Abstract
The primordial follicle (PF) pool (also known as ovarian reserve) is the only source of functional eggs during the reproductive lifespan, which formed at embryonic stage in most female mammals and is no longer renewed. CCAAT/enhancer binding protein beta (C/EBPβ) is a transcription factor (TF) and widely expressed in adult ovarian granulosa cells, but its role in the early ovarian development remains unclear. Here, we showed that C/EBPβ was enhanced during PF formation process in mice, and underwent nuclear translocation in germ cells along with the PF assembly (PFA) process. Importantly, the in vitro knockdown of C/EBPβ could inhibit the related proteins expression, resulting in the obstruction of PF formation. Mechanistically, the chromatin accessibility analysis revealed that C/EBPβ binds to the promoter region of the histone acetylase encoding gene Ep300 to promotes its expression, and enhance neurotrophic tyrosine receptor kinase (NTRK) signaling (required for PF formation) by maintaining chromatin accessibility of Furin promoter region. Interestingly, our results verified that the nuclear translocation of C/EBPβ is regulated by its phosphorylation level, and Lamin B1 acts as a “gatekeeper” molecule for the process. In summary, this report suggests that C/EBPβ is a key regulator for the establishment of ovarian reserve in mice.
Similar content being viewed by others
Introduction
The primordial follicle (PF) pool (also known as ovarian reserve) is formed in the mid-pregnancy or perinatal period in mammals1,2, and then no longer self-renews. Following the follicle recruitment, ovarian follicles grow and mature, finally the functional oocytes are ovulated to wait for fertilization, the PFs are gradually and irreversibly consumed until they are exhausted, and menopause arrives. The number of PFs directly determines the reproductive lifespan of female mammals.
PF formation is a complex regulatory process. The primordial germ cells that migrate to the gonads undergo several rounds of rapid and incomplete mitosis to form germline cysts, which are surrounded by ovarian somatic cells to form nests3. In the following period, the germ cell nests begin to breakdown under the invasion of ovarian somatic cells, and only a small number of oocytes are surrounded by ovarian somatic cells to form PFs, while a large number of germ cells contribute their cytoplasm through the “nursing” process and then undergo irreversible cell death4,5. In mice, primordial follicle assembly (PFA) begins around embryonic day (E) 17.5 and is completed in the following several days, from postnatal day (PD) 3 to PD5, all PFs are assembled and enter a quiescent state waiting for activation2,4. There are two waves of PFs in the mouse ovary that differ in developmental dynamics6. The first wave of follicles develops rapidly and is formed by a single oocyte surrounded by bipotential pregranulosa (BPG) cells in the medullary region, contributing to the onset of puberty7. The second wave of follicles in the cortical region is supported by epithelial pregranulosa (EPG) cells and then enters a quiescent state to maintain lifelong fertility7. Recently, multiple key regulatory factors and signaling pathways have been reported to play an pivotal role in PF formation8, including JNK9, TGF-β10, SRSF111, E-cadherin12, and SF-113. However, the molecular mechanism of ovarian reserve establishment and oocyte fate determination remains largely unclear.
CCAAT/enhancer binding proteins (C/EBPs) belong to the bZIP class of transcription factors (TFs), including C/EBPα, -β, -γ, -δ, -ε, and -ζ, which regulate transcription by binding to specific promoter sequences14,15. Among them, C/EBPβ is localized in the follicular cells of adult ovary, and its expression is reduced in the granulosa cells of polycystic ovary syndrome (PCOS) patients16. Report from mouse models has shown that C/EBPβ deficiency affects the ovulation process and ultimately impairs fertility17. During PF formation, the C/EBPβ expression in germ cell is affected by environmental stress18, but whether C/EBPβ involving in regulating the establishment of ovarian reserve during the early stages of ovarian development is still unknown, especially its molecular mechanism.
Here, we highlight the important function of C/EBPβ in regulating the establishment of ovarian reserve. During PF formation, C/EBPβ is specifically expressed in germ cells and exhibits enhanced transcriptional activity, which depends on the coordinated control of temporal and spatial effects, especially the developmental stage-specific nuclear translocation of C/EBPβ. In vitro knockdown of C/EBPβ results in inhibition of PF formation, mainly due to disruption of NTRK signaling caused by reduced p300-mediated chromatin accessibility. In addition, we show that JNK signaling and Lamin B1 coordinately control the nuclear translocation of C/EBPβ. Overall, our work highlights the key role of C/EBPβ during early ovarian development, especially on PF formation.
Results
Expression pattern of C/EBPβ during establishment of ovarian reserve
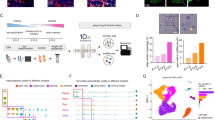
To confirm whether C/EBPβ is associated with the establishment of ovarian reserve, we examined the expression pattern of C/EBPβ in germ cell during PF formation process. Therefore, we selected three consecutive time points with a 3-day interval: E16.5, PD0, and PD3, to characterize the expression pattern of C/EBPβ in the early, middle, and late stages of the formation of ovarian reserve, respectively (Fig. 1A). Compared with the early stage (E16.5) of PF formation, the expression level of C/EBPβ protein in ovaries increased significantly in the middle (PD0) and late (PD3) stages of PF formation (Fig. 1B). During the formation of ovarian reserve, the results of ovarian section staining with DDX4 (a common germ cell cytoplasmic marker) showed that C/EBPβ was specifically localized in germ cells (Fig. 1C), based on the histological analysis, it revealed that the subcellular localization of C/EBPβ in germ cells changed significantly during PF formation. In the early stage of PF formation, C/EBPβ was only weakly expressed in the nuclei of germ cells in nests in the medullary and cortical regions of the ovary (Fig. 1C, D). In the middle stage of PF formation, the nuclear expression of C/EBPβ in the follicular germ cells was significantly stronger than the cytoplasm, and this expression pattern was completely opposite in most of the germ cells in the nests (Fig. 1C, D). As PF formation progressed to the later stage, C/EBPβ was expressed in both the nucleus and cytoplasm of all germ cells, and its nuclear expression was significantly stronger than the cytoplasm (Fig. 1C, D). Thus, as germline nests developed into follicles, the nuclear expression of C/EBPβ in germ cells during ovarian reserve establishment process was enhanced and was accompanied by nuclear translocation (Fig. 1D) suggesting the potential role of C/EBPβ in the regulation of germ cell fate. Based on these results, we determined the temporal pattern (expression in developmental time) and spatial pattern (nuclear translocation) of C/EBPβ expression during the establishment of ovarian reserve.
A Schematic diagram of PF formation in mice. This figure was originally created by the authors using Adobe Illustrator. B Representative images (left) and expression level analysis (right) of C/EBPβ protein in E16.5, PD0, PD3 ovaries. C Representative images (left) and line scan analysis (right) of the cellular localization of C/EBPβ in E16.5, PD0, and PD3 ovaries. White arrow indicates the direction from the medulla (m) to the cortex (c); yellow arrow indicates the direction of the line scan. Details of the protein subcellular localization analysis methods are shown in the “Methods” section. D Fluorescence intensity analysis of the nuclei and cytoplasm of C/EBPβ in germ cells of nest and follicles in E16.5, PD0, and PD3 ovaries. E Cebpa (grey) and Cebpb (blue) abundance in Mus musculus (up) and Homo sapiens (down). The ovarian development events marked in the figure are based on literature reports. Data are means ± SEM. N = 8 for the biological repeats of WB experiment; n = 5 for the biological repeats of cellular localization and fluorescence intensity analysis experiment, a dot in (D) represents a germ cell.
We further examined the expression pattern of Cebpb (the gene encoding C/EBPβ) during PFA in multiple species (including mouse, human, rabbit, chicken, and opossum) using the Evo-Devo database [https://apps.kaessmannlab.org/evodevoapp/]19. The results showed that Cebpb was expressed at high levels during PFA in all five species (Figs. 1E and S1A). In sharp contrast, Cebpa, a TF of the same family as Cebpb, was expressed at low levels or not detected during PFA (Figs. 1E and S1A). The striking species conservation of Cebpb expression patterns during the ovarian reserve establishment stage led us to speculate that this factor may play a regulatory role in the process.
C/EBPβ is required for normal PF formation in mice
To explore the role of C/EBPβ in the establishment process of ovarian reserve, we constructed an in vitro knockdown model, and small interfering RNA (siRNA) specific for C/EBPβ mRNA was used to knock down its expression during the in vitro culture of ovaries from newborn mice (Fig. 2A), it was verified that the expression of C/EBPβ was significantly reduced in the model (Fig. 2B). Knockdown of Cebpb did not cause significant change of Cebpa gene expression (Fig. S2). Next, we measure the effect of C/EBPβ depletion on PF formation, histological results showed that C/EBPβ knockdown led to a decrease in the proportion of PFs in the ovaries, while the number of germ cells was not affected (Fig. 2C–E), indicating that C/EBPβ depletion inhibited the process of PF formation. Notably, knockdown of C/EBPβ suppressed the expression of key factors for PF formation, Nobox, Lhx8, and Sohlh1 (Fig. 2F, G). DDX4 expression did not change significantly after C/EBPβ knockdown, indicating that CEBPβ depletion had little effect on germ cell number, which is consistent with the histological results (Fig. 2E, G). In summary, we show that C/EBPβ is essential for the establishment of ovarian reserve.
A Schematic diagram of the C/EBPβ knockdown model construction during in vitro culture of newborn ovarian. This figure was originally created by the authors using Adobe Illustrator. B Representative images (left) and expression level analysis (right) of C/EBPβ protein between control (Ctrl) and C/EBPβ knockdown (siCebpb) groups. C IF staining of DDX4 in C/EBPβ knockdown model. DDX4 (yellow) indicates oocytes. Hoechst (blue) indicates nuclei (Nuc). White arrow indicates the direction from the medulla (m) to the cortex (c). D, E Violin plots showing the percentage of oocytes in follicles (D) and the total number of oocytes (E) in each ovarian section of each group. F Analysis of mRNA expression levels of Nobox, Lhx8, and Sohlh1 in the Ctrl and siCebpb groups. (G) Representative images (left) and expression level analysis (right) of DDX4, LHX8, SOHLH1, and NOBOX proteins in the Ctrl and siCebpb groups. Data are means ± SEM. N = 4–6 indicated the biological repeats, as shown on graphs.
C/EBPβ is a transcriptional regulator of histone acetylase p300
Previously, C/EBPβ may act as an upstream regulator of histone modification modifying enzymes18. As reported, histone acetylation potentially is also involved in the regulation of PF formation20. Here, we focused on the histone acetyltransferase p300, which has been shown to play a role in follicle activation after the establishment of ovarian reserve21. Interestingly, our results showed that C/EBPβ binds to the promoter of Ep300 (the gene encoding p300) (Fig. 3A), suggesting that C/EBPβ may participate in the regulation of histone modifications by p300 during the establishment of ovarian reserve. Consistent with our expectation, in vitro knockdown of C/EBPβ significantly weakened the expression of p300 (Fig. 3B, C). Histological results showed that C/EBPβ depletion reduced the expression of p300 in the nucleus and cytoplasm of germ cells, but had no effect on the cellular localization of p300 (Fig. 3D, E). Interestingly, the expression of p300 in the germ cells of the follicles was significantly stronger than that in the nests, indicating that the PF formation process is accompanied by the enhanced expression of p300 (Fig. 3D and E). Importantly, under physiological conditions, the enhanced expressions of p300 in the middle and late stages of PF formation were observe, and it also showed an expression pattern similar to that of C/EBPβ (Fig. 1B and Fig. 3F). These experimental evidences prompted us to identify p300 as a downstream factor of C/EBPβ.
A Detection of the enrichment level of the Ep300 promoter fragment bound to C/EBPβ using CUT&RUN-qPCR. B Analysis of mRNA expression levels of Ep300 in the Ctrl and siCebpb groups. C Representative images (left) and expression level analysis (right) of p300 in the Ctrl and siCebpb groups. D Representative images (left) and line scan analysis (right) of the cellular localization of p300 in the Ctrl and siCebpb groups. White dashed lines indicate the approximate outlines of the nests; yellow arrow indicates the direction of the line scan. E Fluorescence intensity analysis of the nuclei and cytoplasm of germ cells in the nests and follicles in the Ctrl and siCebpb groups. F Representative images (left) and expression level analysis (right) of p300 protein in the E16.5, PD0, PD3 ovaries. G Representative images and expression level analysis of p300 in the Ctrl and siEp300 groups. H IF staining of DDX4 in p300 knockdown model. DDX4 (yellow) indicates oocytes. Hoechst (blue) indicates nuclei (Nuc). White arrow indicates the direction from the medulla (m) to the cortex (c). Violin plots showing the percentage of oocytes in follicles (I) and the total number of oocytes (J) in each ovarian section of each group. K Analysis of mRNA expression levels of Nobox, Lhx8, and Sohlh1 in the Ctrl and siEp300 groups. L Representative images (left) and expression level analysis (right) of DDX4, LHX8, SOHLH1, and NOBOX proteins in the Ctrl and siEp300 groups. Data are means ± SEM. N = 3–6 indicated the biological repeats, as shown on graphs; n = 5 for the biological repeats of cellular localization analysis experiment.
To further confirm whether p300 is a regulatory factor in the establishment of ovarian reserve, we constructed an in vitro model with targeted knockdown of p300 (Fig. 3G). In the result, p300 knockdown inhibited PF formation but did not affect the number of germ cells (Fig. 3H–J). Meanwhile, p300 depletion reduced the expression levels of Nobox, Lhx8, and Sohlh1 (Fig. 3K, L). The similar phenotypes of p300 and C/EBPβ knockdown indicated that p300 may serve as a downstream factor of C/EBPβ during PF formation.
p300 enhanced NTRK signaling through chromatin accessibility during establishment of ovarian reserve
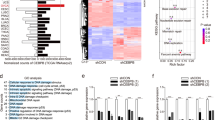
Considering the important role of p300 in regulating chromatin accessibility, the assay for transposase-accessible chromatin by sequencing (ATAC-seq) was employed to explore the possible epigenetic mechanism of p300 during ovarian reserve establishment (GSA: CRA021387). In the results, the proportion of promoter region in the total enrichment signals was reduced in group of p300 knockdown, indicating that p300 may affect gene expression by affecting the open chromatin of promoter region (Fig. 4A). Compared with the control group, 13 upregulated differentially accessible regions (DARs) and 127 downregulated DARs were identified in the p300 knockdown group (Fig. 4B and Supplementary Data 1). Notably, most DARs are concentrated in the promoter region, accounting for 76.47% (Fig. 4C and Supplementary Data 2). It is generally believed that p300 reduces chromatin accessibility by reducing histone acetylation level22. Therefore, we focused on DARs located in the promoter region that were downregulated by p300 (Supplementary Data 2). Next, we obtained differentially expressed genes (DEGs) that were upregulated in germ cells of PD0 vs E16.5 and PD3 vs PD0 (Supplementary Data 3 and 4), which were generated from the reported single-cell transcriptome datasets during PF formation stage8. By jointly analyzing the above three datasets, we obtained 47 candidate genes containing downregulated DARs, which may play a regulatory role in PF formation process (Fig. 4D and Table S1). Enrichment analysis of these candidate genes showed that p300 affected pathways such as NTRK signaling, neurotrophin signaling, and cell growth regulation (Fig. 4E). Notably, neurotrophin signaling and NTRK signaling are involved in regulating follicle development23,24. Thus, the NTRK-related genes obtained by enrichment analysis were subjected to display their expression during the establishment of ovarian reserve (Fig. 4F). Subsequently, we focused on three core genes of the NTRK pathway: Furin, Pcsk6, and Rap1a, whose chromatin accessibility and expression levels were significantly reduced after p300 knockdown (Fig. 4G, H). In particular, the protease encoded by Furin can convert the precursor of nerve growth factor (NGF, an important neurotrophin) into NGF25. Considering the key role of Furin in NTRK signaling pathway, we further explored the TF binding mechanism of p300-mediated chromatin regulation around Furin. Based on Cistromic (ChIP-Seq) regulation report from SPP (The Signaling Pathways Project) [http://www.signalingpathways.org/], Furin-related upstream TFs were obtained, which were ranked according to the binding score (Fig. 4I). Interestingly, C/EBPβ showed strong binding activity to the Furin promoter region (Fig. 4I). To confirm this, we analyzed the binding activity of C/EBPβ to Furin promoter before and after p300 knockdown. The results showed that the binding of C/EBPβ to the Furin promoter was weakened after p300-mediated reduction of chromatin accessibility (Fig. 4J). In addition, we also detected the binding activity of p300 to the Furin promoter region (Fig. 4K).
A The distribution of genomic peaks in the Ctrl and siEp300 groups. B Column graph of DARs between Ctrl and siEp300 groups. C Genomic distribution of downregulated DARs in the siEp300 vs. Ctrl groups. D Venn diagram of the gene set with down-regulated DARs in the promoter region, the DEG set up-regulated at PD0, and the DEG set up-regulated at PD3. E Metascape enrichment analysis of candidate gene sets (Gene set acquisition has been described in the text). F Dot plot shows the expression of NTRK signaling-related genes in metascape enrichment analysis in E16.5, PD0, and PD3 oocytes. G The normalized reads of NTRK core-related genes Furin, Pcsk6, and Rap1a shown by ATAC-seq. H Analysis of mRNA expression levels of Furin, Pcsk6, and Rap1a in the Ctrl and siEp300 groups. I Ranking of TFs binding to the Furin promoter region obtained using The Signaling Pathways Project by binding score. J Detection of the binding activity of C/EBPβ to the Furin promoter region in the Ctrl and siEp300 groups using CUT&RUN-qPCR. K Detection of the enrichment level of Furin promoter fragment bound to p300 using CUT&RUN-qPCR. L Representative images (left) and expression level analysis (right) of FURIN, DDX4, LHX8, and SOHLH1 proteins in the Ctrl and siFurin groups. Violin plots showing the percentage of oocytes in follicles (M) and the total number of oocytes (N) in each ovarian section of each group. Data are means ± SEM. N ≥ 2 per group for the biological repeats of ATAC-seq; n = 5 to 6 for the biological repeats of CUT&RUN-qPCR, qPCR, WB, and oocyte count experiments, as shown on graphs.
Considering the dramatic changes in chromatin dynamics and expression of Furin after p300 knockdown, the role of FURIN in the establishment of follicular reserve was explored (Figs. 4L and S3A). In the results, the in vitro knockdown of FURIN impaired the expressions of LHX8 and SOHLH1, and led to the inhibition of PF formation, but did not affect the number of germ cells (Figs. 4L–4N and S3B), which is similar to the abnormal reproductive phenotype caused by C/EBPβ or p300 depletion. Furthermore, we also showed that C/EBPβ knockdown impaired the expression of FURIN (Fig. S3C). In summary, we proposed that p300 likely activated NTRK signaling by enhancing Furin expression, which is necessary for PF formation.
Activation of the neurotrophin receptor safeguards ovarian reserve against detrimental effects induced by p300 inhibition
As results showed above, NTRK signaling may serve as a downstream pathway of p300 during ovarian reserve establishment (Figs. 4H and S3B). To clarify the role of p300 and NTRK signaling in PF formation, we constructed an in vivo mouse model with p300 inhibition using curcumin (one p300 inhibitor)26 and NGF signaling activation using B-355252 (an NGF receptor agonist)27, respectively (Fig. 5A). Curcumin inhibited the expression of Furin, a downstream gene of p300 (Fig. 5B), while B-355252 enhanced the expression of Fshr (Fig. 5C), a downstream gene of NTRK signaling24. At the same time, the use of curcumin and B-355252 did not produce obvious systemic toxic effects, nor did it cause abnormal expression of other epigenetic regulatory factors (Nr5a113, Lsd128) for ovarian reserve establishment (Fig. S4A, B), indicating the effectiveness and safety of the in vivo model. We found that Curcumin (or p300 inhibition) in vivo reduced serum NGF levels in pregnant mice after delivery, and this inhibitory effect was relieved by activating NTRK signaling with B-355252 (Fig. 5D). In addition, we also noticed that the serum NGF level of newborn pups continued to increase during the period of ovarian reserve establishment, which was similar to the expression trend of C/EBPβ and p300 in the ovary (Figs. 1B, 3F, and S4C). This emphasizes that NTRK signaling is associated with C/EBPβ and p300 expression. Analysis of protein expression in the in vivo model showed that NTRK activation restored the expression of key factors for PF formation, including Lhx8, Sohlh1 and Nobox, that were impaired by p300 inhibition (Figs. 5E, 5F, and S4B). Here, we demonstrated through an in vivo model that NTRK signaling serves as a downstream effector of p300 and that activation of NTRK signaling can protect ovarian reserve from the adverse effects of p300 inhibition.
A Schematic diagram of the mouse model of p300 inhibition and neurotrophin receptor activation. This figure was originally created by the authors using Adobe Illustrator. B Analysis of Furin mRNA levels in ovarian tissues of newborn mice treated with different concentrations of curcumin. C Analysis of Fshr mRNA levels in ovarian tissues of newborn mice treated with different concentrations of B-355252. D NGF levels in the serum of postpartum female mice in the Ctrl, Curcumin, Curcumin+B-355252, and B-355252 groups. E Representative images (left) and expression level analysis (right) of DDX4, LHX8, and SOHLH1 proteins in the Ctrl, Curcumin, Curcumin+B-355252, and B-355252 groups. F IF staining of DDX4 in the Ctrl, Curcumin, Curcumin+B-355252, and B-355252 groups. DDX4 (yellow) indicates oocytes. Hoechst (blue) indicates nuclei (Nuc). G, H Violin plots showing the percentage of oocytes in follicles (G) and the total number of oocytes (H) in each ovarian section of each group. Data are means ± SEM. N = 4 to 6 indicated the biological repeats, as shown on graphs.
Nuclear translocation of C/EBPβ is regulated by the JNK signaling
The phosphorylation level of TF is highly correlated with their transcriptional activity29. Therefore, we examined the phosphorylation level of C/EBPβ during PF formation. The results showed that the phosphorylation level of C/EBPβ increased during PF formation (Fig. 6A), suggesting a potential connection between the phosphorylation modification of C/EBPβ and the establishment of ovarian reserve. In addition, c-Jun amino-terminal kinase (JNK), a member of the mitogen-activated protein kinase (MAPK, an important pathway involved in the regulation of protein phosphorylation) family, was identified as a key regulator of PF formation, and its expression increased during the formation of ovarian reserve9. Here, we speculated that JNK signaling may be involved in the phosphorylation regulation of C/EBPβ in mice. Next, SP600125, a JNK inhibitor9, was applied to explore the effect of JNK inhibition in vitro on C/EBPβ phosphorylation (Fig. 6B). Consistent with our speculation, SP600125 treatment effectively reduced the phosphorylation level of C/EBPβ at Thr188, but did not affect its total protein level (Fig. 6C–E). Meanwhile, p300 expression was significantly reduced after JNK inhibition, especially in the nucleus and cytoplasm of oocytes (Figs. 6C, F, and S5). FURIN expression was also affected after JNK inhibition (Fig. 6C, G). Histological results showed that JNK inhibition suppressed PF formation (Fig. 6H–J), which is consistent with previous reports9. Notably, the histological results also showed dramatic changes in the subcellular localization and expression pattern of C/EBPβ before and after JNK inhibition. The strong positive signal of C/EBPβ in the nucleus was weakened after JNK inhibition, while the positive signal of C/EBPβ in the cytoplasm was increased (Fig. 6K, L). Further analysis of the nuclear and cytoplasmic expression of C/EBPβ showed that the proportion of oocytes with a high nuclear-to-cytoplasmic (N/C) ratio of C/EBPβ was significantly reduced after JNK inhibition (Fig. 6M). Obviously, the nuclear translocation process of C/EBPβ was blocked after JNK inhibition. In summary, it’s concluded that the nuclear translocation process of C/EBPβ is highly correlated with its phosphorylation level, and this process is regulated by JNK signaling.
A Representative images (left) and expression level analysis (right) of p-C/EBPβT188 protein in the E16.5, PD0, PD3 ovaries. B Schematic diagram of the JNK inhibition (JNKi) model construction during in vitro culture of newborn ovarian. This figure was originally created by the authors using Adobe Illustrator. C Representative images of p-C/EBPβT188, C/EBPβ, p300, and FURIN proteins in the Ctrl and JNKi groups. Expression level analysis of p-C/EBPβT188 (D), C/EBPβ (E), p300 (F), and FURIN (G) proteins in the Ctrl and JNKi groups. H IF staining of DDX4 in the Ctrl and JNKi groups. DDX4 (yellow) indicates oocytes. Hoechst (blue) indicates nuclei (Nuc). White arrow indicates the direction from the medulla (m) to the cortex (c). Violin plots showing the percentage of oocytes in follicles (I) and the total number of oocytes (J) in each ovarian section of each group. K Representative images (left) and line scan analysis (right) of the cellular localization of C/EBPβ in the Ctrl and JNKi groups. White dashed lines indicate the approximate outlines of the nests; white arrow indicates the direction from the medulla (m) to the cortex (c); yellow arrow indicates the direction of the line scan. L Fluorescence intensity analysis of the nuclei and cytoplasm of germ cells in the nests and follicles in the Ctrl and JNKi groups. M Proportion of oocytes with C/EBPβ N/C ratio > 1 in the total number of oocytes in the same ovarian section. Data are means ± SEM. N = 4–9 for the biological repeats of WB and oocyte count experiments, as shown on graphs; n = 6 for the biological repeats of cellular localization and fluorescence intensity analysis experiment, a dot in (L) represents a germ cell.
Lamin B1 functions as a regulator of C/EBPβ nuclear translocation
The phosphorylation mediated by JNK inhibition did not seem to promote C/EBPβ to move into the nucleus. The most intuitive manifestation was that C/EBPβ still aggregated near the nuclear-cytoplasmic junction after JNK inhibition, rather than being evenly distributed in the cytoplasm (Fig. 6M). This similar phenomenon has also been reported in other transcription factors30. In order to further clarify the molecular details of C/EBPβ nuclear translocation, we used Lamin B1 to mark the nuclear lamina adjacent to the nuclear membrane to characterize the nuclear entry process of C/EBPβ. We found that after JNK inhibition, the colocalization of C/EBPβ and Lamin B1 was significantly enhanced, suggesting that the reduction in phosphorylation levels caused C/EBPβ to be trapped in the nuclear lamina and unable to complete the nuclear translocation process (Fig. 7A, B). Further analysis revealed that C/EBPβ and Lamin B1 had protein interactions, and this interaction was enhanced after C/EBPβ was dephosphorylated (Fig. 7C). These results collectively indicate that Lamin B1 plays an important role in the regulation of C/EBPβ nuclear translocation. It acts as a “gatekeeper” molecule for C/EBPβ to enter the nucleus and coordinates the spatial effect-dependent regulation of C/EBPβ transcriptional activity with the phosphorylation level of C/EBPβ.
A Representative images (left) and line scan analysis (right) of colocalization of C/EBPβ and Lamin B1 in the Ctrl and JNKi groups. Lamin B1 (blue) indicates nuclear lamina; dashed white arrow indicates the direction of the line scan. B Schematic diagram of the effect of SP600125 treatment on the nuclear translocation of C/EBPβ in oocytes. C Co-IP showed the interaction between C/EBPβ and Lamin B1 in the Ctrl and JNKi groups. Whole ovarian lysate was used as input. D Summary of C/EBPβ regulation during ovarian reserve establishment. Coordinated control of C/EBPβ transcriptional activity by temporal and spatial effects (a); chromatin accessibility dynamics mediated by C/EBPβ downstream molecule p300 participates in regulating the NTRK signaling required for ovarian reserve establishment (b); terminal effects of C/EBPβ regulation during ovarian reserve establishment (c). Data are means ± SEM. N = 5 for the biological repeats of cellular localization analysis experiment; n = 4 for the biological repeats of Co-IP experiment.
Discussion
Ovarian reserve in mammals is directly related to reproductive lifespan, the establishment of which is a complex and finely regulated multicellular process. Actually, a growing number of studies have demonstrated numerous regulatory factors regulate the establishment of ovarian reserve, such as LHX8, NOBOX, SOHLH1, and ELVAL2; mutation of all the genes can cause the phenotype of primary ovarian insufficiency (POI). Our work shows that C/EBPβ is a key regulator of oocyte, during the process, two different pathways, temporal and spatial effect, jointly enhance the transcriptional activity of C/EBPβ, especially the nuclear translocation of C/EBPβ coordinated by Lamin B1 and its own protein phosphorylation. The downstream effect of enhanced C/EBPβ transcriptional activity is manifested as increased p300-mediated chromatin accessibility, which maintains ovarian reserve establishment by promoting NTRK signaling (Fig. 7D).
In the study, C/EBPβ is proved to regulate the establishment of ovarian reserve. Multiple reports from medaka31, mouse17, rat32, pig33, and human16 have demonstrated the important role of C/EBPβ in adult granulosa cells. Multi-species gene expression analysis showed that C/EBPβ expression was highly active and conserved during the ovarian reserve establishment stage, suggesting a potential role of C/EBPβ in PF formation. Here, we provide experimental evidence that depletion of C/EBPβ inhibits PF formation. PF formation is accompanied by a large loss of germ cells, which is a direct result of programmed cell death of nurse cells4,5. C/EBPβ depletion showed a significant effect on the process of PF formation but did not affect the number of germ cells, indicating that C/EBPβ is not directly involved in regulating programmed cell death of germ cells. Notably, reports from genetically manipulated mice are surprisingly consistent with our experimental evidence, showing that disruption of NTRK signaling inhibits PFA but rarely causes perinatal oocyte death in Ntrk1/2 knockout mice24. This suggests that there is some independence between cell death and PFA during PF formation. How NTRK signaling affects PFA through non-cell death pathways will be of interest in our future work. There are three different isoforms of C/EBPβ: LIP (liver-enriched inhibitory protein; 20 kDa), LAP (liver-enriched activator protein; 34 kDa), and LAP* (38 kDa)34. We found that LAP* was significantly more expressed in the ovaries during the establishment of ovarian reserve than the other two isoforms, so we focused on the LAP* isoform, which can promote the downstream transcriptional activation of C/EBPβ35. We showed that the high expression of the C/EBPβ transcription-promoting isoform during the establishment of ovarian reserve enhanced the transcriptional activity of C/EBPβ, which was reflected by the temporal effect.
Importantly, nuclear translocation regulation during oogenesis or follicle development is not uncommon36,37,38. Our results strongly suggested that nuclear translocation of C/EBPβ was highly correlated with the developmental progression from nests to follicles. Specifically, the C/EBPβ expression pattern of germ cells in JNK-inhibited ovaries was similar to that of germ cells in nests at the mid-stage of PF formation in vivo. The phosphorylation level of C/EBPβThr188 is regulated by MAPK (ERK1/2) and is positively correlated with the activity of C/EBPβ39. At the same time, MAPK signaling also plays a role in the regulation of the subnuclear distribution of C/EBPβ40. Here, we report for the first time that MAPK (JNK) regulates phosphorylation of C/EBPβThr188, and the dynamics of this phosphorylation affect C/EBPβ nuclear translocation rather than nuclear redistribution. Interestingly, similar to the perinuclear localization of other TFs30, the perinuclear localization of C/EBPβ led us to note that it is only blocked in nuclear import, as JNK inhibition did not change the nuclear translocation trend of C/EBPβ. It is important to note that C/EBPα has been reported to interact with Lamin B1 in mice41. Lamin A has also been previously identified as a regulator of nuclear translocation42. Here, we identified Lamin B1 as a “gatekeeper” molecule that regulates the nuclear entry of C/EBPβ, which allows phosphorylated C/EBPβ to enter the nucleus, while unphosphorylated C/EBPβ is trapped in the nuclear lamina and accumulates near the nuclear membrane. In summary, we showed that JNK signaling coordinates with Lamin B1 to control the nuclear translocation of C/EBPβ, which is a specific manifestation of the spatial effect regulation of C/EBPβ transcriptional activity. The spatial effect targeted interference of C/EBPβ showed similar effects on p300 and Furin expression and ovarian reserve establishment, which makes it easy for us to confirm that these two pathways are jointly involved in the regulation of C/EBPβ transcriptional activity.
The chromatin accessibility mediated by C/EBPβ via p300 is identified as the potential upstream regulator of NTRK signaling. NTRK signaling is mainly mediated by NTRK1/2/3 receptors and corresponds to several different neurotrophic factor ligands: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4)43. Interestingly, previous studies have shown that NTRK1 and NTRK2 are required for PFA24. Our results suggest that the increased C/EBPβ transcriptional activity enhances p300-mediated chromatin accessibility, which further promotes C/EBPβ binding to Furin promoter region. Chromatin accessibility in the Furin promoter region may increase NGF biosynthesis and enhance NTRK signaling in a short period of time, which perhaps explains the rapid increase in NGF levels during PF formation. Considering the interaction between NGF and NTRK1, as well as the changes in chromatin accessibility of Rap1a promoter region, a downstream signaling molecule of NGF44, we believe that C/EBPβ/p300 possibly promotes early folliculogenesis through NTRK1 signaling. However, there may be redundant effects between multiple NTRK signals; for instance, BDNF is reported to activate Rap145. Thus, we cannot rule out the possibility that NTRK2/3 signaling plays a role in p300 mediated follicle formation, but it is certain that NTRK signaling is an important mediator of C/EBPβ/p300 function during ovarian reserve establishment. In addition, we found that the chromatin accessibility of polyubiquitination-related genes was reduced after p300 knockdown, suggesting that p300 may be involved in the regulation of ovarian reserve through the protein degradation pathway. Besides, p300 also is capable of histone acetyltransferase activity. It is worth noting that histone deacetylase 6 (Hdac6) maintains PF quiescence by promoting NGF ubiquitination and reducing its protein stability46. These results suggest that ubiquitination and acetylation regulation may play a key role in ovarian development, which is a direction we need to pay attention to in the future.
In terms of potential limitations of the study, we note that C/EBPβ is involved in the expression regulation of multiple histone modifications. Therefore, the effect of p300 knockdown on chromatin accessibility may be only one aspect of C/EBPβ regulation of chromatin structure. In addition, in the in vitro model, we were unable to completely eliminate the interest protein, which may have lost the opportunity to observe more profound reproductive phenotypes. The use of conditional depletion models may help to further restore the early reproductive phenotypes of C/EBPβ deficiency.
To our knowledge, this study firstly reported that C/EBPs play a key regulatory role in development of female germ cells. In this work, we showed that both temporal and spatial effects are involved in the regulation of C/EBPβ transcriptional activity during the establishment of ovarian reserve and it enhances NTRK signaling, which is essential for ovarian reserve formation, by maintaining p300-mediated chromatin accessibility. The nuclear translocation of C/EBPβ is a specific manifestation of spatial -dependent transcriptional activity regulation, which is coordinated by the “gatekeeper” molecule Lamin B1 and C/EBPβ phosphorylation. This provides a new perspective for understanding the activity regulation of TFs. In summary, our results showed that C/EBPβ is an essential regulator of ovarian reserve formation and provide unique insights into the TF and epigenetic crosstalk mechanism underlying the establishment of ovarian reserve.
Methods
Animal Model
The mice used in the study had CD1 genetic background, housed in the laboratory animal facility (12 h light-dark cycle) at Qingdao Agricultural University, and free access to water and food. At 18:00, the estrous female mice were placed in the same cage with the male mice, the vaginal plug was checked at 8:00 on the next morning, the presence of the vaginal plug indicated successful mating in female and the day was regarded as E0.5. Euthanasia was performed using CO2 asphyxiation, subsequently followed by neck dislocation. All animal experiments were performed in accordance with the guidelines established by the Animal Ethics Committee of Qingdao Agricultural University and were approved by the Animal Ethics Committee of Qingdao Agricultural University (approval no. 2023-0021).
Reagents and animal treatment
Curcumin (MedChemExpress, HY-N0005, USA) and B-355252 (MedChemExpress, HY-120553) were purchased from MedChemExpress company. Curcumin is stored in sterile water and shaken before injection; B-355252 was prepared in DMSO to produce stock solution, and the working solution was diluted with sterile water before injection. From E16.5 to PD3, pregnant mice were intraperitoneally injected twice a day with curcumin or sterile water at 10:00 and B-355252 or sterile water containing DMSO at 16:00 to produce ovarian samples of pups in each treatment group. The PD3 pups were sacrificed for the isolation of ovarian tissue. The drug treatment doses for mice were obtained by referring to literature reports on other model animals (cited in the results section of the main text) and converted. The safety and efficacy of the drugs at daily working doses based on mouse body weight (3 mg/kg Curcumin; 0.1 mg/kg B-355252) have been verified and are described in the results section of the main text.
Ovarian Culture
The ovaries were isolated under a dissecting microscope and washed with 0.9% sodium chloride solution for in vitro culture. The ovaries were cultured in a 37 °C, 5% CO2 incubator for 3 days.
For RNA interference, ovaries were placed in a 24-well plate containing ovarian basal medium and cultured in suspension for 8 h. The basal medium contains (in volume fraction): DMEM/F12 (HyClone, SH30023.01B, Beijing, China; 49.5%), α-MEM (HyClone, SH30265.01B; 49.5%),100 mM Sodium Pyruvate solution (HyClone, SH40003-12; 1%). During the suspension culture stage, LipofectamineTM 3000 Transfection Reagent (Thermo Fisher Scientific, L3000008, Germany) was used for transfection, and the transfection system was established according to previous reports47,48. After transfection, the ovaries were transferred to agar blocks in complete ovarian culture medium for culture. The complete medium contains (in volume fraction): DMEM/F12 (44%), α-MEM (44%), fetal bovine serum (FBS; Gibco, 10099-141, USA; 10%), 100 mM Sodium Pyruvate solution (1%), Penicillin-Streptomycin 100X solution (HyClone, SV30010; 1%). Half of the culture medium was replaced after 32 h, and samples were collected after 64 h. The siRNA sequences used for RNA interference are listed in Table S2.
For JNK inhibition, the in vitro culture was performed using complete ovarian culture medium containing SP600125 (MedChemExpress, HY-12041; 5 μM), and the drug concentration was selected based on literature reports cited in the results section of the main text. Half of the culture medium was replaced after 36 h from the start of culture, and samples were collected after 72 h culture.
qPCR
Collected ovaries were placed in cryogenic centrifuge tubes and stored at -80°C until RNA was obtained. cDNA synthesis and qPCR were performed as previously reported49. RNA extraction was performed according to the manufacturer’s instructions using the SPARKeasy Improved Tissue/cell RNA kit (Sparkjade, AC0202, Shandong, China). The cDNA synthesis was performed using the SPARKscript II RT plus kit (Sparkjade, AG0304). The Light Cycler 480 II apparatus (Roche, Germany) was used to perform RT-qPCR utilizing SYBR Premix Ex Taq™ II (Vazyme, Q711-02, Nanjing, China). The 2−ΔΔCt method was used to quantify of gene expression levels, which was normalized using Gapdh. Primer sequences used for qPCR are listed in Table S3.
Western blotting
Western blotting was performed as reported previously47,50. Ovarian mixed samples were treated on ice for 20 min with RIPA lysis buffer (Beyotime, P0013C, Shanghai, China) to obtain protein lysates. Proteins were separated by SDS-PAGE and transferred to PVDF membrane (Millipore, IPVH00010, Germany) by electroblotting. The membrane was blocked and incubated with the primary antibodies: DDX4 (ab13840, Abcam), C/EBPβ (ab32358, Abcam), LHX8 (ab137036, Abcam), SOHLH1 (orb158448, Biorbyt), NOBOX (NBP2-46193, Novus), GAPDH (AF7021, Affinity), p300 (ab275378, Abcam), FURIN (R382231, Zen-bioscience), p-C/EBPβThr188 (3084, Cell signaling technology), Lamin B1 (250010, Zen-bioscience) overnight at 4 °C. After washing, the membrane was incubated with the secondary antibody for 90 min at room temperature. Subsequently, Tanon 5200 multi-imager (Tanon Science & Technology, China) was used to visualize the bands utilizing ChemistarTM High-sig ECL Western Blotting Substrate (Tanon Science & Technology, 180-5001, Shanghai, China). Antibodies information are listed in Table S4.
Immunofluorescence staining and follicle quantification
Immunofluorescence staining and follicle quantification were performed as reported previously8,18. Paraffin-embedded ovarian samples were prepared into 5-μm-thick serial sections by sectioning procedure. The sections were rehydrated in gradients, antigen retrieval, blocked, and then incubated with primary antibodies overnight at 4 °C according to standard procedures. The next day, sections were incubated with secondary antibodies at 37 °C for 45 min and then counterstained with Hoechst 33342 (Beyotime, C1026) for 5 min to label cell nuclei. Antibodies information is listed in Table S4.
The number of germ cells in each ovary was counted in five consecutive sections with an interval of 25 μm, and the number of germ cells in the sample was finally quantified as the mean of the five sections. Aggregates of two or more germ cells were considered as germ cell nests, and isolated germ cells surrounded by somatic cells were germ cells in follicles. The percentage of germ cells within follicle was calculated with number of germ cells in follicles vs total number of germ cell.
Protein subcellular localization analysis
To quantify the subcellular localization of C/EBPβ, we performed dual fluorescence staining of DDX4 and C/EBPβ and imaged them on the same microscope using consistent imaging parameters. Based on DDX4 labeling, the cytoplasm and nucleus of germ cells can be easily distinguished. Image J (version 1.53f51) was then used to obtain the mean fluorescence intensity of C/EBPβ in the same area of the cytoplasm and nucleus of germ cells. For line scan analysis, we set a straight line through the nucleus with both the starting and ending points in the cytoplasm in germ cells. Image J was applied to sequentially obtain the fluorescence intensity of each pixel on the line to establish a fluorescence intensity change curve. Line scan analysis of different proteins used the “maximum-minimum” normalization method to scale the fluorescence intensity to between 0 and 1.
ATAC-seq
At least 8 ovaries were collected from 4 litters of PD0 pups in each group. After 3 days of in vitro culture, the ovaries were placed in 2 mg/ml collagenase (Sigma-Aldrich, C4-22-1G, MO, USA) and treated at 37°C for 20 min. The reaction was terminated with complete medium containing 10% serum, and single cell suspension was obtained by filtering through the 40 µm cell strainers. Cell counting and viability detection were performed to ensure that each group contained about 50,000 cells with normal cell viability to meet the requirements of ATAC-seq. ATAC-seq is based on the Illumina platform (Annoroad Gene Technology Co., Ltd.). Cell nuclei were extracted from single-cell suspensions, and ATAC-seq libraries were prepared using the Hyperactive ATAC-Seq Library Prep Kit for Illumina (Vazyme, TD711). After library preparation, sample sequencing was conducted using Illumina NovaSeq 6000 instrument with paired-end strategy of 150 bp reads).
The ATAC-seq analysis was conducted by Biomarker Technologies, which handled the library preparation and sequencing (http://www.biomarker.com.cn/technology-services/medicine-atac). Raw sequencing data were processed to remove adapter sequences and low-quality reads, resulting in Clean Reads. The trimmed reads were aligned to the reference genome using Bowtie2 (version 2.2.6) and reads mapping to the mitochondrial genome were discarded using removeChrom51. PCR duplicates were removed, and the number of mapped reads was downsampled to approximately 20 million per sample using Picard (version 1.126). Peak calling was performed on replicates and self-pseudoreplicates using MACS252. DARs were identified using the R package DESeq2 (version 1.28.1). GO enrichment analysis was carried out using the “ClusterProfiler” R package with default parameters. The ATAC-seq tracks were visualized and analyzed in IGV software (http://software.broadinstitute.org/software/igv/).
Cleavage under targets and release using nuclease and qPCR detection (CUT&RUN-qPCR)
CUT&RUN-qPCR were performed as reported previously18. At least 20 ovaries were collected from 4 litters of PD3 pups (or ovaries of PD0 pups cultured in vitro for 3 days) in each group. Ovaries were placed in 2 mg/ml collagenase and treated at 37 °C for 20 min. The reaction was terminated with complete medium containing 10% serum, and single cell suspension was obtained by filtering through a 40 µm cell strainer. Cell counting and viability detection were performed to ensure that each group contained about 500,000 cells with normal cell viability to meet the requirements of CUT&RUN. CUT&RUN assay was performed using the Hyperactive pG-MNase CUT&RUN Assay Kit for PCR/qPCR (Vazyme, HD-101) according to the manufacturer’ s instructions. Briefly, the cells were incubated with ConA Beads Pro at room temperature for 10 min, then the beads were collected. Primary antibodies were added to the centrifuge tube containing the beads and incubated at 4 °C overnight. The beads were collected and incubated with pG-MNase Enzyme at 4 °C for 1 h. Subsequently, CaCl2 was added into samples, which were incubated on ice for 2 h for DNA fragmentation. After the fragmentation, Stop Buffer (containing Spike in DNA for normalization and calibration of qPCR data) was added at 37 °C for 20 min to terminate the reaction and obtain chromatin enrichment products. After DNA extraction from the collected chromatin enrichment products, qPCR procedures were performed. Antibodies used in CUT&RUN were listed as follows: C/EBPβ (Abcam, ab32358, 1:30), p300 (Zen-bioscience, 347220, 1:50), control rabbit IgG (Beyotime, A7016, 1:30). Primer sequences used for CUT&RUN-qPCR are listed in Table S3.
NGF level detection
The concentration of NGF in serum was measured using enzyme-linked immunosorbent assay kits (Fankew, Shanghai, China) according to the manufacturers’ instructions.
Co-immunoprecipitation (Co-IP)
Antibodies used in Co-IP were listed as follows: C/EBPβ (Abcam, 1:30), control rabbit IgG (Beyotime, 1:30). Each group included 50 mixed ovary samples from at least 10 litters of PD0 pups. After washing in ice-cold PBS, the samples were lysed on ice with RIPA lysis buffer containing protease inhibitor (Beyotime, P1008) for 20 min, and the samples were gently stirred to accelerate the lysis process. The supernatant was collected by centrifugation at 14,000 × g for 5 min at 4 °C. The supernatant was divided into three equal parts, one of which was added with SDS-PAGE sample loading buffer (Beyotime, P0015) and incubated at 95 °C for 5 min, which was stored at −20 °C for use as a positive control (Input) for WB. The other two supernatants were added with the corresponding Co-IP antibodies according to the manufacturer’s instructions and incubated at 4 °C overnight. Protein A/G magnetic beads (Thermo Fisher Scientific, #88803) were then added and incubated with the samples at 4 °C for 3 h. After washing the beads three times with PBST, the proteins were eluted with SDS-PAGE sample loading buffer for 5 min. The eluate was stored at −20 °C until use. For the subsequent WB, a conformation-specific secondary antibody was used to avoid the influence of the light chain and heavy chain formed by the antibody used for Co-IP.
Statistical analysis
Data are presented as mean ± standard error of mean (SEM), and significance was determined using t test (two groups) or one-way ANOVA (more than two groups). P < 0.05 was considered statistically significant.
Ethics statements
All procedures involved in this experiment conformed to the standards of the Animal Ethics Committee of Qingdao Agricultural University (approval no. 2023-0021).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
ATAC-seq data have been deposited in National Genomics Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA021387). The scRNA-seq upstream analysis code and germ cell dataset extraction reference for this article8 (https://github.com/WangLab401/2020scRNA_murine_ovaries). All ATAC-seq codes (https://github.com/WangLab401/Data-code-for-CEBP-beta/blob/main/ATAC-seq%20_main-codes) and scRNA-seq differential expression analysis codes (https://github.com/WangLab401/Data-code-for-CEBP-beta/blob/main/scRNA-seq%20_main-codes) have been stored in Github. Uncropped and unedited blot images are available in the Supplementary Information. Numerical source data for all figures can be found in Supplementary Data 5.
References
Grive, K. J. & Freiman, R. N. The developmental origins of the mammalian ovarian reserve. Development 142, 2554–2563 (2015).
Pepling, M. E. & Spradling, A. C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 234, 339–351 (2001).
Lei, L. & Spradling, A. C. Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development 140, 2075–2081 (2013).
Lei, L. & Spradling, A. C. Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science 352, 95–99 (2016).
Niu, W. & Spradling, A. C. Mouse oocytes develop in cysts with the help of nurse cells. Cell 185, 2576–2590 e2512 (2022).
Zheng, W. et al. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum. Mol. Genet. 23, 920–928 (2014).
Niu, W. & Spradling, A. C. Two distinct pathways of pregranulosa cell differentiation support follicle formation in the mouse ovary. Proc. Natl. Acad. Sci. USA 117, 20015–20026 (2020).
Wang, J. J. et al. Single-cell transcriptome landscape of ovarian cells during primordial follicle assembly in mice. PLoS Biol. 18, e3001025 (2020).
Niu, W. et al. JNK signaling regulates E-cadherin junctions in germline cysts and determines primordial follicle formation in mice. Development 143, 1778–1787 (2016).
Wang, Z. P. et al. Transforming growth factor-beta signaling participates in the maintenance of the primordial follicle pool in the mouse ovary. J. Biol. Chem. 289, 8299–8311 (2014).
Sun, L. et al. SRSF1 regulates primordial follicle formation and number determination during meiotic prophase I. BMC Biol. 21, 49 (2023).
Yan, H. et al. Oocyte-derived E-cadherin acts as a multiple functional factor maintaining the primordial follicle pool in mice. Cell Death Dis. 10, 160 (2019).
Hughes, C. H. K. et al. Steroidogenic factor 1 (SF-1; Nr5a1) regulates the formation of the ovarian reserve. Proc. Natl. Acad. Sci. USA 120, e2220849120 (2023).
Tolomeo, M. & Grimaudo, S. The “Janus” role of C/EBPs family members in cancer progression. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21124308 (2020).
Niehrs, C. & Calkhoven, C. F. Emerging role of C/EBPbeta and epigenetic DNA methylation in ageing. Trends Genet. 36, 71–80 (2020).
Zhou, R. et al. Up-regulated FHL2 inhibits ovulation through interacting with androgen receptor and ERK1/2 in polycystic ovary syndrome. EBioMedicine 52, 102635 (2020).
Fan, H. Y., Liu, Z., Johnson, P. F. & Richards, J. S. CCAAT/enhancer-binding proteins (C/EBP)-alpha and -beta are essential for ovulation, luteinization, and the expression of key target genes. Mol. Endocrinol. 25, 253–268 (2011).
Wang, J. J. et al. Melatonin alleviates the toxic effect of di(2-ethylhexyl) phthalate on oocyte quality resulting from CEBPB suppression during primordial follicle formation. J. Hazard Mater. 465, 132997 (2024).
Cardoso-Moreira, M. et al. Gene expression across mammalian organ development. Nature 571, 505–509 (2019).
Liu, W. X. et al. Maternal vitamin B1 is a determinant for the fate of primordial follicle formation in offspring. Nat. Commun. 14, 7403 (2023).
He, M. et al. p300 maintains primordial follicle activation by repressing VEGFA transcription. Am. J. Physiol. Cell Physiol. https://doi.org/10.1152/ajpcell.00198.2024 (2024).
Ortega, E. et al. Transcription factor dimerization activates the p300 acetyltransferase. Nature 562, 538–544 (2018).
Nilsson, E., Dole, G. & Skinner, M. K. Neurotrophin NT3 promotes ovarian primordial to primary follicle transition. Reproduction 138, 697–707 (2009).
Kerr, B., Garcia-Rudaz, C., Dorfman, M., Paredes, A. & Ojeda, S. R. NTRK1 and NTRK2 receptors facilitate follicle assembly and early follicular development in the mouse ovary. Reproduction 138, 131–140 (2009).
Yan, R. et al. The structure of the pro-domain of mouse proNGF in contact with the NGF domain. Structure 27, 78–89 e73 (2019).
Zhu, X. et al. Curcumin alleviates neuropathic pain by inhibiting p300/CBP histone acetyltransferase activity-regulated expression of BDNF and COX-2 in a rat model. PLoS One 9, e91303 (2014).
Wang, H. K. et al. A novel NGF receptor agonist B355252 ameliorates neuronal loss and inflammatory responses in a rat model of cerebral ischemia. J. Inflamm. Res. 14, 2363–2376 (2021).
He, M. et al. LSD1 contributes to programmed oocyte death by regulating the transcription of autophagy adaptor SQSTM1/p62. Aging Cell 19, e13102 (2020).
Haraguchi, S., Ikeda, M., Akagi, S. & Hirao, Y. Dynamic changes in pStat3 are involved in meiotic spindle assembly in mouse oocytes. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21041220 (2020).
Guastafierro, T. et al. ADP-ribose polymer depletion leads to nuclear Ctcf re-localization and chromatin rearrangement(1). Biochem J. 449, 623–630 (2013).
Ogiwara, K. & Takahashi, T. Involvement of the nuclear progestin receptor in LH-induced expression of membrane type 2-matrix metalloproteinase required for follicle rupture during ovulation in the medaka, Oryzias latipes. Mol. Cell Endocrinol. 450, 54–63 (2017).
Shi, Y. et al. A spatiotemporal gene expression and cell atlases of the developing rat ovary. Cell Prolif. 56, e13516 (2023).
Gillio-Meina, C., Hui, Y. Y. & LaVoie, H. A. Expression of CCAAT/enhancer binding proteins alpha and beta in the porcine ovary and regulation in primary cultures of granulosa cells. Biol. Reprod. 72, 1194–1204 (2005).
Descombes, P. & Schibler, U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67, 569–579 (1991).
Qiu, X., Aiken, K. J., Chokas, A. L., Beachy, D. E. & Nick, H. S. Distinct functions of CCAAT enhancer-binding protein isoforms in the regulation of manganese superoxide dismutase during interleukin-1beta stimulation. J. Biol. Chem. 283, 25774–25785 (2008).
Habara, O., Logan, C. Y., Kanai-Azuma, M., Nusse, R. & Takase, H. M. WNT signaling in pre-granulosa cells is required for ovarian folliculogenesis and female fertility. Development 148, https://doi.org/10.1242/dev.198846 (2021).
Sheng, X. et al. The mitochondrial protease LONP1 maintains oocyte development and survival by suppressing nuclear translocation of AIFM1 in mammals. EBioMedicine 75, 103790 (2022).
Sarkar, K. et al. A feedback loop between heterochromatin and the nucleopore complex controls germ-cell-to-oocyte transition during Drosophila oogenesis. Dev. Cell 58, 2580–2596 e2586 (2023).
Piwien-Pilipuk, G., MacDougald, O. & Schwartz, J. Dual regulation of phosphorylation and dephosphorylation of C/EBPbeta modulate its transcriptional activation and DNA binding in response to growth hormone. J. Biol. Chem. 277, 44557–44565 (2002).
Pilipuk, P. iwien, Galigniana, G. & Schwartz, M. D. J. Subnuclear localization of C/EBP beta is regulated by growth hormone and dependent on MAPK. J. Biol. Chem. 278, 35668–35677 (2003).
Grebien, F. et al. Pharmacological targeting of the Wdr5-MLL interaction in C/EBPalpha N-terminal leukemia. Nat. Chem. Biol. 11, 571–578 (2015).
Lobo, V. et al. Loss of Lamin A leads to the nuclear translocation of AGO2 and compromised RNA interference. Nucleic Acids Res. 52, 9917–9935 (2024).
Huang, E. J. & Reichardt, L. F. Trk receptors: roles in neuronal signal transduction. Annu Rev. Biochem. 72, 609–642 (2003).
Wu, C., Lai, C. F. & Mobley, W. C. Nerve growth factor activates persistent Rap1 signaling in endosomes. J. Neurosci. 21, 5406–5416 (2001).
Hisata, S. et al. Rap1-PDZ-GEF1 interacts with a neurotrophin receptor at late endosomes, leading to sustained activation of Rap1 and ERK and neurite outgrowth. J. Cell Biol. 178, 843–860 (2007).
Zhang, T. et al. HDAC6-dependent deacetylation of NGF dictates its ubiquitination and maintains primordial follicle dormancy. Theranostics 14, 2345–2366 (2024).
Kong, L. et al. Maternal Zearalenone exposure impacted ovarian follicle formation and development of suckled offspring. Sci. Total Environ. 788, 147792 (2021).
Tian, Y. et al. Single-cell transcriptomic profiling provides insights into the toxic effects of Zearalenone exposure on primordial follicle assembly. Theranostics 11, 5197–5213 (2021).
Feng, Y. Q. et al. Impaired primordial follicle assembly in offspring ovaries from zearalenone-exposed mothers involves reduced mitochondrial activity and altered epigenetics in oocytes. Cell Mol. Life Sci. 79, 258 (2022).
Han, B. et al. Changes in seminal plasma microecological dynamics and the mechanistic impact of core metabolite hexadecanamide in asthenozoospermia patients. Imeta 3, e166 (2024).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Acknowledgements
This work was supported by the National Nature Science Foundation of China (32200699 and 32270903), the Start-up Fund for High-level Talents of Qingdao Agricultural University (6651122011), and the Inner Mongolia Autonomous Region Postgraduate Research Innovation Project (KC2024015B and KC2024014B).
Author information
Authors and Affiliations
Contributions
X.Z., Y. Zhou., J.W. and W.S. designed research; X.Z., Y. Zeng., K.Q., P.L. and J.Y. performed research; X.Z., and Z.Q. analyzed data; T.Z., Y. Zhou., J.W. and W.S. edited the manuscript; and X.Z. and Y. Zeng. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Patrícia Rodrigues and the other anonymous reviewer for their contribution to the peer review of this work. Primary Handling Editor: Kaliya Georgieva.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, X., Zeng, Y., Qiao, Z. et al. CCAAT/enhancer binding protein beta (C/EBPβ) regulates the formation of the ovarian reserve. Commun Biol 8, 1394 (2025). https://doi.org/10.1038/s42003-025-08798-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08798-y