Abstract
Ischemia-reperfusion injury (IRI)-induced acute kidney injury (AKI) could be potentially lethal in clinic. We found lipoxygenases-5 (ALOX5) was upregulated in renal tubular cells in IRI-induced AKI; however, its underlying mechanism is not clear. In this study, we explore the function of ALOX in IRI-induced AKI using human renal proximal tubular epithelial cells (HK-2) and C57BL/6 mice. Alox5 knock-out mouse is constructed. Products of polyunsaturated fatty acids metabolized by ALOX5 in the kidney tissue are measured by UHPLC-HRMS/MS platform. Ferroptosis is measured upon ALOX5 intervention and Benzbromarone (BBR) intervention. The results indicate that in IRI-induced AKI, ALOX5 is up-regulated in the renal tubular epithelial cells and exacerbates the accumulation of lipid peroxides, which in turns enhances ferroptosis in the renal tubular cells and finally contributes to cell injury. BBR specifically interacts with ALOX5 and reduces the accumulation of lipid peroxide, thus alleviating the cell damage caused by IRI and improving the renal function of experimental mice. In conclusion, ALOX5 enhances the accumulation of lipid peroxide in the renal tubular cells in IRI-induced AKI and targeting ALOX5 might ameliorate renal tubular injury via inhibiting ferroptosis in this clinical setting.

Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is highly prevalent, affecting more than 13.3 million patients worldwide and causing about 1.7 million cases of death annually1. The close association between AKI with significantly increased mortality2,3,4,5 and its lack of specific treatments underscored the importance of this clinical issue. AKI can result from various causes, among which ischemia-reperfusion injury (IRI) is one of the most common causes5,6. The key pathophysiology of IRI-induced AKI is renal tubular cell injury and death, accompanied by peritubular endothelial dysfunction and inflammatory cell infiltration7,8,9. Elucidation of the underlying mechanisms of renal tubular cell injury in AKI is of great significance to identify potential molecular targets that subsequently help develop effective therapies.
Regulated cell deaths are a crucial mechanism of renal tubular cell injury in AKI, such as apoptosis, pyroptosis, ferroptosis, autophagy, etc.10, among which ferroptosis, characterized by iron dependence and abnormal accumulation of lipid peroxidation, has been recently reported to be involved in a variety of diseases, including neurological degenerative diseases, cancer, and AKI11,12,13. The typical morphological changes of ferroptosis are mitochondrial abnormalities, for example, reduced mitochondrial size, increased bilateral membrane density, rupture of the outer membrane, and disappearance of mitochondrial cristae14. GPX4 is the main defending mechanism of ferroptosis in eukaryotic cells15. Targeting ferroptosis has been reported to be a potential strategy to treat AKI16; however, the specific mechanism has not been fully studied.
Lipoxygenases (LOXs) are dioxygenases that are widely distributed in human cells and can catalyze polyunsaturated fatty acids (PUFAs) such as linoleic acid and arachidonic acid to generate corresponding hydroperoxides, which are involved in a variety of biological reactions in diseases such as asthma and neurological diseases17,18,19,20,21. ALOX5 metabolizes arachidonic acid to produce toxic 5-hydroxyeicosatetraenoic acid (5-HETE), leading to ferroptosis15,22. Although the important role of ALOX5 in ferroptosis has been gradually verified, the specific molecular mechanism of ALOX5 in AKI is still unclear. Moreover, it is not known whether clinical drugs used in kidney disease can target this molecule to treat AKI.
In this study, we proposed a hypothesis that the accumulation of lipid peroxides metabolized by ALOX5 exacerbates renal tubular injury in IRI-induced AKI via ferroptosis. We explored the role and underlying mechanisms of ALOX5 in this setting and found that benzbromarone (BBR), which is commonly used to lower serum uric acid, could target ALOX5 and protect renal tubular cells from IRI-induced injury by inhibiting ferroptosis. Our findings contribute to understanding the role of ALOX5 in AKI and demonstrate that BBR might be a novel strategy to treat AKI.
Results
ALOX5 increased in the renal tubular cells in IRI-induced AKI and was associated with ferroptosis
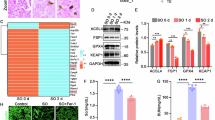
To explore potential DEGs in IRI-induced AKI, we first performed bioinformatics analysis and verified the results in mice model (Fig. 1A). The GSE192532 chip transcript was selected from the searching results in the GEO database using “AKI” or “I/R-AKI” as the keyword. Difference analysis was performed on the sham and IR sample groups (Suppl. Fig. 1). The complex heatmap showing an intersection of the above differentially expressed genes (DEGs) and the ferroptosis-related differential genes in the FerrDb database suggested that upregulated ALOX5 in IRI-induced AKI was also a ferroptosis-related target (Fig. 1B). The KEGG enrichment analysis of the differential genes derived from the intersection results showed five metabolic pathways and ten signaling pathways were significantly enriched by IRI-induced AKI, including ferroptosis (Fig. 1C).
A The schematic graph showing the experimental process. B The heatmap showing the DEGs between the control and IRI-induced AKI groups in the chipset “GSE192532,” which were also ferroptosis-related genes in the FerrDb database. C KEGG analysis showing the enrichment of the above DEGs in the ferroptosis pathway. D H&E and PAS staining showing the renal tubular injury in the IRI–AKI mouse model. E The IHC staining of Alox5 and the immunofluorescence (IF) staining to assess the up-regulated expression of Alox5 following IRI treatment. F Representative EM images of morphological changes in mitochondria cristae shape associated with ferroptosis (red arrow head) in renal tubular cells. Abbreviations: IHC immunohistochemistry, IF immunofluorescence, I/R ischemia-reperfusion, LM light microscopy, EM electron microscopy.
To verify the increased expression of ALOX and the potential involvement of ferroptosis from bioinformatic analysis, we then examined the kidney tissue of the IRI-induced AKI mice model. Renal tubular injury evidenced by enlarged renal tubule lumen, missing brush border, and disorderly arranged nuclei was observed in H&E and PAS staining sections (Fig. 1D). The IHC and IF staining confirmed the upregulation of Alox5 in proximal renal tubular cells (Fig. 1E and Suppl. Fig. 2). Typical morphological characteristics of ferroptosis in the renal tubular cells were observed under electron microscopy, including increased mitochondrial double-layer membrane density and disappearance of mitochondrial cristae (Fig. 1F).
Pathological examination and transmission electron microscopy of renal tissue from the ATN patients showed the increased expression of ALOX5 in renal tubular cells and morphological characteristics of mitochondria relevant to ferroptosis (Fig. 2 and Suppl. Fig. 3). Collectively, these findings aligned with the observations in IRI mice model, confirming ALOX5 expression increased in the renal tubular cells in IRI-induced AKI and was closely associated with ferroptosis. The clinical characteristics of the patients are shown in Supplementary Tables 2 and 3.
A The schematic graph showing the experimental process. B The pathological examinations of adjacent non-tumorous kidney tissue in one patient with renal tumor and kidney tissue obtained via renal biopsy in one patient with acute tubular necrosis, respectively, including the H&E staining showing the renal tubular injury, the IHC and IF staining to detect the expression of ALOX5, and the representative EM images of morphological changes in mitochondria cristae shape associated with ferroptosis (red arrow head) in renal tubular cells. Abbreviations: ATN acute tubular necrosis, IF immunofluorescence, IHC immunohistochemistry, EM electron microscopy, LM light microscopy.
ALOX5 contributed to renal tubular cell injury upon ischemia–reperfusion treatment
To explore the role of ALOX5 on IRI-induced AKI, we used Alox5 knockout (Alox5−/−) mice to construct the IRI-AKI model (Fig. 3A). After ischemia-reperfusion treatment, the blood creatinine and blood urea nitrogen levels increased to a much lesser extent in the Alox5−/− mice than those in the wild-type mice (Fig. 3B), suggesting the deficiency of ALOX5 might help to alleviate renal injury in the IRI-induced AKI mice model. In order to evaluate the enzymatic activity of ALOX5, we quantitatively measured the concentration of 5-HETE, the main product of arachidonic acid metabolized by ALOX5, in the kidney tissue and observed after ischemia reperfusion treatment, the concentration of 5-HETE decreased in the Alox5−/− mice in comparison to that in the wild-type mice (Fig. 3C). This benefit was further confirmed by the improvement in the morphological damages of renal tubular cells in Alox5−/− mice upon ischemia reperfusion treatment (Fig. 3D and Suppl. Fig. 4). These results indicated knocking out ALOX5 significantly reduced the production of toxic 5-HETE in IRI-induced AKI. As we expected, the typical morphological changes of ferroptosis such as increased density of mitochondrial double-layer membranes and disappearance of mitochondrial cristae after IRI treatment were significantly reduced in the Alox5−/− mice in comparison to those in the wild-type mice, evidenced by the increased expression of protective proteins including xCT and GPX4 in the Alox5−/− mice than that in the wild-type animals (p < 0.05; Fig. 3E, F). Taken together, these findings indicate that ALOX5 contributes to ferroptosis in renal tubular epithelium by producing toxic peroxides upon ischemia–reperfusion treatment.
A The schematic graph showing the experimental process. B Increased serum creatinine and urea nitrogen levels in IRI mice, confirming renal function impairment following IRI treatment. n = 6 animals. C Arachidonic acid (AA) and its metabolic product 5-hydroxyeicosatetraenoic acid (5-HETE) detected by UHPLC-MS/MS. n = 6 animals. D The pathological examinations of kidney tissues, including the H&E and PAS staining showing the renal tubular injury, the IHC staining to detect the expression of ALOX5, the immunofluorescence staining to assess the existence of ALOX5, and the representative EM images of morphological changes in mitochondria cristae shape associated with ferroptosis (red arrow head) in renal tubular cells. E, F Western blot results showing the relative protein levels of GPX4 and xCT in the kidney tissues of mice. n = 3 independent experiments. Densitometric quantification normalized to β-actin is shown to the right of the immunoblots. All data are presented as mean ± standards errors. * p < 0.05. **p < 0.01. Abbreviations: IHC immunohistochemistry, I/R ischemia–reperfusion, EM electron microscopy, WT wild-type.
Knocking down ALOX5 in HK-2 cells inhibited hypoxia/reoxygenation-induced ferroptosis
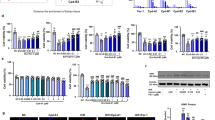
To further confirm the role of ALOX5 in mediating ferroptosis in renal tubular epithelial cells, we changed the expression of ALOX5 in HK-2 cells and detected cellular lipid peroxide accumulation and the expression of ferroptosis-related proteins (Fig. 4A). Laser confocal analysis showed the accumulation of lipid peroxides after hypoxia/reoxygenation treatment, which alleviated after ALOX5 knocking down via siRNA and exacerbated after ALOX5 overexpression (Fig. 4B). WB analysis of ferroptosis-related proteins including GPX4 and xCT indicated the expression of ALOX5 increased, whereas the expression of GPX4 and xCT significantly decreased after hypoxia/reoxygenation treatment (p < 0.05; Fig. 4C–F). Silencing ALOX5 by siRNA mitigated the decreasing trend in the expression of GPX4 and xCT in HK2 cells after hypoxia/reoxygenation treatment, while overexpression of ALOX5 exhibited the opposite effect (Fig. 4G–J).
A The schematic graph showing the experimental process. B Confocal microscopy results showing lipid peroxides detected by Liperfluo staining after ALOX5 knockdown or overexpression in HK2 receiving H/R treatment. C–F Western blot results showing the relative protein levels of GPX4 and xCT after ALOX5 knockdown in HK2 receiving H/R treatment. n = 3 independent experiments. G–J Western blot results showing the relative protein levels of GPX4 and xCT after ALOX5 overexpression in HK2 receiving H/R treatment. n = 3 independent experiments. Densitometric quantification normalized to β-actin is shown to the right of the immunoblots. All data are presented as mean ± standards errors. *p < 0.05. **p < 0.01. Abbreviations: CTRL control, H/R hypoxia/reoxygenation, NC negative control, oe overexpression.
Benzbromarone interacts with ALOX5
To identify small molecules that interacted with ALOX5, we used ALOX5 recombinant protein to conduct molecular interaction experiments with agents in the compound library (Fig. 5A). The results showed that among the screened compounds, benzbromarone (BBR) interacted with the ALOX5 recombinant protein and had the best affinity (Suppl. Fig. 5). In addition, the interaction between BBR and the ALOX5 recombinant protein exhibited a concentration-dependent manner (Fig. 5B). Based on the steady state analysis, the dissociation constant (KD) value measuring the binding affinity was 8.1E−05 ± 1.1E−05, with an R2 of 0.9945 (Fig. 5C). ALOX5 was also among the six potential target genes shared by the three conditions (IRI-induced AKI, BBR, and ferroptosis) based on the network pharmacology analysis (Suppl. Fig. 6). Molecular docking and dynamics simulations supported solid binding between BBR and ALOX5, with a binding free energy of −29 kcal/mol (Fig. 5D). The key amino acid residues that bound to BBR included ILE366, LEU289, LEU245, ALA440, ARG371, ARG458, SER448, and ALA454 (Fig. 5E, F).
A The schematic graph of the kinetic binding study using biolayer interferometry. B Molecular interaction experiment showing the combination between ALOX5 recombinant protein and BBR in different concentrations. C The steady-state analysis showing the degree of fitting of the molecular interaction curve (KD = 8.1E−05 ± 1.1E−05, R2 = 0.9945). D Molecular docking results showing the combination between BBR and ALOX5. E The 2D image of the potential binding sites of ALOX5 to BBR. F The 3D image of the potential binding sites of ALOX5 to BBR. Abbreviations: BBR benzbromarone.
BBR protects renal tubular cells from IRI-induced injury by improving ferroptosis in vivo
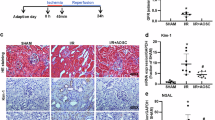
In order to verify the effect of BBR in IRI-induced AKI, we tested the efficacy of BBR treatment in the ischemia-reperfusion model (Fig. 6A). The serum creatinine level significantly increased in IRI mice and significantly improved upon BBR or DEX treatment (Fig. 6B), although the BUN level did not improve significantly upon BBR or DEX treatment (Fig. 6). The biochemical changes were confirmed by morphological changes of renal tubular epithelium, showing renal tubular injury after IRI stress which was mitigated by BBR or DEX treatment (Fig. 6D and Suppl. Fig. 7). The electron microscopy examination showed the typical morphological changes of ferroptosis in the proximal tubular epithelial cells in the IRI mice model, such as mitochondria shrinking, disappearance of cristae, and increased double-layer membrane density, which significantly improved upon BBR treatment (Fig. 6E). The expression of ferroptosis-related proteins xCT and GPX4 decreased in the kidney tissue of IRI mice model, and the treatment of BBR and DEX could both mitigated the downward trend of both proteins (Fig. 6F–H). Collectively, these results indicated that BBR protects renal tubular cells from IRI-induced injury by improving ferroptosis in the mouse model.
A The schematic graph showing the experimental process. B, C Serum creatinine and blood urea nitrogen levels in the I/R mice model receiving BBR treatment. n = 6 animals. D The pathological examinations of kidney tissues, including the H&E and PAS staining showing the renal tubular injury and the IHC staining to detect the expression of ALOX5. E Representative EM images of morphological changes in mitochondria cristae shape associated with ferroptosis (red arrow head) in renal tubular cells. F–H Western blot results showing the relative protein levels of GPX4 and xCT after ALOX5 knockdown in the kidney tissue of I/R mice model receiving BBR treatment. n = 3 independent experiments. Densitometric quantification normalized to β-actin is shown to the right of the immunoblots. All data are presented as mean ± standards errors. *p < 0.05, **p < 0.01. Abbreviations: BBR benzbromarone, DEX dexamethasone, EM electron microscopy, I/R ischemia–reperfusion.
BBR improved ferroptosis by reducing lipid peroxide accumulation mediated by ALOX5
To further explore the underlying mechanism of the protective effect of BBR, we examined the downstream effect of the interaction between BBR and ALOX5 in HK-2 cells (Fig. 7A). Cytotoxicity experiments showed that HK-2 cell proliferation was inhibited upon the BBR treatment, starting from 32 μM to an IC50 of 41.89 μM (Suppl. Fig. 8). The protective effect of BBR for H/R-induced cell death was maximal at the concentration of 2 μM (Fig. 7B), which was used for the subsequent cell experiments. In addition, BBR treatment could significantly ameliorate the cell death induced by RSL3 (Fig. 7C). Confocal microscopy analysis verified the accumulation of lipid peroxide upon H/R treatment, which was resolved evidently after BBR treatment (Fig. 7D). Interventions of ALOX5 expression also showed an effect on the accumulation of lipid peroxide. After overexpression of ALOX5, cellular lipid peroxide accumulation increased, and this change became even more obvious after H/R treatment (Fig. 7E). BBR treatment could effectively reduce the lipid peroxidation in HK2 cells caused by overexpression of ALOX5, as well as the combination treatment of H/R and the overexpression of ALOX5. The expression of ferroptosis-related proteins in HK-2 cells decreased upon H/R treatment but returned to nearly normal levels after BBR treatment (Fig. 7F, G). In addition, the expression of ferroptosis-related proteins in HK2 cells also decreased upon H/R treatment combined with or without ALOX5 overexpression. These changes could be lessened by BBR treatment, suggesting the interaction between BBR and ALOX5 had a protective effect on renal tubular cells (Fig. 7H–K). Taken together, these findings suggested that BBR improved ferroptosis by reducing lipid peroxide accumulation mediated by ALOX5 in renal tubular cells upon H/R treatment.
A The schematic graph showing the experimental process. B CCK8 analysis results showing the cell viability of HK2 cells after BBR and H/R treatments. n = 5 independent experiments. C Cell viability of HK2 cells receiving treatment of RSL3 (1 μM), Fer1 (1 μM), and BBR (2 μM). n = 5 independent experiments. D Confocal microscopy results showing lipid peroxides detected by Liperfluo staining in HK2 cells receiving BBR (2 μM) and H/R treatments. E Confocal microscopy results showing lipid peroxides detected by Liperfluo staining in H/R-HK2 cells receiving BBR (2 μM) and ALOX5 interventions. F, G Western blot results showing the relative protein levels of ALOX5, GPX4, and xCT in HK2 cells receiving BBR (2 μM) and H/R treatments. n = 3 independent experiments. H–K Western blot results showing the relative protein levels of ALOX5, GPX4, and xCT in H/R-HK2 cells receiving BBR (2 μM) and ALOX5 interventions. n = 3 independent experiments. Densitometric quantification normalized to β-actin is shown to the right of the immunoblots. All data are presented as mean ± standards errors. *p < 0.05; **p < 0.01. Abbreviations: BBR benzbromarone, DEX dexamethasone, H/R hypoxia/reoxygenation.
Discussion
In this study, we found that in IRI-induced AKI, ALOX5 was up-regulated in the renal tubular epithelial cells and exacerbated the accumulation of lipid peroxides, which in turn enhanced ferroptosis in the renal tubular cells and finally contributed to the cell injury. BBR was able to interact with ALOX5 and reduced the accumulation of lipid peroxide, thus alleviating the cell damage caused by IRI via inhibiting ferroptosis and improving the renal function of experimental mice.
The mammalian lipoxygenase family consists of six isoforms (ALOXE3, ALOX5, ALOX12, ALOX12B, ALOX15, and ALOX15B) with different substrate specificities. ALOX5 is an immunoreactive metabolic enzyme that is widely expressed in neutrophils, macrophages, and dendritic cells. Lipoxygenase oxidizes PUFAs and is involved in a variety of biological reactions, including inflammation and lipid peroxidation23,24. Beyond the well-studied function of catalyzing the conversion of arachidonic acid into leukotrienes and other pro-inflammatory lipid mediators in immune responses, allergic reactions, and airway inflammation, ALOX5 may also influence inflammatory processes through the regulation of lipid peroxidation, which is the essence of ferroptosis.
Lipid substances are both metabolites of the ferroptosis pathways and toxic molecules that promote ferroptosis25,26. Recent studies have revealed the role of ALOX5-mediated ferroptosis in the pathogenesis of diseases27,28,29. ALOX5 inhibitors can prevent ALOX5 nuclear translocation, thereby reducing the toxic lipid 5-HETE and preventing ferroptosis in dopaminergic neurons29. This study reports for the first time that ALOX5-mediated lipid peroxidation can contribute to renal tubular injury during renal IRI by promoting ferroptosis, and targeting ALOX5 alleviates this damage. Therefore, ALOX5 might be expected to become a new target for the prevention and treatment of AKI in the future. However, it should be noted that the regulation of ferroptosis is a complex network30,31,32, and ALOX5 might be one of the many involved pathways. In addition, the potential interplay between ALOX5 and other cell death pathways warrants attention, since ALOX5 mediates lipid peroxidation, which might relate to other cell death pathways via disturbance in energy metabolism and oxidative stress. The overall benefits of targeting ALOX5 to improve renal IRI need further clarification.
BBR is a widely used pharmacological agent for reducing serum uric acid levels. It functions by inhibiting the expression of urate transporter 1 in the renal proximal tubule, thereby decreasing the reabsorption of uric acid by the kidneys, enhancing uric acid excretion, and consequently lowering blood uric acid levels33. Despite reports indicating that this excretion process may impose an increased burden on the kidneys, potentially elevating creatinine levels34, research has demonstrated that BBR may mitigate cisplatin-induced nephrotoxicity35. In this study, we observed that the protective effect of BBR against H/R injury in HK-2 cells was maximal at 2 μM, only around one order of magnitude lower than the toxic concentration, which started from 32 μM, suggesting that we should be cautious when considering BBR as a potential therapeutic strategy in this AKI setting. These findings might also indicate the need to develop small-molecule inhibitors with greater specificity for ALOX5 and a wider therapeutic window in the future.
In addition to its ability to lower serum uric acid levels, BBR demonstrates therapeutic efficacy through a variety of mechanisms33. It binds to HSP47 to inhibit the interaction between HSP47 and collagen, thereby reducing collagen production and secretion in fibroblasts36. It is also a potent inhibitor of Cytochrome P450 enzymes and can lead to increased activity of medications that are metabolized by these enzymes, such as coumarin oral anticoagulants37. Consequently, the potential for off-target effects should be carefully considered when applying BBR in the treatment of AKI. Further clarification of the specific binding sites of ALOX5 and BBR might facilitate the development of less toxic small molecules, which constitutes the next phase of our research.
There are several limitations to be mentioned. First, we did not perform conditional gene knockout in animal experiments. The development of renal tubular epithelial cell-specific ALOX5 conditional knockout mice can more accurately illustrate the role of ALOX5 in the renal tubular injury in IRI-induced AKI. Second, at this stage, the target binding sites between ALOX5 and BBR were derived through molecular simulation and have not been validated by bench experiments, for example, site-specific knockout methods. Further clarification of the speculative interaction between ALOX5 and BBR might help to develop safer and more specific molecules, and will be our next step of work. Third, this study exclusively examined the IRI-induced AKI model, as the initial bioinformatics analysis indicating ALOX5 upregulation was derived from proteomic analysis of IRI-induced AKI. Therefore, we utilized this model to investigate the role and mechanism of ALOX5 in tubular injury in AKI. It would be advantageous to validate whether ALOX5 exhibits similar effects in other AKI models, as this would contribute to a comprehensive profiling of ALOX5 in AKI and represents a future direction. Fourth, this study serves as an initial exploration into the role of ALOX5 in renal IRI; however, it lacks comprehensive validation from a clinical standpoint. Therefore, it is premature to conclusively identify ALOX5 as a therapeutic target for AKI. Our subsequent objective is to establish a clinical cohort to investigate the potential of ALOX5 as a specific target for ameliorating renal tubular injury in AKI. Fifth, known side effects of BBR, including liver toxicity and coumarin interactions, were not investigated; however, these were not within the scope of this work.
Materials and methods
Bioinformatics analysis
We searched for keywords ‘AKI’ or ‘IR-AKI’ in the GEO database of the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/geo/) and selected the GSE192532 microarray. Data processing was conducted on samples GSM5750692, GSM5750693, GSM5750694, GSM5750695, GSM5750696, and GSM5750697 (Sham and IRI groups, n = 3 each) from this microarray using R software (version 4.3.2, Austria). The limma package (version 3.62.1)38 was utilized for sample data normalization, and differential analysis was performed under the conditions of a log2 fold change greater than 1 and a p-value below 0.05. Ferroptosis-related genes were obtained from the FerrDb database (http://www.zhounan.org/ferrdb/current/)39. The intersection of the two gene sets was considered as the differentially expressed AKI genes associated with ferroptosis. The data was visualized using complex heatmaps constructed with online bioinformatics tools (https://www.bioinformatics.com.cn). The clusterProfiler R package (version 4.14.4)40 and online bioinformatics tools were used to perform Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis on the differentially expressed genes related to AKI and ferroptosis, respectively, and the intersections of these analyses were taken. The resulting KEGG pathways from the intersection represent the pathways involved in AKI that are associated with ferroptosis. Subsequently, target genes of BR were identified using the Swiss Target Prediction (http://swisstargetprediction.ch) and Comparative Toxicogenomic Database (CTD) (https://ctdbase.org) (accessed on 1 December 2022), followed by a network pharmacology analysis.
Reagents
Benzbromarone powder (#S4221), Ferrostatin-1 (Fer-1, #S7243), and RSL3 (#S8155) were purchased from Selleck Chemicals (USA). The ALOX5 antibody (#ab169755), GPX4 antibody (#ab125066), and xCT antibody (#ab175186) were obtained from Abcam (USA). ALOX5 recombinant protein (H00000240-P01) was purchased from Abnova (USA).
Cell culture and treatment
Human Kidney-2 (HK-2) cells were purchased from the cell bank of the Typical Culture Preservation Committee of the Chinese Academy of Sciences, and were cell lines rather than primary cells. The purchased HK-2 cells were cultured in DMEM/F-12 (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) and 1% penicillin-streptomycin (Hyclone, USA) in a cell incubator at 37 °C and 5% CO2. Human renal epithelial cell line HK-2 cells contain the recombinant retroviral vector pLXSN 16 E6/E7 carrying the HPV-16 E7/E7 gene, and retain the functional characteristics of proximal renal tubular epithelial cells41. When the cells grew to 80% confluence, the hypoxia/reoxygenation (H/R) model was established according to a modified protocol based on the previously described methods42 using the Mini Station Plus anaerobic/Low Oxygen Workstation (Chongqing, China). Briefly, HK-2 cells were treated under hypoxic conditions (37 °C, 0% O2, 5% CO2) for 4 h, then transferred to the 37 °C, 5% CO2 incubator for 24 h. The cells were pretreated with BBR solution for 24 h before H/R treatment.
Cell viability
Cell viability was evaluated with CCK8 assay. 10 μl of CCK8 solution (DOJINDO, Japan) was added to each well of a 96-well plate with cells ready for test and incubated at 37 °C for 2 h. The absorbance at 450 nm was measured.
Transfection
The HK-2 cells were transiently transfected with ALOX5siRNA (RiboBio, Guangzhou, China) using the transfection kit (RiboBio, Guangzhou, China) or an overexpression plasmid target ALOX5 (Yunzhou Biosciences, Guangzhou, China) according to the manufactures’ instructions. Cells were harvested at 48 h after transfection.
Lipid peroxidation assay
HK2 cells were evenly distributed on 24-well slide plates. After appropriate treatment, lipid peroxidation was detected using liperfluo reagent (DOJINDO, L248, Japan) at a concentration of 2 μM according to the instructions. The nuclei were stained with DAPI. Fluorescence imaging was performed under a confocal microscope (Zeiss L900).
Human subjects
Kidney biopsy tissue samples were obtained from three patients diagnosed with acute tubular necrosis (ATN). The inclusion and exclusion criteria are presented in Supplementary Table 1. Control samples of adjacent non-tumorous kidney tissue were obtained from the resected kidneys of three patients who underwent unilateral nephrectomy for renal cell carcinoma. Written informed consents of the human subjects were obtained prior to procedures. The study was approved by the Institutional Review Board of Sichuan Provincial People’s Hospital (No. 2018.76) and conducted in compliance with the essentials of the Helsinki Declaration.
Animals and ischemia/reperfusion (I/R) modeling
Wild-type male C57BL/6 mice (weighing 20–25 g, aged 8–10 weeks) were purchased from Chengdu DOSSY Experimental Animal Co., Ltd. (Chengdu, China). Male Alox5 full knockout (Alox5 - / -) mice (weighing 20-25 g, aged 8-10 weeks) were obtained from Cyagen Biology company (Suzhou, China). The mice were randomly assigned to each experimental group (n = 6 each) without controlling for potential confounders. A mouse model for I/R injury-induced AKI was constructed through unilateral nephrectomy and renal artery clamping43. Briefly, the mouse was anesthetized by intraperitoneal injection of 1% sodium pentobarbital solution (50 mg/kg). After disinfection, we made an incision on the right back, dissected the right kidney and renal pedicle, ligated the right renal pedicle, and then removed the right kidney. The left renal pedicle was exposed by the same procedure and occluded for 45 min using microvascular forceps (ROBOZ, RS-5420). The color change from pink to dark purple indicated successful ischemia. Then the vascular clip was released, and the color of the left kidney returned to pink, indicating successful reperfusion. Then we sutured the incision by layers and kept the mice warm until they regained consciousness and were transferred to a housing cage. After reperfusion for 24 h, the mice were anesthetized by intraperitoneal injection of 1% sodium pentobarbital solution (50 mg/kg) and euthanized using the cervical dislocation method, and a blood sample and the left kidney were collected. Serum creatinine and blood urea nitrogen studies were measured using detection kits. The renal tissue was sectioned into blocks for subsequent examinations of renal pathology, electron microscopy, and protein analyses. The details of animal modeling are provided in the Supplementary Materials and Methods.
Animal BBR treatment
BBR powder (Selleck, S4221, USA) was dissolved in a mixture of 95% corn oil and 5% DMSO (v/v) (10 mg/ml) and diluted into the needed concentrations. The DMSO concentration in the final solutions administered to mice was 0.1% DMSO (v/v). For the BBR treatment, the mice were injected intraperitoneally with BBR at a fixed time once daily for two consecutive days (25 or 50 mg/kg/d), and the surgery was performed after the second drug injection44. The mice in the control group were injected with the same volume of the mixed corn oil and DMSO solution. Dexamethasone (DEX) (5 mg/kg) has been reported to be used as a positive control for treatment of IRI-AKI43,45,46. The animal study protocol received ethical approval from the Institutional Review Board of Sichuan Provincial People’s Hospital (No. 2018.76), and all experiments were conducted in accordance with laboratory standards at the Experimental Animal Center of Sichuan Provincial People’s Hospital. We have complied with all relevant ethical regulations for animal use.
Renal function testing
The collected blood samples were centrifuged to obtain serum. According to the manufacturer’s instructions, blood urea nitrogen (BUN) and creatinine (CREA) were measured with the corresponding reagent kit (Nanjing Jiancheng Company, China).
Pathological examinations
Pathological examinations include light microscopy and TEM examinations. All pathological outcomes were assessed blindly by two senior renal pathologists (co-authors: C.S.P. and Y.Q.), and the discrepancies were resolved via discussion.
H&E, PAS, immunohistochemistry, and immunofluorescent staining
The kidney specimens were fixed with 4% paraformaldehyde, embedded in paraffin, and serially sectioned at a thickness of 2 μm for hematoxylin-eosin (H&E) and periodic acid-schiff (PAS) staining. For immunohistochemistry (IHC), sections of kidney tissue were incubated with antibodies against ALOX5 (A2877, 1:200) at 4 °C overnight, then incubated with isotype-matched rabbit IgG (PV-9001) at 37 °C for 1 h, and incubated with 3, 3′-diaminobenzidine (DAB) chromogenic liquid (Zsbio, China). The nuclei were re-stained with hematoxylin (Solarbio, China). Semiquantitative assessments of the immunohistochemical staining intensities were conducted using the ImageJ software as reported before47. For immunofluorescence, sections were permeabilized in immunostaining permeabilization buffer with Triton X-100 and blocked with goat serum for 30 minutes at room temperature. The sections were then incubated with ALOX5 (A2877, 1:200) at 4 °C overnight. After incubating the secondary antibody, the cell nucleus was stained with DAPI, and the sections were sealed, and images were obtained under confocal microscopy.
Oxidized fatty acid metabolite analysis by ultra-high performance liquid chromatography (UHPLC) and mass spectrometry (MS)
Fresh mouse kidney samples were harvested and frozen immediately in a −80 °C refrigerator. Ultra-high performance liquid chromatography–tandem quadrupole mass spectrometry was performed to detect oxylipins in the kidney tissues. Raw data were processed using MassHunter workstation software (version B.08.00, Agilent). The details of UHPLC-MS analysis are provided in the Supplementary Materials and Methods.
Western blot analysis
Kidney tissues and cells were lysed with a mixture of RIPA and PSMF (RIPA:PMSF = 100:1). Subsequently, 5× protein loading buffer (Soleibao, Chengdu, China) was added to the lysed liquid and boiled at 100 °C for 5 minutes. After electrophoresis and transfer onto PVDF membranes, the prepared samples were blocked with 5% milk for 2 h. The membranes then incubated with primary antibodies to ALOX5 (1:1000, Abcam, AB169755), xCT (1:1000, Abcam, AB175186), and GPX4 (1:1000, Abcam, AB125066) at 4 °C overnight. The next day, the membranes were incubated with secondary antibodies (1:5000, ZENBO, 511203), and signaling was obtained with chemiluminescence reagents and measured using the Image J software.
Molecular interactions
We used the Octet RED96e system (ForteBio, USA) to identify small molecules that interact with ALOX5 in commercial compound libraries (Selleck L3300 Compound Libraries) by compound screening. ALOX5 recombinant protein (Abnova, Catalog # H00000240-P01) was labeled with biotin using the Biotinylation kit (Genemore, Prod#G-MM-IGT) and immobilized on the SSA sensor (Cat# 18-5057). Then we used a mixture of PBS, 0.02% Tween 20, and 1% DMSO to dissolve small molecules in the drug library to 100 μM and preliminarily screened the interactions between small molecules and the ALOX5 protein. Drugs showing interactions with ALOX5 evidenced by Kd values less than 10−5 were selected and diluted to different concentrations (3.125, 6.25, 12.5, 25, 50, and 100 μM) for further molecular interaction experiments to verify the binding.
Molecular docking
The molecular docking was used to predict the potential binding of BBR and ALOX5. Briefly, we loaded the sdf structure of BBR from the PubChem database as a ligand. The receptor protein ALOX5 (UniProt ID: P09917) has no crystal structure. After Protein Data Bank (https://www.rcsb.org/) database comparison, the target protein was found to have the highest sequence identity of 98% with the crystal structure (PDB ID: 3V92). This crystal structure was used as a template to construct a 3D model of the receptor protein. Molecular docking was performed with AutoDock Vina (version 1.1.2)48, and dynamics simulations with Amber 1849. The details of molecular docking are provided in the Supplementary Materials and Methods.
Statistics and reproducibility
Statistical analysis was performed using Graphpad Prism (version 9.3.1). Descriptive data were expressed by mean ± standard deviation (SD). Differences were compared using an unpaired Student’s t-test between groups or an analysis of variance (ANOVA) test among multiple groups. If ANOVA showed significance, the post-hoc Student–Newman–Keuls (SNK) method was used for pairwise comparisons. A two-tailed p < 0.05 indicated statistical significance.
In conclusion, this study reported that ALOX5 is upregulated in the renal tubular cells and exacerbates the accumulation of lipid peroxides, which in turn enhances ferroptosis in the renal tubular cells and finally contributes to the tubular injury in IRI-induced AKI. BBR is able to specifically interact with ALOX5 and reduces the accumulation of lipid peroxide, thus alleviating the cell damage caused by IRI via inhibiting ferroptosis and improving renal function. Our findings indicate that ALOX5 might be a novel target for the prevention and treatment of IRI-induced AKI.
Ethics statement
The study was approved by the institutional review board and ethics committee of Sichuan Provincial People’s Hospital (number 2018.76). The institutional review board waived the need for consent due to the retrospective and observational nature of this study. The study was performed in compliance with the essentials of the Helsinki Declaration.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Supplementary Fig. and tables are presented in the Supplementary Information, and unedited blot images for plots are presented as Supplementary Fig. 9 at the end of the Supplementary Information. The numerical data for the graphs are presented in Supplementary Data 1. Other data analyzed or generated during the study are available from the corresponding author on reasonable request.
References
Brown, J. R. et al. Acute kidney injury severity and long-term readmission and mortality after cardiac surgery. Ann. Thorac. Surg. 102, 1482–1489 (2016).
Hoste, E. A. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423 (2015).
Kaddourah, A., Basu, R. K., Bagshaw, S. M. & Goldstein, S. L. Epidemiology of acute kidney injury in critically ill children and young adults. N. Engl. J. Med. 376, 11–20 (2017).
Koulouridis, I., Price, L. L., Madias, N. E. & Jaber, B. L. Hospital-acquired acute kidney injury and hospital readmission: a cohort study. Am. J. Kidney Dis. 65, 275–282 (2015).
Schulman, I. H. et al. Readmission and mortality after hospitalization with acute kidney injury. Am. J. Kidney Dis. 82, 63–74.e61 (2023).
Feng, Y. et al. Characterization of risk prediction models for acute kidney injury: a systematic review and meta-analysis. JAMA Netw. Open 6, e2313359 (2023).
Lu, Q. et al. Rheb1 protects against cisplatin-induced tubular cell death and acute kidney injury via maintaining mitochondrial homeostasis. Cell Death Dis. 11, 364 (2020).
Scholz, H. et al. Kidney physiology and susceptibility to acute kidney injury: implications for renoprotection. Nat. Rev. Nephrol. 17, 335–349 (2021).
Zhao, Z. et al. XJB-5-131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury. Cell Death Dis. 11, 629 (2020).
Sanz, A. B., Sanchez-Nino, M. D., Ramos, A. M. & Ortiz, A. Regulated cell death pathways in kidney disease. Nat. Rev. Nephrol. 19, 281–299 (2023).
Chen, X., Kang, R., Kroemer, G. & Tang, D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 18, 280–296 (2021).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
Wang, Y. et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 51, 102262 (2022).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Shi, Z. et al. Liproxstatin-1 alleviated ischemia/reperfusion-induced acute kidney injury via inhibiting ferroptosis. Antioxidants (Basel) https://doi.org/10.3390/antiox13020182 (2024).
Gilbert, N. C. et al. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nat. Chem. Biol. 16, 783–790 (2020).
Karuppagounder, S. S. et al. N-acetylcysteine targets 5 lipoxygenase-derived, toxic lipids and can synergize with prostaglandin E(2) to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Ann. Neurol. 84, 854–872 (2018).
Prevete, N. et al. New perspectives in cancer: modulation of lipid metabolism and inflammation resolution. Pharm. Res 128, 80–87 (2018).
Sun, Q. Y., Zhou, H. H. & Mao, X. Y. Emerging roles of 5-lipoxygenase phosphorylation in inflammation and cell death. Oxid. Med. Cell Longev. 2019, 2749173 (2019).
Yang, W. S. et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 113, E4966–E4975 (2016).
Li, K. et al. ALOX5 inhibition protects against dopaminergic neurons undergoing ferroptosis. Pharm. Res. 193, 106779 (2023).
Li, C. et al. Inhibition of ALOX12-12-HETE alleviates lung ischemia-reperfusion injury by reducing endothelial ferroptosis-mediated neutrophil extracellular trap formation. Research (Washington D. C.) 7, 0473 (2024).
Yang, X. et al. miR-18a promotes glioblastoma development by down-regulating ALOXE3-mediated ferroptotic and anti-migration activities. Oncogenesis 10, 15 (2021).
Deng, F. et al. The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut Microbes 13, 1–21 (2021).
Mou, Y. et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J. Hematol. Oncol. 12, 34 (2019).
Li, W. et al. 6-Gingerol ameliorates ulcerative colitis by inhibiting ferroptosis based on the integrative analysis of plasma metabolomics and network pharmacology. Food Funct. 15, 6054–6067 (2024).
Liu, T. et al. ALOX5 deficiency contributes to bladder cancer progression by mediating ferroptosis escape. Cell Death Dis. 14, 800 (2023).
Song, S. et al. ALOX5-mediated ferroptosis acts as a distinct cell death pathway upon oxidative stress in Huntington’s disease. Genes Dev. 37, 204–217 (2023).
Mao, X. et al. Critical involvement of lysyl oxidase in seizure-induced neuronal damage through ERK-Alox5-dependent ferroptosis and its therapeutic implications. Acta Pharm. Sin. B 12, 3513–3528 (2022).
Shan, K. et al. Free docosahexaenoic acid promotes ferroptotic cell death via lipoxygenase dependent and independent pathways in cancer cells. Eur. J. Nutr. 61, 4059–4075 (2022).
Wang, M. et al. ALOX5 promotes autophagy-dependent ferroptosis by activating the AMPK/mTOR pathway in melanoma. Biochem. Pharm. 212, 115554 (2023).
Yan, F. et al. Superiority of low-dose benzbromarone to low-dose febuxostat in a prospective, randomized comparative effectiveness trial in gout patients with renal uric acid underexcretion. Arthritis Rheumatol. 74, 2015–2023 (2022).
Liang, N. et al. Baseline urate level and renal function predict outcomes of urate-lowering therapy using low doses of febuxostat and benzbromarone: a prospective, randomized controlled study in a Chinese primary gout cohort. Arthritis Res. Ther. 21, 200 (2019).
Abdel-Razek, E. A., Abo-Youssef, A. M. & Azouz, A. A. Benzbromarone mitigates cisplatin nephrotoxicity involving enhanced peroxisome proliferator-activated receptor-alpha (PPAR-alpha) expression. Life Sci. 243, 117272 (2020).
Park, J. G. et al. Benzbromarone induces targeted degradation of HSP47 protein and improves hypertrophic scar formation. J. Invest. Dermatol. 144, 633–644 (2024).
Felser, A. et al. Hepatocellular toxicity of benzbromarone: effects on mitochondrial function and structure. Toxicology 324, 136–146 (2014).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Zhou, N. & Bao, J. FerrDb: a manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database (Oxford) https://doi.org/10.1093/database/baaa021 (2020).
Xu, S. et al. Using clusterProfiler to characterize multiomics data. Nat. Protoc. 19, 3292–3320 (2024).
Ryan, M. J. et al. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 45, 48–57 (1994).
Liu, H. et al. Inhibition of Brd4 alleviates renal ischemia/reperfusion injury-induced apoptosis and endoplasmic reticulum stress by blocking FoxO4-mediated oxidative stress. Redox Biol. 24, 101195 (2019).
Zhang, J. et al. HMGB1-TLR4 signaling participates in renal ischemia reperfusion injury and could be attenuated by dexamethasone-mediated inhibition of the ERK/NF-kappaB pathway. Am. J. Transl. Res. 8, 4054–4067 (2016).
Qin, Z. et al. Antihyperuricemic effect of mangiferin aglycon derivative J99745 by inhibiting xanthine oxidase activity and urate transporter 1 expression in mice. Acta Pharm. Sin. B 8, 306–315 (2018).
Cabezon, R., Ricart, E., Espana, C., Panes, J. & Benitez-Ribas, D. Gram-negative enterobacteria induce tolerogenic maturation in dexamethasone conditioned dendritic cells. PLoS ONE 7, e52456 (2012).
Vollmer, T. R., Stockhausen, A. & Zhang, J. Z. Anti-inflammatory effects of mapracorat, a novel selective glucocorticoid receptor agonist, is partially mediated by MAP kinase phosphatase-1 (MKP-1). J. Biol. Chem. 287, 35212–35221 (2012).
Anderle, N. et al. Breast cancer patient-derived microtumors resemble tumor heterogeneity and enable protein-based stratification and functional validation of individualized drug treatment. J. Exp. Clin. Cancer Res. 42, 210 (2023).
Eberhardt, J., Santos-Martins, D., Tillack, A. F. & Forli, S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and Python bindings. J. Chem. Inf. Model. 61, 3891–3898 (2021).
Case, D. A. et al. AmberTools. J. Chem. Inf. Model. 63, 6183–6191 (2023).
Acknowledgements
This work is supported in part by the National Natural Science Foundation of China (81800613, U21A20349, 82270729, and 82070690).
Author information
Authors and Affiliations
Contributions
L.Y. and F.Y.L. designed the study. H.L.M. conducted the experiments, collected and analyzed the data, and drafted the paper. L.G.S., L.Y., and F.Y.L. analyzed and interpreted the data and revised the paper. T.Q., L.G.L., H.Y.P., C.S.P., Y.Q., L.Y.W., X.L., Z.Y.L., Y.H.S., and W.L. revised the paper critically to incorporate important intellectual content. All authors gave final approval of the version to be submitted.
Corresponding authors
Peer review
Peer review information
Communications Biology thanks Hailong Zhou and Qisheng Lin for their contribution to the peer review of this work. Primary Handling Editors: Dr Ashwani Kumar Gupta and Dr Joao Valente. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, L., Tang, Q., Li, G. et al. Targeting ALOX5 ameliorates renal tubular injury in ischemia–reperfusion-induced acute kidney injury via inhibition of ferroptosis. Commun Biol 8, 1403 (2025). https://doi.org/10.1038/s42003-025-08803-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08803-4