Abstract
Ultrastrong gels possess generally ultrahigh modulus and strength yet exhibit limited stretchability owing to hardening and embrittlement accompanied by reinforcement. This dilemma is overcome here by using hyperhysteresis-mediated mechanical training that hyperhysteresis allows structural retardation to prevent the structural recovery of network after training, resulting in simply single pre-stretching training. This training strategy introduces deep eutectic solvent into polyvinyl alcohol hydrogels to achieve hyperhysteresis via hydrogen bonding nanocrystals on molecular engineering, performs single pre-stretching training to produce hierarchical nanofibrils on structural engineering, and fabricates chemically cross-linked second network to enable stretchability. The resultant eutectogels display exceptional mechanical performances with enormous fracture strength (85.2 MPa), Young’s modulus (98 MPa) and work of rupture (130.6 MJ m−3), which compare favorably to those of previous gels. The presented strategy is generalizable to other solvents and polymer for engineering ultrastrong organogels, and further inspires advanced fabrication technologies for force-induced self-reinforcement materials.
Similar content being viewed by others
Introduction
One pursuit in the development of soft materials is to engineer fascinating mechanical performances with seamless combination of mutually incompatible mechanical properties, including ultrahigh modulus, ultrahigh strength, superior toughness, and ultrafast resilience1,2,3. Over the past two decades, extensive pioneering advancements were devoted to engineer mechanically strong and tough synthetic elastomers and gels. Recently, we demonstrated that synergistic methodology for mechanical regulation of polymer gels is to incorporate dynamic interactions into the molecular design (i.e., hydrogen bonding domains and metal coordination bonds) and to introduce high-order structure into the structural design (i.e., phase separation and hierarchical structure)4. Specifically, this elaborated incorporation of molecular and structural design endows energy dissipation multimechanisms from molecular to mesoscopic scales, contributing to the improved mechanical properties5,6. By combining the molecular and structural engineering of synergistic methodology, ultrarobust polymer gels can be fabricated for excellently high-bearing, or high impact-resistance engineering soft materials in extreme environmental conditions.
Mechanical training, as structural engineering strategy, is inspired by hypertrophying and strengthening of muscle and tendon through repeated physical exercise like walking, running and lifting heavy loads. This training/force-induced toughening strategy contributes to physical rearrangement and higher crystallinity of chains, or chemical reconstruction and mechanochemical reactions of moieties during the loading, resulting in aligned nanofibrillar network architectures of polymer network7,8,9,10. Mechanical training is performed under the loading of mechanical forces, which typically cause bond scission in polymer network, resulting in catastrophic failure. Particularly, mechanical forces produce mechanical reinforcement of synthetic polymer via strain-induced crystallization and highly aligned nanofibrillar structures. For the former, force-induced failure polymers, i.e., high-molecular-weight polyethylene (PE) and thermoplastic polyurethane (PU), fail to perform mechanical training for enhancing mechanical properties. Whereas for the latter, force-induced self-reinforcement polymers, i.e., tetra-arm poly(ethylene glycol) (PEG)11,12, poly(ethylene oxide) (PEO)13,14 and polyvinyl alcohol (PVA)7,15, are capable of remolding to strengthen itself upon cyclic pre-stretching within appropriate tensile stress, substantially strengthening and toughening these soft materials. Gong, Zhao and co-workers revealed that mechanical force-induced hierarchical structure is difficult to maintain the orientation of polymer chains after single pre-stretching, requiring substantially repetitive pre-stretching in the absence of chemically cross-linked network to permanently fix the physical rearrangement7,16, or requiring additional fabrication procedures, i.e., dried in air under confined conditions, annealed at high temperature and re-swelled in water17. As representative example, mechanically trained PVA hydrogel produces oriented nanofibrillar architecture and nanocrystalline domains, which, however, requires external aquatic environment and repetitive training, resulting in limited mechanical stretchability, albeit with improved strength and modulus. Another major challenge is challenging to control reconfigurable intrachain/interchain noncovalent interactions for mechanical force-induced crystallization in terms of molecular engineering18. Clearly, the structural advantages of mechanical training are not effectively incorporated with molecular engineering, simply to create hierarchical structures.
Deep eutectic solvents (DES), as hydrogen-bonding supramolecular complexes, manipulate the interaction, conformation, and structure of polymer networks, giving mechanical properties such as self-healing19, strong and tough, as well as other physical properties such as high temperature-resistance20 (Supplementary Table 1). In this work, of particular interest to us is to advance high-efficiency mechanical training strategy to engineer mechanically ultrastrong and tough gels, with DES as hyperhysteretic intermediate to form mechanical hyperhysteresis, resulting in high-efficiency hyperhysteresis-mediated mechanical training with only single pre-stretch. DES, as substituting solvent, incorporates three attributes to perform mechanical training efficiently and enhance mechanical properties substantially: (i) reforming and refining nanocrystals by inducing remodeling hydrogen bonds between PVA chains during solvent substituting to enable hyperhystersis; (ii) producing intrinsically higher strong hydrogen bonds density for sufficiently hyperhysteresis and dissipative energy to perform mechanical training; and (iii) providing structural retardation to maintain the conformations of chains after training, and to build chemically cross-linked second network. Molecularly, hyperhysteresis-mediated mechanical training arranges the highly-ordered, refined nanocrystalline domains, with nanocrystalline dimension of about 1.2 nm and adjustable intercrystal spacing of about 12–9 nm. Structurally, hyperhysteresis-mediated mechanical training engineers hierarchically-aligned nanofibrillar microstructures. Without external environment bath and repetitive pre-stretching, hyperhysteresis-mediated mechanical training exhibits the remarkable structural retardation of hierarchical nanofibrils, with negligible structural recovery in simply single pre-stretch and even with changes in surroundings, i.e., second network precursor. In contrast to previously reported polymer gels, eutectogels engineered via hyperhysteresis-mediated mechanical training—which are an order of magnitude higher in terms of modulus and strength—achieve remarkable mechanical properties and convenient fabrication procedures owing to the combination of molecular and structural engineering (Supplementary Table 2).
Results
Ultrastrong eutectogels via hyperhysteresis-mediated mechanical training
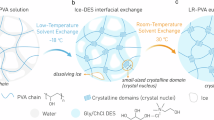
Mechanical training is an efficient mechanical regulation strategy. However, environmental baths and repetitive pre-stretching are required, and the hierarchical structures fail to sustainably maintain after loading for further reinforcements. In order to advance mechanical training, we introduce mechanical hyperhysteresis to conduct single pre-stretch and second network construction, resulting in high-efficiency mechanical training. The hyperhysteresis is essentially mechanical hysteresis, i.e., plastic deformation, which contributes to the training-induced hierarchical structure, where we call structural retardation. Specifically, ultrastrong eutectogels are engineered via hyperhysteresis-mediated mechanical training, which incorporates DES as hyperhysteretic intermediate within semicrystalline PVA hydrogel to conduct mechanical training. We firstly used freezing-thawing method to synthesize physically cross-linked single-network PVA hydrogels (SN–H). Then, we used DES as the solvent to carry out solvent displacement of SN–H. After solvent substitution, SN–H transforms into single-network eutectogels (SN–E). Because of abundant hydrogen bonds and nanocrystals, SN–E embodies mechanical hyperhysteresis, that is, the pre-stretched gel exhibits negligible recovery after unloading. This hyperhysteresis allows mechanical training to be completed with only single pre-stretch. During pre-stretching process, SN–E can adopt reorientation of nanofibrils to form anisotropic structures driven by uniaxial force. To further lock the nanofibrils in the perpendicular to nanofibrils, we introduced chemically cross-linked second network into mechanically trained SN–E to synthesize double-network eutectogels (DN–E), which enable stretchability after training. The DN–E exhibits exceptional mechanical properties compared to SN–H and SN–E owing to anisotropic hierarchical nanofibrillar structure and long-range ordered nanocrystalline domain (Fig. 1A, B and Supplementary Figs. 1, 2).
A Schematic illustration of hyperhysteresis-mediated mechanical training strategy, incorporating three key steps: solvent substituting, mechanical training and fabricating 2st network. The network structure features SN–H with isotropous nanofibrils, i.e., PVA chains, before mechanical training, SN–E with hierarchically aligned nanofibrillar network, i.e., aligned PVA chains, after mechanical training, and DN–E with hierarchically aligned PVA chains interpenetrated by chemically cross-linked network, i.e., P(AAm-co-AAc). B Schematic illustration long-range ordered nanocrystalline domain and anisotropic hierarchical nanofibrillar network solvated by DES. Optical image demonstrates the remarkably ultrahigh strength of the mechanically trained DN–E, bearing 25 kg weight. C The advantage of hyperhysteresis-mediated mechanical training strategy is remarkable structural retardation in only single pre-stretching, displaying negligible structural recovery after training. A comparison of SN–H, SN–E and DN–E in terms of (D) stress–strain curves and (E) Young’s moduli and tensile strengths, exhibiting substantially reinforced mechanical performances. Inset, magnification of the tensile curve of SN–H. Data were presented as mean ± SD (n = 5 independent samples). F Comparison chart in the Ashby plot of Young’s modulus versus fracture strength of ultrastrong eutectogels via hyperhysteresis-mediated mechanical training with other reported strong and tough polymer gels. These gels include NCP hydrogels35, SALT hydrogels36, CRC organo-hydrogels37, PPF hydrogels38, PNS hydrogels3, BIN hydrogels6, HLMN hydrogels39, PAL hydrogels40, FCSO hydrogels25, SMT organo-hydrogels8, FICS ionogels41, HBN hydrogel microfibers42, ISR hydrogel fibers43, HBMC hydrogels44, In situ PS ionogels45, FCSS hydrogels5, ASE eutectogels20, FC DN-hydrogels46, Glassy gels47, SEWA hydrogels48, CSS ionogels29, PCP hydrogels49, APS hydrogels50, HE hydrogels51, RSE hydrogels10, SIC SR-hydrogels12, and HEPC hydrogels52. See Supplementary Table 2 for a complete list of data and abbreviations.
The hyperhysteresis-mediated mechanical training endows significant changes in chain structures and internal interactions. Specifically, DES molecules, formed from choline chloride (ChCl) and hydrogen bond donors of glycerol (Gly) and acrylic acid (AAc), can spontaneously reconstruct the hydrogen bonds to enhance crystallization during solvent substituting, and regulate the nanocrystalline dimension during mechanical training, and form interpenetrated network structure during second network constructing20. Importantly, during the construction of the second network, although AAc in DES was also polymerized to form network as a monomer, ChCl and Gly still effectively maintained the gel state as DES. Specifically, when AAc polymerizes to PAAc, Gly/ChCl as a solvent will form a supramolecular gel owing to the self-assembly of PAAc in the DES system, which is not solidifying. (Fig. 1B and Supplementary Note 1). Alternatively, abundant hydrogen bonds/nanocrystals allow energy dissipation and hyperhysteresis after DES replacement. Furthermore, mechanical training, imposed on the SN–E with randomly chains and nanocrystals, is accompanied by the destruction of randomly distributed nanocrystalline domains, simultaneously follow by the formation of hierarchical alignment of nanofibrils and highly ordered reformed nanocrystalline domains. Compared with mechanical training with repetitive loading, hyperhysteresis-mediated mechanical training shows remarkable structural retardation with only single pre-stretch, resulting in negligible structural recovery of hierarchically-arranged nanofibrils after training (Fig. 1C). Such structural retardation facilitates the reinforcement of polymer network after training, i.e., building a second network.
As shown in Fig. 1D, E, by synergistically deploying solvent substitution and subsequent mechanical training, weak and soft SN–H (i.e., fracture strength of 0.25 MPa, Young’s modulus of 0.2 MPa and work of rupture of 0.11 MJ m−3) is facilely transformed into strong SN–E (i.e., fracture strength of 13.8 MPa, Young’s modulus of 10.5 MPa and work of rupture of 38.2 MJ m−3) via solvent substitution, and is significantly reinforced into ultrastrong DN–E (i.e., fracture strength of 85.2 MPa, Young’s modulus of 98 MPa and work of rupture of 130.6 MJ m−3) via mechanical training and covalent network. Taken in sum, one merit of hyperhysteresis-mediated mechanical training strategy is that it does not require any additional supply of building blocks (i.e., various monomers), or environmental bath (i.e., water bath) during pre-stretching process. Another advantage is that hyperhysteresis-mediated mechanical training is efficient, single pre-stretch, rather than tedious, repetitive pre-stretch. The DN-E achieve exceptional mechanical performances, outperforming all reported strong tough polymer gels (Fig. 1F, Supplementary Fig. 4 and Supplementary Table 2). This superior mechanical property can coexist with electrical conductivity in hopes of achieving bioelectronics (Supplementary Note 2).
Mechanical behaviors of mechanically trained ultrastrong eutectogels
To evaluate the achievement of hyperhysteresis-mediated mechanical training, we systematically demonstrate remarkable mechanical properties of mechanically trained DN–E. The mechanical properties of mechanically trained eutectogels are modulated by adjusting the cycle number of freezing-thawing process, the parameters of mechanical training, and the monomer concentrations in the second network precursor.
DES substitution guarantees mechanical hyperhysteresis, therefore, we firstly measured optimal substituting time before mechanical training. Pure freeze-thawed SN–H failed to carry out tensile experiment attributed to poor mechanical performances (strength and modulus <0.3 MPa, Fig. 1C). As the times of freeze-thawed cycles increase, hydrogen bonding density of SN–H accumulates, and may further influence the solvent substitution dynamics and mechanical training of SN–E. To optimize solvent substitution time, we quantitatively compared the mechanical properties of SN–E undergone different substitution times. We synthesized a series of SN–H with the nomenclature aT–bH, where a represents the times of freeze-thawed cycles, b represents the time required for solvent substitution. Solvent substitution time optimizes the mechanical properties of SN–E without mechanical training (Supplementary Fig. 4). This probably results from the cross-linking density of hydrogen bonds in SN–H, which determines the nucleation kinetics of nanocrystals during substituting and enables SN–E exhibiting optimum mechanical properties. Specifically, the most appropriate substituting time are 24H, 12H, 12H, 5H, and 5H for various SN–H with the freeze-thawing cycles of 3 T, 4 T, 5 T, 6 T and 7 T, respectively (Supplementary Table 3), in which solvent substitution maximized mechanical properties. Remarkably, the moduli, strengths and optimal toughness of 3T–24H, 4T–12H, 5T–12H, 6T–5H and 7T–5H are performed nearly at ~7.5–10.6 MPa, ~7–13.8 MPa and ~27.6–38.2 MJ m−3 for SN–E (Fig. 2A, B and Supplementary Table 4), respectively, and outperform greatly that of SN–H. This reinforcement indicates that abundant hydrogen bonds and higher crystallinity in chains are formed via solvent substituting, which act as an effective energy dissipative mechanism.
A Typical stress‒strain curves under tension and (B) summarized average results of Young’s moduli and fracture strengths for the tested SN–E at different times of freeze-thawed cycles and at optimal solvent substituting time. C Typical strain‒stress curves under tension and (D) summarized average results of Young’s moduli and fracture strengths for the tested DN–E undergone the six times of freeze-thawed cycles at various trained time (1 min, 5 min, 10 min, 30 min and 60 min). E Typical strain‒stress curves under tension and (F) summarized average results of Young’s moduli and fracture strengths for DN–E undergone the six times of freeze-thawed cycles at various trained strain (100%, 150%, 200% and 250%). G Typical strain‒stress curves under tension and (H) summarized average results of Young’s moduli and fracture strengths for DN–E undergone the various times of freeze-thawed cycles (3 T, 4 T, 5 T, 6 T and 7 T) at trained strain of 250% and trained times of 30 min. Data were presented as mean ± SD (n = 5 independent samples). Comparison chart in the Ashby plot of (I) Young’s modulus versus work of rupture and (J) fracture strengths versus work of rupture of ultrastrong eutectogels via hyperhysteresis-mediated mechanical training with other reported strong and tough polymer gels. See Supplementary Table 2 for a complete list of data.
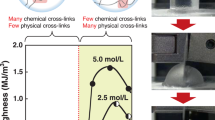
With confirmation of solvent substitution time, we subsequently demonstrated that mechanical trained time and strain can significantly reinforce mechanical properties. We fabricated a series of mechanically trained DN–E with the nomenclature aT–cmin, where c represents the training time (i.e., 1 min, 5 min, 10 min, 30 min and 60 min), and with the nomenclature aT–d%, where d represents training strain (i.e., 100%, 150%, 200% and 250%,). We investigated how the mechanical properties of DN–E were affected by increasing training time. Unless otherwise stated, trained strain in the various aT–cmin is set to be 200%. Our experimental results indicate that moduli and strengths gradually increased with the extension of trained time. The reinforcement using various trained time is also verified in other DN–E subjected to different freeze-thawed cycles. Intriguingly, as the trained time extends from 30 min to 60 min, the improvement in mechanical properties is not significantly noticeable over the same trained strain, thereby confirming the optimal trained time of 30 min. Taking 6T–1 min, 30 min and 60 min as examples, moduli, strengths and optimal toughness are 57.6 ± 4.7 MPa, 49.7 ± 4.2 MPa and 82.0 MJ m−3, 66.0 ± 5.0, 54.8 ± 4.4 MPa and 94.0 MJ m−3, as well as 68.2 ± 1.5 MPa, 55.2 ± 2.7 MPa and 101.1 MJ m−3, respectively (Fig. 2C, D, Supplementary Fig. 5 and Supplementary Table 5). To further study the role of trained strain in the enhancement of mechanical properties, we investigated the mechanical properties of aT–d% DN–E applied various trained strain with fixed training time of 30 min. As the trained strain increases from 100% to 250%, the corresponding moduli, strengths and optimal toughness improve remarkably from 32.3 ± 2.1 to 89.5 ± 6.9 MPa, 24 ± 0.7 to 74.3 ± 9.2 MPa and 38.2 to 130.6 MJ m−3, respectively, with 6T–250% exhibiting the highest modulus of 98 MPa, strength of 85.2 MPa and optimal toughness of 130.6 MJ m−3 (Fig. 2E, F, Supplementary Fig. 6 and Supplementary Table 6). Particularly, this ultra-high modulus does not embrittlement and harden eutectogels. The mechanically trained DN–E is stretchable, with the elongation of ~1.5–3.0, and displays excellent work of rupture from 46.4 MJ m−3 to 130.6 MJ m−3 (Supplementary Tables 5, 6). While potent, this training strategy also results in the decrease in tensile strain (Supplementary Fig. 5 and Supplementary Fig. 6). These experimental results indicate trained stain/time can significantly reinforce the mechanical properties, originating from hierarchical structure and ordered nanocrystals. Freezing-thawing cycles are appreciably associated with this enhancement, but much weaker than that of training strain/time (Fig. 2G, H). In short, we have drawn the conclusion that training strain plays a dominant role in the large-scale regulation of mechanical properties, while the trained time mainly contributes to fine adjustment of mechanical properties.
We next investigated whether monomer concentration in second network precursor can influence the mechanical properties of DN–E. We engineered mechanically trained DN–E infiltrated with the second network precursor of 3 M, 4 M and 5 M. Taking 6T–200% as an example, the tensile measurements of 3 M, 4 M and 5 M show significant diversities in mechanical performances in which the moduli and strengths of 4 M are substantially greater than that of 3 M and 5 M. In particular, the moduli, strengths and optimal toughness of 6T–200%–4 M are 61.44 ± 2.03 MPa, 52.18 ± 3.87 MPa and 86.24 MJ m−3. Similar trend is observed in 5T–200% and 7T–200% (Supplementary Fig. 7 and Supplementary Table 7). These characterizations probably result from solvent diffusion and molecular mobility achieving equilibrium at 4 M. In addition, the composition of DES also significantly affects the mechanical properties of DN–E, for example, AAc plays a dominant role for mechanical properties of DN–E in the subsequent construction of chemically cross-linked networks (Supplementary Fig. 8). Moreover, to investigate the humidity sensitivity and environmental tolerance of DN–E, we set up two opposing environments in order to simulate practical application scenario. We performed tensile measurements after exposure to both conditions for 7 days for quantitatively evaluating mechanical stability (Supplementary Fig. 9 and Supplementary Table 8). Particularly, DN–E treated at dry, high temperature surroundings (60 °C) exhibits an exceptional mechanical stability owing to negligible vapor pressure of DES, underscoring application advantage21,22. While the mechanical properties of DN–E treated at humid, low-temperature surroundings (–20 °C) show mechanical instability compared with the original, dropping by about two folds in moduli and strengths, which may be attributed to the diffusion-induced solvent exchanging between DES and water molecules23.
To compare our work with existing polymer gels, we summarized Young’s moduli, fracture strengths, and work of ruptures of various strong and tough polymer gels on Ashby plots. Young’s modulus–work of rupture plane and fracture strengths–work of rupture plane demonstrates that the mechanical performances of mechanically trained DN–E outperform greatly that of most current polymer gels, and can be easily customized over a broad range (Fig. 2I, J and Supplementary Fig. 10). Collectively, we reported dramatic improvement in mechanical properties using hyperhysteresis-mediated mechanical training, achieving a record-high modulus, strength and toughness.
Structural retardation of hyperhysteresis-mediated mechanical training
As we demonstrated before, trained strain/time significantly improve mechanical properties. Therefore, we further investigated physical mechanisms behind structural retardation of hyperhysteresis-mediated mechanical training. Considering that DES as a eutectic mixture via charge delocalization, we speculated that DES may enhance crystallization, resulting in the structural retardation originated from hyperhysteresis after training24. This is supported by the mechanical performances and training behaviors of SN–E before and after training. Hyperhysteresis or structural retardation presents two manifestations: one is the negligible structural recovery after mechanical training. Second, further immersion in the solution still has a very low structural recovery after training (Supplementary Figs. 11–15). To quantitatively characterize structural retardation, we proposed to use two indices, mechanical training ratio (MTR) of SN–E and mechanical training effectiveness (MTE) of DN–E.
We first studied MTR determined by length variation before and after training to visualize the recovery of SN–E after unloading. Specifically, MTR is obtained by the equation MTR = (lunloading – l0)/l0 × 100%, where l0 is the effective length before training and lunloading is the effective length after unloading (Supplementary Fig. 11). The MTR gradually increases and eventually plateaus when the trained time is between 30 min and 60 min, which is consistent with the reinforcement tendency of mechanical properties (Figs. 2C, 3A, and Supplementary Fig. 16). Such variation tendency indicates that hyperhysteresis-induced structural retardation endows optimal trained parameters for achieving the best mechanical performances, above which the mechanical properties reach plateaus at a slight lower yet still very high level. In addition to trained time, we also investigated the effect of trained strain on MTR. The relationship between MTR and trained strain indicates that each trained strain corresponds to a definite MTR, which is basically unchanged as freeze-thawed times increase. In particular, the MTR is about 50%, 90%, 140% and 190% at the corresponding training strain of 100%, 150%, 200% and 250%, respectively. As trained strains increase, the MTR and the plastic deformation acutely increases. Such dramatically increases are consistent with the enhancements of mechanical performances (Figs. 2E, 3B and Supplementary Fig. 16). Furthermore, the rangeability of MTR with trained strain is significantly stronger than that of training time, suggesting that hyperhysteresis facilitates structural retardation with high time insensitivity, and high-efficiency mechanical training with only single pre-stretch, and further confirming that large-scale/refined mechanical regulation of trained strain/time.
A Summarized average results of MTR index of SN–E with various trained time at trained strain of 200%. B Summarized average results of MTR index of SN–E with various trained time strains at trained time of 30 min. C Summarized average results of MTE index of DN–E with various trained time at trained strain of 200%. D Summarized average results of MTE index of DN–E with various freeze-thawed times and trained strain at trained time of 30 min. Data were presented as mean ± SD (n = 5 independent samples). Stress/strain as a function of training time is divided into three regimes (i–iii) for (E) various trained time at trained strain of 200% and F various trained strains at trained time of 30 min to show the mechanical behaviors during mechanical training. Notably, various trained strains are to be 100%, 150%, 200% and 250%, respectively; trained time are to be 1 min, 5 min, 10 min, 30 min and 60 min, respectively. G Schematic illustration of the mechanical behaviors during mechanical training in original and three regimes of i, ii, and iii. Mechanical training causes disordered polymer chains to be oriented hierarchically and the reformation of highly ordered nanocrystalline domains.
Considering the trained SN–E after training is subsequently submersed in the second network precursor, in which the diffusions of monomer into network also affect the conformation of chains, and further influence the achievement of mechanical training. Therefore, we also assessed the MTE of DN–E after constructing second network. We determined MTE by the equation, MTE = lDN–E/l0 ÷ lunloading/l0 × 100%, where lDN–E is the effective length of the DN–E (Supplementary Fig. 11). The influence of trained time and strain on MTE is negligible in which MTE is been plateauing, suggesting that hyperhysteresis-induced structural retardation exhibits chemical and physical passivation, even with changes in surroundings, and is similar to inherent physical property (Fig. 3C, D and Supplementary Fig. 16). Intriguingly, similar trends are observed in the monomer concentration of second network, which further indicates the high-efficiency of structural retardation after mechanical training (Supplementary Fig. 17). Taken together, these findings further confirm that hyperhysteresis plays a decisive role in maintaining the structural retardation after unloading, even after immersion in precursor.
To further understand the structural retardation during training, we also plotted stress/strain as a function of training time to show the mechanical behaviors during training (Fig. 3E, F). Figure 3G depicts the characteristic behaviors of hyperhysteresis-mediated mechanical training in various regimes. At the beginning of training (regime I), the randomly distributed, entangled chains start to gradually elongate to pre-stretching limit within the stretching direction in which stress increases rapidly owing to the destruction of strong, disordered nanocrystalline domains and hydrogen bonds. In regime II, the prestretched chains experience stress relaxation; and the stress rapidly drops and eventually plateaus in which the breakdown of the nanocrystalline domains no longer occurs again until relaxation ends. In regime III, the reoriented chains are fixed by the stress and the stress tends to plateau in which the solvent molecules begin to remigrate, contributing to permanent alignment of the fibrillar network, and the reformation and rearrangement of highly ordered nanocrystalline domains.
Structural evolution and transformation of internal interactions
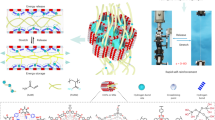
As confirmed by mechanical properties, ultrastrong DN–E utilizes progressive strengthening mechanism for network structures and internal interactions (Fig. 4A and Supplementary Note 1). To further validate structural evolution, we investigated the microstructure and nanocrystalline morphology of SN–H, SN–E and DN–E through scanning electron microscopy (SEM) images and X-ray scattering measurements, respectively. The freeze–thawed SN–H features only low-density nanocrystalline domains or pore walls, indicating the formation of an isotropic, physically cross-linked network (Fig. 4B and Supplementary Fig. 18A)25, whereas the solvent-substituted and subsequently mechanically trained SN–E shows and high-density nanocrystalline domains and highly oriented nanofibrillar network (Fig. 4C and Supplementary Fig. 18B, C). DN–E features hierarchical fibrillar network interpenetrated by chemically cross-linked network, and ordered nanocrystals (Fig. 4D and Supplementary Fig. 18D). Furthermore, SN–E and DN–E showed a significant scattering peak at 2θ = 19.2° in comparison to SN–H, manifesting drastic crystallization driven by solvent substituting26. Small peaks at 2\({{{\rm{\theta }}}}\) = 11.2° and 22.6° are also observed in SN–E and DN–E, suggesting higher crystallinity after solvent substituting, which is consistent with the x-ray diffraction (XRD) measurements (Supplementary Fig. 19)27. To quantify the evolution of nanocrystalline morphology in both processes, we assessed nanocrystalline dimension (D) through wide-angle X-ray scattering (WAXS) and intercrystal spacing (L) through small-angle X-ray scattering (SAXS). The D of SN–H, SN–E and DN–E markedly decreases from 5.165 to 1.055, to 1.054 nm, suggesting that solvent substitution remarkably refines nanocrystalline dimension, whereas mechanical training fails to further refine nanocrystals (Fig. 4E). The L of SN–H, SN–E and DN–E is around 17.542, 12.485 and 9.102 nm, respectively, suggesting the much denser and long-range ordered nanocrystals of DN–E (Fig. 4F and Supplementary Fig. 20)28. In sum, these experimental observations suggest that the reinforcement of mechanical properties is accompanied with nano- and microstructural evolution, i.e., nanocrystalline domains and hierarchical fibrillar network.
A Schematic illustration of the hyperhysteresis-mediated mechanical training strategy ranging from isotropous nanofibrils to hierarchically aligned nanofibrillar network via mechanical training, to hierarchical fibrillar network interpenetrated by chemically cross-linked network via UV light-initiated radical polymerization. SEM images and corresponding 2D WAXS patterns of (B) SN–H, C SN–E before and after mechanical training and (D) mechanically trained DN–E (n = 3 independent samples). E The 1D WAXS profiles of SN–H, SN–E and DN–E. The WAXS intensity profiles develop remarkable crystalline peaks of PVA after solvent substituting and mechanical training. F The 1D SAXS profiles of corrected scattering intensity Iq2 versus vector q for SN–H, SN–E with mechanical training and mechanically trained DN–E. The scattering peak emerged in the low q region gradually increases, indicating that the intercrystal spacing between neighboring nanocrystalline domains decreases after solvent substituting and mechanical training. G Schematic diagram of the structural arrangement of water cluster-PVA, DES ball-PVA, PAAc-PVA and PVA-PVA obtained by DFT optimizations. H Comparison of interaction energy of water cluster and DES ball, water cluster-PVA, DES ball-PVA, PAAc-PVA and PVA-PVA obtained by DFT calculations. I Structure and electrostatic potential distribution of water cluster and DES ball obtained by DFT optimizations. J Representative DSC thermographs of SN–H, SN–E with mechanical training and mechanically trained DN–E to measure the crystallinities. Compared with SN–H, SN–E and DN–E show significant endothermic peaks of PVA nanocrystalline domains. K Measured average crystallinities of SN–H in the dry states, SN–E without mechanical training and mechanically trained DN–E. L Schematics indicate the structural evolution and enhanced nanocrystalline domains in the process of carrying out hyperhysteresis-mediated mechanical training strategy.
Furthermore, using density functional theory (DFT) optimizations and calculations, we studied dynamic internal interactions (i.e., solvent-solvent, solvent-chain, and chain-chain) and electrostatic potential distribution of solvent ball, where DES ball is composed of 4 Gly molecules, 2 AAc molecules and 1 ChCl molecule, water cluster is composed of 7 H2O, PVA and PAAc segment is composed of 7 individual units (Supplementary Fig. 21). The intermolecular interaction of PVA segment-DES ball is obviously greater than that of PVA segment-water cluster, indicating energetically favorable for solvent substituting (Fig. 4G, H). Additionally, we also assessed the interactions of PVA segment-PAAc segment and PVA segment-PVA segment, respectively, where these binding energies are highlighted and comparable to or above that of PVA segment-DES ball, indicating the feasibility of nanocrystals reformation during DES substituting and the contribution of chemically cross-linked networks to mechanical properties. Another noticeable feature is that the internal interactions of DES ball are substantially stronger than that of water cluster, providing hyperhysteresis (Fig. 4H). As expected, DES ball shows enhanced electrostatic potential compared to that of water cluster, making the binding between PVA and DES energetically favorable, which is consistent with interactions between various solvent-chain (Fig. 4I)29. Collectively, these energetic observations suggest that DES serves as the best candidate of hyperhysteretic intermediate, and facilitates internal interactions and nanocrystalline domains.
Thereafter, we performed differential scanning calorimetry (DSC) measurements to evaluate the crystallinities of PVA chains. SN–H, SN–E and DN–E show significant endothermic peaks of PVA crystalline, with measured average crystallinities of 8.12, 38.36 and 36.18 wt%, respectively (Fig. 4J, K, and Supplementary Fig. 22). The enhanced crystallinity implies that substantial nanocrystals nucleate during DES substituting, which is consistent with WAXS, SAXS and DFT calculations. Mechanistically, solvent substitution significantly facilitates the nucleation and refinement of nanocrystals accompanied with the formation of hydrogen bonds, and mechanical training not only constructs hierarchical alignment of nanofibrous, but also reconstructs interfibrillar interactions, leading to the highly ordered nanocrystals under confined configurations (Fig. 4L).
Long-range hierarchical alignment of nanocrystalline morphology
Taking advantage of the training time/strain, the mechanical properties of DN–E can be effectively regulated, originating from training-induced hierarchical structure and ordered nanocrystals in comparison to SN–H without hyperhysteresis-mediated mechanical training (Supplementary Fig. 23). Consequently, to further investigate nanocrystalline morphology during mechanical training, we tracked nanocrystalline dimension (D), intercrystal spacing (L), intermolecular spacing (LM) and interlamellar spacing (LL) using WAXS and SAXS measurements on DN–E.
Similar to the mechanical characterizations, we first analyzed nanocrystalline morphology treated by various trained times for DN–E–6 T. The WAXS profiles at various trained times show anisotropic peaks, indicating enhanced nanocrystalline domains, with the reflection plane of PVA occurring at (100), (10\(\bar{1}\)) and (200), and the strongest peak occurring at a d-spacing of 0.464 nm corresponding to LM (Fig. 5A, C, and Supplementary Fig. 24)30,31. LL also is estimated to be about 0.844 nm (Fig. 5C and Supplementary Fig. 24). Further, the average size of D approximately calculated using Scherrer’s equation form WAXS profiles shows negligible difference as trained time increases, with smaller nanocrystals of 1.20–1.29 nm (Fig. 5E). There results further investigate that trained time fails to remarkably further refine nanocrystalline domains, whereas solvent substituting dominates this refinement. Intriguingly, the observed peak in the low-q regime of the SAXS profiles reveals that trained times significantly influence nanocrystalline morphology (Fig. 5B, D). As shown in Fig. 5E, the average distance of L estimated from the Bragg expression shows progressive reduction as trained time increases, with 1 min of 9.544 nm to 60 min of 8.845 nm), suggesting that trained time produces highly ordered, dense nanocrystals32. Notably, L shows insignificant reduction from 30 to 60 min, this insensitivity of L to trained time is consistent with mechanical properties, and further confirms hyperhysteresis-induced structural retardation.
A The 1D WAXS profiles and (B) the 1D SAXS profiles of DN–E–6 T undergone the six times of freeze-thawed cycles, with various trained time (1 min, 5 min, 10 min, 30 min and 60 min) at the trained strain of 200%. C The 2D WAXS patterns and (D) the 2D SAXS patterns of DN–E–6 T undergone the six times of freeze-thawed cycles, with various trained time (1 min, 5 min, 10 min, 30 min and 60 min) at the trained strain of 200%. E The nanocrystalline dimension D from WAXS measurements, and intercrystal spacing L from SAXS measurements as a function of trained time. F The 1D WAXS profiles and (G) the 1D SAXS profiles of DN–E–6 T undergone the six times of freeze-thawed cycles, with various trained strain (0%, 100%, 150%, 200% and 250%; where 0% represents DN–E–6T without mechanical training) at trained time of 30 minutes. H The 2D WAXS patterns and (J) the 2D SAXS patterns of DN–E–6 T undergone the six times of freeze-thawed cycles, with various trained strain (0%, 100%, 150%, 200% and 250%; where 0% represents DN–E–6T without mechanical training) at trained time of 30 minutes. K The nanocrystalline dimension D from WAXS measurements, and intercrystal spacing L from SAXS measurements as a function of trained strain. L Schematic illustration of the long-range ordered nanocrystalline morphology with low-aspect-ratio alignment in which mechanical training regulates both the arrangement of nanofibrils and the aspect ratio of nanocrystals.
In addition to nanocrystals parallel to aligned nanofibrils, we also demonstrated highly ordered nanocrystals perpendicular to nanofibrils. The SAXS profiles of SN–H show negligible difference in comparison to parallel to nanofibrils, whereas the SAXS profiles of DN–E show difference in both directions, with L∥ of 9.373 nm and L⊥ of 9.035 nm, revealing that nanocrystals rearrange in training direction (Supplementary Fig. 25). Remarkably, as trained strain increases, ΔL gradually decreases, indicating that neighboring nanocrystals, parallel and perpendicular to aligned nanofibrils, show rearrangement from high-aspect-ratio to low-aspect-ratio, suggesting denser nanocrystals (Supplementary Fig. 26). Thereafter, we studied the nanocrystalline morphology perpendicular to aligned nanofibrils treated by elevating trained strains. Similarly, D, LM and LL of nanocrystals shows insensitivity to trained strain. whereas L shows significantly sensitivity to trained strain (Fig. 5F, J and Supplementary Fig. 22). Interestingly, we observed that the long-range alignment of nanocrystalline is strongly sensitive to trained strain, with hierarchical ordered structure observed from 0% of 12.314 up to 250% of 8.845 nm, but is weakly sensitive to trained time from 1 min of 9.544 to 60 min of 8.845 nm, which is consistent with mechanical properties and structural retardation (Fig. 5D, I, E, J). These observations strongly suggest that mechanical training regulates mechanical properties by rearranging nanocrystals to achieve higher long-range order. Specifically, the increase in trained time/strain results in the higher orderly array of nanocrystals between hierarchical nanofibrils, with denser, lower-aspect-ratio nanocrystals (Fig. 5K).
Taken together, these observations allow us to draw conclusions about nanocrystalline morphology. Collectively, structural retardation and evolution demonstrate that mechanical training results in the highly ordered nanocrystals as nanofibrils reorient. WAXS and SAXS measurements further reveal long-range ordered nanocrystalline morphology attributing to training. Specifically, mechanical training parameters, i.e., trained strain and time, have negligible effect on the reformation and refinement of nanocrystalline domain in comparison to solvent substituting, but play a decisive role in the highly oriented, ordered alignment of nanocrystalline domain. The higher the long-range oriented alignment, the stronger the mechanical properties.
Universality of hyperhysteresis-mediated mechanical training strategy
Hyperhysteresis-mediated mechanical training occurs with solvent substituting-triggered strong nanocrystals. Based on this mechanical understanding, we therefore extended the universality of the hyperhysteresis-mediated mechanical training strategy to other organic solvents, in which solvent molecules contain abundant functional groups to form strong hydrogen bonding nanocrystals, e.g., Gly and PEG. (Supplementary Note 3). Similar to DES, PVA hydrogels (SN–H) is introduced into hyperhysteresis induced by strong hydrogen bonds to form organogels (SN–O) via solvent substituting, and is subsequently performed hyperhysteresis-mediated mechanical training and second network to engineer organogels (DN–O) (Fig. 6A, E and Supplementary Fig. 43). Compared with SN–H and SN–O, DN–O are substantially reinforced. Moreover, MTR and MTE indicate that organic solvents also achieve hyperhysteresis-induced structural retardation after training (Fig. 6B, F). Taken PEG as example, weak and soft SN–H is facilely transformed into strong SN–O via solvent substitution, with fracture strength of 11.9 MPa, Young’s modulus of 18.9 MPa and work of rupture of 35.5 MJ m−3. Subsequently, SN–O is significantly reinforced into ultrastrong DN–O via mechanical training and covalent network, with fracture strength of 43.4 MPa, Young’s modulus of 50 MPa and work of rupture of 38.5 MJ m−3 (Fig. 6B, C). Similar trend is observed in Gly as substituted solvents (Fig. 6F, G). These mechanical characteristics demonstrate that the universality of the hyperhysteresis-mediated mechanical training strategy can be successfully extended to other solvents.
A PEG (Mn = 200) as hyperhysteretic intermediate to perform mechanical training. B Summarized average results of MTR index of SN–O and MTE index of DN–O with 6 times freeze-thawed cycles, trained strain of 250% and trained time of 10 min. A comparison of SN–H, SN–O and DN–O for PEG as substituted solvent in terms of (C) stress–strain curves and (D) Young’s moduli and tensile strengths, exhibiting substantially reinforced mechanical performances. Inset, magnification of the tensile curve of SN–H. Data were presented as mean ± SD (n = 5 independent samples). E Gly as hyperhysteretic intermediate to perform mechanical training. F Summarized average results of MTR index of SN–O and MTE index of DN–O with 6 times freeze-thawed cycles, trained strain of 300% and trained time of 10 min. A comparison of SN–H, SN–O and DN–O for Gly as substituted solvent in terms of (G) stress–strain curves and (H) Young’s moduli and tensile strengths, exhibiting substantially reinforced mechanical performances. Inset, magnification of the tensile curve of SN–H. Data were presented as mean ± SD (n = 5 independent samples). Application demonstrations in (I) thermal environments and (J) piezoinoic machines for high-bearing, or high impact-resistance engineering soft materials. Voltage (K) and current responses (L) under repeated step impact at a pressure of 1.5 MPa.
Discussion
An ultrastrong eutectogel of highly oriented nanofibrillar network and nanocrystalline domain can be engineered using hyperhysteresis-mediated mechanical training strategy. This study has demonstrated that this mechanical training strategy substantially reinforces mechanical properties of gels, necessitating unique solvents capable of hydrogen bonding as hyperhysteretic intermediate to increase PVA crystallinities and induce nanocrystalline domains in PVA hydrogels. The proposed hyperhysteresis-mediated mechanical training strategy takes advantage of hyperhysteresis arisen from DES substituting-induced dense nanocrystals/hydrogen bonds, which leads to only single pre-stretch training without environment bath. The concept of hyperhysteresis-mediated mechanical training has been introduced; that is, structural retardation originates from hyperhysteresis to resist structural recovery of hierarchical nanofibrils after training, enabling the reinforcement of network. Interestingly, this structural retardation contributes the sensitivity to training stain and insensitivity to training times, providing a platform for regulating mechanical properties. Considering that hydrogen bonding interactions exist for various polymers and solvent systems, such as DES, PEG and Gly, the presented strategy is generalizable to the other organic solvents to engineer ultrastrong organogels, and we are convinced that not restricted to these polymers and solvents presented here. Given the emerging opportunities in excellently high-bearing, or high impact-resistance engineering soft materials in extreme environmental conditions, we demonstrate the mechanical stability in high temperature environments and the piezoionic machine in response to pressure-driven electrical generation (Fig. 6I, J, K, L, Supplementary Fig. 44 and Supplementary Note 4).
The reinforcing mechanism of hyperhysteresis-mediated mechanical training is demonstrated to be attributed to refined nanocrystalline domain reformed in DES substituting and hierarchically aligned nanofibrils and long-range ordered nanocrystals oriented in training. Consequently, the resultant eutectogels simultaneously achieve ultrahigh fracture strength (20.1 to 85.2 MPa) and Young’s modulus (27.5 to 98.0 MPa). Particularly, such ultra-high modulus does not embrittlement and harden gels, displaying excellent toughness (46.5 MJ m−3 to 130.6 MJ m−3). By incorporating DES substitution and subsequent mechanical training, the mechanical performances of eutectogels outperform that of existing strong and tough gels in terms of the combination of ultrahigh strength and ultrahigh modulus. Within our hyperhysteresis-mediated mechanical training strategy, the strengthening and toughening mechanistic understanding is demonstrated in detail, which provide insight into the designing of mechanically trained gels. Following the same training strategy, we anticipate that there will be other force-induced self-reinforcement polymers performed hyperhysteresis-mediated mechanical training, which will further aid in the extreme mechanical properties of engineering soft materials.
Methods
Preparation of DES
Glycerol (Gly, J&K Scientific Ltd), acrylic acid (AAc, J&K Scientific Ltd) and choline chloride (ChCl, J&K Scientific Ltd) were first mixed in a predetermined molar ratio of 4:2:1, and then the mixture was stirred for 1 h at 60 °C and sonicated for 30 min until achieving homogeneity and transparency.
Synthesis of mechanically trained eutectogels
Firstly, PVA powder (Mw 89,000 to 98,000, 99 + % hydrolyzed, Sigma Aldrich) was dissolved in deionized water under vigorous stirring with an oil bath at 90 °C for 2 h to make a 10 wt% solution. After cooled to room temperature, the obtained solution was poured into a glass mold with a thickness of 2 mm, and was frozen first in a refrigerator at −20 °C for 24 h to synthesis prefabricated PVA hydrogel. After that, the resultant sample is thawed at 40 °C for 3 h. The thawed sample is refrozen in a refrigerator at −20 °C for 24 h and thawed again at 40 °C for 3 h to repeat freeze-thawing process for 3–7 times. Finally, the resultant freeze-thawed sample was swelled in deionized water for 24 h to obtain pure SN − H hydrogel. Then, the SN − H hydrogel prepared above was immersed preprepared DES at 25 °C at the scheduled time to conduct solvent substitution, and formed a SN − E eutectogel. To fabricate the mechanically trained SN − E eutectogels, the SN − E eutectogels were stretched to controlled strain (100%, 150%, 200% and 250%), and maintained for 1 min, 3 min, 5 min, 10 min, 30 min and 60 min. After that, the prestreched SN − E eutectogels were further immersed preprepared covalently precursor leveraging the DES prepared above as solvent and containing acrylamide (AAm, J&K Scientific Ltd) varied from 3.0 to 5.0 M, 0.1 mol% N, N-methylenebisacrylamide (MBAA, J&K Scientific Ltd), and 0.1 mol% 2-Hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959, Sigma Aldrich) at 30 °C for 24 h. The covalent network of eutectogels were formed by UV light-initiated radical polymerization with a wavelength of 365 nm, and completed within 10 min, to obtain mechanically trained DN − E eutectogels.
Characterization of eutectogels
The micro- and nanostructures of various gels were characterized on a SEM (FESEM, QUANTA FEG 650, FEI, USA). XPS (ESCAlab250 Thermal Scientific, USA) was used to determine the element composition of the various gels. XRD was analyzed through a Bruker D8 ADVANCE instrument using Cu Kα radiation (λ = 1.5418 Å) in the 2θ range from 5 to 90° with a scan rate of 10° min−1 to determine the crystalline phase of the gels. The chemical components of gels were investigated by FTIR spectra using Fourier transform infrared spectrometer (FTIR, V70, Brooker, Germany). Temperature-variable IR spectra were collected in the transmission mode on a Bruker INVENIO-S spectrometer equipped with an MCT detector. The temperature range was set from 20 to 200 °C with a ramp rate of 5 °C min−1 in the Harrick praying mantis of diffuse reflection spectroscopy. The storage modulus E' and the loss modulus E'' of the various gels were measured on Dynamic thermomechanical analyzer (DMA, TA Q800) in Tensile mode (1 Hz) over a temperature range of −50 to 100 °C with a rate of 5 °C/min. The DES was dissolved in DMSO to form a homogeneous solution. 1H NMR spectrum was recorded at 25 °C by the Bruker AV400 spectrometer (400 MHz).
Mechanical properties characterization
The mechanical properties of the polymer gels were was mainly evaluated via standard stress-stain experiments by using a testing machine (Shimadzu AGS-X-5000 N). The whole sample is machined into cylindrical shape for tensile tests. Both ends of the dumbbell-shaped sample were connected to the clamps with the lower clamp fixed. All the testing rate was set at 50 mm min−1. Young’s modulus (E [MPa]) was determined from the initial slope of the stress-strain curves. Fracture strengths (Ef [MPa]) was appointed to represent the stresses at which the ultimate tensile failure occurs in the uniaxial tensile test. Toughness (Γ [MJ m−3]), a mechanical parameter that characterizes the work required to fracture the sample per unit, was obtained by integrating the area below stress-strain curve until fracture. Each mechanical test was repeated with at least five individual samples.
Crystallinity content measurement
Differential scanning calorimetry (DSC, TA 250) were adopted to test crystallinity of PVA hydrogels. Before testing, the SN − H hydrogel samples were processed following reference7,33,34, which used excess chemical cross-links to stabilize the amorphous polymer chains before air-drying in order to minimize further formation of crystalline domains during the drying process. Firstly, the freeze-thawed PVA hydrogels were immersed in a chemical crosslinking agent consisting of 55 mL of 50 vol.% glutaraldehyde, 500 μL of 36.5 to 38 wt.% hydrochloric acid and 50 mL deionized water for 2 h. Thereafter, chemically cross-linked PVA hydrogels were soaked in deionized water bath to remove residual hydrochloric acid for 2 h. Finally, the resultant hydrogels were dried at 50 °C for 24 h. For the DSC measurements of SN − E and DN − E eutectogels, the samples were not processed owing to the trace moisture in polymer network of SN − E and DN − E. In a typical DSC measurement, we first weighed the total mass of the dry sample (mtotal). The dry sample was heated up from −50 to 300 °C at a heating rate of 5 °C/min under a nitrogen atmosphere with a flow rate of 20 mL/min. The curve of heat flow shows a broad melting endothermic transition peak at below 200°, indicating that the sample contains a small amount of residual water. The integration of the broad endothermic transition peak gives the enthalpy for evaporation of the residual water per unit mass of the sample (Hresidual water). Therefore, the mass of the residual water (mresidual water) can be calculated as:
where \({H}_{{water}}^{0}=2260J/g\) is the latent heat of water evaporation. The curve of heat flow shows another narrow melting endothermic transition peak ranging from 200° to 250 °C, corresponding to melting of the PVA crystalline domains. The integration of another narrow endothermic transition peak ranging from 200 to 260 °C gives the melting enthalpy for the crystalline domains per unit mass of the sample (Hcrystalline). Therefore, the mass of the crystalline domains (mcrystalline) can be calculated as:
where \({H}_{{PVA}}^{0}=138.6J/g\) is the standard melting enthalpy of 100% crystallized PVA. Therefore, the crystallinity (ξcrystalline) in the ideally sample without residual water can be calculated as:
SAXS and WAXS measurement
To quantify the dimension, structure, and evolution of nanocrystalline domains during the fabrication, X-ray scattering measurements were carried out on a modified Xeuss system (Xenocs 2.0, France) equipped with a semiconductor detector (Pilatus 3 R 100 K, DECTRIS, Switzerland) using Cu Kα radiation. Quantification of 2D SAXS and WAXS patterns was performed with scattering vector (q) and azimuthal angle (2θ) as coordinates. The wavelength of the X-ray radiation was 0.154 nm. The inter-crystal spacing (L), crystal dimension (D), intermolecular spacing (LM) and interlamellar spacing (LL) of nanocrystalline domains were calculated according to the 1D scattering profiles. Specifically, SAXS was used to characterize the inter-crystal spacing, and WAXS was utilized to characterize the crystal dimension, interlamellar spacing and intermolecular spacing30,31,34.
The small-angle x-ray scattering (SAXS) measurements for L. The average distance between adjacent crystalline domains L can be estimated from the scattering maxima corresponding to the peak intensity qmax according to the Bragg expression:
where scattering vector (q) is defined as:
where λ is the x-ray wavelength, and 2θ is the scattering angle.
The wide-angle x-ray scattering (WAXS) measurements for D. By identifying the half width of the maximum diffraction peak β after subtracting the instrumental baseline, the average size of crystalline domains D can be approximately calculated using Scherrer’s equation:
where k is a dimensionless shape factor varying with the actual shape of the crystalline domain, λ is the wavelength of X-ray diffraction, and θ is half of the diffraction angle. The dimensionless shape factor k is set as 1, approximating the spherical shape of the crystalline domains. The intermolecular spacing (LM) and the interlamellar spacing (LL) were determined from the 1D scattering curve of intensity vs. scattering vector (q) obtained by 2D WAXS patterns using Bragg expression.
Density functional theory simulation
Density functional theory (DFT) optimizations and calculations were performed using Gaussian 09 software. Becke’s three-parameter hybrid exchange function, the Lee-Yang-Parr correlation function (B3LYP) was employed to investigate binding energies, and 6–31 G (d) basis set was chosen for C, H, O, N and Cl atoms. All the molecules were separately constructed, geometrically optimized and energy calculations. For calculations of the solvent-solvent interactions, the molar ratio of Gly, AAc and ChCl in a DES ball was defined as 4:2:1, which is close to the experimentally determined molar ratio of our eutectogels. In comparison, a water cluster also contains 7 water molecules. For calculations of the solvent-chain and chain-chain interactions, PVA segment was composed of 7 vinyl alcohol molecules to avoid the chain from being surrounded by solvents. The energy difference was obtained by subtracting the total energy of the system from the energy of each component in the complexes, reflecting the intermolecular interactions energy:
Water content measurement
The water content of the freeze-thawed SN − H hydrogels was measured by comparing their weights before and after drying. The drying possess was carried out at 80 °C for 48 h in vacuum. These weight of the sample before and after drying were measured as mswollen and mdry, and \(\chi\) can be obtained from the following equation:
The trace moisture of the SN − E eutectogels and mechanically trained DN − E eutectogels was measured by the integration of endothermic transition peak of water in DSC. \(\chi\) can be obtained from the following equation:
Swelling ratio measurement
The swelling ratio of the freeze-thawed SN − H hydrogels was measured by comparing their weights before and after immersing in deionized water form 0 h up to 168 h. The immersing possess was carried out at 25 °C. Excess surface water was wiped from the gel surface before weighing. These weight of the sample before and after immersing were measured as m0 and mt, and η can be obtained from the following equation:
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. Raw data are provided as a Source Data file. Source data are provided with this paper.
Change history
17 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41467-025-59056-y
References
Fu, L. et al. Cartilage-like protein hydrogels engineered via entanglement. Nature 618, 740–747 (2023).
Neumann, S. E. et al. The propensity for covalent organic frameworks to template polymer entanglement. Science 383, 1337–1343 (2024).
Chen, L. et al. A hyperelastic hydrogel with an ultralarge reversible biaxial strain. Science 383, 1455–1461 (2024).
Xu, C. et al. Mechanical regulation of polymer gels. Chem. Rev. 124, 10435–10508 (2024).
Guo, X., Dong, X., Zou, G., Gao, H. & Zhai, W. Strong and tough fibrous hydrogels reinforced by multiscale hierarchical structures with multimechanisms. Sci. Adv. 9, eadf7075 (2023).
Bao, B. et al. Rapid fabrication of physically robust hydrogels. Nat. Mater. 22, 1253–1260 (2023).
Lin, S., Liu, J., Liu, X. & Zhao, X. Muscle-like fatigue-resistant hydrogels by mechanical training. Proc. Natl. Acad. Sci. USA 116, 10244–10249 (2019).
Li, T., Qi, H., Dong, X., Li, G. & Zhai, W. Highly robust conductive organo-hydrogels with powerful sensing capabilities under large mechanical stress. Adv. Mater. 36, 2304145 (2024).
Matsuda, T., Kawakami, R., Namba, R., Nakajima, T. & Gong, J. P. Mechanoresponsive self-growing hydrogels inspired by muscle training. Science 363, 504–508 (2019).
Wang, Z. et al. Toughening hydrogels through force-triggered chemical reactions that lengthen polymer strands. Science 374, 193–196 (2021).
Hartquist, C. M. et al. An elastomer with ultrahigh strain-induced crystallization. Sci. Adv. 9, eadj0411 (2023).
Liu, C. et al. Tough hydrogels with rapid self-reinforcement. Science 372, 1078–1081 (2021).
Lang, C. et al. Nanostructured block copolymer muscles. Nat. Nanotechnol. 17, 752–758 (2022).
Hashimoto, K. et al. Strain-induced crystallization and phase separation used for fabricating a tough and stiff slide-ring solid polymer electrolyte. Sci. Adv. 9, eadi8505 (2023).
Sun, M. et al. Multifunctional tendon-mimetic hydrogels. Sci. Adv. 9, eade6973 (2023).
Li, X. Y. et al. Role of hierarchy structure on the mechanical adaptation of self-healing hydrogels under cyclic stretching. Sci. Adv. 9, 12 (2023).
Mredha, M. T. I. et al. A facile method to fabricate anisotropic hydrogels with perfectly aligned hierarchical fibrous structures. Adv. Mater. 30, 1704937 (2018).
Jin, J.-N. et al. Mechanical training enabled reinforcement of polyrotaxane-containing hydrogel. Angew. Chem. Int. Ed. 62, e202218313 (2023).
Li, C. et al. Photoswitchable and reversible fluorescent eutectogels for conformal information encryption. Angew. Chem. Int. Ed. 62, e202313971 (2023).
Tang, N. et al. Evolutionary reinforcement of polymer networks: a stepwise-enhanced strategy for ultrarobust eutectogels. Adv. Mater. 36, 2309576 (2024).
Hansen, B. B. et al. Deep eutectic solvents: a review of fundamentals and applications. Chem. Rev. 121, 1232–1285 (2021).
Mota-Morales, J. D. & Morales-Narváez, E. Transforming nature into the next generation of bio-based flexible devices: new avenues using deep eutectic systems. Matter 4, 2141–2162 (2021).
Zhang, Y., Wang, Y., Guan, Y. & Zhang, Y. Peptide-enhanced tough, resilient and adhesive eutectogels for highly reliable strain/pressure sensing under extreme conditions. Nat. Commun. 13, 6671 (2022).
Smith, E. L., Abbott, A. P. & Ryder, K. S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 114, 11060–11082 (2014).
Hua, M. et al. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 590, 594–599 (2021).
Yang, X. et al. Reinforcing hydrogel by nonsolvent-quenching-facilitated in situ nanofibrosis. Adv. Mater. 35, 2303728 (2023).
Wan, H., Wu, B., Hou, L. & Wu, P. Amphibious polymer materials with high strength and superb toughness in various aquatic and atmospheric environments. Adv. Mater. 36, 2307290 (2024).
Xu, L. et al. A solvent-exchange strategy to regulate noncovalent interactions for strong and antiswelling hydrogels. Adv. Mater. 32, 2004579 (2020).
Li, L. et al. Ultra-tough and recyclable ionogels constructed by coordinated supramolecular solvents. Angew. Chem. Int. Ed. 61, e202212512 (2022).
Christoff-Tempesta, T. et al. Self-assembly of aramid amphiphiles into ultra-stable nanoribbons and aligned nanoribbon threads. Nat. Nanotechnol. 16, 447–454 (2021).
Liang, X. et al. Impact-resistant hydrogels by harnessing 2D hierarchical structures. Adv. Mater. 35, 2207587 (2023).
Zhu, S. et al. Bioinspired structural hydrogels with highly ordered hierarchical orientations by flow-induced alignment of nanofibrils. Nat. Commun. 15, 118 (2024).
Lin, S. et al. Anti-fatigue-fracture hydrogels. Sci. Adv. 5, eaau8528 (2019).
Liang, X. et al. Anisotropically fatigue-resistant hydrogels. Adv. Mater. 33, 2102011 (2021).
Li, W. et al. Nanoconfined polymerization limits crack propagation in hysteresis-free gels. Nat. Mater. 23, 131–138 (2024).
Sun, X., Mao, Y., Yu, Z., Yang, P. & Jiang, F. A Biomimetic “salting out—alignment—locking” tactic to design strong and tough hydrogel. Adv. Mater. 36, 2400084 (2024).
Liu, Q. et al. 3D printable strong and tough composite organo-hydrogels inspired by natural hierarchical composite design principles. Nat. Commun. 15, 3237 (2024).
Xue, B. et al. Strong, tough, rapid-recovery, and fatigue-resistant hydrogels made of picot peptide fibres. Nat. Commun. 14, 2583 (2023).
Li, T. et al. Robust and sensitive conductive nanocomposite hydrogel with bridge cross-linking–dominated hierarchical structural design. Sci. Adv. 10, eadk6643 (2024).
Wang, X. et al. Stretch-induced conductivity enhancement in highly conductive and tough hydrogels. Adv. Mater. 36, 2313845 (2024).
Li, W. et al. Supramolecular ionogels tougher than metals. Adv. Mater. 35, 2301383 (2023).
Shi, Y., Wu, B., Sun, S. & Wu, P. Aqueous spinning of robust, self-healable, and crack-resistant hydrogel microfibers enabled by hydrogen bond nanoconfinement. Nat. Commun. 14, 1370 (2023).
Wu, S. et al. Spider-silk-inspired strong and tough hydrogel fibers with anti-freezing and water retention properties. Nat. Commun. 15, 4441 (2024).
Jiang, Y. et al. Strong and ultra-tough ionic hydrogel based on hyperbranched macro-cross-linker: influence of topological structure on properties. Angew. Chem. Int. Ed. 62, e202310832 (2023).
Wang, M. et al. Tough and stretchable ionogels by in situ phase separation. Nat. Mater. 21, 359–365 (2022).
Fang, Y.-H. et al. Toughening hydrogels with fibrillar connected double networks. Adv. Mater. 36, 2402282 (2024).
Wang, M. et al. Glassy gels toughened by solvent. Nature 631, 313–318 (2024).
Wu, Y. et al. Solvent-exchange-assisted wet annealing: a new strategy for superstrong, tough, stretchable, and anti-fatigue hydrogels. Adv. Mater. 35, 2210624 (2023).
Xiong, J. et al. Low-hysteresis and high-toughness hydrogels regulated by porous cationic polymers: the effect of counteranions. Angew. Chem. Int. Ed. 63, e202316375 (2024).
Zhang, G., Steck, J., Kim, J., Ahn, C. H. & Suo, Z. Hydrogels of arrested phase separation simultaneously achieve high strength and low hysteresis. Sci. Adv. 9, eadh7742 (2023).
Kim, J., Zhang, G., Shi, M. & Suo, Z. Fracture, fatigue, and friction of polymers in which entanglements greatly outnumber cross-links. Science 374, 212–216 (2021).
Liu, P., Zhang, Y., Guan, Y. & Zhang, Y. Peptide-crosslinked, highly entangled hydrogels with excellent mechanical properties but ultra-low solid content. Adv. Mater. 35, 2210021 (2023).
Acknowledgements
Thanks for the financial support of the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB0470103) for Y.F., the National Natural Science Foundation of China (52275219 for Y.F., U21A2046 for D.W.), the Program for Taishan Scholars of Shandong Province (No. ts20190965) for D.W., the Western Light Project of CAS (xbzg-zdsys−202118) for D.W., Major Science and Technology Projects in Gansu Province (No. 22ZD6GA002) for D.W., the Key Research and Development Program in Shandong Province (No. SYS202203) for D.W. and the Major Program of the Lanzhou Institute of Chemical Physics, CAS (No. ZYFZFX-5) for D.W.
Author information
Authors and Affiliations
Contributions
Conceptualization: W.L., D.W., Y.F., and C.X.; Methodology: Y.F., C.X., A.X., H.H., and Z.W.; Investigation: W.L., D.W., and C.X.; Visualization: Y.F., C.X., A.X., and H.H.; Supervision: W.L., D.W., and Y.F.; Writing—original draft: Y.F. and C.X.; Writing—review & editing: W.L. and D.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Wei Zhai and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, C., Xie, A., Hu, H. et al. Ultrastrong eutectogels engineered via integrated mechanical training in molecular and structural engineering. Nat Commun 16, 2589 (2025). https://doi.org/10.1038/s41467-025-57800-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-57800-y
This article is cited by
-
Continuous piezoionicity-driven electromechanical coupling in mechanically robust conductive eutectogels
Nature Communications (2025)