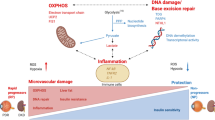
Abstract
Type 2 diabetes (T2D) is a global health issue characterized by abnormal blood glucose levels and is often associated with excessive hepatic gluconeogenesis. Increased circulating non-essential amino acids (NEAAs) are consistently observed in individuals with T2D; however, the specific contribution of each amino acid to T2D pathogenesis remains less understood. Here, we report an unexpected role of the NEAA proline in coordinating hepatic glucose metabolism by modulating paraspeckle, a nuclear structure scaffolded by the long non-coding RNA Neat1. Mechanistically, proline diminished paraspeckles in hepatocytes, liberating the retained mRNA species into cytoplasm for translation, including the mRNAs of Ppargc1a and Foxo1, contributing to enhanced gluconeogenesis and hyperglycaemia. We further demonstrated that the proline–paraspeckle–mRNA retention axis existed in diabetic liver samples, and intervening in this axis via paraspeckle restoration substantially alleviated hyperglycaemia in both female and male diabetic mouse models. Collectively, our results not only delineated a previously unappreciated proline-instigated, paraspeckle-dependent mRNA-retention mechanism regulating gluconeogenesis, but also spotlighted proline and paraspeckle as potential targets for managing hyperglycaemia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The lncRNA–seq and RIP–seq data have been deposited in the NCBI’s Gene Expression Omnibus (GEO) under accession number GSE252452 (including GSE252451 and GSE252450). This paper does not report original code. Any additional information required to reanalyse the data reported in this paper is available from the lead contact on request. Source data are provided with this paper.
References
GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402, 203–234 (2023).
Titchenell, P. M., Lazar, M. A. & Birnbaum, M. J. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol. Metab. 28, 497–505 (2017).
Lin, H. V. & Accili, D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 14, 9–19 (2011).
Rines, A. K., Sharabi, K., Tavares, C. D. & Puigserver, P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat. Rev. Drug Discov. 15, 786–804 (2016).
Wang, S. et al. Amino acids, microbiota-related metabolites, and the risk of incident diabetes among normoglycemic Chinese adults: findings from the 4C study. Cell Rep. Med. 3, 100727 (2022).
Morze, J. et al. Metabolomics and type 2 diabetes risk: an updated systematic review and meta-analysis of prospective cohort studies. Diabetes Care 45, 1013–1024 (2022).
Handzlik, M. K. et al. Insulin-regulated serine and lipid metabolism drive peripheral neuropathy. Nature 614, 118–124 (2023).
Newgard, C. B. et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 (2009).
Okun, J. G. et al. Liver alanine catabolism promotes skeletal muscle atrophy and hyperglycaemia in type 2 diabetes. Nat. Metab. 3, 394–409 (2021).
Gheni, G. et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep. 9, 661–673 (2014).
Wang, Y. & Chen, L. L. Organization and function of paraspeckles. Essays Biochem. 64, 875–882 (2020).
Clemson, C. M. et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol. Cell 33, 717–726 (2009).
Wang, Y. et al. Genome-wide screening of NEAT1 regulators reveals cross-regulation between paraspeckles and mitochondria. Nat. Cell Biol. 20, 1145–1158 (2018).
Knott, G. J. et al. Structural basis of dimerization and nucleic acid binding of human DBHS proteins NONO and PSPC1. Nucleic Acids Res. 50, 522–535 (2022).
Kaare, M. et al. High-fat diet induces pre-diabetes and distinct sex-specific metabolic alterations in Negr1-deficient mice. Biomedicines 9, 1148 (2021).
Guo, H. et al. Oat beta-glucan ameliorates diabetes in high fat diet and streptozotocin-induced mice by regulating metabolites. J. Nutr. Biochem. 113, 109251 (2023).
Tsutsui, H. et al. Biomarker discovery in biological specimens (plasma, hair, liver and kidney) of diabetic mice based upon metabolite profiling using ultra-performance liquid chromatography with electrospray ionization time-of-flight mass spectrometry. Clin. Chim. Acta 412, 861–872 (2011).
Pradas-Juni, M. et al. A MAFG–lncRNA axis links systemic nutrient abundance to hepatic glucose metabolism. Nat. Commun. 11, 644 (2020).
Statello, L., Guo, C. J., Chen, L. L. & Huarte, M. Author correction: gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22, 159 (2021).
Naveed, A. et al. NEAT1 polyA-modulating antisense oligonucleotides reveal opposing functions for both long non-coding RNA isoforms in neuroblastoma. Cell. Mol. Life Sci. 78, 2213–2230 (2021).
Alvarez, M. E., Savoure, A. & Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 27, 39–55 (2022).
Turbitt, J. et al. NKCC transport mediates the insulinotropic effects of taurine and other small neutral amino acids. Life Sci. 316, 121402 (2023).
Sun, Y., Gao, H. Y., Fan, Z. Y., He, Y. & Yan, Y. X. Metabolomics signatures in type 2 diabetes: a systematic review and integrative analysis. J. Clin. Endocrinol. Metab. 105, dgz240 (2020).
Guasch-Ferre, M. et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39, 833–846 (2016).
Lai, M. et al. Amino acid and lipid metabolism in post-gestational diabetes and progression to type 2 diabetes: a metabolic profiling study. PLoS Med. 17, e1003112 (2020).
Chen, Y. et al. Associations between serum amino acids and incident type 2 diabetes in Chinese rural adults. Nutr. Metab. Cardiovasc. Dis. 31, 2416–2425 (2021).
Xiao, F. et al. Effects of essential amino acids on lipid metabolism in mice and humans. J. Mol. Endocrinol. 57, 223–231 (2016).
Yamaguchi, N. et al. Plasma free amino acid profiles evaluate risk of metabolic syndrome, diabetes, dyslipidemia, and hypertension in a large Asian population. Environ. Health Prev. Med 22, 35 (2017).
Gunther, S. H. et al. Serum acylcarnitines and amino acids and risk of type 2 diabetes in a multiethnic Asian population. BMJ Open Diabetes Res. Care 8, e001315 (2020).
Tai, E. S. et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53, 757–767 (2010).
Thalacker-Mercer, A. E. et al. BMI, RQ, diabetes, and sex affect the relationships between amino acids and clamp measures of insulin action in humans. Diabetes 63, 791–800 (2014).
An, H., Tan, J. T. & Shelkovnikova, T. A. Stress granules regulate stress-induced paraspeckle assembly. J. Cell Biol. 218, 4127–4140 (2019).
Benegiamo, G. et al. The RNA-binding protein NONO coordinates hepatic adaptation to feeding. Cell Metab. 27, 404–418 (2018).
Petersen, M. C. & Shulman, G. I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98, 2133–2223 (2018).
Maeder, M. L. et al. CRISPR RNA-guided activation of endogenous human genes. Nat. Methods 10, 977–979 (2013).
Ji, Y. X. et al. A kinome screen reveals that Nemo-like kinase is a key suppressor of hepatic gluconeogenesis. Cell Metab. 33, 1171–1186 e1179 (2021).
Arnberg, N. Adenovirus receptors: implications for targeting of viral vectors. Trends Pharmacol. Sci. 33, 442–448 (2012).
Zhou, Y. et al. Obesity and diabetes related plasma amino acid alterations. Clin. Biochem. 46, 1447–1452 (2013).
Menni, C. et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 62, 4270–4276 (2013).
Qiu, G. et al. Plasma metabolomics identified novel metabolites associated with risk of type 2 diabetes in two prospective cohorts of Chinese adults. Int. J. Epidemiol. 45, 1507–1516 (2016).
Lu, Y. et al. Metabolic signatures and risk of type 2 diabetes in a Chinese population: an untargeted metabolomics study using both LC–MS and GC–MS. Diabetologia 59, 2349–2359 (2016).
de Mello, V. D. et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 7, 46337 (2017).
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (32200960 to Y. Zhao, 32071289 to J.S., 91957105 to J.S.), Fundamental Research Funds for the Zhejiang Provincial Universities (226-2024-00206 to J.S., 226-2024-00134 to J.S.) and the Leading Innovation and Entrepreneur Team of Hangzhou (TD2020006 to J.S.). We thank Y. Mao at the Liangzhu Laboratory of Zhejiang University for assistance with RIP–seq data analysis. We thank S. Liu and G. Xiao at the Core Facilities of Zhejiang University School of Medicine for technical assistance in microscopy analyses.
Author information
Authors and Affiliations
Contributions
J.S., C.L., Z.X. and Y. Zhao conceptualized the project, designed the experiments and wrote the manuscript. Y. Zhao, X.C., Y. Zhu, R.D., J.H., L.X. and J.C. performed the experiments and analysed the data. Y. Zhao and J.P. conducted bioinformatics analysis. Y. Zhao and S.L. collected the diabetes-risk cohort data and did statistical analysis. All of the authors edited the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Shaodong Guo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Revati Dewal, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 NEAA inhibits Neat1 expression in hepatocytes.
a, Differential lncRNAs expression analysis in NEAA-treated (NEAA) vs. control (Ctrl) hepatocytes. b, Expression levels of the top ten significantly altered lncRNAs in NEAA-treated hepatocytes, as determined by BioGPS analysis (www.biogs.org). c, Schematic diagram illustrating the two splice variants of Neat1, with the positions of primers used for qPCR or probes for FISH indicated. d, Neat1_1/2 and Neat1_2 levels in NEAA-treated hepatocytes relative to controls, n = 4 independent experiments. e, Representative fluorescence images of cells stained for Neat1 (green) RNA in control and NEAA-treated hepatocytes. Nuclei are stained with DAPI (blue). f, Quantification Neat1 dots in hepatocytes, n = 40 cells per group. Box plots are as described in Fig. 1. g, The dose-dependent effect of proline on Neat1 level in hepatocytes from male and female mice, n = 3 independent experiments. h, Nono mRNA levels in control siRNA (siScr) and Nono knockdown (siNono) hepatocytes, n = 3 independent experiments. i, Control or Nono knockdown hepatocytes were treated with or without proline for 24 h and then exposed to actinomycin D (ActD) at indicated time points to assess the half-lives of Neat1, n = 3 independent experiments. Data are presented as means ± SD. Statistical significance was determined using a two-tailed Student’s t test in (a), (b), (d), (f) and (h); one-way ANOVA in (g); two-way ANOVA in (i).
Extended Data Fig. 2 NEAA and proline do not alter the total mRNA levels of Ppargc1a and Foxo1.
a, Total Ppargc1a and Foxo1 mRNA levels in hepatocytes treated with NEAA or proline, n = 4 independent experiments. b, Total Ppargc1a and Foxo1 mRNA levels in hepatocytes treated with different concentrations of proline, n = 3 independent experiments. c, U1 and Gapdh mRNA levels in nuclear and cytoplasmic fractions, n = 4 independent experiments. d, Representative immunoblots for Lamin B1 and GAPDH protein levels. e, Nuclear-to-cytoplasmic ratio of Ppargc1a and Foxo1 in hepatocytes treated with different concentration of proline, n = 3 independent experiments. f,g, Neat1, Ppargc1a, and Foxo1 mRNA levels in hepatocytes, n = 4 independent experiments. Data are presented as means ± SD. Statistical significance was determined using one-way ANOVA in (a), (b), and (e); two-tailed Student’s t test in (c), (f), and (g).
Extended Data Fig. 3 PMO-mediated increase Neat1_2 level enhances paraspeckle formation and inhibits gluconeogenesis.
a, Schematic diagram illustrating the binding site of PMO within the Neat1 RNA transcript. b,c, Neat1_2 level (b) and ratio of Neat1_2/Neat1 (c) in hepatocytes transfected with PMO control sequence (Ctrl) and PMO Neat1 target sequence (PMO), n = 3 independent experiments. d, Representative fluorescence images of cells stained for total Neat1 (green, upper panel) and Neat1_2 (green, lower panel) in hepatocytes. e, Quantification of Neat1 and Neat1_2 foci in hepatocytes, n = 40 cells per group. Box plots are as described in Fig. 1. f, Representative images showing cellular distribution of Neat1_2 (green) and NONO (red) in hepatocytes. Nuclei are stained with DAPI (blue). The white boxes show enlarged areas for detailed observation. The graph plots the fluorescence intensity of each probe or protein (y-axis) vs. distance (x-axis) for the corresponding white line. g, Quantification of paraspeckle numbers in hepatocytes, n = 40 cells per group. Box plots are as described in Fig. 1. h,i, Total Ppargc1a and Foxo1 mRNA levels (h) and Nuclear-to-cytoplasmic ratio of Ppargc1a and Foxo1 (i) in hepatocytes, n = 3 independent experiments. j, Promoter activities of G6pc and Pck1 in hepatocytes, n = 3 independent experiments. k, G6pc and Pck1 mRNA levels in hepatocytes, n = 3 independent experiments. l, Glucose production in hepatocytes, n = 3 independent experiments. Data are presented as means ± SD. Statistical significance was determined using a two-tailed Student’s t test in (b), (c), (e), (g), (h), (i), (j), (k), and (l).
Extended Data Fig. 4 Knockdown of Ppargc1a alleviates proline-induced gluconeogenesis.
a, Ppargc1a mRNA and protein level in control siRNA (siScr) and Ppargc1a knockdown (siPpargc1a) hepatocytes, n = 3 independent experiments. b, Representative immunoblots for PGC1α protein levels. α-Tubulin as a loading control. c,f, G6pc and Pck1 mRNA levels in hepatocytes, n = 4 independent experiments. d,e,g, Glucose production in hepatocytes, n = 3 independent experiments in (d) and (e); n= 4 independent experiments in (g). Data are presented as means ± SD. Statistical significance was determined using a two-tailed Student’s t test in (a); two-way ANOVA in (c), (d), (f), and (g); one-way ANOVA in (e).
Extended Data Fig. 5 Silencing of Neat1 promotes gluconeogenesis through the paraspeckle mRNA retention axis in vivo.
a, Schematic diagram illustrating the target site for knockdown of Neat1 (shNeat1#1/sh#1 and shNeat1#2/sh#2), n = 5 mice per group. b, Neat1 level in control mice (shScr) and Neat1 knockdown mice, n = 5 mice per group. c, Water intake and food consumption, n = 5 mice per group. d, Body weight, n = 5 mice per group. e, Liver weight, n = 5 mice per group. f, Representative image of hematoxylin and eosin (H&E) staining of liver sections. g, Serum AST and ALT level, n = 5 mice per group. h, mRNA levels of Neat1, U1 and Gapdh in nuclear and cytoplasmic fractions, n = 5 mice per group. i, Representative immunoblots for Lamin B1 and GAPDH protein levels. j, Gpt and Gpt2 mRNA levels, n = 5 mice per group. k,l, G6PC and PCK1 protein levels (k) or enzymatic activity (l), n = 5 mice per group. m, Urine glucose levels, n = 5 mice per group. Data are presented as means ± SD. Statistical significance was determined using a two-tailed Student’s t test in (b), (c), (d), (e), (g), (h), (j), (k), (l), and (m).
Extended Data Fig. 6 Neat1 overexpression suppresses gluconeogenesis through the paraspeckle mRNA retention axis in vivo.
a,b,c,d, Water intake and food consumption (a), body weight (b), liver weight (c), and representative image of hematoxylin and eosin (H&E) staining of liver sections (d), caScr, n = 7 mice; caNeat1, n = 7 mice; caScr+Pro, n = 7 mice; caNeat1+Pro, n = 6 mice. e, Neat1 RNA level, n = 4 mice. f, Representative images showing cellular localization of Neat1 (green) in liver. Nuclei are stained with DAPI (blue). g, Quantification of fluorescence intensity of Neat1 in (f), n = 4 mice. h,i, G6PC and PCK1 protein levels (h) or enzymatic activity (i), n = 4 mice. Data are presented as means ± SD. Statistical significance was determined using a two-way ANOVA in (a), (b), (c), (e), (g), (h), and (i).
Extended Data Fig. 7 Paraspeckle ameliorates hyperglycaemia by modulating hepatic gluconeogenesis in diabetic mice.
a, Neat1 level in the liver from WT or ob/ob mice, n = 6 mice. b, Representative images showing cellular localization of Neat1 (green) in the liver from WT or ob/ob mice. Nuclei are stained with DAPI (blue). c, Quantitation of fluorescence intensity of Neat1, n = 4 mice. d, mRNA levels of Sfpq, Nono, and Pspc1 in liver from WT or ob/ob mice, n = 6 mice. e, Representative immunoblots for SFPQ, NONO, and PSPC1 protein levels in liver from WT or ob/ob mice. α-Tubulin as a loading control. f, Total Ppargc1a and Foxo1 mRNA levels in liver from WT or ob/ob mice, n = 6 mice. g, Nuclear and cytoplasmic Ppargc1a and Foxo1 mRNA levels, n = 4 mice. h, Representative immunoblots for PGC1α and FOXO1 protein levels. α-Tubulin as a loading control. i, G6pc and Pck1 mRNA levels in WT and ob/ob mice, n = 4 mice. Data are presented as means ± SD. Statistical significance was determined using a two-tailed Student’s t test in (a), (c), (d), (f), (g), and (i).
Extended Data Fig. 8 Neat1 overexpression ameliorates hyperglycaemia in obesity mice.
a, Water intake and food consumption of ob/ob (n = 5 per group) or db/db (n = 7 per group) mice, with and without Neat1 activation. b-, Body weight, n = 5 ob/ob mice per group; n = 7 db/db mice per group. c, Liver weight, n = 5 ob/ob mice per group; n = 7 db/db mice per group. d, Representative image of hematoxylin and eosin (H&E) staining of liver sections. e, Serum AST and ALT levels, n = 5 ob/ob mice per group; n = 7 db/db mice per group. f, Hemoglobin A1c (HbA1c) levels in whole blood, n = 5 ob/ob mice per group; n = 7 db/db mice per group. g,h, Ppargc1a, Foxo1 (g) Gpt and Gpt2 mRNA levels (h) in liver, n = 5 ob/ob mice per group; n = 7 db/db mice per group. i,j, G6PC and PCK1 protein levels (i) or enzymatic activity (j), n = 5 ob/ob mice per group; n = 7 db/db mice per group. k, Urine glucose levels, n = 7 mice per group. l, Random blood glucose levels, n = 7 mice per group. m, Insulin tolerance tests (ITT) after fasting and statistical analysis of AOC (Area of the curve; subtracting the baseline) for the ITT, n = 5 mice per group. n, Glucose tolerance tests (GTT) after fasting and statistical analysis of AOC for the GTT, n = 7 mice per group. Data are presented as means ± SD. Statistical significance was determined using a two-tailed Student’s t test in (a), (b), (c), (e), (f), (g), (h), (i), (j), (k), (l), (m), and (n).
Extended Data Fig. 9 Liver gluconeogenic metabolite tracing and gene expression profiles.
a, Isotopologue abundances of liver TCA cycle and gluconeogenesis-related metabolites in liver from ob/ob mice, n = 4 mice. b, mRNA levels of genes associated with inflammation and lipid metabolism in liver from ob/ob or db/db mice, with and without Neat1 activation, ob/ob, n = 5 mice; db/db, n = 7 mice. Data are presented as means ± SD. Statistical significance was determined using a two-tailed Student’s t test in (a) and (b).
Extended Data Fig. 10 Paraspeckle ameliorates hyperglycaemia by modulating hepatic gluconeogenesis in female ob/ob mice.
a, Schematic illustration of the experimental design to evaluate the effect of Neat1 overexpression in ob/ob female mice, n=5 per group. b, Water intake and food consumption, n = 5 mice per group. c, Body weight, n = 5 mice per group. d, Liver weight, n = 5 mice per group. e, Representative image of hematoxylin and eosin (H&E) staining of liver sections. f, Serum AST and ALT level, n = 5 mice per group. g, HbA1c levels in whole blood, n = 5 mice per group. h, Neat1 RNA level in liver, n = 5 mice per group. i, Representative images showing cellular localization of Neat1 (green) in liver. DAPI staining (blue) marks the nucleus. Quantitation of fluorescence intensity of Neat1 (right panel). j,k, Total Ppargc1a and Foxo1 mRNA levels (j), Nuclear-to-cytoplasmic ratio of Ppargc1a and Foxo1 (k) in liver, n = 5 mice per group. l, Representative immunoblots for PGC1α and FOXO1 protein levels. α-Tubulin as a loading control. m,n, G6pc, Pck1 (m), Gpt, and Gpt2 (n) mRNA levels, n = 5 mice per group. o,p, G6PC and PCK1 protein levels (o) or enzymatic activity (p) in liver, n = 5 mice per group. q, PTT after fasting and statistical analysis of AUC for the PTT, n = 5 mice per group. r, Fasting blood glucose levels, n = 5 mice per group. s, Urine glucose levels, n = 5 mice per group. Data are presented as means ± SD. Statistical significance was determined using a two-tailed Student’s t test in (b), (c), (d), (f), (g), (h), (i), (j), (k), (m), (n), (o), (p), (q), (r), and (s).
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Supplementary Tables 1–7.
Supplementary Data 1
Statistical source data.
Supplementary Data 2
Statistical source data.
Supplementary Data 3
Statistical source data.
Supplementary Data 4
Statistical source data.
Supplementary Data
Unprocessed western blots for Supplementary Fig. 4.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Source Data Figs. 3, 5 and 6 and Extended Data Figures 2, 4, 5, 7 and 10
Unprocessed western blots including Zhao_Fig3., Zhao_Fig5., Zhao_Fig6., Zhao_ED_Fig2., Zhao_ED_Fig4., Zhao_ED_Fig5., Zhao_ED_Fig7. and Zhao_ED_Fig10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Chai, X., Peng, J. et al. Proline exacerbates hepatic gluconeogenesis via paraspeckle-dependent mRNA retention. Nat Metab 7, 367–382 (2025). https://doi.org/10.1038/s42255-024-01206-5
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s42255-024-01206-5