D8 uterine from the PBS and LiCl treatment (60 mg/kg) groups; scale bar = 1 mm. g Number of implantation sites from the PBS and LiCl treatment (60 mg/kg) groups (n = 5). h Weight of implantation sites from the PBS and LiCl treatment (60 mg/kg) groups (n = 5). i Immunostaining of Ki67 expression in D8 implantation sites from the PBS and LiCl treatment (60 mg/kg) groups, and the ratio of the length of Ki67-positive site to the Ki67-negative site (n = 3); scale bar = 200 μm. j qRT-PCR analysis of Prl8a2 expression in D8 uterine from the PBS and LiCl treatment (60 mg/kg) groups (n = 5). k Schematic representation of the stimulated decidualization procedures with LiCl treatment. l Gross morphology of the unstimulated or stimulated uterine from the PBS and LiCl treatment (60 mg/kg) groups; scale bar = 1 cm. m Hematoxylin-eosin staining of the unstimulated or stimulated uterine from the PBS and LiCl treatment (60 mg/kg) groups; scale bar = 1 mm. n Ratio of stimulated to unstimulated uterine weight from the PBS and LiCl treatment (60 mg/kg) groups (n = 5). o qRT-PCR analysis of Prl8a2 expression in stimulated uterine from the PBS and LiCl treatment (60 mg/kg) groups (n = 5). Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t test and one-way ANOVA.
MEN1 knockdown aberrantly activated the WNT/β-catenin signaling pathway by downregulating SFRP2 and DKK1 through H3K4me3
Menin interacts with methyltransferases such as MLL to add H3K4me3 modification, which is a chromatin-based modification associated with transcriptional activation22. β-catenin is encoded by CTNNB1, and MEN1 knockdown showed no effect on CTNNB1 transcription (Supplementary Fig. 4a). The RNA-seq results revealed that MEN1 knockdown significantly downregulated classical WNT inhibitors, including SFRP2, DKK1, TSKU, and FAM53B, and significantly upregulated WNT agonists, such as MACF1, DKK2, and TNIK (Fig. 6a). Based on these findings, we hypothesized that Menin activates the WNT signaling pathway by modulating the expression of WNT repressors through H3K4me3. WB assay showed a reduction in the total H3K4me3 level following MEN1 knockdown (Fig. 6b, Supplementary Fig. 5h). MEN1 knockdown also decreased the mRNA and protein expression levels of SFRP2 and DKK1 (Fig. 6c, d, Supplementary Fig. 5i). In contrast, the mRNA and protein expression levels of SFRP2 and DKK1 were significantly elevated in primary hESCs following overexpressing MEN1 (Fig. 6e, f, Supplementary Fig. 5j). These results suggested that SFRP2 and DKK1 are the probable downstream target genes of Menin. To confirm the regulatory mechanism of MEN1, we performed a ChIP-qPCR analysis of H3K4me3. Compared to the shCtrl group, shMEN1 hESCs exhibited marked reduction in H3K4me3 levels on the promoters of both SFRP2 and DKK1 (Fig. 6g). These findings confirmed that Menin influences SFRP2 and DKK1 expression through H3K4me3 (Fig. 7). IWR-1, an inhibitor of the WNT signaling pathway, promotes β-catenin destruction in the β-catenin destruction complex, thereby blocking WNT/β-catenin signaling. Following the addition of IWR-1 to the differentiation medium in the shMEN1 group, both the transcription levels of PRL, IGFBP1 and HAND2 and the protein level of IGFBP1 were partially restored (Fig. 6h, i, Supplementary Fig. 4b). This finding indicated that decidualization impairment by MEN1 depletion can be partially reversed by suppressing the WNT signaling pathway through appropriate inhibitors.
a Heatmap of differentially expressed WNT signaling-related genes after MEN1 knockdown in decidualized hESCs (n = 3). b Western blotting assay of the H3K4me3 protein in the shCtrl and shMEN1 groups treated with E2, MPA, and 8Br-cAMP for 4 days (n = 3); H3 served as a control. c qRT-PCR analysis of WNT signaling-related genes in the shCtrl and shMEN1 groups treated with E2, MPA, and 8Br-cAMP for 0 and 4 days (n = 3). d Western blotting assay of SFRP2 and DKK1 expression in the shCtrl and shMEN1 groups (n = 3), GAPDH served as a control. e Western blotting assay of Menin, SFRP2, DKK1, IGFBP1, and H3K4me3 expression in the OE-Ctrl and OE-MEN1 groups treated with E2, MPA, and 8Br-cAMP for 4 days (n = 3). GAPDH and H3 served as controls. f qRT-PCR analysis of SFRP2 and DKK1 expression in the OE-Ctrl and OE-MEN1 groups treated with E2, MPA, and 8Br-cAMP for 4 days (n = 3). g Quantitative ChIP analysis of H3K4me3 at SFRP2 and DKK1 promoters in the shCtrl and shMEN1 groups treated with E2, MPA, and 8Br-cAMP for 4 days (n = 3). h qRT-PCR analysis of PRL and IGFBP1 expression in the shCtrl and shMEN1 groups treated with or without IWR-1. i Western blotting assay of Menin and IGFBP1 expression in the shCtrl and shMEN1 groups treated with or without IWR-1 on day 2 of decidualization. GAPDH served as a control. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t test and one-way ANOVA.
Menin in stromal cells maintains the gene expression of WNT repressors SFRP2 and DKK1 through H3K4me3, thereby effectively limiting the overactivation of WNT signaling pathway and supporting the normal decidualization. HAND2 mediates stromal-epithelial crosstalk via FGF signaling, while stromal Menin deficiency disrupts this paracrine axis by suppressing HAND2, thereby impairing epithelial differentiation. Thus, Menin plays a crucial role in regulating endometrial receptivity and facilitating embryo implantation.
Discussion
Endometrial epithelial and stromal cells undergo complex molecular reprogramming during the WOI to achieve a receptive state and facilitate effective communication with the blastocyst1. Disorders associated with endometrial receptivity or alterations in the receptive phase are considered significant contributors to unexplained recurrent implantation failure (RIF)23. Several studies utilizing single-cell sequencing techniques have reported changes in cellular subpopulations and communication networks within the WOI-phase endometrium of RIF24,25. Lai.Z et al. highlighted the increased TOP2A+MKI67+CDC20+ endometrial fibroblast-like cells (FIBs) and decreased MMP14+IGF2+COL6A1+IGFBP2+ FIBs during WOI of RIF in comparison with the control group24. This finding suggested that excessive proliferation of stromal cells and insufficient extracellular matrix remodeling may contribute to the pathogenesis of RIF.
In the present study, MEN1 showed high expression in stromal cells during WOI. We observed hyperproliferative epithelial and stromal cells along with stromal Menin deficiency in the endometrium of RIF patients. MEN1 knockdown impaired decidualization and then disrupted stromal-epithelial crosstalk, thereby potentially driving epithelial cell over-proliferation and hampered endometrial receptivity. Transcriptomic analysis revealed the aberrant activation of the WNT signaling pathway in MEN1-deficient hESCs. Mechanistic investigations further demonstrate that Menin epigenetically activates WNT antagonists SFRP2 and DKK1 through H3K4me3 modification, thereby suppressing β-catenin nuclear accumulation. Notably, the appropriate inhibition of the WNT signaling pathway in MEN1-deficient hESCs appears to be a promising target for restoring the decidualization functionality of stromal cells.
The WNT signaling pathway plays an important role in decidualization both in mouse and humans. This is evidenced by the dynamic expression of the transcriptional co-activator β-catenin, WNT ligands (Wnt4, Wnt6, Wnt7a, etc.), and inhibitors (DKK1, etc.) during decidualization26,27,28,29. Mice with uterine deficient of Ctnnb1 exhibited a complete absence of decidualization and severe fertility defects30. During decidualization, Wnt4 showed elevated expression, functions as a downstream molecule of Bmp2, and mediates cytoplasmic shuttling of Foxo113,27,31. Wnt6 deletion suppressed stromal cell polyploidization and downregulates Cyclin B1 expression, resulting in the slowdown of cell proliferation29. These findings showed that the inhibition of the WNT signaling pathway is deleterious to endometrial decidualization. Aberrant activation of the WNT signaling pathway, however, is also detrimental to decidualization. This is demonstrated by CDC42 deficiency, which results in accelerated cell senescence through β-catenin overactivation32. The expression of DKK1, a WNT signaling antagonist, was also remarkably elevated during decidualization28. A recent high-resolution single-cell reference atlas of human endometrium states that the secretory phase in stromal cells is initiated following WNT inhibition by DKK133. These findings highlighted the importance of maintaining a finely tuned balance in WNT signaling during decidualization.
We found aberrant WNT activation and excessive proliferation in MEN1 knockdown decidual cells. WNT activation has been reported to promote cell proliferation34,35. Upon LiCl-induced WNT activation, we also observed excessive proliferation of hESCs. These results suggested that the decidualization defects in MEN1 knockdown decidual cells were due to aberrant proliferation driven by WNT activation. Nuclear translocation of β-catenin is a hallmark of WNT activation. When WNT signaling is switched on, the classical WNT ligand binds to the receptor complex comprising the Frizzled (FZD) receptor and its co-receptor LRP5/6. This binding recruits AXIN to the cell membrane, leading to the disassembly of the β-catenin degradation complex (APC/AXIN/GSK3β), which allows cytoplasmic β-catenin to translocate to the nucleus35,36. SFRP2 binds to WNT ligands and triggers their sequestration, which prevents the binding of these ligands to FZD receptors37. DKK1 is also a secreted WNT inhibitor and removes the LRP6 protein from the cell surface by endocytosis38. SFRP2 and DKK1, acting through distinct mechanisms, have been shown to promote cytoplasmic β-catenin degradation and suppress the WNT signaling pathway. We found that Menin activated the expression of SFRP2 and DKK1 through H3K4me3, inhibiting the accumulation of nuclear β-catenin and preventing excessive WNT activation. Notably, in mice Sfrp2 has also been identified as a Menin-H3K4me3 typical target gene19, implying that this mechanism is conserved between humans and mice. Previous studies have documented that Menin can directly interact with β-catenin, promoting its nuclear export and ubiquitin-mediated degradation39,40. Our results provided an alternative mechanism for β-catenin nuclear accumulation. However, it cannot be excluded that Menin also facilitates β-catenin degradation through direct interaction in decidual cells.
The paracrine signals from stromal cells play a pivotal role in epithelial cell remodeling, which is considered a critical factor for uterine receptivity20,41,42. For instance, COUP-TFII activation in stromal cells promotes HAND2 expression in stromal cells, thereby suppressing the FGF-ERK pathway and halting epithelial cell proliferation13,43,44. Similarly, stromal Stat3 restricts the over-proliferation of luminal epithelial cells by modulating the E2 signaling pathway45. Uterine stromal Ezh2 knockout blocked the proliferation-differentiation switching (PDS) in epithelial cells46. Pbrm1 regulates Hand2 through epigenetic histone modifications/coactivator recruitment and looping with its promoter, ensuring balanced epithelial proliferation and remodeling47. However, research on human endometrial stromal-epithelial crosstalk has been constrained by the lack of reliable in vitro models. Following recent advancements in organoid technology, novel tools have been developed to address this gap. Turco et al. initially developed stable endometrial epithelial organoids comprising glandular and ciliated epithelial cells with self-renewal capacity and hormone responsiveness48. Rawlings et al. constructed a collagen gel-embedded cell co-culture system, called “assembloid”, which combines epithelial organoids with stromal cells and can mimic hormonal transformation49. By optimizing this assembloid model, our study demonstrated that MEN1 knockdown in stromal cells resulted in hyperproliferation of epithelial cells. The MEN1-HAND2-FGFs axis functions as a critical molecular mechanism underlying the disruption of endometrial transition from the proliferative state to the secretory state in RIF patients. Notably, direct activation of the WNT signaling pathway significantly decreased HAND2 expression in stromal cells and modest inhibition of the WNT signaling pathway restored HAND2 expression in the shMEN1 group. This suggested that HAND2 is a downstream effector molecule of the aberrantly activated WNT signaling pathway following MEN1 knockdown. Interestingly, epithelial WNT signaling silencing determines its differentiation into glandular epithelium. Glandular epithelial WNT receptor expression, including LRP5/6, FZD3, FZD5, and FZD6, shows a dramatic decline during the secretory phase, potentially limiting this pathway activity50. We proposed that SFRP2 and DKK1 secreted by decidual cells likely bind to these receptors, further suppressing surrounding glandular epithelial WNT signaling. This hypothesis highlights that Menin guarantees the expression of SFRP2 and DKK1, which act in a paracrine manner to inhibit glandular epithelial WNT signaling. However, this hypothesis requires further experimental validation in future studies.
Despite these useful findings, the present study has several limitations. While our innovative assembloid model provided valuable insights into the effects of stromal MEN1 knockdown on epithelial function, the contribution of immune cells, blood vessels, and other elements was not considered in this model. Future studies should examine ways to refine this model to better represent the endometrial microenvironment and explore the broader implications of the model. The sample size was also limited because of the complexity and heterogeneity of RIF etiology. A larger sample size can further validate the obtained conclusions.
In summary, our study provided compelling evidence for the indispensable function of Menin in regulating endometrial receptivity. MEN1 deficiency dysregulated the expression of multiple key decidualization-related genes, disrupted stromal-epithelial interactions through the HAND2-FGFs-FGFR-MAPK axis. The key mechanism underlying this is the aberrant activation of the WNT/β-catenin pathway. Menin maintained the expression of SFRP2 and DKK1 through H3K4me3, thereby restricting the nuclear accumulation of β-catenin. These findings highlighted the critical role of Menin in endometrial receptivity and suggested its potential as a therapeutic target in RIF patients.
Materials and methods
Sample collection
The study patients were recruited from the IVF unit of the Reproductive Center of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Zhejiang, China. Secretory endometrial biopsies were obtained from women aged 20–35 years with regular menstrual cycles (25–32 days) and no history of hormone therapy for at least three months prior to hysteroscopy. We defined the 15th - 19th days of the menstrual cycle as the early secretory phase (corresponding to days 1–5 after ovulation suggested by ultrasound), the 20th - 23rd days as the mid-secretory phase (corresponding to days 6-9 after ovulation suggested by ultrasound), and the 24th - 28th days as the late secretory phase (corresponding to days 10-14 after ovulation suggested by ultrasound). Six RIF patients and six control patients were enrolled in the study. The control group patients received assisted reproductive technology for the male factor. All patients received estradiol valerate 6 mg every day for 12-14 days, 40 mg progestin injection daily was added when the endometrium reached maximal thickness. Hysteroscopy was performed on day 6 after progestin injection. The inclusion criteria for RIF patients were as follows: (1) < 40 years of age and (2) failure to achieve clinical pregnancy after at least 3 embryo transfers or transfer of ≥4 good-quality embryos. The exclusion criteria were as follows: other potential causes of RIF, such as chromosomal abnormalities, autoimmune diseases, or chronic endometritis. The design and conduct of this study were approved by the Ethics Committee of Sir Run Shaw Hospital, Zhejiang University School of Medicine (Approval number: 20240356). All ethical regulations relevant to human research participants were followed. All the included participants provided informed consent prior to tissue collection.
Isolation, culture, and in vitro decidualization of hESCs
Freshly obtained endometrial tissue samples were cut into approximately 1 mm3 pieces and digested with 1% type I collagenase (17100017, Thermo ScientificTM) for 2 h. Primary hESCs were obtained by passing the samples through a 40-μm filter (352340, Falcon) and resuspended in a proliferation medium containing Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F12 nutrient mixture (1:1) (PWL005, Meilunbio) with 10% charcoal-stripped fetal bovine serum (CA-FBS) (CMS003.02, easyallTM) and 1% antibiotic (BL505A, Biosharp). The proliferation medium was replaced every 2 days. The differentiation medium was prepared by adding 10 nM estrogen E2 (E2758, Sigma-Aldrich), 1 μM medroxyprogesterone acetate (MPA) (HY-B0648, MedChemExpress), and 0.5 mM 8-bromo-cAMP (cAMP) (HY-12306, MedChemExpress) to phenol red-free DMEM/F12 (MA0214, Meilunbio) supplemented with 2% CA-FBS and 1% antibiotic. To induce decidualization, primary hESCs were cultured in the differentiation medium for 48–96 h. shMEN1 cells were successfully obtained by infecting primary hESCs with a lentivirus for 8–12 h at the density of approximately 50–70%; shCtrl cells were also obtained by the same method. After achieving a cell density of 90–100%, the cells were screened with a proliferative medium containing 2 μg/ml puromycin (MA0318, Meilunbio) for 48 h. The shRNA sequence targeting human MEN1 was 5’-CTGTACCTGAAAGGATCATAC-3’ (ViGene Biosciences).
Immunohistochemical staining
Tissues were fixed with 4% paraformaldehyde (MA0192, Meilunbio) overnight, embedded in paraffin, and cut into 4-μm-thick sections. Dewaxing and rehydration processes for the embedded sections were performed as follows: xylene I for 10 min, xylene II for 10 min, xylene in anhydrous ethanol for 5 min, anhydrous ethanol I for 5 min, anhydrous ethanol II for 5 min, 95% ethanol for 5 min, and 75% ethanol for 5 min. The sections were then placed in 1× Tris-EDTA solution (pH 9.0) (ab93684, Abcam) and boiled for 5 min to achieve antigen retrieval. After natural cooling to room temperature (RT), the sections were blocked with 5% bovine serum albumin (BSA; MB0004, Meilunbio) in PBS at RT for 1 h and then incubated overnight with primary antibodies. Peroxidase activity was blocked with 3% hydrogen peroxide (P0100A, Beyotime) for 10 min at RT. The sections were then incubated with secondary antibodies (GK500710, Gene Tech) for 30 min at RT. Staining with DAB (3,3ʹ-diaminobenzidine) and hematoxylin (H8070, Solarbio) was then performed for nuclear and antigen visualization. Following staining, the slides were subjected to dehydration and rehydration processes and then sealed with neutral gum. Supplementary Table 1 provides details of the antibodies. Menin protein expression levels were evaluated by quantifying the positive rate in three randomly selected fields of identical area from each of three endometrial sections per control and RIF group. The relative expression levels of t-ERK1/2 and p-ERK1/2 in endometrial sections were determined by quantifying integrated optical density (IOD) using Fiji software.
Immunofluorescence (IF) staining
hESCs were fixed with 4% paraformaldehyde for 15 min at RT and then permeabilized with 0.3% Triton X-100 (HFH10, Sigma-Aldrich) and 5% BSA for 10 min. The cells were blocked with 5% BSA for 1 h at RT and incubated overnight with primary antibodies at 4 °C. Subsequently, the cells were incubated with fluorescence-conjugated secondary antibodies (A11001/A10037/A11034/A11036, Invitrogen) and the nuclear stain Hoechst 33342 (H3570, Invitrogen) for visualizing fluorescence signals. Digital images were captured using a confocal laser scanning microscope (LSM800, Zeiss) and processed using ImageJ software. β-catenin nuclear translocation was quantified by calculating the ratio of nuclear positive area to total nuclear area in three randomly selected endometrial sections. Supplementary Table 1 provides details of the antibodies.
Western blotting (WB) assay
Tissues and cells were lysed with high-potency RIPA lysis solution (R0020, Solarbio) containing protease inhibitors and phospho-protease inhibitors (P1260, Solarbio) and 1% phenylmethylsulfonyl fluoride (P0100, Solarbio). Protein supernatants were obtained after high-speed centrifugation at 4 °C. Nuclear-cytoplasmic fractionation was performed according to the manufacturer’s protocol (78833, Thermo ScientificTM). Protein concentration was quantified using the bicinchoninic acid protein assay kit (23225, Thermo ScientificTM). A 5× loading buffer (containing dithiothreitol) (P1040, Solarbio) was added to the protein supernatant; the mixture was then boiled at 100 °C for 10 min and stored at -80°C. Proteins were separated on a 10% or 12.5% gel (PG113, Epizyme) by SDS-PAGE, and the separated proteins were then transferred to a 0.45-µm polyvinylidene fluoride (PVDF) membrane (IPVH00010, Merck). The PVDF membranes were blocked with 5% skimmed milk for 2 h and further incubated overnight with primary antibodies on a shaker at 4 °C. The membranes were then incubated with HRP-conjugated secondary antibodies (111-035-003, Jackson ImmunoResearch) in 5% BSA for 1 h at RT. Subsequently, digital images were captured using enhanced chemiluminescence reagents (WBKLS0500, Millipore). The expression of each protein was normalized to GAPDH or β-actin expression in the corresponding sample, and the relative abundance of the target proteins was estimated by densitometric quantification of the signal intensities using ImageJ software. Supplementary Table 1 provides details of the antibodies.
RNA isolation and quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (15596018CN, Thermo ScientificTM) or the RNA-Quick Purification Kit (RN001, ES Science). The OD260/280 ratio was used to evaluate RNA purity. Next, 1 µg of RNA was used to synthesize cDNA (R223-01, Vazyme). The gene expression levels were analyzed by real-time PCR with SYBR Premix (Q711, Vazyme) using appropriate primers (see Supplementary Table 2). Relative gene expression levels were calculated with the 2-∆∆Ct method by using GAPDH or β-actin RNA as the internal control.
RNA-seq and data analysis
Primary hESCs (3 biological samples) were transfected with either shMEN1 or shCtrl and then subjected to artificial induction of decidualization for 96 h. Total RNA was extracted using TRIzol reagent. Next, 1 µg of the extracted RNA was used to synthesize cDNA, and the remaining RNA was sent to Novozymes Biologicals Ltd. for sequencing and data analysis. Differential expression analysis of two groups was performed using the DESeq2 R package (1.20.0). Gene Ontology (GO) and KEGG enrichment analysis of differentially expressed genes was implemented by the clusterProfiler R package, with corrected P value less than 0.05 were considered significantly. Gene Set Enrichment Analysis (GSEA) was conducted on the GSEA analysis tool (http://www.broadinstitute.org/gsea/index.jsp).
Dual-luciferase reporter assay
Primary hESCs were seeded onto 24-well plates at the density of approximately 60% and transfected with either TOPflash (#12456, Addgene) or FOPflash (#12457, Addgene) as an internal control. Subsequently, after 48 h of artificial decidualization, luciferase assays were conducted using the Firefly Luciferase Assay System (RG005, Beyotime) and measured using Promega GloMax20/20.
Enzyme-linked immunosorbent assay (ELISA)
Spent medium was collected after 2 days of decidualization. Commercial ELISA kits (E-EL-H0141, Elabscience) were used to detect the PRL level. Assays were performed in accordance with the manufacturer’s instructions. The absorbance value was measured at 450 nm by using an ELX800 Universal Microplate Reader, with background subtraction from absorbance measured at 450 nm.
Isolation and culture of endometrial epithelial organoids
Freshly obtained endometrial tissue samples were cut into approximately 1 mm3 pieces and digested with 1% type I collagenase (17100017, Thermo ScientificTM) for 2 h. Tissue debris was removed by filtration using a 100-μm cell filter (352360, Falcon). The primary cells were passed through a 40-μm filter (352340, Falcon), and epithelial fragments located on the top of the filter were collected. The obtained fragments were centrifuged at 600 × g for 5 min and resuspended in a solution containing 70% ice-cold Matrigel (356231, Corning) and 30% DMEM/F12 (PWL005, Meilunbio). The Matrigel/cell suspension (40 μL) was added to each well of a 48-well plate. Following solidification of the Matrigel, 300 μL of organoid expansion medium (ExM) was added. ExM comprised DMEM/F12 supplemented with 2% B27 (12587010, Thermo ScientificTM), 1% N2 (17502048, Thermo ScientificTM), 1% insulin-transferrin-selenium (41400045, Thermo ScientificTM), 1 mM Nicotinamide (HY-B0150, MedChemExpress), 50 ng/ml Epidermal Growth Factor (315-09, PeproTech), 50 ng/ml FGF10 (751006, Biolegend), 100 ng/ml Noggin (250-38, PeproTech), 0.5 μM Transforming Growth Factor-beta/Alk inhibitor A83-01 (HY10432, MedChemExpress), 9 μM ROCK inhibitor Y27632 (HY--10071, MedChemExpress), and 200 ng/ml R-Spondin-1 (315-32, PeproTech). Human endometrial epithelial organoids with low passage numbers (3–6 passages) were used for the experiments.
Establishment of assembloid cultures
At passage 3, hESCs and endometrial epithelial organoid pellets were mixed at the ratio of 3:1 (v:v), and an ice-cold solution of 70% Matrigel + 30% DMEM/F12 was added at the ratio of 1:20 (cell pellet: solution). The Matrigel/cell suspension (40 μL) was added to each well of a 48-well plate. Following solidification of the Matrigel, 300 μL of assembloid culture medium (50% ExM + 50% hESC culture medium) was added. The medium was refreshed every 24–48 h. For decidualization experiments, the assembloid culture medium was supplemented with 10 nM E2 for 2 days and 10 nM E2, 1 μM MPA, and 0.5 mM cAMP for 2 days.
Animals and artificially induced in vivo decidualization
The experimental procedures were approved by the Zhejiang University Institutional Animal Care and Use Committee (Approval number: ZJU202220453). We have complied with all relevant ethical regulations for animal use. Specific pathogen-free (SPF) ICR mice were housed under a controlled environment (22 ± 2 °C, 50-60% humidity, 12 h light/dark cycle, lights on at 7 A.M.) in the Animal Experiment Centre of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine.
Normal 8-week-old female ICR mice were housed with males in a ratio of 2:1 at 5 P.M. to induce pregnancy. Vaginal plugs were detected on the second day at approximately 7 AM. Plug-positive females were labeled as day 1 of pregnancy (D1) and kept separately for experiments. 10 mice were randomly selected for the LiCl and PBS groups. The number of animals used was the minimum necessary to achieve statistically robust results. LiCl (L9650, Sigma-Aldrich) at 60 mg/kg concentration was administered intraperitoneally in LiCl group, and the corresponding volume of PBS was administered for three consecutive days starting from D5 in the control group. On D8, mice and their uterine implantation sites were weighed and preserved for freezing and fixation. Non-pregnant and pseudopregnant mice were excluded from the study. Experimental procedures were conducted between 8:00 and 9:00 AM daily, with testing order randomized to minimize bias.
Vasectomized male mice were prepared as follows: Male mice were anesthetized via intraperitoneal injection of tribromoethanol (1.25% w/v, 0.01 mL/g body weight). Bilateral 1-cm paravertebral skin incisions were made dorsally. The fat pad containing the testis was exteriorized, exposing the vas deferens (identified as a white, firm tubular structure adjacent to a blood vessel). Approximately 1 cm of the vas deferens was excised bilaterally using microscissors. Skin incisions were then sutured closed. Vasectomized males were allowed to recover for ≥2 weeks prior to mating. To generate pseudopregnant female mice, normal ICR females aged 8 weeks were housed with vasectomized males in a 2:1 ratio at 5 P.M. Vaginal plugs were detected on the second day at approximately 7 A.M. and plug-positive females were labeled as day 1 of pregnancy (D1). On D4, following general anesthesia (tribromoethanol, 1.25% w/v, 0.01 mL/g body weight), a midline lower abdominal incision was made in female mice. The uterine horn was bluntly exposed. One uterine horn was gently elevated with forceps, and approximately 15 µL of sesame oil (C24100, Thermo Scientific™) was injected using a insulin syringe, while the other side was used as a blank control. On D5, mice were intraperitoneally administered 60 mg/kg LiCl (n = 5) or PBS (n = 5) for three consecutive days. On D8, the mice were sacrificed by CO₂ inhalation, and the weight of the uterus on the treated side and the control side was accurately measured to determine the extent of decidualization.
The Men1-tm1Zqw (Men1f/f; MGI: 2664869) mouse line51 was maintained on a C57BL/6 J background (>10 generations). Uterine-specific knockout mice (Men1d/d; Men1f/f PgrCre/+) were generated by crossing Men1f/f mice with Pgr-tm2(cre)Lyd (Pgr-Cre; MGI: 3576366) mice52. All experimental mice were compared to wild-type littermate controls (Men1f/f Pgr+/+).
All animal experiments were conducted in accordance with ethical guidelines. In both the experimental and control groups, all animals were included in the data analysis without exclusion.
Chromatin immunoprecipitation (ChIP)-quantitative PCR (qPCR)
The SimpleChIP Enzymatic Chromatin IP Kit with magnetic beads (#9003, Cell Signaling Technology) was used in accordance with the manufacturer’s protocol. Cells (4 × 106) transfected with shMEN1 in 2–3 100 mm culture dishes were treated with formaldehyde to cross-link proteins to DNA. Next, 1× glycine was added to terminate the cross-linking process. Subsequently, 10 μg of sheared chromatin was incubated overnight at 4 °C with 1 μg of anti-H3K4me3 antibodies (#9751, Cell Signaling Technology) or anti-IgG antibodies. Chromatin fragments (1%) were stored at −20 °C for later use as nonprecipitated total chromatin (input) for normalization. Next, ChIP-grade protein G magnetic beads were added to chromatin fragments and incubated for 2 h at 4 °C with rotation. Immunoprecipitated chromatin was then washed with low- and high-salt ChIP buffer. qPCR was performed after reverse cross-linking of protein/DNA complexes to free DNA. Primers used for qPCR are shown in Supplementary Table 2.
Statistics and reproducibility
Biological replicates (n) are defined as experiments performed on independent biological sources. Key experiments were independently repeated at least three times to ensure reliability, including morphological results. The sample size (n, number of biological replicates per group) is explicitly stated in each figure legend. Data presented are representative of ≥3 independent experimental repeats. GraphPad Prism 10.0 was used for statistical analysis. Student’s t test was used to compare the data between two groups, while one-way ANOVA with Tukey’s multiple comparison test was used for comparing data from more than two groups. Two-way ANOVA with Tukey’s correction for multiple comparisons was applied for data from two groups with three time points. All data were expressed as mean ± standard error (X ± SD). A P value of <0.05 indicated a significant difference in all cases, and “ns” denoted a nonsignificant difference.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNA sequencing data used in this study are accessible with the following link: https://www.ncbi.nlm.nih.gov/sra/PRJNA1259970. Uncropped western blot images are available in Supplementary information. The source data for graphs are available in Supplementary Data. All other data are available from the corresponding author on reasonable request.
Code availability
Details of publicly available software used in the study are given in the “Methods”. No novel computational tools or proprietary algorithms were developed for this study.
References
Diedrich, K., Fauser, B. C. J. M., Devroey, P. & Griesinger, G. The role of the endometrium and embryo in human implantation. Hum. Reprod. Update 13, 365–377 (2007).
Franasiak, J. M. et al. A review of the pathophysiology of recurrent implantation failure. Fertil. Steril. 116, 1436–1448 (2021).
Craciunas, L. et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum. Reprod. Update 25, 202–223 (2019).
Arian, S. E. et al. Endometrial receptivity array before frozen embryo transfer cycles: a systematic review and meta-analysis. Fertil. Steril. 119, 229–238 (2023).
Mahajan, N. Endometrial receptivity array: clinical application. J. Hum. Reprod. Sci. 8, 121–129 (2015).
Riestenberg, C. et al. Endometrial compaction does not predict live birth rate in single euploid frozen embryo transfer cycles. J. Assist. Reprod. Genet. 38, 407–412 (2021).
Cha, J., Sun, X. & Dey, S. K. Mechanisms of implantation: strategies for successful pregnancy. Nat. Med. 18, 1754–1767 (2012).
Tu, Z. et al. Molecular determinants of uterine receptivity. Int. J. Dev. Biol. 58, 147–154 (2014).
Wang, W. et al. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med. 26, 1644–1653 (2020).
Gellersen, B. & Brosens, J. J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 35, 851–905 (2014).
Critchley, H. O. D., Maybin, J. A., Armstrong, G. M. & Williams, A. R. W. Physiology of the endometrium and regulation of menstruation. Physiol. Rev. 100, 1149–1179 (2020).
Whitby, S., Zhou, W. & Dimitriadis, E. Alterations in epithelial cell polarity during endometrial receptivity: a systematic review. Front. Endocrinol. 11, 596324 (2020).
Li, Q. et al. The Antiproliferative Action of Progesterone in Uterine Epithelium Is Mediated by Hand2. Science 331, 912–916 (2011).
Dreijerink, K. M. A. et al. Enhancer-mediated oncogenic function of the menin tumor suppressor in breast cancer. Cell Rep. 18, 2359–2372 (2017).
Feng, Z., Ma, J. & Hua, X. Epigenetic regulation by the menin pathway. Endocr. Relat. Cancer 24, T147–T159 (2017).
Gurung, B. et al. Menin epigenetically represses Hedgehog signaling in MEN1 tumor syndrome. Cancer Res 73, 2650–2658 (2013).
Qiu, H. et al. MEN1 deficiency leads to neuroendocrine differentiation of lung cancer and disrupts the DNA damage response. Nat. Commun. 11, 1009 (2020).
Ye, Z. et al. MEN1 promotes ferroptosis by inhibiting mTOR-SCD1 axis in pancreatic neuroendocrine tumors. Acta Biochim. Biophys. Sin. 54, 1599–1609 (2022).
Liu, M. et al. Menin directs regionalized decidual transformation through epigenetically setting PTX3 to balance FGF and BMP signaling. Nat. Commun. 13, 1006 (2022).
Hantak, A. M., Bagchi, I. C. & Bagchi, M. K. Role of uterine stromal-epithelial crosstalk in embryo implantation. Int. J. Dev. Biol. 58, 139 (2014).
Zhang, Q. & Yan, J. Update of Wnt signaling in implantation and decidualization. Reprod. Med. Biol. 15, 95–105 (2016).
Matkar, S., Thiel, A. & Hua, X. Menin: a scaffold protein that controls gene expression and cell signaling. Trends Biochem. Sci. 38, 394–402 (2013).
Moustafa, S. & Young, S. L. Diagnostic and therapeutic options in recurrent implantation failure. F1000Res. 9, F1000 Faculty Rev-208 (2020).
Lai, Z.-Z. et al. Single-cell transcriptome profiling of the human endometrium of patients with recurrent implantation failure. Theranostics 12, 6527–6547 (2022).
Zhang, H., Zhang, C. & Zhang, S. Single-cell RNA transcriptome of the human endometrium reveals epithelial characterizations associated with recurrent implantation failure. Adv. Biol. 8, 2300110 (2024).
Dunlap, K. A. et al. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol. Reprod. 85, 386–396 (2011).
Franco, H. L. et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. Publ. Fed. Am. Soc. Exp. Biol. 25, 1176–1187 (2011).
Macdonald, L. J. et al. Prokineticin 1 induces Dickkopf 1 expression and regulates cell proliferation and decidualization in the human endometrium. Mol. Hum. Reprod. 17, 626–636 (2011).
Wang, Q. et al. Wnt6 is essential for stromal cell proliferation during decidualization in mice. Biol. Reprod. 88, 5 (2013).
Jeong, J.-W. et al. β-catenin mediates glandular formation and dysregulation of β-catenin induces hyperplasia formation in the murine uterus. Oncogene 28, 31–40 (2009).
Li, Q. et al. WNT4 acts downstream of BMP2 and functions via β-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology 154, 446–457 (2013).
Tang, X. et al. CDC42 deficiency leads to endometrial stromal cell senescence in recurrent implantation failure. Hum. Reprod. deae246. https://doi.org/10.1093/humrep/deae246 (2024).
Marečková, M. et al. An integrated single-cell reference atlas of the human endometrium. Nat. Genet. https://doi.org/10.1038/s41588-024-01873-w (2024).
MacDonald, B. T., Tamai, K. & He, X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 (2009).
Nusse, R. & Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13, 767–779 (2012).
van Loon, K., Huijbers, E. J. M. & Griffioen, A. W. Secreted frizzled-related protein 2: a key player in noncanonical Wnt signaling and tumor angiogenesis. Cancer Metastasis Rev. 40, 191–203 (2021).
Wo, D. et al. Opposing roles of Wnt inhibitors IGFBP-4 and Dkk1 in cardiac ischemia by differential targeting of LRP5/6 and β-catenin. Circulation 134, 1991–2007 (2016).
Cao, Y. et al. Nuclear-cytoplasmic shuttling of menin regulates nuclear translocation of {beta}-catenin. Mol. Cell. Biol. 29, 5477–5487 (2009).
Xu, J. et al. MEN1 Deficiency-driven activation of the β-Catenin-MGMT axis promotes pancreatic neuroendocrine tumor growth and confers temozolomide resistance. Adv. Sci. 2308417. https://doi.org/10.1002/advs.202308417 (2024).
Fukui, Y. et al. Uterine receptivity, embryo attachment, and embryo invasion: Multistep processes in embryo implantation. Reprod. Med. Biol. 18, 234–240 (2019).
Large, M. J. & DeMayo, F. J. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol. Cell. Endocrinol. 358, 155–165 (2012).
Chung, D., Gao, F., Jegga, A. G. & Das, S. K. Estrogen mediated epithelial proliferation in the uterus is directed by stromal Fgf10 and Bmp8a. Mol. Cell. Endocrinol. 400, 48–60 (2015).
Lee, D.-K. et al. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Mol. Endocrinol. Baltim. Md 24, 930–940 (2010).
Hiraoka, T. et al. Differential roles of uterine epithelial and stromal STAT3 coordinate uterine receptivity and embryo attachment. Sci. Rep. 10, 15523 (2020).
Fukui, Y. et al. The EZH2–PRC2–H3K27me3 axis governs the endometrial cell cycle and differentiation for blastocyst invasion. Cell Death Dis. 14, 320 (2023).
Xin, Q., Feng, I., Yu, G. & Dean, J. Stromal Pbrm1 mediates chromatin remodeling necessary for embryo implantation in the mouse uterus. J. Clin. Investig. 134, e174194 (2024).
Turco, M. Y. et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 19, 568–577 (2017).
Rawlings, T. M. et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. eLife 10, e69603 (2021).
Garcia-Alonso, L. et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 53, 1698–1711 (2021).
Bertolino, P. et al. Genetic ablation of the tumor suppressor menin causes lethality at mid-gestation with defects in multiple organs. Mech. Dev. 120, 549–560 (2003).
Soyal, S. M. et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41, 58–66 (2005).
Acknowledgements
We are grateful to Prof. Francesco DeMayo (National Institute of Environmental Health Sciences), Prof. ChangXian Zhang (Centre de Recherche en Cancérologie de Lyon, Université Lyon 1) for providing the Pgr-Cre and Men1-loxp transgenic mice. This work was supported by the Natural Science Foundation of Zhejiang Province (LQ23H040002), the Medicine and Health Science and Technology Project of Zhejiang Province (2024KY1116), the National Natural Science Foundation of China (Nos.82271652), Key Medical Discipline of Hangzhou (2025HZPY08), the Science and Technology plan Project of Jiaxing (2023AY31023). We acknowledge other members of Zhejiang Key Laboratory of Precise Protection and Promotion of Fertility, the Research Office, and the Department of Pathology at Sir Run Run Shaw hospital for their technical support and assistance in this study. We would like to thank TopEdit (www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript. Thanks to Biorender.com for assisting in the creation of Fig. 3d (https://BioRender.com/h87w299), Fig. 5d (https://BioRender.com/g10a347), Fig. 5k (https://BioRender.com/uz1eyq3), Fig. 7 (https://BioRender.com/tdt86ad) and Supplementary Fig. 1a (https://BioRender.com/566ykx3).
Author information
Authors and Affiliations
Contributions
The project was initiated and supervised by HB.W., MY.L., and XN.L. The experiments were designed by MY.L. and X.X., with X.X. performing the experiments and MY.L. providing the bioinformatic analyses. YC.H., KE.J., and HY.Z. helped to perform part of the experiments. WN.X. and YN.M. contributed to the collection of clinical samples. XJ.L., MY.L., and XN.L. participated in the quality control of the study. The manuscript was written by X.X. and MY.L. All authors participated in the discussion, revision, and approval of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Sudhansu Dey, Sascha Ott and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Wee-Wei Tee and Rosie Bunton-Stasyshyn. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, X., Han, Y., Jin, K. et al. Endometrial stromal Menin supports endometrial receptivity by maintaining homeostasis of WNT signaling pathway through H3K4me3 during WOI. Commun Biol 8, 995 (2025). https://doi.org/10.1038/s42003-025-08434-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08434-9