Abstract
Macrophages adopt distinct phenotypes in response to environmental cues, with type-2 cytokine interleukin-4 promoting a tissue-repair homeostatic state (M2IL4). Glucocorticoids (GC), widely used anti-inflammatory therapeutics, reportedly impart a similar phenotype (M2GC), but how such disparate pathways may functionally converge is unknown. We show using integrative functional genomics that M2IL4 and M2GC transcriptomes share a striking overlap mirrored by a shift in chromatin landscape in both common and signal-specific gene subsets. This core homeostatic program is enacted by transcriptional effectors KLF4 and the glucocorticoid receptor, whose genome-wide occupancy and actions are integrated in a stimulus-specific manner by the nuclear receptor cofactor GRIP1. Indeed, many of the M2IL4:M2GC-shared transcriptomic changes were GRIP1-dependent. Consistently, GRIP1 loss attenuated phagocytic activity of both populations in vitro and macrophage tissue-repair properties in the murine colitis model in vivo. These findings provide a mechanistic framework for homeostatic macrophage programming by distinct signals, to better inform anti-inflammatory drug design.
Similar content being viewed by others
Introduction
Macrophages are ubiquitous innate immune cells, named for their ability to engulf pathogens, dead cells, cell debris, and other foreign objects and initiate inflammatory and immune responses1. More recently, macrophages were recognized to broadly contribute to the maintenance of homeostasis through numerous, often tissue-specific functions, including phagocytosis of apoptotic cells in diverse tissues, iron recycling in the spleen and liver, and erythropoiesis in the bone marrow and fetal liver2. Macrophages differ ontogenically and principally originate from three sources during embryonic and fetal development: the yolk sac, the fetal liver, and the bone marrow3. In addition to developmental imprinting, macrophages are extremely plastic, with various signals in their microenvironment4, such as cytokines and growth factors, shaping distinct populations4,5,6. The ability of macrophages to adopt specialized phenotypes in response to different stimuli has been termed polarization7 and is central to many physiological and pathological processes, including wound healing, metabolic abnormalities, and cancer8.
A seminal study of macrophage gene expression patterns upon exposure to a diverse range of stimuli in vitro revealed that their transcriptomes exist along a spectrum of states; the opposite poles were represented by those enacted by proinflammatory lipopolysaccharide (LPS) and interferon-gamma (IFNγ) vs. type-2 cytokines IL4, 9 and 13, corresponding to historically described M1- (inflammatory) and M2-like (homeostatic) states, respectively9. One of the most well-studied pathways leading to the M2 phenotype is triggered by the cytokine IL4 (hereafter, M2IL4)10,11. IL4 binds to the receptor subunit IL4Rα12, leading to the IL4 receptor complex assembly and phosphorylation, followed by phosphorylation and nuclear translocation of the transcription factor STAT6 and induction of many STAT6 targets12,13. These include transcription factors EGR2 and PPARγ, as well as effector molecules: Chil3, involved in nematode clearance, Arg1, necessary for arginine to ornithine conversion, thus supporting collagen production and repair, and Mrc1, needed for phagocytosis of pathogens14. Kruppel-like factor 4 (KLF4), another transcriptional regulator and a downstream target of STAT6, is a key player in M2 polarization, critical for the upregulation of M2 and downregulation of inflammatory M1 genes15. Consistently, myeloid cell-specific KLF4 deletion in mice led to metabolic inflammation, predisposing them to diet-induced obesity, glucose intolerance, and insulin resistance, as well as impaired wound healing in vivo15. More recently, it has been shown that EGR2 bridges the early and late gene expression programs during M2 polarization14.
A distinct stimulus that promotes the homeostatic phenotype in macrophages is the glucocorticoids (GCs) – steroid hormones produced by the adrenal cortex that have remained the cornerstone of anti-inflammatory therapies for over 70 years16,17,18,19. GCs activate the glucocorticoid receptor (GR), a ligand-dependent transcription factor of the nuclear receptor superfamily, which, among hundreds of targets, represses pro-inflammatory and activates anti-inflammatory genes20,21. GR functions by engaging various coregulators, including GR-interacting protein 1 (GRIP1, NCoA2), which participates in both processes22,23,24,25. With respect to GC-polarized M2 (hereafter M2GC), glucocorticoid-induced leucine zipper (GILZ, Tsc22d3), a well-known GR target, enhances efferocytosis by macrophages, a hallmark of M2 activity, both in vitro and in vivo26. Several other genes – CD206, CD163, MerTK – known to be highly expressed in M2 – are reported GR transcriptional targets27,28,29. Nonetheless, the precise roles of GR or GRIP1 in generating the M2GC state have not been studied. Interestingly, KLF4 is itself a GR-regulated gene in macrophages30, suggesting that the M2IL4 and M2GC macrophage states are inherently connected. However, the interplay between KLF4 and GR in establishing the homeostatic state remains unexplored.
Here, we evaluate the transcriptomic and epigenomic landscape of the M2GC and M2IL4 populations of primary mouse macrophages. We comprehensively assess the extent of the functional convergence vs. distinctions between these differentially polarized macrophages at the level of transcription factor binding, chromatin state, and gene expression. Finally, we establish a shared transcriptional cofactor utilization as the underlying mechanism in the programming of homeostatic macrophages ex vivo and in vivo, in the mouse model of tissue damage.
Results
Overlapping yet distinct transcriptomic profiles of the M2IL4 and M2GC macrophage populations
To evaluate the transcriptomic make-up of M2IL4 and M2GC macrophages, we performed RNAseq on mouse primary bone marrow-derived macrophages (BMDM) exposed to IL4 or GCs: corticosterone (Cort, endogenous GC in rodents) and dexamethasone (Dex, highly potent, synthetic, clinically used GC) for 24 h. Transcriptome analysis revealed 720, 154, and 307 differentially expressed genes (DEGs) in M2IL4, M2Cort, and M2Dex, respectively, relative to M0 (fold change (FC) ≥ 2; FDR < 0.05) (Fig. 1a). As expected, the M2Cort transcriptome was fully included in that of M2Dex, and even though 157 of Dex-regulated genes failed to pass the significance threshold in M2Cort at n = 3, they displayed a similar pattern of regulation, as shown for representative genes (Supplementary Fig. 1a). This is consistent with Dex being a more stable and potent GR ligand than Cort. Importantly, whereas 628 and 215 genes were specific to M2IL4 and M2Dex macrophages, respectively, 92 genes – 30% of all DEGs in the M2Dex population – were also differentially expressed and changing in the same direction (36 up, 56 down) in the M2IL4.
a Transcriptomic changes in M0 macrophages polarized with IL4 or GCs corticosterone (Cort) or dexamethasone (Dex) for 24 h were determined by RNAseq (n = 3 biological replicates [mice]). Venn diagrams show DEGs in M2IL4 and M2GC relative to M0 (FC ≥ 2; FDR < 0.05); 92 of 133 genes are regulated in both M2IL4 and M2Dex in the same direction. b Volcano plots show DEGs in M2IL4 (left) and M2Dex (right) relative to M0 (LogFC ≥ 1; FDR < 0.05). Selected upregulated genes are highlighted in orange (M2IL4) and green (M2Dex). Downregulated genes are highlighted in red in both populations. Examples of the M2IL4:M2Dex shared DEGs are underlined. c RT-qPCR validation of genes upregulated selectively in M2IL4 (top), M2GC (middle), or both (bottom). One-tailed unpaired t test; p-values are shown; ns, non-significant (p ≥ 0.05). n = 4 biological replicates (mice), error bars are SEM. d Differentially regulated pathways (unadjusted p < 0.01; see ref. 71. equations 14-15) identified using QuSAGE and MsigDB canonical pathways (c2.cp.v7.3, Broad Institute) are shown for M2IL4 (top) and M2Dex (bottom). Underlined are the shared pathways between M2IL4 (upregulated, orange; downregulated, red) and M2Dex (upregulated, green; downregulated, red). Circle size is proportional to the number of genes in the pathway, and color signifies the p-value. e Genes from indicated pathways induced or repressed in M2IL4, M2Dex, or both are plotted as LogFC ± SD. Source data are provided as a Source data file.
Among DEGs upregulated in M2IL4 (n = 431) and M2Dex (n = 161), we found shared genes characteristic of the M2-like phenotype, encoding proteins with the anti-inflammatory, tissue homeostasis and repair functions (Fig. 1b, underlined: Klf4, Klf9, Chil3, Ccl24, and Mrc1). However, in each population, we also noted genes that were more signal-specific: Arg1, Chil4, Pparg, Egr2, and Retnla for M2IL4 and Fkbp5, Hif3a, Tsc22d3, and Zbtb16 for M2Dex (Fig. 1b and Supplementary Fig. 1b). The M2IL4-specific targets are well-established markers of the M2IL4 state; the M2Dex-specific genes include canonical GR targets. We validated genes induced selectively in M2IL4 (Arg1, Pparg, and Chil4), M2GC (Tsc22d3 (or Gilz), Hif3a, and Zbtb16) or both (Klf4, Klf9, and Mrc1) by quantitative PCR (Fig. 1c). The specificity of regulation for some genes was evident as early as 2 h of IL4 and GC treatment (see, for example, Arg1 vs. Hif3a and Zbtb16 in Supplementary Fig. 1c), but target gene transcripts either stabilized or continued to accumulate over time, consistent with the gradual establishment of the phenotype. M2 polarization often involves the suppression of the pro-inflammatory arm of gene expression. Indeed, the pool of downregulated genes (289 in M2IL4 and 146 in M2Dex) included components of the type 1 interferon network, chemokines, and pro-inflammatory mediators (Tnf, Cxcl14, Ifit1 and Irak3, common for M2IL4 and M2GC, and Ccl4 and Ccl5, specific for M2GC) typically upregulated in inflammatory macrophages (Fig. 1b and Supplementary Fig. 1b).
Concomitant induction of shared homeostatic genes and dampened expression of M1-specific ones suggests that macrophage polarization by both IL4 and GCs may rely on overlapping sets of regulatory pathways. Quantitative Set Analysis of Gene Expression (QuSage) revealed 677 and 364 pathways regulated in M2IL4 and M2Dex populations relative to M0 (p < 0.05; Supplementary Fig. 1d and Supplementary Data 1). Of those, 502 and 189, respectively, were M2IL4- and M2Dex-specific, whereas 142 pathways differentially regulated in M2Dex were also regulated in M2IL4 and in the same direction. Upon stratifying the shared pathways by the direction of change, 49 pathways were upregulated in both M2IL4 and M2Dex, and 93 pathways were downregulated in both (Supplementary Fig. 1d), indicating that an overlapping set of pathways were engaged during polarization by IL4 or GCs. Shared upregulated pathways in M2IL4 and M2GC included Lipid Metabolism, Regulation of Autophagy, Negative Regulation of FLT3 and MET activities and MTOR signaling (Fig. 1d, Supplementary Fig. 1e, underlined and Supplementary Data 1). Among signal-specific pathways, the IL4 and Arginine and Proline metabolism-related pathways were selectively upregulated in M2IL4, whereas EGFR_SMRTE pathway and Transcriptional Regulation of Pluripotent Stem Cells were specific to M2Dex and M2Cort (Fig. 1d, Supplementary Fig. 1e and Supplementary Data 1). As expected, multiple inflammatory pathways, including the NLRP3 inflammasome, Toll-like receptor (TLR) cascades, FLMP, and LTF danger signal response pathways, were downregulated in both M2IL4 and M2GC (Fig. 1d, e and Supplementary Fig. 1e), consistent with the immuno-suppressive and anti-inflammatory phenotypes of M2 macrophages (or the effect of IL4 or GC treatment). Previously validated signal-specific genes were found in M2-specific pathways. For example, Arg1 and Egr2 were upregulated specifically in M2IL4, whereas genes involved in transcriptional regulation of pluripotent stem cells such as Hif3a and Klf4 were upregulated in the M2Dex, confirming that the key population-specific markers are associated with functionally relevant pathways (Fig. 1e). Notably, downregulated pathways that were signal-specific, such as the NFkB-related NTHI pathway in the M2IL4 (encompassing Tnf, Nfkbia, Myd88, and RelA) or Chemokine and Chemokine receptors in M2GC population (including Ccl3, Ccl4, Ccl5, Cx3cr1, Ccr7, Cxcl2) (Supplementary Fig. 1f) were still functionally related to inflammation and innate immune responses. Collectively, these findings indicate the existence of overlapping gene expression programs elicited in the M2IL4 and M2GC broadly related to anti-inflammatory, homeostatic, and tissue reparative functions.
IL4 and GCs drive overlapping changes in the macrophage enhancer landscape
To investigate the mechanism underlying transcriptomic overlap between M2IL4 and M2GC macrophage populations, we surveyed accessible chromatin regions using ATACseq. The genomic distribution of ATACseq peaks was comparable in M0, M2IL4, and M2Dex with accessible chromatin mainly observed in the gene body (introns and exons), intergenic regions and promoters (Supplementary Fig. 2a). Out of 71,942 peaks total, we detected 31,654 and 16,379 differential peaks in the M2IL4 and M2Dex populations, respectively, relative to M0 (FC ≥ 2; FDR = 0.05; Fig. 2a). A two-fold higher number of the differential sites in M2IL4 is as expected based on the larger transcriptomic changes occurring in response to IL4 compared to GCs. Out of all differential sites, 23,441 and 8166, respectively, were M2IL4- and M2Dex-specific (Fig. 2a). Interestingly, 4913 differentially regulated peaks in the M2Dex population were also regulated in M2IL4 and in the same direction (Fig. 2a), consistent with an observed overlap in the transcriptomic profiles of the M2IL4 and M2GC populations.
a Venn diagram shows the number of differential ATACseq peaks in M2IL4 and M2Dex relative to M0 (n = 4 biological replicates, FC ≥ 2; FDR < 0.05). 4913 of 8213 peaks differential in both populations were regulated in the same direction. b Heatmaps and numbers of the induced (red) and suppressed (blue) M2IL4 and M2Dex signal-specific and shared ATACseq peaks. c, d Enriched transcription factor binding motifs with associated p-values were identified by HOMER known motif analysis with JASPAR 2022 motif database in signal-specific and shared up- (c) and downregulated (d) ATACseq peaks in M2IL4 and M2Dex. Binomial distribution was used for p-value calculation, and no adjustments were made for multiple comparisons. e Polarization-induced increases in the 5’ Tn5 cut site counts within indicated binding motifs in the M2IL4 and M2Dex populations. The error band is a 95% confidence interval for LOESS smoothing; span = 0.025. f Venn diagram shows the differential M2IL4 and M2Dex H3K27ac ChIPseq peaks relative to those in M0 (n = 3 biological replicates, FC ≥ 2; FDR < 0.05) with 1304 of shared 1667 peaks regulated in the same direction in both populations. g Heatmaps of induced (red) and suppressed (blue) signal-specific and shared filtered H3K27ac peaks (FC ≥ 2; FDR < 0.05) in M2IL4 and M2Dex. h Volcano plots show differential H3K27ac ChIPseq peaks for M2IL4 (left) and M2Dex (right) relative to those in M0 (LogFC ≥ 1; FDR < 0.05); the location of selected hyperacetylated sites relative to the TSS of the closest gene are shown in orange (M2IL4) or green (M2Dex). Hypoacetylated sites are shown in red for each population. Sites overlapping in M2IL4 and M2Dex are underlined. i Non-promoter 12,211 H3K27ac peaks after excluding 1650 promoter-proximal peaks (− 300/ + 200 relative to TSS) are shown as a Venn diagram with the number of differential, relative to M0, M2 population-specific, and shared peaks (FC ≥ 2; FDR < 0.05) indicated. j Average H3K27ac ChIPseq signals at M2IL4 (top), M2Dex (middle), and shared (bottom) differential non-promoter peaks from (I) represented as violin plots. Box-plot insets show median and interquartile range (IQR), and whiskers are extended to 1.5*IQR.
Specifically, we identified 13,039 and 4807 chromatin regions that became more accessible only in M2IL4 or M2Dex, respectively and 2127 peaks that ‘opened’ in both M2-like populations (Fig. 2b, red). At the same time, 10,442 peaks in M2IL4, 3359 peaks in M2Dex, and 2786 peaks in both populations (blue) ‘closed’. These findings indicate that IL4 and GC impart broad changes in chromatin accessibility, with a subset of sites affected in both populations. Transcription factor binding motif enrichment analysis was performed for the ATACseq peak subsets separated by the direction of change and stimulus. A group of motifs from the AP1, ETS, and C/EBP families were enriched in all peak subsets (Fig. 2c, d), likely representing lineage-determining transcription factors involved in cell proliferation and differentiation31. Binding motifs for transcription factors that orchestrate the M2 polarization in response to IL4, namely STAT6, KLF4, and EGR1/2, were enriched in M2IL4 accessible peaks (Fig. 2c). Similarly, the GR-specific GRE motif is enriched, as expected, in the M2Dex peak set with increased accessibility. In shared upregulated peaks, both STAT6 and GRE motifs were enriched. Furthermore, a binding motif for NFIL3, a transcription factor upregulated by both IL4 and GC signaling, was enriched in all 3 peak subsets with increased accessibility in polarized macrophages. Importantly, analysis of Tn5 cut site distribution near overrepresented motifs (Fig. 2e) revealed changes in site accessibility in both M2IL4 (STAT6, EGR2) and M2Dex (NR3C2/GRE, CEBPE), indicating that the observed enrichment was functional. Interestingly, the NFkB-p65 motif appeared in M2Dex and M2IL4:M2Dex shared downregulated clusters (Fig. 2d), consistent with the well-established repression of the NFkB activity by GR21. Together, motif enrichment analysis suggested enhanced binding of M2-specific transcription factors in opening chromatin sites, while motifs for inflammation-driving factors were found in less accessible chromatin in both signal-specific and shared peak subsets.
Histone modifications are typically more stable than transcription factor binding and indicate longer-lived activating or repressive changes in chromatin state32. Among them, acetylation of histone H3 lysine 27 (H3K27ac) is commonly recognized as an enhancer mark found in active chromatin regions33. Thus, we performed chromatin immunoprecipitation (ChIPseq) for H3K27ac in M0, M2IL4, and M2GC to investigate polarization-induced acetylation changes at enhancers and/or promoters. Differential analysis of H3K27ac data shows that 12,298, 1192, and 3222 peaks were regulated in M2IL4, M2Cort, and M2Dex populations, respectively, relative to M0 (Supplementary Fig. 2b). As expected, nearly all differential peaks detected in M2Cort overlapped with those in M2Dex, consistent with transcriptomic changes, and we therefore used only M2Dex for subsequent analyses. Polarization with IL4 and Dex produced 10,639 and 1555, respectively, treatment-specific differential H3K27ac peaks (Fig. 2f). In addition, we identified 1667 peaks overlapping in M2IL4 and M2Dex populations, of which 1304 (40% of total M2Dex differential peaks) were regulated in the same direction (Fig. 2f), consistent with the ATACseq and transcriptomic data.
Figure 2g shows the distribution of M2IL4-specific, M2Dex-specific, or shared peaks when split into hyper-(red) or hypoacetylated (blue) subsets. Reflecting the trend seen in the ATACseq data, many more of the shared sites were hypoacetylated. Importantly, putative shared enhancers included those near ‘hallmark’ M2 genes (Klf9, Klf4, and Chil3; underlined), whereas M1-like genes (Irak3, Card11, Slamf9, and CD300e) lost associated H3K27ac marks (Fig. 2h). At the same time, other peaks associated with M2 genes gained H3K27ac marks in a signal-specific manner (Pparg and Egr2 for M2IL4 and Tsc22d3 and Ms4a4a for M2Dex), suggesting that the distinctions in chromatin landscape between the two populations correlate with population-specific transcription profiles. We validated hyperacetylation at the enhancer regions of key M2 target genes Arg1, Klf9, and Chil3 by ChIP-qPCR (Supplementary Fig. 2c).
To focus specifically on enhancer elements potentially controlling transcriptional output in M2IL4 and M2Dex populations, we excluded 1650 differential acetylation sites that overlapped transcription start sites (TSS: − 300/ + 200, Fig. 2i). The analysis of the remaining 12,211 peaks revealed an even greater overlap between M2IL4 and M2Dex: 42% of all M2Dex differential enhancers overlapped with those seen in the IL4-polarized macrophages. We then stratified signal-specific and shared enhancers by the direction of regulation relative to those in M0 (Fig. 2j). Interestingly, and further corroborating unfiltered acetylation data in Fig. 2g, among the M2IL4:M2Dex common enhancers, we detected over twice as many hypoacetylated enhancers as the hyperacetylated ones (827 vs. 390).
To better understand upward and downward changes in H3K27 acetylation, we performed rank shift analysis conditional on treatment. We ranked combined M2Dex and M2IL4 differential peaks by mean H3K27ac signal (inverse log2CPM) in M0 and M2IL4 macrophages and plotted peak rank in M0 vs. the difference in peak ranks between M2IL4 and M0 (Supplementary Fig. 2d). We used LOESS regression to visualize the relationships between acetylation strength in M0 and signal change in M2IL4 (green line). Although the rank gain in M2IL4 macrophages is larger in magnitude, a larger fraction of peaks lost acetylation signal upon polarization. Interestingly, peaks with large rank change were associated with genes with large expression change in M2IL4 (Supplementary Fig. 2d, right panel). Finally, as also indicated in Fig. 2f, g, i, j, and consistent with transcriptomic and chromatin accessibility data, most shared differential peaks in M2IL4 and M2Dex change in the same direction, suggesting that genome-wide convergence of IL4- and GC-dependent programming occurs at multiple regulatory levels.
Epigenomic and transcriptomic programming are tightly correlated in both M2IL4 and M2GC macrophage populations
The expression of M2IL4- and M2Dex-specific (e.g., Chil4 and Hif3a) and the M2IL4:M2Dex shared (e.g., Klf9) genes tracked closely with changes in chromatin accessibility and H3K27 acetylation (Fig. 3a), which prompted us to systematically examine genome-wide relationships between gene expression and chromatin state during macrophage polarization by IL4 and GC. We, therefore, selected all differential ATACseq and H3K27ac ChIPseq peaks in − 20 Kb to + 20 Kb windows centered on TSS. We first stratified genes (TSS windows) by the gene status in our RNAseq (Fig. 1a) into differentially expressed in M2IL4 and/or M2Dex vs. non-differential. We subsampled non-differential genes to create random sets of the same size as differential gene sets (n = 10). For each gene in either group, we determined the number of associated ATACseq or H3K27ac peaks and selected peaks with the largest (positive or negative) logFC from M0 to either M2IL4 or M2Dex. We observed that polarization-driven changes in both ATACseq and H3K27ac peaks, associated with differential genes, corresponded to the direction of change in gene expression (Fig. 3b). The upregulated genes in M2IL4 and M2Dex populations showed larger signal gain in ATACseq and H3K27ac peaks compared to non-differential genes, whereas downregulated genes exhibited greater loss of ATACseq and H3K27ac ChIPseq signal (Fig. 3b).
a Correlation between RNAseq, ATACseq and H3K27ac ChIPseq signals in IL4-specific Chil4, GC-specific Hif3a and shared Klf9 in each population. b Genome-wide changes in up- or downregulated differential ATACseq (left) and H3K27ac ChIPseq (right) peaks located within – 20 K + 20 K window centered on the TSS of RNAseq-differential or -non-differential genes in M2IL4 and M2Dex, as indicated. c, d Comparison of TSS window-associated ATACseq (c) or H3K27ac ChIPseq (d) peaks in differential (teal) vs. non-differential (gray) genes in M2IL4 (left panels) and M2Dex (right panels), as indicated, stratified by the number of gene-associated peaks. Only peaks with the largest positive (max) or negative (min) changes relative to M0 are shown. A subset of 92 shared up- (red) and downregulated (blue) DEGs identified by RNAseq (Fig. 1) are labeled in each of the 4 panels. e Correlation between normalized H3K27ac ChIPseq and ATACseq signals in the TSS-proximal – 1 K + 1 K windows for differential (teal) and non-differential (gray) genes. A subset of 92 shared upregulated (red) and downregulated (blue) DEGs identified by RNAseq (Fig. 1) are labeled.
We further stratified genes (TSS windows) by the number of associated ATACseq and H3K27ac ChIPseq peaks and plotted them against the largest / smallest normalized peak signals for the corresponding genes in M2IL4 and M2Dex populations. Notably, both M2IL4 (Fig. 3c, d and Supplementary Fig 3a, b, left) and M2Dex (Fig. 3c, d and Supplementary Fig. 3a, b, right) DEGs (teal) were overwhelmingly associated with the larger number of dynamic ATACseq and H3K27ac peaks compared to non-differential genes (gray). Indeed, many of the M2IL4:M2Dex-shared (92) as well as M2IL4 (587)- and M2Dex (174)-specific DEGs identified by RNAseq (Fig. 1a) including shared upregulated (Fig. 3c, d: Ms4a4a, Mrc1, Ccr1, Klf9 and Klf4), shared downregulated (Fig. 3c, d: Cd69, Tnf, Igsf9), M2IL4-unique (Supplementary Fig. 3a and b: Itgax, Msx3, Il1r1) and M2Dex-unique (Supplementary Fig. 3a and b: Fkbp5, Pi3ip1, Il15ra, Tsc22d3) were associated with dynamic ATACseq and H3K27ac peaks. Although not all DEGs were associated with differential ATACseq and H3K27ac peaks, these data reveal a remarkable level of convergence between polarization-driven chromatin accessibility and H3K27 acetylation vs. transcriptomic programming.
To compare M2 polarization-driven chromatin and transcriptional changes directly, we selected all 9323 genes expressed in any condition in our RNAseq experiment and counted the reads overlapping TSS-centered − 1Kb /+ 1Kb regions in both ATACseq and H3K27ac ChIPseq experiments. Using this generated TSS-proximal count matrices, we performed separate differential, relative to M0, analyses in M2IL4 and M2Dex for ATACseq and H3K27ac to identify genes whose TSS-proximal regions undergo changes in accessibility and acetylation status during M2 polarization. This analysis revealed a striking correlation between chromatin accessibility, H3K27 acetylation, and gene expression in both M2IL4 and M2Dex (Fig. 3e and Supplementary Fig. 3c–f). Indeed, M2-like M2IL4:M2Dex-shared (Klf9, Mrc1, and Nfil3) and signal-specific (Arg1 and Chil4 for M2IL4 and Zbtb16 and Hif3a for M2Dex) genes gained H3K27ac mark that also corresponded to more open chromatin, whereas M1-like genes (Tnf, Ifit3, and Pilra) lost both ATACseq peaks and associated H3K27ac mark (Fig. 3e and Supplementary Fig. 3c–f). Furthermore, the Ingenuity Pathway Analysis (QIAGEN) of all M2IL4 and M2Dex DEGs associated with gain in the ATACseq and H3K27ac signals, revealed enrichment of pathways linked to known M2 functions such as cellular movement, immune cell trafficking, and cellular function and maintenance (Supplementary Fig. 3g and Supplementary Data 2). Collectively, these analyses suggest that transcriptomic changes that occur during macrophage polarization by IL4 and Dex are tightly correlated with changes in enhancer landscape and overall chromatin accessibility.
Inducible co-localization of GR, KLF4, and transcriptional coregulator GRIP1 across the genome
IL4 and GCs act through distinct receptors in unrelated protein families and initiate signaling events that have little in common. Therefore, we reasoned that the striking overlap in the transcriptomic profiles of M2IL4 and M2GC is generated through the common utilization of cofactors at the level of downstream effector transcription complexes. GR is a classic ligand-dependent nuclear receptor that relies on the p160 family of coregulators – NCoA1, 2, and 334 – of which NCoA2 (GRIP1/TIF2/SRC2 – hereafter GRIP1) has been implicated both in GR-driven gene activation and repression in various cell types including macrophages20,35,36. Interestingly, we previously described an unexpected interaction between GRIP1 and KLF4, and their co-recruitment to Arg1 and Klf4 genes in IL4-treated macrophages37. We therefore asked whether GRIP1 functioned globally to integrate GC- and IL4-driven M2 polarization. To begin to dissect genome-wide relationships between GR, KLF4, and GRIP1, we first performed GR ChIPseq in M0, M2IL4, M2Dex, and M2Cort populations. As expected, GR peaks in M2Dex and M2Cort fully overlapped, and GR binding was strictly GC ligand-dependent with no specific peaks detected in M2IL4 (Fig. 4a and Supplementary Fig. 4a), including near genes that were transcriptionally activated in response to both IL4 and GCs (e.g., Klf9 in Fig. 4c). Transcription factor binding motif enrichment analysis showed that the dominant motif in both M2Dex and M2Cort GR cistromes was the GRE/ARE (NR3C nuclear receptor binding motif; Supplementary Fig. 4b).
a Venn diagram shows the numbers and overlap of GR ChIPseq peaks in M2Dex and M2Cort macrophages relative to M0 baseline (n = 4 biological replicates [mice]; FC ≥ 2, FDR < 0.05). b Venn diagram shows the numbers and overlap of (left) GRIP1 CUT&RUN peaks across populations (n = 3 biological replicates; FC ≥ 1.5, FDR < 0.05) or GRIP1 peaks in M2IL4 (middle) or M2Dex (right) with all KLF4 peaks (n = 2 biological replicates), as indicated. c GR (ChIPseq), KLF4 (CUT&RUN) and GRIP1 (CUT&RUN) read density distribution over Chil4, Hif3a and Klf9 loci in M0, M2IL4 and M2Dex. d Average profiles of GR, KLF4, and GRIP1 signal from each macrophage population centered on GRIP1 CUT&RUN M2IL4- (left), M2Dex- (middle) specific or invariant ‘static’ (right) peaks (n = 3 biological replicates; FC ≥ 2, FDR < 0.05). e, f Transcription factor binding motif enrichment Z-scores in KLF4 (e) and GRIP1 (f) peak subsets in M2IL4, M2Dex, and M0 macrophage populations, as indicated, were determined in chromVAR from the HOMER list of motifs. Motifs with high variability among the subsets (padj ≤ 1*10−6) were plotted, and indicated motif families are highlighted in boxes.
We then employed CUT&RUN to assess the genomic occupancy of GRIP1 and KLF4 in differentially polarized macrophage populations. GRIP1 bound extensively across the genome in M0 macrophages, with the majority of peaks maintained during polarization (Fig. 4b, left; 347 present in M0 only of the 16,372 total GRIP1 peaks). Polarization induced thousands of additional GRIP1 binding sites (Fig. 4b, left; 2216 for M2IL4 and 1133 for M2Dex with 37 peaks overlapping). We generated a KLF4 peak set from those present in either polarized population (19,217 peaks) to assess its overlap with the GRIP1 binding. Strikingly, the vast majority of GRIP1 peaks, 10,617 of a total of 14,702 in M2IL4 and 9345 of 12,866 in M2Dex, overlapped with at least one KLF4 peak (Fig. 4b, right). Although the total putative binding sites for GRIP1 and KLF4 generated by CUT&RUN considerably exceeded the ChIPseq dataset for GR, we still found that 44% of KLF4 intersections and 27% of GR intersections involve GRIP1 peaks (Supplementary Fig. 4c).
Given the dramatic overlap between GRIP1 occupancy and that of the two transcription factors driving the M2 phenotype in our differentially polarized macrophages, we evaluated the binding of all three proteins near M2IL4-specific Chil4, M2Dex-specific Hif3a and shared Klf9 in all three populations. No GR or GRIP1 peaks and minimal KLF4 binding were detected near these genes in M0 macrophages, demonstrating the signal specificity of these binding events (Fig. 4c). As expected, in M2Dex, GRIP1 was recruited to GR-bound sites of Hif3a and Klf9 – the same sites that gained H3K27ac mark and peaks of chromatin accessibility (Fig. 3a). Also consistent with chromatin activation, in M2IL4, KLF4 was recruited to the acetylated Chil4 enhancer, together with GRIP1 (Fig. 4c, orange tracks for KLF4 and GRIP1). Similarly, KLF4 is also bound to two sites upstream of Klf9, and the promoter-proximal site was co-occupied by GRIP1. Of note, the upstream KLF4 peak in M2IL4 appeared to co-localize with the GR peak in M2Dex, perhaps pointing to the adjacent binding sites for the two factors. Surprisingly, KLF4 was also recruited in Dex-polarized macrophages (Fig. 4c, green tracks for KLF4) to the GR:GRIP1-co-occupied sites of Hif3a and Klf9, as well as promoter-proximal Klf9 site not bound by GR, yet still recruiting GRIP1. These observations suggest that, unlike strictly GC-dependent actions of GR, KLF4 and GRIP1 occupancy is much more dynamic and ‘permissive’, and that upregulation of KLF4 expression by both signals, combined with GRIP1 binding by both GR and KLF4 creates the chromatin environment that facilitates the shared epigenetic and transcriptional programs induced by the two pathways.
Consistent with this idea, when quantified in condition-specific GRIP1 peak subsets, both KLF4 and GRIP1 signals were stimulation-specific for both M2IL4 and M2Dex, and the GR signal was only enriched in M2Dex macrophages and was present in Dex-induced GRIP1 peaks only (Fig. 4d, left and middle columns). In contrast, no obvious bias for KLF4 or GR enrichment in any population was seen in ‘static’ GRIP1 peaks (Fig. 4d, right column).
Taken at face value, our data suggested that long-term exposure to GCs induces KLF4 upregulation and recruitment to DNA, potentially contributing to polarization with IL4 should both signals be present together. To test this idea, we exposed macrophages to IL4, Cort, Dex vs. IL4 + Cort or IL4 + Dex together and assessed the expression of the candidate signal-specific and shared DEGs by RT-qPCR. Interestingly, GR-specific targets Gilz and Per1 were similarly upregulated in M2GC and M2IL4+GC populations with no apparent contribution of IL4 (Supplementary Fig. 4d). In contrast, both shared (Klf9 and Chil3) and, surprisingly, M2IL4-specific (Arg1 and Msx3) genes were induced better in the M2 IL4+Cort and M2IL4+Dex than in the M2IL4 population (Supplementary Fig. 4d), implicating GR signaling in acting cooperatively with IL4 to induce the M2 phenotype.
We then focused on transcription factor motifs enriched in the KLF4 and GRIP1 cistromes. As expected, KLF family binding motifs were broadly over-represented in the KLF4 peaks independent of stimulus origin (Supplementary Fig. 4e). Importantly, binding sites for distinct families of transcription factors were enriched in different macrophage populations. In both KLF4 and GRIP1 cistromes, the NR3C/GRE motif was enriched specifically in GC-polarized macrophages (Fig. 4e, f and Supplementary Fig. 4f). This finding is particularly interesting for the KLF4 cistrome, as it further underscores the co-localization of KLF4 and GR binding sites. A similar pattern was seen for lineage-determining ETS family and, perhaps surprisingly, IRF (Fig. 4e, f). Conversely, the KLF motif was over-represented in M2IL4 and to a lesser degree in M2Dex GRIP1 peaks and colocalized with GREs in GRIP1 M2Dex peaks (Fig. 4f and Supplementary Fig. 4f). Interestingly, a binding motif for STAT6 – a direct downstream effector of IL4 signaling – was enriched selectively in M2IL4 GRIP1 peak subsets and was found in M2Dex GRIP1 peaks containing both GR and KLF4 motifs (Fig. 4f and Supplementary Fig. 4f). Together, these experiments reveal broad, yet specific co-localization of GR:GRIP1 and KLF4:GRIP1 transcription complexes across the genome in differentially polarized macrophage populations.
GRIP1 facilitates the establishment of M2IL4 and M2GC transcriptomes
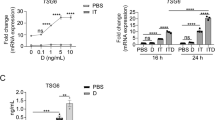
Given that GRIP1 is a transcriptional coregulator that colocalized with KLF4 and GR at regulatory regions associated with many M2-enriched genes, we examined its contribution to IL4- and GC-induced transcriptional programming. We assessed gene expression in M0, M2IL4, M2Dex and M2Cort derived from mice of two genotypes: WT (LysM-Cre+/+; GRIP1wt/wt) and GRIP1 cKO (LysM-Cre+/+; GRIP1fl/fl) lacking GRIP1 in the myeloid lineage37 and, hence, in all four polarized macrophage populations (Fig. 5a). RT-qPCR revealed (Fig. 5b) that in GRIP1-deficient macrophages, the induction of individual candidate M2 genes was dramatically reduced irrespective of whether they were M2IL4-specific (top), M2GC-specific (middle) or shared (bottom).
a, b Expression of (a) GRIP1 or (b) IL4- (top) GC- (middle) or shared (bottom) M2 target genes in WT and GRIP1 cKO M0, M2IL4, M2Cort, and M2Dex populations as assessed by RT-qPCR. The average relative expression of each transcript in WT M0 is arbitrarily set to 1. n = 4 biological replicates [mice]; One-tailed unpaired t test; p-values are indicated; ns, non-significant (p ≥ 0.05). Error bars are SEM. c Stacked bar plot shows the number of DEGs (FC ≥ 1.5) in GRIP1 cKO as a fraction of WT M2IL4, M2Dex, or shared DEGs relative to M0 (RNAseq, WT: n = 3, cKO: n = 4 biological replicates). d GRIP1 peaks from Fig. 4b were annotated to their closest gene using ChIPseeker, and overlap with GRIP1-independent (n = 1516) or -dependent (n = 432) DEGs from (c) was determined for all unique peaks. A bar graph shows the % (and number) of genes in each subset associated with IL4- and/or Dex-induced peaks. The P-value for overrepresentation was determined by bootstrapping samples from the whole GRIP1 peak atlas to determine how often a set of N peaks would contain the observed overlap with each gene list by chance. e Volcano plot shows DEGs in GRIP1 cKO vs. WT in M2IL4 (left) and M2Dex (right) relative to M0 of each genotype. (FC ≥ 1.5, unadjusted p-value < 0.05). Selected inflammation-related genes expressed at higher levels in the cKO M2IL4 and M2Dex relative to WT are highlighted in red. Key M2 target genes downregulated in the cKO are highlighted in both M2IL4 (orange) and M2Dex (green). Examples of the shared M2IL4:M2Dex GRIP1 target genes are underlined. f Differentially regulated pathways (unadjusted p < 0.01 defined as in Fig. 1d) identified using QuSAGE with MsigDB canonical pathways subset (Broad Institute) are shown for GRIP1 cKO vs. WT in M2IL4 (left) and M2Dex (right). Select pathways are labeled (upregulated, red; downregulated, orange for IL4 and green for Dex); M2IL4:M2Dex-shared pathways are underlined. Circle size is proportional to the number of genes in the pathway, and color signifies the p-value. Source data are provided as a Source data file.
To obtain a more accurate assessment of the genome-wide impact of GRIP1 deficiency and focus on genes that lose most of their transcriptional response to polarizing signals, we adjusted the RNAseq cut-off from 2-fold (log2FC ≥ 1) to 1.5-fold (log2FC ≥ 0.6). Under these conditions, we identified 1288 and 465 DEGs, relative to M0, in WT M2IL4 and WT M2Dex, respectively, and 269 genes that were shared (Fig. 5c). Loss of GRIP1 attenuated the regulation of 184 (or 14%) in the M2IL4 transcriptome. A much greater fraction − 151 genes (or 32%) - in the M2Dex transcriptome was GRIP1-dependent. This is consistent with a well-established function of GRIP1 as a GR cofactor, implicated in both GR-mediated repression and activation in vivo20,36. Interestingly, the fraction of GRIP1-dependent genes was highest for the M2IL4:M2Dex-shared transcriptome: 95 (35%) of the shared DEGs required GRIP1 for their regulation. The same pattern was seen at log2FC ≥ 1 (2-fold) cut-off, with the fraction of GRIP1-dependent genes now reaching 42% of the shared transcriptome (Supplementary Fig. 5a).
To relate the requirement for GRIP1 in macrophage transcriptomic changes during polarization to GRIP1 genome-wide binding, we assessed the frequency of association between IL4- and/or GC-inducible GRIP1 peaks with GRIP1-independent (n = 1516) and -dependent (n = 432) DEGs using peak annotation to the closest gene. We found that genes annotated to IL4- and/or Dex-inducible GRIP1 peaks were highly enriched for GRIP1-dependent genes (p < 10−5); overall, 83 (19%) of the GRIP1-dependent genes were associated with inducible GRIP1 peaks (Fig. 5d). The enrichment of GRIP1-independent genes annotating to inducible GRIP1 peaks was statistically marginal (p = 0.02; Fig. 5d); 15% of these genes had inducible GRIP1 peaks. Interestingly ‘static’ GRIP1 peaks were associated with 64% of either GRIP1-dependent or GRIP1-independent genes. Thus, genes whose up- or down-regulation during M2 polarization required GRIP1 were more likely to be associated with sites of inducible GRIP1 recruitment.
The differential gene expression analysis revealed that GRIP1 contributes to the expression of key orchestrators of M2 programming in each population. Among M2IL4-specific DEGs were major transcription factors known to confer the M2IL4 state (e.g., Egr2), as well as other signature M2IL4 genes (Arg1, Retnla) (Fig. 5e, left), suggesting the functional significance of this gene-set despite its relatively modest size. Similarly, GRIP1 was required for the full induction of M2GC-specific genes, e.g., Htr1b and Zbtb16 (Fig. 5e right and Supplementary Fig. 5b). Importantly, many overlapping M2 signature DEGs, e.g., Klf4 and Mrc1 were GRIP1-dependent, consistent with GRIP1 contributing to the regulation of the large portion of the shared M2IL4:M2GC transcriptome (Fig. 5e and Supplementary Fig. 5b, underlined). Interestingly, the expression of a subset of genes related to the inflammatory M1 phenotype, such as Irak3, Tlr2, and Tnf, was higher in GRIP1 cKO M2IL4, M2Dex, and M2Cort transcriptomes relative to WT (Fig. 5e and Supplementary Fig. 5b), indicating that in all three cases, GRIP1 contributed to the repression of these genes.
GRIP1-regulated genes form distinct pathways along the spectrum of macrophage polarization, encompassing both the homeostatic and pro-inflammatory functions. QuSAGE analysis demonstrated dampening of the M2 pathways in M2IL4 and M2GC in the GRIP1 cKO (Fig. 5f and Supplementary Fig. 5c and Supplementary Data 3). For the M2IL4 population, the Arginine and Proline Metabolism (hallmark of metabolic activity of M2IL4) and Regulation of Autophagy (associated with M2 phagocytic activity) were downregulated (Fig. 5f, left). The GC-specific EGFR SMRTE_Pathway was downregulated in the cKO M2GC macrophages (Fig. 5f, right and Supplementary Fig. 5c). In contrast, the pathways associated with inflammatory signatures were upregulated in all three cKO populations (Fig. 5f and Supplementary Fig. 5c).
Because both IL4 and Dex induced dramatic changes in macrophage chromatin accessibility during M2 polarization (Fig. 2a), we asked whether GRIP1 contributes to alteration in the chromatin environment in M2IL4 and/or M2Dex. We performed ATACseq in WT and GRIP1 cKO which showed that overall peak distribution across the genome was similar in WT and cKO in both M2IL4 and M2Dex populations (Supplementary Fig. 5d). Genome-wide comparison of ATACseq peaks in M2IL4 and M2Dex between the two genotypes yielded relatively modest subsets of uniquely IL4- and Dex-regulated peaks (329 and 457, respectively) and 400 shared differential peaks that were affected by loss of GRIP1 (Supplementary Fig. 5e). Among those, 395 and 414 sites were ‘upregulated’ (failed to close) in cKO M2IL4 and cKO M2Dex, respectively, compared to matching WT populations; conversely, 334 and 443 peaks, respectively, were ‘downregulated’ (failed to open) in cKO M2IL4 and cKO M2Dex (Supplementary Fig. 5f). Collectively, these data suggest that GRIP1 is required for establishing the transcriptional state in M2IL4 and M2Dex populations; its exact contribution to de novo IL4- and GC-driven changes in chromatin accessibility remain to be determined.
GRIP1 contributes to the phagocytic activity of M2IL4 and M2GC in vitro and tissue healing in the mouse dextran sulfate sodium (DSS)-induced colitis in vivo
Phagocytic activity is a hallmark function of M2 macrophages, needed for engulfing apoptotic cells and cell debris38. We set out to test if GRIP1 is necessary for phagocytosis in M2IL4, M2Dex, and M2Cort. When exposed to bioparticles that fluoresce when internalized into the acidic phagosome, WT macrophages polarized with either IL4 or GCs exhibited approximately 20% relative increase of phagocytosis in vitro (Fig. 6a). Interestingly, GRIP1 deletion dramatically reduced their phagocytic activity compared to the WT. Indeed, M2IL4 GRIP1 cKO macrophages reverted to the baseline phagocytic activity (equal to that of M0), whereas M2GC showed an attenuated induction. We conclude that GRIP1 contributes to the upregulation of phagocytic activity in both M2IL4 and M2GC.
a Relative fluorescence intensity of WT and GRIP1-cKO M2IL4, M2Cort, and M2Dex relative to that in WT M0 (set to 100%) after exposure to pHrodo Green S. aureus BioParticles (see “Methods”). Shown are mean ± SEM (n = 3). b Histopathological changes assessed by H&E staining of colons from WT and cKO mice 2 wks after initiating DSS-induced colitis. Grades 1, 2, and 3 are defined in Methods. Scale bar = 20 μm. Plotted is the total area representing grade 3 for colon specimens of WT and GRIP1-cKO. Shown are mean ± SEM (n = 4). c The expression of indicated genes in total RNA from colons of WT and cKO mice was measured by RT-qPCR. The expression of each gene was normalized to β-actin, and then the fold difference to baseline, non-treated (calibrant) value was calculated for WT or cKO. Shown are mean ± SEM (WT n = 7 (Il23) 10 (Mrc1), 11 (Cd163), 12 (Il10, Tnf), 13 (Klf4, Arg1, Il1b), 14 (Pparg) or 15 (Grip1, Klf9); cKO n = 5 (Il23), 6 (Mrc1, Il10), 7 (Pparg, Arg1), 8 (the rest)). d, e Cells were isolated from the large intestine lamina propria at day 11, and single-cell suspensions incubated in FACS buffer with conjugated antibodies to CD45-PE, CD11b-PE-Cy7, Ly6C-PerCP-Cy5.5, Ly6G-APC, and CD206-AF700. Dead cells were excluded using DAPI. Cell sorting was performed on Symphony S6 (BD Biosciences; see Supplementary Fig. 6d for FACS strategy), and (d) data analyzed with FlowJo v10.10 software. Ly6C+, CD206+, and Ly6C+CD206+ cells were pooled, total RNA was isolated, and expression of indicated genes was assessed by RT-qPCR (e). Shown are mean ± SEM (n = 4). P-values are indicated; ns, non-significant (p ≥ 0.05). In a–e, n = the number of independent biological replicates (mice), and significance is determined by a one-tailed unpaired t test. Source data are provided as a Source data file.
We next tested whether GRIP1 promotes the homeostatic tissue-repair macrophage phenotype in vivo in an acute inflammatory setting. We chose the DSS-induced colitis model, since virtually all macrophages in the colon are derived from monocytic progenitors39, rendering the model consistent with our reliance on BMDM in vitro. In addition, over the duration of the model, both inflammatory and tissue-repair macrophages function simultaneously, circumventing the complexities of interpreting changes in the sequential recruitment of macrophage populations. When exposed to 2% DSS-containing water for 10–14 days, both WT and GRIP1 cKO mice displayed loss of body weight and colon shortening (Supplementary Fig. 6a). The histopathological features of colitis were graded as: (1) well defined crypts and villi with preserved mucus secretion and a mild lymphocytic inflammation; (2) the villi are short and distorted, crypts and goblet cells are diminished, apparent hyperplasia of glandular and superficial epithelium; (3) loss of crypts, ulceration of mucosa, severe inflammation and heavy infiltration of lymphocytes, macrophages and granulocytes (Fig. 6b). Importantly, GRIP1 cKO mice developed significantly greater tissue destruction as total area occupied by the most severe grade 3 damage was doubled over that seen in the WT (Fig. 6b). Consistently, gene expression analysis of total colonic RNA revealed that expression of many typical M2 genes including Klf4, Klf9, Mrc1 and Arg1 was significantly attenuated in the GRIP1 cKO mice relative to their WT counterparts (Fig. 6c), resembling the results of GRIP1 deficiency in vitro in the M2IL4 and M2GC populations (Fig. 5b and c). Unexpectedly, the inflammatory genes known to be involved in colitis progression (Tnf, Il1b, Il23) were expressed at similar levels in both genotypes (Fig. 6c). No differences in body weight, colon length, histology, and baseline colonic gene expression were seen between WT and GRIP1 cKO mice at homeostasis (Supplementary Fig. 6a–c). To focus specifically on immune cells responsible for the differences between the DSS-treated WT and cKO mice, we have prepared single-cell suspensions from their colons at the peak of the disease (day 11) and stained them with conjugated antibodies to compare individual cell populations between genotypes using fluorescence-activated cell sorting (FACS). Our analysis revealed an expansion of Ly6C+ inflammatory (M1-like) macrophages among the CD45+CD11b+Ly6G- myeloid cells, whereas CD206+ homeostatic (M2-like) macrophages were present at very low numbers at this late stage of the disease and trended further down in the GRIP1 cKO mice (Fig. 6d and Supplementary Fig. 6e), as predicted. We observed no genotype-specific difference in the fraction of Ly6G+ neutrophils (Fig. 6d and Supplementary Fig. 6e); and immunofluorescence revealed no difference in total CD3+ T-cells and B220+ B-cells – cell populations not affected by the myeloid-specific Cre (Supplementary Fig. 6d). Strikingly, gene expression analysis in the pool of Ly6C+ and CD206+ macrophages revealed a dramatic reduction in the M2 genes Arg1, Klf9, and Klf4 with no increase in inflammatory Il1a or Il1b (Fig. 6e), corroborating our data from total colons (Fig. 6c). We conclude that the greater susceptibility of GRIP1 cKO mice to DSS-mediated colonic tissue destruction, at least at the end-point of the disease, is due to the failure of cKO macrophages to repair the damage, rather than increased inflammation in the colon. Thus, GRIP1's contribution to extensive transcriptomic rewiring of macrophages during the establishment of their homeostatic state is required for their adequate tissue repair properties in vivo.
Discussion
Phenotypic similarity between macrophages exposed to type-2 cytokines and those treated with GCs, including their phagocytic activities40,41, wound healing, and tissue repair functions42,43, has been recognized for a long time in both human and murine systems. This was further supported by early microarray studies, which juxtaposed the transcriptomic features of these in vitro produced ‘M2’ populations to more traditionally inflammatory ‘M1’ macrophages19,44. The binary model of macrophage activation was later challenged in a seminal study by Xue et al., which highlighted the remarkable plasticity of macrophage responses, whereby dozens of stimuli yielded a wide spectrum of transcriptomic states9,45. Nonetheless, macrophages elicited by type-2 cytokines and GCs still populated the same ‘pole’ of the spectrum, farthest away from that elicited by inflammatory stimuli such as IFNγ and LPS19. Here, we aimed to understand whether and how two highly divergent biological signals – IL4 and GC – trigger converging polarization programs, the epigenomic wiring that enforces these programs and the mechanisms that underlie their establishment.
We focused on stable changes that occur by 24 h of IL4/GC exposure. We reasoned that, unlike rapid transcriptional responses of genes in open chromatin, often preloaded with fully engaged, initiated RNA Polymerase II21,46,47, epigenomic changes accumulate more slowly and are more indicative of the macrophage transition into a novel phenotypic state. Indeed, a recent study classified enhancers in IL4-treated macrophages into ‘early transient,’ ‘early sustained,’ and ‘late’14. All three groups exhibited p300 and H3K27ac gain, detectable at 24 h of IL4 exposure only for the sustained and late enhancers. Interestingly, after filtering out promoters from the H3K27ac dataset, we obtained 5248 IL4-induced enhancers, comparable to the total of 7309 sustained and late enhancers reported by Daniel et al. 14. Furthermore, that study showed that a subset of the activated enhancers are occupied by the histone reader BRD4 and elongating RNA Polymerase II, suggesting that histone bookmarking and active enhancer transcription both signify the establishment of the M2IL4 state. In addition, the transcription factor EGR2 was proposed to be required for the acquisition of the late enhancer networks10,14 – and we detected EGR2 upregulation, the enrichment of EGR2 binding sites in the opening chromatin and activation of Egr2-associated enhancers specifically in M2IL4. Interestingly, the STAT6 motif was overrepresented among the open motifs in M2IL4 and showed increased 5’ Tn5 cut upon IL4-induced polarization. This was unexpected in light of earlier observations that recruitment of STAT6 – a direct effector of IL4 signaling – largely fades away by 24 h, while EGR2 remains bound14. This may indicate that the chromatin changes near STAT6 binding sites persist long beyond transcription factor occupancy and that STAT6 provides a layer of specificity early on during the IL4-induced polarization process. The observed enrichment of STAT6 motifs, specifically in the M2IL4 GRIP1 peak subset, would be consistent with this idea.
Unlike these exciting developments in our understanding of the epigenomic pathways driving macrophage responses to IL4, little is known about the long-term impact of GCs on macrophage programming. GC signaling elicits profound anti-inflammatory effects in vivo, which made synthetic GCs some of the top prescribed medications worldwide48. In the context of macrophages, the therapeutic efficacy of these molecules has been historically attributed to broad transcriptional repression of genes encoding inflammatory cytokines, chemokines, tissue degrading enzymes, and other mediators of inflammation and tissue destruction49. Indeed, many pro-inflammatory genes were downregulated in our M2Dex and hundreds of associated chromatin sites were losing accessibility and the H3K27ac mark. Surprisingly, however, an overall greater number of genes and associated enhancers, including those with established M2-related functions (e.g., Cebpb50, Jdp251, NOTCH452 and MerTK53) were uniquely activated in M2Dex. In motif enrichment analysis, the GRE and the binding site for CEBP – an established GR-cooperating factor54 were not only over-represented in open peaks in the M2Dex but were more enriched in the Dex-polarized population relative to M0, indicating that many of the associated genes were direct transcriptional targets of GR – an observation further supported by GR ChIPseq.
Perhaps a larger surprise was the level of convergence between the M2IL4 and M2GC populations, both in their chromatin states and their transcriptomes. Out of all the DEGs, acetylated enhancers, and sites of opening chromatin in M2Dex, 30–50% overlapped with those regulated by IL4 and included hallmark M2 genes that confer the homeostatic macrophage phenotype, e.g., Mrc1, Chil3, and Nfil3. In addition, we report a direct correlation between the transcriptomic and epigenomic changes in a 3-way comparison, which held true for both shared and signal-specific DEGs in each population. This suggests that the core M2 transcriptome is relatively stable, with the transcriptomic changes likely to persist for extended periods of time.
Some population-specific DEGs orchestrate population-specific functions. Arg1, encoding arginase 1, drives the hydrolysis of arginine to urea and ornithine, thus promoting polyamine synthesis and decreasing nitric oxide production (a hallmark of M1-like phenotype)55. Since Arg1 upregulation was strictly IL4-specific, we anticipate that this metabolic shift does not occur in M2Dex. Conversely, several M2GC-specific DEGs are canonical GR targets, known to suppress inflammation (e.g., Tsc22d3) or regulate GR activity (e.g., Fkbp5)21. At the same time, shared targets, like Mrc1, are directly involved in phagocytosis, consistent with the increase in phagocytic activity in both M2IL4 and M2GC macrophages.
Given that IL4 and GC signaling pathways are dramatically different, we reasoned that their functional convergence likely occurs at the level of chromatin and target gene regulation. Indeed, GR and KLF4 binding sites have been reported to colocalize throughout the genome30,56; KLF4 itself is upregulated by both IL4 and GC. Here we show that GRIP1, a nuclear receptor cofactor, mediates the transcriptional integration of the two states with 35–42% of the DEGs shared between M2IL4 and M2Dex requiring GRIP1 for their regulation. We also observed a striking overlap between the GRIP1 and KLF4 cistromes which, unexpectedly, extended to GRIP1 peaks and DEGs specific to the M2Dex population. Several mechanisms can potentially account for GRIP1-assisted KLF4 binding in GC-polarized macrophages. Given the proximity of GR and KLF binding sites, GRIP1 recruitment by liganded GR may result in an increase in the local GRIP1 concentration and cofactor ‘spill-over’, enabling GRIP1 to bind and stabilize KLF4 on the DNA (Supplementary Fig. 7). Moreover, like other p160 family members, GRIP1 serves as a platform for many histone modifiers, such as acetyltransferases (HATs) CBP/p300 or arginine methyl transferases (CARM1)57,58, which would extend the regions of permissive chromatin to KLF4 binding sites (Supplementary Fig. 7). In fact, such cooperative recruitment may in turn further facilitate nucleosome remodeling59. In addition, we envision such mechanisms to be broadly applicable to distant higher-order 3D chromatin interactions, thus expanding the network of GRIP1-regulated genes.
One unexpected observation across different readouts was that the overlap between the M2IL4 and M2Dex included more downregulated DEGs and inactivated enhancers than the upregulated ones. Thus far, all our comparisons were relative to unstimulated M0, whose characteristics may not fall into the center of the imaginary spectrum separating fully inflammatory ‘M1’ macrophages from the homeostatic ‘M2’. Many actively expressed M2-like genes and enhancers are likely to already be acetylated. This baseline bias is also supported by the lack of prominent inflammatory signature among downregulated genes, relatively mild enrichment of the NFkB motif in deacetylated peaks, and the absence of NFkB binding site over-representation in the GR cistrome, which in M1-like macrophages are populated by GR through physical interactions with p6521. Thus, our analysis likely underestimates the extent of transcriptional and epigenomic shift between the M1 and M2 populations, and the true overlap between the M2IL4 and M2Dex populations when they undergo this transition.
Finally, we acknowledge that the goal of this study – as well as its limitation – was to obtain a granular view of how a single stimulus, IL4 or GCs, rewires the epigenomic and transcriptional landscape. In vivo, macrophages encounter multiple signals, including type-1 vs. type-2 cytokines, TLR ligands, hormones, or dead cells simultaneously, so their responses would reflect the compounded effects of disparate stimuli. Some of these scenarios have already been modeled in vitro38,60,61,62. In our transcriptomic studies, a subset of genes (n = 41) were regulated by IL4 and Dex in opposite directions, implying a potential antagonism, whereas the rest of the shared genes may display additive or synergistic relationships, as has been recently proposed63. The degree of cooperativity, which we illustrate with a few examples, will ultimately depend on the strength of each signal (e.g., ligand and cytokines concentration), its duration, and the prior macrophage state. Our DSS-colitis transcriptional data suggest that the defective tissue repair function of GRIP1 cKO macrophages rather than the uncontrolled inflammation was a dominant contributor to the phenotype at the end-stage of the disease. We do not, however, rule out the possibility that failure to contain inflammatory responses – consistent with the increased frequency of the Ly6C+ macrophages in GRIP1 cKO mice, with our in vitro data and with established function of GRIP1 in the context of GC signaling20 – aggravates earlier stages of disease. Future studies are required to untangle the complex and potentially timed relationships between macrophage responses and to determine the stability of their epigenomically programmed functional states. In particular, whether pharmacological manipulation of GR or other key regulators can impart to a macrophage a long-lived non-inflammatory phenotype remains an important question for clinical care.
Methods
Mice
C57BL/6 mice (National Cancer Institute, Charles River Laboratories) and their transgenic derivatives were maintained in the Weill Cornell Animal Facility in compliance with the protocol approved by the Institutional Animal Care and Use Committee. All mice were housed in separate ventilated cages (maximum 5 mice/cage) and maintained on a 12/12-h light/dark cycle, 22–26 °C, 40–70% humidity with sterile pellet food and water ad libitum. Homozygous LysM-WT (LysM-Cre;GRIPwt/wt) and GRIP1-cKO (LysM-Cre;GRIPfl/fl) mice were generated previously37. For all tissue and cell collection, mice were euthanized via CO2 inhalation. C57BL/6 mice were used for ChIPseq experiments. WT and GRIP1-cKO mice were used for RT-qPCR, RNAseq, ATACseq, phagocytosis, and colitis experiments. All mice used were 8- to 10-week-old males.
Cell culture
BMDM were prepared as previously described46. Briefly, bone marrow was flushed from the femurs and tibiae of mice and differentiated for 5 days in the DMEM (1 g/l glucose) supplemented with 20% bovine serum and 10% L-cell conditioned media. Adherent cells were scraped, seeded into 150 mm or 6-well plates, and polarized for 24 h with 10 ng/ml IL4, 500 nM Cort, or 100 nM Dex unless otherwise indicated.
RNA isolation and RT-qPCR
Total RNA from macrophages treated as described in Figure legends or from FACS-sorted colonic immune cells was isolated using the Qiagen RNAeasy Plus Mini kit. Total colon RNA was isolated using Trizol (Life Technologies) reagent, and gene expression was assessed using the standard curve method for relative quantification. RNA samples were subjected to random-primed cDNA synthesis (using High-Capacity cDNA Reverse Transcription kit [Thermo Fisher] for sorted colonic immune cells), and gene expression was analyzed by qPCR with Maxima Sybr Green/ROX/2x master mix (Thermo ScientificTm) on StepOne Plus real-time PCR system (Applied Biosystems) using standard protocols, and data was analyzed using δδCt method with b-actin transcript as housekeeping control. Primers are listed in Supplementary Table 1.
RNAseq
The Quality of total RNA was assessed using Agilent BioAnalyzer 2100 for RNA integrity. Samples with RIN > 8 were used for library preparations. Sequencing libraries were prepared using the NEBNext Ultra II Directional RNA Library Prep Kit for Illumina, following the manufacturer’s instructions. Libraries quality was evaluated using Agilent BioAnalyzer 2100, and the libraries were sequenced by a NovaSeq 6000 system (50 bp, paired-end) at the Genomics Resources Core Facility at Weill Cornell College of Medicine at 15–25 mln reads per sample. All experiments were performed in at least triplicates.
ATACseq
ATACseq was performed as described64,65 with minor modifications. Briefly, 105 cells were lysed in ATAC lysis buffer [10 mM Tris-HCl (pH 7.4), 10 mM NaCl, 3 mM MgCl2, and IGPAL CA-630] to isolate nuclei. The resultant nuclei were resuspended in the transposase reaction mix (25 μL x TD buffer (Nextera DNA Sample Preparation Kit), 5 μL Illumina Tn5 transposase, and 22.5 μL nuclease-free water and incubated at 37 °C on a thermomixer at 1000 rpm for 30 min. The transposed DNA was purified using a QIAGEN MinElute Purification Kit, then amplified using NEBNext High-Fidelity 2X PCR Master Mix and a unique ATAC indexing primer for 10 cycles. Post-amplification libraries were double-size-selected for the removal of primer dimers and DNA fragments (> 1000 bp). The amplified libraries were quality-controlled by Agilent BioAnalyzer and pooled. 50-bp paired-end sequencing was performed on an Illumina NovaSeq 6000 system at the Genomics Resources Core Facility at Weill Cornell Medicine. All experiments were performed in quadruplicates.
ChIP-qPCR and ChIPseq
Macrophages were crosslinked with 1% methanol-free formaldehyde (Thermo) for 10 min (H3K27ac) or double-crosslinked with 2 mM disuccinimidyl glutamate (Proteochem c1104) for 30 min, followed by 1% methanol-free formaldehyde for 10 min at RT (GR)36. Nuclear extracts were prepared21, and chromatin DNA was sonicated using Bioruptor Pico (Diagenode, 8 cycles, 30 s on/off bursts) to generate DNA fragments ~ 200–700 bp. After sonication, 10% of nuclear extract was saved as input and rest incubated with protein A/G agarose (Santa Cruz) for 30 min at 4 °C. Precleared extracts were incubated with 40 μL of 50% protein A/G agarose and 5 μg of normal rabbit IgG (Santa Cruz Biotech), H3K27ac (Active Motif 39133), GR (Thermo Fisher Scientific, PA1-511A) at 4 °C overnight. The following day, beads were washed 8x with modified RIPA buffer containing 500 mM LiCl and 2x with TE + 50 mM NaCl buffer. DNA was extracted after reversing crosslinks and purified with a Qiagen PCR purification kit. The resulting DNA was analyzed by qPCR (primer sequences are listed in Supplementary Data 1) with 28S as an internal control, and occupancies expressed relative to the normal IgG baseline. For ChIPseq, libraries were prepared using the NEBNext Ultra II Library Prep Kit for Illumina (NEB), size selected (150–500 bp), and PCR-amplified for 15 cycles. Libraries were sequenced at the Genomics Resources Core Facility at Weill Cornell Medicine using NextSeq500 (75 bp single-end).
CUT&RUN
CUT&RUN assay was performed using the EpiCypher CUTANATM ChIC/CUT&RUN kit v2.1 according to the manufacturer’s protocol. In brief, 106 cells were harvested for each condition, washed with 1X PBS, and resuspended in wash buffer, followed by incubation at RT for 10 min with activated Concanavalin A (ConA) beads. Cells were then permeabilized and incubated overnight on a nutator at 4 °C with 1 μg of normal rabbit IgG (EpiCypher), KLF4 (Proteintech, 1180-1-AP), or GRIP1 (Novus Biologicals, NB100-1756) antibody. Cell-bead antibody complex was then washed with cell permeabilization buffer and incubated with pAG-MNase (EpiCypher), followed by addition of 2 mM CaCl2 and incubation for 2 h at 4 °C. Stop buffer was added to the reaction mixture and DNA fragments were released and purified with a CUTANA DNA purification kit (EpiCypher). DNA library construction was performed using the NEBNext Ultra II DNA Library Prep Kit from NEB (E7645L). The libraries were sequenced on the Illumina NovaSeq 6000 system (50 bp paired-end) at the Genomics Resources Core Facility at Weill Cornell Medicine.
Phagocytosis assay
Macrophages were seeded into a 96-well plate (80,000 cells/well) and polarized with IL4, Cort, or Dex for 24 h. After removing the media, 0.75 mg/ml pHrodo S. aureus Green BioParticles (ThermoFisher Scientific, P35367) were added for 1 h at 37 °C. Before imaging, macrophages were stained with 1 μM Hoescht dye for 5 min at 37 °C. The plates were read on the ImageXpress Micro Confocal Imager at the Weill Cornell Microscopy and Image Analysis Core. Fluorescence puncta count and intensity were measured and normalized to the macrophage count for each image. Background fluorescence intensity and puncta count from macrophages alone and BioParticles alone were subtracted.
Acute DSS colitis histopathology
Mice were given 2 % dextran sulfate sodium (DSS) (MP Biomedicals) in drinking water, evaluated daily for body weight changes, and sacrificed when at least 1 mouse lost 20 % of body weight (11–20 days). Colons were harvested and processed for histopathology or total RNA isolation. The colon tissues were fixed in 10 % formalin, paraffin-embedded, and histopathological changes of colons were assessed (Tania Pannellini, HSS) using H&E staining (Laboratory of Comparative Pathology, MSKCC) and graded as follows: Grade 1 – well-defined crypts and villi; mucus secretion is preserved; mild lymphocytic inflammation; Grade 2 – villi are short and distorted, crypts are progressively lost, goblet cells are diminished, hyperplasia of glandular and superficial epithelium; Grade 3 – crypts are lost, ulceration of mucosa, severe inflammation of mucosa and submucosa, heavy infiltration of lymphocytes, macrophages and granulocytes. The surface area occupied by histopathological changes of each grade was quantified. Immunofluorescence and quantitative analysis for B220+ B-cells and CD3+ T-cells was performed at the Molecular Cytology core facility at MSKCC using anti-CD3 (Abcam, ab135372) and anti-B220 (BD Biosciences, 553086) antibodies.
Immune cell isolation from colon tissue and FACS
Cells were isolated from the large intestine lamina propria as previously described66. Briefly, the mouse's large intestine was washed in cold PBS and cut into 1-2 cm pieces. Subsequently, the tissue was washed with 1 mM DTT (Sigma-Aldrich) and 30 mM EDTA with shaking (200 rpm for 10 min at 37 °C). The tissue was then digested in RPMI-10% FBS containing 200 U/ml collagenase VIII (Sigma-Aldrich) and 150 µg/ml DNase I (Sigma-Aldrich) on a shaker for 1 h at 37 °C. After digestion, the tissue and cells were centrifuged at 600 × g for 5 min at room temperature, resuspended in 40% Percoll (Sigma-Aldrich), and layered on top of 80% Percoll, followed by centrifugation at 1300 × g for 20 min at room temperature. The cells at the interface of the 40%/80% Percoll gradient were collected and washed with FACS buffer (PBS-2% FBS).
For cell sorting, single-cell suspensions were incubated with conjugated antibodies [CD45-PE (BioLegend 103106), CD11b-PE-Cy7 (BioLegend 101215), Ly6C-PerCP-Cy5.5 (BioLegend 128011), Ly6G-APC (BD Pharmingen 560599), and CD206-AF700 (BioLegend 141733)] in FACS buffer and Fc receptors were blocked with anti-CD16/32 antibody (BioLegend 101301) for 30 min at 4 °C. Dead cells were excluded using DAPI. Sorting was performed on Symphony S6 (BD Biosciences, Weill Cornell Flow Cytometry Core Facility), and data was analyzed with FlowJo v10.10 software.
Data analysis
RNAseq
Read quality filtering and adapter trimming were performed with fastp 0.23.267. Filtered reads were mapped to the mouse genome (mm10) with the STAR aligner 2.7.068, and exonic reads were counted against GENCODE release 27 annotations with featureCount69 using a customized pipeline available at https://gitlab.com/hssgenomics/pipelines/RNAseq. Differential gene expression analysis was performed with edgeR 3.38.470 using the quasi-likelihood framework. Genes with low expression levels (< 2 counts per million in at least one group) were filtered from all downstream analyses. P-values were adjusted to correct for multiple testing using the Benhamini-Hochberg FDR procedure. Genes with adjusted p-values < 0.05 and log2 fold change > 1 were considered differentially expressed. Downstream analyses were performed in R using a Shiny-driven visualization platform (RNAseq DRaMA, 1.4.2, https://gitlab.com/hssgenomics/Shiny) developed at the HSS David Z. Rosensweig Genomics Research Center. Differentially regulated pathways were identified using R QuSAGE 2.30.0 package71 with MSigDB C2 set (curated gene sets c2.cp.v7.3). All gene sets with less than 10 genes were not included in the analysis. Pathways with unadjusted p < 0.05 were selected for initial analysis and were further filtered with FDR < 0.1 to correct for multiple testing.
ATACseq
Paired-end reads are pre-processed using fastp, which supports adapter trimming, low-quality base trimming, and calculation of additional quality control metrics. Trimmed reads were aligned to the mm10 genome using bowtie272 with --very-sensitive-local -q -p options set. Low quality, improper, multimapping alignments, and PCR duplicates are filtered using SAMtools73. Peaks are called on each sample (replicas are not pooled) with MACS274 using macs2 callpeak --nomodel --shift −100 --extsize 200 -q 0.05 options. After peak calling, all peaks from all samples called separately were combined, and adjacent peaks (within 10 bp) were merged. Reads from each sample within the combined set of peaks are then counted using featureCounts to produce an initial reads-per-peak count matrix. The ATACseq/ChIPseq pipeline is publicly available at https://gitlab.com/hssgenomics/pipelines/ATACseq.
The quality of individual experiments was evaluated by calculating the Fraction of reads in Peaks (FRiP), fragment size distribution, and analyzing MACS2 peak statistics distributions (peak width, pileup, and enrichment over the background). Furthermore, to ensure consistency, read-in-peak pairwise Spearman correlations were determined within experimental groups. The average within-group correlation was larger than 0.95 (Supplementary Data 4).
The reads-per-peak counts were analyzed for differential peaks using the edgeR quasi-likelihood framework, which allows for building complex modeling solutions not attainable by peak calling algorithms at the peak calling step (e.g., with MACS2 directly). Before the analysis, we removed poorly reproducible peaks containing zero reads in more than 3 samples within a group and peaks with a length of more > 1000 base pairs. Modeling the replicated ATACseq data using the counts-per-peak framework allows us to calculate logFC, p-values, and FDR for each peak. All peaks were annotated to the nearest gene and TSS, if the peak overlaps an annotated Fantom575 enhancer, that enhancer is reported. Peaks with FDR < 0.05 were considered differential.
We used HOMER31 to identify overrepresented transcription factor motifs in peak assemblages. To identify genomic locations of individual binding motif identified by HOMER we used matchMotifs function from R motifmatchr package and position weight matrices (PWM) from JASPAR 202276. The 5’ Tn5 cut site counts within binding motifs of interest and surrounding genomics region (− 250/ + 250) were normalized to the library size and to the local background outside motif-containing peaks using bamProfile function R bamsignals package77.
ChIPseq
Single-end reads were preprocessed as described in the ATACseq section. Peaks are called on individual samples without replica pooling using macs2 callpeak macs2 --broad --bw 180 --keep-dup all --q 0.05 with IgG input as a control. Following peak calling, peaks were merged, and reads, and peaks were counted with featureCount as described in the ATACseq section. Differential peaks and overrepresented binding motifs were identified as described in the ATACseq section. Sequencing QC is provided in Supplementary Data 4.
CUT&RUN
Samples were processed using an in-house nextflow-based pipeline available at https://github.com/michaelbale/jlabflow. Briefly, reads were trimmed using the Cutadapt wrapper trim-galore and mapped to mm10 with bowtie2 using --very-sensitive-local with a maximum insert size of 1000 bp. The initial bam file was then filtered for a minimum MAPQ of 30. Subsequently, PCR duplicates and reads mapping to the mitochondrial chromosome or within regions from a unified forbidden list from ENCODE and78,79. Finally, only correctly mated mapped reads were retained. Peak calls were made using MACS2 on each individual sample and merged using the IDR framework within sample conditions in an iterative manner, then merged across sample conditions for GRIP1 or retaining peaks that reciprocally overlapped each other’s lengths by at least 30% for KLF4. Finally, TMM-normalized signal tracks were visualized using deeptools bamCoverage with a calculated scaleFactor derived from the edgeR package calcNormFactors function.
Differential peak analysis was carried out using DESeq2, and all peaks with an FDR ≤ 0.05 were called differential. Average profile plots within differential regions were generated using deeptools computeMatrix and plotProfile with the TMM-normalized signal track bigwigs. The upset plot was generated using the BedSect web app with input peak sets as indicated.
Global peak-set motif enrichment was done as described with HOMER. The motif heatmaps were generated by calculating deviation z-scores from the chromVAR package with the homer_pwms HOMER motifs from chromVARmotifs. Motifs with significant variability (padj ≤ 1*10−6) were retained for heatmaps. Sequencing QC is provided in Supplementary Data 4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequencing data discussed in this study have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE247223 (RNA-seq); [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE247447] (ChIPseq); GSE248042 (ATACseq); GSE250376 (CUT&RUN). Source data are provided in this paper.
References
Germic, N., Frangez, Z., Yousefi, S. & Simon, H.-U. Regulation of the innate immune system by autophagy: monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 26, 715–727 (2019).
Davies, L. C., Jenkins, S. J., Allen, J. E. & Taylor, P. R. Tissue-resident macrophages. Nat. Immunol. 14, 986–995 (2013).
Perdiguero, E. G. & Geissmann, F. The development and maintenance of resident macrophages. Nat. Immunol. 17, 2–8 (2016).
Locati, M., Curtale, G. & Mantovani, A. Diversity, mechanisms and significance of macrophage plasticity. Annu. Rev. Pathol. 15, 123 (2020).
Wynn, T. A. & Vannella, K. M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462 (2016).
Glass, C. K. & Natoli, G. Molecular control of activation and priming in macrophages. Nat. Immunol. 17, 26–33 (2015).
Murray, P. J. Macrophage polarization. Annu. Rev. Physiol. 79, 541–566 (2017).
Shapouri-Moghaddam, A. et al. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 233, 6425–6440 (2018).
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014).
Hoeksema, M. A. et al. Mechanisms underlying divergent responses of genetically distinct macrophages to IL−4. Sci. Adv. 7, eabf9808 (2021).
Daniel, B. et al. The nuclear receptor PPARγ controls progressive macrophage polarization as a ligand-insensitive epigenomic ratchet of transcriptional memory. Immunity 49, 615–626.e616 (2018).
Junttila, I. S. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 9, 888 (2018).
Czimmerer, Z. et al. The transcription factor STAT6 mediates direct repression of inflammatory enhancers and limits activation of alternatively polarized macrophages. Immunity 48, 75–90.e76 (2018).
Daniel, B. et al. The transcription factor EGR2 is the molecular linchpin connecting STAT6 activation to the late, stable epigenomic program of alternative macrophage polarization. Genes Dev. 34, 1474–1492 (2020).
Liao, X. et al. Kruppel-like factor 4 regulates macrophage polarization. J. Clin. Invest. 121, 2736–2749 (2011).
Coutinho, A. E. & Chapman, K. E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell Endocrinol. 335, 2–13 (2011).
Vandewalle, J., Luypaert, A., De Bosscher, K. & Libert, C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 29, 42–54 (2018).
Barnes, P. J. Glucocorticosteroids. Handb. Exp. Pharm. 237, 93–115 (2017).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 (2004).
Chinenov, Y. et al. Role of transcriptional coregulator GRIP1 in the anti-inflammatory actions of glucocorticoids. Proc. Natl. Acad. Sci. USA 109, 11776–11781 (2012).
Sacta, M. A. et al. Gene-specific mechanisms direct glucocorticoid-receptor-driven repression of inflammatory response genes in macrophages. Elife 7, e34864 (2018).
Rogatsky, I., Zarember, K. A. & Yamamoto, K. R. Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J. 20, 6071–6083 (2001).
Chinenov, Y., Sacta, M. A., Cruz, A. R. & Rogatsky, I. GRIP1-associated SET-domain methyltransferase in glucocorticoid receptor target gene expression. Proc. Natl. Acad. Sci. USA 105, 20185–20190 (2008).
Hong, H., Kohli, K., Garabedian, M. J. & Stallcup, M. R. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol. Cell. Biol. 17, 2735–2744 (1997).
Voegel, J. J. et al. The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J. 17, 507–519 (1998).
Vago, J. P. et al. Glucocorticoid-induced leucine zipper modulates macrophage polarization and apoptotic cell clearance. Pharm. Res. 158, 104842 (2020).
van de Garde, M. D. et al. Chronic exposure to glucocorticoids shapes gene expression and modulates innate and adaptive activation pathways in macrophages with distinct changes in leukocyte attraction. J. Immunol. 192, 1196–1208 (2014).
Garabuczi, E., Sarang, Z. & Szondy, Z. Glucocorticoids enhance prolonged clearance of apoptotic cells by upregulating liver X receptor, peroxisome proliferator-activated receptor-delta and UCP2. Biochim. Biophys. Acta 1853, 573–582 (2015).
Shepherd, V. L., Konish, M. G. & Stahl, P. Dexamethasone increases expression of mannose receptors and decreases extracellular lysosomal enzyme accumulation in macrophages. J. Biol. Chem. 260, 160–164 (1985).
Chinenov, Y., Coppo, M., Gupte, R., Sacta, M. A. & Rogatsky, I. Glucocorticoid receptor coordinates transcription factor-dominated regulatory network in macrophages. BMC Genomics 15, 656 (2014).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Bannister, A. J. & Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 21, 381–395 (2011).
Creyghton, M. P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 107, 21931–21936 (2010).
Johnson, A. B. & O’Malley, B. W. Steroid receptor coactivators 1, 2, and 3: critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol. Cell Endocrinol. 348, 430–439 (2012).
Rogatsky, I., Luecke, H. F., Leitman, D. C. & Yamamoto, K. R. Alternate surfaces of transcriptional coregulator GRIP1 function in different glucocorticoid receptor activation and repression contexts. Proc. Natl. Acad. Sci. USA 99, 16701–16706 (2002).
Rollins, D. A. et al. Glucocorticoid-induced phosphorylation by CDK9 modulates the coactivator functions of transcriptional cofactor GRIP1 in macrophages. Nat. Commun. 8, 1739 (2017).
Coppo, M., Chinenov, Y., Sacta, M. A. & Rogatsky, I. The transcriptional coregulator GRIP1 controls macrophage polarization and metabolic homeostasis. Nat. Commun. 7, 12254 (2016).
Bosurgi, L. et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076 (2017).
Yip, J. L. K., Balasuriya, G. K., Spencer, S. J. & Hill-Yardin, E. L. The role of intestinal macrophages in gastrointestinal homeostasis: Heterogeneity and Implications in Disease. Cell Mol. Gastroenterol. Hepatol. 12, 1701–1718 (2021).
McColl, A. et al. Glucocorticoids induce protein S-dependent phagocytosis of apoptotic neutrophils by human macrophages. J. Immunol. 183, 2167–2175 (2009).
Balce, D. R. et al. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood 118, 4199–4208 (2011).
Galuppo, P. et al. The glucocorticoid receptor in monocyte-derived macrophages is critical for cardiac infarct repair and remodeling. FASEB J. 31, 5122–5132 (2017).
Jayme, T. S. et al. Human interleukin-4-treated regulatory macrophages promote epithelial wound healing and reduce colitis in a mouse model. Sci. Adv. 6, eaba4376 (2020).
Martinez, F. O., Gordon, S., Locati, M. & Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 (2006).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Adelman, K. et al. Immediate mediators of the inflammatory response are poised for gene activation through RNA polymerase II stalling. Proc. Natl. Acad. Sci. USA 106, 18207–18212 (2009).
Yu, L. et al. Negative elongation factor complex enables macrophage inflammatory responses by controlling anti-inflammatory gene expression. Nat. Commun. 11, 2286 (2020).
Reichardt, S. D. et al. The role of glucocorticoids in inflammatory diseases. Cells 10, 2921 (2021).
Escoter-Torres, L. et al. Fighting the fire: Mechanisms of inflammatory gene regulation by the glucocorticoid receptor. Front. Immunol. 10, 1859 (2019).
Ruffell, D. et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 106, 17475–17480 (2009).
Puig-Kroger, A. et al. Folate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 69, 9395–9403 (2009).
Lopez-Lopez, S. et al. NOTCH4 potentiates the IL-13 induced genetic program in M2 alternative macrophages through the AP1 and IRF4-JMJD3 axis. Int. Immunol. 35, 497–509 (2023).
Li, Z. H. et al. Mesenchymal stem cells promote polarization of M2 macrophages in mice with acute-on-chronic liver failure via Mertk/JAK1/STAT6 signaling. Stem Cells 41, 1171–1184 (2023).
Grontved, L. et al. C/EBP maintains chromatin accessibility in liver and facilitates glucocorticoid receptor recruitment to steroid response elements. EMBO J. 32, 1568–1583 (2013).
Rath, M., Muller, I., Kropf, P., Closs, E. I. & Munder, M. Metabolism via Arginase or Nitric Oxide synthase: Two competing arginine pathways in macrophages. Front. Immunol. 5, 532 (2014).
Sevilla, L. M. et al. Glucocorticoid receptor and Klf4 co-regulate anti-inflammatory genes in keratinocytes. Mol. Cell Endocrinol. 412, 281–289 (2015).
Lee, Y. H., Koh, S. S., Zhang, X., Cheng, X. & Stallcup, M. R. Synergy among nuclear receptor coactivators: selective requirement for protein methyltransferase and acetyltransferase activities. Mol. Cell Biol. 22, 3621–3632 (2002).
Xu, J. & Li, Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol. Endocrinol. 17, 1681–1692 (2003).
Comoglio, F. et al. Dissection of acute stimulus-inducible nucleosome remodeling in mammalian cells. Genes Dev. 33, 1159–1174 (2019).
Piccolo, V. et al. Opposing macrophage polarization programs show extensive epigenomic and transcriptional cross-talk. Nat. Immunol. 18, 530–540 (2017).
Munoz-Rojas, A. R., Kelsey, I., Pappalardo, J. L., Chen, M. & Miller-Jensen, K. Co-stimulation with opposing macrophage polarization cues leads to orthogonal secretion programs in individual cells. Nat. Commun. 12, 301 (2021).
Czimmerer, Z. et al. The epigenetic state of IL-4-polarized macrophages enables inflammatory cistromic expansion and extended synergistic response to TLR ligands. Immunity 55, 2006–2026 e2006 (2022).
Caratti, G. et al. Glucocorticoid activation of anti-inflammatory macrophages protects against insulin resistance. Nat. Commun. 14, 2271 (2023).
Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: A method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21–29 (2015).
Corces, M. R. et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods 14, 959–962 (2017).
Zhou, Y. et al. Intestinal toxicity to CTLA-4 blockade driven by IL-6 and myeloid infiltration. J. Exp. Med. 220, e20221333 (2023).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Yaari, G., Bolen, C. R., Thakar, J. & Kleinstein, S. H. Quantitative set analysis for gene expression: a method to quantify gene set differential expression including gene-gene correlations. Nucleic Acids Res. 41, e170 (2013).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The sequence alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Kawaji, H., Kasukawa, T., Forrest, A., Carninci, P. & Hayashizaki, Y. The FANTOM5 collection, a data series underpinning mammalian transcriptome atlases in diverse cell types. Sci. Data 4, 170113 (2017).
Castro-Mondragon, J. A. et al. JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 50, D165–D173 (2022).
Mammana, A. & Helmuth, J. bamsignals: Extract read count signals from bam files. (2023).
Nordin, A., Zambanini, G., Pagella, P. & Cantu, C. The CUT&RUN suspect list of problematic regions of the genome. Genome Biol. 24, 185 (2023).
Ogata, J. D. et al. excluderanges: exclusion sets for T2T-CHM13, GRCm39, and other genome assemblies. Bioinformatics 39, btad198 (2023).
Acknowledgements
We thank Dr. S. Mimouna, D. Kim, and K. Seide for technical assistance with tissue harvest and advise in DSS colitis experiments. All sequencing for this study was performed at the Weill Cornell Genomics Resources Core Facility. We are grateful to the Center of Comparative Medicine and Pathology of Memorial Sloan-Kettering Cancer Center as well as the Laboratory of Comparative Pathology at Weill Cornell for histopathology analysis. Tania Pannellini (HSS) assisted in the analysis of H&E staining (Laboratory of Comparative Pathology, MSKCC). Immunohistochemistry was performed at the Molecular Cytology core facility at MSKCC. FACS sorting was performed at Weill Cornell Medicine Flow Cytometry Core. This work was supported by the grants to I.R.: NIH R01DK099087, NIH R01AI148129, and The Hospital for Special Surgery David Rosensweig Genomics Center, to S.Z.J.: NIH R01AI148416, and the NIH fellowship F31 HL152706-01A1 to A.W.D.
Author information
Authors and Affiliations
Contributions
D.K.D., M.D., and I.R. designed experiments, D.K.D. and M.D. performed all wet bench experiments, M.J.B., D.O., and Y.C. wrote the code, D.K.D., M.D., M.J.B., A.W.D., V.C., Y.C., and I.R. analyzed data, D.K.D., M.D., M.J.B., Y.C., S.Z.J., and I.R. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jan Tuckermann, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Deochand, D.K., Dacic, M., Bale, M.J. et al. Mechanisms of epigenomic and functional convergence between glucocorticoid- and IL4-driven macrophage programming. Nat Commun 15, 9000 (2024). https://doi.org/10.1038/s41467-024-52942-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-52942-x