Abstract
Nurse cell (NC) dumping, a process essential for oocyte development, involves the rapid cytoplasmic transfer from germline-derived NCs into the oocyte. However, its regulatory mechanism remains unclear. Here, we report that ecdysone signaling in stretch follicle cells (SFCs) regulates NC dumping through dumpless1, a ZAD-C2H2 zinc finger transcription factor, in Drosophila. Ecdysone induced dumpless1 expression in SFCs, and CRISPR/Cas9-mediated knockout of dumpless1 or its functional domain ZAD suppresses NC dumping. Depletion of dumpless1 upregulates integrin βPS expression in SFC plasma membrane, while reducing cortical enrichment of Rho1 signaling-dependent phosphorylated myosin light chain (p-MLC) and disrupting actin cables organization in NCs. SFC-specific overexpression of integrin βPS reduces p-MLC enrichment in the NC cortex, whereas its knockdown in SFCs of dumpless1-/- mutants partially rescues NC dumping defect. Our findings identify dumpless1 as a critical effector of ecdysone signaling, bridging somatic-germline communication through the integrin βPS-Rho1-p-MLC axis, revealing a multicellular regulatory mechanism in Drosophila oogenesis.
Similar content being viewed by others
Introduction
Cellular coordination is vital for multicellular organisms and depends on effective cell-cell communication among diverse cell types and tissues1,2,3. This intricate communication network is crucial for maintaining homeostasis, guiding development and influencing disease processes4. Traditionally, cell-cell communication has been primarily attributed to secreted ligands and plasma membrane receptors. However, it also encompasses a broader array of mechanisms, including secretases, extracellular matrix proteins, transporters, and direct cell-contact mechanisms5. Investigating these complex interactions in vivo remains methodologically challenging. Drosophila oogenesis serves as an excellent model for investigating the spatial and temporal regulation of signaling, given its well-defined anatomy and compact structure.
In Drosophila oogenesis, the oocyte develops within a specialized multicellular structure known as the egg chamber. This chamber contains a central germline cyst, which consists of one oocyte and 15 nurse cells (NCs) interconnected via ring canals. The cyst is surrounded by a monolayer of somatic follicle cells (FCs). Throughout its development, the egg chamber progresses through 14 morphologically distinct stages before reaching maturation6,7. Precise regulation of cell-cell communication is essential for each stage of oocyte development. A key example is “NC dumping”, a process during stages 10B-12 in which NCs rapidly transfer their cytoplasmic contents to the oocyte via ring canals in preparation for embryonic development8. Disruption of NC dumping results in short eggs and sterility9. Although it is well-established that NC nuclear positioning mediated by actin cables, actomyosin contractility regulated by small GTPase Rho1 and the characteristics of ring canals are critical for this process10,11,12,13, the signal involved in NC dumping remains unclear. A recent study shows that genetic ablation of stretch FCs (SFCs) surrounding NCs disrupts this process14, indicating that SFC signals may play a role in NC dumping. Nonetheless, the exact mechanisms of SFC-NC communication that facilitate NC dumping are still unknown.
The steroid hormone ecdysone is known for its role in regulating developmental transitions in insect molting and metamorphosis. Recent studies have revealed that ecdysone is biosynthesized in the adult ovary15,16 and regulates multiple aspects of oogenesis, including early germ cell differentiation17,18, egg chamber progression19, morphogenesis of follicular epithelium20, follicle cells migration21, oocyte’s lipid accumulation22 and eggshell formation23. Ecdysone signaling operates via a heterodimeric nuclear receptor complex consisting of Ecdysone receptor (EcR) and Ultraspiracle (Usp)24,25. Notably, expression of a dominant negative (DN) form of EcR in border cells and SFCs during stages 10-14 inhibits NC dumping21, implicating that ecdysone signaling is involved in this process. However, the mechanisms by which ecdysone signaling regulates NC dumping remain unknown.
Our recent work elucidates the regulatory mechanism by which ecdysone regulates vitellogenesis in Bombyx mori oogenesis26. Here, we reveal the regulatory mechanism underlying ecdysone signaling-mediated SFC to NC communication during NC dumping in Drosophila oogenesis. In response to ecdysone stimulation, an uncharacterized zinc finger-associated domain-C2H2-zinc finger (ZAD-ZNF) transcription factor, named dumpless1, suppresses integrin βPS expression in SFCs. This, in turn, activates myosin-driven contraction in NCs by enhancing Rho1 signaling-dependent phosphorylated myosin light chain (p-MLC) cortical enrichment. Additionally, dumpless1 also facilitates actin cables organization in NCs, thus contributing to NC dumping. This study not only reveals how hormone regulation influences somatic-germ cell communication but also highlights the specific signaling pathways involved in NC dumping during oogenesis.
Results
Ecdysone signaling in SFCs regulates nurse cell dumping
To investigate in which cells ecdysone signaling might be transcriptionally active in regulating NC dumping, we first detected its activity within the egg chambers. We observed high levels of EcR protein accumulation in both SFCs and MFCs using antisera targeting a common domain of EcR throughout stages 10–12, coinciding with NC dumping. In contrast, an undetectable expression level of EcR was observed in NCs and the oocyte (Fig. 1a–f’). Among the three EcR isoforms (EcRA, EcRB1 and EcRB2), which differ in their N-terminal regions27, EcRB1 showed a similar localization pattern to EcR in the egg chambers at stages 10–12 (Supplementary Fig. 1a–f’), indicating that EcRB1 may serve as the primary mediator of ecdysone signaling in SFCs. Additionally, EcRE-lacZ, a transgenic reporter gene construct expressing β-galactosidase under the control of ecdysone response elements (EcREs)28, exhibited high expression levels of β-galactosidase in SFCs at stages 10–12, with undetectable expression levels in MFCs, NCs and oocyte (Fig. 1g–l’). These results indicate high ecdysone activity in SFCs during NC dumping.
a–f’ Confocal images of surface (a-a’, c-c’ and e-e’) and medial view (b-b’, d-d’ and f-f’) of EcR expression (green) in egg chambers at stages 10–12, detected by an anti-EcR antibody. g–l’ Confocal images of surface (g-g’, i-i’ and k-k’) and medial view (h-h’, j-j’ and l-l’) of ecdysone signaling activity (red) in the egg chambers at stages 10–12 of EcRE-lacZ reporter constructs, detected by an anti-β-galactosidase antibody. m-n” EcRDN overexpression in SFCs impedes NC dumping. Egg chambers at stage 13 are dissected from the controls (Gal80ts; c329b > ) (m-m”) and EcRDN overexpressed ovaries (Gal80ts; c329b > EcRDN) (n-n”). Nuclei are marked by DAPI in cyan. The yellow dashed line in (n-n’) marks untransferred NC cytoplasm and intact NC nuclei. The scale bar represents 100 µm. NC nurse cell, SFC stretch follicle cell, MFC mainbody follicle cell, BC border cell, OC oocyte, DA dorsal appendage.
To further determine whether ecdysone signaling in SFCs regulates NC dumping, EcR function in SFCs was disrupted by overexpressing a dominant negative form of EcR (EcRDN) driven by c329b-Gal4, a SFCs-driver29. Overexpressing EcRDN in SFCs resulted in “dumpless” egg chambers, showing retention of NC cytoplasm and intact NC nuclei (marked by a yellow dashed line) even by stage 13, when dorsal appendages (black arrow) formed (Fig. 1n-n”), compared to the control (Fig. 1m-m”). Collectively, these results suggest that ecdysone signaling regulates NC dumping through SFC-NC communication.
Screening of transcription factors in response to ecdysone signaling in SFCs during NC dumping
Transcription factors (TFs) are key mediators of the signaling pathways. To identify TFs that participate in NC dumping and are mediated by ecdysone signaling, we performed two comparative transcriptome analyses: (1) between stage 9 egg chambers (before NC dumping) and stages 10–12 (during NC dumping), and (2) between ovaries of Gal80ts; c329b and Gal80ts; c329b > EcRDN females at 40 h after eclosion (Supplementary Fig. 2a–c). A total of 41 upregulated TFs were identified during NC dumping (Stages 10–12 vs. Stage 9; |log2 (Stage 10–12/Stage 9)| > 1 and p-value < 0.05; Supplementary Fig. 2d), indicating their potential roles in regulating NC dumping. Conversely, 30 TFs were downregulated in EcRDN-expressing ovaries (|log2 (Gal80ts; c329b > EcRDN/Gal80ts; c329b > )| > 1 and p-value < 0.05; Supplementary Fig. 2e), implying that these TFs may be positively regulated by ecdysone signaling. Strikingly, 10 TFs were upregulated in the egg chambers during NC dumping, but downregulated in the ovaries upon EcRDN overexpression in SFCs (Fig. 2a), indicating their possible involvement in ecdysone-mediated NC dumping regulation. In Drosophila, stages 10–12 egg chambers consist of one oocyte localized at the posterior, surrounded by mainbody FCs (MFCs), terminal FCs (TFCs), polar cells (PCs) and dorsal appendage forming FCs (DAFFCs), and 15 NCs surrounded by SFCs, which are differentiated at stage 9 (Fig. 2b and30). Among the candidate TFs, six of these TFs (grau, CG12942, Sry-beta, Hmg-2, dwg and Nf-YA) exhibited relatively high expression levels in SFCs, and in particular, CG12942, an uncharacterized TF, displayed the highest and specific expression as detected by Fly Cell Atlas single-cell RNA-seq (http://flybase.org/) (Fig. 2c), indicating that SFCs might facilitate NC dumping via these TFs. Previous studies showed that Grau was mainly expressed in NCs and early follicle cells using Grau::GFP transgenic line31 and Sry-beta was mainly expressed in NCs and oocyte using Sry-β-LacZ transgenic line32. Mutation of Hmg-2 and Nf-YA causes pupal and late larval lethality, respectively, based on the FlyBase data (http://flybase.org/). Therefore, RNA interference (RNAi)-mediated knockdown of CG12942 and dwg in SFCs was then performed using the c329b-Gal4 driver, respectively. Knockdown of CG12942 in SFCs resulted in short eggs, consistent with “dumpless” phenotype9, whereas knockdown of dwg did not (Fig. 2d, e and Supplementary Data 1). Additionally, CG12942 expression showed specifically high levels in the ovary by Fly Cell Atlas microarray data (http://flybase.org/) (Supplementary Fig. 2f), implying its specific function in female reproduction.
a A Venn diagram representation of TFs that are upregulated in egg chambers during NC dumping (red circle) but downregulated in the ovaries after ecdysone signaling disruption in SFCs (blue circle). b Schematic representation of the structure of a stage 10 egg chamber in Drosophila. c Heatmap showing relatively expressed levels of differentially expressed TFs in different types of cells in an egg chamber at stage 10. d, e Changes in mature egg length after RNAi knockdown of differentially expressed TFs in SFCs driven by c329b-Gal4 (n = 36). Two-tailed t-test was performed. Data are presented as mean values ± SD. f–i’ Confocal medial view images of CG12942 expression (red) in egg chambers of w1118 at stages 9–12, detected by an anti-CG12942 antibody. The yellow arrow indicates newly formed SFCs. j–m’ Confocal images of medial view presenting CG12942-GFP fusion protein (green) in the egg chambers of CG12942::GFP line at stages 9–12. n, o CG12942 expression (red) in the egg chambers at stages 10–11, detected by an anti-CG12942 antibody, in the flip-out Gal4 clones of EcRDN overexpression (green). p EcRE consensus sequence and predicted binding sequence of EcR/USP complex upstream of CG12942. Nuclei are marked by DAPI in cyan. The scale bar represents 50 µm, respectively. SFC stretch follicle cell, MFC mainbody follicle cell, OC oocyte, NC nurse cell, PC polar cell, TFC terminal follicle cell, DAFFC dorsal appendage forming follicle cell, RC ring canal.
To determine the role of CG12942 in ecdysone signaling-induced regulation of NC dumping, spatiotemporal expression pattern of CG12942 in the egg chambers was detected using an anti-CG12942 antibody, which detected a prominent protein band, along with a faint secondary band by western blot analysis (Supplementary Fig. 4e). At stage 9, weak nuclear CG12942 expression was observed in anterior FCs, which are newly formed SFCs (Fig. 2f-f’ and Supplementary Fig. 3a-a’; yellow arrow). Strikingly, CG12942 expression was increased in SFC nuclei by stage 10 and remained high expression through stages 11–13 (Fig. 2g–i’and Supplementary Fig. 3b–e’). To validate these observations, we analyzed a C-terminal GFP-tagged CG12942 knock-in reporter line (CG12942::GFP, BDSC, stock #66394), generated by the model organism Encyclopedia of Regulatory Network (modERN) Project. The anti-CG12942 antibody recognized both endogenous CG12942 (red arrow) and the CG12942-GFP fusion protein (black arrow) in ovaries of the CG12942::GFP line (Supplementary Fig. 4f), confirming the line’s integrity. Consistent with antibody staining, CG12942-GFP localized in SFC nuclei at stages 10-12, with undetectable expression in other cell types (Fig. 2j–m’ and Supplementary Fig. 3f–j’). Subsequently, CG12942 promoter-driven GFP expression was localized in SFCs at stages 10-13 (Supplementary Fig. 3k–o’), confirming that CG12942 is specifically expressed in SFCs. These findings are consistent with the data of Fly Cell Atlas single-cell RNA-seq (http://Flybase.org) (Fig. 2c), collectively demonstrating that CG12942 is a nuclear-localized protein specifically enriched in SFCs during NC dumping.
To further investigate the role of ecdysone signaling in regulating CG12942 expression, we disrupted ecdysone signaling using the Flip-out Gal4 system to overexpress EcRDN. The EcRDN-overexpressing SFCs (marked by GFP) showed a complete loss of CG12942 expression (red) at stages 10–11, compared to the neighboring GFP-negative control cells (Fig. 2n, o), suggesting that CG12942 expression in SFCs is indeed ecdysone-dependent. Additionally, two putative EcREs (-1728 nt-1714 nt, AATTAGTTGAAATA; -1671 nt-1657 nt, ACTTCGGTGAAATT) were predicted in the 2000 bp upstream regulatory region of CG12942 (Fig. 2p), based on the consensus EcRE sequence (5’-(A/G)G(G/T)TCANTGA(C/A)C(C/T)-3’) for 20E/EcR/USP binding33. The presence of these potential EcREs suggests that ecdysone signaling may directly induce CG12942 transcription through EcR/USP complex binding to its promoter region.
CG12942 (dumpless1) in SFCs controls NC dumping
To further confirm that CG12942 functions in NC dumping, the D. melanogaster CG12942 mutant line was established using CRISPR/Cas9-mediated genome editing. CG12942 encodes a protein characterized by a zinc finger-associated domain (ZAD), unique to arthropods, at its N-terminus and ten ZnF-C2H2 domains at the C-terminus (Supplementary Fig. 4a). Two small guide RNA (sgRNA) target sites were identified on exon 1 of CG12942 (Supplementary Fig. 4b). The mixture of the synthesized sgRNA and Cas9 protein was injected into preblastoderm embryos of flies. Two mutant genotypes were identified: one with a 1-bp deletion in exon 1, leading to a premature stop codon at the 34th amino acid, and the other with a 351-bp deletion in exons 1 and 2, resulting in a loss of 117 amino acids (positions 38-154), including most of the ZAD (Supplementary Fig. 4c, d). Western blot analysis with the anti-CG12942 antibody confirmed a significantly reduced CG12942 expression in CG12942-/- mutants and a smaller protein product in CG12942ΔZAD mutants (Supplementary Fig. 4e and Supplementary Data 1). The developmental duration of CG12942-/- mutants was not obviously different from the control flies w1118 (Supplementary Fig. 4g, h and Supplementary Data 1), indicating that CG12942 does not function in general development. Although early egg chambers (stages 1-10 A) appeared normal, the proportion of oocytes in stage 10B egg chambers was significantly reduced in the mutants, compared to the control flies w1118 (Supplementary Fig. 5a–j and Supplementary Data 1). By stage 13, egg chambers of CG12942-/- and CG12942ΔZAD mutants retained substantial NC cytoplasm (marked by a yellow dashed line) despite dorsal appendage formation (red arrow), producing shorter eggs than control w1118 flies (Fig. 3a–i). Quantitative analysis revealed that the mature eggs of CG12942-/- mutants were both shorter and wider than the controls, while the mature eggs of CG12942ΔZAD were only shorter (Fig. 3j, k and Supplementary Data 1). To further confirm that NC dumping was arrested by CG12942 null allele, the egg chambers at stage 10 were dissected from w1118 and CG12942-/- mutants, and cultured in vitro for live imaging. Strong and rapid cytoplasmic flow was observed in the cultured egg chambers isolated from the control flies, with average NC cytoplasmic transfer rates of 0.19% per minute. By contrast, slow and weak NC cytoplasmic flow occurred, with average NC cytoplasmic transfer rates of 0.09% per minute (Fig. 3l, Supplementary Movie 1 and Supplementary Data 1), indicating a defect in NC dumping. Crucially, SFC-specific expression of CG12942 in CG12942-/- mutants, driven by c329b-Gal4, partially rescued egg length defects (Fig. 3m, n and Supplementary Data 1), confirming that the defective egg length phenotype was caused by CG12942 mutation. These results reveal that CG12942 plays an important role in NC dumping, and its mutation results in a “dumpless” phenotype. Hence, CG12942 is named dumpless1.
a–i Egg chamber at stage 13, stained with phalloidin to visualize F-actin (red) at the medial focal plane, and mature eggs in control flies w1118 (a–c), CG12942-/- (d–f) and CG12942∆ZAD mutants (g–i). The yellow dashed line marks dumping uncompleted NCs. The scale bar represents 100 µm. NC nurse cell, OC oocyte, DA dorsal appendage. j, k Measurements of mature egg length (j) and width (k) in w1118, CG12942-/- and CG12942∆ZAD mutants (x-axis) (w1118, n = 102; CG12942-/-, n = 108; CG12942∆ZAD, n = 121). Two-tailed t-test was performed. Data are presented as mean values ± SD. l Average NC (Nurse cell)/EC (Egg chamber) ratio during NC dumping in w1118 and CG12942-/- mutants. Ex vivo NC dumping rates in control and CG12942 mutant egg chambers are determined. Time zero is defined as the time when the egg chamber reaches stage 10 (n = 5). Data are presented as mean values ± SEM. m Overexpression of CG12942 in SFCs rescues the egg length defects in CG12942-/- mutants. The scale bar represents 100 µm. n Quantification of length of mature eggs from the controls (CG12942-/-; c329b > ) and overexpressing CG12942 with HA-tag in SFCs of CG12942 mutants (CG12942-/-; c329b>: n = 62; CG129421-/-; c329b > CG12942-3×HA: n = 99). Two-tailed t-test was performed. Data are presented as mean values ± SD. o-p” The ZAD enhances the nuclear localization of dumpless1. Kc cells were transfected with pdumpless1-EGFP (o-o”) or pdumpless1∆ZAD-EGFP (p-p”). The scale bar represents 10µm. Nuclei are marked by DAPI in cyan. N nuclei, C cytoplasm.
The similar phenotypes between CG12942-/- and CG12942ΔZAD mutants (Fig. 3a–k and Supplementary Fig. 5a–i) suggest that ZAD is essential for dumpless1 function. To further investigate the specific function of ZAD, the subcellular localization of EGFP-fused dumpless1 and EGFP-fused dumpless1ΔZAD was examined in Kc cells. EGFP-dumpless1 was localized exclusively to the nuclei (Fig. 3o-o”), whereas EGFP-dumpless1ΔZAD was present in both the nuclei and cytoplasm (Fig. 3p-p”), implying that ZAD may function in enhancing the nuclear localization of dumpless1.
Collectively, dumpless1 is necessary for the rapid transfer of NC cytoplasm to the oocyte starting from stage 10B during oogenesis, and its mutation or even ZAD region deletion would cause incomplete NC dumping, leading to short eggs.
dumpless1 regulates Rho1 signaling-dependent p-MLC cortical enrichment and actin cable formation during NC dumping
To elucidate the mechanism underlying the arrest of NC dumping in dumpless1-/- mutants, we performed RNA-seq analysis comparing ovaries from w1118 and dumpless1-/- 7-day-old adults. A total of 1,403 differentially expressed genes (DEGs) (|log2 (dumpless1-/-/w1118)| > 1.5 and p-value < 0.05) were identified (Fig. 4a), and they were enriched in GO catalogs related to Rho protein signal transduction, regulation of Rho protein signal transduction, regulation of actomyosin structure organization, cortical actin cytoskeleton and myosin light chain binding (Fig. 4b). The actomyosin cytoskeleton, consisting of filamentous actin (F-actin) and the motor protein, myosin II, is evolutionarily conserved and essential for generating mechanical forces in biological process34. Rho1, a small GTPase, is critical for the phosphorylation of myosin light chain (p-MLC) via its effector ROK and for F-actin polymerization, thereby driving actomyosin contraction35. Notably, Rho1 signaling-activated p-MLC is crucial for NC dumping, and the mutation of phosphorylated sites results in “dumpless” phenotype10,13,36. Immunofluorescence analysis revealed stage-dependent Rho1 localization in NCs of the w1118 flies: minimal at stage 9 (Fig. 4c-c’), but strongly enriched at the cortical region (submembrane; white arrow) and cytoplasm (cyan arrow) during stages 10–12 (Fig. 4f-f’, i-i’ and l-l’). In contrast, both dumpless1-/- and dumpless1ΔZAD mutants showed significantly reduced cortical (white arrow) and cytoplasmic (cyan arrow) Rho1 accumulation in NCs at stages 10–12, compared to the control (Fig. 4f–n’), with quantitative analysis confirming these defects (Fig. 4o–p and Supplementary Data 1). These results suggest that dumpless1 plays a regulatory role in both cortical and cytoplasmic Rho1 enrichment in NCs during NC dumping.
a Analysis of DEGs and PCA of RNA-seq data from the 7-day-old adult ovaries of w1118 and dumpless1-/- mutants. b Barplots of GO-enriched biological processes for DEGs associated with Rho1 signaling. c–n’ Confocal images of Rho1 accumulation in NCs at stages 9–12 in w1118 (c-c’, f-f’, i-i’ and l-l’), dumpless1-/- (d-d’, g-g’, j-j’ and m-m’) and dumpless1∆ZAD mutants (e-e’, h-h’, k-k’ and n-n’), stained with an anti-Rho1 antibody. The white and cyan arrows indicate the cortex and cytoplasm of NCs, respectively. F-actin is marked by phalloidin in magenta. The bar represents 50 µm. NC nurse cell, OC oocyte. o Quantified fluorescence intensity of Rho1 in NC cortex at stages 9–12 of w1118, dumpless1-/- and dumpless1∆ZAD mutants. From left to right: n = 4, 6, 4, 11, 11, 15, 16, 15, 16, 15, 16, 16, respectively. p Quantified fluorescence intensity of Rho1 in NC cytoplasm at stages 10–12 of w1118, dumpless1-/- and dumpless1∆ZAD mutants. From left to right: n = 12, 11, 13, 15, 15, 15, 12, 15, 15, respectively. Two-way ANOVA was made for multiple comparisons. Data are presented as mean values ± SD.
Furthermore, the immunofluorescence analysis using an anti-p-MLC antibody demonstrated an undetectable level of p-MLC in NCs at stage 9 (Fig. 5a-a’), but progressive cortical enrichment (yellow arrow) from stages 10–12 in the w1118 flies (Fig. 5d-d’, g-g’ and j-j’), which was attenuated in both mutants (Fig. 5e, f’, h, i’, k, l’, m and Supplementary Data 1). These results suggest that dumpless1 regulates Rho1 signaling-dependent p-MLC cortical enrichment in NCs during NC dumping.
a–l’ Confocal images of p-MLC in NCs at stages 9–12 in w1118 (a-a’, d-d’, g-g’ and j-j’), dumpless1-/- (b-b’, e-e’, h-h’ and k-k’) and dumpless1∆ZAD mutants (c-c’, f-f’, i-i’ and l-l’), stained with a p-MLC antibody regonized Ser 21 p-MLC. m Quantified fluorescence intensity of p-MLC in NC cortex at stages 10–12 of w1118, dumpless1-/-and dumpless1∆ZAD mutants. From left to right: n = 15, 15, 13, 20, 20, 16, 15, 16, 15, respectively. Two-way ANOVA was made for multiple comparisons. n–s’ Confocal images of p-MLC in NC cortex at stages 10–12 of Gal80ts; c329b > (n–p’) and Gal80ts; c329b > EcRDN (q–s’) females. t Quantified fluorescence intensity of p-MLC in NC cortex at stages 10–12 of Gal80ts; c329b> and Gal80ts; c329b > EcRDN females. From left to right: n = 13, 13, 13, 15, 12, 15, respectively. Two-tailed t-test was performed. Nuclei are marked by DAPI in cyan. The yellow arrows indicate the cortex of NCs. The bar represents 50 µm. NC nurse cell, SFC stretch follicle cell, MFC mainbody follicle cell, OC oocyte. Data are presented as mean values ± SD.
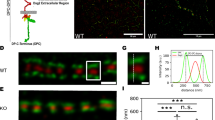
Since NC nuclear repositioning mediated by actin cables is also critical for NC dumping12, the actin cables in NCs were also observed. F-actin levels in the NC cortex (yellow arrow) at stages 9–12 of both dumpless1-/- and dumpless1ΔZAD mutants appeared normal, compared to the controls (Fig. 6a–l’, m and Supplementary Data 1). However, the actin cables intensity (pink arrow) showed reduction in NCs at stages 11–12 of both dumpless1-/- and dumpless1ΔZAD mutants, compared to the controls (Fig. 6a–l’, n and Supplementary Data 1), implying that the disruption of actin cables in NCs at stages 11–12 of dumpless1-/- and dumpless1ΔZAD mutants may represent an additional factor contributing to the obstruction of NC dumping.
a–l’ Images of F-actin (red) in NCs at stages 9–12 of w1118 (a-a’, d-d’, g-g’ and j-j’), dumpless1-/- (b-b’, e-e’, h-h’ and k-k’) and dumpless1∆ZAD mutants (c-c’, f-f’, i-i’ and l-l’). m, n Quantified fluorescence intensity of F-actin in the NC cortex and actin cables in NCs at stages 10–12 of w1118, dumpless1-/- and dumpless1∆ZAD mutants, respectively. m From left to right: n = 14, 14, 15, 11, 12, 13, 9, 12, 15, respectively. n From left to right: n = 11, 14, 13, 11, 12, 13, 9, 13, 14, respectively. Two-way ANOVA was made for multiple comparisons. o–t’ Confocal images of F-actin in NCs at stages 10–12 of Gal80ts; c329b > (o–q’) and Gal80ts; c329b > EcRDN (r–t’) females. u, v Quantified fluorescence intensity of F-actin in the NC cortex and actin cables in NCs at stages 10–12 of Gal80ts; c329b> and Gal80ts; c329b > EcRDN. u From left to right: n = 13, 13, 13, 15, 11, 14, respectively. v From left to right: n = 13, 13, 11, 11, 9, 14, respectively. Two-tailed t-test was performed. F-actin is marked by phalloidin in red. Nuclei are marked by DAPI in cyan. The yellow and pink arrows indicate cortical F-actin and cytoplasmic actin cables, respectively. The scale bar represents 50 µm. NC nurse cell, OC oocyte. Data are presented as mean values ± SD.
Given that the dumpless1 expression is ecdysone-dependent, we examined whether ecdysone signaling affects p-MLC cortical enrichment and actin cable organization in NCs. Indeed, EcRDN overexpression in SFCs significantly reduced cortical p-MLC enrichment (Fig. 5n–s’, t and Supplementary Data 1) and actin cable intensity (Fig. 6o–t’, u, v and Supplementary Data 1) in NCs at stages 10–12, compared to the controls, demonstrating that ecdysone signaling regulates p-MLC cortical enrichment and actin cable formation in NCs via dumpless1.
dumpless1 regulates Rho1 signaling-dependent p-MLC cortical enrichment in NCs via integrin βPS
To investigate how ecdysone-induced dumpless1 expression in SFCs regulates Rho1 signaling-dependent p-MLC enrichment in the NC cortex, we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of DEGs between the Gal80ts; c329b > EcRDN and Gal80ts; c329b > , as well as between w1118 and dumpless1-/- ovaries. Notably, the top 10 enriched pathways in both RNA-seq datasets included the extracellular matrix (ECM)-receptor interaction pathway (Fig. 7a, b). Within this pathway, the expression levels of integrin βPS, also known as myospheroid (mys) in Drosophila, were upregulated in both RNA-seq datasets (Fig. 7c, d). Further analysis using an anti-integrin βPS antibody revealed undetectable integrin βPS levels in SFCs of stage 9 egg chambers (Fig. 7e-e’ and Supplementary Fig. 6c-c’), consistent with the previous observation37. From stage 10 to stage 12 in w1118 flies, integrin βPS signals gradually appeared in the SFC plasma membrane (white arrow), where they colocalized with a membrane-tethered GFP signal specially expressed in FCs, but were absent in NCs (Fig. 7f-f’, i-i’, l-l’, o, Supplementary Fig. 6a, d-d’, g-g’, j-j’ and Supplementary Data 1). In contrast, integrin βPS signals were significantly elevated in the SFC plasma membrane of dumpless1-/- and dumpless1ΔZAD mutants at stages 10–12, compared to controls (Fig. 7f–n’, p, Supplementary Fig. 6d–l’ and Supplementary Data 1). Notably, integrin βPS was also detected on both the outer and NC-facing membranes of SFCs (Supplementary Fig. 6b). Furthermore, overexpression of EcRDN in SFCs driven by c329b-Gal4> increased integrin βPS expression in SFCs (white arrow), compare to the controls (Fig. 7q–s and Supplementary Data 1). Given that the integrins regulate Rho1 signaling38, we propose that the elevated expression levels of integrin βPS may reduce Rho1 signaling-dependent p-MLC in the NC cortex, contributing to the defective NC dumping phenotype observed in dumpless1-/- and dumpless1ΔZAD mutants in a cell-nonautonomous manner.
a, b Barplots of the KEGG pathways enriched for the DEGs in the ovaries between Gal80ts; c329b> and Gal80ts; c329b > EcRDN (a) and between w1118 and dumpless1-/- mutant females (b), respectively. The red asterisk indicates the shared KEGG pathways. c A Venn diagram showing the overlapping genes in the ECM-receptor interaction pathway enriched in both RNA-Seq datasets. d Differences in the expression of ECM-receptor integrin βPS in the ovaries of two pairs of comparison. e–n’ Integrin βPS expression (green) in the egg chambers at stages 9–12, detected by anti-integrin βPS antibody, from w1118 (e-e’, f-f’, i-i’ and l-l’), dumpless1-/- (g-g’, j-j’ and m-m’) and dumpless1∆ZAD (h-h’, k-k’ and n-n’) mutant. o Quantified fluorescence intensity of integrin βPS in the plasma membrane of SFCs at stages 9–12 of w1118 (From left to right: n = 6, 14, 14, 13, respectively). Different lowercase letters represent statistical significance by one-way ANOVA. p Quantified fluorescence intensity of integrin βPS in the plasma membrane of SFCs at stages 10–12 of w1118, dumpless1-/- and dumpless1∆ZAD mutants. From left to right: n = 14, 15, 12, 14, 16, 14, 13, 12, 14, respectively. Two-way ANOVA was made for multiple comparisons. q-r’ Integrin βPS expression (green) in egg chambers at stage 11 from the control (Gal80ts; c329b > ) and ecdysone-disrupted ovaries (Gal80ts; c329b > EcRDN). s Quantification of fluorescence intensity of integrin βPS in the plasma membrane of SFCs at stage 11 of the control (n = 6) and ecdysone-disrupted ovaries (n = 8). Two-tailed t-test was performed. F-actin is marked by phalloidin in magenta. The white arrow indicates the plasma membrane of SFCs. Nuclei are marked by DAPI in cyan. The scale bar represents 50 µm. NC nurse cell, SFC stretch follicle cell, MFC mainbody follicle cell, OC oocyte. Data are presented as mean values ± SD.
To verify this hypothesis, overexpression of integrin βPS in SFCs was conducted, and this resulted in the decreased Rho1 signaling-dependent p-MLC enrichment in the NC cortex (yellow arrow) at stages 10–11 (Fig. 8a–f and Supplementary Data 1). Conversely, Rho1 signaling-dependent p-MLC enrichment in the NC cortex (yellow arrow) was increased after integrin βPS expression was knocked down in SFCs of dumpless1-/- mutants, compared with the controls (Fig. 8g–n and Supplementary Data 1), indicating that dumpless1 negatively regulates integrin βPS to maintain proper Rho1 signaling. Importantly, reducing integrin βPS expression in SFCs of dumpless1-/- mutants partially restored the length of mature eggs (Fig. 8o and Supplementary Data 1), suggesting a partial rescue.
a–d’ Changes of p-MLC (red) enrichment in the NCs at stages 10–11 after overexpression of integrin βPS (green) in SFCs. e, f Quantified fluorescence intensity of integrin βPS in the plasma membrane of SFCs and p-MLC in the NC cortex at stages 10-11 of Gal80ts; c329b> and Gal80ts; c329b>mys lines, respectively. e From left to right: n = 12, 13, 13, 14, respectively. f From left to right: n = 15, 13, 14, 11, respectively. g–l’ Changes of p-MLC (red) enrichment in NCs at stages 10–12 after knockdown of integrin βPS (green) in SFCs of dumpless1-/- mutants. m, n Quantified fluorescence intensity of integrin βPS in the plasma membrane of SFCs and p-MLC in the NC cortex at stages 10–12 of dumpless1-/-; Gal80ts; c329b>and dumpless1-/-; Gal80ts; c329b>mysRNAi line, respectively. m From left to right: n = 6, 6, 11, 14, 13, 10, respectively. n From left to right: n = 8, 6, 11, 14, 13, 10, respectively. o Knockdown of integrin βPS in SFCs partially rescues the egg length defects in dumpless1-/- mutants (n = 52). Two-tailed t-test was performed. The yellow arrows indicate the cortex of NCs. The scale bar represents 50 µm. NC nurse cell, SFC stretch follicle cell, MFC mainbody follicle cell, OC oocyte. Two-tailed t-test was performed. Data are presented as mean values ± SD.
Taken together, these results demonstrate that dumpless1, induced by ecdysone signaling in SFCs, modulates Rho1 signaling-dependent cortical p-MLC enrichment in NCs by suppressing integrin βPS expression levels in SFCs, thereby ensuring proper NC dumping in oogenesis.
Discussion
Oogenesis requires precise coordination of signaling among multiple cell types. NC dumping, which occurs from stage 10B to stage 12, is essential for egg production and maturation, and its failure can lead to sterility39. Ecdysone, particularly 20-hydroxyecdysone (20E), is primarily synthesized in MFCs of the adult ovary15,16, and disruption of its signaling in SFCs inhibits NC dumping (Fig. 1m-n”). Here, we identify dumpless1, a ZAD-ZNF transcription factor, as a key mediator of ecdysone signaling in SFCs, which orchestrates NC dumping. We propose a model wherein ecdysone signaling induces dumpless1 expression in SFCs, which suppresses integrin βPS expression to activate Rho1 signaling-dependent p-MLC cortical enrichment, thereby promoting myosin-driven contraction of NCs to rapidly transfer NC cytoplasm to the developing oocyte (Fig. 9). In addition, dumpless1 also plays an important role in the organization of actin cables in NCs that contribute to dumping. This complex interaction involves communication among MFC, SFC, NC and the oocyte.
In responding to MFC-synthesized 20E stimulation, dumpless1 in SFCs suppresses ECM-receptor integrin βPS expression, which in turn activates Rho1-dependent cortical p-MLC enrichment to initiate actomyosin contraction. Additionally, dumpless1 also facilitates actin cables organization in NCs, thus contributing to NC dumping.
An interesting finding of this study is that ecdysone signaling mediates SFC-NC-oocyte communication to support oocyte development. We observe active ecdysone signaling in SFCs during stages 10–12, as evidenced by the presence of EcR, EcRB1 and EcRE-lacZ (Fig. 1a–l’ and Supplementary Fig. 1). Disrupting EcR function in SFCs results in the retention of NC cytoplasm (Fig. 1m-n”), confirming that ecdysone signaling is required for NC dumping. These results align with known roles of ecdysone in Drosophila oogenesis, including early germ cell differentiation17,18, egg chamber progression19,40,41, lipid metabolism and storage42, follicle cell polarity21, border cell migration43, and the endocycle-to-gene-amplification switch at stage 10B44. While these studies primarily focus on cell-autonomous effects, our findings emphasize the importance of ecdysone signaling in coordinating somatic-germ cell communications during NC dumping.
A key mechanism underlying ecdysone-dependent control of NC dumping relies on SFC-expressed dumpless1. Ecdysone synthesis rises at stage 9, peaks at stage 1045, and activates strong signaling in anterior FCs at stage 9, including newly formed SFCs43. dumpless1 expression initiates in newly formed SFCs at stage 9 (Fig. 2f-f’ and Supplementary Fig. 3a-a’), and reaches its peak in SFCs at stages 10-13 (Fig. 2g–m’ and Supplementary Fig. 3). Disruption of ecdysone signaling in SFCs results in the reduced dumpless1 expression (Fig. 2n, o), confirming that specific expression of dumpless1 in SFCs is controlled by ecdysone signaling. While these findings establish dumpless1 as an ecdysone signaling target, its precise spatiotemporal expression pattern in SFCs remains to be fully characterized. We speculate that this regulation may involve negative modulators of ecdysone signaling originating from other cell types, similar to the BTB protein Abrupt, which spatially restricts EcR activity during border cell migration43. Consistent with its regulatory role, both of EcRDN overexpression in SFCs and dumpless1 mutants result in a “dumpless” phenotype (Fig. 1m-n”, Fig. 3 and Supplementary Movie. 1). Additionally, we notice that the proportion of oocytes in stage 10 egg chambers was significantly reduced in the mutants, compared to the control flies w1118 (Supplementary Fig. 5a–j and Supplementary Data 1). Given that oocyte growth depends heavily on cytoplasmic contributions from interconnected NCs46, and considering the two phases of cytoplasmic streaming during Drosophila oogenesis, slow streaming (stages 7–10 A) and fast streaming (NC dumping, stages 10B-12)47, we speculate that dumpless1 expression in stages 9–10 A SFCs may additionally regulate slow cytoplasmic streaming. Thus, these results establish dumpless1 as a downstream effector of ecdysone signaling to regulate cytoplasmic streaming.
dumpless1 belongs to the ZAD-ZNF gene family (Supplementary Fig. 4a), which has expanded significantly in insect lineages and is the most abundant class of TFs in many genomes, including D. melanogaster48. Over 90 ZAD-ZNF genes are present in Drosophila, with many expressed in the ovaries and early embryos, suggesting lineage-specific adaptations in reproductive biology49. Interestingly, functional screening indicates that only 8 of 68 tested ZAD-ZNF genes are required in germ cells for oogenesis50. Our study reveals that dumpless1 regulates oocyte development through somatic-germline interactions. This suggests that highly expressed ovarian ZAD-ZNF genes may frequently function in a cell-nonautonomous manner to coordinate oocyte development.
We further reveal that ecdysone signaling-induced dumpless1 regulates Rho1 signaling-dependent p-MLC cortical enrichment and actin cables organization in NCs via integrin βPS in SFCs (Figs. 4–8), linking ecdysone signaling to cytoskeletal remodeling. This parallels findings that inhibition of EcR affects the actin cytoskeleton51, further emphasizing its influence on cytoskeletal dynamics. Interestingly, integrin βPS expression is increased in the plasma membrane of SFCs at stages 10–12, but it does not do so in NCs of dumpless1-/- and dumpless1ΔZAD mutants compared to the control (Fig. 7f–n’, p). This finding is consistent with a recent study reporting undetectable integrin βPS levels in NCs37. Together, these results suggest that integrin βPS regulates NC dumping in a non-cell-autonomous manner. Integrins are well-established mediators of bidirectional signaling-transmitting outside-in signals through ECM-activated FAK/Src, MAPK and Rho GTPase pathways, and inside-out signals via talin/kindlin regulation52. Rho1 activity can be activated by integrin engagement in epithelial cells53 or transiently suppressed following integrin stimulation54,55. Our data reveal that SFC-specific integrin βPS overexpression decreases cortical p-MLC enrichment in adjacent NCs (Fig. 8a–f), whereas its knockdown in SFCs of dumpless1-/- mutants partially restores p-MLC cortical enrichment in NCs (Fig. 8g–o). This indicates that dumpless1 normally suppresses integrin βPS in SFCs to maintain proper Rho1/p-MLC activation in NCs, though the precise inhibitory mechanism remains unclear. Given that mechanical signals regulate Rho1 function to control pulsatile actomyosin contraction and coordinate cell behavior across the epithelium56, we speculate that mechanical tension shielding, whereby enhanced integrin βPS-ECM adhesion in SFCs reduces mechanical stress transmission to NCs, attenuating Rho1 signaling activation. Additionally, a recent study has shown that β3-integrin in endothelial cells regulates communication with tumor cells, and the loss of β3-integrin expression in endothelial cells stimulates the MEK1-ERK1/2-ROCK2-dependent signaling to activate p-MLC in tumor cells via FAK-p-Akt-p65-driven cytokine production CCL257. Thus, paracrine suppression, wherein integrin βPS overexpression, may inhibit secretion of cytokines, such as TGF-β1, which regulates Rho signaling during pulp repair58, to attenuate Rho1-activating signals in NCs. Future studies should employ tension biosensors and cell-specific genetic perturbations to dissect the dominant mechanism.
Finally, we find that the ZAD governs the in vivo function of dumpless1. The ZAD, found at the N-terminus of many ZNF TFs in arthropods48, facilitates the formation of homodimers or heterodimers59,60. Our results show that deletion of the ZAD region in dumpless1 increases integrin βPS expression in SFCs (Fig. 7f–p) and reduces Rho1 signaling-dependent p-MLC enrichment in the NC cortex (Fig. 5d-m), leading to the “dumpless” phenotype (Fig. 3a–i). This strongly suggests that ZAD is critical for dumpless1’s function in vivo, possibly by influencing its nuclear localization (Fig. 3o–p”), which might be necessary for its transcriptional regulatory activity.
In conclusion, this study identifies dumpless1, an ecdysone signaling-induced ZAD-ZNF TF in Drosophila, as a key regulator of NC dumping through the integrin βPS-Rho1 signaling-p-MLC signaling pathway, which is vital for oocyte development. These shed light on the molecular mechanisms non-autonomously regulating NC dumping and somatic-germ cell communication in Drosophila oogenesis.
Methods
Drosophila stocks and genetics
All fly stocks were maintained on standard cornmeal-yeast-agar medium at 25 °C, unless otherwise specified. The Drosophila line w1118 was used as a wild-type control. The following strains were used to knockdown or overexpress genes of interest: UAS-dwg-RNAi (THU5807, TsingHua Fly Center), CG12942-RNAi (Bloomington Drosophila Stock Center (BDSC), stock #27077), UAS-EcR-DN (BDSC, stock #6869), UAS-mys-RNAi (BDSC, stock #27735), UAS-mys (BDSC, stock #68158), UAS-nls.GFP (BDSC, stock #4776), UAS-CG12942-3×HA (FlyORF, F001836) and UAS-mCD8-GFP (BDSC, stock #5131). The Tj-Gal4 strain, a gift from Dr W. Luo, was used to drive UAS-nls.GFP expression in MFCs, SFCs and border cells for RNA-seq experiments to identify TFs involved in NC dumping. The c329b-Gal4 (BDSC, stock #3746) was used to overexpress or knock down genes in SFCs. For enhanced RNAi expression, all RNAi experiments were performed at 29 °C. The EcRE-LacZ (BDSC, stock#4517), a transgenic reporter gene construct expressing β-galactosidase under the control of ecdysone response elements (EcREs), was used to assess EcR activity. The CG12942::GFP protein trap lines (BDSC, stock #66394) were obtained from BDSC by the model organism Encyclopedia of Regulatory Network (modERN) Project, aiming to generate GFP-tagged TF transgenic lines in D. melanogaster and Caenorhabditis elegans for the investigation of the function of single TFs61. The C-terminal “GFP. FPTB” tag, a superfolder GFP-FLAG-PreScission-TEV-BLRP tag combination, was introduced into clones from one of two P[acman] BAC libraries with average insert sizes of 30 or 80 kb containing TF genes of interest. The tagged clones were injected into embryos carrying the φC31 integrase system and attP docking sites on the second or third chromosome, facilitating target genomic integration of the entire BAC into docking sites. After screening, the transgenic lines were verified by PCR analysis to ensure the correct insertion of GFP-tagged TF62. To control temporal gene expression, flies carrying tublin-Gal80ts (BDSC, stock #7017 and #7019) were raised at 18 °C and then shifted to 29 °C after eclosion for 3–5 days or as mentioned otherwise. Mosaic analysis was performed by crossing the flip-out Gal4 line hsFLP; actin > CD2>Gal4, UAS-GFP/TB to the transgene lines of interest. Female adults were heat-shocked at 37 °C for 15 min after eclosion, and then recovered at 25 °C for 2 days before dissection of the mosaic egg chambers.
CRISPR/Cas9-mediated depletion of CG12942
Two sgRNA targeting sites (5’-CCGCCGTACCGGATCCCGCGCTC-3’ and 5’-GTGCCGAGCCTGTCTATCGGTGG-3’) in the first exon of the CG12942 gene were designed and synthesized for CRISPR/Cas9-mediated depletion. A mixture of sgRNA and Cas9 protein was injected into fertilized eggs of w1118 within 1 h after oviposition. Progenies with successful deletion of CG12942 were screened by sequencing and then crossed to a strain carrying a balancer to maintain the selected mutant lines. Primers used for sequencing are listed in Supplementary Data 1.
Construction of CG12942 promoter-Gal4 line
The DNA fragment that spans 2000 bp upstream and 144 bp downstream of the transcription start site of the dumpless1 gene was synthesized based on the nucleotide sequence from Flybase (FBgn0033569) and cloned into the pChs-Gal4 vector (Drosophila Genomics Resource Center, DGRC, #1218) using KpnI and Sac II restriction enzyme sites to generate a CG12942 promoter-Gal4 construct. A mixture of donor plasmid and helper plasmid was injected into fertilized eggs of w1118 and transgenic flies were produced by P-element-mediated insertion of the CG12942 promoter-Gal4 construct.
RNA-Seq and data analysis
To identify TFs involved in NC dumping, virgin Tj-Gal4 females were crossed to UAS-nls.GFP males, marking FCs with green fluorescence signals. Then ovaries from 3–5 day-old female progeny were dissected, and egg chambers were staged based on border cell migration and oocyte size. Staging criteria: Stage 9: Border cells migrate within the nurse cell cluster or contact the surface of the oocyte; Stage 10: Border cells migrate to the anterior surface of the oocyte, occupying half the egg chamber volume; Stage 11: The oocyte occupies over half of the volume of the egg chamber, while cytoplasm remains in NCs; Stage 12: The oocyte occupies almost the entire egg chamber, and no visible cytoplasm remains in NCs (Supplementary Fig. 2a). At least 100 egg chambers were collected in each biological replicate for RNA-seq analysis. To identify ecdysone signaling-mediated TFs during NC dumping, both Gal80ts; c329b> and Gal80ts; c329b > EcRDN flies were raised at 18 °C and shifted to 29 °C after eclosion for 40 h before dissection for RNA-seq. To investigate how dumpless1 regulated NC dumping, the ovaries of 7-day-old w1118 and dumpless1-/- females were dissected for RNA-Seq. RNA extraction and sequencing were performed by Majorbio Bio-pharm Technology Co., Ltd (Shanghai, China) using the Illumina Novaseq X Plus system. Clean reads were aligned to the Drosophila BDGP6.32 reference genome (http://asia.ensembl.org/Drosophila_melanogaster/Info/Index), and differential expression analysis was conducted using DESeq2 with criteria |Log2FoldChange | > 1 or 1.5 and p-value < 0.05. Data were analyzed using the Majorbio Cloud Platform (www.majorbio.com).
Developmental timing analysis
Eggs were collected at 25 °C within 2–4 h and incubated for 72 h to reach the 2nd instar larvae stage. Twenty larvae were transferred to a new vial and maintained at 25 °C. The number of pupariation and eclosion events was recorded daily for 6 independent experiments.
Egg size measurement
Virgin females were collected and kept in vials with fresh food. Yeast was added daily for 7 days before dissection. Ovaries were dissected in phosphate-buffered saline (PBS), and egg length was measured using Fiji software. All images were captured using a Nikon SMZ25 stereomicroscope.
Preparation of anti-CG12942 antibody
The anti-CG12942 antibody was generated for immunofluorescence and western blot analysis. To enable recombinant expression and purification, a DNA fragment encoding the first 280 amino acid residues of CG12942 was cloned into the pET28a-SUMO vector using EcoR I and Kpn I restriction sites. The specific primers used for cloning are provided in Supplementary Data 1. The purified protein was emulsified with Freund’s complete adjuvant and injected subcutaneously into New Zealand White rabbits. After four immunizations, serum was collected, and the polyclonal antibody was purified using affinity chromatography.
Immunofluorescence and microscopy
Newly eclosed virgin females of the desired phenotype were maintained with fresh food and yeast for 3–5 days before dissection. Ovaries were dissected in PBS and fixed in 4% paraformaldehyde (Cat#AR-0212, DINGGUO) at room temperature for 30-45 min, and blocked/permeabilized in PBT (0.5% BSA (Cat#9036-19-5, Sigma), 0.3% Triton X-100 (Cat#9048-46-8, Sigma) in PBS). Primary antibodies were incubated overnight at 4 °C. The following primary antibodies were used: anti-CG12942 antibody (1:2000, lab-made), anti-integrin βPS antibody (1:100, CF.6G11, Developmental Studies Hybridoma Bank (DSHB)), anti-Rho1 antibody (1:50, P1D9, DSHB), anti-p-MLC antibody (recognize Ser 21 p-MLC in Drosophila) (1:50, 3671S, Cell Signaling Technology), anti-EcR antibody (1:30, Ag10.2, DSHB), anti-EcRB1 antibody (1:50, AD4.4, DSHB) and anti-β-galactosidase (1:200, Z3781, Promega),anti-GFP antibody (1:200, YM3009, Immunoway). Secondary antibodies, including Alexa Fluor 488 goat anti-mouse IgG (1:1000, A-11029, Invitrogen), Alexa Fluor 488 goat anti-rabbit IgG (1:1000, A-11008, Invitrogen) and Alexa Fluor 594 goat anti-rabbit IgG (1:1000, A-11037, Invitrogen), were incubated for 2 hours at room temperature. Rhodamine-phalloidin (1:40, 40734ES75, Yeasen) was used to visualize actin fibers and DAPI (1:1000, Cat#4GD3410-1MG, Genview) for nuclear staining. Images were acquired with an Olympus Fluoview FV3000 confocal microscope.
Measurement method for fluorescence intensity of the cell cortex and plasma
Egg chambers of dumpless1-/- and dumpless1ΔZAD could not be characterized by the volume of NCs, so stages were defined based on the morphology of centripetal cells, roof cells and dorsal appendage tube mentioned as followed: stage 10B: a subset of follicle cells begins to elongation and migrate centripetally to invade between NCs and oocyte, cytoplasmic actin cables become visible; stage 11: centripetally cells completely cover the anterior of oocyte, while roof cells intercalate and constrict apically and expand basally; stage 12: dorsal appendage tube become visible.
All images in the same experiment were acquired with consistent parameters on the Olympus FV3000 confocal microscope. The quantification of fluorescence intensity was conducted by Image J. Actin in the NC cortex was used as an indicator of the NC cortex. After removing the noise of each image by applying Gaussian blur filters in ImageJ, the Morphological Segmentation tool of the plugin MorphoLibJ was utilized to segment individual NCs or SFCs and create the “mask” of each NC or SFC. The ROI of each NC cortex and plasma was acquired separately by the Morphological Filter tool of the plugin MorphoLibJ based on the “mask”, while for the ROI of SFCs, plasma was created directly by the “mask”. Mean fluorescence intensity was measured using the ROI created.
Protein extraction and western blot
Virgin females were maintained with fresh food and yeast for 3–5 days, and mated with males of relevant genotypes for 18-20 h. Ovaries were dissected in cold PBS. At least 450 egg chambers of stages 10-13 were collected and lysed in RIPA lysis buffer containing Phenylmethanesulfonyl fluoride (PMSF) (1:100, WB-0072, DINGGUO), followed by 2 min grinding and 3 min sonication. Protein concentration was determined by the BCA Assay Kit (20201ES86, Yeasen). Protein from egg chambers (80 μg total protein/lane) was separated by SDS-PAGE (20324ES62, Yeasen) and transferred to 0.45 μm PVDF membranes (IPVH00010, Millipore). Membranes were blocked using 5% (w/v) skimmed milk in Tris-buffered saline (ST661, Beyotime) with Tween 20 (TBST) (CAS#9005-64-5, Sigma), then incubated overnight with primary antibody at 4 °C. After washing with TBST, membranes were incubated with relevant horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. Chemiluminescence reaction was conducted using SuperPico ECL Chemiluminescence Kit (E422-02, Vazyme), and membranes were exposed on BIO-RAD ChemiDocTM Touch Imaging System. The following antibodies were used: anti-CG12942 antibody (1:2000, lab-made), anti-α tubulin (1:3000, AF5012, Beyotiome Biotechnology), HRP-labeled goat anti-rabbit IgG (H + L) (1:2000, A0208, Beyotiome Biotechnology).
Live imaging of NC dumping
Preparation of live imaging medium: Supplement Schneider’s Drosophila Medium (21720024, Gibco) with 15% vol/vol FBS (A5256901, Gibco) and 0.6×penicillin/streptomycin (15140122, Gibco). Adjust solution pH to 6.95-7.00. Just before use, add insulin (Cat#11061-68-0, Sigma) to the medium to a final concentration of 0.2 mg/mL and warm up the medium to room temperature. 3-day ovaries were dissected in live imaging medium within 20 minutes. Egg chambers at stage 10 were stained with CellMask Plasma Membrane Stains (1:1000, C10046, Invitrogen) in live imaging medium for 10 min at 30 °C. After rinsing in live imaging medium 3 times, egg chambers were mounted on glass-bottom dishes covered with a coverslip to prevent evaporation of live imaging medium and imaged immediately. Time-lapse movies were recorded every 2 minutes. The ratio of NC volume to total egg chamber volume (NC/EC) was calculated at 6-minute intervals for a total duration of 180 minutes using the Fiji software package. Live imaging protocol refers to Reference 63.
Subcellular localization of dumpless1 in the Kc cell line
For the subcellular localization of dumpless1, the coding sequence of dumpless1 fused with GFP and the ZAD deleted form of dumpless1 fused with GFP sequence were cloned into pAc5.1/V5-His vector (#V4410-20, Invitrogen) to generate two expression vectors: pdumpless1-GFP and pdumpless1ΔZAD-GFP. Primers used for cloning are listed in Supplementary Data 1. Kc cells were transfected with recombinant plasmids using FuGENE HD reagent (E2311, Promega), and cells were fixed in 4% paraformaldehyde for 30 minutes, washed in PBS and stained with DAPI post 60 h transfection. Images were acquired using an Olympus Fluoview FV3000 confocal microscope.
Statistics and reproducibility
All experiments included at least 3 individual biological repeats. Statistical analysis of the data was performed using Student’s t-test or two-way ANOVA. For the t-test: *p < 0.05; **p < 0.01; ***p < 0.001. The data are presented as mean ± SD or ±SEM. The statistical data of all images are represented in Supplementary Data 1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA sequencing data generated in this study have been deposited in the NCBI Sequence Read Archive (SRA) under accession numbers PRJNA1182949, PRJNA1183546 and PRJNA1183641. Additional datasets, including individual values, mean values, and exact p-values for all charts, are provided in Supplementary Data 1. All other relevant data and materials are available from the corresponding authors upon reasonable request.
References
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Rouault, H. & Hakim, V. Different cell fates from cell-cell interactions: core architectures of two-cell bistable networks. Biophys. J. 102, 417–426 (2012).
Zhou, X. et al. Circuit design features of a stable two-cell system. Cell 172, 744–757 (2018).
Dimitrov, D. et al. Comparison of methods and resources for cell-cell communication inference from single-cell RNA-Seq data. Nat. Commun. 13, 3224 (2022).
Armingol, E., Officer, A., Harismendy, O. & Lewis, N. E. Deciphering cell-cell interactions and communication from gene expression. Nat. Rev. Genet. 22, 71–88 (2021).
Spradling, A. The Development of Drosophila melanogaster. Vol.I (Cold Spring Harbor Laboratory Press, 1993).
McLaughlin, J. M. & Bratu, D. P. Drosophila melanogaster oogenesis: an overview. Methods Mol. Biol. 1328, 1–20 (2015).
Nicolas, E., Chenouard, N., Olivo-Marin, J. C. & Guichet, A. A dual role for actin and microtubule cytoskeleton in the transport of Golgi units from the nurse cells to the oocyte across ring canals. Mol. Biol. Cell 20, 556–568 (2009).
Mahajan-Miklos, S. & Cooley, L. Intercellular cytoplasm transport during Drosophila oogenesis. Dev. Biol. 165, 336–351 (1994).
Wheatley, S., Kulkarni, S. & Karess, R. Drosophila nonmuscle myosin II is required for rapid cytoplasmic transport during oogenesis and for axial nuclear migration in early embryos. Development 121, 1937–1946 (1996).
Tan, C., Stronach, B. & Perrimon, N. Roles of myosin phosphatase during Drosophila development. Development 130, 671–681 (2003).
Huelsmann, S., Ylänne, J. & Brown, N. H. Filopodia-like actin cables position nuclei in association with perinuclear actin in Drosophila nurse cells. Dev. Cell. 26, 604–615 (2013).
Imran Alsous, J. et al. Dynamics of hydraulic and contractile wave-mediated fluid transport during Drosophila oogenesis. Proc. Natl Acad. Sci. USA 118, e2019749118 (2021).
Timmons, A. K. et al. Phagocytosis genes nonautonomously promote developmental cell death in the Drosophila ovary. Proc. Natl Acad. Sci. USA 113, E1246–E1255 (2016).
Domanitskaya, E., Anllo, L. & Schüpbach, T. Phantom, a cytochrome P450 enzyme essential for ecdysone biosynthesis, plays a critical role in the control of border cell migration in Drosophila. Dev. Biol. 386, 408–418 (2014).
Knapp, E. & Sun, J. Steroid signaling in mature follicles is important for Drosophila ovulation. Proc. Natl Acad. Sci. USA 114, 699–704 (2017).
Ables, E. T. & Drummond-Barbosa, D. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell 7, 581–592 (2010).
König, A., Yatsenko, A. S., Weiss, M. & Shcherbata, H. R. Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation. EMBO J. 30, 1549–1562 (2011).
Buszczak, M. et al. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development 126, 4581–4589 (1999).
Romani, P. et al. Cell survival and polarity of Drosophila follicle cells require the activity of ecdysone receptor B1 isoform. Genetics 181, 165–175 (2009).
Hackney, J. F., Pucci, C., Naes, E. & Dobens, L. 2007. Ras signaling modulates activity of the ecdysone receptor EcR during cell migration in the Drosophila ovary. Dev. Dyn. 236, 1213–1226 (2007).
Uryu, O., Ameku, T. & Niwa, R. Recent progress in understanding the role of ecdysteroids in adult insects: germline development and circadian clock in the fruit fly Drosophila melanogaster. Zool. Lett. 1, 32 (2015).
Bernardi, F., Romani, P., Tzertzinis, G., Gargiulo, G. & Cavaliere, V. EcR-B1 and Usp nuclear hormone receptors regulate expression of the VM32E eggshell gene during Drosophila oogenesis. Dev. Biol. 328, 541–551 (2009).
Koelle, M. R. et al. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell 67, 59–77 (1991).
Yao, T. P., Segraves, W. A., Oro, A. E., McKeown, M. & Evans, R. M. Drosophila ultraspiracle modulates ecdysone receptor function via heterodimer formation. Cell 71, 63–72 (1992).
Zhu, Z. et al. 20E-mediated regulation of BmKr-h1 by BmKRP promotes oocyte maturation. BMC Biol. 19, 39 (2021).
Talbot, W. S., Swyryd, E. A. & Hogness, D. S. Drosophila tissues with different metamorphic responses to ecdysone express different ecdysone receptor isoforms. Cell 73, 1323–1337 (1993).
Kozlova, T. & Thummel, C. S. Essential roles for ecdysone signaling during Drosophila mid-embryonic development. Science 301, 1911–1914 (2003).
Manseau, L. et al. GAL4 enhancer traps expressed in the embryo, larval brain, imaginal discs, and ovary of Drosophila. Dev. Dyn. 209, 310–322 (1997).
Lebo, D. P. V. & McCall, K. Murder on the ovarian express: a tale of non-autonomous cell death in the Drosophila ovary. Cells 10, 1454 (2021).
Chen, B., Harms, E., Chu, T., Henrion, G. & Strickland, S. Completion of meiosis in Drosophila oocytes requires transcriptional control by grauzone, a new zinc finger protein. Development 127, 1243–1251 (2000).
Yanicostas, C., Ferrer, P., Vincent, A. & Lepesant, J. A. Separate cis-regulatory sequences control expression of serendipity beta and janus A, two immediately adjacent Drosophila genes. Mol. Gen. Genet. 246, 549–560 (1995).
Antoniewski, C., Laval, M. & Lepesant, J. A. Structural features critical to the activity of an ecdysone receptor binding site. Insect Biochem. Mol. Biol. 23, 105–114 (1993).
Agarwal, P. & Zaidel-Bar, R. Principles of actomyosin regulation in vivo. Trends Cell Biol. 29, 150–163 (2019).
Schmidt, A., Li, L., Lv, Z., Yan, S. & Großhans, J. Dia- and Rok-dependent enrichment of capping proteins in a cortical region. J. Cell Sci. 134, jcs258973 (2021).
Jordan, P. & Karess, R. Myosin light chain-activating phosphorylation sites are required for oogenesis in Drosophila. J. Cell Biol. 139, 1805–1819 (1997).
Huang, Y. C. et al. βPS-Integrin acts downstream of Innexin 2 in modulating stretched cell morphogenesis in the Drosophila ovary. G3 11, jkab215 (2021).
Qin, X. et al. Cell-matrix adhesion and cell-cell adhesion differentially control basal myosin oscillation and Drosophila egg chamber elongation. Nat. Commun. 8, 14708 (2017).
Minestrini, G., Máthé, E. & Glover, D. M. Domains of the Pavarotti kinesin-like protein that direct its subcellular distribution: effects of mislocalisation on the tubulin and actin cytoskeleton during Drosophila oogenesis. J. Cell Sci. 115, 725–736 (2002).
Carney, G. E. & Bender, M. The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics 154, 1203–1211 (2000).
Terashima, J. & Bownes, M. E75A and E75B have opposite effects on the apoptosis/development choice of the Drosophila egg chamber. Cell Death Differ. 13, 454–464 (2006).
Sieber, M. H. & Spradling, A. C. Steroid signaling establishes a female metabolic state and regulates SREBP to control oocyte lipid accumulation. Curr. Biol. 25, 993–1004 (2015).
Jang, A. C., Chang, Y. C., Bai, J. & Montell, D. Border-cell migration requires integration of spatial and temporal signals by the BTB protein abrupt. Nat. Cell Biol. 11, 569–579 (2009).
Sun, J., Smith, L., Armento, S. & Deng, W. M. Regulation of the endocycle/gene amplification switch by Notch and ecdysone signaling. J. Cell Biol. 182, 885–896 (2008).
Schwartz, M. B., Kelly, T. J., Woods, C. W. & Imberski, R. B. Ecdysteroid fluctuations in adult Drosophila melanogaster caused by elimination of pupal reserves and synthesis by early vitellogenic ovarian follicles. Insect Biochem. 19, 243–249 (1989).
Lu, W., Lakonishok, M., Serpinskaya, A. S. & Gelfand, V. I. A novel mechanism of bulk cytoplasmic transport by cortical dynein in Drosophila ovary. Elife 11, e75538 (2022).
Quinlan, M. E. Cytoplasmic Streaming in the Drosophila Oocyte. Annu. Rev. Cell Dev. Biol. 32, 173–195 (2016).
Chung, H. R., Schäfer, U., Jäckle, H. & Böhm, S. Genomic expansion and clustering of ZAD-containing C2H2 zinc-finger genes in Drosophila. EMBO Rep. 3, 1158–1162 (2002).
Chung, H. R., Löhr, U. & Jäckle, H. Lineage-specific expansion of the zinc finger associated domain ZAD. Mol. Biol. Evol. 24, 1934–1943 (2007).
Shapiro-Kulnane, L., Bautista, O. & Salz, H. K. An RNA-interference screen in Drosophila to identify ZAD-containing C2H2 zinc finger genes that function in female germ cells. G3 11, jkaa016 (2021).
Romani, P., Gargiulo, G. & Cavaliere, V. The ecdysone receptor signalling regulates microvilli formation in follicular epithelial cells. Cell Mol. Life Sci. 73, 409–425 (2016).
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Gimond, C. et al. Induction of cell scattering by expression of beta1 integrins in beta1-deficient epithelial cells requires activation of members of the rho family of GTPases and downregulation of cadherin and catenin function. J. Cell Biol. 147, 1325–1340 (1999).
Arthur, W. T., Petch, L. A. & Burridge, K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719–722 (2000).
Tomar, A. & Schlaepfer, D. D. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr. Opin. Cell Biol. 21, 676–683 (2009).
Rosa-Birriel, C., Malin, J. & Hatini, V. Medioapical contractile pulses coordinated between cells regulate Drosophila eye morphogenesis. J. Cell Biol. 223, e202304041 (2024).
Wong, P. P. et al. Cancer burden is controlled by mural cell-β3-integrin regulated crosstalk with tumor cells. Cell 181, 1346–1363.e21 (2020).
Shao, M. Y. et al. β-Catenin and Rho GTPases as downstream targets of TGF-β1 during pulp repair. Cell Biol. Int. 35, 105–109 (2011).
Jauch, R. et al. The zinc finger-associated domain of the Drosophila transcription factor grauzone is a novel zinc-coordinating protein-protein interaction module. Structure 11, 1393–1402 (2003).
Bonchuk, A. et al. Structural basis of diversity and homodimerization specificity of zinc-finger-associated domains in Drosophila. Nucleic Acids Res. 49, 2375–2389 (2021).
Kudron, M. M. et al. The ModERN Resource: genome-wide binding profiles for hundreds of Drosophila and Caenorhabditis elegans transcription factors. Genetics 208, 937–949 (2018).
Venken, K. J. et al. BAC libraries for transgenesis studies in Drosophila melanogaster. Nat. Methods 6, 431–434 (2009). acman.
Prasad, M., Jang, A. C., Starz-Gaiano, M., Melani, M. & Montell, D. J. A protocol for culturing Drosophila melanogaster stage 9 egg chambers for live imaging. Nat. Protoc. 2, 10 (2007).
Acknowledgements
We thank Dr. Sheng Li and Dr. Suning Liu of South China Normal University, Dr. Xuan Guo for sharing fly stocks, and Dr. Wei Luo of South China Normal University for technical support. We also thank Bloomington Drosophila Stock Center, Vienna Drosophila Resource Center and Tsinghua Fly Center for providing fly stocks. We thank the Core Facility of Drosophila Resource and Technology (CEMCS, CAS) and Qidong Fungene Biotechnology for generating transgenic flies. This study was supported by a research grant from the Chinese National Natural Science Foundation (No. 32170494) to H. Deng.
Author information
Authors and Affiliations
Contributions
H.D. designed and monitored the project. J.L. performed the most experiments. Z.P. performed the localization of EcR, EcRB1 and EcRE-lacZ experiments. X.P. performed the RNAi screening experiment. F.L. performed the subcellular localization of Dumpless1 in Kc cells. J.L., J.W. and Y.F. interpreted the data. Q.F. and X.Y. supervised the project or related experiments and revised the manuscript. H.D. wrote and finalized the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Pan, Z., Peng, X. et al. Ecdysone signaling-induced dumpless1 expression controls nurse cell dumping in Drosophila oogenesis. Nat Commun 16, 8917 (2025). https://doi.org/10.1038/s41467-025-63973-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63973-3