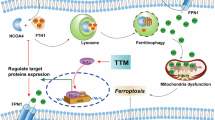
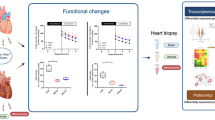
Abstract
Heart transplantation is the gold standard treatment for patients with end-stage heart failure. However, there is a shortage of donor hearts available. The short tolerable cold ischemic time for delivering donor hearts to matching recipients is closely responsible for this shortage. Here we uncover the phenomenon of mineralocorticoid receptor (MR) phase separation, which exacerbates injury to the murine and human donor heart during cold storage and can be modulated with pharmacological inhibition to improve preservation quality. Interestingly, donor cardiomyocytes strongly expressed MR, which undergoes preservation-related phase separation. The phenomenon of macromolecular phase separation is not limited to the heart or MR during preservation. Cold preservation of the lung, liver and kidney also displays phase separation of other transcriptional regulators including histone deacetylase 1 (HDAC1), bromodomain-containing 4 (BRD4) and MR. Our results reveal an understudied area of preservation biology that may be further exploited to improve the preservation of multiple solid organs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Raw sequencing and processed data generated in this study are deposited in the NCBI Gene Expression Omnibus under accession number GSE261124. Raw and processed mass spectral data are available on the MassIVE server under accession number MSV000096745. Material derived from this work may be requested from P.C.T. (tang.paul2@mayo.edu), and human samples can be shared with the scientific community via a material transfer agreement. Source data are provided with this paper.
References
Singh, S. S. A., Dalzell, J. R., Berry, C. & Al-Attar, N. Primary graft dysfunction after heart transplantation: a thorn amongst the roses. Heart Fail. Rev. 24, 805–820 (2019).
Schroder, J. et al. Successful utilization of extended criteria donor (ECD) hearts for transplantation — results of the OCS™ Heart EXPAND Trial to evaluate the effectiveness and safety of the OCS Heart System to preserve and assess ECD hearts for transplantation. J. Heart Lung Transplant. 38, S42 (2019).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Ruff, K. M., Roberts, S., Chilkoti, A. & Pappu, R. V. Advances in understanding stimulus-responsive phase behavior of intrinsically disordered protein polymers. J. Mol. Biol. 430, 4619–4635 (2018).
Brangwynne, C. P., Tompa, P. & Pappu, R. V. Polymer physics of intracellular phase transitions. Nat. Phys. 11, 899–904 (2015).
Mittag, T. & Pappu, R. V. A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol. Cell 82, 2201–2214 (2022).
Pappu, R. V., Cohen, S. R., Dar, F., Farag, M. & Kar, M. Phase transitions of associative biomacromolecules. Chem. Rev. 123, 8945–8987 (2023).
Jimenez-Panizo, A. et al. The multivalency of the glucocorticoid receptor ligand-binding domain explains its manifold physiological activities. Nucleic Acids Res. 50, 13063–13082 (2022).
Frank, F., Liu, X. & Ortlund, E. A. Glucocorticoid receptor condensates link DNA-dependent receptor dimerization and transcriptional transactivation. Proc. Natl Acad. Sci. USA 118, e2024685118 (2021).
Ban, F. et al. Development of an androgen receptor inhibitor targeting the N-terminal domain of androgen receptor for treatment of castration resistant prostate cancer. Cancers 13, 3488 (2021).
Saravanan, B. et al. Ligand dependent gene regulation by transient ERα clustered enhancers. PLoS Genet. 16, e1008516 (2020).
Graham, J. D., Hanson, A. R., Croft, A. J., Fox, A. H. & Clarke, C. L. Nuclear matrix binding is critical for progesterone receptor movement into nuclear foci. FASEB J. 23, 546–556 (2009).
Hancock, R. Crowding, entropic forces, and confinement: crucial factors for structures and functions in the cell nucleus. Biochemistry 83, 326–337 (2018).
Pitt, B. et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 348, 1309–1321 (2003).
McMurray, J. J. & O’Meara, E. Treatment of heart failure with spironolactone—trial and tribulations. N. Engl. J. Med. 351, 526–528 (2004).
Funder, J. W. Aldosterone and mineralocorticoid receptors—physiology and pathophysiology. Int. J. Mol. Sci. 18, 1032 (2017).
Seo, K. et al. Improved cardiac performance and decreased arrhythmia in hypertrophic cardiomyopathy with non-β-blocking R-enantiomer carvedilol. Circulation 148, 1691–1704 (2023).
Kelu Bisabu, K. et al. Novel gain-of-function variant in CACNA1C associated with Timothy syndrome, multiple accessory pathways, and noncompaction cardiomyopathy. Circ. Genom. Precis. Med. 13, e003123 (2020).
Uoselis, L. et al. Temporal landscape of mitochondrial proteostasis governed by the UPRmt. Sci. Adv. 9, eadh8228 (2023).
Pei, S. et al. AMPK/FIS1-mediated mitophagy is required for self-renewal of human AML stem cells. Cell Stem Cell 23, 86–100 (2018).
Xue, B., Dunbrack, R. L., Williams, R. W., Dunker, A. K. & Uversky, V. N. PONDR-FIT: a meta-predictor of intrinsically disordered amino acids. Biochim. Biophys. Acta 1804, 996–1010 (2010).
Wagh, K., Garcia, D. A. & Upadhyaya, A. Phase separation in transcription factor dynamics and chromatin organization. Curr. Opin. Struct. Biol. 71, 148–155 (2021).
Goehring, N. W., Chowdhury, D., Hyman, A. A. & Grill, S. W. FRAP analysis of membrane-associated proteins: lateral diffusion and membrane–cytoplasmic exchange. Biophys. J. 99, 2443–2452 (2010).
Shin, Y. et al. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell 168, 159–171 (2017).
Dabrowski, R. et al. Clinical efficacy of potassium canreonate-canrenone in sinus rhythm restoration among patients with atrial fibrillation — a protocol of a pilot, randomized, double -blind, placebo-controlled study (CANREN-AF trial). Trials 21, 397 (2020).
Boccanelli, A. et al. Anti-remodelling effect of canrenone in patients with mild chronic heart failure (AREA IN-CHF study): final results. Eur. J. Heart Fail. 11, 68–76 (2009).
Fernandez, M. D., Carter, G. D. & Palmer, T. N. The interaction of canrenone with oestrogen and progesterone receptors in human uterine cytosol. Br. J. Clin. Pharmacol. 15, 95–101 (1983).
Izzo, L. T. et al. Acetylcarnitine shuttling links mitochondrial metabolism to histone acetylation and lipogenesis. Sci. Adv. 9, eadf0115 (2023).
Liu, J., Marchase, R. B. & Chatham, J. C. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J. Mol. Cell. Cardiol. 42, 177–185 (2007).
Ekholm, S., Ruskoaho, H. & Karppanen, H. Depressant effects of l-tyrosine on isolated perfused rat and rabbit hearts. Pharmacol. Toxicol. 66, 209–212 (1990).
Watanabe, K. et al. Cells recognize osmotic stress through liquid–liquid phase separation lubricated with poly(ADP-ribose). Nat. Commun. 12, 1353 (2021).
Minasian, S. M., Galagudza, M. M., Dmitriev, Y. V., Karpov, A. A. & Vlasov, T. D. Preservation of the donor heart: from basic science to clinical studies. Interact. Cardiovasc. Thorac. Surg. 20, 510–519 (2015).
Schroder, J. N. et al. Transplantation outcomes with donor hearts after circulatory death. N. Engl. J. Med. 388, 2121–2131 (2023).
Keidar, S. et al. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation 109, 2213–2220 (2004).
Pitt, B. et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 341, 709–717 (1999).
Harmon, T. S., Holehouse, A. S., Rosen, M. K. & Pappu, R. V. Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. eLife 6, e30294 (2017).
Stortz, M., Pecci, A., Presman, D. M. & Levi, V. Unraveling the molecular interactions involved in phase separation of glucocorticoid receptor. BMC Biol. 18, 59 (2020).
Weikum, E. R., Liu, X. & Ortlund, E. A. The nuclear receptor superfamily: a structural perspective. Protein Sci. 27, 1876–1892 (2018).
Zhang, F. et al. Dynamic phase separation of the androgen receptor and its coactivators key to regulate gene expression. Nucleic Acids Res. 51, 99–116 (2023).
Korkmaz, F. & Severcan, F. Effect of progesterone on DPPC membrane: evidence for lateral phase separation and inverse action in lipid dynamics. Arch. Biochem. Biophys. 440, 141–147 (2005).
Stefanadis, C. et al. Aortic function in patients during intra-aortic balloon pumping determined by the pressure–diameter relation. J. Thorac. Cardiovasc. Surg. 116, 1052–1059 (1998).
Dhalla, A. K., Hill, M. F. & Singal, P. K. Role of oxidative stress in transition of hypertrophy to heart failure. J. Am. Coll. Cardiol. 28, 506–514 (1996).
Ruff, K. M., Dar, F. & Pappu, R. V. Ligand effects on phase separation of multivalent macromolecules. Proc. Natl Acad. Sci. USA 118, e2017184118 (2021).
Pinheiro, E. D. S., Preato, A. M., Petrucci, T. V. B., Dos Santos, L. S. & Glezer, I. Phase-separation: a possible new layer for transcriptional regulation by glucocorticoid receptor. Front. Endocrinol. 14, 1160238 (2023).
Du, M. & Chen, Z. J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 361, 704–709 (2018).
Marzolla, V. et al. Mineralocorticoid receptor in adipocytes and macrophages: a promising target to fight metabolic syndrome. Steroids 91, 46–53 (2014).
Xie, J. et al. Targeting androgen receptor phase separation to overcome antiandrogen resistance. Nat. Chem. Biol. 18, 1341–1350 (2022).
Silvestre, J. S. et al. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J. Biol. Chem. 273, 4883–4891 (1998).
Klein, I. A. et al. Partitioning of cancer therapeutics in nuclear condensates. Science 368, 1386–1392 (2020).
Lei, I. et al. Metabolic reprogramming by immune-responsive gene 1 up-regulation improves donor heart preservation and function. Sci. Transl. Med. 15, eade3782 (2023).
Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell 175, 1842–1855 (2018).
Zheng, G. X. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2018).
Vandenbon, A. & Diez, D. A clustering-independent method for finding differentially expressed genes in single-cell transcriptome data. Nat. Commun. 11, 4318 (2020).
Skinnider, M. A. et al. Cell type prioritization in single-cell data. Nat. Biotechnol. 39, 30–34 (2021).
Litvinukova, M. et al. Cells of the adult human heart. Nature 588, 466–472 (2020).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Van den Berge, K. et al. Trajectory-based differential expression analysis for single-cell sequencing data. Nat. Commun. 11, 1201 (2020).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Ramirez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Wancewicz, B. et al. Comprehensive metabolomic analysis of human heart tissue enabled by parallel metabolite extraction and high-resolution mass spectrometry. Anal. Chem. 96, 5781–5789 (2024).
Goerlich, C. E. et al. Blood cardioplegia induction, perfusion storage and graft dysfunction in cardiac xenotransplantation. Front. Immunol. 12, 667093 (2021).
Saemann, L. et al. Monitoring of perfusion quality and prediction of donor heart function during ex-vivo machine perfusion by myocardial microcirculation versus surrogate parameters. J. Heart Lung Transplant. 40, 387–391 (2021).
Targosova, K. et al. Cardiac nicotinic receptors show β-subunit-dependent compensatory changes. Am. J. Physiol. Heart Circ. Physiol. 320, H1975–H1984 (2021).
Aksentijevic, D. et al. Intracellular sodium elevation reprograms cardiac metabolism. Nat. Commun. 11, 4337 (2020).
Lei, I. et al. ‘The secret life of human donor hearts’: an examination of transcriptomic events during cold storage. Circ. Heart Fail. 13, e006409 (2020).
Lei, I. et al. Differential inflammatory responses of the native left and right ventricle associated with donor heart preservation. Physiol. Rep. 9, e15004 (2021).
Acknowledgements
We are very grateful for the generosity of donors and the ‘Gift of Life Michigan’ organ procurement organization, which provided hearts for research through the procurement facility. We also thank Essential Pharmaceuticals for the gift of the commercial HTK preservation solution for our studies. Key administrative and technical support by M. McCotter, A. Malis, S. Marshall and G. Rising at the University of Michigan was greatly appreciated. We thank A. Sarcon at Mayo Clinic for the artwork. This study was supported by the National Institutes of Health grants HL164416, HL166140 (P.C.T.), U01-AI132895 (J.S.P.), AI151588, AI173950 (J.L.P., M.C.), HL163672, HL139735 (Z.W.), HL159871, HL134569 and HL109946 (Y.E.C.), the Thoracic Surgery Foundation — Southern Thoracic Surgery Association (P.C.T.), a Gardner Surgical Investigator Award, American Association for Thoracic Surgery (P.C.T.), a McKay Research Grant (P.C.T.), the Frankel Cardiovascular Center, University of Michigan—Ann Arbor (to P.C.T.), Mayo Clinic (to P.C.T.) and an American Heart Association Career Development Award (930124 to I.L.). M.R.P. acknowledges the T32 Training Program in Translational Cardiovascular Science (T32 GM135119). Y.G. acknowledges NIH R01HL109810. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
I.L., Z.W., Y.E.C. and P.C.T. were involved in conceptualization of the study. I.L., H.S., W.G., W.H., P.E.N., M.R.P., M.C.W., A.L., L.L. A.A.E.E., M.J., J.L.P., J.S.P., M.C., F.D.P., Y.E.C., B.P., Z.W., R.M.M. and Y.G. designed and implemented the methodology. I.L., H.S., W.G., W.H., P.E.N., M.R.P., M.C.W., A.L., A.A.E.E., M.J. and P.C.T. contributed to the investigations. I.L., H.S., W.G., W.H., P.E.N., M.R.P., M.C.W., A.L. and P.C.T. visualized the data. I.L. and P.C.T. acquired funding for the studies. I.L. and P.C.T. administered the project. J.L.P., J.S.P., Z.W., Y.E.C., R.M.M., Y.G. and P.C.T. provided supervision for the study. I.L. and P.C.T. wrote the original draft. All authors contributed to data interpretation and discussion of results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
I.L., Z.W., F.D.P., B.P., Y.E.C. and P.C.T. have filed a US provisional patent (title: Histone-acetylation-modulating agents for the treatment and prevention of organ injury, no. 63/045,784, international application no. PCT/US2021/039650). A.A.E.E. has a consulting agreement with TransMedics. F.D.P. is an ad hoc, noncompensated scientific advisor for Medtronic, Abbott, FineHeart and CH Biomedical and a noncompensated medical monitor for Abiomed. F.D.P. is also a member of the data safety monitoring board for Carmat and the NHLBI PumpKIN clinical trial as well as the chair of data safety and management for the DCD heart national FDA clinical trial and the EXPAND heart-Continuous access protocol national FDA trial. P.C.T. is a noncompensated member of the data safety monitoring board for the XVIVO Perfusion PRESERVE trial. The other authors declare no competing interests.
Peer review information
Nature Cardiovascular Research thanks Jason P. Fine, Sarah L. Longnus, Lori West and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Single-cell transcriptomic expression of cell type markers and differential cardiomyocyte transcriptomes.
a, Violin plot constructed from integrated dataset displaying cell markers genes for the respective cell population clusters including endothelial cells (EC), fibroblasts (FB) smooth muscle cells (SMC), neuronal cells (NC), leukocytes (LK) as well as cardiomyocyte (CM) clusters 1 and 2. UMAP plot of single-cell transcriptomic analysis from cold (4 °C) preserved human hearts showing cell population expression of b, cardiac muscle action potential pathway genes and c, autophagy related pathway transcripts. UMAP plot of cell population expression of d, RYR2 and e, CACNA1C as well as f, PINK1 and g, FIS1.
Extended Data Fig. 2 Donor heart preservation is associated with increased MR protein expression.
Increased MR protein expression is seen with normalization to baseline preservation time in a, human donor hearts at baseline, 4 and 10 h of 4 °C static preservation with HTK without reperfusion (n = 3/group) and b, murine donor heart after baseline and 16 h of 4 °C HTK preservation followed by ex-vivo perfusion (n = 4/group). c, MR immunostaining (purple) with DAPI (blue) in MRfl/fl versus Myh6CreERT2;MRfl/fl hearts with tamoxifen injection. Representative fluorescence images of n = 4/group. d, QRT-PCR analysis of cytokine transcript expression in transplanted donor hearts preserved for 16 h normalized to control MRfl/fl hearts at baseline time (n = 3). e, Immunostaining of CD45 (red) and Ly6C (red) with DAPI (blue) in transplanted hearts. Representative fluorescence images of n = 4/group. f. Quantitation of positive staining cell counts expressed as percentage of total cells averaged over 4 high powered (200x) fields (n = 4). Data are presented as means ± SD. *P < 0.05 and **P < 0.01 by two-sided Mann-Whitney test, Tukey’s corrections were used for multiple comparisons using one-way ANOVA.
Extended Data Fig. 3 MR dynamics in living cells show propensity for LLPS and condensate formation that is driven by the intrinsic disordered protein region.
a, Neonatal rat cardiomyocytes (NRCM) expressing green fluorescent protein (GFP)-MR using adenovirus were bathed in cold (4°C) HTK preservation solution under hypoxic conditions (1% O2) for 10 h. A laser was used to perform fluorescence recovery after photobleaching (FRAP) within the nucleus. The dark region in the middle represents the nucleoli. Photobleached regions are circled and fluorescence recovery was quantified in adjoining graph. Data are presented as means ± SD (n = 3 biological replicates). b, We designed constructs that used lipofectamine to transfect Human Embryonic Kidney (HEK-293) cell cultures to express a mCherry-optoDrop (OptoDroplets) vector with either a full length MR (MR_FL) protein or a mutant MR without the IDR at the N-terminal domain (MRΔIDR). c. The HEK-293 cells were then immersed in histidine-tryptophan-ketoglutarate preservation solution under normoxic conditions and exposed to blue light for up to 60 s to induce MR condensate formation (white arrow). MR_FL is seen throughout the entire cell whereas MRΔIDR remained mostly within the cytoplasm thus outlining the nucleus. Data shown represent n = 4 biological replicates. d, Laser “photobleaching” of MR_FL condensates in live HEK-293 cells followed by fluorescence recovery. Photobleached region is circled. Image shown represent n = 4 biological replicates.
Extended Data Fig. 4 Cryopreservation is ligand independent.
Adeno-associated virus 9 (AAV) was administered to Myh6CreERT2;MRfl/fl mice 28 days prior to cardiac preservation. AAV9 was used to deliver cardiac-specific constructs containing MR with a deleted ligand binding domain (LBD, MRΔLBD). a, After 16 h of preservation followed by ex-vivo perfusion, construct expression in-vivo was verified by western blot. b, Murine donor hearts from wild type control, Myh6CreERT2;MRfl/fl mice infected with AAV-Luciferase (AAV-Luc) control, and Myh6CreERT2;MRfl/fl mice infected with AAV-MRΔLBD (MR missing the LBD) were cold (4 °C) preserved for 16 h followed by ex-vivo perfusion with Kreb buffer (n = 8). c, Immunostaining of MR condensates (red) with DAPI (blue). Representative fluorescence images of n = 4/group. d, Transcript expression of MR target genes ZFP219, CAMK2D, and PER1 in ex-vivo perfused murine hearts cold preserved for 16 h (n = 4 per group). Murine donor hearts were cold preservation with HTK only versus HTK + aldosterone (50 μM) (n = 8/group) for 12 h followed by ex-vivo perfusion. The cardiac functions e, contractility and f, relaxation were assessed by conduction catheter. Data are presented as means ± SD. *P < 0.05 and **P < 0.01 by two-sided Mann-Whitney test. Tukey’s corrections were used for multiple comparisons using one-way ANOVA for multiple group comparisons.
Extended Data Fig. 5 Canrenone improves donor heart function with ex-vivo perfusion and transplantation after prolonged preservation.
a, Canrenone dose titration with murine donor hearts cold (4 °C) preserved for 16 h followed by ex-vivo perfusion with Kreb buffer (n = 6/group). Tested canrenone dosages of 12.5 μM, 50 μM, and 200 μM are indicated.Preservation time titration of canrenone (50 μM) versus vehicle control (n = 5/group) with ex-vivo perfusion and conductance catheter assessment of cardiac b, contractility and c, relaxation. Transplanted donor heart d, contractility and e, relaxation (n = 8 for HTK+Veh, n = 9 for HTK+Canr) as well as f, circulating cardiac troponin I levels were compared following 16 h of cardiac preservation with canrenone versus vehicle control (n = 8/group). Cold preservation with HTK only, HTK + finerenone (50 μM) versus HTK + spironolactone (50 μM) (n = 8/group) with ex-vivo perfusion and conductance catheter assessment of cardiac g, contractility and h, relaxation. Data are presented as means ± SD. *P < 0.05 and **P < 0.01 by two-sided Mann-Whitney test.
Extended Data Fig. 6 Canrenone treatment improved porcine donor heart function after 4 h of preservation.
Ex-vivo assessment of pig hearts preserved with HTK ± Canrenone (Canr) treatment for 10 h. Hemodynamic and coronary measurements recorded in working mode after 1 h of perfusion include a, contractility (max dP/dt), b, relaxation (min dP/dt), c, cardiac output (ml/min/g) and d, coronary blood flow, e, Cardiac troponin I (cTnI) were quantified in ex-vivo perfusate for porcine heart (n = 8 per group). f. Western blot of BAX, Cleaved CASPASE 3 and BCL2 in ex-vivo perfused human heart. g. Quantification of western blots (n = 4). Data are presented as means ± SD. *P < 0.05 and **P < 0.01 by two-sided Mann-Whitney test.
Extended Data Fig. 7 Canrenone reverses cold (4 °C) preservation-induced transcriptomic changes in Cardiomyocytes Cluster 1 (CM1) and Cardiomyocytes Cluster 2 (CM2) within human donor hearts during storage.
a. Cold preservation induced gene expression was identified by comparing human donor heart with 10 h of preservation compared to baseline. Red dots indicate upregulated genes in CM1 population during cold storage while blue dots show the effect of canrenone on genes represented by the red dots. Light blue dots indicate downregulated genes in CM1 population during cold storage while purple dots show the effect of canrenone on genes represented by the light blue dots. Volcano plot demonstrates that Canrenone reverses the cold preservation-induced global up- and downregulated genes in CM1. b. Heatmap of gene expression in CM1 of human donor hearts cold (4 °C) preserved for 10 h versus baseline (n = 3/group). Juxtaposed to the right is the HTK+Canr versus HTK+Veh effect on gene expression following 10 h of cold (4°C) preservation of human donor hearts (n = 3/group). c. Cold preservation induced gene expression was identified by comparing human donor heart with 10 h of preservation compared to baseline. Red dots indicate upregulated genes in CM2 population during cold storage while blue dots show the effect of canrenone on genes represented by the red dots. Light blue dots indicate downregulated genes in CM2 population during cold storage while purple dots show the effect of canrenone on genes represented by the light blue dots. Volcano plot demonstrates that Canrenone reverses the cold preservation-induced global up- and downregulated genes in CM2. d. Heatmap of gene expression in CM2 of human donor hearts cold (4 °C) preserved for 10 h versus baseline (n = 3/group). Juxtaposed to the right is the HTK+Canr versus HTK+Veh effect on gene expression following 10 h of cold (4 °C) preservation of human donor hearts (n = 3/group). Baseline is where samples were collected in the back table immediately following preservation solution administration and removal of the donor heart from the surgical field.
Extended Data Fig. 8 Canrenone reverses cold (4°C) preservation induced transcriptomic changes in fibroblasts (FB) and endothelial cells (EC) within human donor hearts during storage.
a. Cold preservation induced gene expression was identified by comparing human donor heart with 10 h of preservation compared to baseline. Red dots indicate upregulated genes in FB population during cold storage while blue dots show the effect of canrenone on genes represented by the red dots. Light blue dots indicate downregulated genes in FB population during cold storage while purple dots show the effect of canrenone on genes represented by the light blue dots. Volcano plot demonstrates that Canrenone reverses the cold preservation-induced global up- and downregulated genes in FB. b. Heatmap of gene expression in FB of human donor hearts cold (4 °C) preserved for 10 h versus baseline (n = 3/group). Juxtaposed to the right is the HTK+Canr versus HTK+Veh effect on gene expression following 10 h of cold (4 °C) preservation of human donor hearts (n = 3/group). c. Cold preservation induced gene expression was identified by comparing human donor heart with 10 h of preservation compared to baseline. Red dots indicate upregulated genes in EC population during cold storage while blue dots show the effect of canrenone on genes represented by the red dots. Light blue dots indicate downregulated genes in EC population during cold storage while purple dots show the effect of canrenone on genes represented by the light blue dots. Volcano plot demonstrates that Canrenone reverses the cold preservation-induced global up- and downregulated genes in EC. d. Heatmap of gene expression in EC of human donor hearts cold (4 °C) preserved for 10 h versus baseline (n = 3/group). Juxtaposed to the right is the HTK+Canr versus HTK+Veh effect on gene expression following 10 h of cold (4 °C) preservation of human donor hearts (n = 3/group). Baseline is where samples were collected in the back table immediately following preservation solution administration and removal of the donor heart from the surgical field.
Extended Data Fig. 9 Canrenone reverses cold (4 °C) preservation induced transcriptomic changes in smooth muscle cells (SMC) and leukocytes (LK) within human donor hearts during storage.
a. Cold preservation induced gene expression was identified by comparing human donor heart with 10 h of preservation compared to baseline. Red dots indicate upregulated genes in SMC population during cold storage while blue dots show the effect of canrenone on genes represented by the red dots. Light blue dots indicate downregulated genes in SMC population during cold storage while purple dots show the effect of canrenone on genes represented by the light blue dots. Volcano plot demonstrates that Canrenone reverses the cold preservation-induced global up- and downregulated genes in SMC. b. Heatmap of gene expression in SMC of human donor hearts cold (4 °C) preserved for 10 h versus baseline (n = 3/group). Juxtaposed to the right is the HTK+Canr versus HTK+Veh effect on gene expression following 10 h of cold (4 °C) preservation of human donor hearts (n = 3/group). c. Cold preservation induced gene expression was identified by comparing human donor heart with 10 h of preservation compared to baseline. Red dots indicate upregulated genes in LK population during cold storage while blue dots show the effect of canrenone on genes represented by the red dots. Light blue dots indicate downregulated genes in LK population during cold storage while purple dots show the effect of canrenone on genes represented by the light blue dots. Volcano plot demonstrates that Canrenone reverses the cold preservation-induced global up- and downregulated genes in LK. d. Heatmap of gene expression in LK of human donor hearts cold (4 °C) preserved for 10 h versus baseline (n = 3/group). Juxtaposed to the right is the HTK+Canr versus HTK+Veh effect on gene expression following 10 h of cold (4 °C) preservation of human donor hearts (n = 3/group).Baseline is where samples were collected in the back table immediately following preservation solution administration and removal of the donor heart from the surgical field.
Extended Data Fig. 10 Pseudotime analysis showing the modulatory effect of canrenone on cold donor heart preservation associated gene expression.
Density distribution plot showing the distribution of cells having differential transcriptome expression profile along pseudotime for cold (4 °C) preserved human hearts without reperfusion at a, baseline versus 10 h of preservation (n = 3/group) or b, for human hearts cold (4 °C) preserved for 10 h without reperfusion using HTK alone versus HTK + canrenone (CANR, n = 3/group). c, “Rolling wave” heatmap plot along pseudotime trajectory showing the differentially expressed genes ordered according to hierarchical clustering following 10 h of human heart preservation without reperfusion using HTK + VEH versus HTK + CANR (n = 4/group). P-values for pseudotime dependency using the Kolmogorov-Smirnov test are shown.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Supplementary Table 1
Supplemental data for metabolomic analysis of human perfused hearts.
Supplementary Video 1
Representative videos of pig hearts preserved with HTK ± canrenone for 4 and 10 h followed by ex situ perfusion.
Supplementary Video 2
Representative videos of human hearts preserved with HTK ± canrenone for 10 h followed by ex situ perfusion.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Full blot image.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Table 1
Statistical source data.
Source Data Table 2
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Full blot image.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Full blot image.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Full blot image.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lei, I., Sicim, H., Gao, W. et al. Mineralocorticoid receptor phase separation modulates cardiac preservation. Nat Cardiovasc Res 4, 710–726 (2025). https://doi.org/10.1038/s44161-025-00653-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44161-025-00653-x