Abstract
Staphylococcus aureus remains a leading global cause of bacterial infection-associated mortality and has eluded prior vaccine development efforts. S. aureus α-toxin (Hla) is an essential virulence factor in disease, impairing the T cell response to infection. The anti-Hla antibody response is a correlate of human protective immunity. Here we observe that this response is limited early in human life and design a vaccine strategy to elicit immune protection against Hla in a neonatal mice. By targeted disruption of the interaction of Hla with its receptor ADAM10, we identify a vaccine antigen (HlaH35L/R66C/E70C, HlaHRE) that elicits an ~100-fold increase in the neutralizing anti-Hla response. Immunization with HlaHRE enhances the T follicular helper (TFH) cell response to S. aureus infection, correlating with the magnitude of the neutralizing anti-toxin response and disease protection. Furthermore, maternal HlaHRE immunization confers protection to offspring. Together, these findings illuminate a path for S. aureus vaccine development at the maternal-infant interface.
Similar content being viewed by others
Introduction
Staphylococcus aureus infection results in over 1 million deaths globally per year1. While infection prevention is of public health importance, prior vaccine development efforts have failed2. Each candidate vaccine to reach clinical trials has been highly immunogenic as defined by the vaccine-elicited antibody response, however these responses were not defined as a correlate of human immunity against S. aureus. Further, recent studies have indicated that the pre-existing immune response to S. aureus that develops in the context of naturally occurring exposure throughout life may be detrimental to vaccine efficacy3,4. Novel approaches to vaccine development are needed, grounded in an understanding of human immunity and cognizant of the demand for a population-level solution.
S. aureus α-toxin (Hla) is a conserved pore-forming toxin required for disease pathogenesis5. Expressed by nearly all clinical isolates, Hla utilizes A Disintegrin and Metalloprotease 10 (ADAM10) as a cellular receptor to inflict host cell injury6,7,8,9,10. ADAM10 is a type I transmembrane protein that is widely expressed on human cells (Supplementary Fig. 1a). Characterized by an N-terminal metalloprotease domain, ADAM10 contributes to cellular and tissue development, homeostasis, and response to injury, dependent on proteolytic cleavage of ADAM10 substrates in a cell-type specific manner11. Extensive knowledge of both Hla and ADAM10 has enabled a detailed understanding of the molecular mechanisms by which the toxin-receptor interaction contributes to tissue injury in S. aureus infection. Upon binding of the Hla monomer to ADAM10, assembly of the heptameric toxin pore is initiated6. Pore formation on the host cell membrane results in the upregulation of the catalytic activity of ADAM10 and concomitant cleavage of native ADAM10 substrates, including E-cadherin, VE-cadherin, and platelet glycoprotein VI6,7,9,12,13,14. These cleavage events, together with cellular injury attributable to Hla pore formation, culminate in tissue-specific insults that are manifest in S. aureus disease.
Hla has emerged as a key target for vaccine development. Pre-clinical studies have revealed that active and passive immunization targeting Hla affords disease protection15,16. Among staphylococcal virulence factors, Hla is unique in its ability to impair the antigen-specific T cell response, promoting recurrent infection17. Neutralization of Hla leads to preservation of the T cell response in animal models17, and enhances recovery of the T cell response following S. aureus infection in children18. The anti-Hla antibody titer is elevated among pediatric subjects who exhibit protection against recurrent infection19, however a study by Wu et al., revealed that infants <1 year of age harbor a lower serum anti-Hla titer and neutralizing capacity than children 2–10 years of age20. Consistent with this finding, up to 50% of infants and children with S. aureus skin and soft tissue infection (SSTI) experience disease recurrence21. Together, these findings define the anti-Hla response as a correlate of human protective immunity, suggesting that elicitation of a highly neutralizing anti-Hla antibody response early in life is a strategic target for vaccine development. In this study, we characterize a fully detoxified structural variant of Hla as a vaccine antigen, and reveal the mechanism by which this antigen amplifies T cell-mediated protection against S. aureus disease early in life.
Results
Hla antigen screen to enhance the toxin-neutralizing vaccine response
To refine our knowledge of anti-Hla response development, we evaluated 343 subjects aged <1 to 18 years (Supplementary Table 1). Analysis of the anti-Hla titer by age revealed a gradual increase in the half-maximal effective concentration (EC50) over the first 5 years of life, with titers in children under 2 being most distinct from those of older children (Fig. 1a). Paired examination of the serum Hla neutralizing activity in a rabbit red cell (rRBC) protection assay confirmed that subjects <2 years exhibit response immaturity (Fig. 1b). S. aureus exposure through colonization is documented to occur in the first days and weeks of life, with mortality from S. aureus infection over the first 4 years of life rivaling that seen in older adults1. Together, these data suggest that the risk of both S. aureus disease and modification of the host response to this microbe occur early in life.
a Half-maximal effective concentration of anti-Hla reactive antibodies in sera from children within the designated age groups (Log EC50). b Serum-based protection against Hla-mediated (2 nM) rRBC lysis. Protection noted as percent compared to toxin-only control. For a, b, 75th and 25th (black) and 50th (red) percentiles noted. Data were analyzed by one-way ANOVA with Tukey’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
We, therefore, undertook a vaccine development approach designed to elicit immunity against S. aureus infection through neonatal vaccination. We hypothesized that the Hla-ADAM10 interaction and subsequent conformational changes that induce pore formation may influence vaccine efficacy. We generated a series of modified Hla antigens based on structural determinants and correlates of toxin function, in part based on the expected immunogenic properties derived from a combination of prior observations and T cell epitope prediction algorithms. Six distinct antigens were generated and evaluated including three full-length Hla variant antigens appended with a 6x-histidine tag to facilitate affinity purification (Supplementary Fig. 1b): HlaH35L (purple) has been extensively studied as a toxoid vaccine that binds ADAM10 yet fails to form a stable pore6,7,22, HlaD45A/Y118F (orange), predicted to interfere with β-barrel stem domain unfolding23, and HlaR66C/E70C (blue), which abrogates cell binding24. We also examined a panel of peptide antigens (Supplementary Fig. 1c): Hla50, harboring five tandem arrays of the first 50 amino acids of Hla that elicit protective immunity in animal models25; HlaP1, a synthetic antigen harboring five tandem arrays of the immune epitope database-predicted T cell epitope Hla36–50; and HlaP2, a synthetic antigen harboring three distinct predicted T cell epitopes from Hla (Hla36–50, Hla51–65, and Hla161–175). Each antigen was screened by delivery to groups of mice to examine vaccine efficacy compared to Freund’s adjuvant control. Upon a screening subcutaneous challenge with S. aureus, mice receiving HlaH35L, HlaD45A/Y118F, and HlaR66C/E70C vaccines exhibited protection against abscess formation and dermonecrosis (Supplementary Fig. 1d). In contrast, mice receiving peptide vaccines exhibited similar or larger lesions than those observed in sham-vaccinated mice.
To further examine vaccine properties of the three full-length antigens, HlaD45A/Y118F and HlaR66C/E70C were modified to incorporate the H35L mutation to ensure full detoxification, which was verified in a rabbit red cell lysis assay (Supplementary Fig. 1e, HlaH35L denoted HlaH, HlaH35L/D45A/Y118F denoted HlaHDY, and HlaH35L/R66C/E70C denoted HlaHRE). As an initial functional immunologic screen, we delivered each candidate antigen to mice in a prime-boost regimen formulated with Freund’s adjuvant. We confirmed that each candidate antigen enhanced the antigen-specific memory T cell response to infection utilizing the ovalbumin-specific OT-II T cell system and an ovalbumin-expressing S. aureus strain (Fig. 2a)17. To assess the B cell response to immunization, we defined the serum toxin-neutralizing capacity in a rabbit red blood cell (rRBC) lysis protection assay and quantified the half-maximal anti-Hla titer (EC50) in serum from vaccinated mice. The vaccine-elicited antibody response varied in an antigen-specific manner, with HlaHRE eliciting a high degree of toxin neutralization (Fig. 2b). These findings suggested that nearly identical antigens based on primary sequence can exhibit distinct biological properties that modify antigenicity and host immune response.
a OT-II CD4+ T cell subset analysis of OT-II CD4+ T cells harvested from the draining lymph nodes of mice immunized with adjuvant alone (sham) or candidate Hla vaccines HlaH, HlaHDY, HlaHRE. Tissue harvest occurred 7 days following subcutaneous infection with 1 × 108 CFU of wild-type S. aureus USA300/LAC strain compared by non-parametric one-way ANOVA with Dunnette’s multiple comparisons test. Data represents biological replicates (n = 5 per group) with mean ± SD. b Correlation between protection against rRBC lysis upon treatment with 2 nM Hla and serologic anti-Hla titer (Log EC50) from mice following immunization with HlaH35L (purple), HlaHDY (orange), HlaHRE (blue). Data represents the mean of biological replicates (n = 5) c Vaccine antigen Hla variant binding to rRBC as compared to wild-type Hla by non-parametric one-way ANOVA with Tukey’s multiple comparisons test, HlaHDY p = 0.0005, HlaHRE p < 0.0001. Data represents the median of technical replicates (n = 3 per group). d Melting curve analysis of HlaH, HlaHDY, and HlaHRE compared to wild-type Hla. e SDS-PAGE analysis of 35S-methionine labeled Hla, HlaH, HlaHDY, and HlaHRE produced by coupled in vitro transcription and translation. f Analysis of heptamer assembly and stability for Hla, HlaH, HlaHDY, and HlaHRE, evaluated following incubation of radiolabeled toxin with rRBCs for one hour at room temperature followed by incubation at 37, 62, or 80 °C for 10 min. g Analysis of HlaHRE heptamer capability with increasing concentrations of radiolabeled toxin. The radiolabeled toxin was incubated with rRBCs for one hour at room temperature, followed by incubation at 37 °C. Relative densitometric units of monomeric and heptameric toxin was quantified using ImageJ software. SDS-PAGE separation was followed by phosphor-image detection of labeled toxin; for e–g, toxin monomers (Hla1) and oligomers (Hla7) are noted. h Disulfide bond identified in antigen HlaHRE by mass spectrometry. Disulfide bond (S-S) peptide linking of Cys66 and Cys70 within the HlaHRE protein was identified by LC-MS/MS analysis and validated by manual alignment of y or b-ion matching. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
To characterize the biochemical properties of candidate antigens, we evaluated ADAM10 binding in a sensitive rRBC binding assay. HlaH exhibited preservation of binding compared to wild-type Hla, with diminution of HlaHDY binding and near-complete loss of HlaHRE binding (Fig. 2c). Melt curve analysis of each antigen revealed that the HlaHDY variant is structurally distinct from Hla, HlaH, and HlaHRE (Fig. 2d). This finding was confirmed by SDS-PAGE analysis where the HlaHDY variant formed the oligomeric toxin (Hla7) in solution without exposure to a cell membrane (Fig. 2e), while wild-type Hla and other variants were present solely in monomeric form (Hla1). Examining the biological properties of these Hla variants on rRBCs, we observed that the wild-type Hla oligomer exhibits temperature stability up to 62 °C (Fig. 2f, left), illuminating the functional defect in the HlaH variant that is unable to form a temperature-stable oligomer (Fig. 2f, left). HlaHDY retains the ability to oligomerize on the cell membrane proportionate to its binding capability (Fig. 2f, right), whereas the HlaHRE variant exhibited limited monomer binding and oligomer formation (Fig. 2f, right). To assess the oligomerization efficiency of HlaHRE on the rRBC surface, we examined increasing concentrations of HlaHRE until achieving an equivalent amount of monomeric Hla rRBC binding (Fig. 2g). Densitometric quantification of the oligomer:monomer ratio revealed a 3.6:1 ratio for Hla compared to 1.9:1 ratio for HlaHRE signifying a functional oligomerization defect in addition to the binding defect observed in the HlaHRE antigen. As Hla undergoes an ordered series of intramolecular movements upon binding to enable heptameric pore formation, we hypothesized that this defect in functional oligomerization of HlaHRE may represent a broader conformational restriction of this antigen based on the incorporation of two cysteine residues that may contribute to disulfide bond formation. To examine whether HlaHRE has a disulfide bond between C66 and C70, we subjected the antigen to mass spectrometry-based analysis, which revealed that the majority of purified HlaHRE does indeed incorporate an intramolecular disulfide bond (Fig. 2h).
Protective efficacy of the HlaHRE candidate vaccine antigen
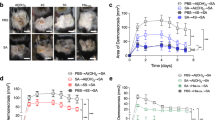
We sought to evaluate each antigen’s protective efficacy in a prime-boost neonatal vaccination model (Fig. 3a), recognizing that neonates and infants represent the vaccine-targetable population in which to enhance the anti-Hla response in humans (Fig. 1). Several immunologic challenges arise in vaccine design for the neonatal population26,27. The neonatal immune response is characterized by delayed formation of the germinal center (GC) with a resultant decrease in the quantity and quality of the affinity-matured antibody response28. Moreover, an increased frequency of FoxP3+ T regulatory (TREG) cells in neonates, together with the presence of maternally-derived antibodies that can modulate vaccine antigen availability and presentation, amplifies the challenge of vaccine formulation for this population28. We thus performed an initial survey of each candidate antigen formulated with four adjuvants: Alum (Alhydrogel), AddaS03 (AS03-like), MPLA+Alum (AS04-like), and CpG-ODN1585. This array of adjuvants was selected for initial studies owing to their distinct immunostimulatory properties, reasoning that a broad-based approach to vaccine formulation would enable us to specifically evaluate the relevance of antigenic variation. To rigorously examine vaccine protection, we utilized a high inoculum of S. aureus (1 × 108 CFU) to elicit primary SSTI in 5–6-week-old mice with a secondary challenge performed on the contralateral flank 21 days following the primary infection (Fig. 3a). All antigens elicited protection against primary abscess formation (Fig. 3b, left) and dermonecrosis (Fig. 3b, right) when formulated with Alum. Upon recurrent infection, HlaHRE elicited protection against abscess formation and dermonecrosis (Fig. 3c), while HlaH and HlaHDY vaccines afforded limited protection. The diminution of vaccine-mediated protection was prominent for HlaH (76.9 ± 9.3% to 28.4 ± 11%) and HlaHDY (76.1 ± 13% to 11.5 ± 4.6%) compared to that observed for HlaHRE (94.4 ± 2.6% to 76.5 ± 8.3%). Enhanced protective immunity was observed with the HlaHRE vaccine candidate during secondary infection irrespective of the adjuvant utilized (Supplementary Fig. 2, AddaS03 (a), MPLA + Alum (b), CpG-ODN1585 (c)). Analysis of the anti-Hla neutralizing antibody titer in serum from each group of vaccinated mice prior to primary infection revealed a marked benefit in those mice receiving the HlaHRE antigen irrespective of adjuvant (Fig. 3d). Correlation analysis of the serologic anti-Hla antibody response and toxin-neutralizing activity from each group of immunized mice reflected the importance of the vaccine antigen as the principal determinant of vaccine-mediated protection, as the HlaHRE vaccine elicited an increased anti-toxin-neutralizing antibody response within each adjuvant group compared to the HlaH vaccine (Fig. 3e, Alum, 92.3-fold; AddaS03, 23.8-fold; MPLA+Alum, 84.2-fold; CpG-ODN1585, 14.6-fold).
a Schematic showing the timeline of neonatal vaccination and SSTI modeling (BioRender). Skin abscess (left) and dermonecrosis (right) area following primary (b) (n = 10 per group) and re-infection (c) of immunized mice (n = 10 for sham and HlaH, and n = 9 for HlaHDY and HlaHRE) with 1 × 108 CFU S. aureus, noting the statistical significance of HlaHRE compared to each vaccine condition designated by color. Data analyzed by two-way ANOVA with Tukey’s multiple comparisons test, mean ± SD. Independent experiments were repeated twice. d Serum dilution from mice in (b, c) that affords 50% protection against rRBC lysis (IC50) when exposed to 0.8 pM recombinant Hla, noting the statistical significance of HlaHRE compared to HlaH by one-way ANOVA with Tukey’s multiple comparisons test. Data represents the median of biological replicates, n = 5 per group for all antigen-adjuvant combinations, except n = 4 for CpG-ODN1585 HlaHRE. e Correlation between serum dilution that affords 50% protection against Hla-mediated (0.8 pM) rRBC lysis and anti-Hla titer from mice following immunization. Data represents the mean from biological replicates in (d). Independent experiments were repeated twice. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file. Figure 3a Created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
HlaHRE augments the germinal center response to vaccination
Amplification of the neutralizing antibody response suggested that the HlaHRE antigen promotes affinity maturation of the antibody response. Affinity maturation is the product of the germinal center (GC) response, characterized by a dynamic interaction between antigen-presenting cells, B cells, and antigen-specific T cells within the draining lymph node (dLN)29. While T cell activation is required to initiate the vaccine response, differentiated antigen-specific T follicular helper (TFH) cells are essential for affinity-based positive selection of GC B cells30. CXCR5 upregulation in a subset of activated T cells within the interfollicular (or mantle) zone of the dLN enables T cell migration to the nascent GC and subsequent upregulation of Bcl6, the lineage-defining transcription factor of TFH cells31. The essential role of the TFH compartment in B cell maturation in the GC is evident in Bcl6 knockout mice which are devoid of GCs, thereby unable to generate a mature antibody response32. To evaluate whether the GC response distinguishes the HlaH and HlaHRE antigens, we first examined the kinetics of the developing anti-Hla neutralizing response to vaccination. Owing to blood volume and tissue limitations in neonatal mice, we subjected 5-week-old mice to prime-boost vaccine delivery with each antigen adjuvanted with Alum. As early as 1-week post-boost, a significant increase in the Hla-neutralizing antibody response was elicited by HlaHRE relative to that of HlaH, p < 0.0001 (Fig. 4a). By 3 weeks post-boost when the elicited antibody response is expected to be fully matured, an increase in the neutralizing antibody titer was seen for both HlaH and HlaHRE antigens, however, the HlaH-elicited response remained lower in magnitude than that of the HlaHRE response, p = 0.0002 (Fig. 4a). The functional attributes of these elicited antibodies were evaluated by assessing blockade of radiolabelled Hla binding (Fig. 4b) and oligomerization (Fig. 4c) on rabbit red cells. In both studies, HlaHRE-elicited antibodies exhibited enhanced performance compared to those elicited by HlaH (p = 0.0002 and p = 0.004, respectively).
a Serum dilution from immunized mice that affords 50% protection against rRBC lysis (IC50) by 0.8 pM Hla, noting statistical significance of HlaHRE versus HlaH by two-tailed unpaired t-test. Data represents the median of biological replicates (1 week, n = 8/group, 3 weeks, n = 7/group). b Serum neutralization of Hla binding to rRBC comparing sera from mice in (a), noting statistical analysis by two-tailed unpaired t-test. Data represented as the median of biological samples (n = 8) represented as the mean of two technical replicates/samples. c Densitometric analysis of Hla oligomer formation on rRBC comparing immunized sera from mice in (a). Data represents the median of biological replicates (n = 5/group), analyzed by a two-tailed unpaired t-test. Analysis of left inguinal germinal center cells 1-week following immunization of mice with HlaH (n = 15) or HlaHRE (n = 15) comparing: d GL7+Fas+ B cells, e Ki67+GL7+Fas+ B cells, f CD4+ T cells, g CXCR5 median fluorescence intensity (MFI) on CD4+ T cells, and h CXCR5 median fluorescence intensity (MFI) on CD4+Ki67+ T cells. Data represents the median of biological replicates, analyzed by a two-tailed paired t-test. i Gross pathology of skin lesions (2 days post-infection) in mice vaccinated as neonates with sham, HlaH, or HlaHRE (n = 5). Analysis of CXCR5hiBcl6hi in Ki67++TFH cell compartment in mice in (i), data represents the median of biological replicates (n = 6). k Analysis of CXCR5hiBcl6hi TFH cell compartment in mice subjected to primary infection with wild-type (WT) (n = 6), Δhla (n = 6), or Δhla::phla (n = 3) variants of S. aureus. Statistical analysis by paired, two-tailed t-tests (d–h) and two-way ANOVA with Tukey’s multiple comparisons test (j, k) *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
We extended these studies to directly evaluate the cellular response within the vaccine-draining iliac lymph node response at day 7 following prime-boost vaccination with either HlaH or HlaHRE. We did not observe differences in either the total population of CD19+ GC B cells marked by co-expression of GL7/Fas (Fig. 4d and Supplementary Fig. 3a) or the proliferating (Ki67+) CD19+GL7+Fas+ B cells (Fig. 4e and Supplementary Fig. 3a). Similarly, no significant difference was observed in the total CD4+ T cell compartment between the two vaccines (Fig. 4f and Supplementary Fig. 3b). In contrast, we observed an increase in median CXCR5 fluorescence intensity in the bulk CD4+ population, p = 0.0143 (Fig. 4g and Supplementary Fig. 3b) and Ki67+ CD4+ cells, p = 0.032 (Fig. 4h and Supplementary Fig. 3b) in HlaHRE-immunized mice. As CXCR5 expression is required for early TFH differentiation to render these cells capable of migration to the nascent germinal center, this finding suggests that the HlaHRE antigen amplifies the pre-TFH response.
We hypothesized that vaccine-mediated protection by HlaHRE would elicit an enhanced TFH response to infection. We thus examined the CXCR5+ compartment in the dLN of vaccinated mice 7 days following secondary S. aureus SSTI (as in Fig. 3). Following infection, clinical lesions remained prominent in the sham and HlaH vaccinated mice, however, were limited in the HlaHRE vaccine recipients (Fig. 4i). As the TFH population is relatively rare in the dLN, we first utilized mass cytometry to analyze pooled dLN to gate on CXCR5+ cells that co-express ICOS and PD1, revealing an increase in the CD4+ CXCR5+ ICOS+ PD1+ TFH compartment in HlaHRE-vaccinated mice relative to that in HlaH-vaccinated mice (Supplementary Fig. 4a). Recent studies have illustrated that the GC TFH compartment is differentiated from the pre-TFH population by high-level expression of CXCR5 and Bcl6 in response to infection31,33,34. To enhance the specificity of our analysis, we utilized a flow cytometric approach to detect CXCR5hi Bcl6hi CD4+ T cells in individual dLN harvested following S. aureus infection (Supplementary Fig. 3c). Compared to sham and HlaH vaccinated mice, animals receiving HlaHRE exhibited expansion of the proliferating CD4+ CXCR5hi Bcl6hi TFH population, p = 0.0012 and p = 0.0033, respectively (Fig. 4j).
We then examined whether the beneficial effects of HlaHRE vaccination reflected the role of Hla in the modulation of the T cell compartment. While our prior studies revealed that Hla blunted the antigen-specific T cell response17, these studies did not encompass the evaluation of the functional specificity of the T cell response. Groups of mice were infected with wild-type S. aureus or its isogenic Δhla variant and dLN subjected to mass cytometry 7 days post-primary infection. Deletion of Hla was associated with an increase in the CXCR5+ ICOS+ PD1+ TFH population (Supplementary Fig. 4b). Analysis of individual mice infected with WT S. aureus, Δhla, and the Δhla variant complemented with a plasmid that enables restoration of Hla expression (Δhla::phla) confirmed that Hla expression negatively impacts the CXCR5hi Bcl6hi GC TFH population, WT vs Δhla p = 0.0326, Δhla vs Δhla::phla p = 0.0236 (Fig. 4k).
HlaHRE efficacy is preserved in the setting of prior antigen exposure
There is an increasing appreciation in the S. aureus field that vaccine failures may, in part, result from vaccine delivery to a non-naive host that harbors a pre-existing immune response that becomes amplified by vaccination3,4. We thus addressed the protective efficacy of the HlaHRE vaccine in two clinical contexts in which pre-existing immunity is relevant: passive transfer of maternal antibodies that may limit vaccine antigen bioavailability and/or antigenicity35, and in the setting of prior S. aureus infection. We first examined whether HlaHRE-immunized dams conferred protection against S. aureus infection to their offspring (Fig. 5a). Pups from HlaHRE-immunized dams exhibited protection against skin abscess and dermonecrosis (Fig. 5b, blue) compared to offspring of Alum-immunized dams (black), reflecting the neutralizing anti-Hla response (Fig. 5c). To evaluate whether passive transfer of maternal antibodies impaired the protective response to subsequent infant vaccination35, we examined whether circulating anti-Hla antibodies in offspring adversely influenced the clinical outcome of active vaccination. Pups from sham (gray) and HlaHRE-immunized (green) dams received HlaHRE vaccination followed by S. aureus infection. Both groups manifest protection against S. aureus infection, as observed in pups born to mice that received passive immunity from maternal immunization (blue), suggesting that prior maternal immunity does not impair the functional outcome of active immunization. We extended these maternal-infant studies by evaluating the duration of the serologic response in offspring, recognizing that passively transferred antibodies are reported to exist in murine offspring up to ~15 weeks of life. Mice from each of the vaccine groups were sampled at 6, 8, 10, and 16 weeks of life and assessed for serum Hla-neutralizing properties. We observed a robust passive transfer of anti-Hla neutralizing function, consistent with the observed protection following maternal immunization (Supplementary Fig. 5, blue). Active immunization of pups following maternal HlaHRE immunization (green) was indistinguishable from that observed in HlaHRE-vaccinated pups born to dams that received sham vaccination with alum alone (gray), signifying that exposure to maternal anti-Hla antibody does not incur functional loss of the HlaHRE vaccine response in offspring. A progressive decrease in the neutralizing antibody titer occurred in all groups over 10–16 weeks, as expected based on known kinetics of the vaccine response (Supplementary Fig. 5). We were unable to examine the duration of clinical protection against S. aureus infection in mice that were 16 weeks old owing to inter-mouse heterogeneity in the hair follicle cycle which directly effects re-epithelialization in response to injury36.
a Schematic showing the timeline for maternal and neonatal vaccination followed by SSTI. b Skin abscess (left) and dermonecrosis (right) area of mice following primary infection with 1 × 108 CFU S. aureus. Mice were born to dams that received Alum or the Alum-HlaHRE vaccine; pups were either unimmunized (black n = 4 and blue n = 8, respectively), Alum immunized (gray, n = 5), or Alum-HlaHRE immunized (green, n = 5), noting statistical significance of Alum maternal immunization compared to each vaccine condition designated by color by two-way ANOVA with Tukey’s multiple comparisons test. Data represented as the mean ± SD. c Serum dilution from mice in (b), that affords 50% protection against rRBC lysis (IC50) when exposed to 0.8 pM Hla. Statistical analysis by one-way ANOVA with Tukey’s multiple comparisons test. Data represents the median of biological replicates (black n = 5, blue n = 5, gray n = 3, and green n = 3). d Schematic showing the timeline for a model in which juvenile mice are exposed to S. aureus infection prior to vaccination. e Skin abscess (left) and dermonecrosis (right) area of mice (sham n = 13, HlaHRE n = 14) following secondary infection with 1 × 108 CFU S. aureus. Data represents mean ± SD, analyzed by two-way ANOVA with Sidak’s multiple comparisons test. f Serum dilution from mice in (e), that affords 50% protection against rRBC lysis (IC50) when exposed to 0.8 pM Hla. Data represents the median, analzyed by one-way ANOVA with Tukey’s multiple comparisons test (primary infection group n = 7, sham n = 6, and HlaHRE n = 6). Source data are provided as a Source Data file. Figure 5a, d Created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
To directly evaluate whether prior S. aureus infection modulated the protective efficacy of HlaHRE vaccination, groups of naive 5-week-old mice were subjected to S. aureus SSTI followed by a prime-boost vaccine regimen with Alum alone or Alum-adjuvanted HlaHRE vaccines (Fig. 5d). HlaHRE conferred near-complete protection against infection on the contralateral flank two weeks following boost (Fig. 5e), reflecting the neutralizing anti-Hla response (Fig. 5f). Together, these findings suggest that vaccination with the HlaHRE antigenic variant may not be as susceptible to the deleterious impact of pre-existing immunity on vaccine outcomes. It remains to be determined if this property reflects the biochemical nature of the HlaHRE antigen itself, the specificity of the elicited antibody response, or is simply related to an antibody response generated against a secreted protein antigen.
TFH amplification correlates with HlaHRE-mediated protection
To understand the potential scope of clinical applicability of the HlaHRE vaccine, we assessed protective efficacy in a series of distinct biological settings. S. aureus skin infection is commonly modeled in male mice owing to both the epidermal structure of the skin, which facilitates histopathologic analysis, and the magnitude of the lesional differences that result from male hormone-mediated enhancement in S. aureus virulence37. We first demonstrated HlaHRE vaccine efficacy in protecting female mice against severe SSTI following neonatal vaccination (Supplementary Fig. 6a), consistent with the elicitation of the anti-Hla neutralizing antibody response (Supplementary Fig. 6b). Second, we evaluated protective efficacy in young mice that were not exposed to vaccination as neonates. Groups of 3-week-old naive mice received a priming immunization with either Alum alone or Alum-adjuvanted HlaHRE followed by a boost 14 days later (Supplementary Fig. 6c). When subjected to primary S. aureus infection, HlaHRE-vaccinated mice exhibited protection from infection (Supplementary Fig. 6d), also reflecting a productive neutralizing anti-Hla antibody response (Supplementary Fig. 6e) and corresponding vaccine-elicited TFH response (Supplementary Fig. 6f). Finally, we evaluated whether a non-tagged variant of the HlaHRE antigen was functional. Neonatal vaccination with both the tagged HlaHRE antigen and the native, untagged HlaHRE antigen (HlaHRE-NATIVE) elicited significant protection against S. aureus skin infection (Supplementary Fig. 6g). While a minor improvement in the clinical efficacy of HlaHRE-NATIVE was observed early in the course of infection, the neutralizing IC50 was nearly identical for both antigens (Supplementary Fig. 6h).
Successful vaccine design, evaluation, and implementation in the human population requires both antigen optimization and knowledge of the mechanism by which distinct adjuvants amplify desired host immune responses. We therefore assessed whether the observed adjuvant-specific differences (Alum, AddaS03, MPLA + Alum, and CpG-ODN1585) in potency of HlaHRE vaccine-mediated protection (Fig. 3 and Supplemental Fig. 2) reflected the magnitude of the TFH response in the dLN. Evaluating the relationship between TFH recovery from the dLN and clinical abscess size, we observed an inverse correlation (Fig. 6a). In accord with this finding, we observed a direct correlation between the IC50 of the neutralizing anti-Hla response and TFH response (Fig. 6b). Together, these findings underscore the importance of the TFH response as an immune correlate of protective efficacy of the HlaHRE vaccine, and suggests that the magnitude of this response can be favorably modulated by vaccine formulation.
a Inverse correlation of TFH cell recovery and clinical abscess size from immunized mice after S. aureus SSTI infection. b Direct correlation between the anti-Hla neutralizing IC50 and TFH response from immunized mice after S. aureus SSTI infection. All mice were subjected to priming immunization as neonates with a boost at the time of weaning. Data represents the mean of biological replicates (n = 7 per group for TFH cell analysis and Alum-HlaHRE n = 11, AddaS03-HlaHRE n = 10, and n = 12 for MPLA+Alum-HlaHRE and CpG-ODN1585-HlaHRE groups). Data and R2 values were analyzed by non-linear regression, semi-log line. Source data are provided as a Source Data file.
Discussion
Our findings in this study leverage the observation that the anti-Hla response is a defined correlate of human protective immunity to S. aureus in the pediatric population19. Through population-level analysis characterizing the development of anti-Hla response in childhood, we define a specific window of opportunity in the first two years of life to augment this response through vaccination. We describe a candidate Hla antigen, HlaHRE, distinguished by its ability to elicit a robust toxin-neutralizing response. When delivered as a pre-exposure vaccination of neonatal mice or in a maternal vaccine strategy, HlaHRE affords pre-clinical protection from severe S. aureus skin infection. Moreover, we have demonstrated that prior exposure to Hla is not an a priori limitation to successful amplification of the host immune response to the HlaHRE vaccine. An analysis of the mechanism of protective immunity elicited by HlaHRE revealed the importance of the TFH response as a functional immunologic correlate of HlaHRE vaccine-mediated protection.
These studies address several critical challenges within the S. aureus vaccine field. It has been recognized that vaccine development to target S. aureus has suffered from a lack of understanding of the T cell response to this pathogen. Consequently, vaccines have not been designed to elicit T cell-specific correlates of immune protection38. For the first time, we propose and evaluate a vaccine specifically designed to protect the T cell response to S. aureus early in life. Hla neutralization contributes to the protection of the antigen-specific T cell response during infection17,18, thus providing mechanistic insight into the observed pre-clinical efficacy of the HlaHRE vaccine. Detoxified Hla variants (including HlaH35L, HlaH35L/H48L) have previously been considered or are being evaluated for vaccine development39. Our studies illustrate that the specific antigenic form of Hla influences the magnitude of the neutralizing antibody response, revealing that some Hla variants may only generate modest vaccine protection. While it is possible that this response difference may be overcome by delivery of higher quantities of antigen or alternatively through booster regimens, the potency of the HlaHRE antigen in amplification of the host immune response may be advantageous to enable antigen sparing and in settings such as the neonate and the elderly where the vaccine-elicited response may be limited by inherent attributes of host immunity.
As S. aureus is a leading cause of bacterial infection-associated mortality worldwide in the first year of life1, our findings establish the scientific rationale for considering maternal immunization and/or neonatal HlaHRE vaccination. Maternal immunization offers the attractive opportunity to afford protection of neonates against disease and modulation of the T cell response to S. aureus by Hla prior to the ability of active immunization to engender protection. As passive immunity will predictably wane, a durable approach to active immunization of the infant/child will be required to ensure long-term protection of the T cell response against this pathogen. It will be important to rely upon analysis of the epidemiology of S. aureus disease to inform ongoing vaccine development for this population.
At present, the precise molecular mechanism by which the HlaHRE antigen amplifies the TFH response to promote affinity maturation of anti-Hla antibody response is not fully understood. The increase in CXCR5 expression on pre-TFH CD4+ T cells following delivery of HlaHRE compared with that observed upon HlaH immunization suggests that HlaHRE may more effectively promote a cDC2-dependent response, favoring TFH differentiation40. While it was unexpected that two amino acid substitutions in HlaHRE relative to HlaH would alter the outcome of vaccination, a precedent for this level of antigen specificity in defining the host response to vaccination was observed in respiratory syncytial virus (RSV) vaccine development. Utilization of a pre-fusion-locked F antigen conformation elicits a highly neutralizing anti-viral antibody relative to that observed with post-fusion F antigen41,42. These two vaccine examples illustrate the exquisite specificity of antigen recognition by the host immune system, and indicate that a detailed analysis of antigen trafficking kinetics, cellular uptake and presentation, and duration of antigen availability in the GC will be required to fully elucidate the biological differences that underlie antigen specificity observed with the HlaHRE variant. As HlaH and HlaHRE are functionally distinguished by ADAM10 binding capability, it will also be of interest to understand whether cellular trafficking of the Hla-ADAM10 complex on both professional and non-professional antigen-presenting cells modulates antigen distribution and processing.
Our findings have important implications for S. aureus vaccine development. HlaHRE-elicited protective immunity is robust upon formulation with multiple distinct adjuvants (Fig. 3 and Supplemental Fig. 2), compelling the inclusion of this specific antigenic variant of Hla to optimize vaccination outcomes. Whether HlaHRE is considered for use in a monovalent context or in the presence of other vaccine antigens, its biological properties are expected to augment the Hla-neutralizing antibody response. The use of HlaHRE may simultaneously enhance the functional T cell and GC response to other antigens, especially as this antigen augments the TFH response. Our studies, for the first time, indicate that the TFH response is a key target of Hla in S. aureus pathogenesis. It is of interest to note that in recurrent human Streptococcus pyogenes (group A Strep) infection, modulation of the TFH response has been implicated as a principal mechanism of immunoevasion33. While the TFH response during S. aureus infection can be rigorously examined in modeling systems, it will be essential to translate these studies to human immunity to S. aureus in order to inform vaccine development. Variation in the magnitude of the TFH response as a function of the adjuvant utilized (Fig. 6) indicates that it may be possible to tailor the vaccine formulation to the target population of interest, simultaneously providing a framework on which to evaluate novel adjuvants to further optimize vaccine performance.
Together, these studies underscore the importance of Hla neutralization by a S. aureus vaccine. As a toxin that interferes with the development of T cell-mediated immunity to S. aureus17, Hla neutralization is expected to not only protect against toxin-mediated cellular injury in disease, but, perhaps more importantly, ensure that the T cell compartment remains viable and poised throughout life to support the generation of a diverse immune response to the pathogen. Delivery of an HlaHRE-containing vaccine early in life may thus afford protection against S. aureus infection in infants and children, but also enable the success of S. aureus vaccines developed for administration later in life to protect against disease-specific virulence factors. The HlaHRE vaccine may thereby represent an opportunity to advance the development of a population-level S. aureus vaccine.
Methods
Study approval
All experimental procedures and reagents were approved by Washington University in St. Louis Institutional Biosafety Committee (protocol 14083). All animals in this study were housed according to Washington University in St. Louis IACUC guidelines, (ventilation sufficient to maintain appropriate temperature (68–79 °F) and humidity (30–70%) ranges and to control odor; standard diurnal light cycle 12-h light: 12-h dark) and all experimental procedures were approved by the IACUC of Washington University in St. Louis (protocol 22-0377). The collection and use of human samples in this study was approved by the University of Chicago Institutional Review Board. Written informed consent was obtained for all participants, with assent obtained from children of a developmentally appropriate age (~7 years of age or older). Schematic figures were created with BioRender and licensed for use in publication.
Participant population and sample and data collection
Participants were children 18 years and younger undergoing sedated procedures or imaging studies at Comer Children’s Hospital. Upon enrollment, study personnel collected swab samples (CultureSwab Liquid Stuart, Becton Dickinson, Franklin Lakes, NJ) from the anterior nares, axillae, and inguinal folds of each participant to determine S. aureus colonization status via culture-based methods. Participants or their parents/legal guardians were asked to complete a survey regarding the subject’s current and past medical history, and history of S. aureus infection or known skin and soft tissue infection in the participant or a household member. Lastly, serum samples were obtained from each participant. Samples were de-identified and stored in a secure repository at −80 °C. Gender was not considered in this study, which aimed to broadly classify the serologic response to S. aureus based on age without any other discriminatory or stratifying features of the subject population.
ELISA and antibody neutralization assays
Human serum analysis
Nunc Maxisorp 384-well plates (Fisher Scientific) were coated with HlaH35L at 1 μg/ml in PBS and incubated overnight at 4 °C. Plates were blocked with 0.1% bovine serum albumin (BSA) in PBS for 2 h at room temperature. Patient sera samples were diluted 1:10 and then twofold for a total of 24 dilutions and transferred to the 384 Maxisorp plates for 1 h at room temperature. Plates were washed three times with PBS/0.05% Tween-20 and incubated with goat anti-human HRP conjugated antibody (Southern Biotech) at a 1:20,000 dilution for 1 h at room temperature. Plates were washed three times and developed for 30 min at room temperature with QuantaBlu Fluorogenic Peroxidase Substrate Kit, and reactions were stopped with QuantaBlu Stop Solution. Fluorometric detection was measured using a Tecan Infinite M200 Pro plate reader at excitation 320 nm and emission 400 nm. For the neutralization assay, patient sera samples were diluted 1:10 in 0.1% BSA/PBS and incubated with purified toxin for 1 h at room temperature. After incubation, 5 × 107 of prewashed rRBCs were added and incubated for 1 h at room temperature on a platform shaker. The final concentration of toxin for the assays was 2 nM. Cells were pelleted, and supernatant absorbance readings (450 nm) were used to generate non-linear regression curves with log (inhibitor) vs. response—variable slope curves using GraphPad Prism software.
Juvenile murine serum analysis
Sera was diluted 1:25 and then by fourfold serial dilutions for eight dilutions total. Anti-Hla titers were determined by ELISA as previously described and ELISA absorbance readings (450 nm) used for generation of four-parameter log(dose)-response curves using Prism software. For the neutralization assay, serum was diluted 1:100 and then twofold for eight dilutions total. The diluted serum was incubated with 2 nM purified toxin for 15 min at room temperature before 5 × 107 rRBCs were added then incubated at room temperature for 1 h. Cells and debris were pelleted, and supernatant absorbance readings (450 nm, Tecan Infinite M200 Pro) were used to generate non-linear regression curves with log (inhibitor) vs. response—variable slope curves using GraphPad Prism software. Both the ELISA and neutralization assays were performed in triplicate.
Neonatal murine serum analysis
384-well Maxisorp plates (Thermo Fisher) were coated with 50 µL of 1 µg/mL HlaH35L and incubated overnight at 4 °C. Plates were blocked with 0.1% BSA in PBS for 2 h at room temperature, following incubation with 15 µL of neonatal pre-infection sera for 2 h at room temperature. Plates were washed three times with PBS/0.05% Tween-20 and then incubated with 15 µL of goat anti-mouse IgG-HRP antibody (Southern Biotech) at a 1:20,000 dilution for 1 h at room temperature. Plates were washed three times and developed for 15 min at room temperature with 20 µL of TMB substrate kit (Thermo Scientific Pierce PI34021), and the reaction stopped with 20 µL of 2 N sulfuric acid (Fisher Scientific). Absorbance values were read at 450 nm using a Tecan Infinite M200 Pro plate reader. For neutralization assays, neonatal pre-infection sera was diluted twofold for a total of 16 dilutions and incubated for 1 h at room temperature with 0.8 pM purified Hla. After incubation, 30 µL of prewashed rabbit red cells (1 × 107 total cells) was added to the toxin/sera and incubated for 1 h at room temperature in a v-bottom 384-well plate (Thomas Scientific). Cells and debris were pelleted, and supernatant absorbance readings (450 nm, Tecan Infinite M200 Pro) were used to generate non-linear regression curves with log (inhibitor) vs. response—variable slope curves using GraphPad Prism software.
Adult murine serum analysis with high-dose immunization
Sera was collected 1 week, 2 weeks, and 3 weeks post prime and boost 20 µg vaccinations. Sera was diluted 1:4 in PBS, and assays were performed as described above.
Plasmid construction and Hla antigen purification
Toxin variants were cloned into the pET24b expression vector containing a C-terminal polyhistidine tag. HlaH35L was previously generated as described7. HlaD45A/Y118F was generated via site-directed mutagenesis with template DNA from a pET24b construct containing wild-type Hla cDNA previously generated. The following oligos were utilized: D45A sense – 5′ GTATTTTATAGTTTTATCGATGCTAAAAATCACAATAAAA 3′; D45A antisense – 5′ TTTTATTGTGATTTTTAGCATCGATAAAACTATAAAATAC 3′; Y118F sense – 5′ GTATATGAGTACTTTAACTTTTGGATTCAACGGTAATGTTA 3′; Y118F antisense – 5′ TAACATTACCGTTGAATCCAAAAGTTAAAGTACTCATATAC 3′. HlaR66C/E70C was previously generated in the Bubeck Wardenburg lab as a GST fusion protein in pGEX6P1. To generate a polyhistidine-tagged protein, template DNA from the pGEX6P1 construct containing R66C/E70C cDNA was used with primers for restriction site cloning using XbaI and XhoI (New England Biolabs) into pET24b vector.
The following primers were used: sense – 5′ CGGCGGCTCGAGATTTGTCATTTCTTCTTT 3′;
antisense – 5′ CGGCGGTCTAGAAGGAGGATATATATGGCAGATTCTGATATAATATT 3′.
Site-directed mutagenesis was used to incorporate the H35L mutation in HlaD45/Y118F and HlaR66C/E70C constructs with the following primers: sense – 5′
CTTATGATAAAGAAAATGGCATGCTCAAAAAAGTATTTTATAGTTTTATCGATG 3′;
antisense –5′ CATCGATAAAACTATAAAATACTTTTTTGAGCATGCCATTTTCTTTATCATAAG 3′. Mutagenesis reactions were DpnI (New England Biolabs) digested and transformed into Escherichia coli DH5α on selection agar. Each construct was sequenced (Azenta Life Sciences) for verification. Confirmed clones were then transformed into E. coli BL21 for recombinant protein expression and purification.
Peptide sequences Hla50, HlaP1 and HlaP2 constructs were generated by SynBio Technologies, and constructs transformed into BL21 for protein expression and purification. Recombinant toxin variants were purified using standard protocols described previously, LPS extracted and evaluated by 10% SDS-PAGE followed by Coomassie blue staining.
Characterization assays for Hla antigens
Hemolysis assay
Rabbit red blood cell (rRBC) hemolysis was assayed by incubation of rRBCs (Hemostat Labs) with purified toxin variants ranging from 0.08 to 10 µg/mL for 1 h at room temperature. Following incubation, cells were pelleted by centrifugation, and supernatant absorbance at 450 nm was measured using a Tecan Infinite M200 Pro plate reader. Percent rRBC hemolysis were calculated relative to 1% Triton X-100 maximal lysis controls. The assays were performed in triplicate and repeated for reproducibility on separate days.
Radiolabeled toxin binding and oligomerization assay
Hla was synthesized by in vitro transcription and translation in an E. coli S30 extract (Promega) supplemented with T7 RNA polymerase, rifampin (rifampicin), and [35S] methionine according to the manufacturer’s instructions. For the binding assay, 125 µL of 12.5% rabbit red blood cells (rRBC) in K-PBSA/βME (20 mM potassium phosphate [monobasic], 150 mM NaCl pH 7.4, 1 mg/ml bovine serum albumin, 1 mM β-mercaptoethanol) was incubated with 10 µl of radiolabeled Hla mixture diluted 1:10 (~1 nM) for 5 min at room temperature. Cells were pelleted, washed twice with ice-cold PBS, resuspended in 200 μl of PBS, and added to scintillation fluid (Research Products International, Econo-Safe). Radioactivity from cell-bound toxin was quantified on a Beckman LS6000 scintillation counter. For oligomerization assays, rRBCs and radiolabelled Hla were used as described above for 1 h at room temperature. Following incubation, cells were pelleted and washed with 500 μl K-PBSA/βME and then resuspended in 90 μl 1× Laemmli buffer. Samples were divided into 30 μl aliquots and incubated at 37, 62, and 80 °C for 10 min before samples were loaded onto 10% sodium dodecyl sulfate (SDS)-PAGE gels for electrophoresis. The gels were dried, and results were visualized using a phosphorimager (GE Healthcare Typhoon Trio Imager). To analyze HlaHRE oligomerization capability, rRBCs were treated as described above with 10 μl of increasing concentration of radiolabeled Hla mixture (1x = ~1 nM; 2x = ~2 nM, and 5x = ~5 nM) and incubated at 37 °C before samples were separated by SDS-PAGE and prepared as above. For binding and oligomerization assays using vaccinated mouse sera, each sera sample was diluted 1:5 in PBS, then incubated with 10 µl of radiolabeled Hla diluted 1:10 for 10 min at room temperature before adding the rabbit red cells. Assays were performed as described above. Binding assays were performed in triplicate and repeated for reproducibility on separate days. Oligomerization assays were performed with a single replicate and repeated for reproducibility on separate days.
Proteomics disulfide bond analysis of HlaHRE
The purified protein sample was digested with 250 ng trypsin for overnight incubation at 37 °C. The resulting peptides were desalted using the C18 column, and the eluates were dried under a SpeedVac vacuum concentrator. Peptides were analyzed by LC-MS/MS using a Vanquish Neo UHPLC System coupled to an Orbitrap Eclipse Tribrid Mass Spectrometer with FAIMS Pro Duo interface (Thermo Fisher Scientific). The sample was loaded on a Neo trap cartridge coupled with an analytical column (75 µm ID × 50 cm PepMapTM Neo C18, 2 µm). Samples were separated using a linear gradient of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile) over 120 min. For MS acquisition, FAIMS switched between CVs of −35 and −65 V with cycle times of 1.5 s per CV. MS1 spectra were acquired at 60,000 resolution with a scan range from 380 to 1400 m/z, normalized AGC target set at Standard mode, and maximum injection time set at Auto mode. Precursors were filtered using monoisotopic peak determination set to peptide, charge state 3 to 8, dynamic exclusion of 60 s with ±10 ppm tolerance. For the MS2 analysis, the isolated ions were fragmented by assisted higher-energy collisional dissociation (HCD) at 25 and 30% and acquired in an orbitrap at 30,000 resolution. The AGC and maximum IT were 200% and 70 ms, respectively.
The acquired MS/MS data was queried for disulfide cross-link identification against a target protein sequence using MeroX 2.0 software. The Disulfide bond was selected as the cross-linker, and the maximum number of missed cleavages was set to 4. The precursor mass tolerance was set to 20 ppm, and the fragment mass tolerance was set to 50 ppm. The search results were validated with 5% FDR and a precise scoring setting. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [1] partner repository with the dataset identifier PXD047859.
Thermal shift assay
The stability of each toxin variant was measured by quantifying the melting temperature of each toxin variant (50% denaturation point) compared to the wild-type toxin. Each well contained a final concentration of 5 μM protein and a 1:20 dilution of 5x Sypro Orange stock (Thermo Fisher S6650) in 20 mM Tris pH 7.5 and 150 mM NaCl buffer. The plates were heated in StepOnePlus™ Real-Time PCR System (Thermo Fisher) from 20 to 90 °C in increments of 0.3 °C. The wavelengths for excitation and emission were 490 and 575 nm, respectively.
Bacterial strains
S. aureus strains USA300/LAC, and the isogenic USA300/LAC Δhla mutant were grown in tryptic soy broth (Bacto TSB, Fisher Scientific) overnight at 37 °C with agitation for 14–16 h. The Hla plasmid complemented S. aureus strain (Δhla::phla) and the ovalbumin-expressing S. aureus strains were grown as described above with 20 µg/mL chloramphenicol selection. The strains were subcultured 1:100 in TSB the following day and grown to exponential phase (OD600 0.5) and washed once with PBS, then resuspended in PBS to deliver 1 × 108 CFU in 50 µL via subcutaneous injection15.
Plasmid construction for bacterial strains
The hla locus was deleted from the USA300 strain (Δhla) using the pJB38 plasmid and the complemented strain (Δhla::phla) was created with the pOS1 plasmid encoding hla, resulting in restoration of Hla expression43. A previously generated ovalbumin-expressing USA300 strain was utilized for the OT-II CD4+ T cell subset analysis. In brief, this strain carries a plasmid engineered with an improved translation initiation region, optimized Shine-Dalgarno, and translation enhancer sequences, and an S. aureus codon-optimized chicken egg ovalbumin sequence that includes the S. aureus Hla signal sequence for secretion16.
Animal modeling
For all studies, animals were allocated randomly to experimental groups. Animals utilized for serum and tissue harvesting were randomly selected within each group. The Investigators were not blinded to allocation during experiments and outcome assessment.
Vaccine preparation and immunization
Vaccine antigens were LPS extracted and prepared as a 1:1 antigen to adjuvant formulation using the following: Alhydrogel 2% (InvivoGen, vac-alu-250), AddaS03 (InvivoGen, 10253-42-02), AS04-like formulation with antigen plus 500 μg/mL Alhydrogel 2% and 50 μg/mL of MPLA-SM (InvivoGen, vac-mpla), or CpG-ODN1585 (InvivoGen, 2 µg of CpG-ODN1585). For initial antigen screening and germinal center studies, 20 μg of each antigen was delivered via intramuscular (IM) injection to cohorts of 3-week-old or 5-week-old C57BL/6J male or female mice (offspring from C57BL/6 purchased from The Jackson Laboratory) on days 0 and 14. For the immunization of neonatal mice, 2 μg of each antigen was delivered in a total volume of 10μl IM to litters of C57BL/6 pups between P1-P3 with a boost delivered on P21 (day of weaning) with 50 μl total volume. Immunization of juvenile mice was conducted by delivery of 2 μg antigen in a total volume of 50 μl.
Skin and soft tissue infection modeling
All animal experiments were reviewed, approved, and supervised by the Institutional Animal Care and Use Committee (IACUC) at Washington University in St. Louis. To determine antibody titers, serum was collected from 5 to 10 mice per group, 1 week after boost immunizations and prior to the S. aureus challenge. C57BL/6 male and female mice 5–6 weeks of age were anesthetized via intraperitoneal injection of ketamine (20 mg/kg) and xylazine (5 mg/kg) followed by right flank subcutaneous challenge with 1 × 108 CFU S. aureus strain USA300/LAC in 50 µL PBS 71. Lesional abscess and dermonecrosis area (mm2) were measured at 24-h intervals for 10 days44. Abscesses were demarcated as the nodular and sometimes fluctuant area of subepithelial infection. Dermonecrosis area was measured independently as the area overlying the abscess where the epithelial injury was visible. Mice received a secondary infection on the left flank, 21–28 days after the start of the primary infection. Abscess and dermonecrosis size for all studies were determined according to the formula A = (π/2)(length mm)(width mm)44. All mice within each experiment were randomly selected for immunizations and age-matched.
Cell-based immunologic assays
Mass cytometry analysis
Ten days following either primary or secondary infection as indicated, the inguinal draining lymph node (dLN) was harvested from the ipsilateral side of S. aureus infected mice and processed to obtain single cell suspensions. A minimum of three dLN from independent mice were pooled together for each condition. Cells (3 × 106 per sample) were subjected to cisplatin labeling followed by staining for cell surface markers and intracellular antigens of interest with the antibody reagents, as noted in Supplementary Table 3. Fixation and cell permeabilization for intracellular staining were performed with the eBioscience Permeabilization Buffer and FoxP3 Transcription Factor Staining Buffer Set according to the manufacturer’s protocol. Stained cells were washed, barcoded by sample, pooled, and analyzed on a Fluidigm CyTOF2 Helios mass cytometer by the Bursky Center for Human Immunology and Immunotherapy Programs at Washington University. De-barcoded samples were analyzed using Cytobank software (Beckman Coulter).
Flow cytometric analysis
Draining lymph nodes were processed as above to obtain a single-cell suspension. About 1 × 106 cells were then subjected to fluorescent antibody labeling, as noted in Supplementary Table 4 for extra- and intracellular staining. Viable cells were detected with Live/Dead Fixable Blue (Invitrogen L23105). Purified Hla antigen was labeled with Alexa Fluor 594 (Invitrogen A20104). These samples were run on a Cytek Aurora (spectral five lasers) and analyzed using Cytobank (Beckman Coulter).
Statistics and reproducibility
Sample sizes for each experiment were determined based on prior published data. The number of animals utilized in each experiment was based on the minimal number needed to achieve results of statistical significance. Statistical analysis for serologic studies and all in vitro assays was performed with GraphPad Prism 10 software using one-way ANOVA with Tukey’s or Dunnette’s multiple comparisons test or unpaired, two-tailed independent t-test. Assessment of statistical significance in skin infection studies was performed with GraphPad Prism 10 software using two-way ANOVA with Tukey’s or Sidak’s multiple comparisons test. Assessment of statistical significance in T cell analysis studies was performed with GraphPad Prism 10 software using either paired, two-tailed T-test or two-way ANOVA with Tukey’s or Dunette’s multiple comparisons test. Data results were considered statistically significant at a P value of 0.05 or less (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). No data were excluded from the analysis except two male mice in Fig. 5g due to inconsistent hair growth cycle compared to other mice in group. All data is representative of at least two independent experiments. SDS-PAGE data are shown as representative gels of two independent experiments with similar results.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data generated in this study are provided in the Source Data file. The mass spectrometry proteomics data in this study have been deposited in the ProteomeXchange Consortium via the PRIDE [1] partner repository which can be accessed at http://www.ebi.ac.uk/pride/archive/projects/PXD047859. A reporting summary is available for this study. Source data are provided with this paper.
References
Ikuta, K. S. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 400, 2221–2248 (2022).
Clegg, J. et al. Staphylococcus aureus vaccine research and development: the past, present and future, including novel therapeutic strategies. Front. Immunol. 12, 705360 (2021).
Tsai, C. M. et al. Non-protective immune imprint underlies failure of Staphylococcus aureus IsdB vaccine. Cell Host Microbe 30, 1163–1172.e1166 (2022).
Caldera, J. R. et al. The characteristics of pre-existing humoral imprint determine efficacy of S. aureus vaccines and support alternative vaccine approaches. Cell Rep. Med. 5, 101360 (2024).
Berube, B. J. & Bubeck Wardenburg, J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins 5, 1140–1166 (2013).
Wilke, G. A. & Bubeck Wardenburg, J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc. Natl Acad. Sci. USA 107, 13473–13478 (2010).
Inoshima, I. et al. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med. 17, 1310–1314 (2011).
Inoshima, N., Wang, Y. & Bubeck Wardenburg, J. Genetic requirement for ADAM10 in severe Staphylococcus aureus skin infection. J. Invest. Dermatol. 132, 1513–1516 (2012).
Powers, M. E., Kim, H. K., Wang, Y. & Bubeck Wardenburg, J. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J. Infect. Dis. 206, 352–356 (2012).
Becker, R. E., Berube, B. J., Sampedro, G. R., DeDent, A. C. & Bubeck Wardenburg, J. Tissue-specific patterning of host innate immune responses by Staphylococcus aureus alpha-toxin. J. Innate Immun. 6, 619–631 (2014).
Rosenbaum, D. & Saftig, P. New insights into the function and pathophysiology of the ectodomain sheddase A Disintegrin And Metalloproteinase 10 (ADAM10). FEBS J. 291, 2733–2766 (2023).
Inoshima, N., Wang, Y. & Wardenburg, J. B. Genetic requirement for ADAM10 in severe Staphylococcus aureus skin infection. J. Invest. Dermatol. 132, 1513–1516 (2012).
Powers, M. E., Becker, R. E., Sailer, A., Turner, J. R. & Bubeck Wardenburg, J. Synergistic action of Staphylococcus aureus alpha-toxin on platelets and myeloid lineage cells contributes to lethal sepsis. Cell Host Microbe 17, 775–787 (2015).
Alfano, D. N., Miller, M. J. & Bubeck Wardenburg, J. Endothelial ADAM10 utilization defines a molecular pathway of vascular injury in mice with bacterial sepsis. J. Clin. Invest. 133, e168450 (2023).
Sampedro, G. R. et al. Targeting Staphylococcus aureus alpha-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. J. Infect. Dis. 210, 1012–1018 (2014).
Tkaczyk, C. et al. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin. Vaccin. Immunol. 19, 377–385 (2012).
Lee, B., Olaniyi, R., Kwiecinski, J. M. & Wardenburg, J. B. Staphylococcus aureus toxin suppresses antigen-specific T cell responses. J. Clin. Invest. 130, 1122–1127 (2020).
Kleinhenz, M. et al. Toxin-neutralizing Abs are associated with improved T cell function following recovery from Staphylococcus aureus infection. JCI Insight 9, e173526 (2024).
Fritz, S. A. et al. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin. Infect. Dis. 56, 1554–1561 (2013).
Wu, Y. et al. Prevalence of IgG and neutralizing antibodies against Staphylococcus aureus alpha-toxin in healthy human subjects and diverse patient populations. Infect. Immun. 86, e00671–17 (2018).
Hogan, P. G. et al. Impact of systemic antibiotics on Staphylococcus aureus colonization and recurrent skin infection. Clin. Infect. Dis. 66, 191–197 (2018).
Menzies, B. E. & Kernodle, D. S. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect. Immun. 62, 1843–1847 (1994).
Sugawara, T. et al. Structural basis for pore-forming mechanism of staphylococcal alpha-hemolysin. Toxicon 108, 226–231 (2015).
Walker, B. & Bayley, H. Key residues for membrane binding, oligomerization, and pore forming activity of staphylococcal alpha-hemolysin identified by cysteine scanning mutagenesis and targeted chemical modification. J. Biol. Chem. 270, 23065–23071 (1995).
Ragle, B. E. & Bubeck Wardenburg, J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect. Immun. 77, 2712–2718 (2009).
Pichichero, M. E. Challenges in vaccination of neonates, infants and young children. Vaccine 32, 3886–3894 (2014).
Basha, S., Surendran, N. & Pichichero, M. Immune responses in neonates. Expert Rev. Clin. Immunol. 10, 1171–1184 (2014).
Sakala, I. G., Eichinger, K. M. & Petrovsky, N. Neonatal vaccine effectiveness and the role of adjuvants. Expert Rev. Clin. Immunol. 15, 869–878 (2019).
Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu. Rev. Immunol. 40, 413–442 (2022).
Crotty, S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50, 1132–1148 (2019).
Zhu, F. et al. Spatiotemporal resolution of germinal center Tfh cell differentiation and divergence from central memory CD4(+) T cell fate. Nat. Commun. 14, 3611 (2023).
Fukuda, T. et al. Disruption of the Bcl6 gene results in an impaired germinal center formation. J. Exp. Med. 186, 439–448 (1997).
Dan, J. M. et al. Recurrent group A Streptococcus tonsillitis is an immunosusceptibility disease involving antibody deficiency and aberrant T(FH) cells. Sci. Transl. Med. 11, eaau3776 (2019).
Dan, J. M. et al. A cytokine-independent approach to identify antigen-specific human germinal center T follicular helper cells and rare antigen-specific CD4+ T cells in blood. J. Immunol. 197, 983–993 (2016).
Vono, M. et al. Maternal antibodies inhibit neonatal and infant responses to vaccination by shaping the early-life B cell repertoire within germinal centers. Cell Rep. 28, 1773–1784.e1775 (2019).
Ansell, D. M., Kloepper, J. E., Thomason, H. A., Paus, R. & Hardman, M. J. Exploring the “hair growth-wound healing connection”: anagen phase promotes wound re-epithelialization. J. Invest. Dermatol. 131, 518–528 (2011).
John, M. S. et al. Androgens at the skin surface regulate S. aureus pathogenesis through the activation of agr quorum sensing. Preprint at bioRxiv (2024).
Armentrout, E. I., Liu, G. Y. & Martins, G. A. T cell immunity and the quest for protective vaccines against Staphylococcus aureus infection. Microorganisms 8, 1936 (2020).
Venkatasubramaniam, A. et al. Safety and immunogenicity of a 4-component toxoid-based Staphylococcus aureus vaccine in rhesus macaques. Front. Immunol. 12, 621754 (2021).
Krishnaswamy, J. K., Alsen, S., Yrlid, U., Eisenbarth, S. C. & Williams, A. Determination of T follicular helper cell fate by dendritic cells. Front. Immunol. 9, 2169 (2018).
McLellan, J. S. et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340, 1113–1117 (2013).
Simoes, E. A. F. et al. Prefusion F protein-based respiratory syncytial virus immunization in pregnancy. N. Engl. J. Med. 386, 1615–1626 (2022).
Yang, F. et al. Staphylococcus aureus alpha-toxin impairs early neutrophil localization via electrogenic disruption of store-operated calcium entry. Cell Rep. 42, 113394 (2023).
Bunce, C., Wheeler, L., Reed, G., Musser, J. & Barg, N. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 60, 2636–2640 (1992).
Acknowledgements
We thank Jacqueline Ansted and the Pediatric Sedation Service faculty and nurses at the University of Chicago for their support of pediatric clinical sample collections. This work was supported, in part, by the Bursky Center for Human Immunology and Immunotherapy Programs at Washington University, Immunomonitoring Laboratory. We thank Diane Bender, Roderick Lin, Olga Malkova, and Stephen Oh from the Bursky Center for support of Mass Cytometry Studies, Jaclyn Wright for insightful discussion on flow cytometric studies, and Lydia Wheat for review of the manuscript and editorial suggestions. This work was supported by NIH grants R01 AI097434 and R41 AI167137 (to J.B.W.) and R01 AI159677 and UL1RR024999 (to S.A.F. and J.B.W.).
Author information
Authors and Affiliations
Contributions
K.L.T., R.O., S.A.F., and J.B.W. designed the studies. K.L.T., M.B., R.O., H.R.B., and J.B.W. performed a laboratory-based analysis of Hla vaccine candidates, including all animal studies. H.R.B., S.H., A.C.D., S.A.F., and J.B.W. contributed to the collection, processing, and analysis of all human-derived samples. F.F., G.K.A., B-K.C., and Y.A.G. led studies to evaluate structural and biochemical attributes of the Hla antigens. K.L.T., R.O., S.A.F., and J.B.W. wrote the paper, which was reviewed and edited by all authors.
Corresponding author
Ethics declarations
Competing interests
J.B.W. has a financial agreement with Aridis Pharmaceuticals related to patents owned by the University of Chicago. K.L.T., R.O., and J.B.W. may receive royalty income based on technology that is currently owned by Washington University and subject to licensing by Forward Defense, LLC. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tomaszewski, K.L., Blanchard, M., Olaniyi, R. et al. Enhanced Staphylococcus aureus protection by uncoupling of the α-toxin-ADAM10 interaction during murine neonatal vaccination. Nat Commun 15, 8702 (2024). https://doi.org/10.1038/s41467-024-52714-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-52714-7